Summary
Regenerative medicine affords a promising therapeutic strategy for the treatment of patients with chronic kidney disease. Nephron progenitor cell populations exist only during embryonic kidney development. Understanding the mechanisms by which these populations arise and differentiate is integral to the challenge of generating new nephrons for therapeutic purposes. Pluripotent stem cells (PSCs), comprising embryonic stem cells, and induced pluripotent stem cells (iPSCs) derived from adults, have the potential to generate functional kidney cells and tissue. Studies in mouse and human PSCs have identified specific approaches to the addition of growth factors, including Wnt and fibroblast growth factor, that can induce PSC differentiation into cells with phenotypic characteristics of nephron progenitor populations with the capacity to form kidney-like structures. Although significant progress has been made, further studies are necessary to confirm the production of functional kidney cells and to promote their three-dimensional organization into bona fide kidney tissue. Human PSCs have been generated from patients with kidney diseases, including polycystic kidney disease, Alport syndrome, and Wilms tumor, and may be used to better understand phenotypic consequences of naturally occurring genetic mutations and to conduct “clinical trials in a dish”. The capability to generate human kidney cells from PSCs has significant translational applications, including the bioengineering of functional kidney tissue, use in drug development to test compounds for efficacy and toxicity, and in vitro disease modeling.
Keywords: Pluripotent stem cell, embryonic stem cell, iPS cell, kidney on a chip, kidney development, kidney cell differentiation
Approximately 2,000,000 people worldwide suffer from end-stage renal disease. Existing strategies for renal replacement therapy include dialysis and transplantation. Although these treatment options can prolong survival, both have serious limitations. Dialysis is associated with significant morbidity, and mortality rates are 6.5 to 7.9 times greater than the general population. Although dialysis functions to remove waste products and help maintain electrolyte, acid-base, and fluid balance, it cannot substitute all of the kidneys’ functions. Kidneys are the most frequently transplanted organs, but demand greatly outweighs supply and the waiting period is typically several years. Acute rejection occurs in approximately 15% of transplant cases, and successfully transplanted kidneys require life-long immunosuppression to reduce the rate of chronic rejection.1 For these reasons, kidney regeneration from human stem cells, ideally patient immunocompatible, is an attractive solution for renal replacement.
FEATURES OF NEPHROGENIC STEM CELLS
The adult mammalian kidney lacks the capacity to generate entirely new nephrons or to regenerate segments from resected nephrons. Nephrogenesis ceases before or shortly after birth in human beings and mice, concomitant with the disappearance of the nephron progenitor pool.2–4 Nevertheless, adult kidney epithelial cells are capable of extensive dedifferentiation, proliferation, and repopulation of damaged tubules after injury.5,6 These processes make it possible for existing nephrons to recover after acute kidney injury but do not provide a source for new nephrons. Clinically, the ramifications of this are that human beings are born with a limited number of nephrons, which can be damaged irreversibly. The loss of a significant number of nephrons is therefore an event with important long-term consequences.
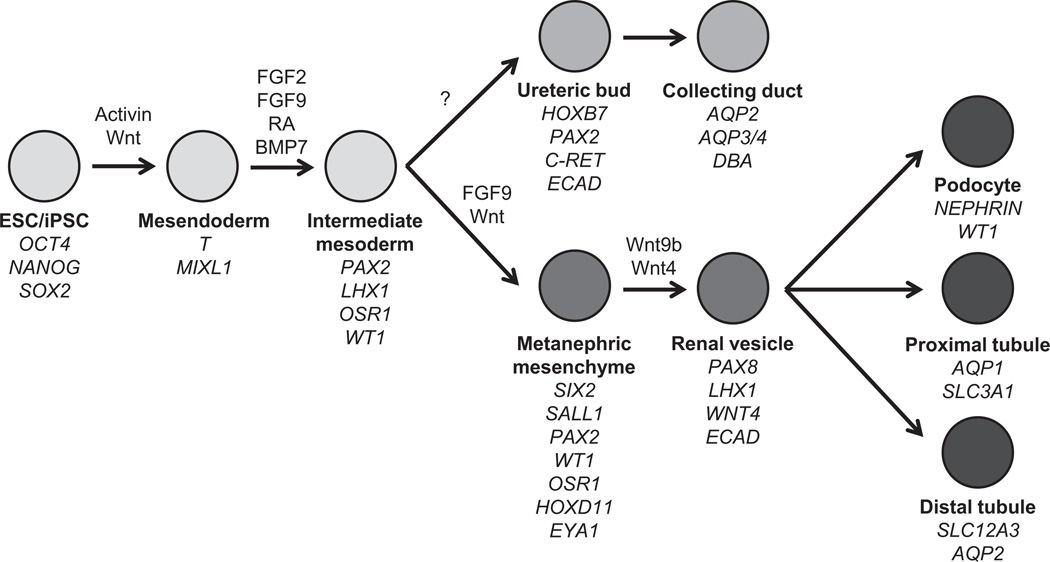
Because embryonic kidney cells are the only cells capable of forming new nephrons, derivation of such cells is likely to be required to create new nephrons for patients with kidney disease. Understanding nephron development in the embryo therefore is essential to designing and interpreting experiments that aim to generate new nephrons in the adult. Mammalian kidney development has been summarized elsewhere in greater detail.3,7 The pronephric duct arises from the intermediate mesoderm (IM) of the embryo at approximately day 22 of gestation in human beings, or day 8 in mice. The duct iteratively invades the adjacent mesenchyme, giving rise sequentially to the pronephros, mesonephros, and, finally, the metanephros, which in amniotes will develop into the adult kidney. Formation of the complete nephron tubule involves reciprocal interactions between two tissues, the ureteric bud (UB) and the metanephric mesenchyme (MM).8 Molecular analysis over the past 60 years has identified a variety of signaling molecules that specify these tissues (including Wnts, fibroblast growth factors [FGFs], bone morphogenetic proteins [BMPs], glial cell-derived neurotrophic factor, and hepatocyte growth factor [HGF]), as well as transcription factors (WT1, SIX1, SIX2, PAX2, PAX8, and H0XD11) that regulate branching morphogenesis and maintain the nephron progenitor cell pool.3,7,9 In addition to the tubular components, the patterned kidney also includes a significant population of vascular and interstitial cells in a complex three-dimensional (3D) configuration.
Decades of experiments on isolated metanephroi have established a framework for understanding the characteristics of these cells and their potential for regeneration. When cultured together ex vivo, UB and MM can undergo branching nephrogenesis. This ability to self-organize makes it feasible to envision nephron regeneration from these embryonic cell types. When implanted into living hosts, fetal kidney cells isolated from various mammals, including human beings, have been reported to form new vascularized nephrons that can produce urine and prolong survival after nephrectomy.10–3 Notably, the ability of meta-nephric tissue to undergo branching nephrogenesis requires both the UB and the MM; when separated from each other, the UB does not branch, and the MM does not survive unless it is co-cultured with appropriate inducing tissues such as embryonic spinal cord.14 Various survival and differentiation factors have been identified for metanephric cells, notably BMPs and FGFs, although long-term cultivation of these cells in vitro remains a challenge for the field.3,15,16
PLURIPOTENT STEM CELLS
Pluripotent stem cells (PSCs) are cultured populations of early embryonic progenitors, believed to represent blastocyst or epiblast cell types from which the entire soma is derived. They are defined by two characteristics: pluripotency, the ability to give rise to diverse and complex tissues from each of the three embryonic germ layers; and self-renewal, the capacity to proliferate and expand indefinitely in culture without transformation. Because adult mammalian kidney cells cannot regenerate new nephrons,5 and fetal kidney cells undergo apoptosis or differentiate in culture,3,16 PSCs are currently the only long-term cultivatable cell type capable of neonephrogenesis. The most convincing demonstration of this has been in cloned mice, in which the entire soma, including the kidneys, can be derived from cultured PSCs via tetraploid complementation.17,18
PSCs include both embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs). ESCs are grown in primary culture after derivation from embryos, whereas iPSCs are cells derived from the adult that transcriptionally have been reprogrammed to an ESC-like state. iPSCs greatly resemble ESCs, although some differences may exist between the two cell types.19 Patient-derived iPSCs have dual value as a laboratory resource for studying the cellular basis of human disease and as a potential source of immunocompatible replacement tissue.20–23 Because they are pluripotent and self-renewing, iPSCs provide a practically unlimited opportunity to obtain diverse cell types and tissues with naturally occurring human disease mutations for experimental investigation. This constitutes a great advantage over many primary cell types in culture, which are difficult to obtain and often senesce or dedifferentiate rapidly.21 Studies in mice show that somatic cells derived from iPSCs are immunocompatible with the hosts from which they derive.24 In theory, iPSCs could be used to generate tissues or organs that would be immunocompatible with the individual from whom they were derived. These characteristics were considered so significant that the discovery of iPSCs was co-awarded the Nobel Prize in Medicine in 2012, only 6 years after its initial publication.25 Although the application of iPSCs to kidney research is still in its infancy, recent progress has suggested utility for both disease modeling26 and regenerative27–31 approaches.
METHODS OF DIFFERENTIATION OF PSCs
By using biochemical treatment regimens, PSCs have been differentiated successfully into various types of cells and tissues, including hepatic, neural, hematologic, pancreatic, and cardiac lineages. The various approaches have been reviewed elsewhere.32 When PSCs are dissociated and deprived of growth factors that sustain pluripotency (FGFs and activin in human PSCs under standard growth conditions, or leukemia-inhibitory factor in mouse PSCs), they undergo stochastic differentiation into embryoid bodies (EBs) in vitro or teratomas in vivo.21,33 These 3D growths include diverse somatic cell types and tissues representative of the three embryonic germ layers and can be used as an indicator of pluripotency of the PSCs. Histologic and immunohistochemical examination of these stochastic growths suggests the possibility that PSCs can differentiate stochastically into kidney cells and tissues,33,34 although the markers and structures observed in these studies also may be observed in cells from other somatic lineages.
Directed differentiation approaches involve the sequential application of chemicals or growth factors to PSCs to reproducibly obtain differentiated descendants, ideally in high yield and of significant purity (Fig. 1). The majority of existing protocols use embryonic development as a roadmap for differentiation, subdividing organogenesis into a series of discrete stages. When known, developmental growth factor signals or inhibitors are used to manipulate cells from one stage to the next, and the progress of differentiation is tracked by monitoring the expression of markers that are characteristic of each stage of differentiation. Unbiased, large-scale chemical screens also have identified novel potent factors that can be used to substitute or complement the use of exogenous growth factors.21 Directed differentiation is more reproducible and efficient than stochastic differentiation and is preferable both for molecular investigations as well as for therapeutic applications. Ultimately, successful directed differentiation must be tested and optimized empirically using an assay readout that has been proven to characterize the cell type of interest reliably. Technically, directed differentiation approaches can be applied to two-dimensional monolayers or to 3D embryoid bodies. Monolayer approaches offer an increased measure of reproducibility and control, whereas EB approaches are ideal for long-term cultures and the formation of complex 3D tissues.35
Figure 1.
Directed differentiation of hPSCs into cells of the kidney lineage. Stepwise differentiation of hPSCs into embryonic and adult kidney cell types. Growth factors that have been shown to induce differentiation toward specific stages and markers characteristic of each stage of are shown.
DIFFERENTIATION OF MOUSE ESCs INTO CELLS OF KIDNEY LINEAGE
The earliest studies to generate cells of the kidney lineage were performed using mouse ESCs (mESCs) (Table 1). EBs formed from mESCs, differentiated in the presence of serum, expressed multiple kidney developmental genes, including Pax2, WT1, Lhxl, Emx2, c-ret, and Salll.36 In vivo transplantation of the cells from these EBs into the mouse retroperitoneum yielded teratoma-like growths containing structures co-expressing DBA and Pax2.36 The ability of undifferentiated mESCs to respond to developmental signals also was tested by microinjecting them into E12 to E13 mouse metanephric kidney cultures, where they were observed to integrate into tubular-like epithelial structures, some of which bound Lotus tetragonolobus lectin (LTL) and expressed Na+/K+-adenosine triphosphatase.37 Subsequent studies relied less on stochastic differentiation and focused on the use of developmental growth factors to more specifically direct kidney lineage specification. Relatively little is known about the precise signals required to differentiate PSCs into the earliest stages of kidney lineage. Retinoic acid (RA) and activin were selected on the basis of their ability to expand the pronephric field in developmental studies in Xenopus,38 and BMP signals were selected for their mesoderm-inducing properties in ESCs.39,40 In one of the first reports to test combinations of these factors to induce kidney differentiation, mouse EBs treated with RA, activin, and BMP7 expressed the IM markers Pax2, WT1, and Lhxl and formed tubular structures in vitro.41 When micro-injected into E12.5 mouse embryonic kidney cultures, cells from these growth factor-treated EBs integrated into laminin-bound, LTL+ tubular structures, although the investigators noted that cells from untreated control EBs also were capable of tubular integration to a lesser extent.41 Most subsequent studies have tested combinations of one or more of these growth factors, with varying degrees of success.28,31,42–48 Small-molecule chemicals, which frequently have the advantage of being more potent and stable compounds, also have been used to complement or substitute peptide growth factors in more recent efforts at directed differentiation.28,31,49
Table 1.
Directed Differentiation of Mouse PSCs Into Kidney Cells
| Study | Starting Substrate | Culture Method | Differentiation Protocol |
Differentiation End Point |
Findings |
|---|---|---|---|---|---|
| Steenhard et al,37 2005 |
ROSA26 mESCs | Injection into E12–13 kidneys (3–5 d) |
Integration into tubules |
Integration into tubular structures expressing LTL and Na+/K+-adenosine triphosphatase |
|
| Kim and Dressier,41 2005 |
ROSA26 mESCs | EB formation | 10% FBS without LIF (2 d) 10% FBS + RA/ activin/BMP7 (5 d) |
Intermediate mesoderm |
Expression of Pax2, WT1, and Lhx1 in EBs by RT-PCR Expression of Pax2 in EBs by IF Integration into LTL+ tubular structures when injected into E12.5 kidneys |
| Kobayashi et al,42 2005 |
Wnt4-expressing mESCs |
EB formation | 15% FCS without LIF (2 d) 15% FCS + HGF/ activin (20 d) |
AQP2+ cells in EBs | Expression of AQP2 by RT-PCR Expression of AQP2 by IF Formation of tubular- like structures in 3D culture |
| Bruce et al,43 2006 | Pax2-GFP mESCs | EB formation | Serum-free media + BMP4/LIF (16 d) |
Expression of Pax2 in EBs by IF |
|
| Yamamoto et al,36 2006 |
mESCs | EB formation | Teratoma formation (2–4 wk) |
Formation of tubular- like structures expressing Pax2 and DBA |
|
| Vigneau et al,44 2007 |
T-GFP mESCs | EB formation | Serum-free media + activin (4 d) |
T (Brachyury)+ mesoderm |
T+-GFP cells express cadherin-11, WT1, Pax2, and Wnt4 by RT-PCR T+-GFP cells injected into E11.5 kidneys integrate into the nephrogenic zone T+-GFP cells injected into newborn mice kidneys integrate into proximal tubules |
| Nakane et al,50 2009 |
mESCs with tetracycline- inducible Pax2 |
EB formation | Pax2 overexpression | Expression of AQP1 in EBs by IF Expression of Pax2 and integrin α8 by RT-PCR |
|
| Morizane et al,45 2009 |
mESCs mi PSCs |
EB formation | 10% FCS (3 d) 10% FCS + GDNF or BMP7 or activin (15 d) |
GDNF and BMP7 induced expression of Pax2 and WT1 Activin induced expression of KSP |
|
| Ren et al,46 2010 | mESCs | EB formation | 15% FCS without LIF (2 d) 15% FCS + activin/ RA (6 d) Conditioned media from UB (10 d) |
Renal lineage cells | Expression of Pax2 by IF for IM differentiation Expression of WT1, E-cadherin, POD-1, and Pax2 by IF for renal lineage differentiation |
| Mae et al,49 2010 | mESCs | Monolayer | 2% FBS + JAK-inh/ LY294002/ CG1423/RA (8 d) |
Intermediate mesoderm |
Expression of Osr1 and Pax2 by IF |
| Nishikawa et al,47 2011 |
mESCs | Monolayer | 10% FBS + activin (2 d) 10% FBS + activin/ BMP4 (2 d) 10% FBS + activin/ BMP4/LiCl (2 d) 10% FBS + activin/ BMP4/LiCl/RA (2 d) Conditioned media from UB and MM cells |
UB-conditioned media induced MM markers. MM-conditioned media induced UB markers |
Expression of T by RT- PCR for PS differentiation Expression of Pax2 and Lhx1 by RT-PCR for IM differentiation Expression of GDNF, WT, and cadherin-11 by RT-PCR for MM differentiation Expression of HoxB7, Wnt11, and c-Ret9 for UB differentiation |
| Morizane et al,48 2013 |
mESCs | EB formation Monolayer |
10% FCS + activin (3 d) 10% FCS + activin/ HGF or activin/IGF (15 d) |
Ksp+ cells | Ksp+ cells by FACS Formation of tubular- like structures in Matrigel with expression of kidney markers by IF and RT-PCR Integration of Ksp+ cells into tubular structures of E13.5 kidneys |
| Taguchiet al,31 2013 |
mESCs hiPSCs |
EB formation | Serum-free media + activin (1 d) Serum-free media + BMP4/CHIR (2.5 d) Serum-free media + activin/BMP4/ CHIR/ RA/Y27632 (1 d) Serum-free media + CHIR/FGF9/ Y27632 (2 d) |
Metanephric mesenchyme |
Nephron precursors derived from OSR1+ posterior IM and T+ posterior mesoderm Expression of Osr1, WT1, Pax2, Six2, and Sall1 by IF and RT-PCR Co-culture of induced EBs resulted in formation of glomerular-like and tubular-like structures expressing kidney markers Protocol reproducible in hiPSCs with longer time course of differentiation |
FACS, fluorescence-activated cell sorting; FBS, fet al bovine serum; FCS, fet al calf serum; GDNF, glial cell–derived neurotrophic factor; KSP, kidney-specific protein; LIF, leukemia-inhibitory factor; RT-PCR, reverse-transcription polymerase chain reaction.
Two studies have tested conditioned medium from embryonic kidney cells as a supplement to growth factor treatment, with the rationale that this media could contain the correct factors necessary to properly induce kidney differentiation. In one study, mesoderm differentiation was induced with activin and RA, then switched to conditioned media collected from UB cells. After an additional 10 days of differentiation, WT1, PAX2, POD-1, and E-cadherin expression was documented by immunocytochemistry, although the efficiency of marker expression was low.46 Similarly, treatment of mESCs with activin, BMP4, LiCl (to activate Wnt signaling), and RA, followed by conditioned media harvested from UB and MM cell lines, resulted in an up-regulation of MM and UB markers, respectively.47 As an alternative to growth factor or chemical treatment of mESCs, other groups have tried to promote kidney differentiation in mESCs by the overexpression of kidney developmental genes in EBs. EBs formed from Wnt4-transformed mESCs expressed aquaporin-2 in the presence of HGF and activin and could give rise to tubular-like structures in 3D culture.42 Overexpression of Pax2 in EBs resulted in the up-regulation of integrin α8 and aquaporin-1, although this analysis was limited to messenger RNA expression by semiquantitative reverse-transcription polymerase chain reaction and immunostaining only for aquaporin-1 (AQP1).50
Recently, Taguchi et al31 used a developmental strategy by identifying and isolating putative precursor populations in early stages of mouse embryogenesis that could give rise to nephron progenitor cells of the metanephric mesenchyme. By using lineage-tracing methods, the investigators showed that metanephric mesenchyme precursors could be traced back to OSR1+ posterior IM at E9.5 and T (Brachyury)+ posterior mesoderm at E8.5. Importantly, they showed that the MM and UB originate from posterior and anterior regions of the nascent mesoderm, respectively, and that this separation of fate is determined early in the postgastrulation stage. By first identifying sequences of growth factor and small-molecule combinations needed to induce MM differentiation from T+ posterior mesoderm, the investigators then were able to establish multistep protocols to differentiate both mESCs and hiPSCs into EBs that immunostained positive for multiple markers of MM, including PAX2, SIX2, SALL1, and WT1. Despite the well-established differences between the biology and differentiation kinetics of mouse and human PSCs,51–54 protocols were similar and fairly reproducible for both mouse and human PSCs, with the human iPSC (hiPSC) protocol requiring 14 days compared with 8.5 days for mESCs. When the EBs containing nephron progenitors were co-cultured with mouse embryonic spinal cord, a well-established inducer of kidney tubulogenesis, there was formation of 3D tubular structures with marker expression characteristic of renal tubules and glomeruli.31
DIFFERENTIATION OF HUMAN ESCs AND IPSCs INTO CELLS OF THE KIDNEY LINEAGE
Compared with studies using mESCs, only recently have there been a number of reports on the directed differentiation of human ESCs (hESCs) into kidney cells (Table 2). Translation of mESC differentiation protocols for use with hPSCs frequently has required modification owing in part to fundamental differences between the blastocyst-like, leukemia-inhibitory factor maintained “ground state” of naive pluripotency typical of mESCs, and the epiblast-like, FGF/activin-maintained “primed state” of pluripotency typical of hPSCs,51–54 as well as differences in culture media and growth factors required for maintenance. Since the discovery of hiPSCs,20 which has facilitated and promoted more widespread study of hPSCs worldwide, directed differentiation using hESC and hiPSC systems has taken precedence over work with mPSCs. Protocols established using hESCs and hiPSCs derived from healthy individuals have potential clinical application, and readily can be applied to disease-specific hiPSC lines for the purposes of disease modeling in human cells. One of the first reports describing the successful derivation of hESCs from human embryos noted structures in teratomas that appeared to resemble fetal glomeruli.33 In an effort to document the effects of eight different growth factors on the differentiation of hESCs, treatment with factors such as HGF, nerve growth factor, and activin could induce the expression of WT1 and RENIN, providing an early description of the expression of kidney markers in differentiating hESCs.55 By using both EB and monolayer culture methods, growth factor combinations of RA, activin, and BMP7 were tested in the presence of 10% fetal bovine serum and induced an up-regulation of messenger RNA levels of kidney developmental markers, including PAX2, WT1, OSR1, EYA1, LHX1, and CD24 over 8 days of differentiation.56 Small clusters of cells staining positive for PAX2 and vimentin also were observed with monolayer culture, although efficiencies were not reported and co-staining with other pertinent markers of nephron precursor populations was not performed. Rather than using a growth factor approach, Lin et al34 differentiated hESCs in media supplemented with a reduced concentration of fetal bovine serum over 14 days, then used cell sorting to fractionate populations of cells on the basis of expression of three different markers: CD24, a cell surface marker of mouse MM; podocalyxin, a cell surface marker of MM as well as IM; and GCTM2, a marker of pluripotency. The fraction of CD24+podocalyx-in+GCTM2− cells was found to have higher levels of PAX2, LHX1, and WT1 transcripts relative to unfractionated cells, and contained a subpopulation of PAX2+WT1+ cells when assayed by immunocytochemistry.
Table 2.
Directed Differentiation of Human PSCs Into Kidney Cells
| Study | Starting Substrate |
Culture Method |
Differentiation Protocol | Differentiation End Point |
Findings |
|---|---|---|---|---|---|
| Schuldiner et al,55 2000 |
hESCs | EB formation | 20% KOSR without LIF and bFGF (5 d) 20% KOSR + HGF or nerve growth factor or activin (10 d) |
Expression of WT1 and RENIN by RT-PCR |
|
| Batchelder et al,56 2009 |
hESCs | EB formation Monolayer |
10% FBS + RA/activin/ BMP7 (EB, 2 d) 10% FBS + RA/activin/ BMP7 (monolayer, 6 d) |
PAX2+vimentin+ cells |
Expression of PAX2, WT1, OSR1, EYA1, LHX1, CD24 by RT-PCR Expression of PAX2 and vimentin by IF |
| Lin et al,34 2010 | hESCs | Monolayer | 20% FCS (2 d) 5% FCS (12 d) Flow sorting by CD24+podocalyxin+ GCTM2− cells |
Intermediate mesoderm |
Expression of WT1 and PAX2 by IF Up-regulation of renal developmental genes in microarray analysis |
| Song et al,57 2012 | hiPSCs | EB formation Monolayer |
2.5% FBS + activin/ BMP7/RA (EB, 3 d) 2.5% FBS + activin/ BMP7/RA (monolayer, 7 d) |
Podocytes | Expression of podocin, synaptopodin, and PAX2 by IF Integration into WT1+ glomerular structures of dissociated- reaggregated E13.5 kidneys |
| Narayanan et al,59 2013 |
hESCs | Monolayer | REGM + 0.5% FBS + BMP2 + BMP7 Flow sorting for AQP1+ cells |
Proximal tubular-like cells |
Expression of AQP1 by IF and FACS Integration of AQP1+ cells into tubular compartments of ex vivo newborn mouse kidneys Formation of cord-like structures in Matrigel Parathyroid hormone–stimulated cyclic adenosine monophosphate production, GGT activity, ammonia production |
| Mae et al,28 2013 |
OSR1-GFP hiPSCs |
EB formation Monolayer |
Serum-free media + CHIR99021/activin (2 days) Serum-free media + CHIR99021/BMP7 (9–16 d) |
Intermediate mesoderm |
Expression of OSR1-GFP by FACS Expression of PAX2 and WT1 by IF Expression of kidney, adrenal gland, and gonadal markers by IF and RT-PCR Formation of growths with expression of kidney markers when injected into immunocompromised mice Integration into dissociated- reaggregated E11.5 mouse kidneys |
| Xia et al,29 2013 | hESCs hiPSCs |
Monolayer | Serum-free media + BMP4/FGF2 (2 d) Serum-free media + RA/ activin/BMP2 (2 d) |
Intermediate mesoderm |
Expression of PAX2, LHX1, OSR1, and WT1 by IF and RT-PCR Integration into ureteric bud structures of dissociated- reaggregated E11.5 kidneys |
| Taguchi et al,31 2013 | mESCs hiPSCs |
EB formation | Serum-free media + activin (1d) Serum-free media + BMP4/CHIR (2.5 d) Serum-free media + activin/BMP4/CHIR/ RA/Y27632 (1 d) Serum-free media + CHIR/FGF9/Y27632 (2 d) |
Metanephric mesenchyme |
Nephron precursors derived from OSR1+ posterior IM and T+ posterior mesoderm Expression of Osr1, WT1, Pax2, Six2, and Sall1 by IF and RT- PCR Co-culture of induced EBs resulted in formation of glomerular-like and tubular-like structures expressing kidney markers Protocol reproducible in hiPSCs with longer time course of differentiation |
| Takasato et al,30 2013 |
hESCs | Monolayer | Serum-free media + activin/BMP4 or CHIR99021 (2 d) Serum-free media + FGF9 + heparin (4 d) Serum-free media + FGF9 + heparin (6 d) Serum-free media alone (6 d) |
Metanephric mesenchyme |
Expression of PAX2 and LHX1 by IF and RT- PCR (IM) Expression of SIX2, HOXD11, and WT1 by IF (MM) Formation of tubular-like structures expressing ECAD, JAG1, and CADH6 Integration into E12.5–E13.5 kidneys Self-aggregation into tubular-like structures expressing kidney markers |
| Lam et al,27 2013 | hESCs hiPSCs |
Monolayer | Serum-free media + CHIR99021 (1.5 d) Serum-free media + FGF2/RA (1.5 d) Serum-free media alone (6 d for tubular structures) or serum-free media + FGF9/activin (3–6 d for MM) |
Intermediate mesoderm |
Expression of PAX2, LHX1, and WT1 by IF Formation of polarized, apically ciliated tubular-like structures expressing LTL, KSP, and NCAD Tubular cells integrate into laminin-bounded structures of E12.5 kidneys Expression of SIX2, SALL1, and WT1 by IF with FGF9 treatment MM cells form LTL+ laminin+ structures in E12.5kidneys |
| Araoka et al,63 2014 |
OSR1-GFP hiPSCs |
Monolayer | Serum-free media + CHIR99021 + AM580 or TTNPB (2 d) Serum-free media + AM580 or TTNPB (12 d) |
Intermediate mesoderm |
Expression of OSR1-GFP by FACS Expression of PAX2,WT1, LHX1, and SALL1 by IF Expression of kidney, adrenal gland, and gonadal markers by IF and RT-PCR Integration into dissociated- reaggregated E11.5 kidneys Formation of tubular-like structures in cell aggregates |
FACS, fluorescence-activated cell sorting; FBS, fetal bovine serum; KSP, kidney-specific protein; LIF, leukemia-inhibitory factor; RT-PCR, reverse-transcription polymerase chain reaction.
Efforts also have been made to differentiate hESCs and hiPSCs directly into more mature kidney epithelial cells, bypassing detailed characterization of a distinct nephron precursor intermediate. One such study reported differentiation of hiPSCs into what the researchers concluded were kidney podocytes.57 By using a combination of EB and monolayer differentiation methods, hiPSCs were treated with RA, activin, and BMP7 for a total of 10 days, resulting in the emergence of cells with cytoplasmic projections somewhat reminiscent of foot processes. These cells expressed podocyte markers such as podocin and synaptopodin but also were positive for PAX2 and proliferated in culture, suggesting that they might represent immature rather than terminally differentiated podocytes. The investigators also showed some evidence of integration of the iPSC-derived podocyte-like cells into WT1+ glomerular structures of dissociated-reaggregated E13.5 mouse embryonic kidney explant cultures. A weakness of this study was that it is very difficult to confirm podocyte identity in vitro because of the absence of glomerular organization and the highly differentiated foot processes typical of these cells in vivo.58
In another study, AQP1+ proximal tubular-like cells were generated with 38% efficiency by differentiating hESCs on Matrigel (BD Bioscience, San Jose, CA) in Renal Epithelial Cell Growth Media (REGM; Lonza) supplemented with BMP2 and BMP7.59 These cells showed similar gene expression profiles to human primary proximal tubular cells, although some differences in protein expression also were noted. Functional analyses showed that, similar to proximal tubular cells, AQP1+ cells increased cyclic adenosine monophosphate production in response to parathyroid hormone stimulation, displayed GGT activity, and produced ammonia. Integration of the AQP1+ cells into tubular compartments of mouse newborn kidneys ex vivo also was shown, and the cells formed cord-like structures when cultured on Matrigel in vitro or implanted in Matrigel into immunodeficient animals. Although the characterization for these cells was more detailed than in the podocyte study, there remains some concern whether the markers and cellular properties shown are sufficient to conclude that the AQP1+ population represents renal tubular epithelial cells.
Recently, there has been a surge of reports focusing on the generation of human nephron progenitor cells,27–31 including cells of the IM and MM, from which nearly all the epithelial cells of the nephron are derived.60,61 Mae et al28 showed induction of an OSR1+ IM population using a stepwise combination of the GSK-3β inhibitor CHIR99021, activin, and BMP7 signaling in engineered OSR1-GFP hiPSC lines. OSR1-GFP+ cells could give rise to cells expressing markers of mature kidneys, adrenal glands, and gonads in vitro and incorporate with low efficiency into dissociated-reaggregated E1 1.5 mouse metanephric kidneys. Although the investigators reported efficiencies of greater than 90% of OSR1-GFP+ cells after 11 to 18 days of differentiation, the proportion of OSR1+ cells that co-expressed other important IM markers such as PAX2 or WT1 was comparatively low. It should be noted that OSR1 is expressed in both the lateral plate and IM during early mesoderm specification,62 and therefore OSR1 expression alone cannot be used to label a population as being IM. In a follow-up study the same group of investigators reduced the duration of the original protocol to 6 days by substituting activin and BMP7 with either of two RA-receptor agonists, AM580 or TTNPB, which were identified in a high-throughput small-molecule screen for inducers of OSR1-GFP+ cells.63
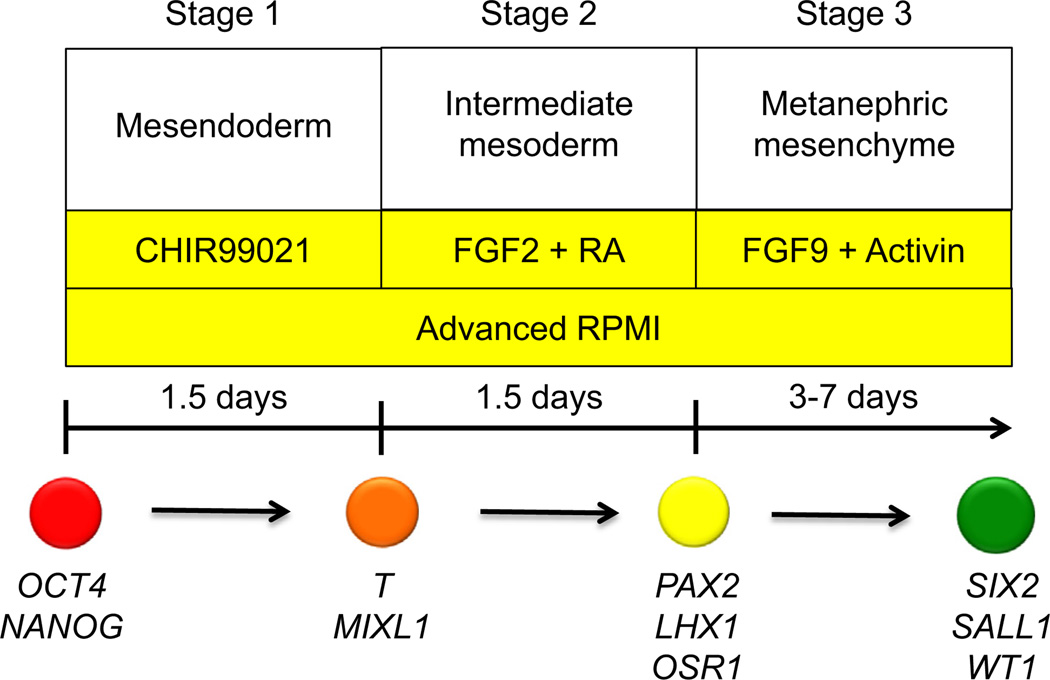
Our laboratory recently established a protocol to rapidly and robustly differentiate hPSCs into IM cells (Fig. 2).27 Mesendoderm differentiation could be achieved with nearly 100% efficiency by treating hESCs or hiPSCs with CHIR99021, establishing a robust platform for the screening of compounds that could promote IM differentiation. CHIR99021 alone was insufficient to induce IM differentiation, as assayed by PAX2 expression, and prolonged treatment with CHIR99021 promoted differentiation into cells expressing FOXF1+, a marker of lateral plate mesoderm. The addition of high-dose activin after CHIR99021 treatment diverted differentiation away from lateral plate mesoderm into SOX17+FOXA2+ definitive endoderm, showing that the precisely timed addition of signaling factors could modulate cell fate within this system. Screening for IM differentiating factors showed FGF2 to be a potent inducer of PAX2 expression, but only in cells that had been pretreated with CHIR99021. Treatment of either hESCs or hiPSCs with CHIR99021 followed by the addition of both FGF2 and RA generated PAX2+LHX1+ with 70% to 80% efficiency within only 3 days of differentiation. Extended culture of these PAX2+LHX1+ cells after withdrawal of FGF2 and RA resulted in the formation of tubular-like structures that expressed the mature proximal tubular markers LTL, kidney-specific protein, and N-cadherin. These tubular structures were polarized and possessed apical cilia expressing polycystin-2 (PC2). Furthermore, we showed that a combination of FGF9 and activin could differentiate PAX2+LHX1+ cells into cells co-expressing multiple markers of MM, including SIX2, SALL1, and WT1. Stimulation of SIX2+ cells with CHIR99021 induced a morphologic change similar to tubulogenesis in the developing mouse kidney with down-regulation of SIX2 and up-regulation of the proximal tubular marker LTL. Incorporation of the SIX2+ cells into dissociated-reaggregated E12.5 mouse kidneys yielded structures expressing LTL and laminin.
Figure 2.
Schematic diagram of protocol to generate nephron progenitor cell populations from hPSCs. Directed differentiation of hPSCs into nephron progenitor cells is divided into three discrete stages, with growth factors, culture media, and duration of treatment shown for each stage. Markers characteristic of each stage of differentiation also are listed.
The study by Takasato et al30 supported the role of Wnt and FGF signaling in the induction of IM and MM cell populations from hPSCs. The combination of CHIR99021 (or activin and BMP4), followed by high-dose FGF9, generated PAX2+LHX1+ cells and SIX2+ cells after 6 days and after 10 to 18 days of treatment, respectively. The investigators showed that the treated hESCs could contribute in part to mouse tubular structures when recombined with dissociated-reaggregated mouse embryonic kidneys. In addition, hESC-derived kidney progenitors could self-aggregate to form 3D structures resembling kidney tubules that expressed markers such as WT1, PAX2, E-cadherin, JAG1, AQP1, AQP2, and SLC3A1. Each of the earlier-described studies differed somewhat in the details and timing of growth factor addition to hPSCs, however, a common theme appears to be that CHIR99021 promotes mesoderm differentiation, which can be specified further into IM via FGF signaling.
Although most attention has been given to producing progenitor cells of the MM, a recent study by Xia et al29 focused instead on the derivation of progenitor cells of the UB. The step wise treatment of hESCs and hiPSCs with BMP4 and FGF2 followed by RA, activin, and BMP2 resulted in IM-like cells expressing PAX2, OSR1, WT1, and LHX1 after 4 days of differentiation. These cells up-regulated UB markers HOXB7, RET, and GFRA1 after an additional 2 days of differentiation, leading the investigators to label them as putative UB progenitor-like cells. Co-culture with dissociated-reaggregated E1 1.5 mouse embryonic kidneys showed some integration of these UB progenitor-like cells into mouse ureteric bud tips and trunks, whereas undifferentiated hESCs or hiPSCs tended to overgrow the cultures and disrupt growth of the mouse metanephroi. As discussed later, the interpretation of this assay remains unclear, particularly when it is taken into consideration that some of the differentiated cells appeared in independent clusters that clearly were negative for UB and MM markers expressed in the neighboring mouse cells.
MODELING KIDNEY DISEASE USING hiPSCs
hiPSCs derived from patients present a valuable resource for studying pathophysiological mechanisms of disease and developing potential therapeutics. Importantly, hiPSCs are human cells and carry naturally occurring human mutations. Therefore, they are an important complement to mouse models, which may not fully recapitulate human genotypes and phenotypes. For example, human beings with autosomal-dominant polycystic kidney disease are germline heterozygotes, whereas mouse models often are germline homozygotes, because germline heterozygote mice develop only mild cystic disease.64 Generation of a mouse model requires construction of recombinatory templates introducing mutations into the genome of wild-type mESCs, followed by screening, selection, blastocyst injection, crossing, genotyping, and back-crossing the progeny. This is a laborious and low-throughput process and therefore it is difficult to create mouse models for the vast majority of disease-causative alleles in human beings,65 of which there may be hundreds for any given gene. Furthermore, the contribution of the complex genomic background to human disease phenotypes would be exceedingly difficult to study in a mouse. In contrast, hiPSCs preserve the naturally occurring genetic mutations and genetic background of their parental somatic cells. With the proper technical training, hiPSCs can be generated in the laboratory in approximately 6 weeks. Multiple hiPSC lines can be created simultaneously, and indeed this is often the most convenient and best-controlled process for studying a set of lines from different patients. Since the establishment of techniques to create iPSCs in 2006,66 hiPSCs have been generated that represent more than 50 diseases and hundreds of patients. In a number of cases disease phenotypes have been identified in these cells.21
hiPSCs also have significant advantages compared with human cells isolated from the kidney and placed into primary culture or immortalized and placed into continuous culture. Many primary cell cultures, including cultures of renal tubular and glomerular epithelia, represent mixed populations that tend to senesce or dedifferentiate rapidly in culture and may express markers of myofibroblasts.58,67 In contrast, undifferentiated hiPSCs can self-renew indefinitely and are a relatively stable and homogenous cell type in culture. Somatic cells for hiPSC reprogramming can be derived from noninvasive samples, such as skin, hair, blood, or urine,68,69 and once obtained do not need to be rederived because of their inherent capacity for self-renewal. hiPSCs created and cryopreserved in one laboratory therefore can be studied by any other laboratory for the reproduction of phenotypes with minimal concern for passage number effects.
Compared with immortalized cell lines, hiPSCs have the advantage that they are considered to be a nontransformed cell type, similar to hESCs, which are derived directly from dissociated embryos. In addition, many immortalized kidney cell types for the kidney are from nonhuman species or of uncertain tissue origin, for example, Madin-Darby Canine Kidney cells and Human Embryonic Kidney 293 cells. In contrast, hiPSCs are a distinct cell type in culture with well-established cell type markers, gene expression patterns, morphology, and cell-cycle properties. hiPSCs from different individuals allow for comparisons between cells derived from different patients and with hESCs. Although kidney regeneration is certainly one important long-term goal, the use of hiPSCs to model disease pathophysiology at the cellular level is a more immediate application that already successfully has been shown for a wide variety of nonkidney organs and cell types.21
PATIENT-DERIVED hPSC METHODOLOGIES FOR THE STUDY OF KIDNEY DISEASE
Patient-specific hPSC methodologies have been applied to the study of kidney disease (Table 3). Genetically characterized hiPSCs or hESCs have been derived from patients with autosomal-dominant polycystic kidney disease (ADPKD) and autosomal-recessive PKD, Alport syndrome, systemic lupus erythematosus, and Wilms tumor.26,70–73 Although successful derivation of hPSCs from patients with kidney disease is relatively straightforward at this time, a much greater challenge is to use these cells successfully to study disease processes and derive potential treatments of human disease. Innovation of human in vitro laboratory models is particularly urgent because, as a result of species-specific differences, existing rodent models do not recapitulate many aspects of human kidney disease accurately.
Table 3.
hPSCs Derived From Patients With Kidney Disease
| Disease | Reference | Cell Type | Gene | Sequenced | Patients | Phenotype |
|---|---|---|---|---|---|---|
| ADPKD | Freedman et al26 | iPSC | PKD1 | Yes | 3 | Reduced ciliary PC2 |
| Thatava et al70 | iPSC | PKD1 | No | 1 | Not determined | |
| Xia et al29 | iPSC | N.D. | No | 1 | Not determined | |
| Autosomal-recessive PKD | Freedman et al26 | iPSC | PKHD1 | Yes | 2 | Not determined |
| Systemic lupus erythematosus | Thatava et al70 | iPSC | N.D. | No | 1 | Not determined |
| Chen et al72 | iPSC | N.D. | No | 4 | Not determined | |
| Wilms tumor | Thatava et al70 | iPSC | N.D. | No | 1 | Not determined |
| Alport syndrome | Frumkin et al71 | ESC | COL4A5 | No | 2 | Not determined |
| Fabry disease | Tropel et al73 | ESC | GLA | Yes | 1 | Not determined |
N.D. = Not determined.
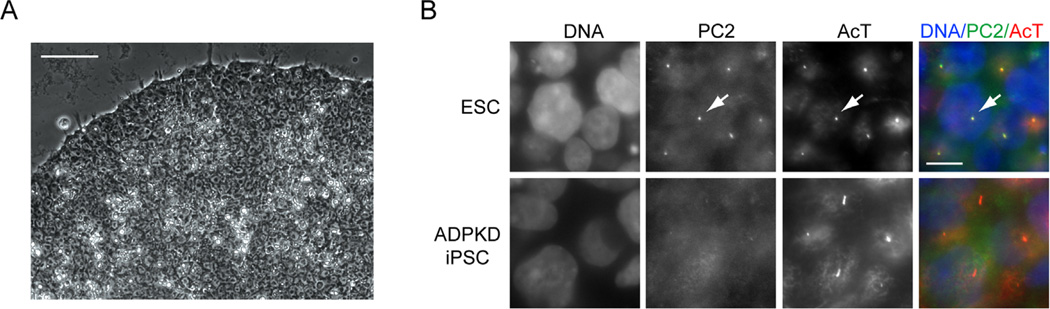
In only one of the aforementioned reports of hPSCs representing various kidney diseases was a disease phenotype described: we have shown that reduced levels of PC2 at the primary cilium consistently was observed in cells from multiple patients with ADPKD.26 This study built on the observation that undifferentiated hESCs are epithelial cells that possess primary cilia,74 which are antenna-like sensory organelles central to PKD etiologies. hiPSCs from patients with ADPKD and autosomal-recessive PKD, as well as healthy controls, were shown to endogenously express PKD disease genes.26 No defects were observed in the ability of PKD hiPSCs to form cilia, but ADPKD iPSCs showed reduced ciliary PC2 levels, as assayed by quantitative immunofluorescence (Fig. 3). To extend this observation to somatic lineages, hiPSCs were either stochastically differentiated into EB epithelial cells, or alternatively directed to differentiate into hepatoblasts, the bipotential progenitor of hepatocytes and cholangiocytes, which give rise to liver cysts and fibrosis in PKD patients. These somatic cell types also showed reduced ciliary PC2 levels. The genetic mutations in all three ADPKD hiPSC lines were found to be in PKD1, suggesting that polycystin-1 (PC1) plays an important role in trafficking PC2 to the cilium. Transient overexpression of wild-type PC1 in ADPKD hiPSC-derived hepatoblasts or IMCD3 cells increased ciliary PC2 levels, suggesting a possible therapeutic approach. Notably, reduced ciliary PC2 also has been observed in primary cells from patients and mouse models with heterozygous PKD1 mutations.75,76 However, because such cells were derived from kidneys with advanced disease, it was not clear in those studies whether the observed loss of PC2 was a primary defect or a secondary consequence of disease. Furthermore, a previous study had argued that PC2 traffics to the cilium independent of PC1.77 Demonstration of reduced ciliary PC2 in hiPSCs derived from fibroblasts, a cell type not known to be affected in PKD patients, suggested that PC1 at least enhances PC2 ciliary trafficking, and that primary defects in this process may occur in cells that appear otherwise normal. This study provides proof of principle for the application of hiPSCs to the study of kidney disease pathophysiology and for identifying possible therapeutic approaches.78 Notably, many of the kidney-directed differentiation protocols described earlier were not yet developed when this work was performed.
Figure 3.
Use of kidney disease iPSCs to investigate molecular pathophysiology. (A) Phase-contrast micrograph showing a colony of undifferentiated iPSCs derived from a patient with ADPKD. Scale bar, 50 µm. (B) Immunolocalization of PC2 and acetylated α-tubulin (AcT) in undifferentiated hPSCs. PC2 localizes to cilia in wild-type ESCs (arrow), but is absent from cilia in ADPKD iPSCs. Scale bar, 10 µm.
An important current goal is to model disease pathophysiology and therapy in hiPSC-derived cells of the kidney lineage, even as directed differentiation techniques continue to improve. As described previously, recently published directed differentiation protocols have been shown using both hESCs and hiPSCs, and human-mouse chimeric UB structures could be replicated using hiPSCs derived from patients with ADPKD. A caveat to such experiments is that hiPSC lines reprogrammed from different patients, tissues, or even from a single batch of primary cells may differ considerably in their propensity to differentiate into different lineages.79,80 It therefore is important that phenotypes be shown reproducibly in multiple patients, and if possible that rescue experiments be performed to show their specificity.21 Experiments comparing hiPSCs reprogrammed from urine cells, kidney epithelial cells, or mesangial cells68,81,82 also should take into account the possibility of injury or secondary mutations in the genitourinary systems of patients with disease, which could affect iPSC function. Recently developed genome editing tools, including transcription activator-like effector nucleases and the Cas9/clustered regularly interspersed short palindromic repeats system, can be applied to hPSCs to introduce or correct genetic mutations for analysis of cellular phenotypes on an isogenic genetic background, and may provide a useful complement to hiPSCs with naturally occurring genetic mutations.83,84
FUTURE DIRECTIONS
Although the field has made significant advances in the past few years toward the generation of functional kidney cells and tissue from PSCs, much work remains to be performed before these efforts can be translated to useful tools for the care of patients with acute and chronic kidney disease and the screening of potential therapeutic agents for efficacy or toxicity. Progress has been made in differentiating hPSCs into populations with properties characteristic of kidney progenitor cells. Now these results must be expanded to maximize the efficiency of these protocols, identify ways to maintain the survival and phenotype of these progenitor cells in extended culture, and establish new protocols for generating terminally differentiated, functional renal epithelial cells. Whenever possible, specific cell surface markers for differentiated cell populations should be identified because this would facilitate their isolation and purification for analysis and downstream application. The efficient generation and purification of hiPSC-derived proximal tubular cells, for example, would be instrumental for bioengineering 3D proximal tubules, high-throughput drug toxicity screening, seeding onto renal assist devices for the treatment of acute and chronic kidney injury, and in vitro disease modeling with patient-specific cell lines.
As we continue to move forward, it also will be increasingly important to establish criteria for the validation of putative hPSC-derived nephron progenitor cells and renal epithelial cells (Table 4), just as criteria have been proposed for the validation of hiPSC lines. It is important that protocols be reproducible in multiple hESC and hiPSC lines. If methods are to be applied to the large-scale production of kidney cell types or to disease modeling in multiple patient cell lines, we cannot afford to use protocols that work in only select cell lines. The well-established variability between individual hESC or hiPSC lines must be taken into account, and adjustments to culture conditions to account for these differences, if needed, should be presented. Second, characterization of putative kidney cell populations must show marker expression that is robust and consistent. Gene expression profiling (by means of reverse-transcription polymerase chain reaction or global gene expression analysis), although integral to the characterization process, must be interpreted cautiously, especially because differentiation protocols often result in heterogeneous populations of cells that can confound the data by reducing the signal-to-noise ratio. Marker analysis should include clear protein expression in differentiated cells by immunocytochemistry, immunoblot, and/or flow cytometry, and quantification of differentiation efficiencies based on protein expression, subcellular localization, and co-localization should be standard. These should be compared with protein expression profiles of endogenous primary cells isolated from adult and embryonic kidneys as positive controls. Because several markers of kidney development are expressed in other non-kidney tissues (eg, PAX2, LHX1, WT1, and SIX2) and in undifferentiated PSCs (E-cadherin, podocalyxin), showing the absence of non–kidney-specific markers is equally important as the presence of kidney markers. Just because a marker has been observed in a developing or adult kidney does not qualify it as kidney-specific. PSCs are capable of turning into any cell in the body and naturally differentiate into heterogenous populations; therefore, the burden of proof for kidney derivation is much higher than for primary cells isolated from specific areas of the embryo. For markers to be considered kidney-specific, it is necessary to show that they are expressed only in the kidney and nowhere else.
Table 4.
Proposed Criteria for the Validation of hPSC-Derived Kidney Cells
| Assay | Comments | References | |
|---|---|---|---|
| Phenotypic characterization Expression of embryonic and/or adult kidney markers |
Specificity of antibodies and probes should be shown using positive and negative controls |
27,29,30,31,34,37,41,42,43,44,45,46,47,48,49,50,56,57,59,63 |
|
| Co-localization of multiple markers within the same cells and in neighboring segments should be shown |
|||
| Quantification of kidney marker expression and absence of expression of nonkidney markers should be performed |
|||
| Gene and protein expression levels should be interpreted cautiously in heterogeneous differentiated cell populations |
|||
| Marker expression should be consistent with a specific cell identity |
|||
| Kidney epithelial structures | Insufficient without accompanying marker analysis because PSCs are epithelial cells and are capable of differentiating into diverse epithelia |
27,30,31,36,42,48,59,63 | |
| Ultrastructural analysis (presence of brush border) could provide more supportive evidence of kidney phenotype |
|||
| Evidence of hollow lumens surrounded by polarized epithelia should be presented |
|||
| Functional analysis In vitro |
Biochemical assays of epithelial cell function |
Epithelial functions should be compared with primary kidney cells as positive controls and nonkidney epithelial cells as negative controls |
59 |
| Integration into embryonic kidney explant cultures |
Results should be compared with stochastically differentiated PSCs as negative controls and primary fetal kidney cells as positive controls |
27,28,29,30,37,41,44,48,57,59,63 | |
| In vivo | Formation of glomeruli and nephron structures |
Should be accompanied by marker analysis, ideally showing nephron segments in serial sections |
31 |
| Urine production | Requires PSC-derived kidney tissue to be vascularized |
Not shown | |
| Functional rescue after kidney injury |
Requires strategy to deliver cells to the kidney effectively |
Not shown |
Ultimately, marker expression must be complemented by stringent functional analysis of the putative kidney cell populations. The establishment of reliable functional assays continues to be a challenge, and the interpretation of currently used assays is controversial. Integration of putative stem cell–derived kidney cells into developing mouse kidneys was proposed as a functional assay by Davies et al85 and continues to be the most widely used test for showing functional capacity. However, it remains unclear as to whether this assay truly tests functional capacity because incorporation of a cell type from one organism into the structure of another has never been the intrinsic property of a cell type. Furthermore, how one defines integration or incorporation is also subject to interpretation. Is the presence of a single human cell in a mouse tubule sufficient evidence of incorporation or merely an accident? Several human cell lines fail to integrate substantially into mesenchymal or epithelial progenitor populations in this assay,86 and it has been reported that undifferentiated hPSCs grow rapidly and disrupt metanephric growth.29 However, incorporation and possibly even transdifferentiation of undifferentiated PSCs and also mesenchymal stromal cells into mouse metanephroi in organ culture also have been reported,37,87 therefore presentation of integration data in the presence of convincing negative controls (both undifferentiated and differentiated) will be necessary. Another approach is to test kidney cells in vitro for biochemical characteristics typical of primary cells. For instance, hPSC-derived AQP1-expressing cells responded to parathyroid hormone stimulation by upregulating cyclic adenosine monophosphate, similar to primary proximal tubular cells.59 It remains unclear, however, whether there is a defined set of properties unique to kidney cells in this regard. In none of the published work on kidney differentiation from PSCs is there a conclusive demonstration of a branching network of human nephrons or even a single, continuous, segmented nephron consisting of a vascularized glomerulus connecting to a proximal tubule and collecting duct, as might be expected to emerge from metanephric cells. Such a demonstration would provide stronger evidence of nephrogenesis than isolated images of cells expressing different markers found in kidneys. In the absence of such evidence, reports of “mini-kidney” (Source: http://www.salk.edu/news/pressrelease_details.php?press_id=648) and “functional kidney” (Source: https://www.fightaging.org/archives/2013/12/generating-functional-kidney-tissue-from-stem-cells.php) generation seem somewhat premature. A conclusive functional demonstration of kidney lineage differentiation also would include physiological metrics, such as urine production or prolongation of life by stem cell administration after injury.
The nephron is by nature a complex 3D structure, and any effort to reproduce this architecture using hPSCs likely will require differentiation within a 3D environment. This partly has been addressed by recent studies, including those from our own laboratory, that have taken advantage of co-culture methods involving the recombination of hPSC-derived kidney cells with dissociated-reaggregated mouse embryonic kidneys into 3D pellets. Although it would appear that the mouse embryonic kidney provides a proper niche and signaling factors for further differentiation and incorporation of these differentiated hPSCs into mouse tubular or glomerular structures, a hybrid mouse-human kidney would provoke an extreme immune rejection and preclude clinical application. One creative approach is to use blastocyst complementation to grow xenobiotic organs from one species in another. This approach recently was used to generate a rat pancreas in a mouse.88 However, interspecific efficiencies were extremely low, and the strategy did not work when attempted for the kidney.89 Interestingly, recent findings have suggested that hPSC-derived nephron progenitors are capable of forming 3D structures on their own in the absence of mouse tissue.27,30 Because such methods could in theory harness the multipotency of nephron progenitor cells to produce the entire complement of renal epithelial cells and their associated structures, it may be unnecessary to define the signals required to generate each individual epithelial cell type. However, the disadvantage lies in the present inability to control which nephron structures will be formed, and there is no guarantee that separately formed tubular structures will find a way to join to form a full-length nephron. Furthermore, a means of incorporating a blood supply, critical to forming functioning glomeruli and supporting the tubular system, has not yet been established, although there are data to suggest that grafting embryonic kidney rudiments onto a vascular source, such as the chick chorioallantoic membrane, possibly could provide a source of blood vessels to the kidney tissue.90 An alternative approach is to use bioengineered 3D microenvironments in which differentiated hPSCs could be encapsulated. Given the importance of mechanical and biophysical cues provided by the surrounding cellular environment to stem cell differentiation,91–95 crafting a 3D niche based on the extracellular matrix composition and properties of the developing or adult kidney could promote the formation of 3D kidney structures from hPSCs. Efforts should be taken to define the biophysical properties of both the developing and adult mouse and human kidneys more comprehensively to provide a more accurate framework for mimicking this environment in vitro. High-throughput bioprinting technologies also could be used to screen for particular combinations of extracellular matrix proteins embedded in hydrogels that induce 3D kidney structure formation.
For clinical applications, safety and efficacy considerations will be prerequisite. Undifferentiated hiPSCs are, by their nature, tumorigenic, and therefore must be eliminated before transplantation, either passively (owing to complete and irreversible differentiation) or actively (by pretreatment with compounds that are selectively toxic to undifferentiated hPSCs, such as oleate synthesis inhibitors96). Because nephron progenitor-like cells have been observed in some renal tumors, the safety of this population also must be shown in preclinical transplant models. The precise route of such administration also will require optimization. How cells might trespass the glomerular filtration barrier from either the bloodstream or from within the nephron is not clear. Human nephrogenic stem cells have been injected successfully into kidneys, but because such an invasive approach would be likely to cause significant damage to the existing tissue, more work is required to show that it would provide benefit to patients.12 To satisfy the requirement for clinical efficacy, nephron progenitor cells must be shown to be functionally equivalent or superior to the existing gold standard of transplanted kidneys from allogeneic hosts. These obviously are major challenges that will require time, effort, and resources to surmount. The reward will be improved survival, outcomes, and quality of life for patients with devastating renal diseases.
Acknowledgments
Financial support: Supported by National Institutes of Health grants R01 DK39773, RC1 DK0864406 (J.V.B.), and F32 DK092036 (B.S.F.), and American Heart Association grant 11FTF7320023 (A.Q.L.).
Footnotes
Conflict of interest statement: none.
REFERENCES
- 1.Greenberg A, Cheung AK. Primer on kidney diseases. 5th. Philadelphia, PA: Saunders/Elsevier; 2009. [Google Scholar]
- 2.Hartman HA, Lai HL, Patterson LT. Cessation of renal morphogenesis in mice. Dev Biol. 2007;310:379–387. doi: 10.1016/j.ydbio.2007.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harbor Perspect Biol. 2012;4:1–18. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faa G, Gerosa C, Fanni D, et al. Marked interindividual variability in renal maturation of preterm infants: lessons from autopsy. J Matern Fetal Neonatal Med. 2010;23(Suppl 3):129–133. doi: 10.3109/14767058.2010.510646. [DOI] [PubMed] [Google Scholar]
- 5.Humphreys BD, Valerius MT, Kobayashi A, et al. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilbert SF. Developmental biology. 9th. Sunderland, MA: Sinauer Associates; 2010. [Google Scholar]
- 8.Grobstein C. Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science. 1953;118:52–55. doi: 10.1126/science.118.3054.52. [DOI] [PubMed] [Google Scholar]
- 9.Carroll TJ, McMahon AP. Secreted molecules in metanephric induction. J Am Soc Nephrol. 2000;11(Suppl 16):S116–S119. [PubMed] [Google Scholar]
- 10.Rogers SA, Hammerman MR. Prolongation of life in anephric rats following de novo renal organogenesis. Organogenesis. 2004;1:22–25. doi: 10.4161/org.1.1.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekel B, Reisner Y. Engraftment of human early kidney precursors. Transplant Immunol. 2004;12:241–247. doi: 10.1016/j.trim.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Harari-Steinberg O, Metsuyanim S, Omer D, et al. Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med. 2013;5:1556–1568. doi: 10.1002/emmm.201201584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammerman MR. Growing new kidneys in situ. Clin Exp Nephrol. 2004;8:169–177. doi: 10.1007/s10157-004-0308-9. [DOI] [PubMed] [Google Scholar]
- 14.Saxen L, Lehtonen E, Karkinen-Jaaskelainen M, Nordling S, Wartiovaara J. Are morphogenetic tissue interactions mediated by transmissible signal substances or through cell contacts? Nature. 1976;259:662–663. doi: 10.1038/259662a0. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert SF. Developmental biology. 10th. Sunderland, MA: Sinauer Associates, Inc; [Google Scholar]
- 16.Barak H, Huh SH, Chen S, et al. FGF9 and FGF20 maintain the sternness of nephron progenitors in mice and man. Dev Cell. 2012;22:1191–1207. doi: 10.1016/j.devcel.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland MJ, Hazen JL, Nazor KL, et al. Adult mice generated from induced pluripotent stem cells. Nature. 2009;461:91–94. doi: 10.1038/nature08310. [DOI] [PubMed] [Google Scholar]
- 18.Zhao XY, Li W, Lv Z, et al. iPS cells produce viable mice through tetraploid complementation. Nature. 2009;461:86–90. doi: 10.1038/nature08267. [DOI] [PubMed] [Google Scholar]
- 19.Cahan P, Daley GQ. Origins and implications of pluripotent stem cell variability and heterogeneity. Nat Rev Mol Cell Biol. 2013;14:357–368. doi: 10.1038/nrm3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Grskovic M, lavaherian A, Strulovici B, Daley GQ. Induced pluripotent stem cells–opportunities for disease modelling and drug discovery. Nat Rev Drug Discov. 2011;10:915–929. doi: 10.1038/nrd3577. [DOI] [PubMed] [Google Scholar]
- 22.Wernig M, Meissner A, Foreman R, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 23.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 24.Kaneko S, Yamanaka S. To be immunogenic, or not to be: that's the iPSC question. Cell Stem Cell. 2013;12:385–386. doi: 10.1016/j.stem.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Lensch MW, Mummery CL. From stealing fire to cellular reprogramming: a scientific history leading to the 2012 Nobel prize. Stem Cell Rep. 2013;1:5–17. doi: 10.1016/j.stemcr.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman BS, Lam AQ, Sundsbak JL, et al. Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol. 2013;24:1571–1586. doi: 10.1681/ASN.2012111089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam AQ, Freedman BS, Morizane R, Lerou PH, Valerius MT, Bonventre JV. Rapid and efficient differentiation of human pluripotent stem cells into intermediate mesoderm that forms tubules expressing kidney proximal tubular markers. J Am Soc Nephrol. 2014;25:1211–1225. doi: 10.1681/ASN.2013080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mae S, Shono A, Shiota F, et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia Y, Nivet E, Sancho-Martinez I, et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol. 2013;15:1507–1515. doi: 10.1038/ncb2872. [DOI] [PubMed] [Google Scholar]
- 30.Takasato M, Er PX, Becroft M, et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nat Cell Biol. 2014;16:118–126. doi: 10.1038/ncb2894. [DOI] [PubMed] [Google Scholar]
- 31.Taguchi A, Kaku Y, Ohmori T, et al. Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell. 2014;14:53–67. doi: 10.1016/j.stem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Cohen DE, Melton D. Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet. 2011;12:243–252. doi: 10.1038/nrg2938. [DOI] [PubMed] [Google Scholar]
- 33.Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 34.Lin SA, Kolle G, Grimmond SM, et al. Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem Cells Dev. 2010;19:1637–1648. doi: 10.1089/scd.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen KG, Mallon BS, McKay RD, Robey PG. Human pluripotent stem cell culture: considerations for maintenance, expansion, and therapeutics. Cell Stem Cell. 2014;14:13–26. doi: 10.1016/j.stem.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamamoto M, Cui L, Johkura K, et al. Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol. 2006;290:F52–F60. doi: 10.1152/ajprenal.00001.2004. [DOI] [PubMed] [Google Scholar]
- 37.Steenhard BM, Isom KS, Cazcarro P, et al. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol. 2005;16:1623–1631. doi: 10.1681/ASN.2004070584. [DOI] [PubMed] [Google Scholar]
- 38.Osafune K, Nishinakamura R, Komazaki S, Asashima M. In vitro induction of the pronephric duct in Xenopus explants. Dev Growth Differ. 2002;44:161–167. doi: 10.1046/j.1440-169x.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 39.Wiles MV, lohansson BM. Embryonic stem cell development in a chemically defined medium. Exp Cell Res. 1999;247:241–248. doi: 10.1006/excr.1998.4353. [DOI] [PubMed] [Google Scholar]
- 40.Johansson BM, Wiles MV. Evidence for involvement of activin A and bone morphogenetic protein 4 in mammalian mesoderm and hematopoietic development. Mol Cell Biol. 1995;15:141–151. doi: 10.1128/mcb.15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim D, Dressier GR. Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol. 2005;16:3527–3534. doi: 10.1681/ASN.2005050544. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi T, Tanaka H, Kuwana H, et al. Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Commun. 2005;336:585–595. doi: 10.1016/j.bbrc.2005.08.136. [DOI] [PubMed] [Google Scholar]
- 43.Bruce SJ, Rea RW, Steptoe AL, Busslinger M, Bertram JF, Perkins AC. In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation. 2007;75:337–349. doi: 10.1111/j.1432-0436.2006.00149.x. [DOI] [PubMed] [Google Scholar]
- 44.Vigneau C, Polgar K, Striker G, et al. Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol. 2007;18:1709–1720. doi: 10.1681/ASN.2006101078. [DOI] [PubMed] [Google Scholar]
- 45.Morizane R, Monkawa T, Itoh H. Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochem Biophys Res Commun. 2009;390:1334–1339. doi: 10.1016/j.bbrc.2009.10.148. [DOI] [PubMed] [Google Scholar]
- 46.Ren X, Zhang J, Gong X, et al. Differentiation of murine embryonic stem cells toward renal lineages by conditioned medium from ureteric bud cells in vitro. Acta Biochim Biophys Sin (Shanghai) 2010;42:464–471. doi: 10.1093/abbs/gmq046. [DOI] [PubMed] [Google Scholar]
- 47.Nishikawa M, Yanagawa N, Kojima N, et al. Stepwise renal lineage differentiation of mouse embryonic stem cells tracing in vivo development. Biochem Biophys Res Commun. 2012;417:897–902. doi: 10.1016/j.bbrc.2011.12.071. [DOI] [PubMed] [Google Scholar]
- 48.Morizane R, Monkawa T, Fujii S, et al. Kidney specific protein-positive cells derived from embryonic stem cells reproduce tubular structures in vitro and differentiate into renal tubular cells. PLoS One. 2013;8:e64843. doi: 10.1371/journal.pone.0064843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mae S, Shirasawa S, Yoshie S, et al. Combination of small molecules enhances differentiation of mouse embryonic stem cells into intermediate mesoderm through BMP7-positive cells. Biochem Biophys Res Commun. 2010;393:877–882. doi: 10.1016/j.bbrc.2010.02.111. [DOI] [PubMed] [Google Scholar]
- 50.Nakane A, Kojima Y, Hayashi Y, Kohri K, Masui S, Nishinakamura R. Pax2 overexpression in embryoid bodies induces upregulation of integrin alpha8 and aquaporin-1. In Vitro Cell Dev Biol Anim. 2009;45:62–68. doi: 10.1007/s11626-008-9151-8. [DOI] [PubMed] [Google Scholar]
- 51.Hanna J, Cheng AW, Saha K, et al. Human embryonic stem cells with biological and epigenetic characteristics similar to those of mouse ESCs. Proc Natl Acad Sci U S A. 2010;107:9222–9227. doi: 10.1073/pnas.1004584107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh AM, Bechard M, Smith K, Dalton S. Reconciling the different roles of Gsk3beta in “naive” and “primed” pluripotent stem cells. Cell Cycle. 2012;11:2991–2996. doi: 10.4161/cc.21110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wray J, Kalkan T, Smith AG. The ground state of pluripotency. Biochem Soc Trans. 2010;38:1027–1032. doi: 10.1042/BST0381027. [DOI] [PubMed] [Google Scholar]
- 54.Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 55.Schuldiner M, Yanuka O, Itskovitz-Eldor J, Melton DA, Benvenisty N. Effects of eight growth factors on the differentiation of cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2000;97:11307–11312. doi: 10.1073/pnas.97.21.11307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Batchelder CA, Lee CC, Matsell DG, Yoder MC, Tarantal AF. Renal ontogeny in the rhesus monkey (Macaca mulatta) and directed differentiation of human embryonic stem cells towards kidney precursors. Differentiation. 2009;78:45–56. doi: 10.1016/j.diff.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song B, Smink AM, Jones CV, et al. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saleem MA, O'Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 59.Narayanan K, Schumacher KM, Tasnim F, et al. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013;83:593–603. doi: 10.1038/ki.2012.442. [DOI] [PubMed] [Google Scholar]
- 60.Mugford JW, Sipila P, McMahon JA, McMahon AP. Osr1 expression demarcates a multi-potent population of intermediate mesoderm that undergoes progressive restriction to an Osr1-dependent nephron progenitor compartment within the mammalian kidney. Dev Biol. 2008;324:88–98. doi: 10.1016/j.ydbio.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kobayashi A, Valerius MT, Mugford JW, et al. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell. 2008;3:169–181. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dressler GR. Advances in early kidney specification, development and patterning. Development. 2009;136:3863–3874. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Araoka T, Mae S, Kurose Y, et al. Efficient and rapid induction of human iPSCs/ESCs into nephrogenic intermediate mesoderm using small molecule-based differentiation methods. PLoS One. 2014;9:e84881. doi: 10.1371/journal.pone.0084881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson PD. Mouse models of polycystic kidney disease. Curr Top Dev Biol. 2008;84:311–350. doi: 10.1016/S0070-2153(08)00606-6. [DOI] [PubMed] [Google Scholar]
- 65.Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–79. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 67.Humphreys BD, Lin SL, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou T, Benda C, Duzinger S, et al. Generation of induced pluripotent stem cells from urine. J Am Soc Nephrol. 2011;22:1221–1228. doi: 10.1681/ASN.2011010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aasen T, Izpisua Belmonte JC. Isolation and cultivation of human keratinocytes from skin or plucked hair for the generation of induced pluripotent stem cells. Nat Protoc. 2010;5:371–382. doi: 10.1038/nprot.2009.241. [DOI] [PubMed] [Google Scholar]
- 70.Thatava T, Armstrong AS, De Lamo JG, et al. Successful disease-specific induced pluripotent stem cell generation from patients with kidney transplantation. Stem Cell Res Ther. 2011;2:48. doi: 10.1186/scrt89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frumkin T, Malcov M, Telias M, et al. Human embryonic stem cells carrying mutations for severe genetic disorders. In Vitro Cell Dev Biol Anim. 2010;46:327–336. doi: 10.1007/s11626-010-9275-5. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, Luo R, Xu Y, et al. Generation of systemic lupus erythematosus-specific induced pluripotent stem cells from urine. Rheumatol Int. 2013;33:2127–2134. doi: 10.1007/s00296-013-2704-5. [DOI] [PubMed] [Google Scholar]
- 73.Tropel P, Tournois J, Come J, et al. High-efficiency derivation of human embryonic stem cell lines following pre-implantation genetic diagnosis. In Vitro Cell Dev Biol Anim. 2010;46:376–385. doi: 10.1007/s11626-010-9300-8. [DOI] [PubMed] [Google Scholar]
- 74.Kiprilov EN, Awan A, Desprat R, et al. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;180:897–904. doi: 10.1083/jcb.200706028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nauli SM, Rossetti S, Kolb RJ, et al. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–1025. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 76.Xu C, Rossetti S, Jiang L, et al. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2007;292:F930–F945. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geng L, Okuhara D, Yu Z, et al. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 78.Hofherr A, Kottgen M. Induced pluripotent stem cells from polycystic kidney disease patients: a novel tool to model the pathogenesis of cystic kidney disease. J Am Soc Nephrol. 2013;24:1507–1509. doi: 10.1681/ASN.2013070767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boulting GL, Kiskinis E, Croft GF, et al. A functionally characterized test set of human induced pluripotent stem cells. Nat Biotechnol. 2011;29:279–286. doi: 10.1038/nbt.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim K, Doi A, Wen B, et al. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–290. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Song B, Niclis JC, Alikhan MA, et al. Generation of induced pluripotent stem cells from human kidney mesangial cells. J Am Soc Nephrol. 2011;22:1213–1220. doi: 10.1681/ASN.2010101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montserrat N, Ramirez-Bajo MJ, Xia Y, et al. Generation of induced pluripotent stem cells from human renal proximal tubular cells with only two transcription factors, OCT4 and SOX2. J Biol Chem. 2012;287:24131–24138. doi: 10.1074/jbc.M112.350413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ding Q, Lee YK, Schaefer EA, et al. A TALEN genome-editing system for generating human stem cell-based disease models. Cell Stem Cell. 2013;12:238–251. doi: 10.1016/j.stem.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mali P, Yang L, Esvelt KM, et al. RNA-guided human genome engineering via Cas9. Science. 2013;339:823–826. doi: 10.1126/science.1232033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Davies JA, Unbekandt M, Ineson J, Lusis M, Little MH. Dissociation of embryonic kidney followed by re-aggregation as a method for chimeric analysis. Methods Mol Biol. 2012;886:135–146. doi: 10.1007/978-1-61779-851-1_12. [DOI] [PubMed] [Google Scholar]
- 86.Hendry CE, Vanslambrouck JM, Ineson J, et al. Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol. 2013;24:1424–1434. doi: 10.1681/ASN.2012121143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yokoo T, Ohashi T, Shen JS, et al. Human mesenchymal stem cells in rodent whole-embryo culture are reprogrammed to contribute to kidney tissues. Proc Natl Acad Sci U S A. 2005;102:3296–3300. doi: 10.1073/pnas.0406878102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kobayashi T, Yamaguchi T, Hamanaka S, et al. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142:787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 89.Usui J, Kobayashi T, Yamaguchi T, Knisely AS, Nishinakamura R, Nakauchi H. Generation of kidney from pluripotent stem cells via blastocyst complementation. Am J Pathol. 2012;180:2417–2426. doi: 10.1016/j.ajpath.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 90.Davies J. Engineered renal tissue as a potential platform for pharmacokinetic and nephrotoxicity testing. Drug Discov Today. 2014;19:725–729. doi: 10.1016/j.drudis.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lesher-Perez SC, Frampton JP, Takayama S. Microfluidic systems: a new toolbox for pluripotent stem cells. Biotechnol J. 2013;8:180–191. doi: 10.1002/biot.201200206. [DOI] [PubMed] [Google Scholar]
- 92.Nikkhah M, Edalat F, Manoucheri S, Khademhosseini A. Engineering microscale topographies to control the cell-substrate interface. Biomaterials. 2012;33:5230–5246. doi: 10.1016/j.biomaterials.2012.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dado D, Sagi M, Levenberg S, Zemel A. Mechanical control of stem cell differentiation. Regen Med. 2012;7:101–116. doi: 10.2217/rme.11.99. [DOI] [PubMed] [Google Scholar]
- 94.Keung AJ, Kumar S, Schaffer DV. Presentation counts: micro-environmental regulation of stem cells by biophysical and material cues. Ann Rev Cell Dev Biol. 2010;26:533–556. doi: 10.1146/annurev-cellbio-100109-104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5:17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ben-David U, Gan QF, Golan-Lev T, et al. Selective elimination of human pluripotent stem cells by an oleate synthesis inhibitor discovered in a high-throughput screen. Cell Stem Cell. 2013;12:167–179. doi: 10.1016/j.stem.2012.11.015. [DOI] [PubMed] [Google Scholar]