Abstract
Neisseria meningitidis , a devastating pathogen exclusive to humans, expresses capsular polysaccharides that are the major meningococcal virulence determinants and the basis for successful meningococcal vaccines. With rare exceptions, the expression of capsule (serogroups A, B, C, W, X, Y) is required for systemic invasive meningococcal disease. Changes in capsule expression or structure (e.g. hypo- or hyperencapsulation, capsule “switching”, acetylation) can influence immunologic diagnostic assays or lead to immune escape. The loss or down-regulation of capsule is also critical in meningococcal biology facilitating meningococcal attachment, microcolony formation and the carriage state at human mucosal surfaces.
Encapsulated meningococci contain a cps locus with promoters located in an intergenic region between the biosynthesis and the conserved capsule transport operons. The cps intergenic region is transcriptionally regulated (and thus the amount of capsule expressed) by IS element insertion, by a two-component system, MisR/MisS, and through sequence changes that result in post transcriptional RNA thermoregulation. Reversible on-off phase variation of capsule expression is controlled by slipped strand mispairing of homo-polymeric tracts and by precise insertion and excision of IS elements (e.g. IS1301) in the biosynthesis operon. Capsule structure can be altered by phase-variable expression of capsular polymer modification enzymes or “switched” through transformation and homologous recombination of different polymerases. Understanding the complex regulation of meningococcal capsule has important implications for meningococcal biology, pathogenesis, diagnostics, current and future vaccine development and vaccine strategies.
INTRODUCTION
Neisseria meningitidis (the meningococcus) is a Gram-negative bacterial pathogen of humans that causes epidemic meningitis and rapidly fatal sepsis in many parts of the world. The reservoir of the meningococcus is the human nasopharynx and the organism is transmitted from person to person by close contact of oral or nasal secretions and/or large aerosolized droplet nuclei that transverse ~1 m. Meningococcal disease has a mortality rate that exceeds 50% without effective antibiotic and supportive therapy, and even with antibiotic therapy and supportive therapy case mortality remains 10–15%. However, in most instances, the acquisition of meningococci in the upper respiratory tract does not result in invasive disease. N. meningitidis is often a commensal inhabitant of the human respiratory tract, isolated from the nasopharynx of 3–20% of healthy individuals in the absence of outbreaks or crowding. Factors determining the establishment of the carriage state versus the development of invasive meningococcal disease following acquisition are not completely clear, but include specific capsule structures, other bacterial virulence determinants and host susceptibility.
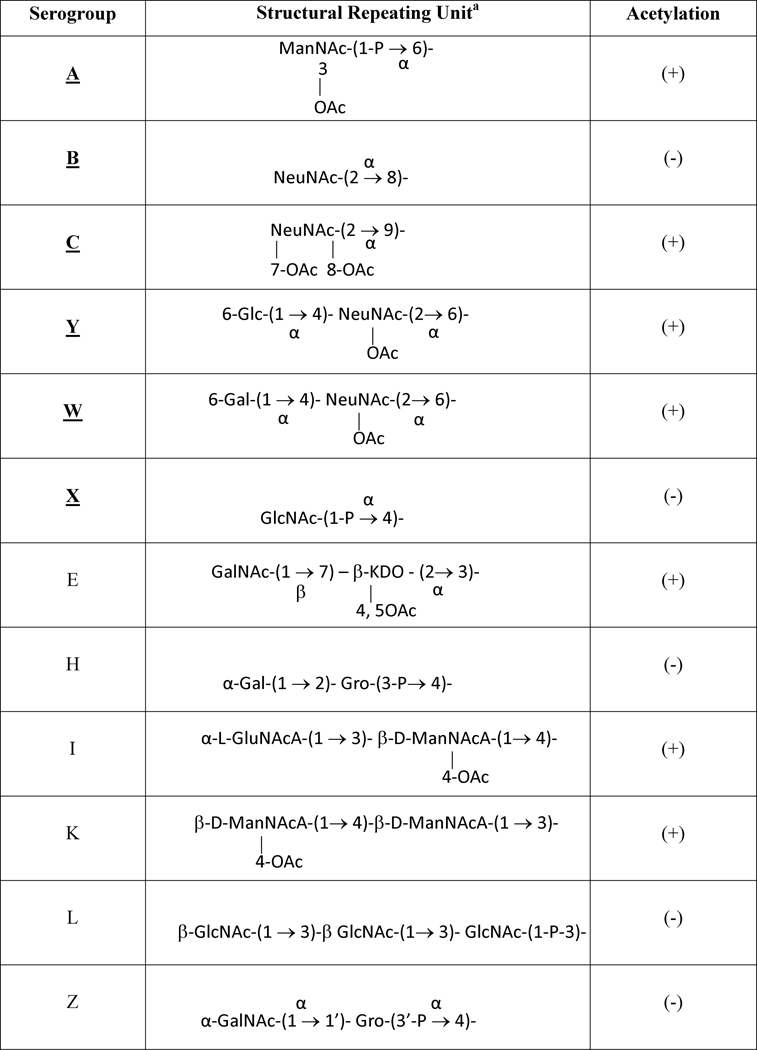
Although there are twelve defined capsular polysaccharides (CPS) expressed by N. meningitidis (Harrison et al., 2013) (which form the basis for serogroups), only six, serogroups A, B, C, W, X, and Y, have been significantly associated with invasive disease (Table 1) and for W, X and Y these have emerged in the last two decades. Serogroup A N. meningitidis, which expresses a homopolymeric (α1→6) N-acetylmannosamine 1-phosphate CPS, has caused large pandemic outbreaks, especially in sub-Saharan Africa. Serogroups B and C meningococci also cause outbreaks throughout the world and have been responsible for most sporadic meningococcal disease in developed countries. Serogroup B and C capsular polysaccharides are both homopolymers of sialic acid (N-acetylneuraminic acid) but with different linkages: (α2→8)- sialic acid for serogroup B and (α2→9)-, and partially O-acetylated sialic acid for serogroup C. In the 1990s, serogroup Y N. meningitidis emerged as a major cause of sporadic disease in the United States (Rosenstein et al., 1999) and has been increasingly a cause of meningococcal disease in other countries; and serogroup W emerged in the early years of the current decade as a cause of epidemic outbreaks associated with the Hajj pilgrimage and in sub-Saharan Africa (Taha et al., 2000). The serogroup Y capsular polymer is composed of alternating D-glucose and partially O-acetylated sialic acid, while the serogroup W capsular polysaccharide is an alternating D-galactose and partially O-acetylated sialic acid. Serogroup X has also recently caused disease in outbreaks and endemic disease in parts of sub-Saharan Africa; the serogroup X capsular polysaccharide is a homopolymer of (α1→4) N-acetylglucosamine 1-phosphate.
Table 1.
Capsular polysaccharide structures of pathogenic (bold) and generally non-pathogenic Neisseria meningitidis
ManNAc, N-acetyl-D-mannosamine; NeuNAc, N-acetyl-neuraminic acid (sialic acid); GalNAc, N-acetyl-galactosamine; GlcNAc, N-acetyl-glucosamine; GluNAcA, N-acetyl-guluronic acid; ManNAcA, N-acetyl-mannosaminuronic acid; Glc, glucosamine; Gal, galactosamine; Gro, glycerol; KDO, 2-keto-3-deoxyoctulosonic acid.
Capsular polysaccharides have historically been the targets for serogroup-specific meningococcal vaccine development. The capsular polysaccharide vaccines developed in the early 1970s were successful in adult populations such as military recruits and for control of some outbreaks (Artenstein et al., 1970). However, capsular polysaccharide vaccines are generally not effective for children less than 2 years of age, the population with the highest incidence of meningococcal disease, induce hypo-responsiveness with repeated vaccination (Granoff et al., 1998) and fail to induce long-term memory response (Lepow et al., 1986; MacLennan et al., 2001; Rosenstein et al., 2001a; Rosenstein et al., 2001b). In general, meningococcal capsular polysaccharides are poorly boostable (T-cell independent) antigens, the immunological basis for which is being unraveled (Li et al., 2014).
The development and introduction of polysaccharide-protein conjugate vaccines have been major advances in the prevention of meningococcal disease. Meningococcal conjugate vaccines are safe, have at least equal efficacy to the polysaccharide vaccines and considerably greater effectiveness, and induce immune memory responses and amnestic antibody levels with repeated doses in contrast to the hypo-responsiveness seen with repeated injection of the polysaccharide. Conjugate vaccines have now largely replaced polysaccharide vaccines in many countries in the prevention of meningococcal disease and a serogroup A conjugate vaccine (Frasch et al., 2012) has been recently introduced into the African meningitis belt with dramatic control of serogroup A meningococcal disease (Collard et al., 2013; Kristiansen et al., 2013). Unlike the polysaccharide vaccines, meningococcal conjugate vaccines have long term effects in reducing transmission by prevention of acquisition, resulting in significant herd protection at modest levels of vaccine coverage. These conjugate vaccines can provide broad long-term protection early in life, through adolescence and into adulthood against the major N. meningitidis serogroups or invasive ST complexes and have the potential to greatly reduce or even eliminate the burden of meningococcal disease. The introduction of the monovalent serogroup C meningococcal conjugate vaccines in 2000 virtually eliminated the incidence of serogroup C meningococcal disease in UK, an effect that has persisted for well over a decade. This vaccine demonstrated 90% effectiveness at 3 years in 11–18 year-olds (Trotter et al., 2004); and herd protection has reduced the rates of serogroup C carriage and disease in non-vaccinated individuals by more than 50% (Ramsay et al., 2003). The serogroup C conjugates also had dramatic reductions in serogroup C disease in other countries. A meningococcal conjugate vaccine for serogroups A, C, Y and W was first licensed in the US for adolescents in early 2005 (Bilukha&Rosenstein, 2005) and now three multivalent meningococcal conjugate vaccines are widely available. In the US these vaccines have been recommended for high risk children over two years of age and one C/Y conjugate is available for use in 2–18 month old children at risk for meningococcal disease.
The serogroup B meningococcal capsule is poorly immunogenic in all populations, even when coupled to carrier proteins (Finne et al., 1983). This is due to structural identity of the serogroup B capsule and polysialic acid moieties of human tissue antigens such as the neural cell adhesion molecule, N-CAM. Thus, strategies for serogroup B vaccine development have been focused on non-capsular surface antigens (Sadarangani&Pollard, 2010; Zimmer&Stephens, 2006). A new serogroup B vaccine using sub-capsular recombinant protein antigens was approved in late 2012 in Europe, is now licensed in the UK, Canada and Australia and has been used in a recent US institutional serogroup B epidemic outbreak (Carter, 2013; Granoff, 2013). A second serogroup B vaccine using sub-capsular recombinant proteins is in late stage clinical trials.
Role of the capsule in meningococcal pathogenesis
The sialic acid-, mannosamine-, or glucosamine-based capsular polysaccharides are major meningococcal virulence factors and are required and/or modulates many aspects of meningococcal pathogenesis. At mucosal surfaces, meningococcal capsules can provide anti-adherent properties through coverage of adherence ligands leading to loss of meningococci from mucosal surfaces and the reduced adherence may facilitate transmission of encapsulated meningococci (Stephens et al., 1993; Virji et al., 1993). A recent study comparing exemplar strains from two hypervirulent clonal complexes (Bartley et al., 2013) showed that the serogroup B capsule was the major inhibitory determinant affecting both attachment to and invasion of host epithelial cells in one meningococcal strain (15-fold and 35-fold increase in the non-encapsulated strain, respectively), but capsule only modestly affected attachment and invasion in another serogroup B strain (≤2-fold difference). Thus, the contribution of capsule to host cell interaction can be influenced by strain to strain differences. Attachment and invasion of human cells are important events in nasopharyngeal colonization, endothelial cell invasion, and possibly transgression of the blood brain barrier by meningococci, events facilitated by pili, outer membrane proteins, and LOS that are unmasked with the loss of capsule (Bartley et al., 2013). Capsules also impart anti-opsonophagocytic and antibactericidal properties to meningococci and enhance survival in the intracellular environment of human cells (Spinosa et al., 2007), and in the bloodstream. Capsules also contribute to antimicrobial peptide resistance, probably by preventing the peptides from reaching the bacterial membrane (Jones et al., 2009). Serogroup C capsule has been shown to inhibit LOS-induced cytokine secretion from monocytes, and block the maturation of dendritic cells by binding to CD14 (Kocabas et al., 2007). Interestingly, encapsulated serogroup B meningococci are reported to escape the phagosome of human epithelial cells, multiply in the cytosol and then spread intracellularly and to surrounding cells by exploiting the microtubule cytoskeleton during late stages of the infectious process (Tala et al., 2008). The serogroup B CPS directly mediates the interactions between bacteria and microtubules, leading to inhibition of tubulin polymerization in vitro (Tala et al., 2014). Curiously, the serogroup C capsule, which differs only by its anomeric linkage from the serogroup B CPS, was not able to interact with microtubules (Tala et al., 2014). Finally, capsular polysaccharides have been proposed to prevent desiccation, thereby supporting meningococcal survival outside the human host. However, encapsulation did not appear to provide a survival advantage to meningococci against desiccation (Tzeng et al., 2013).
The complement system plays a key human host defense role against invasive N. meningitidis (reviewed by Lewis & Ram, 2014). Deficiencies in components of the classical, alternative and terminal complement pathways are strongly associated with an increased incidence of invasive meningococcal infections (Ellison et al., 1983; Figueroa et al., 1993; Lewis&Ram, 2014) and capsules confer increased resistance to complement mediated bactericidal killing. Meningococcal capsules (depending on structure) can limit or enhance complement activation of both classical and alternative pathways on the bacterial surface (Lewis&Ram, 2014). The expression of serogroup B and C sialic acid capsules decrease alternative complement pathway (AP) activation by enhancing the binding of a complement inhibitory protein (factor H) to C3b, thus limiting C3 deposition on bacterial membrane. Capsular polysaccharides of serogroups W and Y paradoxically enhance AP activation and also serve as targets for C3 deposition and foci for subsequent AP amplification (Ram et al., 2011). However, encapsulated serogroups W and Y remain resistant to killing by the AP pathway alone (Ram et al., 2011). Serogroup A CPS also increases resistance to serum bactericidal activities, but does not affect AP-mediated C3b deposition.
Meningococcal CPSs can also inhibit the classical pathway of complement (Agarwal et al., 2014). All major meningococcal capsules (A/B/C/W/Y) interfere with the engagement of C1q by antibody, leading to decreased C4b deposition and inhibition of the classical pathway activation. The attenuation of the classical pathway by capsule is stoichiometric and can be overcome once the titers of antibody and complement exceed a threshold, a likely scenario following immunization (Agarwal et al., 2014).
Terminal complement deficiencies preventing proper formation of the membrane attack complex (MAC) are associated with recurrent meningococcal infections (Figueroa et al., 1993). Further, encapsulated meningococci bind significantly less MAC than non-capsulated strains, prevent efficient insertion of MAC and/or divert MAC to non-bactericidal sites. A kinetic analysis demonstrated minimal amounts of MAC insertions on capsulated strains, while a steady increase was observed on noncapsulated strains (Ram et al., 1999).
Genetic organization of meningococcal capsule locus and capsule gene product functions in disease-causing serogroups
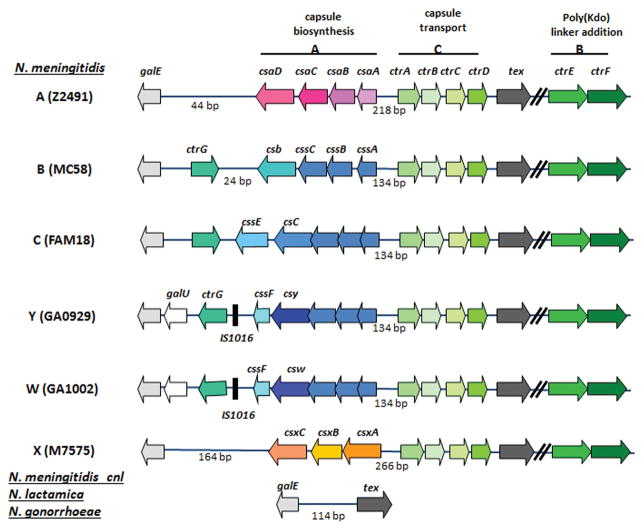
The genetic organization of capsular polysaccharide (cps) locus in all encapsulated meningococcal serogroups has been established by genome sequence analyses and a unified approach to the nomenclature of capsule genes has been published (Harrison et al., 2013). The capsule biosynthesis operon is composed of 3–7 genes in different serogroups (Harrison et al., 2013) and has the highest sequence diversity, consistent with varied capsular polymer compositions. The operon is transcribed divergently from promoters located in the intergenic region between the biosynthesis and the conserved capsule transport (ctr) operons (with the exception of serogroups I and K where the operons are transcribed in the same direction). Both operons have lower GC contents than the overall GC content of meningococcal genomes, indicative of horizontal transfer events (Harrison et al., 2013). Genes encoded independent of the cps locus also influence capsule expression. The meningococcal KpsF is the arabinose 5-phosphate isomerase required for 3-deoxy-D-manno-octulosonic acid (Kdo) biosynthesis. Inactivation of KpsF reduces expression of both sialic acid and non-sialic acid capsules, providing the first demonstration of a role for Kdo in meningococcal capsule expression (Tzeng et al., 2002).
Four genes comprise the capsule biosynthesis operon in sialic acid producing serogroups (Figure 1). The nucleotide sequences of first three biosynthesis genes, cssA/B/C, are highly homologous among the sialic acid capsule-expressing serogroups and these genes encode the N-acetylglucosamine-6-phosphate 2-epimerase, the CMP-sialic acid synthetase, and the sialic acid synthetase, respectively. These three enzymes generate the CMP-sialic acid substrates used for elongation of capsular polymers, which is mediated by the capsule polymerase (named Csb/Csc/Csy/Csw for serogroups B/C/Y/W, respectively). The meningococcal capsule polymerases determine serogroup-specific polymer linkages. The nucleotide sequences of four biosynthetic genes in serogroup A meningococci, csaA-csaD, are unique (Swartley et al., 1998), as is the biosynthesis cassette encoding csxA-csxC in serogroup X (Tzeng et al., 2003).
Figure 1.
Genetic organization of the capsule loci of N. meningitidis serogroups A, B, C, Y, W and X. The capsule transport operon, region C, is highly conserved among all serogroups. The enzymes encoded by cssA-C synthesize CMP-sialic acid, and are conserved among the sialic acid-producing serogroups B, C, Y, and W. The capsule polymerase is encoded by the fourth gene of the css operon, and this gene varies in nucleotide sequence from serogroup to serogroup. The capsules of serogroups A and X are composed of (α1→6)- N-acetylmannosamine 1-phosphate and (α1→4)- N-acetylglucosamine 1-phosphate, respectively, and the biosynthesis genes comprising the biosynthesis operon are unique to those particular serogroups. The length of the intergenic region separating css and ctr operons are indicated. The 134-bp IGR of sialic acid producing serogroups contain two copies of 8-bp direct repeat involved in thermoregulation. Flanking the ctr and syn operons are tex and galE. The nucleotide sequences between the polymerase and galE vary and contain genes encoding the capsule O-acetylation enzymes and ctrG. Two other genes in region B, ctrE and ctrF, are present in encapsulated N. meningitidis, but are not found in N. gonorrhoeae or N. lactamica. In commensal N. meningitidis lacking the cps locus (capsule null locus, cnl), N. gonorrhoeae and N. lactamica, tex and galE are adjacent. The representatives of each serogroup shown are A: Z2491, B: MC58, C: FAM18, Y: GA0929, W: GA1002 and X: M7575.
Several recent reports have defined the biochemical characteristics of meningococcal capsule polymerases and the proteins responsible for O-acetylation of capsule polymers. Csy and Csw, which catalyze heteropolymers, are 98% identical to each other at the nucleotide level, but they are only ~12% identical to Csc and Csb. Csw is a chimeric enzyme comprising two independent sialyl- and galactosyltransferase catalytic domains that work in succession because Csw variants with only one functionality can complement each other in trans when combined in vitro (Romanow et al., 2013; Romanow et al., 2014). Interestingly, amino acid residue 310, located within the predicted hexosyltransferase moiety of the polymerase, accounted for substrate specificity of W and Y capsules: Pro-310 determined galactosyltransferase activity that resulted in a serogroup W capsule, while Gly-310 determined glucosyltransferase activity that yielded a serogroup Y capsule (Claus et al., 2009). Csc and Csb share 75% sequence homology and 64% identity and synthesize sialic acid homopolymers with different linkages. Through chimeric fusion between Csb and Csc, the 107-aa N-terminal sequence was found to be sufficient to determine the α2→8 polymer linkage (Peterson et al., 2011). A 95-aa C-terminal sequence extension of Csb (compared to the E. coli K1 homologue that catalyzes an identical α2→8 linked polysialic acid capsule) was critical for Csb’s activity because deletion of this region completely abolished capsule polymerase enzymatic activity (Freiberger et al., 2007). Recently, an in vitro one-pot synthesis reaction using recombinant CsaA, CsaB, CsaC and their substrates were demonstrated to produce capsule identical to the natural serogroup A polymer by 1H NMR, 31P NMR, and immunoblotting (Fiebig et al., 2014b). The polymerase CsaB is capable of efficiently initiating polymerization and elongating the ManNAc-1-phosphate dimer in the absence of any acceptor. CsaB is also highly selective of non-O-acetylated donor substrates. Thus, O-acetylation of serogroup A capsule takes place on the polysaccharides. Similarly, the serogroup X capsule polymerase, CsxA, is also capable of initiating UDP-GlcNAc polymerization in the absence of any acceptor using short oligosaccharide primers derived from serogroup X capsular polysaccharides (Fiebig et al., 2014a; Muindi et al., 2014). These in vitro biosynthesis systems could potentially serve as alternative strategies to generate capsular polymers for meningococcal conjugate polysaccharide vaccine production.
O-acetylation of capsule polymers is variable among meningococcal capsule serogroups: highest for serogroups A (75–90%), C (88%) and Y (79%), variable for serogroup W (8%-85%) (Longworth et al., 2002), while the serogroup B capsule is not O-acetylated. CssE (OatC) and CssF (OatWY), located downstream of the capsule polymerases, mediate O7-acetylation of sialic acid units in serogroup C and Y/W capsules, respectively (Claus et al., 2004). Critical catalytic residues in CssE and CssF have been defined as well as the crystal structure of CssF (Bergfeld et al., 2009; Lee et al., 2009). On the other hand, O3-acetylation of serogroup A capsule is mediated by CsaC (SacC) encoded within the biosynthesis operon (Gudlavalleti et al., 2004) and CsaC shares no homology with other known O-acetyltransferases or with the sialic acid capsule O-acetyltransferases, CssE and CssF (Claus et al., 2004). O-acetylation is important for immunogenicity and antibody formation following vaccination (Berry et al., 2002; Fusco et al., 2007; Michon et al., 2000), but the immunologic and biologic importance differs by serogroups. The de-O-acetylated serogroup C capsular polysaccharide is more immunogenic than the O-acetylated variant (Richmond et al., 2001); while O-acetylation of serogroup A capsular polysaccharide is critical to immunogenicity of the polysaccharide in both human immune sera and murine antibody response models (Berry et al., 2002). The serogroup A csaC mutant also shows significantly reduced ability to establish colonization in a mouse model of meningococcal colonization (Yi et al., 2003).
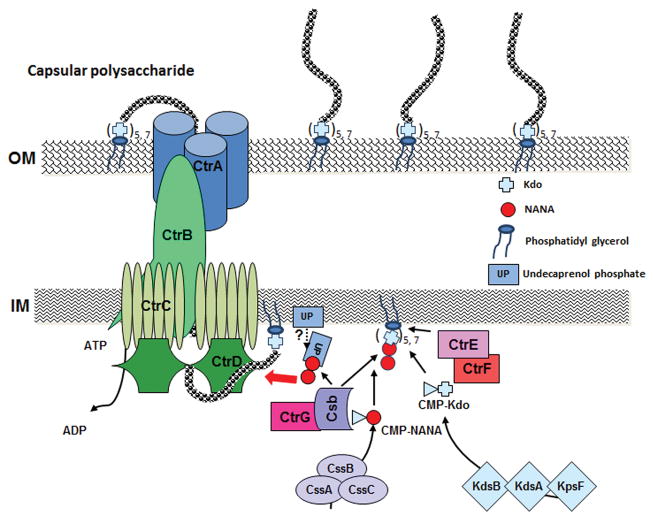
Capsular polysaccharides are anchored to the outer membrane via lipid modification of 1, 2-diacylglycerols at the reducing end of the polymers (Figure 2). Approximately 80–90% of the phospholipid substitution is dipalmitoyl (C16:0) glycerol and the rest is distearoyl (C18:0) glycerol. A recent study utilizing endo-sialidase from E. coli K1 CPS-specific bacteriophages further characterized the detailed structures of the outer membrane anchoring moieties. Digestion of purified CPS with a specific endo-sialidase depolymerizes the CPS to species containing the first few residues of the repeating unit and the terminal lipid. Mass spectrometry analyses revealed the presence of a β-linked poly-Kdo linker (5 or 7 Kdo units) between the polysialic acid polymer and the phosphatidylglycerol moiety with either lyso- or diacyl chains (Willis et al., 2013). The poly-Kdo linker is proposed to be a unifying feature of CPSs synthesized via the ABC transporter-dependent pathway (Willis&Whitfield, 2013b). Subsequently, meningococcal CtrE and CtrF encoded in region B were shown to be the β-Kdo transferases (Willis&Whitfield, 2013a). These two capsule related β-Kdo transferases are not homologous to the α-Kdo transferase, KdtA, responsible for Kdo incorporation in LPS (Willis&Whitfield, 2013a). Cross-complementation can be achieved with the meningococcal CtrE/F proteins and the corresponding E. coli KpsC/S homologues. In vitro, CtrF adds the first Kdo residue to a phosphatidylglycerol acceptor and CtrE then adds additional Kdo residues (Willis&Whitfield, 2013a). However, a functional role of undecaprenol-phosphate (UP), which participates as a lipid carrier in protein glycosylation, O-antigen biosynthesis and peptidoglycan biosynthesis, has also been proposed to act as an intermediate carrier of sialyl residues in capsular polysaccharide biosynthesis in N. meningitidis (Masson&Holbein, 1985) and K1 E. coli (Troy et al., 1975) but has not been well defined. Another gene within the cps locus, ctrG, is exclusively associated with sialic acid-containing serogroups and a homologue can be found in other sialic acid capsule producing pathogens (e.g. K1 E. coli). CtrG was proposed to serve as a priming enzyme that adds the first residue of the CPS repeat unit to the poly-Kdo linker to create an acceptor appropriate for continuing CPS polymerization by capsule polymerases (Willis&Whitfield, 2013b). As the highly conserved CPS transport machinery displays no specificity for the CPS structures, the conserved β-poly Kdo-glycolipid moiety is envisioned as an attractive candidate for an export signal. Curiously, the ctrE, ctrF and ctrG mutations in N. meningitidis caused accumulation of intracellular diacyl phosphatidylglycerol modified CPS at near wild type levels (Hobb et al., 2010; Tzeng et al., 2005). Thus, it appears that in the absence of CtrE/F/G an aberrant poly-Kdo independent lipid modification of capsular polymers is operational in meningococcal CPS production. However, the resulting aberrant lipidated polymers are not engaged with the translocation apparatus for export to the bacterial surface, consistent with the idea of β-poly Kdo-glycolipid functioning as export signal. Alternatively, CtrE/F/G proteins may have additional functions in enabling the proper translocation of completely assembled capsular polysaccharides. The differences in capsule assembly between meningococcal and E. coli systems require further studies.
Figure 2.
A hypothetical model of capsule assembly and translocation apparatus based on homology analysis using serogroup B capsular polysaccharide as an example. UP, undecaprenol phosphate; NANA, sialic acid; Kdo, 3-deoxy-D-manno-octulosonic acid; IM, inner membrane; OM, outer membrane.
Biosynthesis of capsular polymers occurs in the cytoplasm at the cytoplasmic membrane. Four polycistronic genes, ctrA-D, encode proteins that transport CPS across the inner and outer membranes (Figure 2). CtrC and CtrD share amino acid homology with the ATP-dependent transporters. CtrA and CtrB are proposed to form a cell envelope-spanning export complex. CtrB shares homology to membrane fusion proteins thought to create a link between the inner and outer membranes and facilitate substrate transport through the designated porin, CtrA. Membrane topology analyses of CtrB showed that its N terminus is located in the cytoplasm and the central portion of the protein is located in the periplasm. Cryo-EM studies showed that CtrB reconstituted into lipid bilayers forms an oligomeric periplasmic structure with a central cavity (Larue et al., 2011). Cross-complementations of CtrA and CtrB in E. coli mutants with defects in genes encoding the corresponding proteins show that only cognate CtrA/B protein pairs are active in CPS export (Larue et al., 2011).
MECHANISMS AFFECTING CAPSULE EXPRESSION
Clinical, in vitro, and epidemiological studies indicate that capsules are not constitutively expressed on the meningococcal surface. CPS is increased on clinical isolates recovered from patients with invasive disease, and the absence or decreased expression of capsule is noted on meningococci of identical genetic type recovered from the nasopharynx. In an infant mouse intranasal infection model comparing epidemiologically related case and carrier isolates, non-encapsulated carrier strains failed to colonize the nasal passages and were not invasive, supporting capsule as a major virulence factor (Mackinnon et al., 1993). Considering the different environments that meningococci may encounter and the dichotomous effects of the presence or absence of the capsular polysaccharide on host-pathogen interactions, it is not surprising that meningococci have evolved multiple strategies for altering and regulating the expression of capsules.
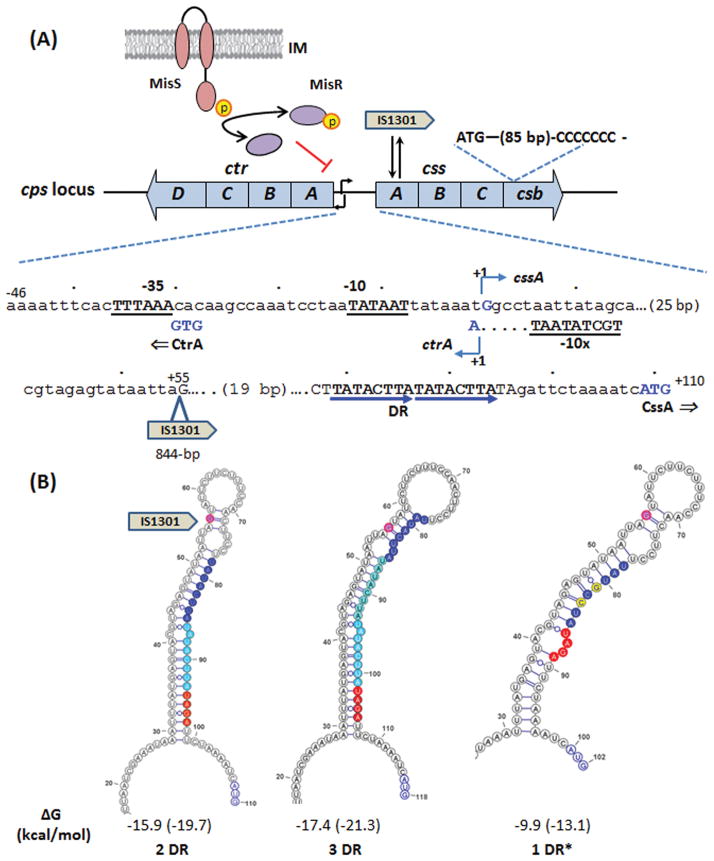
Meningococcal capsule expression is changed through 1) on-off/off-on phase variation of capsule biosynthesis genes, 2) structural alterations of the capsular polysaccharides and 3) modifying the amount of capsular polysaccharide expressed at the bacterial surface. Multiple genetic mechanisms contribute to this variability (Figure 3). However, many of these mechanisms have only been characterized in certain serogroups and may not be universal for all meningococci. For example, on-off phase variation of capsule controlled by slipped strand mispairing of homo polymeric tracts that results in premature translation termination was observed only in serogroup B meningococci (Hammerschmidt et al., 1996b; Martin et al., 2004); while reversible disruption of cssA via the precise insertion and excision of an insertion element was shown in both serogroups B and C meningococci (Hammerschmidt et al., 1996a). Phase-variable expression of capsular polymer acetylation enzymes that modify capsule structures was detected in serogroups W and Y but not serogroup C meningococci (Claus et al., 2004). In contrast, some capsule changes can occur in multiple serogroups, such as the switching of the capsule polymer compositions by transformation and subsequent homologous recombination of different capsule polymerases, and the permanent loss of capsule expression by transformation of allelic variants. The regulatory mechanisms that modulate the amounts of capsule production are best characterized in sialic acid capsule expressing meningococci and operate at both transcriptional and translational levels. These include transcriptional regulation of the divergent promoters of capsule biosynthesis and transport operon, an IS element insertion into the untranslated region of cssA, a two-component regulatory system as well as post transcriptional RNA thermoregulation of cssA translation (Figure 3). The current understanding of the mechanisms that change capsule expression will be described in the following sections.
Figure 3.
Summary of the regulatory events affecting capsule expression. (A) Schematics of the ctr and css operons of the serogroup B cps locus are shown with the partial intergenic sequence displayed below. The transcriptional start site is marked as +1 with the nucleotides G (css), A (ctr) and the start codons of CssA and CtrA shown in blue. The promoter elements are underlined and in bold. The IS1301 insertion within UTR occurred at the +55 position. The 8-bp direct repeat moiety served as RNA thermosensor is marked by blue arrows. The MisRS TCS mediated regulation, the reversible IS1301 insertion/excision event within cssA and the slipped strand mispairing poly(C) track are also indicated. (B) The stem-loop structures with minimal free energy (ΔG in kcal/mol) formed by sequences with two copies (2DR), three copies (3DR) and one copy (1DR) of the 8-bp direct repeat sequence (blue). One of several possible 1DR* structures, which are predicted to have similar ΔG values, is shown here with the A/G and T/C nucleotide changes labeled in yellow. The RBS sequence is shown in red and the translational start codon is colored in blue. The +55 nucleotide position of the IS1301 insertion site is marked in magenta. The folding structures formed by sequences from the transcriptional start site to the ATG start codon are predicted by the RNAfold program. The ΔG calculations using default parameters at 37°C and 30°C (in parentheses) are shown under each structure.
Transformation and capsule switching
N. meningitidis is naturally competent for genetic transformation and the population is shaped via transformation-and-recombination events more than by spontaneous mutations. Transformation plays a major role in the genome plasticity and virulence of meningococci. Based on low GC content, the capsule cps locus (Figure 1) has been designated a putative island of horizontally transferred DNA (Tettelin et al., 2000) and may have originated as one or more transformation events in a primordial meningococcal ancestor (Schoen et al., 2008) whose cps locus resembled that found in capsule negative locus (cnl) meningococci, N. gonorrhoeae or commensal Neisseria species.
Transformation and recombination events influence expression of meningococcal capsule (Frosch&Meyer, 1992). Co-colonization of different serogroup strains in the human nasopharynx and genetic exchange of the cps gene clusters by transformation and allelic-exchange result in “capsule switching” in vivo (Swartley et al., 1997). The meningococcal capsule-switching phenomenon was first reported in 1997 in a serogroup B outbreak in the U.S. Pacific Northwest (Swartley et al., 1997). Closely related serogroup B meningococci of the ST-32 complex were initially isolated, but within several years of onset of the outbreak, cases caused by strains of serogroup C/ST-32 complex were detected (Swartley et al., 1997). Molecular typing and genetic analysis of the strains showed that they were identical except for a transformation event in the capsule biosynthesis locus. In an analysis of invasive N. meningitidis isolates in the US in the pre-meningococcal conjugate vaccine era, 2000–2005 (Harrison et al., 2010), 1.5% of serogroup B isolates, 0.9% of serogroup Y isolates and 12.9% of serogroup C isolates were identified as potential capsule switchers. Most of the serogroup C capsule switches were from virulent serogroup B or serogroup Y lineages. Gene conversion events that change the capsule polymerase from (α2→8)-linked polysialic acid (serogroup B) to (α2→9)-linked polysialic acid (serogroup C) may be facilitated by the high similarity of the DNA coding sequences between these serogroups (Edwards et al., 1994; Swartley et al., 1997). Also, there is evidence for selection processes that favor or restrict transformation events, such as the restriction modification system differences noted between ST-11 and ST-32 isolates (Claus et al., 2000). More recently, surveillance in Spain (Alcala et al., 2002), Portugal (Simoes et al., 2009), Italy (Stefanelli et al., 2003), Czech Republic (Kriz et al., 1999) and Canada (Kertesz et al., 1998; Tsang et al., 2005) have detected C→B capsular switching either after the implementation of mass vaccination of monovalent serogroup C conjugate vaccine or during epidemic situations. There is also a report of capsular switching between serogroups A and C strains in China (Wang et al., 2010). Further, evidence for capsule switching between carriage (W) and invasive meningococci (C) was reported in New Zealand and the exchange of entire cps locus was suggested (Beddek et al., 2009). Capsule switching can occur rapidly. An otherwise identical serogroup C strain was isolated from close contact of a fulminant serogroup B meningococcal septicemia index case within a few days (Vogel et al., 2000). The ability to switch capsules presumably provides advantages to meningococci due to escape of naturally acquired and vaccine-induced anti-capsular immunity.
Transformation of mutated capsule biosynthesis genes between meningococci in vivo can also result in non-encapsulated phenotypes. Many nongroupable isolates recovered from carriers contain point mutations within an otherwise intact serogroup B, C, or Y biosynthesis operon or they contain evidence of major recombination events such as deletion of regions of the cps locus or an insertion element (IS1301 or IS1016-like) either within or in place of the biosynthesis operon (Dolan-Livengood et al., 2003). Also, one-third or more of nongroupable meningococci in the nasopharynx often contain no cps locus, rendering them non-encapsulated (Claus et al., 2002; Dolan-Livengood et al., 2003). These isolates harbor the N. gonorrhoeae/N. lactamica-like genetic configuration (cnl, Figure 1) at the cps locus.
Phase variable expression of capsule or changes in capsule structure mediated by IS elements
While studying the influence of capsule and sialylated lipooligosaccharide (LOS) on adhesion and invasion of epithelial cells using an encapsulated serogroup B isolate, Hammerschmidt et al. detected capsule-negative meningococci at high frequencies in the intracellular population. Further characterization revealed that an insertion element IS1301 was present in the cssA coding sequence (Hammerschmidt et al., 1996a). Under in vitro culture conditions, a precise excision of IS1301 from cssA that restored capsule expression was found to be at a frequency of 4 × 10−4. No IS1301-mediated capsule phase variation was detected in in vitro experiments (Hammerschmidt et al., 1996b), suggesting that IS1301 insertion into cssA might be a rare event (≤ 1×10−5). However, ~60–100% of intracellular bacteria that survived gentamicin treatment were capsule negative, suggesting a selective advantage conferred by un-capsulated meningococci in the infection assays and the IS1301 insertion into cssA was detected in 10–50% of all unencapsulated meningococci recovered from epithelial cells. Thus, IS1301-mediated capsule phase variation might provide a selective advantage under certain in vivo conditions, but it is difficult to assess whether this mechanism is of any major biological relevance during meningococcal infection. IS1301, which has a GC content of 42.2%, is 844 bp in length with 19-bp flanking inverted repeats. Only the sialic acid-producing meningococcal serogroups, but not serogroup A N. meningitidis, N. gonorrhoeae, and commensal Neisseria species, appear to harbor IS1301. In addition to the reversible off-on switching of capsule expression, IS1301 insertion of cssA also results in the loss of sialylation of LOS. Sialylated LOS, in the absence of capsule, can influence adherence of meningococci to epithelial cells (Bartley et al., 2013).
Additional IS1301 insertion events within the cps locus have been identified. IS1301 was found within csb, which would preserve LOS sialylation but eliminate capsule expression. Further, IS1301 was found in the biosynthesis operon of nongroupable meningococcal invasive and carriage isolates from a population-based collection (Dolan-Livengood et al., 2003). In these isolates, the IS1301 insertions were often associated with deletions of the capsule biosynthesis or transport coding sequences, thus permanently inactivating capsule expression. Finally, IS1301 was also found to disrupt cssF in many serogroup W and Y strains, yielding non-acetylated CPS (Claus et al., 2004).
Hyperencapsulation associated with IS elements
Three epidemiologically unlinked serogroup C ST-11 N. meningitidis invasive isolates from Spain were found to be highly resistant to conjugate vaccine induced bactericidal antibodies and complement-mediated killing (Uria et al., 2008). In these isolates, an IS1301 element was inserted in the untranslated region of cssA. This insertion appeared to lead to transcriptional up-regulation of both css and ctr operons with concomitant increased capsule production, consequently evading killing by bactericidal antibodies (Uria et al., 2008). Because the css-ctr intergenic region is highly conserved and IS1301 elements are prevalent in all sialic-acid producing serogroups, the concern about vaccine efficacy and potential vaccine failures due to IS1301 was raised. Curiously, strains containing the same IS1301 insertion within IGR but without capsule up-regulation were subsequently identified in many serogroup B isolates. These isolates have additional polymorphisms, including either an 8-bp deletion within the untranslated region of cssA (which also eliminated RNA thermoregulation, discussed below) or a 69-bp insertion within IS1301 (Kugelberg et al., 2010). Thus, the mechanisms by which IS1301 up-regulates CPS transcription are not fully understood. Interestingly, an IS1016 element is found within the intergenic region separating ctrA and csxA in multiple serogroup X strains without apparent effects on capsule expression (Tzeng et al., 2003).
Phase variation of encapsulation or O-acetylation by slipped strand mispairing
Slipped-strand mispairing, which is believed to occur during DNA replication or possibly during DNA repair, is a common mechanism of phase variation in Neisseria spp. and can affect capsule expression. Comparative whole genome analyses estimate ~ 100 putative phase-variable genes in pathogenic Neisseria spp. (Snyder et al., 2001). Analysis of unencapsulated serogroup B meningococcal variants isolated from the intracellular population that did not contain IS1301 detected re-expression of capsule at a frequency of 10−3 (Hammerschmidt et al., 1996b). Nucleotide sequencing revealed a stretch of seven cytidine residues at position 89 of the csb polysialyltransferase coding sequence (Hammerschmidt et al., 1996b). Insertion or deletion of a single cytidine causes a frame shift and results in a premature translational termination and consequently a switch of capsule expression from on to off. The in vitro frequency of loss of capsule expression due to an insertion of one dC causing out-of-frame switch was 3 × 10−5 (Hammerschmidt et al., 1996b). Studies of several other serogroup B strains showed that the on-to-off switch frequencies were lower than 1 × 10−5, but can be enhanced significantly by a mutS mutation in the mismatch repair system (Martin et al., 2004). The deletion of a dC residue from an 8 dC tract that restored capsule expression, however, occurred at a much higher frequency, 1 × 10−3 (Hammerschmidt et al., 1996b) and the reversion from an 8 dC tract is more frequent than from a 6 dC tract. Curiously, the polycistronic css mRNA in the csb phase-off strains is reduced in size as a result of premature transcription termination at a cryptic Rho-dependent site within csb. This phenomenon links transcription termination to the premature translational termination caused by phase variation (Lavitola et al., 1999). The serogroup Y capsule polymerase also contains a poly(C)7 tract at position 148 and the serogroup W polymerase contains 6 dCs at position 149. However, the slipped-strand mispairing control of encapsulation has thus far only been defined in serogroup B strains. Slipped-strand mispairing is also the cause of phase variable O-acetylation in serogroup C meningococci (Claus et al., 2004). A poly(T)7 and a poly(A)7 homopolymeric tract was found at positions 210 and 458 of cssE, respectively, and variations in either tract results in premature translational termination of acetyltransferases.
Transcriptional regulation of capsule expression
The promoters essential for transcription of the css and ctr operons overlap and reside within a highly homologous 134-bp intergenic region in serogroups B, C, W and Y. The start site of ctrA transcription is one nucleotide from the transcriptional start site of cssA and both start sites were preceded by perfect σ70 −10 boxes. However, the only identifiable −35 region preceded cssA (Fig. 3A). The css promoter was shown to exhibit ~four-fold higher activity than did the ctr promoter, and activities of both promoters were shown to be growth-phase dependent (Tzeng et al., 2001). Mutagenesis involving specific deletions and insertions within the intergenic region indicates that a major control of transcriptional activity is located within the cssA untranslated region (UTR) (Tzeng et al., 2001; Von Loewenich et al., 2001) that contains direct and inverted repeats motifs. The intergenic regions of serogroups A and X, 218-bp and 266-bp, respectively, are distinct from those of sialic acid containing serogroups and share no similarities to other non-pathogenic serogroups.
A transcriptional factor implicated in the regulation of capsule expression is CrgA. CrgA, a LysR family transcriptional regulator, is induced upon meningococcal contact with epithelial cells through a contact regulatory element of Neisseria (CREN) within the crgA promoter (Morelle et al., 2003). This has been proposed as a link between host cell contact and decreased levels of capsule expression because down-regulation of capsule during intimate host cell interaction was observed in the wild type strain, but not in the crgA mutant. However, the role of CrgA in regulating several adherence relevant genes, including cssA, pilC1 and pilE, has been disputed (Ieva et al., 2005). Ieva et al. demonstrated that CrgA is a repressor of its own expression and an activator for the divergently transcribed mdaB encoding a NADPH-quinone oxidoreductase. CrgA responds to α-methylene-γ-butyrolactone, an inducer of MdaB, to further repress or induce expression of crgA and mdaB, respectively, while the inhibitor elicits no effect on the expression of cssA. In addition, induction of CrgA protein in a Ptac::crgA strain by IPTG did not alter cssA transcription. Thus, the mechanism of adherence-mediated capsule regulation remains to be defined.
To successfully colonize and invade hosts, many bacterial pathogens utilize two-component systems (TCSs) to sense the microenvironment and coordinately regulate gene expression in response to signals emanating from the host. Capsules in several bacteria are known to be regulated by TCSs (Deretic&Konyecsni, 1989; Heath et al., 1999; Stout&Gottesman, 1990). Meningococci encode relatively few (4 pairs) TCSs and capsule expression is affected through one of these, the MisR/MisS system. Capsule production is increased in misR and misS mutants, which display a concomitant enhanced serum resistance, thus implying a negative regulatory role mediated by the MisRS TCS (Tzeng et al., 2008). The response regulator, MisR, was shown to bind directly to the divergent promoter region by gel mobility-shift assays (Bartley et al., 2013), but the regulatory sequence has not been defined. Thus, this environmental sensing two-component system plays a role in regulating meningococcal capsule expression, however, the environmental signal detected by the MisRS system remain to be defined.
Altered transcription of capsule genes has been detected in several transcriptome studies investigating various environmental conditions that meningococci presumably encounter during colonization and invasion (Dietrich et al., 2003; Echenique-Rivera et al., 2011; Kurz et al., 2003). Capsule expression was reported to be down-regulated during intimate bacterial adherence to epithelial Hec-1-B cells (Deghmane et al., 2002). However, microarray transcriptome analysis of a non-encapsulated meningococcal strain attached to epithelial HEp-2 cells showed up-regulation of ctrC/D/G and cssA/B (Dietrich et al., 2003). This up-regulation was not detected in meningococci adhered to human brain microvascular endothelial cells (HBMEC) in the same study. Differences in meningococcal strains, cell lines and experimental conditions may explain these variable results. Up-regulation of ctrE and csb are also detected during the intracellular phase of meningococcal infection (Spinosa et al., 2007). Treating meningococci with normal human serum followed by a microarray comparison to bacteria suspended in PBS, detected three capsule genes being differentially expressed (Kurz et al., 2003). In contrast, none of the capsule genes were differentially regulated in another microarray analysis examining transition to growth in human blood (Echenique-Rivera et al., 2011). These data could suggest additional regulatory mechanisms that may be clonal complex-dependent.
Post-transcriptional control of meningococcal capsule expression
The 5′ untranslated region (UTR) of cssA contains certain polymorphisms, which do not appear to be serogroup- or lineage-specific among meningococcal isolates. Recently, in order to identify genetic changes affecting serum resistance other than IS1301 insertions (Uria et al., 2008), Loh et al. subjected a N. meningitidis serum sensitive strain to serial passages in human serum. Within 6 rounds this strain became as resistant to killing as a strain with IS1301 in the UTR of cssA (Loh et al., 2013). Nucleotide sequencing of the cps locus identified the loss of a single copy of a duplicated 8-bp direct repeat sequence within UTRcssA. The UTR of css mRNA is predicted to form a secondary structure that includes the predicted ribosome-binding site (RBS) in the stem, while the 8-bp deletion would result in a destabilized stem–loop with exposed RBS (Figure 3B). The predicted secondary structure of css mRNA is consistent with an RNA thermosensor (Johansson et al., 2002). In RNA thermosensors, the mRNA assumes a hairpin structure at lower temperatures that occludes the RBS, and stalls protein translation; higher temperatures destabilize the secondary structure, allowing translation (Johansson et al., 2002). Consistent with a RNA thermosensor regulation, CssA protein levels are higher in N. meningitidis grown at 37°C than when grown at 30°C. In strains with the 8-bp deletion, although the css mRNA levels are unaffected, CssA protein levels were increased at lower temperatures when compared to the wild type strain and thus had a less pronounced increase at higher temperatures. These data indicated that the loss of one 8-bp repeat disrupts the thermosensor and post-transcriptionally dysregulates capsule biosynthesis. The RNA thermosensor secondary structure is also proposed to modulate two other meningococcal immune defense mechanisms conferring resistance against complement-mediated killing: the expression of a factor H binding protein (McNeil et al., 2013) and the Lst sialyltransferase controlling sialylation of lipopolysaccharide (Gilbert et al., 1996). Thus, by sensing an increase in ambient temperature caused by local inflammation, RNA thermosensor regulation of multiple immune defense mechanisms would presumably offer an adaptive advantage to the meningococcus. For example, in the nasopharynx during co-infection with other respiratory pathogens increased temperature and up-regulation of capsule could promote transmission. In invasive sites (e.g., bloodstream), which is at higher temperatures than the nasopharynx, thermoregulation could also provide an advantage to meningococci.
The prevalence of polymorphisms within the RNA thermosensor sequence was evaluated among a collection of meningococcal disease isolates (Loh et al., 2013). Isolates with two copies of the 8-bp direct repeat sequence (2DR) were most frequently found in this collection (~75%); while strains with the loss of one 8-bp repeat (1DR) represent the remainder. Among the 1DR strains, 78% have two nucleotide substitutions (1DR*, Fig. 3B), 9% with a single substitution and 13% with no changes. Those with either one or two nucleotide substitutions were shown to restore thermoregulation of CssA by Western blots in E. coli (Loh et al., 2013). Strains that carry 3 copies of the 8-bp sequence (3DR) were also identified (Von Loewenich et al., 2001) and a stem-loop structure similar to that of 2DR sequence is predicted (Fig. 3B). The effectiveness of thermoregulation among these polymorphisms remains to be better defined. Curiously, IS1301 insertion events into the UTRcssA described above, which could lead to transcriptional up-regulation of both css and ctr operons, occur within the proposed RNA thermosensor secondary structure (Figure 3B). The insertion would displace the 5′ stem-forming sequence and disrupt the thermosensing mechanism. Thus, clones acquired IS1301 insertion within the UTRcssA that resulted in hyper-encapsulation may incur a fitness cost of losing the presumptive beneficial thermosensing regulation.
CONCLUSIONS
Neisseria meningitidis (the meningococcus) is the cause of epidemic “cerebrospinal fever” including clusters and sporadic cases of acute bacterial meningitis and septicemia. Significant advances have been made in the last decade in the introduction of new vaccines to prevent meningococcal disease. The new meningococcal polysaccharide-protein conjugate vaccines (McIntyre et al., 2012; Vipond et al., 2012) against serogroups A, C, Y, and/or W and the new serogroup B recombinant protein based vaccines achieving licensure, hold great promise for prevention of meningococcal disease globally and in expanded populations (e.g., infants and young children). However, threats to these vaccines are the emergence of previously minor capsular serogroups that now cause significant endemic and epidemic meningococcal disease (e.g., Y, W and X in the last two decades), the escape from capsule specific immune protection by “capsule switching”, and the resistance to bactericidal antibodies produced by these vaccines through “hyperencapsulation”.
To better understand meningococcal pathogenesis and the threats to meningococcal vaccines a thorough understanding of capsule expression and regulation in Neisseria meningitidis is important. As summarized in this review, meningococci have evolved multiple genetic mechanisms to maintain variability in the regulation and expression of capsule. These mechanisms include: 1) transcriptional regulation of capsule synthesis, 2) transformation and recombination of mutated or different capsule genes, 3) on-off switches via insertion/excision of IS elements in the biosynthesis genes and slipped-strand mispairing of the capsule polymerases and acetylation enzymes, 4) transcriptional regulation by control of the intergenic promoter region and 5) post-transcriptional RNA thermosensing regulation of the capsule biosynthesis proteins. Similar to other meningococcal outer membrane components, the presence or absence of capsule can vary at frequencies of up to 10−3. Capsule on/off phase variation can also alter the ability to identify potentially invasive meningococcal clones by standard serogrouping techniques. Routine capsule-based serology for diagnosis and classification will not be effective in identifying capsule-negative meningococci that can presumably revert to encapsulated phenotypes, indicating the need for genetically assessing isolates in outbreaks and when new meningococcal vaccines are introduced. While these genogrouping and genetic typing methods (Bennett&Cafferkey, 2006; Zhu et al., 2012) will identify the correct capsular type and genetic background, they will not reveal whether capsule is expressed. Thus, a combination of both serogrouping and genotyping techniques is the most beneficial approach.
The ability to alter or regulate capsule expression provides significant biologic selective advantages to the meningococcus. Loss or down-regulation of capsule expression is important to enhancing cell attachment, cell entry, microcolony formation and to facilitating a carriage state at human mucosal surfaces. Loss of capsule as well as antigenic switching of the capsular polysaccharide by allelic transformation of the capsule genes, selective modification of capsule polymers (e.g., acetylation) and hyperencapulation mechanisms, may alter recognition by capsule-specific bactericidal antibodies (the correlate of protection for polysaccharide and polysaccharide-protein conjugate meningococcal vaccines) and result in immune escape. The ability of N. meningitidis to switch capsular polysaccharides via transformation highlights the importance of meningococcal vaccines directed against multiple serogroups and against other antigens. Thus, N. meningitidis has evolved a diverse molecular arsenal to regulate the expression of capsular polysaccharides. These mechanisms are important to the biology and pathogenesis of the meningococcus and in the development and implementation of meningococcal diagnostics and vaccines.
References
- Agarwal S, Vasudhev S, DeOliveira RB, Ram S. Inhibition of the classical pathway of complement by meningococcal capsular polysaccharides. J Immunol. 2014;193:1855–63. doi: 10.4049/jimmunol.1303177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcala B, Arreaza L, Salcedo C, et al. Capsule switching among C:2b:P1.2,5 meningococcal epidemic strains after mass immunization campaign, Spain. Emerg Infect Dis. 2002;8:1512–4. doi: 10.3201/eid0812.020081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artenstein MS, Gold R, Zimmerly JG, et al. Prevention of meningococcal disease by group C polysaccharide vaccine. N Engl J Med. 1970;282:417–20. doi: 10.1056/NEJM197002192820803. [DOI] [PubMed] [Google Scholar]
- Bartley SN, Tzeng YL, Heel K, et al. Attachment and Invasion of Neisseria meningitidis to Host Cells Is Related to Surface Hydrophobicity, Bacterial Cell Size and Capsule. PLoS One. 2013;8:e55798. doi: 10.1371/journal.pone.0055798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddek AJ, Li MS, Kroll JS, et al. Evidence for capsule switching between carried and disease-causing Neisseria meningitidis strains. Infect Immun. 2009;77:2989–94. doi: 10.1128/IAI.00181-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DE, Cafferkey MT. Consecutive use of two multiplex PCR-based assays for simultaneous identification and determination of capsular status of nine common Neisseria meningitidis serogroups associated with invasive disease. J Clin Microbiol. 2006;44:1127–31. doi: 10.1128/JCM.44.3.1127-1131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfeld AK, Claus H, Lorenzen NK, et al. The polysialic acid-specific O-acetyltransferase OatC from Neisseria meningitidis serogroup C evolved apart from other bacterial sialate O-acetyltransferases. J Biol Chem. 2009;284:6–16. doi: 10.1074/jbc.M807518200. [DOI] [PubMed] [Google Scholar]
- Berry DS, Lynn F, Lee CH, et al. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect Immun. 2002;70:3707–13. doi: 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2005;54:1–21. [PubMed] [Google Scholar]
- Carter NJ. Multicomponent meningococcal serogroup B vaccine (4CMenB; Bexsero): a review of its use in primary and booster vaccination. BioDrugs. 2013;27:263–74. doi: 10.1007/s40259-013-0029-2. [DOI] [PubMed] [Google Scholar]
- Claus H, Borrow R, Achtman M, et al. Genetics of capsule O-acetylation in serogroup C, W-135 and Y meningococci. Mol Microbiol. 2004;51:227–39. doi: 10.1046/j.1365-2958.2003.03819.x. [DOI] [PubMed] [Google Scholar]
- Claus H, Friedrich A, Frosch M, Vogel U. Differential distribution of novel restriction-modification systems in clonal lineages of Neisseria meningitidis. J Bacteriol. 2000;182:1296–303. doi: 10.1128/jb.182.5.1296-1303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus H, Maiden MC, Maag R, et al. Many carried meningococci lack the genes required for capsule synthesis and transport. Microbiology. 2002;148:1813–9. doi: 10.1099/00221287-148-6-1813. [DOI] [PubMed] [Google Scholar]
- Claus H, Stummeyer K, Batzilla J, et al. Amino acid 310 determines the donor substrate specificity of serogroup W-135 and Y capsule polymerases of Neisseria meningitidis. Mol Microbiol. 2009;71:960–71. doi: 10.1111/j.1365-2958.2008.06580.x. [DOI] [PubMed] [Google Scholar]
- Collard JM, Issaka B, Zaneidou M, et al. Epidemiological changes in meningococcal meningitis in Niger from 2008 to 2011 and the impact of vaccination. BMC Infect Dis. 2013;13:576. doi: 10.1186/1471-2334-13-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deghmane AE, Giorgini D, Larribe M, et al. Down-regulation of pili and capsule of Neisseria meningitidis upon contact with epithelial cells is mediated by CrgA regulatory protein. Mol Microbiol. 2002;43:1555–64. doi: 10.1046/j.1365-2958.2002.02838.x. [DOI] [PubMed] [Google Scholar]
- Deretic V, Konyecsni WM. Control of mucoidy in Pseudomonas aeruginosa: transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol. 1989;171:3680–8. doi: 10.1128/jb.171.7.3680-3688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich G, Kurz S, Hubner C, et al. Transcriptome analysis of Neisseria meningitidis during infection. J Bacteriol. 2003;185:155–64. doi: 10.1128/JB.185.1.155-164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan-Livengood JM, Miller YK, Martin LE, et al. Genetic basis for nongroupable Neisseria meningitidis. J Infect Dis. 2003;187:1616–28. doi: 10.1086/374740. [DOI] [PubMed] [Google Scholar]
- Echenique-Rivera H, Muzzi A, Del Tordello E, et al. Transcriptome analysis of Neisseria meningitidis in human whole blood and mutagenesis studies identify virulence factors involved in blood survival. PLoS Pathog. 2011;7:e1002027. doi: 10.1371/journal.ppat.1002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards U, Muller A, Hammerschmidt S, et al. Molecular analysis of the biosynthesis pathway of the alpha-2-->8 polysialic acid capsule by Neisseria meningitidis serogroup B. Mol Microbiol. 1994;14:141–9. doi: 10.1111/j.1365-2958.1994.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Ellison RTd, Kohler PF, Curd JG, et al. Prevalence of congenital or acquired complement deficiency in patients with sporadic meningocococcal disease. N Engl J Med. 1983;308:913–6. doi: 10.1056/NEJM198304213081601. [DOI] [PubMed] [Google Scholar]
- Fiebig T, Berti F, Freiberger F, et al. Functional expression of the capsule polymerase of Neisseria meningitidis serogroup X: a new perspective for vaccine development. Glycobiology. 2014a;24:150–8. doi: 10.1093/glycob/cwt102. [DOI] [PubMed] [Google Scholar]
- Fiebig T, Freiberger F, Pinto V, et al. Molecular Cloning and Functional Characterization of Components of the Capsule Biosynthesis Complex of Neisseria meningitidis Serogroup A: TOWARD IN VITRO VACCINE PRODUCTION. J Biol Chem. 2014b;289:19395–407. doi: 10.1074/jbc.M114.575142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol Res. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet. 1983;2:355–7. doi: 10.1016/s0140-6736(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Frasch CE, Preziosi MP, LaForce FM. Development of a group A meningococcal conjugate vaccine, MenAfriVac(TM) Hum Vaccin Immunother. 2012;8:715–24. doi: 10.4161/hv.19619. [DOI] [PubMed] [Google Scholar]
- Freiberger F, Claus H, Gunzel A, et al. Biochemical characterization of a Neisseria meningitidis polysialyltransferase reveals novel functional motifs in bacterial sialyltransferases. Mol Microbiol. 2007;65:1258–75. doi: 10.1111/j.1365-2958.2007.05862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosch M, Meyer TF. Transformation-mediated exchange of virulence determinants by co-cultivation of pathogenic Neisseriae. FEMS Microbiology Letters. 1992;79:345–9. doi: 10.1111/j.1574-6968.1992.tb14062.x. [DOI] [PubMed] [Google Scholar]
- Fusco PC, Farley EK, Huang CH, et al. Protective meningococcal capsular polysaccharide epitopes and the role of O acetylation. Clin Vaccine Immunol. 2007;14:577–84. doi: 10.1128/CVI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M, Watson DC, Cunningham AM, et al. Cloning of the lipooligosaccharide alpha-2,3-sialyltransferase from the bacterial pathogens Neisseria meningitidis and Neisseria gonorrhoeae. J Biol Chem. 1996;271:28271–6. doi: 10.1074/jbc.271.45.28271. [DOI] [PubMed] [Google Scholar]
- Granoff DM. Commentary: European Medicines Agency recommends approval of a broadly protective vaccine against serogroup B meningococcal disease. Pediatr Infect Dis J. 2013;32:372–3. doi: 10.1097/INF.0b013e318282942f. [DOI] [PubMed] [Google Scholar]
- Granoff DM, Gupta RK, Belshe RB, Anderson EL. Induction of immunologic refractoriness in adults by meningococcal C polysaccharide vaccination. J Infect Dis. 1998;178:870–4. doi: 10.1086/515346. [DOI] [PubMed] [Google Scholar]
- Gudlavalleti SK, Datta AK, Tzeng YL, et al. The Neisseria meningitidis serogroup A capsular polysaccharide O-3 and O-4 acetyltransferase. J Biol Chem. 2004;279:42765–73. doi: 10.1074/jbc.M313552200. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt S, Hilse R, van Putten JP, et al. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 1996a;15:192–8. [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt S, Muller A, Sillmann H, et al. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996b;20:1211–20. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- Harrison LH, Shutt KA, Schmink SE, et al. Population structure and capsular switching of invasive Neisseria meningitidis isolates in the pre-meningococcal conjugate vaccine era--United States, 2000–2005. J Infect Dis. 2010;201:1208–24. doi: 10.1086/651505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison OB, Claus H, Jiang Y, et al. Description and nomenclature of Neisseria meningitidis capsule locus. Emerg Infect Dis. 2013;19:566–73. doi: 10.3201/eid1904.111799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath A, DiRita VJ, Barg NL, Engleberg NC. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun. 1999;67:5298–305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobb RI, Tzeng YL, Choudhury BP, et al. Requirement of NMB0065 for connecting assembly and export of sialic acid capsular polysaccharides in Neisseria meningitidis. Microbes Infect. 2010;12:476–87. doi: 10.1016/j.micinf.2010.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieva R, Alaimo C, Delany I, et al. CrgA is an inducible LysR-type regulator of Neisseria meningitidis, acting both as a repressor and as an activator of gene transcription. J Bacteriol. 2005;187:3421–30. doi: 10.1128/JB.187.10.3421-3430.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson J, Mandin P, Renzoni A, et al. An RNA thermosensor controls expression of virulence genes in Listeria monocytogenes. Cell. 2002;110:551–61. doi: 10.1016/s0092-8674(02)00905-4. [DOI] [PubMed] [Google Scholar]
- Jones A, Georg M, Maudsdotter L, Jonsson AB. Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J Bacteriol. 2009;191:3861–8. doi: 10.1128/JB.01313-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz DA, Coulthart MB, Ryan JA, et al. Serogroup B, electrophoretic type 15 Neisseria meningitidis in Canada. J Infect Dis. 1998;177:1754–7. doi: 10.1086/517439. [DOI] [PubMed] [Google Scholar]
- Kocabas C, Katsenelson N, Kanswal S, et al. Neisseria meningitidis type C capsular polysaccharide inhibits lipooligosaccharide-induced cell activation by binding to CD14. Cell Microbiol. 2007;9:1297–310. doi: 10.1111/j.1462-5822.2006.00872.x. [DOI] [PubMed] [Google Scholar]
- Kristiansen PA, Diomande F, Ba AK, et al. Impact of the serogroup A meningococcal conjugate vaccine, MenAfriVac, on carriage and herd immunity. Clin Infect Dis. 2013;56:354–63. doi: 10.1093/cid/cis892. [DOI] [PubMed] [Google Scholar]
- Kriz P, Giorgini D, Musilek M, et al. Microevolution through DNA exchange among strains of Neisseria meningitidis isolated during an outbreak in the Czech Republic. Res Microbiol. 1999;150:273–80. doi: 10.1016/s0923-2508(99)80052-7. [DOI] [PubMed] [Google Scholar]
- Kugelberg E, Gollan B, Farrance C, et al. The influence of IS1301 in the capsule biosynthesis locus on meningococcal carriage and disease. PloS one. 2010;5:e9413. doi: 10.1371/journal.pone.0009413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz S, Hubner C, Aepinus C, et al. Transcriptome-based antigen identification for Neisseria meningitidis. Vaccine. 2003;21:768–75. doi: 10.1016/s0264-410x(02)00596-0. [DOI] [PubMed] [Google Scholar]
- Larue K, Ford RC, Willis LM, Whitfield C. Functional and structural characterization of polysaccharide co-polymerase proteins required for polymer export in ATP-binding cassette transporter-dependent capsule biosynthesis pathways. J Biol Chem. 2011;286:16658–68. doi: 10.1074/jbc.M111.228221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavitola A, Bucci C, Salvatore P, et al. Intracistronic transcription termination in polysialyltransferase gene (siaD) affects phase variation in Neisseria meningitidis. Mol Microbiol. 1999;33:119–27. doi: 10.1046/j.1365-2958.1999.01454.x. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Rakic B, Gilbert M, et al. Structural and kinetic characterizations of the polysialic acid O-acetyltransferase OatWY from Neisseria meningitidis. J Biol Chem. 2009;284:24501–11. doi: 10.1074/jbc.M109.006049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepow ML, Beeler J, Randolph M, et al. Reactogenicity and immunogenicity of a quadrivalent combined meningococcal polysaccharide vaccine in children. J Infect Dis. 1986;154:1033–6. doi: 10.1093/infdis/154.6.1033. [DOI] [PubMed] [Google Scholar]
- Lewis LA, Ram S. Meningococcal disease and the complement system. Virulence. 2014;5:98–126. doi: 10.4161/viru.26515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Rouphael N, Duraisingham S, et al. Molecular signatures of antibody responses derived from a systems biology study of five human vaccines. Nat Immunol. 2014;15:195–204. doi: 10.1038/ni.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E, Kugelberg E, Tracy A, et al. Temperature triggers immune evasion by Neisseria meningitidis. Nature. 2013;502:237–40. doi: 10.1038/nature12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longworth E, Fernsten P, Mininni TL, et al. O-Acetylation status of the capsular polysaccharides of serogroup Y and W135 meningococci isolated in the UK. FEMS Immunol Med Microbiol. 2002;32:119–23. doi: 10.1111/j.1574-695X.2002.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Mackinnon FG, Borrow R, Gorringe AR, et al. Demonstration of lipooligosaccharide immunotype and capsule as virulence factors for Neisseria meningitidis using an infant mouse intranasal infection model. Microb Pathog. 1993;15:359–66. doi: 10.1006/mpat.1993.1085. [DOI] [PubMed] [Google Scholar]
- MacLennan J, Obaro S, Deeks J, et al. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J Infect Dis. 2001;183:97–104. doi: 10.1086/317667. [DOI] [PubMed] [Google Scholar]
- Martin P, Sun L, Hood DW, Moxon ER. Involvement of genes of genome maintenance in the regulation of phase variation frequencies in Neisseria meningitidis. Microbiology. 2004;150:3001–12. doi: 10.1099/mic.0.27182-0. [DOI] [PubMed] [Google Scholar]
- Masson L, Holbein BE. Role of lipid intermediate(s) in the synthesis of serogroup B Neisseria meningitidis capsular polysaccharide. J Bacteriol. 1985;161:861–7. doi: 10.1128/jb.161.3.861-867.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre PB, O’Brien KL, Greenwood B, van de Beek D. Effect of vaccines on bacterial meningitis worldwide. Lancet. 2012;380:1703–11. doi: 10.1016/S0140-6736(12)61187-8. [DOI] [PubMed] [Google Scholar]
- McNeil LK, Zagursky RJ, Lin SL, et al. Role of factor H binding protein in Neisseria meningitidis virulence and its potential as a vaccine candidate to broadly protect against meningococcal disease. Microbiol Mol Biol Rev. 2013;77:234–52. doi: 10.1128/MMBR.00056-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michon F, Huang CH, Farley EK, et al. Structure activity studies on group C meningococcal polysaccharide-protein conjugate vaccines: effect of O-acetylation on the nature of the protective epitope. Dev Biol (Basel) 2000;103:151–60. [PubMed] [Google Scholar]
- Morelle S, Carbonnelle E, Nassif X. The REP2 repeats of the genome of Neisseria meningitidis are associated with genes coordinately regulated during bacterial cell interaction. J Bacteriol. 2003;185:2618–27. doi: 10.1128/JB.185.8.2618-2627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muindi KM, McCarthy PC, Wang T, et al. Characterization of the meningococcal serogroup X capsule N-acetylglucosamine-1-phosphotransferase. Glycobiology. 2014;24:139–49. doi: 10.1093/glycob/cwt091. [DOI] [PubMed] [Google Scholar]
- Peterson DC, Arakere G, Vionnet J, et al. Characterization and acceptor preference of a soluble meningococcal group C polysialyltransferase. J Bacteriol. 2011;193:1576–82. doi: 10.1128/JB.00924-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Lewis LA, Agarwal S. Meningococcal group W-135 and Y capsular polysaccharides paradoxically enhance activation of the alternative pathway of complement. J Biol Chem. 2011;286:8297–307. doi: 10.1074/jbc.M110.184838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram S, Mackinnon FG, Gulati S, et al. The contrasting mechanisms of serum resistance of Neisseria gonorrhoeae and group B Neisseria meningitidis. Mol Immunol. 1999;36:915–28. doi: 10.1016/s0161-5890(99)00114-5. [DOI] [PubMed] [Google Scholar]
- Ramsay ME, Andrews NJ, Trotter CL, et al. Herd immunity from meningococcal serogroup C conjugate vaccination in England: database analysis. BMJ. 2003;326:365–6. doi: 10.1136/bmj.326.7385.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond P, Borrow R, Findlow J, et al. Evaluation of De-O-acetylated meningococcal C polysaccharide-tetanus toxoid conjugate vaccine in infancy: reactogenicity, immunogenicity, immunologic priming, and bactericidal activity against O-acetylated and De-O-acetylated serogroup C strains. Infect Immun. 2001;69:2378–82. doi: 10.1128/IAI.69.4.2378-2382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanow A, Haselhorst T, Stummeyer K, et al. Biochemical and biophysical characterization of the sialyl-/hexosyltransferase synthesizing the meningococcal serogroup W135 heteropolysaccharide capsule. J Biol Chem. 2013;288:11718–30. doi: 10.1074/jbc.M113.452276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanow A, Keys TG, Stummeyer K, et al. Dissection of Hexosyl- and Sialyltransferase Domains in the Bifunctional Capsule Polymerases from Neisseria meningitidis W and Y Defines a New Sialyltransferase Family. J Biol Chem. 2014;289:33945–57. doi: 10.1074/jbc.M114.597773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein NE, Fischer M, Tappero JW. Meningococcal vaccines. Infect Dis Clin North Am. 2001a;15:155–69. doi: 10.1016/s0891-5520(05)70273-7. [DOI] [PubMed] [Google Scholar]
- Rosenstein NE, Perkins BA, Stephens DS, et al. The changing epidemiology of meningococcal disease in the United States, 1992–1996. J Infect Dis. 1999;180:1894–901. doi: 10.1086/315158. [DOI] [PubMed] [Google Scholar]
- Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med. 2001b;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- Sadarangani M, Pollard AJ. Serogroup B meningococcal vaccines-an unfinished story. Lancet Infect Dis. 2010;10:112–24. doi: 10.1016/S1473-3099(09)70324-X. [DOI] [PubMed] [Google Scholar]
- Schoen C, Blom J, Claus H, et al. Whole-genome comparison of disease and carriage strains provides insights into virulence evolution in Neisseria meningitidis. Proc Natl Acad Sci U S A. 2008;105:3473–8. doi: 10.1073/pnas.0800151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes MJ, Cunha M, Almeida F, et al. Molecular surveillance of Neisseria meningitidis capsular switching in Portugal, 2002–2006. Epidemiol Infect. 2009;137:161–5. doi: 10.1017/S0950268808001106. [DOI] [PubMed] [Google Scholar]
- Snyder LA, Butcher SA, Saunders NJ. Comparative whole-genome analyses reveal over 100 putative phase-variable genes in the pathogenic Neisseria spp. Microbiology. 2001;147:2321–32. doi: 10.1099/00221287-147-8-2321. [DOI] [PubMed] [Google Scholar]
- Spinosa MR, Progida C, Tala A, et al. The Neisseria meningitidis capsule is important for intracellular survival in human cells. Infect Immun. 2007;75:3594–603. doi: 10.1128/IAI.01945-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanelli P, Fazio C, Neri A, et al. First report of capsule replacement among electrophoretic type 37 Neisseria meningitidis strains in Italy. J Clin Microbiol. 2003;41:5783–6. doi: 10.1128/JCM.41.12.5783-5786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DS, Spellman PA, Swartley JS. Effect of the (a 2-->8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J Infect Dis. 1993;167:475–9. doi: 10.1093/infdis/167.2.475. [DOI] [PubMed] [Google Scholar]
- Stout V, Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990;172:659–69. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartley JS, Liu LJ, Miller YK, et al. Characterization of the gene cassette required for biosynthesis of the (a1-->6)-linked N-acetyl-D-mannosamine-1-phosphate capsule of serogroup A Neisseria meningitidis. J Bacteriol. 1998;180:1533–9. doi: 10.1128/jb.180.6.1533-1539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartley JS, Marfin AA, Edupuganti S, et al. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci U S A. 1997;94:271–6. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taha MK, Achtman M, Alonso JM, et al. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet. 2000;356:2159. doi: 10.1016/S0140-6736(00)03502-9. [DOI] [PubMed] [Google Scholar]
- Tala A, Cogli L, De Stefano M, et al. Serogroup-specific interaction of Neisseria meningitidis capsular polysaccharide with host cell microtubules and effects on tubulin polymerization. Infect Immun. 2014;82:265–74. doi: 10.1128/IAI.00501-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tala A, Progida C, De Stefano M, et al. The HrpB-HrpA two-partner secretion system is essential for intracellular survival of Neisseria meningitidis. Cell Microbiol. 2008;10:2461–82. doi: 10.1111/j.1462-5822.2008.01222.x. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Saunders NJ, Heidelberg J, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–15. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- Trotter CL, Andrews NJ, Kaczmarski EB, et al. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet. 2004;364:365–7. doi: 10.1016/S0140-6736(04)16725-1. [DOI] [PubMed] [Google Scholar]
- Troy FA, Vijay IK, Tesche N. Role of undecaprenyl phosphate in synthesis of polymers containing sialic acid in Escherichia coli. J Biol Chem. 1975;250:156–63. [PubMed] [Google Scholar]
- Tsang RS, Law DK, Tyler SD, et al. Potential capsule switching from serogroup Y to B: The characterization of three such Neisseria meningitidis isolates causing invasive meningococcal disease in Canada. Can J Infect Dis Med Microbiol. 2005;16:171–4. doi: 10.1155/2005/216369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Datta A, Strole C, et al. KpsF is the arabinose-5-phosphate isomerase required for 3-deoxy-D-manno-octulosonic acid biosynthesis and for both lipooligosaccharide assembly and capsular polysaccharide expression in Neisseria meningitidis. J Biol Chem. 2002;277:24103–13. doi: 10.1074/jbc.M200931200. [DOI] [PubMed] [Google Scholar]
- Tzeng YL, Datta AK, Strole CA, et al. Translocation and surface expression of lipidated serogroup B capsular Polysaccharide in Neisseria meningitidis. Infect Immun. 2005;73:1491–505. doi: 10.1128/IAI.73.3.1491-1505.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Kahler CM, Zhang X, Stephens DS. MisR/MisS two-component regulon in Neisseria meningitidis. Infect Immun. 2008;76:704–16. doi: 10.1128/IAI.01007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Martin LE, Stephens DS. Environmental survival of Neisseria meningitidis. Epidemiol Infect. 2013:1–4. doi: 10.1017/S095026881300085X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Noble C, Stephens DS. Genetic basis for biosynthesis of the (alpha 1-->4)-linked N-acetyl-D-glucosamine 1-phosphate capsule of Neisseria meningitidis serogroup X. Infect Immun. 2003;71:6712–20. doi: 10.1128/IAI.71.12.6712-6720.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng YL, Swartley JS, Miller YK, et al. Transcriptional regulation of divergent capsule biosynthesis and transport operon promoters in serogroup B Neisseria meningitidis. Infect Immun. 2001;69:2502–11. doi: 10.1128/IAI.69.4.2502-2511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uria MJ, Zhang Q, Li Y, et al. A generic mechanism in Neisseria meningitidis for enhanced resistance against bactericidal antibodies. J Exp Med. 2008;205:1423–34. doi: 10.1084/jem.20072577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vipond C, Care R, Feavers IM. History of meningococcal vaccines and their serological correlates of protection. Vaccine. 2012;30(Suppl 2):B10–7. doi: 10.1016/j.vaccine.2011.12.060. [DOI] [PubMed] [Google Scholar]
- Virji M, Makepeace K, Ferguson DJ, et al. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- Vogel U, Claus H, Frosch M. Rapid serogroup switching in Neisseria meningitidis. N Engl J Med. 2000;342:219–20. doi: 10.1056/NEJM200001203420319. [DOI] [PubMed] [Google Scholar]
- Von Loewenich FD, Wintermeyer E, Dumig M, Frosch M. Analysis of transcriptional control mechanisms of capsule expression in Neisseria meningitidis. Int J Med Microbiol. 2001;291:361–9. doi: 10.1078/1438-4221-00142. [DOI] [PubMed] [Google Scholar]
- Wang Q, Shao Z, Wang X, et al. Genetic study of capsular switching between Neisseria meningitidis sequence type 7 serogroup A and C strains. Infect Immun. 2010;78:3883–8. doi: 10.1128/IAI.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis LM, Stupak J, Richards MR, et al. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci U S A. 2013;110:7868–73. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis LM, Whitfield C. KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc Natl Acad Sci U S A. 2013a;110:20753–8. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis LM, Whitfield C. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res. 2013b;378:35–44. doi: 10.1016/j.carres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- Yi K, Stephens DS, Stojiljkovic I. Development and evaluation of an improved mouse model of meningococcal colonization. Infect Immun. 2003;71:1849–55. doi: 10.1128/IAI.71.4.1849-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang Q, Wen L, et al. Development of a multiplex PCR assay for detection and genogrouping of Neisseria meningitidis. J Clin Microbiol. 2012;50:46–51. doi: 10.1128/JCM.00918-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer SM, Stephens DS. Serogroup B meningococcal vaccines. Curr Opin Investig Drugs. 2006;7:733–9. [PubMed] [Google Scholar]