Abstract
We observed over 400-fold enhanced fluorescence from single Cy5 molecules assembled on multilayer silver nanoparticle-dielectric-metal (PDM) substrate. This substantial enhancement is due to the near-field enhanced excitation, emission, and interaction of Cy5 with plasmonic nanostructures. Experimental observation is supported by finite-element method calculations.
Graphical Abstract
Single molecule spectroscopy (SMS) is a powerful tool to study the properties of individual fluorophores and the dynamics of biomolecules.1 It is valuable because the measurements bypass the ensemble averaging that occurs in traditional fluorescence measurements. SMS is usually performed in a diffraction limited volume with high illumination power to obtain high rates of excitation and emission. As a result, single fluorophores are typically observed for a short period of time, prior to photobleaching within seconds. Additional requirement for SMS is that the fluorophore must have a relatively high emission brightness and extinction coefficients.
Substantial increase in fluorophore brightness can be accomplished using fluorophore combined with metallic nanostructures. Single molecule studies were used for probing fluorophore-metal coupling which is a through-space interaction in the near-field region.2 For last several years, we have been working in the area of metal-enhanced fluorescence (MEF), which is expected to improve the detectability of single molecules.2c, 2f–h, 2j MEF occurs due to the near-field interaction of fluorophores with metallic colloids or surfaces leading to substantial fluorescence enhancements. This phenomenon results from the combined effects of the creation of an intense excitation field around the metal nanoparticle in the vicinity of the fluorophore, an increase in the intrinsic emission rate of the fluorophore and a strong coupling between the fluorophore and the plasmons in the metal. In recent years, near-field interactions have been widely employed to increase the excitation and emission rates of a fluorophore, enhance fluorescence intensity, improve photostability, and reduce blinking.2f–h It is established that the irradiation of a subwavelength size metal nanoparticle with the light can create an enhanced local electric field. Placement of a fluorophore in this local field within a near-field distance from the metal nanoparticle (NP) can result in significant increases in its excitation or/and emission rates due to near-field interactions leading to significant enhancement. The most notable methods used for fluorescence enhancement on planar substrates include chemical deposition of silver island films on glass, physical adsorption of Ag colloids, and patterning methods based upon electron beam lithography. Defined nanostructured substrates by top-down lithographic fabrication have resulted in moderate improvement in fluorescence3 but a large enhancement of over thousand-fold was reported for single low quantum-yield molecules from lithographically fabricated bowtie nanoantenas.2e
We have fabricated plasmonic substrates consisting of silver nanoparticle – dielectric – mirror (PDM) layers on large area of glass slides using sputtering method. We reported earlier measurements of several streptavidin conjugated organic dyes on PDM substrates showing enhanced emission for ensemble molecules up to 200-folds in the 450–800 nm wavelength range.4 The single molecule studies of fluorophores at distance of ~10 nm from the metal surface is expected to provide more insights into the metal-fluorophore mechanism and a distribution of large enhancement depending on the location of the probe molecules. Current limitation for broader use of the SMS combined with plasmonic nanostructures is the need for precise placing of fluorophore in the near field. This requires chemical attachment of fluorophores to the metal nanoparticles2b, 2d, 2e, 2h or physical placement of fluorophore in desired nanoscale location2e.
In this communication, we report the use of PDM substrates that allows performing the SMS over the large substrate area without consideration for fluorophore localization or exploiting only specific locations. Fluorophores on PDM substrate display highly enhanced fluorescence. We report here a few hundred-fold enhanced fluorescence and up to 10-fold decrease in lifetime from single Cy5 molecules assembled on PDM substrate. We have performed single molecule imaging spectroscopy and time-resolved studies where fluorophores are within about 5 – 10 nm from the surface of plasmonics substrate. We also performed numerical calculations using the finite-element method (FEM) that afford insights on understanding the excitation and emission contributions to the observed fluorescence enhancements and provide guidance for rational design of substrates with a dielectric photonic cavity.
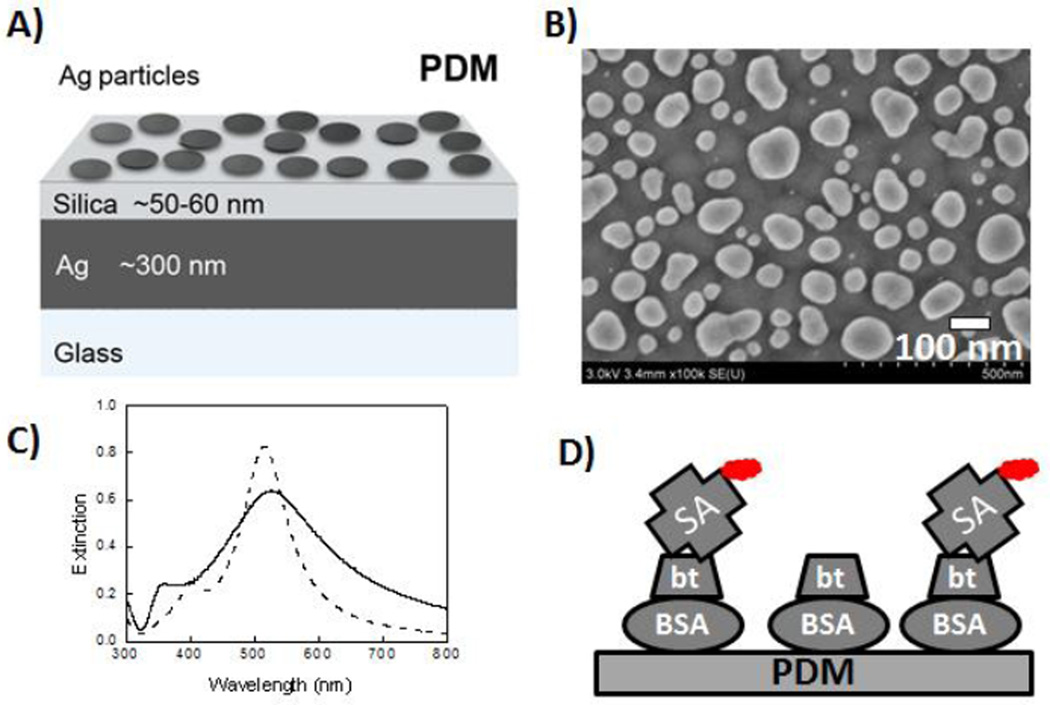
We fabricated the PDM substrate as reported in our previous study4a. Briefly, the silver thin layer nanoparticles were deposited over 50 nm thick silica layer which was deposited on 300 nm thick silver film. Top Ag layer was converted into Ag nanoparticles using thermal annealing. The PDM structure is schematically shown in Figure 1A. Scanning electron microscope (SEM) images of these substrates were recorded with a Hitachi SU-70 SEM instrument. Figure 1B shows the SEM image of the fabricated structure displaying top layer with Ag particles of heterogeneous sizes and shapes in the range of 40 to 100 nm in diameter. The particulate nature of the top layer is further confirmed by recording the extinction spectrum of deposited Ag nanoparticles on silica coated glass (Figure 1C). The single probe immobilization procedure used in the present study is schematically shown in Figure 1D. Briefly, PDM substrates or cover glass slides were incubated in buffer solution of 10 µM biotinylated bovine serum albumin (BSA-bt, Sigma) for 20 h (5°C). After being washed 3 times with PBS buffer, the PDM substrates and glass slides were incubated with buffer solution of 200 pM streptavidin (SA) conjugated Cy5 (GE Amersham) for 2 hrs at 5 °C. Thus, the individual SA-Cy5 molecules were randomly distributed on PDM or glass substrates. The substrates were washed multiple times with PBS buffer and used for subsequent single molecule measurements. Single molecule measurements were performed using a Microtime 200 scanning confocal microscope equipped time-correlated single photon counting (TCSPC) capabilities (PicoQuant, Germany). A pulsed laser diode (635 nm, 100 ps, 40 MHz) was used as the excitation source. The excitation laser was directed through a 10 nm band-pass excitation clean-up filter and reflected by a dichroic mirror into an inverted Microscope (Olympus, IX 71). A water immersion objective (Olympus 60×, 1.2 NA) was used for focusing the laser light onto the sample and for collecting the fluorescence emission from the sample. The fluorescence signal was passed through a dichroic mirror and a band-pass filter (HQ685/70, Chroma) and focused through a 75 µm pinhole to a single photon avalanche photodiode detector. Images were acquired by raster scanning the sample. Intensity-time trajectories and intensity decays were obtained by positioning the incident laser light over individual Cy5-SA molecules. Lifetimes were estimated by multi-exponential fitting. For acquiring single molecule emission spectra, the Microtime 200 system was attached to an Acton spectrograph consisting of high reflectance mirrors and dispersion grating with efficient imaging in the 450–750 nm wavelength region. An electron-multiplied CCD (Princeton Instruments PhotonMax512) was used as the detector. Numerical calculations were performed based on the FEM using COMSOL Multiphysics as described in details.4b
Fig.1.
(A) Schematic representation of PDM substrate, (B) SEM image of top Ag NP layer of planar PDM substrate, (C) Extinction spectrum of Ag NP on silica coated glass (solid line) and the numerically calculated spectrum for Ag array of semi-spherical NP of 60 nm diameter and edge-to-edge spacing of 40 nm (dashed line). (D) Cy5 conjugated SA bound to biotinylated BSA on PDM substrate for SMS.
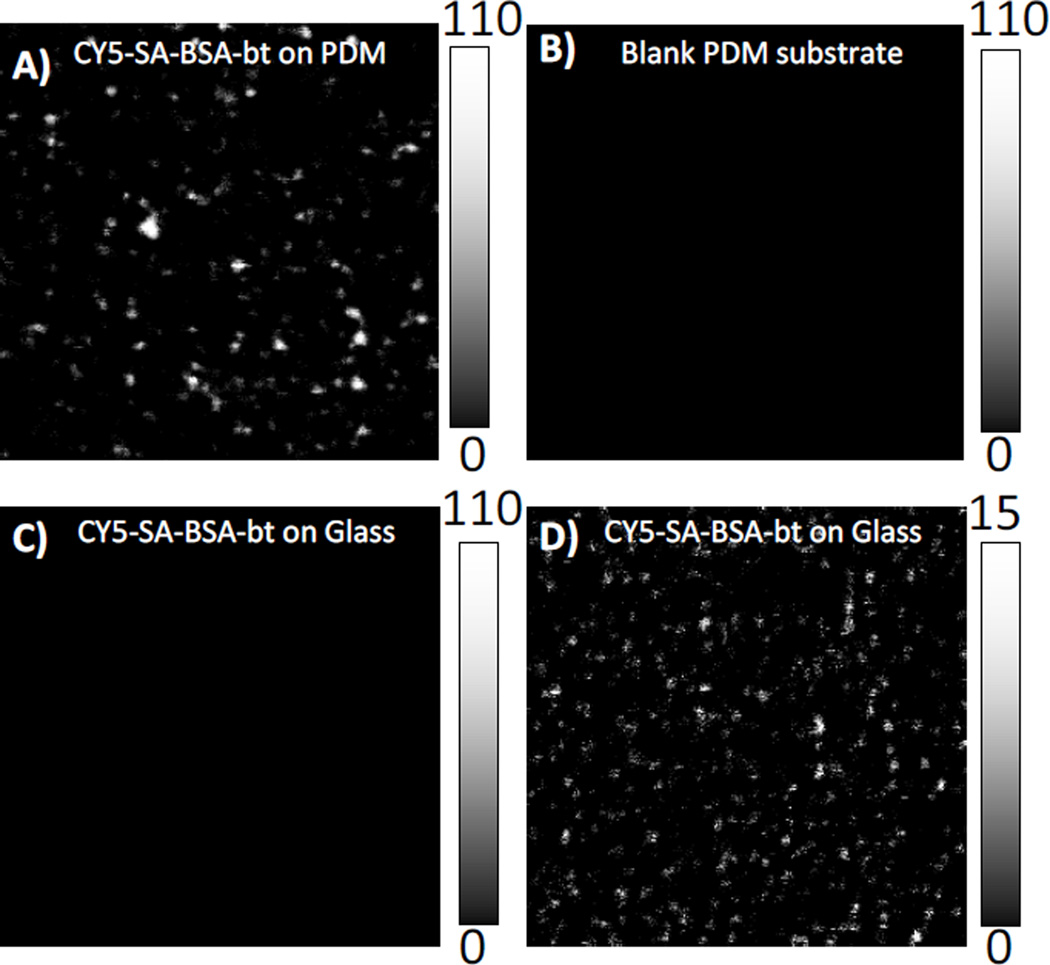
Single molecule fluorescence measurements of Cy5-SA were performed on the PDM substrate and glass. Figure 2A shows 20 × 20 µm fluorescence images of Cy5-SA immobilized on the biotinylated PDM substrate upon excitation with an incident power of 14 nW at the sample. The well-separated bright spots in the images represent emission from single Cy5-SA molecules. No measurable Cy5-SA was observed on glass at the excitation of 14 nW (Figure 2C). To observe the individual Cy5-SA molecules on the glass slide, the incident excitation power was increased 50-fold, to 700 nW (Figure 2D). The substantial increase in the brightness of individual Cy5-SA molecules on the PDM substrate is clearly evident in comparison to the image obtained from Cy5-SA molecules on cover glass slide (Figures 2A & 2D). Taking into account the difference in laser power and the count rate difference between the images (Figures 2A & 2D), the increase in fluorescence is over 400-fold. It is important to note that high fluorescence enhancement is observed over large area. We observed images as shown in Figure 2A over entire PDM substrate of 1.5 × 1.5 mm2. This means that PDM substrates show great potential as solid supports for studies of single molecule photophysics and design of single molecule-based bioassays. Figure 2B shows the background count rate of PDM substrate without any assembly or dye at 14 nW incident laser power.
Fig.2.
Single molecule measurements using scanning confocal microscope. (A) Image of Cy5-SA molecules on PDM substrate and (B) blank PDM substrate at 14nW excitation power. Images of Cy5-SA molecules on glass at (C) 14 nW and (D) 700 nW. The image sizes are 20 µm × 20 µm. Scale bar shows the intensity counts in 1-msec bin.
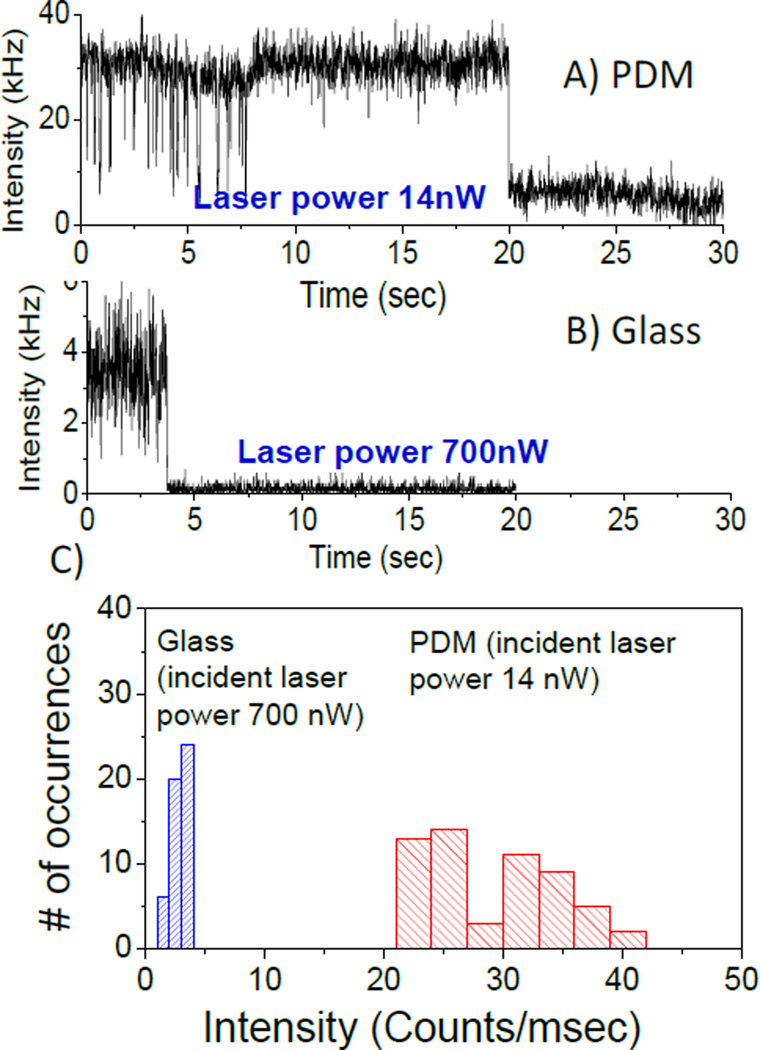
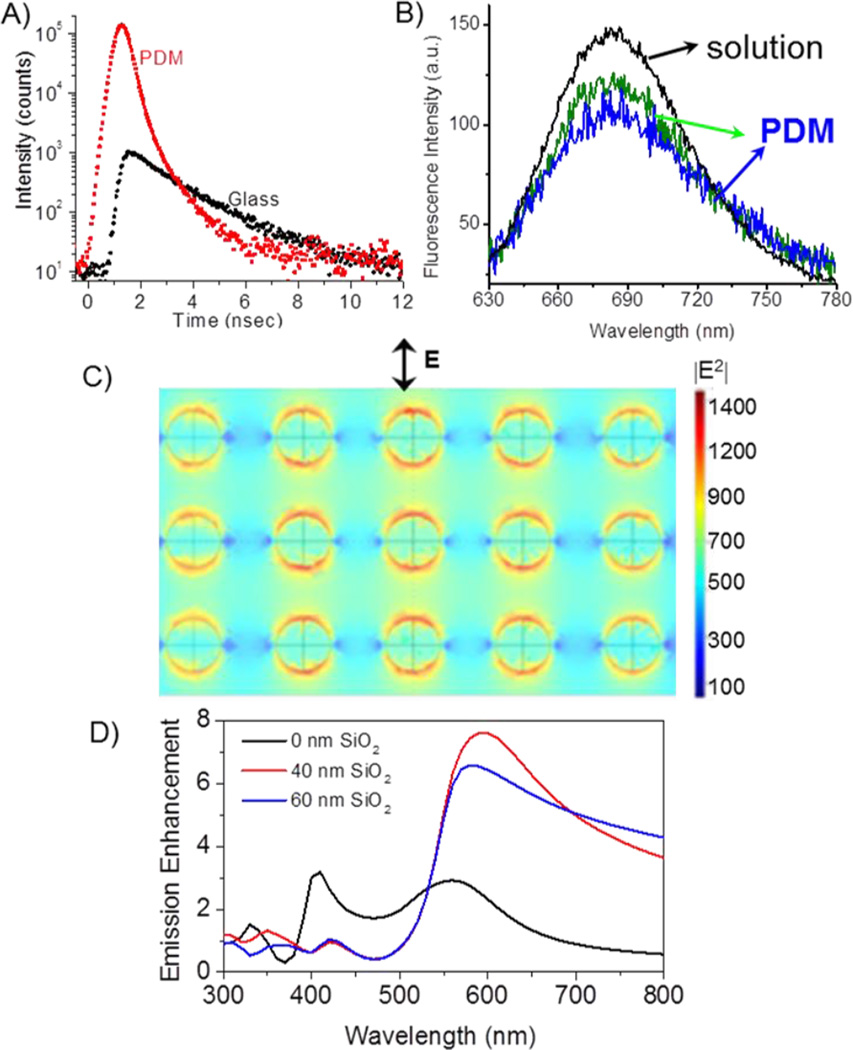
The objectives of single molecule experiments are often to study the dynamics of a single molecule for an extended period of time. The observation times are frequently limited to seconds due to photobleaching. Hence, it would be useful if the single molecule observation times could be increased by PDM substrates. The individual Cy5-SA molecules under continuous illumination showed single-step photobleaching, corresponding to the typical behaviour expected for an individual dye molecule (Figures 3A and B). Observation time was up to 20s on PDM substrate prior photobleachnig of Cy5-SA compared to about 4s on glass. Although, one representative intensity-time trace for glass and PDM are presented, there are many similar intensity spots on images as shown in Figure 2A & D. The higher intensity level after single-step photobleaching of individual Cy5 on PDM compared to glass is possibly due to higher scattering from silver nanostructures. Interestingly, much larger fluctuations in the fluorescence intensities are observed for the Cy5-SA on the PDM substrate in comparison to glass, prior to complete photobleaching. This fluctuation in the fluorescence intensity can be attributed to the flexibility of the Cy5 molecules that are tethered to the surface by the biotin-SA chemistry. It is known that the metal-fluorophore coupling depends on the distance and orientation of the fluorophore transition dipole with respect to the PDM substrate.5 Accordingly, conformational fluctuations can significantly influence the plasmonic coupling and hence the resultant fluorescence enhancement, leading to the variation in the fluorescence intensity. These individual behaviours or heterogeneities are masked in the ensemble measurements. Furthermore, in SMS, we are studying only a single Cy5-SA molecule with a fixed nano-environment and at the fixed orientation with respect to metal surface. Accordingly, to understand the heterogeneity in the brightness of individual Cy5-SA molecules on the PDM and glass substrates, intensity histograms were constructed by observing many dye molecules as shown by number of occurrences (Figure 3C). Count rates of Cy5-SA molecules on glass and PDM substrate are obtained after subtracting the background following single-step photobleaching. Examination of the intensity histograms shows that the Cy5-SA molecules on PDM substrate have an average intensity of about 30 kHz at an incident laser power of 14 nW. On the other hand an average of ~3.5 kHz on glass was observed at an illumination power of 700 nW. This indicates on average 400-fold increase in intensity from over 60 molecules on each substrate (Figure 3C). Intensities of the Cy5-SA molecules on the PDM substrate vary from 22 to 40 kHz which reflects the observed brightness of spots shown in Figure 2A. It is possible that bright molecules reside in the favourable plasmonic hotspots on the PDM substrate where the fluorophore-plasmon coupling is efficient. Such plasmonic hotspots can magnify electromagnetic fields to a large extent.6
Fig.3.
Representative intensity-time trajectories of individual Cy5 molecules assembled on (A) PDM, (B) Glass. For PDM, 50-fold less incident excitation power was used. (C) Comparison of brightness of Cy5 molecules plotted as histograms showing on average ~400 fold increase in intensity on PDM substrate.
To have more insight on the underlying photophysics of single Cy5-SA molecules on PDM and glass substrates, time-resolved measurements were performed. Typical single molecule intensity decays of individual Cy5-SA molecules on the PDM and glass substrate are presented in Figure 4A. The intensity decays clearly display much faster decay rates and higher counts of Cy5 on PDM substrate in comparison to that from glass. The intensity decays of individual Cy5-SA molecules on glass were fitted with a single exponential model resulting in range of lifetime values of 1.7 to 1.9 nsec. The intensity decays of Cy5-SA on PDM were fitted with a bi-exponential model with average amplitude weighted lifetime of 220 psec. A large reduction in the lifetime of single molecule probes on the PDM substrate is in good agreement with the MEF phenomenon and previous observations using single molecule spectroscopy.2c, 2g, 2h Large decrease in the lifetime indicates that the radiative (kr), nonradiative (knr), or both decay rates were modified by the presence of silver nanoparticles. The significant decrease in lifetime and simultaneous large fluorescence enhancement of Cy5 with a quantum efficiency of ~20% imply an increase of the radiative decay rate, which in consequence leads to increased quantum efficiency. Additionally, we detected some molecules are more photostable than the others. More rigorous quantitative study is needed to understand the correlation between the lifetime of individual probes on PDM and the time it takes to photobleach.
Fig. 4.
A) Fluorescence intensity decays of individual Cy5 molecules on PDM and glass. B) Single-molecule spectra of individual Cy5 molecules on PDM. Emission of Cy5-SA molecules in solution is included for comparison. C) Distribution of the excitation field intensity for 630 nm on a multilayer substrate. Calculations are for a Ag array with 40 nm diameter particle and inter-particle spacing of 40 nm. The polarization of incident electric field is in the plane of figure as shown. D) Calculated average emission enhancement for dipoles placed within a 10nm layer on the surface of PDM substrate.
To confirm the fluorescence of single Cy5-SA molecules on PDM substrate we recorded emission spectra using an Acton spectrograph coupled to an EMCCD camera (Fig.4B). It is recognized that silver nanoparticles can be photoactivated with incident light and display characteristic blinking behavior.7 Strong intensity fluctuations and nondestructive blinking have been reported for silver nanoparticles under continuous illumination. This could lead to complications in single molecule spectroscopic studies. However, the blinking behavior from single organic dye molecules is very different from metal nanoparticles and can be distinguished from the latter. The emission spectral shape and maxima of single Cy5-SA molecules on the PDM substrate are similar to those observed from ensemble of Cy5-SA molecules in solution with the same set up. The single molecule spectra clearly indicate that the fluorescence from individual Cy5-SA on the PDM substrate arise from single dye molecules and not from the nanoparticle luminescence.
Two mechanisms of fluorescence enhancement caused by the multilayered PDM substrates were considered. Accordingly, we performed two sets of numerical calculations: (1) excitation enhancement due to coupling of excitation light into a multilayered PDM substrate and (2) emission enhancement of randomly distributed and randomly oriented dipoles (representing fluorophores). For numerical calculations an Ag array of 40 nm diameter particle and 40 nm edge-to-edge spacing was used that closely mimic the surface plasmon spectrum of Ag nanostructures of PDM substrates.4b Average excitation and emission enhancements were calculated for a layer of 10 nm of water above the PDM surface with several of silica layers and compared to bare glass. The excitation enhancement for wavelength of 630 nm and 60 nm silica is shown in Figure 4C. The enhanced excitation on PDM substrates originates from enhanced field due to surface plasmons of Ag nanoparticles and resonance layer of silica. Theoretical studies of single quantum emitter with dielectric-metal configuration have been reported with emphasis on local density of states and nonradiative losses.8
To account for the emission process, we assumed a random distribution of point dipoles (fluorophores) both in terms of the orientation of the transition dipole moments and distances from the surface within 10 nm representing the present sample geometry. Results of FEM calculations providing emission enhancement for dipoles on PDM substrate geometry are shown in Figure 4D without and with SiO2 layers of 40 and 60 nm. The enhancement factors were calculated relative to the reference system of the glass slide without any additional dielectric or metal layers. It is informative that our numerical model including metal-dipole system resulted in strongly wavelength-dependent emission enhancement. Silica thicknesses of 40–60 nm provide constructive enhancements at longer wavelengths but no effect or quenching at shorter wavelengths, below about 520 nm (Figure 4D). The emission enhancement is several folds larger in the presence of silica layers above 600 nm. Calculated total fluorescence enhancement as a product of excitation and emission enhancements was about 150-fold for excitation of 630 nm and emission of 680 nm.4b The observed average fluorescence enhancement of about 400-fold for single Cy5-SA molecules indicate that PDM substrate produce more favourable field enhancements than regular Ag nanoparticle array.
Conclusions
We report several-hundred fold enhanced emission from single Cy5-SA molecules on multilayer PDM substrates. This single molecule studies are focussed on large size planar plasmonic substrates with highly enhanced fluorescence signals. The large enhancement of emission from individual probes on PDM substrate is accompanied with ~10-fold decrease in fluorescence lifetime. A large decrease in the lifetime of the fluorophore could possibly improve fluorophore stability on PDM. Shorter lifetime also enables significant increase of the photon count rate. Numerical calculations using finite element method provided insights into the mechanisms of fluorescence enhancements through the near-field intensity effect on excitation and the effect of interaction of excited fluorophores with localized plasmons of the silver nanostructures, including increased emission collection due to the underlying silver mirror. PDM substrates allow overcoming current limitations of single molecule spectroscopy such as low brightness and low photostability of most fluorophores and provide substantially easier use of planar substrates. These open opportunities for much broader scope of SMS photochemical studies and practical applications.
Supplementary Material
Acknowledgments
The authors acknowledge the financial support by grants from National Institutes of Health (NIH) Grants AI087968 (K.R.); EB006521 and GM107986 (J.R.L.)
Notes and references
- 1.(a) Moerner WE. J. Phys. Chem. B. 2002;106:910. [Google Scholar]; (b) Juette MF, Terry DS, Wasserman MR, Zhou Z, Altman RB, Zheng Q, Blanchard SC. Curr Opin Chem Biol. 2014;20:103. doi: 10.1016/j.cbpa.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Michalet X, Weiss S, Jager M. Chem Rev. 2006;106:1785. doi: 10.1021/cr0404343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Acuna G, Grohmann D, Tinnefeld P. FEBS Lett. 2014;588:3547. doi: 10.1016/j.febslet.2014.06.016. [DOI] [PubMed] [Google Scholar]; (b) Bharadwaj P, Novotny L. Opt Express. 2007;15:14266. doi: 10.1364/oe.15.014266. [DOI] [PubMed] [Google Scholar]; (c) Choudhury SD, Badugu R, Nowaczyk K, Ray K, Lakowicz JR. J Phys Chem Lett. 2013;4:227. doi: 10.1021/jz301867b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Eghlidi H, Lee KG, Chen XW, Gotzinger S, Sandoghdar V. Nano Lett. 2009;9:4007. doi: 10.1021/nl902183y. [DOI] [PubMed] [Google Scholar]; (e) Kinkhabwala A, Yu Z, Fan S, Avlasevich Y, Müllen K, Moerner WE. Nature Photonics. 2009;3:654. [Google Scholar]; (f) Lakowicz JR, Ray K, Chowdhury M, Szmacinski H, Fu Y, Zhang J, Nowaczyk K. Analyst. 2008;133:1308. doi: 10.1039/b802918k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ray K, Badugu R, Lakowicz JR. J Am Chem Soc. 2006;128:8998. doi: 10.1021/ja061762i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Ray K, Zhang J, Lakowicz JR. Anal Chem. 2008;80:7313. doi: 10.1021/ac8009356. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yuan H, Khatua S, Zijlstra P, Yorulmaz M, Orrit M. Angew Chem Int Ed Engl. 2013;52:1217. doi: 10.1002/anie.201208125. [DOI] [PubMed] [Google Scholar]; (j) Donehue J, Wertz E, Talicska C, Biteen J. J. Phys. Chem. C. 2014;118:15027–15035. [Google Scholar]
- 3.Pompa PP, Martiradonna L, Torre AD, Sala FD, Manna L, De Vittorio M, Calabi F, Cingolani R, Rinaldi R. Nat Nanotechnol. 2006;1:126. doi: 10.1038/nnano.2006.93. [DOI] [PubMed] [Google Scholar]
- 4.(a) Szmacinski H, Badugu R, Lakowicz JR. J Phys Chem C Nanomater Interfaces. 2010;114:21142. doi: 10.1021/jp107543v. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Szmacinski H, Badugu R, Mahdavi F, Blair S, Lakowicz JR. J Phys Chem C Nanomater Interfaces. 2012;116:21563. doi: 10.1021/jp3072876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Ray K, Badugu R, Lakowicz JR. J Phys Chem C Nanomater Interfaces. 2007;111:7091. doi: 10.1021/jp067635q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ray K, Szmacinski H, Enderlein J, Lakowicz JR. Appl Phys Lett. 2007;90:251116. doi: 10.1063/1.2751125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Cang H, Labno A, Lu C, Yin X, Liu M, Gladden C, Liu Y, Zhang X. Nature. 2011;469:385. doi: 10.1038/nature09698. [DOI] [PubMed] [Google Scholar]; (b) Brinks D, Castro-Lopez M, Hildner R, van Hulst NF. Proc Natl Acad Sci U S A. 2013;110:18386. doi: 10.1073/pnas.1308652110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wu X, Yeow EK. Nanotechnology. 2008;19:035706. doi: 10.1088/0957-4484/19/03/035706. [DOI] [PubMed] [Google Scholar]; (b) Peyser L, Vinson AE, Bartko AP, Dickson RM. Science. 2001;291:103. doi: 10.1126/science.291.5501.103. [DOI] [PubMed] [Google Scholar]
- 8.Chen XW, Agio M, Sandoghdar V. Phys Rev Lett. 2012;108:233001. doi: 10.1103/PhysRevLett.108.233001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.