Abstract
The search for new treatments against leishmaniasis has increased due to high frequency of drug resistance registered in endemics areas, side effects, and complications caused by coinfection with HIV. Morinda citrifolia Linn., commonly known as Noni, has a rich chemical composition and various therapeutic effects have been described in the literature. Studies have shown the leishmanicidal activity of M. citrifolia; however, its action on the parasite has not yet been elucidated. In this work, we analyzed leishmanicidal activity and ultrastructural changes in Leishmania infantum promastigotes caused by M. citrifolia fruit juice treatment. M. citrifolia fruit extract showed a yield of 6.31% and high performance liquid chromatography identified phenolic and aromatic compounds as the major constituents. IC50 values were 260.5 µg/mL for promastigotes and 201.3 µg/mL for intracellular amastigotes of L. infantum treated with M. citrifolia. Cytotoxicity assay with J774.G8 macrophages showed that M. citrifolia fruit juice was not toxic up to 2 mg/mL. Transmission electron microscopy showed cytoplasmic vacuolization, lipid inclusion, increased exocytosis activity, and autophagosome-like vesicles in L. infantum promastigotes treated with M. citrifolia fruit juice. M. citrifolia fruit juice was active against L. infantum in the in vitro model used here causing ultrastructural changes and has a future potential for treatment against leishmaniasis.
1. Introduction
Due to the continental dimensions of Brazil there are various parts of its territory with difficult access. Consequently, there is a limit to public health resources and a tendency for the inhabitants of these remote regions not to get the necessary government health benefits. This geographical isolation contributes to strengthening the local traditional medical practices and other natural resources to treat diseases, including parasitic diseases such as leishmaniasis [1].
Leishmaniasis is caused by protozoan parasites transmitted through the bites of infected female sandflies (usually Phlebotomus or Lutzomyia). The disease appears in three clinical forms: the visceral form, also known as kala-azar, is usually fatal within 2 years if left untreated; the cutaneous form, causing skin ulcers; and the mucocutaneous form, which invades the mucous membranes of the upper respiratory tract, causing gross mutilation by destroying soft tissues in the nose, mouth, and throat [2]. The disease, which is prevalent in 98 countries and 3 territories on 5 continents, has approximately 1.3 million new cases annually, of which 300,000 are visceral and 1 million are cutaneous or mucocutaneous. These numbers show the importance of this disease in public health, including Brazil [3].
Treatment for leishmaniasis was first introduced by Vianna in 1912. Organic compounds of antimony are the drugs of choice in treating this disease, and amphotericin B was introduced recently. Both treatments present several side effects, are highly toxicity, and have an elevated cost which has led to the search for new alternatives. The search for a leishmanicidal agent of low toxicity and high efficiency is a challenge and has involved several research groups around the world [4]. Herbal remedies have gained a lot of attention in this area as a potential source to obtain new compounds with therapeutic activities.
Morinda citrifolia Linn. is a small plant native to Southeast Asia, commonly known as Noni, and one of the most significant sources of traditional medicine in those countries. Due to the various ethnopharmacological activities associated with this plant, it is now cultivated all over the world, including Brazil. Studies have shown the efficacy of Noni in the treatment of pain and inflammatory reactions [5] and antitumoral activity [6]. Activity against bacteria [7] and fungi [8] has also been observed. Recently, the in vitro activity of morindicone and morinthone isolated from the stem of M. citrifolia was described against L. major. Moreover, a clinical study was carried out to determine the efficiency of a topical ointment with M. citrifolia stem extract against cutaneous leishmaniasis, and there was an excellent response in 50% and a good improvement in 30% of the 40 patients evaluated [9]. Therefore, to demonstrate the action of M. citrifolia against promastigotes of Leishmania and evaluate the ultrastructural changes caused by such treatment, this study evaluated promastigotes forms of Leishmania infantum treated with M. citrifolia fruit juice by electron microscopy.
2. Materials and Methods
2.1. Plant Material
M. citrifolia fruits were collected in November 2011 from São Luís (S2°31 W44°16), Maranhão, in the Brazilian Legal Amazon at 24 m above sea level. Fruits were collected when the exocarp was translucent. The plant material was identified by Ana Maria Maciel Leite, and the voucher specimen number 2000346 was deposited at the Herbário Professora Rosa Mochel, Universidade Estadual do Maranhão. In the laboratory, the fruits were washed with distilled and sterilized water, dried at 25°C, and placed in sterile glass bottles for 3 days to drain off the extract released. This liquid was centrifuged twice at 4000 rpm for 15 minutes; the supernatant was lyophilized and stored at −20°C [8]. The lyophilized M. citrifolia fruit juice was dissolved in DMSO and dilutions with different concentrations in culture medium were made immediately before use. The concentration of DMSO in medium did not exceed 1%.
2.2. High Performance Liquid Chromatography Coupled with Diode Array and Evaporative Light Scattering Detectors (HPLC-DAD-ELSD)
The HPLC chromatographic profile of the M. citrifolia fruit juice was performed on a Shimadzu LC-10Avp equipped with two LC-8Avp pumps, controlled by a CBM-10A interface module, an automatic injector with two detectors, a diode array detector SPD-M10A (DAD), and an evaporative light scattering detector (ELSD) with a drift tube temperature setting of 40°C, using nitrogen as the nebulizer gas and gain at 4.0. HPLC grade solvents and Milli-Q water were used and the analysis was performed on a reversed phase LiChrospher C18 column (4.6 mm × 250 mm; 5 μm, Waters). The mobile phase was water (A) and methanol (B), with the following gradient composition: (0–20 min) 5–20% (B), (20–30 min) 20%–35% (B), and (30–35 min) 35% (B). The UV chromatogram was obtained at 365 nm. The sample injection volume was 10 µL. A constant flow of 1 mL/min was used during the analysis. Before analysis 5.0 mg of the extract was dissolved in 1.0 mL of Milli-Q water and the mixture was centrifuged.
2.3. Parasites
Promastigote forms of L. infantum (MCAN/BR/2008/1112) were cultured at 26°C in Schneider's Insect medium (Sigma-USA) supplemented with 10% fetal bovine sera (Gibco-USA), 100 U/mL of penicillin (Gibco-USA), and 100 µg/mL of streptomycin. The cultures used had a maximum of ten in vitro passages.
2.4. Animals
Female BALB/c mice of 4–6 years old were purchased from Centro de Criação de Animais de Laboratório do Instituto Oswaldo Cruz, Rio de Janeiro, and maintained under pathogen-free conditions. The animals were handled in accordance with Guidelines for Animal Experimentation of the Colégio Brasileiro de Experimentação Animal. The local Ethics Committee on Animal Care and Utilization approved all procedures involving the animals (CEUA FIOCRUZ-LW72/12).
2.5. Cells Culture
The macrophage J774.G8 line was cultured in RPMI 1640 medium (Sigma, USA) supplemented with 10% fetal bovine sera, penicillin (100 U/mL), and (100 µg/mL) streptomycin, at 37°C and 5% CO2. Female BALB/c mice were inoculated with 3 mL of sodium thioglycolate 3% and after 72 hours peritoneal macrophages were harvested with PBS solution. The harvest was centrifuged at 4000 rpm and the cells suspended in RPMI medium supplemented as described before and cultured at 37°C and 5% CO2.
2.6. Activity against Promastigote Forms
Promastigote forms of L. infantum (106 parasites/mL) from a 2–4-day-old culture were placed in 96-well plates in the presence of different concentrations of M. citrifolia fruit juice (960–30 µg/mL), in a final volume of 200 µL per well, for 72 hours. Wells without parasites were used as blank and wells with only parasites were used as control. After the treatment, the viability of parasites was evaluated by the tetrazolium-dye (MTT) colorimetric modified method [10]. MTT (5 mg/mL), a volume equal to 10% of the total, was added to each well. After 2 hours, the plate was centrifuged at 4000 rpm; then the supernatant was removed from each well and 100 µL of DMSO was added to dissolve the formazan. The absorbance was analyzed on a spectrophotometer at a wavelength of 540 nm. The data was normalized according to the formula
| (1) |
The results were used to calculate IC50 (50% inhibition of parasite growth). Amphotericin B was used as the reference drug.
2.7. Activity against Intracellular Amastigotes
Peritoneal macrophages were cultured in 24-well plates (105 cells/well), with coverslips, at 37°C and 5% CO2. The cells were infected with promastigote forms of L. infantum using a ratio of 10 : 1 parasite/cell, and after 2 hours the cells were washed three times with PBS to remove free parasites. The infected cells were treated with different concentrations of M. citrifolia fruit juice (480–30 μg/mL) in triplicate for 24 hours. The coverslips with the infected and treated cells were fixed with Bouin, stained with Giemsa, and examined by light microscopy. The inhibition percentage was calculated and IC50 was calculated with the GraphPad Prism software. Amphotericin was used as the reference drug.
2.8. Cytotoxicity Assay
J774.G8 macrophages were cultured in 96-well plates (5 × 105 cells/mL) with different concentrations of M. citrifolia fruit juice (2000–1.8 µg/mL) to a final volume of 200 µL per well, at 37°C and 5% CO2. Wells without cells were used as blank and wells with only cells were used as control. After 24 hours, the cells were fixed with 10% trichloroacetic acid for 1 hour at 4°C, stained with Sulforhodamine B (Sigma, USA) solution 0.4% in 1% acetic acid for 30 minutes, and washed with 1% acetic acid solution. Sulforhodamine B was solubilized in 200 µL of 10 mM tris-base solution and the plate was read in a spectrophotometer at 540 nm wavelength [11]. The data was normalized following the formula described earlier. The results were used to calculate the cell cytotoxicity by 50% (CC50) with the GraphPad Prism 5.
2.9. Transmission Electron Microscopy
Promastigote forms of L. infantum were treated with M. citrifolia fruit juice at concentrations of 480, 240, 120, 60, and 30 µg/mL for 24 hours. The parasites were fixed with 2.5% glutaraldehyde (Sigma, USA) in 0.1 M sodium-cacodylate buffer, pH 7.2 overnight. Parasites were washed three times with 0.1 M sodium-cacodylate buffer and postfixed in a solution containing 1% osmium tetroxide, 0.8% ferrocyanide, and 5 mM calcium chloride, washed in 0.1 M sodium-cacodylate buffer, dehydrated in graded acetone, and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and examined in a transmission electron microscope JEM-1011 (JEOL, Japan).
2.10. Statistical Analysis
The values were expressed as mean ± SD. The results were analyzed statistically by Analysis of Variance (ANOVA) followed by the Tukey test. The analyses were performed with the software GraphPad Prism 5.0.4. Differences were considered significant when p < 0.05.
3. Results and Discussion
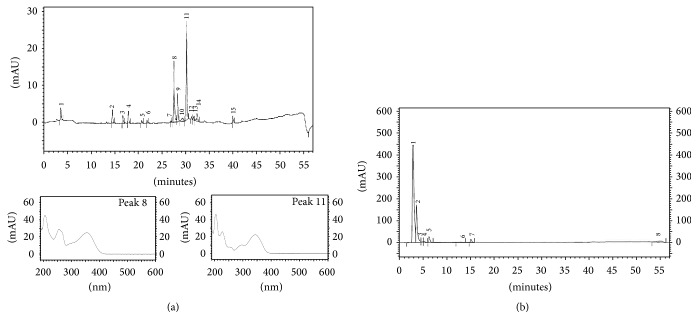
The M. citrifolia fruit juice was brown, translucent, and of medium viscosity, with its characteristic odor and pH 3.94. After lyophilization, the juice yielded 6.31% of a highly hygroscopic powder. The constituents of M. citrifolia fruit juice were analyzed by HPLC-DAD-ELSD and the analysis of the chromatograms obtained from both detectors showed peaks related to compounds with sensitivity in the UV region. The LC-DAD and LC-ELSD chromatograms are presented in Figure 1. The major peaks of the UV-365 nm chromatogram were associated with the characteristic UV spectra of flavonoid (peak 8) and anthraquinones (peak 11). The ELSD fingerprint showed an intense peak at 3.2 min which was not observed in the DAD chromatogram. This signal could be related to the polysaccharides previously reported in M. citrifolia that have significant antitumoral activity [12]. Polysaccharides from Echinacea purpurea showed activity against Leishmania enriettii [13] and the presence of these substances in the phytochemical fingerprint showed the probable chemical potential of the fruit extract against protozoa such as the Leishmania genus. This potential was demonstrated through in vitro leishmanicidal activity of the M. citrifolia fruit juice against L. infantum.
Figure 1.
High Performance Liquid Chromatography coupled with diode array detector (a) and evaporative light scattering detector (b) of Morinda citrifolia fruit juice at 365 nm. (a) Peaks 8 and 11: highlight of the UV spectra.
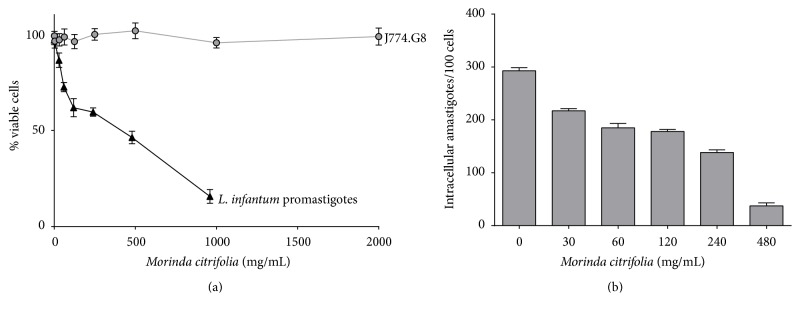
The effect of M. citrifolia fruit juice on the promastigote forms of L. infantum was monitored for 72 hours. M. citrifolia fruit juice produced a dose-dependent reduction in the proliferation of the parasite (Figure 2(a)), with growth inhibition of 50% of the promastigotes at a concentration of 260.5 µg/mL (Table 1). The values are considered promising when compared with other fruit extracts. The crude extract from the fruit Momordica charantia showed an IC50 under 600 µg/mL for L. donovani promastigotes [14].
Figure 2.
Leishmanicidal activity and in vitro cytotoxicity of Morinda citrifolia fruit juice. (a) Viability of Leishmania infantum promastigotes and J774.G8 macrophages treated with M. citrifolia for 72 and 24 hours, respectively. (b) Intracellular amastigotes in BALB/c peritoneal macrophages treated for 24 hours. Data represent mean ± SD of at least three independent experiments realized in quintuplicate.
Table 1.
Activity against promastigotes and intracellular amastigotes of Leishmania infantum, cytotoxicity in peritoneal macrophages from BALB/c and selectivity index of Morinda citrifolia fruit extract treatment and amphotericin B.
| Compounds | IC50 (µg/mL) | CC50 | SI | |
|---|---|---|---|---|
| Promastigote | Intracellular amastigote | J774.G8 | ||
| Morinda citrifolia fruit juice | 260.5 ± 0.044 | 201.3 ± 0.175 | >2000 | >9.9 |
| Amphotericin B | 3.1 ± 0.230 | 0.9 ± 0.121 | 2.7 ± 0.156 | 3.0 |
IC50: inhibitory concentration of 50% parasites. CC50: cytotoxicity concentration of 50% cells. SI: selectivity index. Data are presented as the mean ± SD of three independent experiments realized at least in triplicate.
There are few data about in vitro leishmanicidal activity of M. citrifolia constituents in the literature. A clinical trial on the antileishmanial activity of M. citrifolia showed good activity for two anthraquinones isolated from stem extract, morindicone and morinthone [9]. Anthraquinones also have been isolated from M. lucida, a plant of same gender of M. citrifolia, and presented activity against the growth of Plasmodium falciparum and promastigotes of L. major in vitro [15].
Trying to find compounds responsible for in vitro leishmanicidal activity, the M. citrifolia fruit juice was submitted to a column partition and, interestingly, the partitions showed IC50 values above the value obtained for the full juice (data not shown). This result indicates that various substances present in the M. citrifolia fruit juice contribute to the leishmanicidal activity, probably, synergistically, corroborating previous studies where more than one molecule presented biological activity [16, 17].
Although the leishmanicidal activity in vitro against promastigotes is used by many researchers as a screening test to search for new drugs for the treatment of leishmaniasis, the positive result of this test alone cannot be considered as an indicator of potential drug action. Activity against intracellular amastigotes is necessary and is perhaps the most effective way to relate the in vitro activity of a substance with its possible effectiveness in vivo. Thus we also evaluated M. citrifolia activity against intracellular amastigotes (Figure 2(b)).
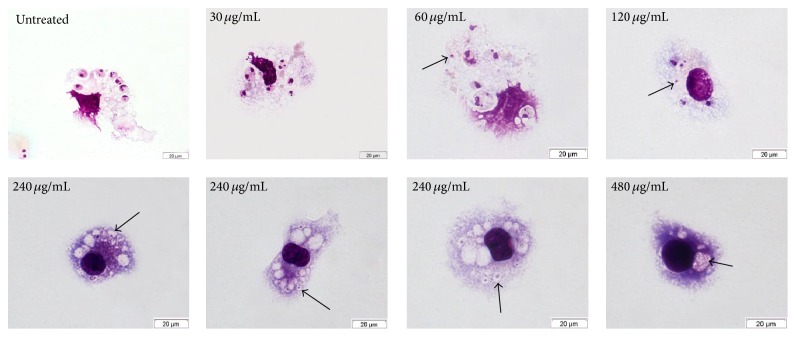
As shown in Table 1, there is an increase in activity of the M. citrifolia fruit juice against intracellular amastigotes compared with the activity against promastigotes. The IC50 value decreased for intracellular amastigotes with a value of 201.3 µg/mL. When observed by light microscopy, macrophages showed vacuoles with probable remains of intracellular amastigotes (Figure 3). This result indicates the possible action of the M. citrifolia fruit juice on macrophage activation and modulation, as already shown in previous works, such as the decreased production of IL-4 and increased production of TNF-α, IL-1β [12], INF-γ, and NO [18].
Figure 3.
Light microscopy of BALB/c peritoneal macrophages infected with Leishmania amazonensis and treated with Morinda citrifolia fruit juice. Arrows indicate vacuoles with probable remains of intracellular amastigotes.
To ensure that M. citrifolia fruit juice was only acting on intracellular amastigotes, without causing damage to the host cell, the cytotoxicity in J774.G8 lineage macrophages was investigated by the Sulforhodamine B method (Figure 2(a)). This colorimetric method is based on the quantification of total protein by binding anionic Sulforhodamine B electrostatic crystals with cellular proteins. No cytotoxicity was observed at the concentrations analyzed and the selectivity index for M. citrifolia fruit juice was at least 3.3-fold higher than amphotericin B (Table 1).
Macrophages were used to assess the toxicity in vitro for being target cells of infection by Leishmania. The analysis of cytotoxicity against macrophages J774.G8 shows the low cytotoxicity of M. citrifolia fruit juice. This data becomes more relevant when analyzed together with IC50 to intracellular amastigote forms, generating SI higher than 9.9 that falls within the generic hit selection criteria of SI to new compounds for infectious diseases from Japanese Global Health Innovative Technology [19]. Indeed, as the generic hit criteria must be applied to phytotherapy with some reservations, the selective index is the most reliable criterion to assess the safety of extracts, essential oils, or others natural products. Besides, the leishmanicidal activity of M. citrifolia must be analyzed in addition to immunomodulatory effects and toxicity in posterior studies.
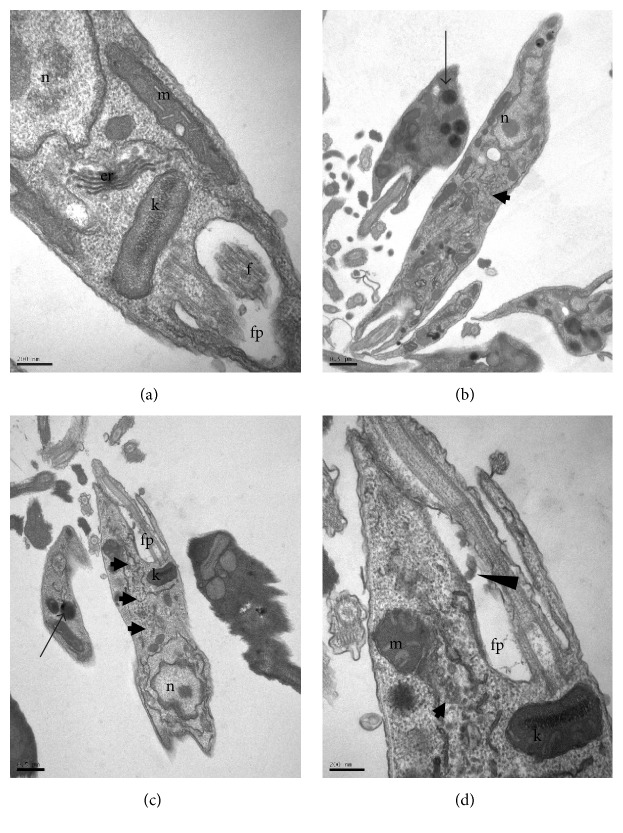
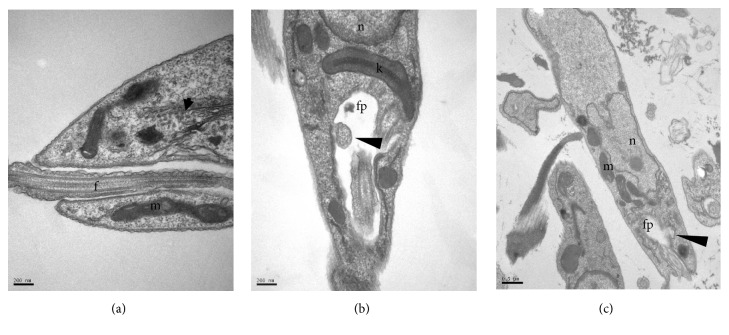
The transmission electron microscopy analysis of L. infantum promastigotes treated with the M. citrifolia fruit juice was performed to determine the ultrastructural changes. Photomicrographs of promastigotes (Figures 4, 5, 6, and 7) showed the degree of damage after 24 hours of treatment. The parasites without treatment showed normal morphology (Figure 4(a)).
Figure 4.
Ultrastructure of Leishmania infantum promastigotes incubated for 24 hours, at 26°C, with Morinda citrifolia fruit juice. (a) Control. (b–d) Promastigote treated with 30 µg/mL. (b-c) Electron-dense vesicles (arrows) and granular material throughout the cytoplasm (block arrows). (d) Membranes in flagellar pocket (arrowhead). k: kinetoplast, m: mitochondria, n: nucleus, pf: flagellar pocket, f: flagellum, and er: endoplasmic reticulum.
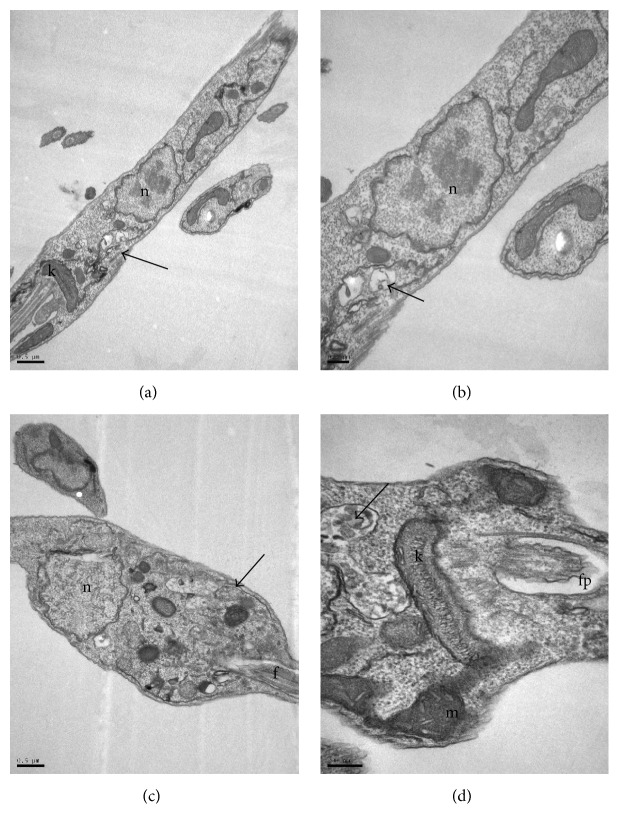
Figure 5.
Ultrastructure of Leishmania infantum promastigotes incubated for 24 hours, at 26°C, with Morinda citrifolia fruit juice. (a–c) Promastigote treated with 60 µg/mL. Electron-dense vesicles (arrows) and granular material throughout the cytoplasm (block arrows). Vesicles breaking up in flagellar pocket (arrowhead). k: kinetoplast, m: mitochondria, n: nucleus, pf: flagellar pocket, and f: flagellum.
Figure 6.
Ultrastructure of Leishmania infantum promastigotes incubated for 24 hours, at 26°C, with Morinda citrifolia fruit juice. (a-b) Promastigotes treated with 120 µg/mL. (c-d) Promastigotes treated with 240 µg/mL. Vesicles with granular material throughout the cytoplasm (arrows). Vesicles with autophagosome-like material (asterisks). k: kinetoplast, m: mitochondria, n: nucleus, pf: flagellar pocket, and f: flagellum.
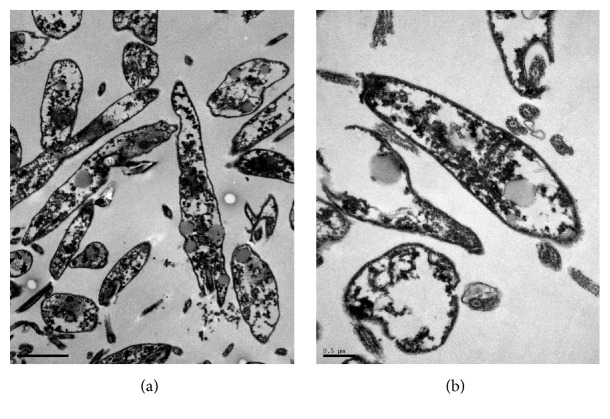
Figure 7.
Ultrastructure of Leishmania infantum promastigotes incubated for 24 hours, at 26°C, with 480 µg/mL of Morinda citrifolia fruit juice. (a-b) Promastigotes with loss of membrane integrity.
The observation of L. infantum promastigotes treated with 30 µg/mL of juice showed vacuolization of the cytoplasm, some with electron-dense regions inside, and this became more evident at higher concentrations (Figures 4(b) and 4(c)). Similar structural changes have also been described in L. amazonensis treated with essential oils [20]. In these, the vacuoles are associated with entry of substances by simple diffusion, caused by increased permeability of the membrane due to the compounds in the essential oils.
Vesicles in the flagellar pocket were observed in promastigotes treated with 30 and 60 µg/mL of M. citrifolia fruit juice (Figures 4(d), 5(b), and 5(c)). The presence of vesicles in flagellar pockets indicates an intense exocytic activity in the region of the flagellar pocket. These changes have also been reported in promastigotes of L. amazonensis treated with inhibitors of ergosterol synthesis, such as 22,26-azasterol [21]. The increased activity in the region of the exocytic flagellar pocket may be the result of an abnormal secretion of lipids, which accumulate as a consequence of drug action or indicate an exacerbated production of proteins by cells in an attempt to survive [22].
Membrane structures in the cytoplasm were observed in treatments with 30, 60, 120, and 240 µg/mL of M. citrifolia fruit juice (Figures 4(b)–4(d), 5(a), 6(a) and 6(b)). These structures are membranes of the endoplasmic reticulum dispersed in the cytoplasm and are probably involved in the recycling of abnormal organelles. The presence of autophagosome-like vesicle material in promastigotes treated with 240 µg/mL of M. citrifolia fruit juice shows an autophagic process (Figures 6(c) and 6(d)), suggesting a remodeling of organelles irreversibly damaged by the treatment. Autophagy can serve as a protective mechanism by recycling macromolecules and removing damaged organelles, but excessive autophagy can result in cell death [23]. To try to survive the effects caused by M. citrifolia fruit juice treatment, parasites may react triggering autophagic events, and this exacerbated autophagic response could lead to death, as observed in parasites treated with the 480 µg/mL of juice.
The treatment with 480 µg/mL of M. citrifolia fruit juice for 24 hours induced severe cellular damage (Figures 7(a) and 7(b)), with the extravasation of cytoplasmic contents and loss of cellular integrity. The progression of ultrastructural changes is related to increasing drug concentrations, reaching its apex with parasite destruction at higher drug concentrations, and showing the direct action of M. citrifolia fruit juice on the parasite and its dose-dependent action. No changes were observed in the nucleus, the mitochondria, the flagellum, the kinetoplast, or the subpellicular microtubules.
4. Conclusion
M. citrifolia fruit juice showed leishmanicidal activity against L. infantum promastigote, causing ultrastructural changes such as cytoplasmic vacuolization, lipid inclusion, increased exocytosis activity, autophagosome-like vesicles, loss of cellular integrity, and death of the parasite. Considering the activity and the alterations observed against promastigote forms of L. infantum, further studies must be conducted to evaluate the potential of M. citrifolia fruit juice in leishmaniasis treatment.
Acknowledgments
This work was supported by FAPEMA (APP-00844/09), CNPq (407831/2012.6), and IOC.
Disclosure
Ana Lúcia Abreu-Silva (CNPq no. 306218/2010-0) is senior researcher.
Competing Interests
The authors report no conflict of interests.
Authors' Contributions
Kátia da Silva Calabrese and Ana Lúcia Abreu-Silva contributed equally to this work.
References
- 1.Rodrigues E. Plants and animals utilized as medicines in the Jaú National Park (JNP), Brazilian Amazon. Phytotherapy Research. 2006;20(5):378–391. doi: 10.1002/ptr.1866. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second Who Report on Neglected Diseases. Lyon, France: World Health Organization; 2013. [Google Scholar]
- 3.Alvar J., Vélez I. D., Bern C., et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE. 2012;7(5) doi: 10.1371/journal.pone.0035671.e35671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miot H. A., Miot L. D., Costa A. L., Matsuo C. Y., O'Dwyer L. H. Avaliação do efeito antiparasitário do omeprazol na prevenção do desenvolvimento de lesões cutâneas em hamsters infectados por Leishmania brasiliensis . Anais Brasileiros de Dermatologia. 2005;80(supplement 3, article 4):S329–S332. doi: 10.1590/s0365-05962005001000011. [DOI] [Google Scholar]
- 5.Basar S., Uhlenhut K., Högger P., Schöne F., Westendorf J. Analgesic and antiinflammatory activity of Morinda citrifolia L. (Noni) fruit. Phytotherapy Research. 2010;24(1):38–42. doi: 10.1002/ptr.2863. [DOI] [PubMed] [Google Scholar]
- 6.Hiramatsu T., Imoto M., Koyano T., Umezawa K. Induction of normal phenotypes in ras-transformed cells by damnacanthal from Morinda citrifolia . Cancer Letters. 1993;73(2-3):161–166. doi: 10.1016/0304-3835(93)90259-c. [DOI] [PubMed] [Google Scholar]
- 7.Kandaswamy D., Venkateshbabu N., Gogulnath D., Kindo A. J. Dentinal tubule disinfection with 2% chlorhexidine gel, propolis, morinda citrifolia juice, 2% povidone iodine, and calcium hydroxide. International Endodontic Journal. 2010;43(5):419–423. doi: 10.1111/j.1365-2591.2010.01696.x. [DOI] [PubMed] [Google Scholar]
- 8.Jainkittivong A., Butsarakamruha T., Langlais R. P. Antifungal activity of Morinda citrifolia fruit extract against Candida albicans. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2009;108(3):394–398. doi: 10.1016/j.tripleo.2009.05.044. [DOI] [PubMed] [Google Scholar]
- 9.Sattar F. A., Ahmed F., Ahmed N., Sattar S. A., Malghani M. A. K., Choudhary M. I. A double-blind, randomized, clinical trial on the antileishmanial activity of a Morinda citrifolia (Noni) stem extract and its major constituents. Natural Product Communications. 2012;7(2):195–196. [PubMed] [Google Scholar]
- 10.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 11.Skehan P., Storeng R., Scudiero D., et al. New colorimetric cytotoxicity assay for anticancer-drug screening. Journal of the National Cancer Institute. 1990;82(13):1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 12.Hirazumi A., Furusawa E. An immunomodulatory polysaccharide-rich substance from the fruit juice of Morinda citrifolia (noni) with antitumour activity. Phytotherapy Research. 1999;13(5):380–387. doi: 10.1002/(SICI)1099-1573(199908/09)13:5<380::AID-PTR463>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Steinmüller C., Roesler J., Gröttrup E., Franke G., Wagner H., Lohmann-Matthes M.-L. Polysaccharides isolated from plant cell cultures of Echinacea purpurea enhance the resistance of immunosuppressed mice against systemic infections with Candida albicans and Listeria monocytogenes . International Journal of Immunopharmacology. 1993;15(5):605–614. doi: 10.1016/0192-0561(93)90078-d. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S., Raychaudhuri B., Banerjee S., Das B., Mukhopadhaya S., Datta S. C. Momordicatin purified from fruits of Momordica charantia is effective to act as a potent antileishmania agent. Parasitology International. 2010;59(2):192–197. doi: 10.1016/j.parint.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 15.Sittie A. A., Lemmich E., Olsen C. E., et al. Structure-activity studies: in vitro antileishmanial and antimalarial activities of anthraquinones from Morinda lucida . Planta Medica. 1999;65(3):259–261. doi: 10.1055/s-2006-960473. [DOI] [PubMed] [Google Scholar]
- 16.Liu C.-H., Xue Y.-R., Ye Y.-H., Yuan F.-F., Liu J.-Y., Shuang J.-L. Extraction and characterization of antioxidant compositions from fermented fruit juice of Morinda citrifolia (Noni) Agricultural Sciences in China. 2007;6(12):1494–1501. doi: 10.1016/s1671-2927(08)60013-9. [DOI] [Google Scholar]
- 17.Furusawa E., Hirazumi A., Story S., Jensen J. Antitumour potential of a polysaccharide-rich substance from the fruit juice of Morinda citrifolia (Noni) on sarcoma 180 ascites tumour in mice. Phytotherapy Research. 2003;17(10):1158–1164. doi: 10.1002/ptr.1307. [DOI] [PubMed] [Google Scholar]
- 18.Palu A. K., Kim A. H., West B. J., Deng S., Jensen J., White L. The effects of Morinda citrifolia L. (noni) on the immune system: its molecular mechanisms of action. Journal of Ethnopharmacology. 2007;115(3):502–506. doi: 10.1016/j.jep.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Katsuno K., Burrows J. N., Duncan K., et al. Hit and lead criteria in drug discovery for infectious diseases of the developing world. Nature Reviews Drug Discovery. 2015;14(11):751–758. doi: 10.1038/nrd4683. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira V. C. S., Moura D. M. S., Lopes J. A. D., de Andrade P. P., da Silva N. H., Figueiredo R. C. B. Q. Effects of essential oils from Cymbopogon citratus (DC) Stapf., Lippia sidoides Cham., and Ocimum gratissimum L. on growth and ultrastructure of Leishmania chagasi promastigotes. Parasitology Research. 2009;104(5):1053–1059. doi: 10.1007/s00436-008-1288-6. [DOI] [PubMed] [Google Scholar]
- 21.Rodrigues J. C. F., Attias M., Rodriguez C., Urbina J. A., De Souza W. Ultrastructural and biochemical alterations induced by 22,26-azasterol, a Δ24(25)-sterol methyltransferase inhibitor, on promastigote and amastigote forms of Leishmania amazonensis. Antimicrobial Agents and Chemotherapy. 2002;46(2):487–499. doi: 10.1128/AAC.46.2.487-499.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tiuman T. S., Ueda-Nakamura T., Garcia Cortez D. A., et al. Antileishmanial activity of parthenolide, a sesquiterpene lactone isolated from Tanacetum parthenium . Antimicrobial Agents and Chemotherapy. 2005;49(1):176–182. doi: 10.1128/aac.49.11.176-182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishikawa T., Tsuno N. H., Okaji Y., et al. Inhibition of autophagy potentiates sulforaphane-induced apoptosis in human colon cancer cells. Annals of Surgical Oncology. 2010;17(2):592–602. doi: 10.1245/s10434-009-0696-x. [DOI] [PubMed] [Google Scholar]