Abstract
AIM: To investigate the long-term oncologic outcomes and prognostic factors in patients with obstructive colorectal cancer (CRC) at multiple Japanese institutions.
METHODS: We identified 362 patients diagnosed with obstructive colorectal cancer from January 1, 2002 to December 31, 2012 in Yokohama Clinical Oncology Group’s department of gastroenterological surgery. Among them, 234 patients with stage II/III disease who had undergone surgical resection of their primary lesions were analyzed, retrospectively. We report the long-term outcomes, the risk factors for recurrence, and the prognostic factors.
RESULTS: The five-year disease free survival and cancer-specific survival were 50.6% and 80.3%, respectively. A multivariate analysis showed the ASA-PS (HR = 2.23, P = 0.026), serum Albumin ≤ 4.0 g/dL (HR = 2.96, P = 0.007), T4 tumor (HR = 2.73, P = 0.002) and R1 resection (HR = 6.56, P = 0.02) to be independent risk factors for recurrence. Furthermore, poorly differentiated cancers (HR = 6.28, P = 0.009), a T4 tumor (HR = 3.46, P = 0.011) and R1 resection (HR = 6.16, P = 0.006) were independent prognostic factors in patients with obstructive CRC.
CONCLUSION: The outcomes of patients with obstructive CRC was poor. T4 tumor and R1 resection were found to be independent prognostic factors for both recurrence and survival in patients with obstructive CRC.
Keywords: Obstructive colorectal cancer, Prognostic factor, Survival
Core tip: Obstructive colorectal cancer (CRC) still have poor prognosis. However, the prognostic factor of obstructive CRC is unclear. The aim of this article is to clarify the long-term outcome and the risk factors for obstructive CRC at multiple institutions. The five-year disease free survival and cancer-specific survival were 50.6% and 80.3%, respectively. T4 tumor and R1 resection were independent prognostic factors for both recurrence and survival.
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in Japan, and the incidence of CRC has been increasing rapidly. CRC is difficult to diagnose due to it early atypical symptoms and signs. Around 7%-16% of patients with colorectal malignancy present with acute colorectal obstruction[1-3]. It is generally accepted that right sided obstructive CRC can best be treated by right hemicolectomy with ileocolic anastomosis. On the other hand, the optimal treatment for left-sided obstructive CRC remains controversial[4-7]. The treatment options range from an emergency radical operation, such as Hartmann’s procedure, to bowel decompression using metallic stents or transanal tube or proximal diversion with a subsequent staged resection. The choices of surgical intervention for obstructive CRC vary greatly, according to the tumor location, general condition of the patients, and the experience level of the surgeons[8]. Therefore, It has been reported that CRC patients with obstruction have an advanced stage and worse long-term survival compared to non-obstructive CRC[3,9-11]. Although the negative impact of obstruction on the postoperative outcomes has been well documented, few studies have examined the outcomes of obstructive CRC patients in Japan[11-13]. Furthermore, the risk factors for recurrence and the prognostic factors are unclear owing to the small number of patients in previous study.
The aim of this study is to investigate the long-term oncologic outcomes and prognostic factors in patients with obstructive CRC at multiple Japanese institutions.
MATERIALS AND METHODS
Three hundred and sixty-two patients who were diagnosed to have obstructive colorectal cancer from January 2002 to December 2012 at the Yokohama Clinical Oncology Group’s Department of Gastroenterological Surgery (10 institutions) were enrolled. Obstructive CRC was diagnosed based on medical history, physical examination, abdominal computed tomography (CT), and colonoscopy, and the surgical findings. We first performed emergency decompression of bowel obstruction by ileostomy/colostomy or the insertion of a decompression tube, or emergency resection of the primary lesion. The type of decompression method was chosen according to the surgeon’s judgment and preference. Patients with distant metastatic lesions (n = 103), who only underwent stoma creation and best supportive care (n = 23), stage I (n = 2) were excluded from this study. As a result, 234 patients who underwent surgical resection were analyzed retrospectively (Figure 1). The prognostic factors influencing survival and risk factors for recurrence were analyzed.
Figure 1.
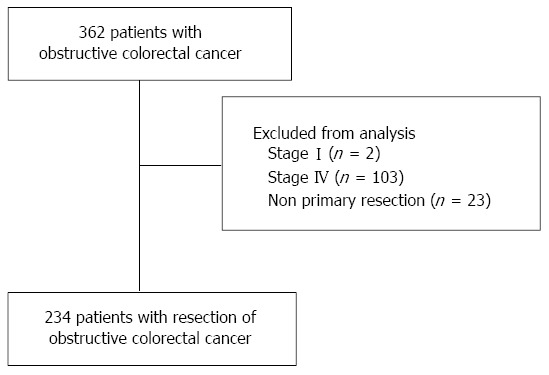
Study flowchart.
Clinicopathological information was obtained from the medical records of the patients including gender, age, The American Society of Anesthesiologists (ASA)-physical status (PS), serum albumin, CEA, preoperative decompression, location of the tumor, tumor size, differentiation of the tumor, depth of the tumor, intramural lymphatic invasion, intramural vascular invasion, lymph node dissection, number of lymph nodes harvested, lymph node involvement, postoperative complication, anastomotic leakage, curability, and adjuvant chemotherapy. There were missing values for BMI in 13 patients, for serum albumin in 14 patients, for CEA in 19 patients, for tumor size in 4 patients, for lymphatic invasion in one patient and for harvested lymph nodes in 10 patients because this was a retrospective study.
Japanese D3 lymphadenectomy is equivalent to complete mesocolic excision (CME) with central vascular ligation (CVL)[14]. D2 lymphadectomy includes pericolic and intermediate nodes region, and D0-1 includes only pericolic nodes region.
Statistical analysis
The disease-free survival (DFS) and cancer-specific survival (CSS) were estimated using the Kaplan-Meier method, and statistical significance was determined by the log-rank test. A multivariate analysis was performed using the Cox proportional hazard model to examine the independent prognostic factors and risk factors of recurrence. A P value of < 0.05 indicated statistical significance. All analyses were performed using the IBM SPSS, version 21 (SPSS Inc., Chicago, IL, United States).
This study received approval from the institutional review board of Yokohama City University.
RESULTS
Characteristic of the patients
The clinicopathological characteristics of the patients are summarized in Table 1. There were 234 patients who underwent surgical resection for obstructive CRC. The median age of the patients was 71 years (range 35-96) and there were 141 (60.3%) men and 93 (39.7%) women. Of these patients, 183 patients (72.2%) received preoperative decompression by colostomy/ileostomy (n = 56) or transanal tube insertion (n = 127)[15]. The most common tumor site was the sigmoid colon (n = 95). Other primary tumors were located in the descending colon (n = 36), ascending colon (n = 33), transverse colon (n = 33), rectum (n = 27), and cecum (n = 10). Among the 234 patients in this study, 165 patients (70.5%) had obstructing cancers at a site distal to the splenic flexure. T4 tumors were found in 125 patients (53.4%). There were 113 stage II patients and 121 stage III patients. In the stage III cases, 90 patients had N1 disease and 31 patients had N2.
Table 1.
Clinicopathological characteristic of patients with obstructive colorectal cancer n (%)
| Variable | Category | n = 234 |
| Gender | Male | 141(60.3) |
| Female | 93 (39.7) | |
| Age (yr)1 | 71 (35-96) | |
| ASA | I | 70 (30) |
| II | 128 (54.7) | |
| III | 36 (15.3) | |
| Location of tumor | Cecum | 10 (4.3) |
| Ascending colon | 33 (14.1) | |
| Transverse colon | 33 (14.1) | |
| Descending colon | 36 (15.4) | |
| Sigmoid colon | 95 (40.6) | |
| Rectum | 15 (10.5) | |
| Decompression | + | 183 (78.2) |
| - | 51 (21.8) | |
| CEA (mg/dL) 1 | 4.9 (0.3-2470) | |
| Serum albumin1 | 3.4 (1.4-4.9) | |
| Tumor size(mm) | 48 (10-140) | |
| Depth of tumor | pT3 | 109 (46.6) |
| pT4a | 93 (39.7) | |
| pT4b | 32 (13.7) | |
| Lymph node involvement | N0 | 113 (48.3) |
| N1 | 90 (38.5) | |
| N2 | 31 (13.2) | |
| R0 resection | + | 219 (93.6) |
| - | 15 (6.4) | |
| Adjuvant chemotherapy | + | 91 (38.9) |
| - | 143 (61.1) |
Median (range).
A total of 219 patients (93.6%) underwent R0 resection of the primary lesion. The reasons of R1 resection (n = 15) were positive surgical margins in 11 patients, other organ involvement in 3 patients and residual lymph node metastasis in 1 patient.
Ninety-one of the 234 patients underwent adjuvant chemotherapy. The chemotherapeutic regimen was oral 5-fluorouracil (5-FU) in 47 patients, oral 5-FU plus leucovorin in 36, oxaliplatin-based chemotherapy in 5, and a Roswell Park Memorial Institute (RPMI) regimen in 3[16].
Long-term outcomes
The median follow-up interval was 39 mo. The five-year DFS and CSS for all patients were 50.6% and 80.3%, respectively. The 5-year DFS of the patients with stage II and stage III disease were 47.4% and 55.1%, respectively. The 5-year CSS were 78.7% and 82.0%, respectively. There were no significantly differences in both the DFS and CSS between stage II and stage III disease (P = 0.856, P = 0.560) (Figures 2 and 3).
Figure 2.
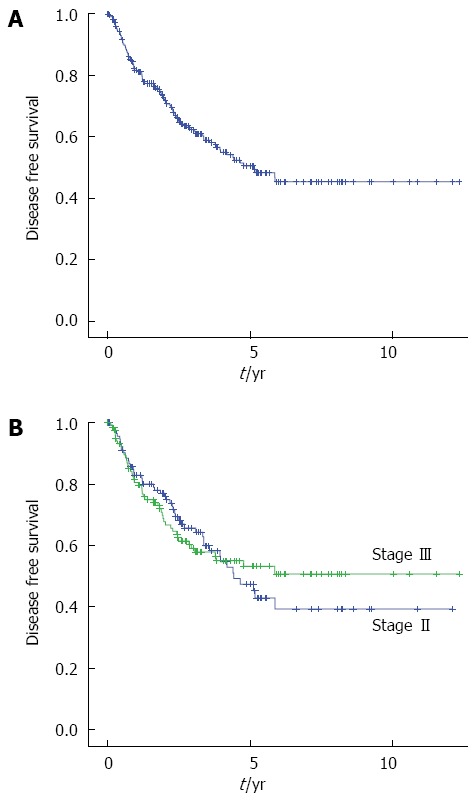
Kaplan-Meier curves showing the disease free survival after primary tumor resection in patients with obstructive colorectal cancer. A: All stage (n = 234); B: Stage II (n = 114, blue line), Stage III (n = 120, green line).
Figure 3.
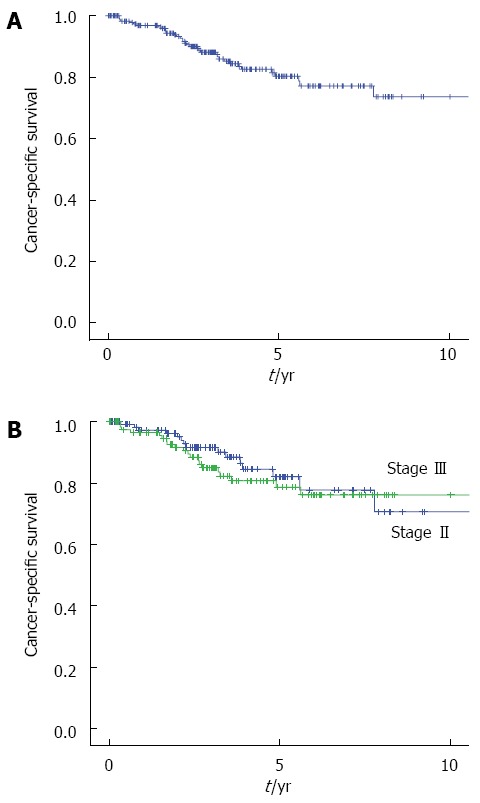
Kaplan-Meier curves showing the cancer-specific survival rates after primary tumor resection in patients with obstructive colorectal cancer. A: All stage (n = 234); B: Stage II (n = 114, blue line), Stage III (n = 120, green line).
Recurrence site
A total 71 patients (30.3%) experienced recurrence during the study follow-up (Table 2). The most common site of recurrence was the liver (n = 27, 11.5%), followed by the lung (n = 22, 9.4%), peritoneum (n = 21, 9.0%), and local recurrence (n = 9, 3.8%). Other sites of recurrence included the non-regional lymph nodes (n = 6), anastomosis (n = 3), abdominal wall (n = 2), and pleural dissemination (n = 2). The rate of the recurrence sites did not differ substantially between stage II and stage III disease (data not shown).
Table 2.
Patterns of recurrence following colorectal resection of obstructive colorectal cancer (n = 71)
| Site of recurrence | n (%) |
| Liver | 27 (11.5) |
| Lung | 22 (9.4) |
| Peritoneal dissemination | 21 (9.0) |
| Local recurrence | 9 (3.8) |
| Lymph node | 6 (2.6) |
| Anastomosis | 3 (1.3) |
| Abdominal wall | 2 (0.9) |
| Pleural dissemination | 2 (0.9) |
Risk factors for recurrence
The risk factors for recurrence according to our analysis are shown in Table 3. According to a univariate analysis, the factors associated with recurrence were age ≥ 75 years (P = 0.011), ASA-PS (P = 0.017), serum albumin ≤ 4.0 g/dL (P = 0.001), T4 tumor (P = 0.001), and R1 resection (P < 0.001). A multivariate analysis of these factors confirmed significant differences for ASA-PS (HR = 2.234, P = 0.026) serum albumin (HR = 2.967, P = 0.007), depth of tumor (HR = 2.728, P = 0.002) and curability (HR = 6.555, P = 0.02). There were no differences in the relapse rate according to whether the patients underwent preoperative decompression or not. Furthermore, lymph node involvement was also not associated with recurrence.
Table 3.
Result of the univariate and multivariate analysis of risk factors for recurrence
| Factor | n |
Univariate analysis |
Multivariate analysis |
||||
| 3-yr RFS | 5-yr RFS | P value | HR (95%CI) | P value | |||
| Gender | M | 141 | 59.2% | 47.6% | 0.409 | ||
| F | 93 | 66.8% | 54.7% | ||||
| Age (yr) | ≥ 75 | 96 | 55.0% | 42.4% | 0.011 | 1.228 (0.659-2.290) | 0.518 |
| < 75 | 138 | 67.3% | 55.9% | ||||
| ASA-PS | 1 | 71 | 75.7% | 64.8% | 0.017 | 2.234 (1.101-4.535) | 0.026 |
| 2-3 | 163 | 56.6% | 44.6% | ||||
| BMI (kg/m2) | ≥ 25 | 28 | 66.0% | 49.7% | 0.951 | ||
| < 25 | 193 | 62.7% | 52.7% | ||||
| Serum albumin (g/dL) | ≤ 4.0 | 172 | 54.9% | 41.3% | 0.001 | 2.967 (1.342-6.560) | 0.007 |
| > 4.0 | 48 | 79.7% | 73.1% | ||||
| CEA (mg/dL) | ≥ 5.0 | 102 | 52.4% | 44.8% | 0.052 | ||
| < 5.0 | 113 | 74.1% | 54.2% | ||||
| Decompression | + | 183 | 62.9% | 50.7% | 0.572 | ||
| - | 51 | 60.5% | 50.4% | ||||
| Location of tumor | Right side | 69 | 62.5% | 55.5% | 0.738 | ||
| Left side | 165 | 62.1% | 48.7% | ||||
| Tumor size (mm) | ≥ 50 | 99 | 61.0% | 48.8% | 0.384 | ||
| < 50 | 121 | 63.9% | 54.1% | ||||
| Differentiation of tumor | tub1,tub2 | 222 | 63.7% | 51.2% | 0.080 | ||
| por, muc | 12 | 36.7% | 36.7% | ||||
| Depth of tumor | T3 | 108 | 73.5% | 64.7% | 0.001 | 2.728 (1.467-5.072) | 0.002 |
| T4 | 126 | 53.0% | 38.7% | ||||
| Intramural lymphatic invasion | + | 157 | 61.4% | 53.2% | 0.600 | ||
| - | 73 | 64.4% | 45.2% | ||||
| Intramural vascular invasion | + | 164 | 60.3% | 49.3% | 0.683 | ||
| - | 70 | 68.1% | 57.4% | ||||
| Lymph node involvement | + | 120 | 65.7% | 47.4% | 0.856 | ||
| - | 114 | 59.1% | 53.2% | ||||
| Lymph node dissection | D0,D1 | 31 | 44.3% | 44.3% | 0.067 | ||
| D2,D3 | 199 | 64.7% | 50.8% | ||||
| No. of lymph nodes harvested | < 12 | 73 | 54.8% | 44.5% | 0.085 | ||
| ≥ 12 | 151 | 66.4% | 52.5% | ||||
| Postoperative complication | + | 78 | 57.3% | 44.7% | 0.071 | ||
| (≥ Grade 2) | - | 156 | 64.7% | 53.5% | |||
| Anastomotic leakage | + | 18 | 71.1% | 56.9% | 0.562 | ||
| - | 191 | 61.7% | 50.0% | ||||
| Curability | R0 | 219 | 66.4% | 54.8% | < 0.001 | 6.555 (1.344-31.970) | 0.020 |
| R1 | 15 | 7.6% | 0.0% | ||||
| Adjuvant chemotherapy | + | 91 | 66.2% | 55.6% | 0.069 | ||
| - | 143 | 59.8% | 47.2% | ||||
Prognostic factors for CSS
The prognostic factors for CSS are shown in Table 4. A univariate analysis identified age ≥ 75 (P = 0.027), poorly or mucinous differentiation(P = 0.001), T4 tumor (P = 0.001), D0 or D1 lymph node dissection(P = 0.0014), R1 resection (P < 0.001) as poor prognostic factors. According to a multivariate analysis, poorly differentiated cancers or mucinous differentiation (HR = 6.282, P = 0.009), T4 tumor (HR = 3.458, P = 0.011) and R1 resection (HR = 6.162, P = 0.006) were independent prognostic factors in patients with obstructive CRC (Figure 4).
Table 4.
Result of the univariate and multivariate analysis of prognostic factors for cancer-specific survival
| Factor | n |
Univariate analysis |
Multivariate analysis |
||||
| 3-yr CSS | 5-yr CSS | P value | HR(95%CI) | P value | |||
| Gender | M | 141 | 88.3% | 80.8% | 0.924 | ||
| F | 93 | 87.8% | 79.7% | ||||
| Age (yr) | ≥ 75 | 96 | 82.2% | 71.6% | 0.027 | 1.464 (0.647-3.310) | 0.360 |
| < 75 | 138 | 91.9% | 85.4% | ||||
| ASA-PS | 1 | 71 | 86.0% | 83.5% | 0.710 | ||
| 2-3 | 163 | 89.0% | 78.7% | ||||
| BMI (kg/m2) | ≥ 25 | 28 | 92.0% | 85.8% | 0.389 | ||
| < 25 | 193 | 87.3% | 80.0% | ||||
| Serum albumin (g/dL) | ≤ 4.0 | 172 | 85.6% | 82.6% | 0.536 | ||
| > 4.0 | 48 | 87.7% | 76.2% | ||||
| CEA (mg/dL) | ≥ 5.0 | 102 | 86.7% | 80.0% | 0.715 | ||
| < 5.0 | 113 | 90.5% | 82.0% | ||||
| Decompression | + | 183 | 88.4% | 81.7% | 0.514 | ||
| - | 51 | 87.1% | 74.8% | ||||
| Location of tumor | Right side | 69 | 82.6% | 70.5% | 0.103 | ||
| Left side | 165 | 90.4% | 84.3% | ||||
| Tumor size (mm) | ≥ 50 | 99 | 84.1% | 76.9% | 0.291 | ||
| < 50 | 121 | 90.4% | 85% | ||||
| Differentiation of tumor | tub1, tub2 | 222 | 89.9% | 82.5% | 0.001 | 6.282 (1.584-24.909) | 0.009 |
| por, muc | 12 | 50.0% | 50.0% | ||||
| Depth of tumor | T3 | 108 | 95.7% | 92.3% | 0.001 | 3.458 (1.324-9.031) | 0.011 |
| T4 | 126 | 81.1% | 69.2% | ||||
| Intramural lymphatic invasion | + | 157 | 90.8% | 82.7% | 0.449 | ||
| - | 73 | 86.9% | 79.4% | ||||
| Intramural vascular invasion | + | 164 | 94.2% | 90.1% | 0.152 | ||
| - | 70 | 85.9% | 76.7% | ||||
| Lymph node involvement | + | 120 | 92.5% | 82.9% | 0.332 | ||
| - | 114 | 84.0% | 77.7% | ||||
| Lymph node dissection | D0, D1 | 31 | 60.7% | 60.7% | 0.014 | 0.958 (0.300-3.056) | 0.942 |
| D2, D3 | 199 | 90.4% | 83.0% | ||||
| No. of lymph nodes harvested | < 12 | 73 | 83.3% | 78.3% | 0.314 | ||
| ≥ 12 | 151 | 91.1% | 81.0% | ||||
| Postoperative complication | + | 78 | 85.9% | 75.6% | 0.644 | ||
| (≥ Grade 2) | - | 156 | 89% | 82.5% | |||
| Anastomotic leakage | + | 18 | 100% | 100% | 0.069 | ||
| - | 191 | 87.1% | 78.7% | ||||
| Curability | R0 | 219 | 91.3% | 84.7% | < 0.001 | 6.162 (1.692-22.445) | 0.006 |
| R1 | 15 | 39.7% | 19.9% | ||||
| Adjuvant chemotherapy | + | 91 | 87.7% | 81.8% | 0.800 | ||
| - | 143 | 88.8% | 79.4% | ||||
Figure 4.
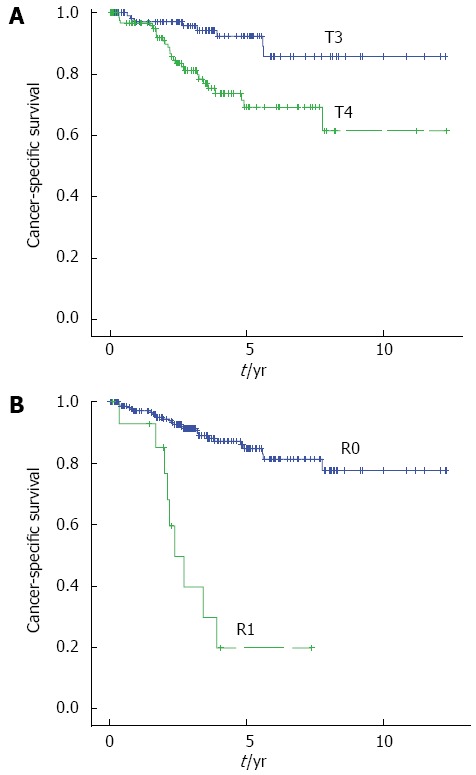
Kaplan-Meier curves showing the cancer-specific survival rates in patients with T4 tumor and R1 resection. A: T3 (n = 108, blue line); T4 (n = 126, green line); B: R0 (n = 219, blue line), R1 (n = 15, green line).
DISCUSSION
In the present study, we evaluated the long-term oncologic outcomes and prognostic factors in patients with obstructive CRC in multiple Japanese institutions. Most previous studies have reported that patients with obstructive CRC have significantly poorer oncologic outcomes than patients with nonobstructive CRC[9,11,12,17]. Obstructive tumors have been reported to have a more advanced stage than nonobstructive tumors[11,18]. The reported 5-year survival ranges between 36% to 64.6% in patients with obstructive CRC[11,17,19,20]. Our retrospective data showed 5-year CSS to be 80.3%, which was higher than the previously reported findings. One reason for the good outcomes might be that Japanese standard surgical procedures include complete tumor resection and extended D2/D3 lymph node dissection, including the pericolic, intermediate and most central lymph nodes. West et al reported that the Japanese surgical procedures as well as CME with CVL eradicates tumors more effectively than the conventional procedures[14]. In our study, D2/D3 lymph node dissection was performed in about 70% of all patients.
Several authors have suggested preoperative obstruction to be a prognostic factor in CRC[12,17]. However, there are few data concerning the prognostic factors associated with obstructive CRC patients[4,19]. Jiang et al[4] reported a delayed resection to provide a better oncologic outcome than a primary resection for obstructive left-sided colorectal cancer. Other authors have showed that decompression followed surgery is better than emergency surgery in terms of the primary anastomosis rate, the stoma rate, the morbidity rate, the successful treatment of the patient’s comorbidities, and preparation for elective surgery[21,22]. According to our results, however, no difference in the prognosis was found in regard to whether patients underwent preoperative decompression or not, It therefore remains inconclusive as to which approach may be superior to the other.
Malignant obstruction can occur in any part of the colon and rectum, however, the risk varies at different locations. In present study, 70.5% of the obstructive CRC occurred in the left-sided colon and most of them occurred in the sigmoid colon. This tumor distribution is similar to what has been reported by other series[11,19]. Our results showed that the prognosis was not different between right-sided and left-sided obstructive CRC.
Obstructive tumors are reported to be associated with a more advanced stage than nonobstruction[11,18]. Our data showed 53.4% to have T4 tumors, and 51.7% had positive lymph nodes. This is one of the reasons why obstructive CRC has a worse prognosis. In present study, especially, a T4 tumor was found to be a risk factor for recurrence in patients with obstructive CRC.
Seventy-one patients had a recurrence of the disease as the first event in our study. The distant metastasis rate is significantly higher in obstructed patients when compared with nonobstructed patients[17]. The common sites of recurrence were the liver (11.5%) and lung (9.4%), which were similar to previous reports[23]. An interesting finding in the present study is that patients with obstructive CRC showed a higher rate of peritoneal dissemination (9.0%) than previously reported (1.9%-3.5%) in nonobstructive CRC[24,25]. These findings suggested that obstructive CRC was locally advanced cancer consisting of T4 tumors and which may be unexpectedly exposed during R1 resection. Therefore, reducing the rate of performing R1 resection might be a key to achieving improved surgical results.
In our study, patients with stage II disease and those with stage III disease had similar poor outcomes in terms of the 5-year DFS and CSS. This finding suggests that lymph node involvement, which is a well-known prognostic factor, does not have any significant impact on the outcomes in patients with obstructive CRC. One of the reasons for this finding is due to the fact that Japanese standard lymph node dissection procedures for advanced colorectal cancer include D2/D3 lymph node dissection, which is nearly the same method as that performed for CME and CVL[14]. In our clinical oncology group, lymphadenectomy for colorectal cancer was routinely performed during the study period. Therefore, a T4 tumor was identified as the most important prognostic factor, in which the 5-year DFS and OS were 38.7% and 60%, respectively.
In the present study, a lower level of albumin was also a predictive factor for survival. The serum albumin levels have recently been studied as the Glasgow Prognostic Score (GPS), based on a combination of albumin and C-reactive protein (CRP). Several authors have revealed the GPS to have prognostic value in patients with advanced colorectal cancer[26,27]. However, we failed to collect data of CRP because this was a retrospective analysis. It is estimated that low albumin levels are associated with a decreased survival time because a low albumin level likely reflects some type of systemic compromise[28].
Our results demonstrated that poorly differentiated tumors or mucinous differentiated tumors are also predictive factors for survival. Histologically, poorly differentiated CRC represents from 4.8% to 23.2% of all colorectal cancers[29]. The rate of poorly differentiated tumors was not higher than that described in previous reports. Poorly differentiated cancers have been linked to adverse prognoses in many studies[30].
Recently, several authors have suggested the feasibility of performing preoperative chemotherapy without the routine use of radiation therapy for locally advanced rectal cancer and a high R0 resection rate[31,32]. Furthermore, the FOxTROT Collaborative Group demonstrated the feasibility of performing preoperative chemotherapy for locally advanced colon cancer[33]. Our result suggested that obstructive colorectal cancer is also locally advanced cancer. Therefore, preoperative chemotherapy after the decompression of bowel obstruction may also be useful for the management of obstructive colorectal cancer.
Our retrospective study had several important limitations. First, there were several missing data and we could not obtain the clinical course related to the treatment of patients after recurrence. Second, the adjuvant therapy, which affects the outcome, was not uniform.
In conclusion, in addition to generally accepted knowledge, we found that T4 tumor and R1 resection were prognostic factors for both recurrence and survival. These results suggested that a curative resection of the tumor is very important and that systemic treatment for preventing distant metastasis, such as peritoneal dissemination associated with T4 tumors, is necessary in patients with obstructive colorectal cancer.
ACKNOWLEDGMENTS
We are grateful to the many members of the Yokohama Clinical Oncology Group and Dr. Mari S Oba (Department of Biostatistics, Yokohama City University) whose comments were extremely valuable throughout the course of our study.
COMMENTS
Background
Colorectal cancer (CRC) is one of most common cancer in the world. Around 7%-16% of patients with colorectal cancer present with acute colorectal obstruction. It has been reported that CRC patients with obstruction have an advanced stage and poor long-term survival compared to non-obstructive CRC. However, the risk factors for recurrence and the prognostic factors of patients with obstructive CRC are unclear.
Research frontiers
The authors often treat the obstructive CRC. However, there are few literatures concerning survival and prognostic factor of obstructive CRC. The research hotspot is to introduce long-term outcome of patients with obstructive colorectal cancer and prognostic factors in Japan.
Innovations and breakthroughs
The present study represents the characteristics and the long-term outcome of obstructive CRC patients in Japan and revealed that T4 tumor and R1 resection are risk factors of recurrence and prognostic factors. These results suggested that a curative resection of the tumor is very important and systemic treatment for preventing distant metastasis, such as peritoneal dissemination associated with T4 tumors, is necessary in patients with obstructive colorectal cancer.
Applications
This study showed the poor survival for obstructive colorectal cancer patients and prognostic factor. The present study provided readers the important information of treatment for patients with obstructive CRC.
Peer-review
The authors demonstrated that T4 tumor status and R1 resection are independent prognostic factors in patients with obstructive colorectal cancer.
Footnotes
Institutional review board statement: This study was reviewed and approved by the institutional review board of Yokohama City University.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: There are no conflicts of interest to report.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 12, 2016
First decision: March 7, 2016
Article in press: April 7, 2016
P- Reviewer: Arigami T S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
References
- 1.Chen HS, Sheen-Chen SM. Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery. 2000;127:370–376. doi: 10.1067/msy.2000.104674. [DOI] [PubMed] [Google Scholar]
- 2.Deans GT, Krukowski ZH, Irwin ST. Malignant obstruction of the left colon. Br J Surg. 1994;81:1270–1276. doi: 10.1002/bjs.1800810905. [DOI] [PubMed] [Google Scholar]
- 3.Ohman U. Prognosis in patients with obstructing colorectal carcinoma. Am J Surg. 1982;143:742–747. doi: 10.1016/0002-9610(82)90050-2. [DOI] [PubMed] [Google Scholar]
- 4.Jiang JK, Lan YT, Lin TC, Chen WS, Yang SH, Wang HS, Chang SC, Lin JK. Primary vs. delayed resection for obstructive left-sided colorectal cancer: impact of surgery on patient outcome. Dis Colon Rectum. 2008;51:306–311. doi: 10.1007/s10350-007-9173-4. [DOI] [PubMed] [Google Scholar]
- 5.Lim JF, Tang CL, Seow-Choen F, Heah SM. Prospective, randomized trial comparing intraoperative colonic irrigation with manual decompression only for obstructed left-sided colorectal cancer. Dis Colon Rectum. 2005;48:205–209. doi: 10.1007/s10350-004-0803-9. [DOI] [PubMed] [Google Scholar]
- 6.Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814–1821. doi: 10.1007/s00464-010-1471-6. [DOI] [PubMed] [Google Scholar]
- 7.Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, et al. Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013;258:107–115. doi: 10.1097/SLA.0b013e31827e30ce. [DOI] [PubMed] [Google Scholar]
- 8.Zorcolo L, Covotta L, Carlomagno N, Bartolo DC. Safety of primary anastomosis in emergency colo-rectal surgery. Colorectal Dis. 2003;5:262–269. doi: 10.1046/j.1463-1318.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 9.Phillips RK, Hittinger R, Fry JS, Fielding LP. Malignant large bowel obstruction. Br J Surg. 1985;72:296–302. doi: 10.1002/bjs.1800720417. [DOI] [PubMed] [Google Scholar]
- 10.McArdle CS, McMillan DC, Hole DJ. The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg. 2006;93:483–488. doi: 10.1002/bjs.5269. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z, Wang L, Kang L, Xiang J, Peng J, Cui J, Huang Y, Wang J. Clinicopathologic characteristics and outcomes of patients with obstructive colorectal cancer. J Gastrointest Surg. 2011;15:1213–1222. doi: 10.1007/s11605-011-1563-1. [DOI] [PubMed] [Google Scholar]
- 12.Katoh H, Yamashita K, Wang G, Sato T, Nakamura T, Watanabe M. Prognostic significance of preoperative bowel obstruction in stage III colorectal cancer. Ann Surg Oncol. 2011;18:2432–2441. doi: 10.1245/s10434-011-1625-3. [DOI] [PubMed] [Google Scholar]
- 13.Hotta T, Takifuji K, Kobayashi Y, Tabuse K, Shimada K, Maeda T, Nakatani Y, Fukiage O, Yamaue H. Management of obstructive colorectal cancer: evaluation of preoperative bowel decompression using ileus tube drainage. Surg Today. 2012;42:1154–1164. doi: 10.1007/s00595-011-0116-2. [DOI] [PubMed] [Google Scholar]
- 14.West NP, Hohenberger W, Weber K, Perrakis A, Finan PJ, Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol. 2010;28:272–278. doi: 10.1200/JCO.2009.24.1448. [DOI] [PubMed] [Google Scholar]
- 15.Nozoe T, Matsumata T. Usefulness of preoperative colonic lavage using transanal ileus tube for obstructing carcinoma of left colon: device to perform one-stage operation safely. J Clin Gastroenterol. 2000;31:156–158. doi: 10.1097/00004836-200009000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Petrelli N, Herrera L, Rustum Y, Burke P, Creaven P, Stulc J, Emrich LJ, Mittelman A. A prospective randomized trial of 5-fluorouracil versus 5-fluorouracil and high-dose leucovorin versus 5-fluorouracil and methotrexate in previously untreated patients with advanced colorectal carcinoma. J Clin Oncol. 1987;5:1559–1565. doi: 10.1200/JCO.1987.5.10.1559. [DOI] [PubMed] [Google Scholar]
- 17.Wang HS, Lin JK, Mou CY, Lin TC, Chen WS, Jiang JK, Yang SH. Long-term prognosis of patients with obstructing carcinoma of the right colon. Am J Surg. 2004;187:497–500. doi: 10.1016/j.amjsurg.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 18.Chin CC, Wang JY, Changchien CR, Huang WS, Tang R. Carcinoma obstruction of the proximal colon cancer and long-term prognosis--obstruction is a predictor of worse outcome in TNM stage II tumor. Int J Colorectal Dis. 2010;25:817–822. doi: 10.1007/s00384-010-0904-y. [DOI] [PubMed] [Google Scholar]
- 19.Lee YM, Law WL, Chu KW, Poon RT. Emergency surgery for obstructing colorectal cancers: a comparison between right-sided and left-sided lesions. J Am Coll Surg. 2001;192:719–725. doi: 10.1016/S1072-7515(01)00833-X. [DOI] [PubMed] [Google Scholar]
- 20.Biondo S, Kreisler E, Millan M, Fraccalvieri D, Golda T, Martí Ragué J, Salazar R. Differences in patient postoperative and long-term outcomes between obstructive and perforated colonic cancer. Am J Surg. 2008;195:427–432. doi: 10.1016/j.amjsurg.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD. The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg. 2004;240:76–81. doi: 10.1097/01.sla.0000130723.81866.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee GJ, Kim HJ, Baek JH, Lee WS, Kwon KA. Comparison of short-term outcomes after elective surgery following endoscopic stent insertion and emergency surgery for obstructive colorectal cancer. Int J Surg. 2013;11:442–446. doi: 10.1016/j.ijsu.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Wolmark N, Rockette H, Fisher B, Wickerham DL, Redmond C, Fisher ER, Jones J, Mamounas EP, Ore L, Petrelli NJ. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol. 1993;11:1879–1887. doi: 10.1200/JCO.1993.11.10.1879. [DOI] [PubMed] [Google Scholar]
- 24.Takakura Y, Ikeda S, Imaoka Y, Urushihara T, Itamoto T. An elevated preoperative serum carbohydrate antigen 19-9 level is a significant predictor for peritoneal dissemination and poor survival in colorectal cancer. Colorectal Dis. 2015;17:417–425. doi: 10.1111/codi.12865. [DOI] [PubMed] [Google Scholar]
- 25.Murono K, Kazama S, Yamaguchi H, Kawai K, Ishihara S, Sunami E, Kitayama J, Satoh Y, Kurihara M, Yatomi Y, et al. Detection of carcinoembryonic antigen mRNA in peritoneal lavage by the transcription-reverse transcription concerted method indicates poor prognosis in patients with stage II and III colon cancer. Surgery. 2015;157:322–330. doi: 10.1016/j.surg.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 26.Ishizuka M, Nagata H, Takagi K, Horie T, Kubota K. Inflammation-based prognostic score is a novel predictor of postoperative outcome in patients with colorectal cancer. Ann Surg. 2007;246:1047–1051. doi: 10.1097/SLA.0b013e3181454171. [DOI] [PubMed] [Google Scholar]
- 27.McMillan DC, Crozier JE, Canna K, Angerson WJ, McArdle CS. Evaluation of an inflammation-based prognostic score (GPS) in patients undergoing resection for colon and rectal cancer. Int J Colorectal Dis. 2007;22:881–886. doi: 10.1007/s00384-006-0259-6. [DOI] [PubMed] [Google Scholar]
- 28.Dixon MR, Haukoos JS, Udani SM, Naghi JJ, Arnell TD, Kumar RR, Stamos MJ. Carcinoembryonic antigen and albumin predict survival in patients with advanced colon and rectal cancer. Arch Surg. 2003;138:962–966. doi: 10.1001/archsurg.138.9.962. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H, Yoon YS, Hong SM, Roh SA, Cho DH, Yu CS, Kim JC. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013;140:341–347. doi: 10.1309/AJCP8P2DYNKGRBVI. [DOI] [PubMed] [Google Scholar]
- 30.Ishihara S, Watanabe T, Akahane T, Shimada R, Horiuchi A, Shibuya H, Hayama T, Yamada H, Nozawa K, Matsuda K, et al. Tumor location is a prognostic factor in poorly differentiated adenocarcinoma, mucinous adenocarcinoma, and signet-ring cell carcinoma of the colon. Int J Colorectal Dis. 2012;27:371–379. doi: 10.1007/s00384-011-1343-0. [DOI] [PubMed] [Google Scholar]
- 31.Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H, et al. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol. 2013;43:964–971. doi: 10.1093/jjco/hyt115. [DOI] [PubMed] [Google Scholar]
- 32.Schrag D, Weiser MR, Goodman KA, Gonen M, Hollywood E, Cercek A, Reidy-Lagunes DL, Gollub MJ, Shia J, Guillem JG, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32:513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Foxtrot Collaborative Group. Feasibility of preoperative chemotherapy for locally advanced, operable colon cancer: the pilot phase of a randomised controlled trial. Lancet Oncol. 2012;13:1152–1160. doi: 10.1016/S1470-2045(12)70348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


