Abstract
AIM: To explore the effects and mechanism of action of antidepressant mirtazapine in functional dyspepsia (FD) patients with weight loss.
METHODS: Sixty depressive FD patients with weight loss were randomly divided into a mirtazapine group (MG), a paroxetine group (PG) or a conventional therapy group (CG) for an 8-wk clinical trial. Adverse effects and treatment response were recorded. The Nepean Dyspepsia Index-symptom (NDSI) checklist and the 17-item Hamilton Rating Scale of Depression (HAMD-17) were used to evaluate dyspepsia and depressive symptoms, respectively. The body composition analyzer was used to measure body weight and fat. Serum hormone levels were measured by ELISA.
RESULTS: (1) After 2 wk of treatment, NDSI scores were significantly lower for the MG than for the PG and CG; (2) After 4 or 8 wk of treatment, HAMD-17 scores were significantly lower for the MG and PG than for the CG; (3) After 8 wk of treatment, patients in the MG experienced a weight gain of 3.58 ± 1.57 kg, which was significantly higher than that observed for patients in the PG and CG. Body fat increased by 2.77 ± 0.14 kg, the body fat ratio rose by 4%, and the visceral fat area increased by 7.56 ± 2.25 cm2; and (4) For the MG, serum hormone levels of ghrelin, neuropeptide Y (NPY), motilin (MTL) and gastrin (GAS) were significantly upregulated; in contrast, those of leptin, 5-hydroxytryptamine (5-HT) and cholecystokinin (CCK) were significantly downregulated.
CONCLUSION: Mirtazapine not only alleviates symptoms associated with dyspepsia and depression linked to FD in patients with weight loss but also significantly increases body weight (mainly the visceral fat in body fat). The likely mechanism of mirtazapine action is regulation of brain-gut or gastrointestinal hormone levels.
Keywords: Mirtazapine, Functional dyspepsia, Weight loss, Depression
Core tip: A part of functional dyspepsia (FD) patients were found with weight loss in recent studies. As an antidepressant, mirtazapine was found not only to alleviate symptoms associated with dyspepsia and depression linked to FD with weight loss, but also to significantly increase body weight (mainly the visceral fat in body fat). Moreover, the likely mechanism of mirtazapine action is the regulation of brain-gut or gastrointestinal hormone levels.
INTRODUCTION
Functional dyspepsia (FD) is a common clinical syndrome characterized by chronic and recurrent symptoms in the gastroduodenal region in the absence of any organic or metabolic disease that explains the symptoms[1]. It impairs the patient’s quality of life and work efficiency, and increases the utilization of medical resources[2-4]. Psychosocial factors may play an important role in FD and lead to the use of antidepressant and anxiolytic agents in FD management[5].
Weight loss is a common symptom of digestive diseases, and may indicate an organic disease[6]. However, the indicators of weight loss for the diagnosis of an organic disease are limited[7-9]. Tack et al[10] found that of 40 FD patients, 55% had a weight loss that was > 5% of initial body weight. In a study investigating laboratory parameters and the nutritional status of 180 patients who were diagnosed with FD, 16.67% of patients had a weight loss from 5% to 10% of their initial body weight, and 4.44% had a weight loss of > 10% of their initial body weight[11]. Our previous multi-center research study of 1057 FD patients showed that with the onset of dyspepsia symptoms, 19.58% had lost ≥ 5% of their initial body weight during the previous 12 mo or less. FD patients with weight loss had lower body mass index, more frequent physician visits, higher psychological disorders, poorer appetite and lower quality of life[12].
Antidepressant mirtazapine is clinically used in the treatment of depression or anxiety disorders. In recent years, many clinical trials associated with antidepressants for FD have indicated that antidepressants are effective in treating FD patients[13-15]. A case report about mirtazapine in the treatment of an FD patient with depression reported that the patient’s indigestive symptoms, appetite, depression, and quality of life were improved after taking mirtazapine for 4 wk[16]. However, studies that have focused on the parts of the body that are involved in weight gain and the underlying mechanisms have been rare.
Clinical observations have shown that increases in appetite and food intake, and consequent weight gain occur in some patients undergoing mirtazapine treatment. Whereas such side effects may limit the general application of mirtazapine in antidepressant therapy, these very same effects proved to be beneficial in treating FD patients with weight loss.
Therefore, expanding upon previous work[12,17], in this study we comprehensively explored the effect of mirtazapine on depressive FD patients with weight loss by dynamic observation not only of the changes in dyspepsia and depressive symptoms but also the modifications of body weight and fat distribution and the levels of serum hormones.
MATERIALS AND METHODS
Ethics statement
This study was a prospective, randomized, controlled trial in depressive FD patients with weight loss and was approved by the hospital ethics committee (Clinical trial registration number: ChiCTR-TRC-13003161). Written informed consent was obtained from the patients according to the Declaration of Helsinki.
Patients
In this prospective study, 60 patients were recruited between September 2011 and June 2013 from the gastroenterology outpatient clinic of Guangzhou Nansha Central Hospital. All the patients fulfilled the following criteria[12]: (1) diagnosed with FD according to Rome III criteria; (2) with a weight loss of ≥ 5% of initial body weight since the onset of symptoms; (3) diagnosed with depression by psychiatrists according to the Chinese Classification of Mental Disorders (CCMD-3) and scores of the Hamilton Rating Scale of Depression (HAMD) over 18; and (4) ranged in age from 18 to 65 years; and (5) signed informed consent statements.
The following exclusion criteria were adopted: (1) organic diseases such as peptic ulcers, atrophy or erosive gastroduodenal lesions, tumors, and esophagitis by gastroscopic examination; (2) liver, gallbladder, pancreas, spleen and bowel organic disease by laboratory, B ultrasonic or X-ray examination; (3) dyspepsia symptoms and weight loss that were explained by metabolic or infectious diseases such as diabetes, hyperthyroidism, or tuberculosis; (4) anorexia nervosa and patients with body weight management problems; (5) age < 18 or > 65 years; (6) pregnancy or breast feeding; (7) disabilities; (8) current use of other drugs in clinical research or use of similar drugs in the last half-month; (9) in a severe anxiety or depressive state, or with suicidal tendencies; (10) current use of non-steroidal anti-inflammatory drugs, steroids, or drugs affecting gastric acid secretion; and (11) contraindications for paroxetine or mirtazapine use including hypersensitivity, liver dysfunction or renal failure.
Grouping
Sixty depressive FD patients with weight loss were randomly divided into a mirtazapine group (MG), a paroxetine group (PG) or a conventional therapy group (CG) with 20 patients in each. The trial period spanned 8 wk. The CG was treated with histamine type 2 receptor antagonists or proton pump inhibitors or prokinetic agents; MG was treated with mirtazapine (Remeron®, N.V. Organon, Holland, 30 mg/d); and PG was treated with paroxetine (Seroxat®, SK&F, China, 20 mg/d). All protocols were based on conventional therapy.
Assessments
Adverse effects and treatment response were recorded and data collected at specific time points. These were before treatment (for baseline determination), 2 wk, 4 wk, 6 wk and 8 wk of treatment for the following assessments: dyspepsia symptoms were evaluated with NDSI; depressive symptoms, with HAMD-17; and the change in body weight and the distribution of body fat with the body composition analyzer (InBody720, Biospace, South Korea). Serum hormone levels were measured at baseline, 4 wk and 8 wk; expression levels of ghrelin, leptin, neuropeptide Y (NPY), 5-hydroxy tryptamine (5-HT), cholecystokinin (CCK), motilin (MTL) and gastrin (GAS) were assayed by ELISA.
NDSI: The NDSI evaluated the frequency, intensity, and practical impediments of 15 GI symptoms (including epigastric pain, epigastric burning, postprandial fullness, and early satiety) over a 2-wk period. We recorded each subscale score concerning daily activities/work (13 items), knowledge and control (7 items), eating/drinking (3 items), and sleep disturbance (2 items)[18]. We added each item score within each subscale to produce a subscale score. Low scores indicate mild symptoms.
HAMD-17: The rating standards of the HAMD[19] were as follows: no depression (0-6), mild depression (7-17), moderate depression (18-24), and severe depression (> 25). A higher score indicates worse depression.
Body composition analyzer (InBody720): Patient requirements included: empty stomach, empty bladder, light clothes and no shoes for measurement in the early morning on the body composition analyzer. Patients with a pacemaker or with metal in the body were excluded from measurement on this instrument.
Treatment response: Treatment response was defined as a > 50% reduction in the NDSI score. The response was calculated as: [(score at treatment - score at baseline)/score at baseline] × 100. The treatment responses of the three groups were calculated independently.
Statistical analysis
Data analyses were performed using SPSS 13.0 software (SPSS Inc., Chicago IL, United States), and measurement data are reported as the mean ± SD, and were compared across groups using one-way ANOVA and the Student-Newman-Keuls test for multiple comparisons. Count data were compared across groups using a χ2 test. All tests were two-tailed and P < 0.05 was considered statistically significant.
RESULTS
Study participants
A total of 60 depressive FD patients with weight loss were enrolled in the study. All patients were randomized to receive mirtazapine, paroxetine or conventional treatment. No patient was lost to follow-up. The baseline characteristics of the patients are shown in Table 1. No differences were observed among the three groups in gender, age, height, weight, body mass index (BMI) or body loss when diagnosed.
Table 1.
General characteristics of study patients
| Variable | MG (n = 20) | PG (n = 20) | CG (n = 20) | P value |
| Gender (M/F) | 8/12 | 11/9 | 7/13 | 0.725 |
| Age (yr) | 43.45 ± 11.50 | 37.75 ± 10.78 | 39.95 ± 6.84 | 0.914 |
| Height (cm) | 163.81 ± 12.36 | 162.36 ± 10.06 | 160.72 ± 10.63 | 0.834 |
| Weight (kg) | 49.77 ± 6.79 | 48.93 ± 5.89 | 48.22 ± 5.57 | 0.973 |
| BMI (kg/m2) | 18.73 ± 5.62 | 18.65 ± 4.73 | 18.84 ± 6.38 | 1.005 |
| Body loss when diagnosed | 3.42 ± 0.54 | 3.72 ± 0.64 | 3.69 ± 0.71 | 0.872 |
MG: Mirtazapine group; PG: Paroxetine group; CG: Conventional group.
Improvement of dyspepsia symptoms
As shown in Figure 1A, the patients’ dyspepsia symptoms gradually improved over the course of treatment in the three groups. After 2 wk, the NDSI score was significantly lower in the MG than in the PG and CG (P < 0.05 for all), and this trend continued until the end of the study. Since 6 wk, NDSI score of the PG was significantly lower than that of the CG (P < 0.05).
Figure 1.
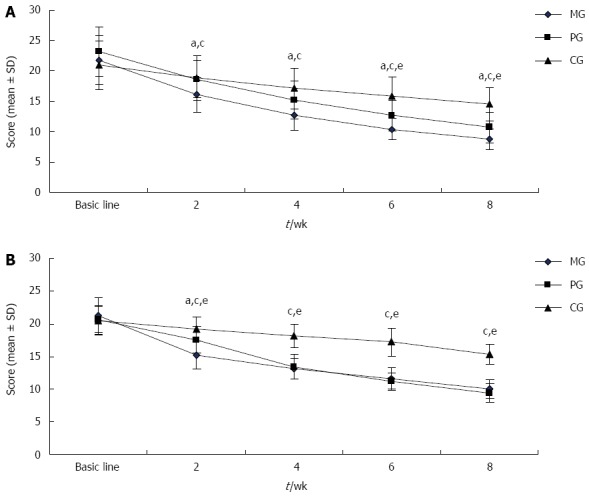
Comparison of Nepean Dyspepsia Index-symptom scores (A) or Hamilton Rating Scale of Depression-17 scores (B). aP < 0.05, MG vs PG; cP < 0.05, MG vs CG; eP < 0.05, PG vs CG. MG: Mirtazapine group; PG: Paroxetine group; CG: Conventional group.
Improvement of depressive symptoms
Patients’ depressive symptoms were improved in the three treatment groups (Figure 1B). At all time points, the HAMD-17 score was significantly lower in the MG and PG than in the CG (P < 0.05). After 2 wk of treatment, the HAMD-17 score was sharply lower in the MG than in the PG (P < 0.05); however, at 4 wk, the score of PG became very close to that of the MG and remained so until the end of the study.
Change of body weight and its composition
As shown in Figure 2, the patients’ body weights were not significantly different before treatment among the three groups. As treatment progressed, body weight of patients in the MG gradually increased. At 6 wk and 8 wk, patients’ body weights were significantly heavier in the MG than those in the PG and CG (P < 0.05 for all); thus, there was no significant body weight change over the test period in either the PG or CG.
Figure 2.
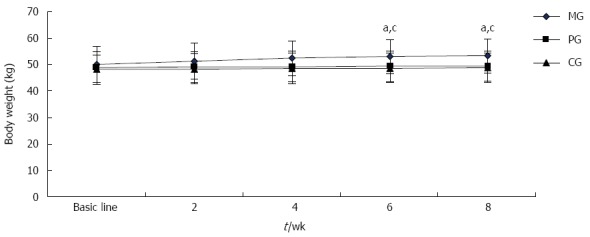
Change of body weight. aP < 0.05, MG vs PG; cP < 0.05, MG vs CG. MG: Mirtazapine group; PG: Paroxetine group; CG: Conventional group.
Further analysis of body weight and its composition is shown in Table 2. After 8 wk of treatment, 19 patients in the MG presented an increase in body weight and BMI. The patients in the MG gained 3.58 ± 1.57 kg, which was significantly higher than that gained in either the PG, at 0.53 ± 0.44 kg or the CG, at 0.56 ± 0.45 kg (P < 0.05). However, no obvious change of body weight was observed in the PG or CG throughout treatment. Body fat is one of the main components contributing to body weight. Over the course of treatment, body fat increased by 2.77 ± 0.14 kg; the body fat ratio rose by 4%; and visceral fat area was increased by 7.56 ± 2.25 cm2 (P < 0.05). No significant change in muscle volume was detected over the treatment period.
Table 2.
Change of body weight and its composition in mirtazapine group
| Variable | Baseline | 2 wk | 4 wk | 6 wk | 8 wk |
| Body weight (kg) | 49.77 ± 6.79 | 51.35 ± 6.80 | 52.36 ± 6.60a | 53.07 ± 6.46a | 53.35 ± 6.52a |
| BMI (kg/m2) | 18.73 ± 5.62 | 18.97 ± 5.43 | 19.70 ± 4.52a | 19.87 ± 4.62a | 20.07 ± 5.23a |
| Body fat (kg) | 8.36 ± 2.53 | 9.28 ± 2.33 | 11.05 ± 1.92a | 11.09 ± 1.87a | 11.13 ± 2.86a |
| Body fat ratio (%) | 0.17 ± 0.02 | 0.18 ± 0.04 | 0.20 ± 0.07a | 0.20 ± 0.09a | 0.21 ± 0.08a |
| Visceral fat area (cm2) | 35.27 ± 8.12 | 36.12 ± 8.05 | 41.68 ± 9.23a | 42.02 ± 9.14a | 42.83 ± 10.64a |
| Muscle volume (kg) | 38.25 ± 7.53 | 38.26 ± 7.49 | 38.46 ± 6.84 | 38.52 ± 6.64 | 38.62 ± 6.77 |
P < 0.05 vs baseline. BMI: Body mass index.
Changes in expression levels of serum hormones
After 4 wk and 8 wk of treatment in MG, the levels of ghrelin, NPY, MTL, and GAS were significantly upregulated, while the levels of leptin, 5-HT and CCK were significantly downregulated (P < 0.05). After 8 wk of treatment, significant differences appeared between the levels in the MG and those in the PG and CG (P < 0.05). Moreover, at 4 wk in the PG, the levels of NPY, MTL and GAS sharply increased, whereas the levels of 5-HT and CCK decreased. There was no obvious difference in CG hormone expression levels over the treatment period (Table 3).
Table 3.
Changes of expression level of serum hormones
| Group | Ghrelin (ng/mL) | Leptin (ng/mL) | NPY (pg/mL) | 5-HT (ng/mL) | CCK (pg/mL) | MTL (pg/mL) | GAS (pg/mL) | |
| MG | Baseline | 4.62 ± 1.53 | 9.87 ± 4.65 | 107.52 ± 26.21 | 237.83 ± 56.94 | 392.36 ± 27.21 | 32.53 ± 6.28 | 56.37 ± 21.15 |
| 4 wk | 6.02 ± 3.43ace | 7.25 ± 3.47ace | 114.56 ± 25.10a | 208.47 ± 52.48a | 221.69 ± 23.77a | 49.46 ± 6.10ace | 48.37 ± 11.93ace | |
| 8 wk | 8.97 ± 3.64ace | 4.03 ± 2.77ace | 149.27 ± 39.53ace | 176.92 ± 53.38ace | 183.85 ± 27.65ace | 66.28 ± 3.97ace | 41.61 ± 10.52ace | |
| PG | Baseline | 4.68 ± 2.12 | 9.07 ± 4.65 | 105.12 ± 29.52 | 232.83 ± 50.94 | 390.82 ± 27.54 | 31.98 ± 9.34 | 54.98 ± 15.24 |
| 4 wk | 5.12 ± 2.23 | 8.75 ± 3.05 | 112.31 ± 15.10a | 215.32 ± 24.91a | 291.57 ± 31.76a | 40.37 ± 8.23a | 52.37 ± 12.97 | |
| 8 wk | 6.01 ± 3.27a | 8.25 ± 2.13 | 114.27 ± 28.53a | 210.17 ± 49.17a | 283.85 ± 47.15a | 53.28 ± 6.84a | 48.61 ± 11.17a | |
| CG | Baseline | 4.89 ± 2.47 | 8.87 ± 2.65 | 104.93 ± 17.95 | 235.81 ± 61.82 | 391.75 ± 24.96 | 36.26 ± 6.22 | 54.23 ± 26.83 |
| 4 wk | 5.48 ± 2.15 | 8.32 ± 3.57 | 108.92 ± 29.64 | 211.26 ± 46.28 | 224.67 ± 23.45 | 43.92 ± 7.24 | 49.21 ± 12.15 | |
| 8 wk | 6.93 ± 2.35 | 8.02 ± 1.45 | 121.43 ± 13.92 | 208.95 ± 38.29 | 303.12 ± 26.76 | 55.53 ± 5.98 | 43.34 ± 13.72 |
P < 0.05 vs baseline in the same group;
P < 0.05, MG vs PG;
P < 0.05, MG vs CG. MG: Mirtazapine group; PG: Paroxetine group; CG: Conventional group; NPY: Neuropeptide Y; 5-HT: 5-hydroxytryptamine; CCK: Cholecystokinin; MTL: Motilin; GAS: Gastrin.
Adverse effects and treatment response
The adverse effects associated with the different protocols were recorded for the 20 patients assigned to each treatment group: in the MG, these were dizziness (10%), lethargy (15%), and fatigue (15%); in the PG, they were dizziness (15%), lethargy (20%), nausea (5%) and fatigue (20%). As these adverse effects were mild, they dissipated without treatment within 1 wk. No obvious adverse effects were reported in the CG.
After 8 wk, 85% of patients in the MG, and 80% in the PG responded positively to treatment, which were significantly higher than that (55%) found in the CG; however, there was no significant difference in the results between the MG and PG.
DISCUSSION
FD is a common psychosomatic disease associated with a variety of mental disorders including anxiety, depression, panic attacks, and post-traumatic stress disorder, of which anxiety and depression are the most common. Negative spiritual, psychological and social factors can accelerate the onset of FD symptoms and exacerbate them and thereby ultimately affect treatment efficacy. However, at present, the impact of such factors on the incidence and progression of FD is not very clear; one intriguing possibility is that they may work to change gastrointestinal motor or sensory function through the brain-gut axis. Weight loss is a common symptom of digestive diseases, and may indicate an organic disease[6], but recently, certain studies have found that patients with functional gastrointestinal diseases often showed weight loss[11,20].
Currently, there is no very effective treatment for depressive FD patients with weight loss, because of the chronic and recurrent characteristics of the disease. Antidepressants are often used to treat patients with depression. One of these, mirtazapine, a serotonin-norepinephrine reuptake inhibitor that is clinically used for the treatment of depression, acts rapidly with positive effects on sleep disorders, appetite loss, depressive symptoms, etc.
Herein, we analyzed the effects of mirtazapine on depressive FD patients with weight loss. Mirtazapine showed higher efficacy in relieving dyspeptic symptoms and lowering NDSI scores when compared to paroxetine and conventional treatment, and was equal to paroxetine in mitigating depressive symptoms. After 8 wk of treatment, 85% of MG patients were classified as treatment responsive, a proportion higher than 80% as observed in the PG, and significantly higher than 55% as seen in the CG. This may be related to specific aspects of mirtazapine action that not only may alleviate depression and improve the function of the nervous system, but also regulate gastrointestinal motor or sensory function.
In this study, we show that mirtazapine treatment of depressive FD patients with weight loss not only effectively treated symptoms of dyspepsia and depression, but also induced significant weight gain, an effect not observed with either paroxetine or conventional treatment. Specifically, 80% of the patients experienced weight gain after 4 wk of treatment with mirtazapine; furthermore, 95% of these patients continued to gain weight until the end of the treatment. The average weight gain was 3.58 ± 1.57 kg, resulting in significantly higher weight than the baseline weight recorded before treatment. In humans, weight is mainly composed of muscle volume, body fat, and inorganic salts, and muscle volume and body fat are the most affected. Through dynamic observation of the weight distribution of the various body components, we found that muscle volume stayed relatively constant throughout treatment, whereas body fat significantly changed. Body fat, which includes subcutaneous fat, visceral fat, muscle clearance fat, proved to be the main contributor to body weight gain. Further analysis of body fat distribution revealed that visceral fat showed a marked increase with mirtazapine treatment at 4 wk and 8 wk, which indicated that visceral fat was the key element responsible for the observed body weight gain.
Generally, significant imbalances of visceral fat are known to increase the incidence of cardiovascular disease, digestive disease, urinary disease, etc. In our study, although visceral fat did indeed increase after 8 wk of mirtazapine treatment, body fat ratios remained at normal levels. We speculate that most of the patients were at a low level of body weight and BMI before treatment, and even underweight according to BMI, whereas muscle volume remained at normal levels throughout; thus, the amount of body fat must have been seriously deficient before treatment. Moreover, through appetite growth and symptom relief, muscle volume may have gradually increased with treatment, whereas overall body fat may have grown at a slower rate.
In recent years, functional gastrointestinal disease has been closely associated with dysregulation of the brain-gut axis. The brain-gut axis, which is regulated by neuroendocrine and immune factors, is a bipolar system between the gastrointestinal tract and brain that is affected by psychosocial factors. The coordination between the central nervous system and gastrointestinal contractility is regulated through a variety of brain-gut peptides and gastrointestinal hormones. In this study, the levels of ghrelin, NPY, MTL, and GAS which may increase appetite, food intake or gastrointestinal dynamic promotion were significantly upregulated, whereas the levels of leptin, 5-HT and CCK which may decrease food intake, block gastrointestinal motility or increase gastrointestinal sensitivity were significantly downregulated.
In conclusion, antidepressant mirtazapine not only improved patients’ conditions concerning indigestive and depressive symptoms, but also increased appetite and body weight (mainly the visceral fat in body fat), much more effectively than either paroxetine or conventional therapy. The clinical efficacy of mirtazapine may be mediated in part through the regulation of brain-gut or gastrointestinal hormones. To clarify these effects and the underlying mechanisms of mirtazapine action in FD patients with weight loss will require bigger sample sizes, and multi-center, randomized controlled trials in future studies.
COMMENTS
Background
Functional dyspepsia (FD) is a common psychosomatic disease associated with a variety of mental disorders, and weight loss was often found in FD patients. Such patients had lower body mass index, more frequent physician visits, higher psychological disorders, poorer appetite and lower quality of life. In recent years, many clinical trials indicated that antidepressant mirtazapine are effective in treating FD patients. Whereas some side effects may limit the general application of mirtazapine in antidepressant therapy, these may prove to be beneficial in treating FD patients with weight loss.
Research frontiers
This study comprehensively explored the effect of mirtazapine on depressive FD patients with weight loss by dynamic observation not only of the changes in dyspepsia and depressive symptoms but also the modifications of body weight and fat distribution and the level of serum hormones.
Innovations and breakthroughs
This study showed that antidepressant mirtazapine not only improved patients’ conditions concerning indigestive and depressive symptoms, but also increased appetite and body weight, mainly the visceral fat in body fat, much more effectively than either paroxetine or conventional therapy. The clinical efficacy of mirtazapine may be mediated in part through the regulation of brain-gut or gastrointestinal hormones.
Applications
The findings can supply the evidence for the clinical application of mirtazapine in FD patients with weight loss.
Terminology
The Nepean Dyspepsia Index-symptom is a scale that evaluates the frequency, intensity, and practical impediments of 15 gastrointestinal symptoms.
Peer-review
This is a good and practical study in which the authors found that the beneficial effects and mechanism of action of antidepressant mirtazapine in FD patients with weight loss. It is believed that the findings can provide a new angle and evidence for the clinical application of mirtazapine in FD patients with weight loss.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Guangzhou First People’s Hospital and Guangzhou Nansha Central Hospital.
Clinical trial registration statement: This registration policy applies to prospective, randomized, controlled trials only.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: Not declared.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 25, 2016
First decision: March 7, 2016
Article in press: April 15, 2016
P- Reviewer: Kobayashi T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
References
- 1.Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada JR, Stanghellini V. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Brook RA, Kleinman NL, Choung RS, Melkonian AK, Smeeding JE, Talley NJ. Functional dyspepsia impacts absenteeism and direct and indirect costs. Clin Gastroenterol Hepatol. 2010;8:498–503. doi: 10.1016/j.cgh.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ. Functional gastrointestinal disorders as a public health problem. Neurogastroenterol Motil. 2008;20 Suppl 1:121–129. doi: 10.1111/j.1365-2982.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 4.Aro P, Talley NJ, Agréus L, Johansson SE, Bolling-Sternevald E, Storskrubb T, Ronkainen J. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. 2011;33:1215–1224. doi: 10.1111/j.1365-2036.2011.04640.x. [DOI] [PubMed] [Google Scholar]
- 5.Miwa H, Ghoshal UC, Gonlachanvit S, Gwee KA, Ang TL, Chang FY, Fock KM, Hongo M, Hou X, Kachintorn U, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150–168. doi: 10.5056/jnm.2012.18.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madsen LG, Bytzer P. The value of alarm features in identifying organic causes of dyspepsia. Can J Gastroenterol. 2000;14:713–720. doi: 10.1155/2000/783950. [DOI] [PubMed] [Google Scholar]
- 7.Hammer J, Eslick GD, Howell SC, Altiparmak E, Talley NJ. Diagnostic yield of alarm features in irritable bowel syndrome and functional dyspepsia. Gut. 2004;53:666–672. doi: 10.1136/gut.2003.021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakil N, Moayyedi P, Fennerty MB, Talley NJ. Limited value of alarm features in the diagnosis of upper gastrointestinal malignancy: systematic review and meta-analysis. Gastroenterology. 2006;131:390–401; quiz 659-660. doi: 10.1053/j.gastro.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 9.Meineche-Schmidt V, Jørgensen T. ‘Alarm symptoms’ in patients with dyspepsia: a three-year prospective study from general practice. Scand J Gastroenterol. 2002;37:999–1007. doi: 10.1080/003655202320378167. [DOI] [PubMed] [Google Scholar]
- 10.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 11.Filipović BF, Randjelovic T, Kovacevic N, Milinić N, Markovic O, Gajić M, Filipović BR. Laboratory parameters and nutritional status in patients with functional dyspepsia. Eur J Intern Med. 2011;22:300–304. doi: 10.1016/j.ejim.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Jia L, Lei XG, Jiang SM, Wang SB, Xu M. The clinical-psychological features of functional dyspepsia patients with weight loss: a multi-center study from China. Digestion. 2015;91:197–201. doi: 10.1159/000375400. [DOI] [PubMed] [Google Scholar]
- 13.Hojo M, Miwa H, Yokoyama T, Ohkusa T, Nagahara A, Kawabe M, Asaoka D, Izumi Y, Sato N. Treatment of functional dyspepsia with antianxiety or antidepressive agents: systematic review. J Gastroenterol. 2005;40:1036–1042. doi: 10.1007/s00535-005-1687-8. [DOI] [PubMed] [Google Scholar]
- 14.Talley NJ, Herrick L, Locke GR. Antidepressants in functional dyspepsia. Expert Rev Gastroenterol Hepatol. 2010;4:5–8. doi: 10.1586/egh.09.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry S, Dinan TG. Functional dyspepsia: are psychosocial factors of relevance? World J Gastroenterol. 2006;12:2701–2707. doi: 10.3748/wjg.v12.i17.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SY, Rho SH, Choi SC. Functional dyspepsia and mirtazapine. Can J Psychiatry. 2002;47:582–583. doi: 10.1177/070674370204700618. [DOI] [PubMed] [Google Scholar]
- 17.Jiang SM, Wu JH, Jia L. Intervention of mirtazapine on gemcitabine-induced mild cachexia in nude mice with pancreatic carcinoma xenografts. World J Gastroenterol. 2012;18:2867–2871. doi: 10.3748/wjg.v18.i22.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talley NJ, Haque M, Wyeth JW, Stace NH, Tytgat GN, Stanghellini V, Holtmann G, Verlinden M, Jones M. Development of a new dyspepsia impact scale: the Nepean Dyspepsia Index. Aliment Pharmacol Ther. 1999;13:225–235. doi: 10.1046/j.1365-2036.1999.00445.x. [DOI] [PubMed] [Google Scholar]
- 19.Calotă DR, Niţescu C, Marinescu S, Cristescu C, Boiangiu I, Florescu IP, Lascăr I. Correlations between morphological appearance and psychosocial difficulties in patients with extensive burns who received allotransplant. Rom J Morphol Embryol. 2012;53:703–711. [PubMed] [Google Scholar]
- 20.Zanini B, Ricci C, Bandera F, Caselani F, Magni A, Laronga AM, Lanzini A. Incidence of post-infectious irritable bowel syndrome and functional intestinal disorders following a water-borne viral gastroenteritis outbreak. Am J Gastroenterol. 2012;107:891–899. doi: 10.1038/ajg.2012.102. [DOI] [PubMed] [Google Scholar]


