Abstract
Background. All-oral combination of direct-acting antivirals could lead to higher sustained virologic response (SVR) in hepatitis C virus (HCV)-infected patients. In the present study, we examined the efficacy and safety of the dual oral treatment with HCV nonstructural protein (NS) 5A inhibitor daclatasvir (DCV) plus HCV NS3/4A inhibitor asunaprevir (ASV) for 24 weeks in real-world HCV genotype 1-infected Japanese individuals.
Methods. After screening for HCV NS5A resistance-associated variants (RAVs) by PCR invader assay, a total of 54 Japanese patients infected with HCV genotype 1 treated with DCV plus ASV were retrospectively analyzed. SVR12 was used for evaluation of the virologic response.
Results. Of the total 54 patients, 46 patients (85.2%) were treated with DCV plus ASV for 24 weeks and achieved SVR12. The other 8 patients (14.8%) discontinued this treatment before 24 weeks due to adverse events. Of these 8 patients, 5 and 3 patients did and did not achieve SVR12, respectively. Finally, 51 of 54 (94.4%) patients achieved SVR12.
Conclusion. Treatment with DCV and ASV after screening for HCV NS5A RAVs by PCR invader assay is effective and safe in the treatment of real-world HCV genotype 1-infected patients in Japan.
Keywords: Asunaprevir, Daclatasvir, HCV NS5A, Interferon-free, Resistance-associated variants
Introduction
Hepatitis C virus (HCV) infection causes acute and chronic hepatitis, resulting in cirrhosis and hepatocellular carcinoma (HCC) 1-3. Retrospective studies have suggested that patients with chronic hepatitis C infection and advanced fibrosis who achieve a sustained virologic response (SVR) with interferon-including regimens have a lower risk of hepatic decompensation and HCC 4,5. A prospective study of Hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) also demonstrated that patients with advanced chronic hepatitis C and SVR achieved by interferon-including regimens had a marked reduction of death/liver transplantation as well as liver-related morbidity/mortality 6, although they are still at risk of developing HCC 6,7. Thus, eradication of HCV could result in the reduction of liver-related deaths in HCV-infected individuals 8.
HCV is a single-stranded positive RNA virus ~9,600 nt. in length, belonging to the Flaviviridae family. The HCV genome encodes at least 10 proteins — 4 structural (core, E1, E2 and p7) and 6 non-structural (NS2, NS3, NS4A, NS4B, NS5A and NS5B) 3. In the interferon-free treatment era, the targets of direct-acting agents are mainly HCV NS3/4A serine protease, NS5A protein and NS5B polymerase 3. The function of HCV NS5A is not well understood, but this protein is involved in HCV replication and hepatocarcinogenesis 3.
In Japan, approximately 1.5 - 2 million HCV carriers might still exist 9. The distribution of HCV genotype 1b, 2a and 2b was reported as 70%, 20% and 10%, respectively. Almost all of HCV genotype 1 in Japan is of the 1b variety 9. HCV NS5A inhibitor daclatasvir (DCV) plus HCV NS3/4A protease inhibitor asunaprevir (ASV) treatment for 24 weeks can result in higher SVR in HCV genotype 1b-infected patients if they are without resistance-associated variants (RAVs) 10,11.
Mice with high frequencies of HCV NS3-D168 variants showed low susceptibility to ASV and failed to respond to the DCV-plus-ASV treatment 12. Direct population sequencing demonstrated that amino-acid substitution resistant to ASV D168N was detected in only 1.1% of Japanese patients with HCV genotype 1b, who are naïve to HCV NS3 inhibitors 13. In Japan, RAVs to HCV NS3/4A inhibitors are usually not measured prior to DCV-plus-ASV treatment if the patients had not used HCV NS3/4A protease inhibitors such as simeprevir or faldaprevir.
Before treatment with DCV plus ASV, RAVs to HCV NS5A inhibitors at positions L31 and Y93 should be measured to avoid treatment failure in HCV genotype 1b-infected individuals 14. These mutations could reduce the efficacy of DCV in vivo 10,11 and in vitro 15. A previous study using direct-sequencing in Japan revealed that L31M, Y93H and Y93H/L31M were detected in 2.7%, 8.2% and 0.3% of the patients, respectively (11.2 % of the total patients) 16. Ultra-deep sequencing analysis is useful for HCV NS5A RAVs, as this method has higher sensitivity, but it is also a costly procedure 14.
The PCR invader assay system consists of nested PCR followed by Invader reaction with well-designed primers and probes. PCR invader assay for the detection of HCV NS5A RAVs showed a lower detection limit than the use of direct sequencing 17 and demonstrated close correlation with the results of deep-sequencing 18. We performed screening for HCV NS5A RAVs by PCR invader assay in a total of 100 HCV genotype 1-infected patients and treated the selected patients with DCV plus ASV. The present study reports the real-world data of the treatment with DCV plus ASV in Japan.
Materials and Methods
Patients
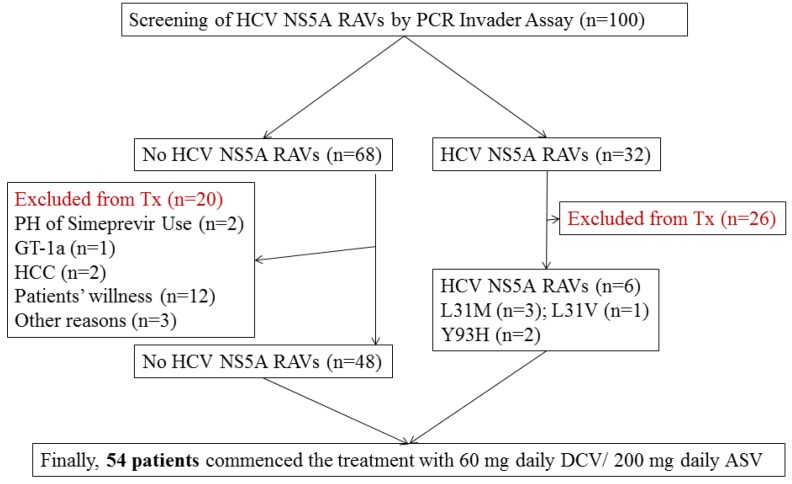
A total of 100 Japanese patients chronically infected with HCV genotype 1 were examined for HCV NS5A RAVs by PCR Invader Assay (BML, Tokyo, Japan) 17. When less than 20% and equal to or more than 20% of HCV NS5A Y93 variants were detected, respectively, the existence of weakly positive and strongly positive RAVs was defined. Mutations at HCV NS5A L31 (L31M, 8; L31F, 1; L31V, 1) were detected in 10 patients (10%). HCV NS5A Y93H was strongly positive in 24 patients (24%), and HCV NS5A Y93H was weakly positive in 24 patients (24%). Finally, 32 of 100 patients (32%) were positive for L31M/F/V and/or strongly positive for Y93H.
The treatment with DCV plus ASV for 24 weeks was commenced for 54 of these 100 patients, and they were retrospectively followed up for at least 12 weeks between October 2014 and March 2016 at the Department of Gastroenterology, Chiba University Hospital (Figure 1). The 54 patients were eligible by meeting the following criteria: (1) infected with HCV genotype 1 alone, (2) age >20 years, (3) diagnosed as chronic hepatitis C, (4) negative for hepatitis B surface antigen, (5) negative for human immunodeficiency virus, (6) no decompensated cirrhosis, (7) no severe renal disease, (8) no severe heart disease, (9) no active drug users, (10) no pregnancy, and (11) no use of drugs having interaction with DCV or ASV.
Figure 1.
Study profile. HCV NS5A, hepatitis C virus non-structural 5A; resistance-associated variants, RAVs; Tx, therapy; PH, past history; GT, genotype; HCC, hepatocellular carcinoma; DCV, daclatasvir; ASV, asunaprevir.
Study design
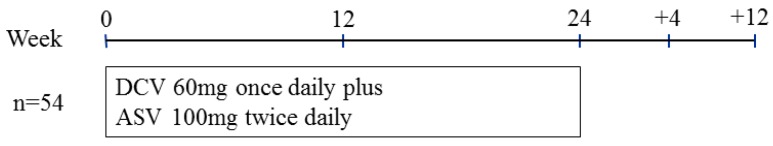
The Ethics Committee of Chiba University School of Medicine approved the study protocol (no. 1753). Informed consent was obtained from all patients. In this retrospective observational study, DCV 60 mg once daily plus ASV 100 mg twice daily were given orally for as long as 24 weeks (Figure 2). Clinical and laboratory assessments were performed every 4 weeks during the treatment and at 12 weeks after the stoppage of treatment. Adverse events were noted by oral inquiry (patient interview), physical examinations and laboratory data.
Figure 2.
Treatment protocol of daclatasvir (DCV) plus asunaprevir (ASV) for real-world HCV genotype 1-infected patients in Japan.
Clinical and laboratory assessments
Hematological and biochemical tests were performed at least every 4 weeks after commencement of treatment, as well as after discontinuation of the treatment. These parameters were measured by standard laboratory techniques at central laboratories, Chiba University Hospital 19. Transient elastography (Fibroscan, Echosens, Paris) was used to measure liver stiffness according to the methods previously described 19. Cirrhosis of the liver was diagnosed by ultrasound, computed tomography, and/or liver stiffness (equal to or more than 12 kPa).
Measurement of HCV RNA and HCV genotyping
HCV RNA was measured by TaqMan HCV Test, version 2.0, real-time PCR assay (Roche Diagnostics, Tokyo, Japan), with a lower qualification limit of 15 IU/mL, and with a quantitation range of 1.2-7.8 log10 IU/mL 19. HCV genotype was determined by direct sequencing methods before treatment.
Assessment of treatment efficacy
SVR12 was defined as HCV RNA negativity at 12 weeks after treatment completion and was used as the evaluation of virologic response. Treatment response was defined as follows: relapse, reappearance of HCV RNA after the end of treatment despite achievement of end-of-treatment response (EOTR), which was defined as undetectable HCV RNA at the end of treatment; virologic breakthrough (VBT) and reappearance of HCV RNA at any time during treatment after virologic response 8; rapid virologic response (RVR) was defined as undetectable HCV RNA after 4 weeks of therapy 8.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). We used univariate analyses, applying the chi-square test or Student's t-test. P<0.05 was considered statistically significant.
Results
Patients
The present study focused on 54 patients who received DCV-plus-ASV therapy, finished the protocol, and were followed for 12 weeks or more after its completion. Patient characteristics at baseline are shown in Table 1. Of the total patients, 53 (98.1%) were infected with HCV genotype 1b. Mean age was ~70 years, and the patient group was female-dominant. Patients with previous treatment experience were also in the majority. Two patients had a history of telaprevir use. Of the total patients, 46.3% had cirrhosis. As for HCV RAVs, 6 patients were positive for RAVs at L31 or strongly positive for Y93H (Table 1).
Table 1.
Characteristics of 54 patients at the commencement of treatment
| Characteristics | Values |
|---|---|
| Age (years) | 69.2 ± 9.1 |
| Gender (male/female) | 19/35 |
| Genotype (1a/1b) | 1/53 |
| HCV RNA (LIU/mL) | 6.0 ± 0.55 |
| HCV RNA (<5.0LIU/mL/5.0LIU/mL≦) | 2/52 |
| Interferon treatment (naïve/experienced) | 17/37 |
| Body Weight (kg) | 56.6 ± 9.8 |
| Body Height (m) | 1.58 ± 0.08 |
| Chronic hepatitis/cirrhosis | 29/25 |
| Liver stiffness (kPa) | 12.6 ± 4.4 |
| AST (IU/L) | 61.2 ± 39.7 |
| ALT (IU/L) | 54.6 ± 37.7 |
| Hemoglobin (g/dL) | 13.2 ± 1.8 |
| Platelets (x104 /μL) | 13.0 ± 5.5 |
| Mutations at L31 (negative/L31M/L31V) | 50/3/1 |
| Mutations at Y93 (negative/weakly positive/strongly positive) | 35/17/2 |
Virologic response
The combination therapy of DCV plus ASV for HCV genotype 1-infected previously treatment-experienced patients or interferon-ineligible/intolerant patients was approved by the Japanese health insurance system in 2014 9,10. In 2015, this combination therapy was also approved for treatment-naïve patients infected with HCV genotype 1 by the Japanese health insurance system 9,20.
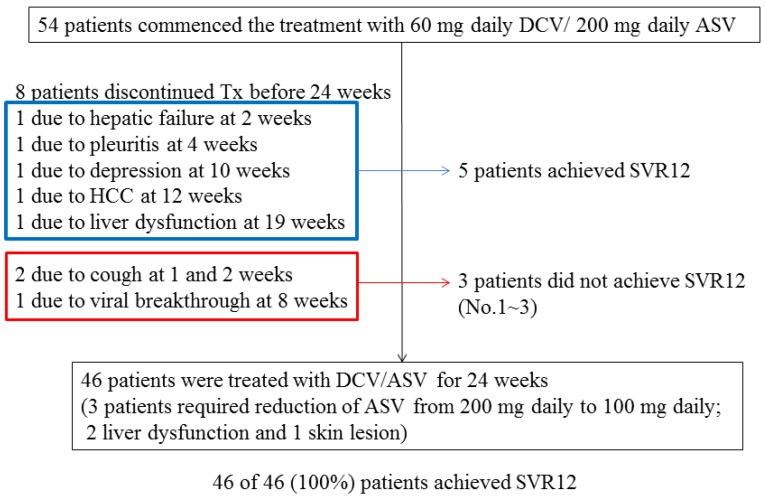
Of the total 54 patients, 46 patients (85.2%) were treated with DCV plus ASV for 24 weeks and achieved EOTR/SVR12, although 3 patients had their ASV dose reduced (from 200 mg to 100 mg daily) due to adverse events (2, mild liver dysfunction; 1, skin lesion). Among the total 54 patients, 8 other patients (14.8%) discontinued the treatment with DCV plus ASV before reaching 24 weeks due to adverse events (Figure 3). Of these 8 patients, 5 achieved EOTR/SVR12 and 3 did not. Finally, 51 (94.4%) of the 54 patients achieved EOTR/SVR12. Interestingly, the single HCV genotype 1a-infected patient achieved SVR12. Five of the 6 HCV genotype 1b-infected patients positive for RAVs at L31 or strongly positive for Y93H also achieved SVR12. Forty (74.1%) patients achieved RVR and 48 (88.9%) achieved less than 1.2 LIU/mL HCV RNA at 4 weeks.
Figure 3.
Patient disposition and sustained virologic response at 12 weeks (SVR12). Of total 54 patients, 51 patients (94.4%) achieved SVR12.
Virologic failure
Three (5.6%) patients had HCV RNA 12 weeks after the discontinuation of treatment. Virologic breakthrough occurred in one 59-year-old female with non-cirrhosis, who had previously received standard interferon, and her HCV NS5A RAVs analysis showed L31 wild type and Y93H weak positivity (case 3 in Table 2). She achieved RVR but then had a virologic breakthrough at 8 weeks. She had rheumatoid arthritis and was taking low-dose prednisolone. Two patients discontinued treatment 1 and 2 weeks after its commencement, and they remained positive for HCV RNA (cases 1 and 2, respectively; Table 2). One was a 77-year-old treatment-naïve female with non-cirrhosis, and her HCV NS5A RAVs analysis showed L31 wild type and Y93H strong positivity. The other was a 78-year-old male with cirrhosis and post-HCC treatment, who had previously received peginterferon but discontinued it due to intestinal pneumonitis, and his HCV NS5A RAVs analysis showed L31 wild type and Y93H weak positivity. He achieved RVR but relapsed at 8 weeks after discontinuing the treatment at 2 weeks.
Table 2.
Resistance-associated variants (RAVs) in 3 patients with virologic failure were analyzed by direct sequencing methods.
| Case | Times | NS3 | NS5A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| V36 | T54 | Q80 | R155 | A156 | D168 | V170 | L31 | Q54 | Y93 | ||
| No.1 | Before Tx | - | - | - | - | - | - | - | - | - | - |
| Stopping Tx (1 week) |
- | - | - | - | - | - | - | - | - | - | |
| No.2 | Before Tx | - | - | - | - | - | - | V/I/M | - | - | - |
| Stopping Tx (2 weeks) |
- | - | - | - | - | - | - | - | - | - | |
| No.3 | Before Tx | - | - | - | - | - | - | - | - | - | - |
| VBT (8 weeks) | - | - | R | - | - | E | I | - | - | - | |
| 24 weeks after Tx | - | - | R | - | - | E | I | - | - | - | |
Tx, therapy; VBT, virologic breakthrough
Population sequencing of HCV NS3 and NS5A RAVs in patients with virologic failure
RAVs in HCV NS3 and HCV NS5A regions were analyzed by Sanger direct-sequencing methods in the 3 patients with virologic failure (Table 2). Fortunately, none showed any HCV NS5A RAVs either before or after treatment. In case 3, we found Q80R, D168E and V170I as RAVs in the NS3 region at the time of virologic breakthrough and at 6 months after discontinuation of treatment. In case 2, we found V170I/M mutation before the commencement of treatment but could not detect this mutation by direct sequencing methods after its stoppage.
Discussion
The present study demonstrated that the combination treatment with DCV plus ASV could lead to 94.4% SVR12 in real-world HCV genotype 1-infected Japanese patients after screening for HCV NS5A RAVs by PCR Invader Assays. A previous study of Japanese phase 3 trials 10 showed that 88.1% of interferon-ineligible/intolerant and 80.5% of interferon-nonresponder patients had achieved SVR12 by the DCV-plus-ASV treatment. Another phase 3 study 20 demonstrated that this treatment led to 89.1% SVR12 in treatment-naïve patients. In the present study of real-world Japanese patients infected with HCV genotype 1, the treatment with DCV plus ASV resulted in 94.1% (16/17) and 94.6% (35/37) SVR12 in treatment-naïve and treatment-experienced patients, respectively. In the present study, we ignored the results of weak positivity for HCV NS5A Y93H on the basis of previous reports from our group regarding the distribution of HCV NS5A RAVs 14,15. In addition, it was shown that PCR invader assay for HCV NS5A RAVs might be useful for selecting patients for the combination DCV-plus-ASV treatment.
In the present study, the direct sequencing method did not detect any HCV NS5A RAVs (L31M/V and/or Y93H) in patients with virologic failure prior to treatment. In one patient with virologic breakthrough, HCV NS3 RAV (D168E) was then detected (Table 2). Kumada et al. 10 also reported that 29 of 34 patients with virologic failure had RAVs to both DCV (predominantly HCV NS5A-L31M/V-Y93H) and ASV (predominantly HCV NS3-D168 variants), detected at failure, and that 22 patients with virologic failure had HCV NS5A RAVs (L31M/V and/or Y93H) prior to treatment.
A previous study 21 demonstrated that DCV-plus-ASV treatment for 24 weeks could lead to 36% (2/9) SVR12 in HCV genotype 1a-infected patients who had no response to previous treatment with peginterferon plus ribavirin. DCV plus ASV provided SVR12 in 90% of treatment-naïve patients, in 82% of non-responders, and in 82% of ineligible/intolerant patients, all infected with HCV genotype 1b 22. Although one female patient infected with HCV genotype 1a and no HCV NS5A RAVs fortunately achieved SVR12 in the present study, it seems better to avoid this combination therapy for HCV genotype 1a-infected patients. HCV genotype 1a is observed in only ~1% of HCV-infected patients in Japan 23.
In Japan, DCV-plus-ASV treatment for 24 weeks was introduced as the earliest interferon-free combination treatment for patients infected with HCV genotype 1. Furthermore, in null responders and patients with viral breakthrough for DCV-plus-ASV treatment, the emergence of viruses resistant to both drugs has been observed 11. In a previous report 24, retreatment with sofosbuvir plus ledipasvir for 24 weeks could lead to only 50% of SVR12 in patients with more than 2 NS5A RAVs, which were induced by HCV NS5A inhibitor-including regimens, and 33% of SVR12 in patients with baseline NS5A Y93 RAVs positive, which was induced by HCV NS5A inhibitor-including regimens, although the total number of patients was also small. We should wait for new drugs for retreatment, or at least the disappearance of these RAVs induced by DCV-plus-ASV treatment. These RAVs should be checked before the retreatment of patients previously treated with HCV NS5A inhibitor-including regimens and advanced liver fibrosis 2,25.
Combination therapy of DCV plus ASV could be used to treat chronic HCV genotype 1-infected patients on hemodialysis or patients with severe renal impairment 26,27. We did not observe any cases with renal dysfunction in this study.
In the present study, mild and severe liver impairment was observed in 3 and 1 patients, respectively (Figure 3). In a 70-year-old female with interferon treatment-experience and compensated cirrhosis, after 2 weeks of this treatment, she had fever and her prothrombin time was 39%. After immediately discontinuing the treatment, she recovered and achieved EOTR/SVR12. In Japan, in phase 3 trial of 222 patients who were interferon-ineligible/intolerant or non-responders 10, 10 of 11 patients discontinued treatment due to alanine aminotransferase (ALT) and aspartate aminotransferase (AST) elevations. In total 35 (15.8%) of 222 patients, increased ALT was observed. In 16 (7.2%) of 222 patients, grade 3-4 ALT abnormalities were observed 10. Attention should be paid to liver function, and should be monitored during this treatment. In the present study, 2 patients also discontinued treatment due to a cough. In the phase 3 trial 10, nasopharyngitis was observed in 67 (30.2%) of the 222 patients.
The overall genetic barrier to resistance of DCV plus ASV was reported to be lower 10,11, compared with that of sofosbuvir plus ledipasvir 28. In conclusion, dual oral therapy with DCV and ASV after screening for HCV NS5A RAVs was proven to be effective and safe in the treatment of real-world HCV genotype 1-infected patients in Japan, although the total number of patients was small.
Acknowledgments
We are all thankful to our colleagues at the liver units of each hospital who cared for the patients described herein.
Funding
This work was supported by Research Grants for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
References
- 1.Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26(3 Suppl 1):34S–8S. doi: 10.1002/hep.510260706. [DOI] [PubMed] [Google Scholar]
- 2.AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62:932–54. doi: 10.1002/hep.27950. [DOI] [PubMed] [Google Scholar]
- 3.Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010;4:548–61. doi: 10.1007/s12072-010-9193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poynard T, McHutchison J, Manns M. et al. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303–13. doi: 10.1053/gast.2002.33023. [DOI] [PubMed] [Google Scholar]
- 5.George SL, Bacon BR, Brunt EM. et al. Clinical, virologic, histologic, and biochemical outcomes after successful HCV therapy: a 5-year follow-up of 150 patients. Hepatology. 2009;49:729–38. doi: 10.1002/hep.22694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morgan TR, Ghany MG, Kim HY. et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology. 2010;52:833–44. doi: 10.1002/hep.23744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanda T, Imazeki F, Mikami S. et al. Occurrence of hepatocellular carcinoma was not a rare event during and immediately after antiviral treatment in Japanese HCV-positive patients. Oncology. 2011;80:366–72. doi: 10.1159/000330549. [DOI] [PubMed] [Google Scholar]
- 8.Omata M, Kanda T, Yu ML. et al. APASL consensus statements and management algorithms for hepatitis C virus infection. Hepatol Int. 2012;6:409–35. doi: 10.1007/s12072-012-9342-y. [DOI] [PubMed] [Google Scholar]
- 9.Omata M, Kanda T, Yokosuka O. et al. Features of hepatitis C virus infection, current therapies and ongoing clinical trials in ten Asian Pacific countries. Hepatol Int. 2015;9:486–507. doi: 10.1007/s12072-015-9630-4. [DOI] [PubMed] [Google Scholar]
- 10.Kumada H, Suzuki Y, Ikeda K. et al. Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 2014;59:2083–91. doi: 10.1002/hep.27113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Ikeda K, Suzuki F. et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J Hepatol. 2013;58:655–62. doi: 10.1016/j.jhep.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 12.Kan H, Hiraga N, Imamura M, Combination therapies with daclatasvir and asunaprevir on NS3-D168 mutated HCV in human hepatocyte chimeric mice. Antivir Ther; 2015. Nov 12. doi: 10.3851/IMP3009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Kanda T, Nakamoto S. et al. Hepatitis C virus protease inhibitor-resistance mutations: our experience and review. World J Gastroenterol. 2013;19:8940–8. doi: 10.3748/wjg.v19.i47.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirotsu Y, Kanda T, Matsumura H. et al. HCV NS5A resistance-associated variants in a group of real-world Japanese patients chronically infected with HCV genotype 1b. Hepatol Int. 2015;9:424–30. doi: 10.1007/s12072-015-9624-2. [DOI] [PubMed] [Google Scholar]
- 15.Nakamoto S, Kanda T, Wu S. et al. Hepatitis C virus NS5A inhibitors and drug resistance mutations. World J Gastroenterol. 2014;20:2902–12. doi: 10.3748/wjg.v20.i11.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki F, Sezaki H, Akuta N. et al. Prevalence of hepatitis C virus variants resistant to NS3 protease inhibitors or the NS5A inhibitor (BMS-790052) in hepatitis patients with genotype 1b. J Clin Virol. 2012;54:352–4. doi: 10.1016/j.jcv.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 17.Tadokoro K, Suzuki F, Kobayashi M. et al. Rapid and high sensitive detection of Y93H amino acid substitution in HCV NS5A region using PCR-Invader assay. Kanzo (in Japanese) 2014;55:720–2. [Google Scholar]
- 18.Yoshimi S, Ochi H, Murakami E. et al. Rapid, Sensitive, and Accurate Evaluation of Drug Resistant Mutant (NS5A-Y93H) Strain Frequency in Genotype 1b HCV by Invader Assay. PLoS One. 2015;10:e0130022. doi: 10.1371/journal.pone.0130022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda T, Imazeki F, Yonemitsu Y. et al. Quantification of hepatitis C virus in patients treated with peginterferon-alfa 2a plus ribavirin treatment by COBAS TaqMan HCV test. J Viral Hepat. 2011;18:e292–7. doi: 10.1111/j.1365-2893.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumada H, Suzuki F, Suzuki Y. et al. Randomized comparison of daclatasvir + asunaprevir versus telaprevir + peginterferon/ribavirin in Japanese hepatitis C virus patients. J Gastroenterol Hepatol. 2016;31:14–22. doi: 10.1111/jgh.13073. [DOI] [PubMed] [Google Scholar]
- 21.Lok AS, Gardiner DF, Lawitz E. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216–24. doi: 10.1056/NEJMoa1104430. [DOI] [PubMed] [Google Scholar]
- 22.Manns M, Pol S, Jacobson IM. et al. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597–605. doi: 10.1016/S0140-6736(14)61059-X. [DOI] [PubMed] [Google Scholar]
- 23.Wu S, Kanda T, Nakamoto S. et al. Prevalence of hepatitis C virus subgenotypes 1a and 1b in Japanese patients: ultra-deep sequencing analysis of HCV NS5B genotype-specific region. PLoS One. 2013;8:e73615. doi: 10.1371/journal.pone.0073615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawitz E, Flamm S, Yang JC. et al. Retreatment of patients who failed 8 or 12 weeks of ledipasvir/sofosbuvir-based regimens with ledipasvir/sofosbuvir for 24 weeks. J Hepatol. 2015;62(suppl 2):S192.. Abstract O005. [Google Scholar]
- 25.Omata M, Kanda T, Wei L, APASL consensus statements and recommendation on treatment of hepatitis C. Hepatology Int; 2016. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suda G, Kudo M, Nagasaka A, Efficacy and safety of daclatasvir and asunaprevir combination therapy in chronic hemodialysis patients with chronic hepatitis C. J Gastroenterol; 2016. Jan 14. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Sorbera MA, Friedman ML, Cope R. New and Emerging Evidence on the Use of Second-Generation Direct Acting Antivirals for the Treatment of Hepatitis C Virus in Renal Impairment. J Pharm Pract; 2016. Feb 22. pii: 0897190016632128. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Mizokami M, Yokosuka O, Takehara T. et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis. 2015;15:645–53. doi: 10.1016/S1473-3099(15)70099-X. [DOI] [PubMed] [Google Scholar]