Abstract
Doxorubicin (DOX) is a potent and widely used anthracycline antibiotic for the treatment of several malignancies. Unfortunately, the clinical utility of DOX is often restricted due to the elicitation of organ toxicity. Particularly, the increased risk for the development of dilated cardiomyopathy by DOX among the cancer survivors warrants major attention from the physicians as well as researchers to develop adjuvant agents to neutralize the noxious effects of DOX on the healthy myocardium. Despite these pitfalls, the use of traditional cytotoxic drugs continues to be the mainstay treatment for several types of cancer. Recently, phytochemicals have gained attention for their anticancer, chemopreventive, and cardioprotective activities. The ideal cardioprotective agents should not compromise the clinical efficacy of DOX and should be devoid of cumulative or irreversible toxicity on the naïve tissues. Furthermore, adjuvants possessing synergistic anticancer activity and quelling of chemoresistance would significantly enhance the clinical utility in combating DOX-induced cardiotoxicity. The present review renders an overview of cardioprotective effects of plant-derived small molecules and their purported mechanisms against DOX-induced cardiotoxicity. Phytochemicals serve as the reservoirs of pharmacophore which can be utilized as templates for developing safe and potential novel cardioprotective agents in combating DOX-induced cardiotoxicity.
1. Introduction
Doxorubicin (DOX) is a potent and widely used anthracycline antibiotic for the treatment of cancers. However, the major impeding issue pertaining to the clinical application of DOX is related to its ability to induce untoward toxicity to the healthy tissues [1]. The occurrence of fatal cardiotoxicity in pediatric as well as in adult patients is characterized by an irreversible cardiomyopathy which compromises the clinical utility of DOX and accounts for the major cause of the chemotherapy related morbidity and mortality [1]. In spite of introducing several less toxic derivatives of DOX, elicitation of cardiotoxicity still remains the major concern [2]. However, with the advent of newer class of monoclonal antibodies revolutionized cancer chemotherapy, still this approach is burdened with myriad adverse effects [3]. Thus, the use of traditional cytotoxic drugs continues to be a preferred mode for the treatment of cancer.
To limit the DOX-induced cardiotoxicity, several molecules, such as beta blockers, angiotensin receptor blockers, amifostine, dexrazoxane, Mesna (2-mercaptoethane sulfonate Na), leucovorin, and erythropoietin, have been evaluated as cardioprotective adjuvants in preclinical studies [4]. Recently, dexrazoxane, when subjected to clinical trial against combating DOX-induced cardiotoxicity, exhibited marked cardioprotection and did not compromise the anticancer activity of DOX [5]. Similarly, carvedilol (beta blocker) has also been demonstrated to confer protection against DOX-induced cardiotoxicity in human subjects [6]. However, large-scale clinical applications of these adjuvants are yet to be established in human subjects. The substantial burden arising from cancer and cardiotoxicity and their interrelationship in imposing morbidity and mortality has emerged as the major driving factor for the academia and pharmaceutical industry to devise and develop strategies that can simultaneously provide long-term cardioprotection from DOX-associated cardiotoxicity without compromising the efficacy of cancer chemotherapy [7]. Since the origin of the human civilization, plants and herbs have been traditionally used in the treatment of various diseases and ailments [8]. In this direction, the prospect of harnessing the potentials of plant-derived small molecules (phytochemicals) appears to provide rich dividends, since phytochemicals have been extensively studied in the preclinical studies and shown to possess anti-inflammatory, antioxidant, and anticancer activities. Phytochemicals are the natural constituents of herbs and plants. Moreover, anticancer drug paclitaxel and antimalarial agent artemisinin are phytochemicals originally extracted from plants; however, these agents are now chemically synthesized [9, 10].
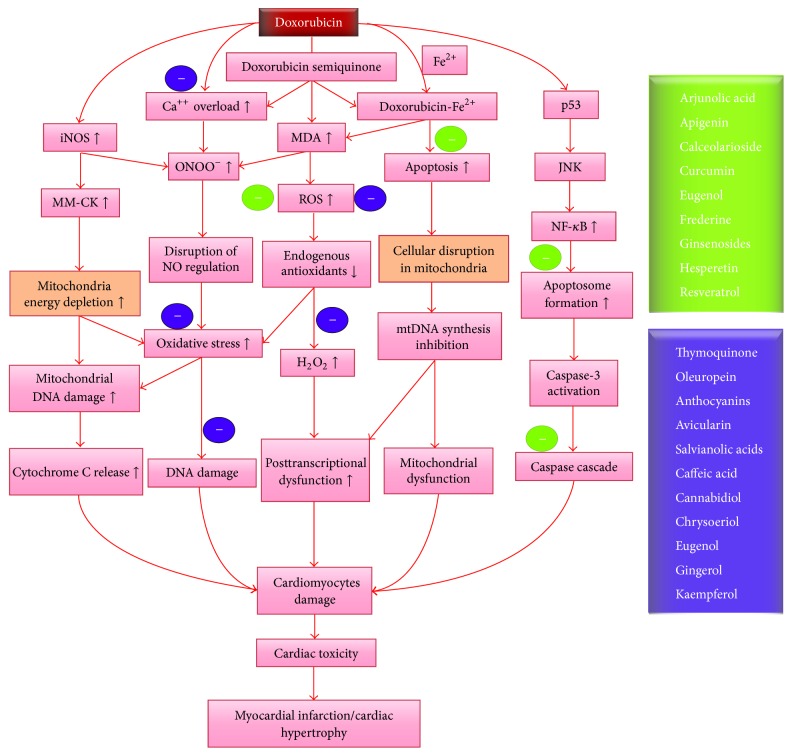
In this review, we have systematically presented the evidence wherein the phytochemicals were investigated for cardioprotective effects against DOX and the relevant mechanisms. Several mechanisms have been postulated for the development of DOX-induced cardiotoxicity. However, oxidative stress driven inflammation, apoptosis, and myocardial remodeling have emerged to be the key players in DOX-induced cardiotoxicity. In-depth description of pathophysiology of DOX-induced cardiotoxicity has been reviewed elsewhere [11, 12]; however, to provide clarity to our discussion, we have provided a simplified scheme for the pathomechanisms in Figure 1.
Figure 1.
This scheme shows the pathways involved in the elicitation of DOX-induced adverse effects in the myocardium and its attenuation by phytochemicals.
2. Phytochemicals Limiting DOX-Induced Cardiotoxicity
In this section, we systematically described the phytochemicals investigated for their cardioprotective activity against DOX-induced toxic effects in the myocardium. Phytochemicals investigated in the animal model of DOX-induced cardiotoxicity are listed in Table 1 and the molecules investigated in cell culture models (in vitro) are presented in Table 2.
Table 1.
Phytochemicals investigated for cardioprotective activity against DOX-induced cardiotoxicity in in vivo studies.
| Phytochemical | DOX-induced cardiomyopathy-animal model (acute or chronic) | Cardiac function determined (yes/no) | References |
|---|---|---|---|
| Arjunolic acid | Chronic | Yes | [13] |
|
| |||
| Berberine | Chronic | Yes | [20] |
|
| |||
| Berberine | Acute | Yes | [19] |
|
| |||
| Baicalein | Chronic | No | [23] |
|
| |||
| Caffeic acid phenethyl ester | Acute | Yes | [25] |
|
| |||
| Cannabidiol | Acute | Yes | [30] |
|
| |||
| Cannabidiol | Chronic | No | [29] |
|
| |||
| Carotenoids | Acute | No | [34] |
|
| |||
| Eugenol | Acute | Yes | [51] |
|
| |||
| Gingerol | Chronic | No | [56] |
|
| |||
| 23-Hydroxybetulinic acid | Chronic | Yes | [60] |
|
| |||
| Hesperetin | Chronic | No | [64] |
|
| |||
| Hesperidin | Acute | No | [62] |
|
| |||
| Isorhamnetin | Chronic | No | [66] |
|
| |||
| Indole-3-carbinol | Chronic | No | [67] |
|
| |||
| Kaempferol | Chronic | No | [69] |
|
| |||
| Lycopene | Acute | No | [70] |
|
| |||
| Lycopene | Acute | No | [71] |
|
| |||
| Lycopene | Acute | Yes | [72] |
|
| |||
| Mangiferin | Acute | No | [79] |
|
| |||
| Mangiferin | Acute | No | [78] |
|
| |||
| Naringenin | Acute | No | [79] |
|
| |||
| Naringenin | Acute | No | [80] |
|
| |||
| Ocotillol | Acute | No | [86] |
|
| |||
| Ocotillol | Chronic | No | [86] |
|
| |||
| Hydroxytyrosol | Chronic | No | [65] |
|
| |||
| Tetrandrine | Chronic | Yes | [140] |
|
| |||
| Periplogenin | Chronic | No | [92] |
|
| |||
| p-coumaric acid | Acute | No | [82] |
|
| |||
| Procyanidins | Chronic | Yes | [100] |
|
| |||
| Robinin | Acute | No | [114] |
|
| |||
| Thymoquinone | Acute | No | [137] |
|
| |||
| Thymoquinone | Acute | No | [138] |
|
| |||
| Silibinin | Chronic | Yes | [126] |
|
| |||
| Sesamin | Acute | No | [124] |
|
| |||
| Sesamol | Chronic | No | [125] |
|
| |||
| Tetrahydroxystilbene glucoside | Acute | No | [135] |
|
| |||
| Oleuropein | Acute | No | [87] |
|
| |||
| Oleuropein | Acute | No | [88] |
|
| |||
| Oleuropein | Chronic | Yes | [89] |
|
| |||
| Frederine | Acute | Yes | [52] |
|
| |||
| Visnagin | Acute | Yes | [146] |
|
| |||
| Visnagin | Chronic | Yes | [146] |
|
| |||
| Schisandrin B | Acute | Yes | [121] |
|
| |||
| Schisandrin B | Chronic | Yes | [122] |
|
| |||
| Salvianolic acid A | Acute | Yes | [117] |
|
| |||
| Tanshinone IIA | Chronic | Yes | [133] |
|
| |||
| Oleuropein | Acute | No | [87] |
|
| |||
| Oleuropein | Acute | No | [88] |
|
| |||
| Oleuropein | Chronic | Yes | [89] |
|
| |||
| Frederine | Acute | Yes | [52] |
|
| |||
| Visnagin | Acute | Yes | [146] |
|
| |||
| Visnagin | Chronic | Yes | [146] |
|
| |||
| Schisandrin B | Acute | Yes | [121] |
|
| |||
| Schisandrin B | Chronic | Yes | [122] |
|
| |||
| Salvianolic acid A | Acute | Yes | [117] |
|
| |||
| Tanshinone IIA | Chronic | Yes | [133] |
Table 2.
Phytochemicals exhibiting cytoprotection in the in vitro models of DOX-induced cardiotoxicity.
| Phytochemical | Concentration of the phytochemical | Cell culture model | DOX dose and time of incubation | References |
|
| ||||
| Arjunolic acid | 100 μg/mL | Neonatal rat cardiomyocytes | 1 μM for 12 h | [13] |
|
| ||||
| Apigenin | 25–100 µM | Rat heart cardiomyocytes | 100 µM for 8 h | [16] |
|
| ||||
| Avicularin | 10–80 μM | H9c2 cells | 20 μM for 24 h | [17] |
|
| ||||
| Berberine | 0.06, 0.25, 1.0, and 4.0 µM | Neonatal rat cardiomyocytes and MCF-7 cells | 1 µM for 2 h | [19] |
|
| ||||
| Baicalein | 25 μM | Chick embryo cardiomyocytes and MCF-7 cells | 1, 10, 50, or 100 μM for 24 h | [22] |
|
| ||||
| Calceolarioside | 40 μM | H9c2 cells | 1, 2, or 5 μM for 30 h | [27] |
|
| ||||
| 23-Hydroxybetulinic acid | 0.2, 2, and 20 μM | H9c2 cells | 5 μM for 18 h | [60] |
|
| ||||
| Isorhamnetin | 3.125 to 25 µg/mL | MCF-7, HepG2, and Hep2 cells | 1 µM for 36 h | [66] |
|
| ||||
| Kaempferol | 5 to 50 μM | H9c2 cells | 1 μM for 24 h | [69] |
|
| ||||
| Morin hydrate | 0.17 mM | ECV304 and HepG2 cells | 6 mM for 12 h | [77] |
|
| ||||
| Naringenin-7-O-glucoside | 10–80 μM | H9c2 cells | 10 μM for 24 h | [83] |
|
| ||||
| Osthole | 10–40 μM | Neonatal rat cardiomyocytes | 1 μmol for 24 h | [90] |
|
| ||||
| Luteolin-7-O-β-D-glucopyranoside | 5, 10, and 20 μM | H9c2 cells | 20 μM for 24 h | [75] |
|
| ||||
| Luteolin-7-O-β-D-glucopyranoside | 5–80 µM | H9c2 cells | 10 µM for 24 h | [76] |
|
| ||||
| Vincristine | 10–30 µM | Adult mouse cardiomyocytes | 15 and 20 µg/mL for 24 h | [144] |
|
| ||||
| Sulforaphane | 2.5 µM | H9c2 cells | 5 µg/mL for 16–18 h | [129] |
|
| ||||
| C-Phycocyanin | 10 μM | Adult rat cardiomyocytes | 10 μM for 4, 24, and 48 h | [94] |
|
| ||||
| Plantainoside D | 1–20 μg/mL | H9c2 cells | 1, 2, and 4 μM for 30 h | [93] |
|
| ||||
| Sesamol | 12.5–50 μM | H9c2 cells | 1 μM for 30 min | [125] |
|
| ||||
| Tetrahydroxystilbene glucoside | 3–300 μM | Neonatal rat cardiomyocytes | 1 μmol/L for 24 h | [143] |
|
| ||||
| Chrysoeriol | 20 µg/mL | H9c2 cells | 1 μM for 24 h | [43] |
|
| ||||
| Visnagin | 20 μM | Neonatal rat and zebrafish cardiomyocytes, cardiac HL-1 cells | 100 μM for 48 h | [146] |
|
| ||||
| Z-Guggulsterone | 1–30 μM | H9C2 cells | 1 μM for 24 h | [143] |
|
| ||||
| Tanshinone IIA | 0.1, 0.3, 1, and 3 μM | Neonatal rat cardiomyocytes | 1 μM for 24 h | [134] |
|
| ||||
| Tanshinone IIA | 1.6–40 μM | H9c2 cells | 1 μM for 24 h | [133] |
|
| ||||
| Tanshinone IIA | 0.5, 1, and 2 μmol/L | Neonatal rat cardiomyocytes | 1 μmol/L for 24 h | [132] |
|
| ||||
| Sodium tanshinone IIA sulphonate | 0.05–0.5 mM | Mice heart mitochondria | 0.2 mmol for 10 min | [131] |
|
| ||||
| Anthocyanidins and anthocyanins | 0–100 μM | H9c2 cells and MCF-7 cells | 1 μM for 24 h | [14] |
|
| ||||
| Caffeic, chlorogenic, and rosmarinic acid | 100 and 200 µM | Rat heart microsomes and mitochondria | 100 μM for 8 h | [116] |
ECV304 cells: human umbilical vein endothelial cells; HepG2 cells: human hepatocellular carcinoma cells; MCF-7 cells: human breast carcinoma; H9c2 cells: rat ventricular cardiomyoblast cells.
2.1. Arjunolic Acid
Arjunolic acid is a chiral triterpenoid saponin, isolated from Terminalia arjuna. Arjunolic acid treatment to adult rat cardiomyocytes in the presence of DOX attenuated caspase-dependent apoptotic signaling by ameliorating proapoptotic p53, p38, and JNK-MAPKs and mitochondrial pathways leading to apoptosis. Furthermore, arjunolic acid when administered to rats significantly inhibited DOX-induced myocardial toxicity by mitigating oxidative stress and apoptotic pathways [13].
2.2. Anthocyanins
Anthocyanins are a group of polyphenolic compounds which are abundantly found in common fruits and vegetables. The effect of six anthocyanidins (cyanidin chloride, delphinidin chloride, malvidin chloride, pelargonidin chloride, peonidin chloride, and petunidin chloride) and seven anthocyanins (cyanidin 3-O-β-galactopyranoside chloride, cyanidin 3-O-β-glucopyranoside chloride, delphinidin 3-O-β-glucopyranoside chloride, malvidin 3-O-β-glucopyranoside chloride, pelargonidin 3-O-β-glucopyranoside chloride, peonidin 3-O-β-glucopyranoside chloride, and petunidin 3-O-β-glucopyranoside chloride) was investigated against DOX-induced cardiotoxicity in H9c2 cardiomyoblasts. All these anthocyanidins improved the cell viability via quenching of reactive oxygen species (ROS) [14]. Delphinidin was found to confer protection against DOX and etoposide inhibition of topoisomerase II, thus warranting careful scrutiny against the use of these agents in combating DOX-induced cardiotoxicity.
2.3. Apigenin
Apigenin is a flavone type flavonoid predominantly found in flowers of chamomile. It is also present in edible plants such as fruits (oranges, apples, cherries, and grape fruits) and vegetables (onions, celery, parsley, broccoli, pepper, barley, and tomatoes) [15]. Apigenin treatment to adult rat cardiomyocytes significantly improved cell viability in the presence of DOX and the mechanism appeared to involve quenching of ROS, mitigation of lipid peroxidation, and myocyte necrosis [16]. However, to date, no studies have demonstrated apigenin efficacy in vivo.
2.4. Avicularin
Avicularin, chemically a biflavonoid and a quercetin glycoside, is isolated from the leaves of a flowering plant, Malus hupehensis, popularly known as Chinese crabapple, Hupeh crab, or tea crabapple [17]. The protective effects of avicularin against DOX-induced cardiotoxicity were demonstrated in H9c2 cells and the mechanism purported was via its antioxidant property [17]. However, the cardioprotective effects of avicularin are yet to be confirmed in vivo.
2.5. Berberine
Berberine is an alkaloid identified in the roots and bark of the Berberis species [18]. For the first time, its protective effects on DOX-induced cardiotoxicity in mice were studied by Lv et al. [19]. Berberine was found to reduce mortality, improve body weight and cardiac function, and restore ECG changes in DOX-treated rats. Furthermore, in neonatal rat cardiomyocytes, berberine inhibited DOX-induced apoptosis via counteracting ROS induced p53 activation, mitochondrial dissipation, executioner caspase activation, and activation of AMPK [19]. Moreover, berberine has also been shown to inhibit DOX-induced cardiotoxicity via mitigation of biotransformation of DOX, thereby limiting the bioavailability of doxorubicinol (a major alcohol metabolite of DOX) in the cytoplasm of rat hearts [20].
2.6. Baicalein
Baicalein is a flavonoid derived from the roots of Scutellaria baicalensis [21]. By using cultured chick cardiomyocytes in vitro, it was shown that baicalein attenuated DOX-induced ROS activation, proapoptotic MAPK, and apoptosis [22]. Recently, in a murine model of DOX-induced cardiotoxicity, baicalein significantly mitigated cardiac injury via augmenting nuclear factor E2-related factor-2 (Nrf2), antioxidant defense, blunting of nitrative stress, inflammation, and apoptosis [23].
2.7. Caffeic Acid Phenethyl Ester (CAPE)
Caffeic acid phenethyl ester is an active component of propolis which is the major hive product of bees and is known to be rich in flavonoids [24]. CAPE has been demonstrated to attenuate DOX-induced cardiotoxicity via attenuation of ROS generation and apoptosis. Furthermore, it also improved cardiac function as assessed by hemodynamic measurements and preserved the myocardial structure [25].
2.8. Calceolarioside
Calceolarioside is a phenylpropanoid glycoside isolated from Calceolaria hypericina known to possess antiplatelet and anticancer activities [26]. Calceolarioside attenuated DOX-induced cardiotoxicity in H9c2 cells via upregulation of antioxidant enzymes and suppression of apoptosis [27]. However, these effects are yet to be confirmed in animal models.
2.9. Cannabidiol
Cannabidiol (CBD) is a major nonpsychoactive constituent of the plant Cannabis sativa, popularly known as Marijuana and used for recreational as well as medicinal purposes [28]. In a chronic model of DOX-induced cardiotoxicity in rats, CBD has been demonstrated to suppress myocardial toxicity via attenuating oxidative stress, inflammation, and cell death pathways [29]. Recently, Hao et al. demonstrated that CBD attenuated DOX-induced cardiotoxicity via augmenting mitochondrial biogenesis and blunting of oxidative and nitrative stress and apoptosis [30]. It is also pertinent to note that CBD also exerts several cardioprotective actions against diabetic cardiovascular complications [31] and it has been approved in Canada and Europe for the management of pain associated with multiple sclerosis [32].
2.10. Carotenoids
Carotenoids are the organic pigments and constitute a large group of more than 600 compounds found in plants, which impart color to the leaves and fruits [33]. These are produced from 8 isoprene molecules and contain 40 carbon atoms and are also known as tetraterpenoids with polyisoprenoid structure having a long conjugated double bond system forming the backbone of the molecule, which may be terminated by cyclic end groups that contain oxygen-bearing substitutes. The electron-rich conjugated system of the polyene is believed to afford antioxidant and free radical scavenging activity and these pharmacological effects were attributed to health benefits offered by carotenoids [33]. Recently, the cardioprotective efficacy of carotenoids was demonstrated in DOX-induced tumor-bearing mice [34]. Specifically, carotenoids were found to improve the antioxidant defense and preserve the myocardial membranes, reflected as reduced leakage of myocyte injury marker enzymes without compromising DOX activity on the tumor growth inhibition [34].
2.11. Chrysin
Chrysin is a flavone class of flavonoid and one of the most important bioactive constituents of different fruits, vegetables, and mushrooms [35]. Recently, chrysin cardioprotective effect against DOX-induced acute cardiotoxicity in rats was demonstrated by Mantawy et al. [36]. Chrysin was found to improve antioxidant defense, attenuate oxidative/nitrative stress, and suppress the generation of inflammatory mediators [36].
2.12. Catechins
Epigallocatechin-3-gallate (EGCG) accounts for 50–80% of catechins in green tea and represents about 200–300 mg in a brewed cup of green tea. Convincing data are available to demonstrate that catechins possess potent antioxidant, anti-inflammatory, immunomodulatory, cardioprotective, and anticancer activities [37]. Green tea leaf extract supplementation in cultured rat cardiomyocytes showed its ability to protect the cells against DOX-induced decreased H9c2 cells viability, via quenching of ROS generation [38]. EGCG in vitro has been demonstrated to protect cardiomyocytes of neonatal rat hearts from DOX-induced cytotoxicity by attenuating ROS production, apoptosis, and increasing activities and protein expression of endogenous antioxidant enzymes [39]. In another study, EGCG treatment to rat cardiomyocytes significantly attenuated DOX-induced ROS generation and alterations in myocyte contractile dynamics via modulation of proteins involved in calcium handling system [40]. In addition, EGCG also elicited cardioprotective effects on a chronic model of DOX-induced cardiotoxicity via attenuation of oxidative stress and apoptosis pathways [41].
It has been reported that supplementation of EGCG in the diet increases the activities of P-450 family of reductase, augments the bioavailability of DOX, and could predispose the subject to increased risk of cardiotoxicity [42]. Hence, further studies are warranted to investigate and extend the cardioprotective benefits of ECGG, without untoward perpetuation of DOX-induced cardiotoxicity.
2.13. Chrysoeriol
Chrysoeriol is a flavone compound isolated from the leaves of Digitalis purpurea, popularly known as Foxglove and well reputed for its cardioprotective actions [43]. Chrysoeriol has been found to reduce cell death and attenuate ROS generated oxidative stress and lipid peroxidation in DOX-induced cardiotoxicity in H9c2 cardiomyoblasts without affecting antitumor activity of DOX [43]. For an evidence based approach, additional studies on its cardioprotective efficacies are warranted.
2.14. Curcumin
Curcumin is a phenolic yellow pigment constituent found in the rhizomatous parts of Curcuma longa (turmeric) [44]. Several in vitro and in vivo studies have demonstrated curcumin cardioprotective actions against DOX-induced myocardial toxicity. The key mechanisms postulated for curcumin cardioprotective activity include diminution of oxidative stress, inflammation, and associated cell death pathways [45–48]. Although curcumin has been reported to elicit several beneficial effects in various preclinical studies, the bioavailability is yet to be established in human subjects. Therefore, derivatives of curcumin are being perused to increase its bioavailability and, in this direction, a recent report suggests that the nanoparticle of curcumin could ameliorate DOX-induced cardiotoxicity [49].
2.15. Eugenol
Eugenol is the active component of essential oil isolated from Syzygium aromaticum, popularly known as clove, which is one of the common ingredients of spice mixtures [50]. Eugenol treatment was shown to significantly improve antioxidant defense mechanisms, decrease lipid peroxidation, and attenuate abnormal Ca2+ transients in the cardiomyocytes along with inhibition of apoptosis in rat hearts following acute DOX administration. Eugenol also preserved the myocardium and restored hemodynamics along with preserved histology [51].
2.16. Frederine
7-Monohydroxyethylrutoside (monoHER2, frederine) is a synthetic flavonoid, and it significantly inhibited DOX-induced myocardial toxicity, via suppression of oxidative stress and apoptosis in a chronic murine model [52, 53]. In addition, monoHER2 did not interfere with DOX anticancer activity in vitro and in vivo [54]. Considering these observations, monoHER2 could be further developed for this clinical application as cardioprotective adjuvant.
2.17. Gingerol
Gingerol is the pungent phenolic constituent of Zingiber officinalis (ginger) [55]. In a chronic model of DOX-induced cardiomyopathy, gingerol inhibited DOX-induced myocardial ROS generation, inflammation via attenuation of NF-κB activation, and downregulation of soluble receptor for advanced glycation end products (sRAGE). In addition, gingerol also inhibited myocardial apoptosis via mitigating caspase-3 activities [56].
2.18. Ginsenosides
Ginsenosides are the saponin constituents of Panax notoginseng, popularly known as ginseng in traditional Chinese medicine [57]. Ginsenosides have been classified into protopanaxatriols (Rg1, Rh1, and PPT) and protopanaxadiols (Rg3, Rh2, and PPD). Of the ginsenosides, protopanaxadiols such as ginsenoside Rb1, ginsenoside Rh2, and compound K have been shown to exhibit anticancer and anti-inflammatory activities [57].
Ginsenoside Rh2 (Rh2) has been shown to elicit cardioprotective effects against DOX-induced cardiotoxicity in H9c2 cell line, as well as in vivo in an acute mouse and chronic rat model of DOX-induced cardiomyopathy. Rh2 enhanced cell viability of H9c2 cells and ameliorated DOX-induced release of the CK-MB, LDH. Furthermore, Rh2 ameliorated DOX-induced myocardial toxicity in mouse and rats via suppressing oxidative stress and improved the indices of cardiac function as determined by ECG [58].
2.19. Diosgenin
Diosgenin is a steroid saponin found abundantly in several plants including Solanum and Dioscorea species and Costus speciosus. In a chronic model of DOX-induced cardiomyopathy, diosgenin elicited cardioprotective effects, via activation of prosurvival kinase, protein kinase A (PKA), diminution of p38-MAPK, caspase-3 activities, and generation of free radicals along with attenuation of inflammatory mediators. Mechanistically, it was found to improve myocardial fibrosis and increase the cardiac levels of cGMP via modulation of phosphodiesterase-5 activity [59].
2.20. Hydroxybetulinic Acid
23-Hydroxybetulinic acid is isolated from Pulsatilla chinensis. In a chronic murine model of DOX-induced cardiomyopathy, 23-hydroxybetulinic acid significantly improved the survival of the animals and inhibited apoptosis mainly via inhibition of DOX metabolism in the mitochondria. Similar results were also obtained in H9c2 cells [60].
2.21. Hesperidin
Hesperidin is a bioflavonoid abundantly found in vegetables and citrus fruits such as oranges, lemons, and grapefruits [61]. Citrus flavonoids have been shown to reduce risk of cardiovascular diseases prominently due to their antioxidant and anti-inflammatory effects involving numerous cell signaling pathways [61]. It has been found to improve antioxidant status, inhibit lipid peroxidation, and reduce myocardial enzyme leakage by salvaging myocardium in DOX-induced cardiotoxicity in acute toxicity model conducted rats [62]. Additionally, it sensitized cancer cells to DOX-induced apoptosis and showed synergism in inhibiting P-gp and multidrug resistance, thus appearing to be effective as an adjunct to enhance the efficacy and attenuate the resistance to DOX during chemotherapy [63]. Thus, the potential of citrus flavonoids as cochemotherapeutic and cardioprotective agents is encouraging but further studies are warranted for conclusive evidence in cancer as well as chemotherapy associated cardiotoxicity. In addition, hesperetin (aglycone) derivative of hesperidin also ameliorated chronic DOX treatment associated cardiotoxicity in rats, via attenuation of p38-MAPK, caspase-3, and NF-κB activation and oxidative stress in the myocardial tissues [64].
2.22. Hydroxytyrosol
Hydroxytyrosol is a polyphenolic constituent in Olea europaea, popularly known as olive oil, which is widely used in food and medicine [65]. Hydroxytyrosol has been shown to improve cardiac function by maintaining homeostasis at mitochondrial level, by preserving mitochondrial electron transport chain complexes I–IV and inhibiting apoptosis-inducing factor, and oxidative stress markers in chronic DOX-induced cardiotoxicity in rats harboring breast cancer [65]. Furthermore, hydroxytyrosol did not compromise the DOX antitumor activity against the implanted tumor cells and also improved the survival of the animals [65].
2.23. Isorhamnetin
Isorhamnetin is a flavonol aglycone abundantly found in several medicinal plants, such as Hippophae rhamnoides (sea buckthorn) [66]. Isorhamnetin significantly conferred cardioprotection in a chronic model of DOX-induced cardiotoxicity. The mechanism of cardioprotection involves suppression of oxidative stress and activation of mitochondrial apoptotic pathway and mitogen-activated protein kinase pathways, suggesting antioxidant mediated cardioprotective mechanism. Similar results were obtained in H9c2 cells [66]. Furthermore, it also synergistically improved the DOX anticancer activity in tumor cell lines [66].
2.24. Indole-3-carbinol
Indole-3-carbinol is a natural indole compound predominantly found in cruciferous vegetables [67]. In a chronic DOX-infusion associated cardiotoxicity murine model, indole-3-carbinol was found to reduce solid Ehrlich tumor size and volume, augment antioxidant defense, and inhibit lipid peroxidation, leading to stabilization of cell membrane and reduced leakage of myocyte injury marker enzymes. It was also found to decrease sphingosine kinase 1 (SphK1) activity and inflammatory mediators along with mitigating histological perturbations and modulating cell death mediators [67].
2.25. Kaempferol
Kaempferol is one of the most common dietary flavonoids and it is well studied for its antiapoptotic, cardioprotective, antioxidative, anti-inflammatory, chemopreventive, and anticancer properties as well as modulation of chemoresistance [68]. The cardioprotective effects of kaempferol against DOX-induced cardiotoxicity in rats were demonstrated using a chronic model. Kaempferol counteracted cardiotoxicity by inhibiting p53 expression in mitochondrion-dependent apoptotic signaling and ERK-dependent mitogen-activated protein kinase pathway following binding to the promoter region of the Bax proapoptotic gene. It also effectively suppressed DOX-induced extracellular signal-regulated kinase (ERK1/2) activation but had no effect on p38 and JNK [69].
2.26. Lycopene
Lycopene is a carotenoid and nonprovitamin A found abundantly in Lycopersicum esculentum (tomatoes) and known to impart color to tomatoes [33]. Karimi et al., in a very early study, demonstrated the protective effect of tomato extract and lycopene on acute DOX-induced cardiotoxicity in mice [70]. Tomato extract and lycopene prevented rise in myocyte injury marker enzyme, CK-MB, in serum and ameliorated cardiomyocytes injury evidenced by histopathological examination. Furthermore, Yilmaz et al. studied the protective role of lycopene in DOX-induced heart and kidney toxicities using biochemical and histopathological assessments and reported that lycopene has potential to inhibit lipid peroxidation and improve antioxidants evidenced by reduced lipid peroxides and improved GSH in both the heart and the kidneys. The protective effect was further substantiated by histopathological changes. The authors concluded that treatment with lycopene might prevent cardiac and renal toxicities in rats [71]. However, the results were not further substantiated by functional improvement as lycopene did not prevent left ventricular systolic dysfunction induced by DOX [72]. However, it suppressed DOX-induced myocyte damage without preventing interstitial collagen accumulation increase [72].
Lycopene supplementation also increased lycopene absorption in heart, liver, and plasma and, in another study, the same group of authors showed that there was no depletion of lycopene from myocardium of lycopene-supplemented rats treated with DOX and that higher antioxidant capacity in myocardium and less oxidative cleavage of lycopene in intestinal mucosa of DOX-treated rats suggest an antioxidant role of DOX rather than acting as a prooxidant [73]. The authors further showed that tomato-oleoresin enhances the chemotherapeutic effect of DOX. It maintained lycopene levels in heart and protected against cardiac oxidative DNA damage induced by DOX in rats [73]. The lycopene protected the heart against DOX associated cardiotoxicity by several mechanisms including the quenching of singlet oxygen, peroxy radicals, reaction with free radicals, restoring levels of vitamin E and vitamin C, reducing DNA damage, restoring cellular antioxidants, and preventing depletion of glutathione. Several studies showed the potential role of lycopene in the prevention of side effects of antineoplastic drugs in cell culture and animal models [74]. These results suggest that tomato extract and lycopene inhibit DOX cardiotoxicity and collectively might serve as a novel combination chemotherapeutic agent with DOX to limit free radical-mediated organ injury. However, further studies are required to investigate the role of lycopene in mitigating the side effects of chemotherapy in human subjects.
2.27. Luteolin-7-O-β-D-glucopyranoside
Luteolin-7-O-β-D-glucopyranoside is a flavonoid isolated from the plant Dracocephalum tanguticum that is widely used in Chinese and Tibetan traditional medicine [75]. The cytoprotective activities were demonstrated on DOX-induced cytotoxicity in H9c2 cardiomyocytes. Among several isolated compounds, luteolin-7-O-β-D-glucopyranoside was found to show antioxidant effect and a potent cytoprotective activity against DOX-induced toxicity as evidenced by decreased death of H9c2 cells, reduced myocyte injury marker enzymes, and reduced intracellular concentration of ROS and Ca2+ [75]. Recently, Yao et al. also demonstrated protective effects of luteolin-7-O-glucoside on DOX cytotoxicity in H9c2 cells [76]. It was found to improve cell viability and ameliorate ROS generation and mitochondrial depolarization. Furthermore, it enhanced the expression of prosurvival kinases and diminished ROS generation [76].
2.28. Morin Hydrate
Morin hydrate is a biflavonoid commonly found in fruits such as guava, fig, almond, grapes, and apple and vegetables such as onion, seed weeds, and several other members of Moraceae family [77]. It has been observed to enhance antioxidant defense against oxidative stress in human umbilical vein endothelial cells (ECV304) and HepG2 cells and minimize DOX toxicity in ECV304 and primary mouse cardiomyocytes [77]. However, in vivo studies regarding morin efficacy in combating DOX-induced cardiotoxicity are still lacking.
2.29. Mangiferin
Mangiferin is a xanthonoid structure with C-glucosyl linkage and polyhydroxy component found in many plant species; however, Mangifera indica (mango tree) is the major source [78]. Using a chronic model of cardiomyopathy in rats, mangiferin has been shown to exert cardioprotective action against DOX-induced cardiotoxicity by inhibition of proinflammatory mediators and proapoptotic genes and regulating calcium homeostasis modulating proteins [79].
2.30. Naringin
Naringin is a flavanone glycoside abundantly found in citrus fruits such as lemon, oranges, and grapefruits and in tomatoes. It has been documented to possess numerous biological properties such as antioxidant, anti-inflammatory, and antiapoptotic activities [80]. In an acute model of DOX-induced cardiotoxicity, naringin treatment improved antioxidant defense and inhibited lipid peroxidation along with histopathological preservation, thereby reducing leakage of myocyte marker enzymes. It also decreased the levels of inflammatory mediators and restored the mitochondrial complexes (I–IV) activities in the heart tissues along with histopathological salvage [80]. The studies indicate cardioprotective effects; however, further clinical research is required to provide significant insights into the mechanisms underlying the effects of naringin on human subjects.
2.31. Naringenin and Its Derivatives
Naringenin is a flavanone commonly found in citrus fruits such as grapefruit, orange, and lemon [80]. Arafa et al. demonstrated that naringenin elicited antioxidant mediated protection against DOX-induced cardiac toxicity in Swiss albino rats [81]. Recently, the combination of p-coumaric acid and naringenin was found to be superior in exerting antioxidant mediated cardioprotection against DOX-induced cardiotoxicity in rats [82]. Additionally, naringenin enhanced antitumor effect of DOX by selectively modulating drug efflux pathways; that is, it inhibited the activity of multidrug resistance-associated protein and did not affect the in vivo pharmacokinetics of intravenously administered DOX [80].
Naringenin-7-O-glucoside is a flavanone glycoside isolated from Dracocephalum rupestre. It has been demonstrated to protect against DOX-induced cardiotoxicity in H9c2 cells [83]. Particularly, naringenin-7-O-glucoside improved cell viability, prevented the release of myocyte injury marker enzymes LDH and CK, and augmented antioxidant defense [84]. Furthermore, naringenin-7-O-glucoside was observed to enhance NAD(P)H: quinone oxidoreductase (NQO1) and ERK activation and Nrf2 protein levels in DOX stressed H9c2 cells. These phenotypic changes brought about by naringenin-7-O-glucoside are attributed to the induction of antioxidant defense and attenuation of cell death pathways [85]. However, in vivo studies are yet to be performed.
2.32. Ocotillol
Ocotillol is an aglycone derivative of pseudoginsenoside-F11, which is devoid of sugar moiety and is found in American ginseng, Panax quinquefolius. Fu et al. reported that ocotillol enhances survival rate of animals in both acute and chronic models of DOX-induced cardiotoxicity. Ocotillol prevented depletion of glutathione and lipid peroxidation along with restoration of myocyte injury marker enzymes following preservation of cardiomyocytes cell membrane. Furthermore, ocotillol also improved cardiac function and hence was suggested as an adjuvant for counteracting DOX-induced cardiotoxicity [86].
2.33. Oleuropein
Oleuropein is a phenolic constituent of Olea europaea (olive oil) [87]. Andreadou et al. have shown the protective effect of oleuropein against DOX-induced acute cardiotoxicity in rats. Oleuropein was found to restore the myocardial necrosis marker enzyme levels and attenuation of oxidative stress and apoptosis [88]. Moreover, the same group of authors in a separate study demonstrated that oleuropein treatment aids the compensation of distressed energy metabolic pathways mechanistically by restoration of metabolites to the normal levels as DOX generated free radicals nonenzymatically convert pyruvate to acetate and alpha-ketoglutarate to succinate [87]. The cardioprotective role of oleuropein in chronic DOX-induced cardiomyopathy has also been demonstrated [89]. Particularly, oleuropein treatment significantly suppressed DOX-induced oxidative/nitrative stress, augmented prosurvival kinases, and improved the cardiac functions [89].
2.34. Osthole
Osthole is a coumarin compound found in several medicinal plants such as Cnidium monnieri and Angelica pubescens [90]. In rat neonatal cardiomyocytes, osthole significantly improved the survival of the cells by abrogating apoptosis, wherein the mechanism appeared to involve the suppression of mitochondrial pathway of apoptosis triggered by DOX [90]. However, in vivo studies have not been conducted, and it is warranted to recapitulate the in vitro cardioprotective actions of osthole.
2.35. p-coumaric Acid
p-coumaric acid is a phenolic acid that serves as a precursor of other phenolic compounds and is found in plants such as peanut, tea, and coffee [82]. p-coumaric acid has been shown to attenuate oxidative stress and inhibit myocyte injury in DOX-induced myocardial injury in rats [91]. In a separate study, p-coumaric acid in combination with naringenin showed cardioprotection by augmentation of antioxidant defense against DOX-induced cardiotoxicity in rats [82].
2.36. Periplogenin
Periplogenin is a cardenolide isolated from Aegle marmelos, commonly known as Bael in the traditional Indian system of medicine [92]. Periplogenin has been shown to decrease lipid peroxide levels, improve antioxidant defense, and salvage cardiomyocytes in a chronic model of DOX-induced cardiotoxicity in rats [92].
2.37. Plantainoside D
Plantainoside D is an iridoid glucoside isolated from Picrorhiza scrophulariiflora (Picrorhiza). The chemopreventive and antioxidant activities encouraged evaluating the cardioprotective effect of plantainoside D against DOX-induced apoptosis in H9c2 cells. Plantainoside D was found to inhibit oxidative stress and proinflammatory cytokines expression and attenuate apoptosis in H9c2 cardiomyoblasts [93].
2.38. Phycocyanin
C-Phycocyanin is a biliprotein found in Spirulina platensis, blue-green algae. Phycocyanin has been found to protect against DOX-induced oxidative stress and apoptosis in adult rat cardiomyocytes as evidenced by reduced ROS formation, DNA fragmentation, and attenuation of Bax as well as release of cytochrome C and increase in the activity of caspase-3 [94]. However, these in vitro findings are yet to be confirmed in rodent model of DOX-induced cardiomyopathy.
2.39. Proanthocyanidin and Derivatives
Proanthocyanidins are a mixture of structurally and functionally diverse chemicals which are predominantly found in grape seed and show high bioavailability and protect the organs from toxic chemicals used to induce diseases in in vitro and in vivo studies [95, 96]. Ray et al. reported the bioavailability and protective property of grape seed proanthocyanidin and a novel IH636 grape seed proanthocyanidin extract against DOX-induced cardiotoxicity as well as multiorgan protection in mice [97]. Bagchi et al. further reported that IH636 proanthocyanidin extract afforded protection was superior to vitamin C, vitamin E, and beta-carotene and demonstrated significant cytotoxicity towards human breast, lung, and gastric adenocarcinoma cells, while enhancing the growth and viability of normal cells in both in vitro and in vivo studies [98]. In another study, proanthocyanidins enhanced DOX-induced antitumor effect and reversed drug resistance and mechanisms attributed partially to the promotion of DOX-induced apoptosis through elevation of intracellular DOX, Ca2+, and Mg2+ concentration and reduction of pH value and mitochondrial membrane potential in DOX-resistant K562/DOX cells [99]. Furthermore, proanthocyanidin strongly enhanced the antitumor activity of DOX and ameliorated chronic DOX-induced myocardial oxidative stress and immunosuppression in tumor-bearing mice [99]. In addition, grape seed proanthocyanidin also showed antioxidant mediated cardioprotection against both high and low dose DOX-induced cumulative chronic cardiotoxicities in rats [100].
2.40. Resveratrol
Resveratrol, a natural phytoalexin, is commonly found in Vitis vinifera (grapes) [101]. Resveratrol induced antioxidants and phase 2 enzymes in the H9c2 cells, accompanied by increased resistance to oxidative and electrophilic cell injury [102]. Additionally, there was no significant effect of resveratrol on NADPH-cytochrome P-450 reductase (P-450 reductase), which plays an important role in the metabolism of many endogenous compounds and xenobiotics including DOX. The enzyme P-450 reductase activates them to their more toxic metabolites via one electron reduction which triggers free radical cascade. In some cases, however, such transformation is essential to produce therapeutic effect of anticancer drugs [42].
In DOX-induced cardiotoxicity in rats, resveratrol has been shown to ameliorate the severity of cardiac dysfunction and prevent oxidant stress responses [103, 104]. Furthermore, resveratrol was found to confer cardioprotection and reduce cardiac fibrosis in acute as well as chronic in vivo models of DOX-induced cardiomyopathy in rats. Mechanistically, resveratrol has been demonstrated to protect against DOX-induced oxidative stress through changes in mitochondrial function, specifically the Sirt1 pathway, leading to cardiac cell survival [105]. Resveratrol attenuated DOX-induced cardiomyocyte apoptosis in mice via upregulation of Sirt1-mediated p53 deacetylation and activation of Sirt1, a NAD+-dependent deacetylase, resulting in improved mitochondrial function, which culminates in activation of the transcription factors which coordinate expression of key antioxidant proteins by binding to the antioxidant response elements that regulate cell survival [106]. The overexpression of Sirt1 inhibited cell apoptosis by suppression of p38-MAPK phosphorylation and caspase-3 activation along with amelioration of ROS generation and prevented DOX-induced functional loss in DOX-induced cardiomyocyte injury [106]. DOX induces autophagy in cardiomyocytes which is a degradation system for eukaryotic cells to turn over organelles and long-lived proteins, thereby maintaining cellular homeostasis. Thus, aberrant autophagy activity impairs basal cardiac structure and function, making animals more sensitive to stress-induced heart failure. The ability of resveratrol to inhibit autophagy is mediated by inhibition of p70S6 kinase 1 (S6 K1) that is essential for resveratrol to suppress DOX-induced autophagy and cytotoxic effects [107].
DOX inhibits AMP-activated protein kinase (AMPK), resulting in Sirt1 dysfunction and p53 accumulation in mouse embryonic fibroblasts, and pharmacological activation of AMPK by resveratrol has been shown to alleviate the side effects of DOX in H9c2 cells [108]. Furthermore, resveratrol has been shown to confer cardioprotection in DOX-induced cardiomyocyte apoptosis in nude mice by induction of heme oxygenase-1 (HO-1) mediated mechanisms [109, 110]. Resveratrol was also reported to aid the differentiation of adipose-derived mesenchymal stem cells to cardiomyocytes and protected against noxious effects of DOX to the myocardium [111]. Furthermore, resveratrol supplement along with exercise training was found to be more effective in preventing DOX-induced LV remodeling associated with the reduction of DOX-induced oxidative stress [112]. In spite of these advances made in preclinical studies, resveratrol bioavailability is seldom established in human subjects and this warrants further approaches to extend its beneficial effects to mankind [113].
2.41. Robinin
Robinin is a flavonoid glycoside isolated from leaves of Vigna unguiculata, a dietary plant used in traditional cuisine in India [114]. Treatment with robinin was found to improve endogenous antioxidants, reduce ROS generation, and inhibit lipid peroxidation and proinflammatory mediators such as cyclooxygenase (COX2) and lipoxygenase (LOX15) along with restoring myocyte injury marker enzymes. The improvement in the level of transforming growth factor-β1 (TGF-β1), Smad2, murine double minute (Mdm2), Smad3, cyclin-dependent kinase inhibitor 2A, Smad4, and Smad7 in addition to favorable modulation of p53, Bcl-2, and Bax revealed the cardioprotective mechanism of robinin in combating DOX-induced cardiotoxicity [114].
2.42. Rosmarinic Acid
Rosmarinic acid is an ester of caffeic acid abundantly found in numerous plants, being most common in Boraginaceae and Lamiaceae families [115]. Rosmarinic acid showed remarkable cytoprotection against DOX toxicity in neonatal rat cardiomyocytes and DOX-induced lipid peroxidation of heart membranes, mitochondria, and microsomes and effects were found to be comparable to dexrazoxane [116]. Furthermore, it inhibited DOX-induced oxidative stress and apoptosis in H9c2 cardiomyoblasts by improving cell viability, inhibiting the production of ROS, and activation of prosurvival kinases [115]. However, in vivo cardioprotective actions against DOX-induced cardiotoxicity are hitherto unknown.
2.43. Salvianolic Acids
Salvianolic acids especially salvianolic acid A and salvianolic acid B are the most abundant water-soluble compounds extracted from Salvia miltiorrhiza (Danshen or red sage) [117]. Salvianolic acid A has been shown to protect against DOX-induced mitochondrial toxicity in vitro in rat cardiomyocytes due to its antioxidant action, without antagonizing effect on the antitumor activity of DOX [118]. The protective effects of salvianolic acid were reconfirmed in vivo in another study, against DOX cardiotoxicity in mice [117], via abrogation of oxidative stress and inflammation.
2.44. Schisandrin B
Schisandrin B, a dibenzocyclooctadiene lignin, is isolated from the fruit of Schisandra chinensis. It has been shown to salvage cardiomyocytes, via conferring antioxidant defense by restoring glutathione flux in an acute animal model of DOX-induced cardiotoxicity [119]. Furthermore, it also mitigated DOX-induced cardiotoxicity in rabbits [120]. Recently, cardioprotective effects of schisandrin B against DOX-induced cardiotoxicity were reconfirmed and the mechanism of protection was evidenced by amelioration of proinflammatory cytokines, lipid peroxidation, DNA damage, apoptosis, and MAPK activation in the myocardial tissues [121, 122].
2.45. Salidroside
Salidroside, a phenylethanoid glycoside, has been isolated from the roots of Rhodiola rosea (Roseroot). Wang et al. demonstrated that treatment of salidroside to either H9c2 cells or mice stressed with acute DOX administration conferred cardioprotective effects. The mechanism was defined to involve antioxidant and suppression of proapoptotic mediators [123]. The cardioprotective effects of salidroside were reconfirmed in a placebo controlled clinical trial wherein sixty patients with breast cancer receiving epirubicin were given salidroside (600 mg/day) or placebo starting 1 week before chemotherapy and assessed at baseline and 7 days after each new epirubicin dose of 100 mg/m2. Decline in strain rate peak was observed at an epirubicin dose of 200 mg/m2, with no significant differences between salidroside and placebo. At increasing cumulative doses of epirubicin, the strain rate normalized only with salidroside, showing a significant difference in comparison with placebo at epirubicin doses of 300 mg/m2. The authors concluded that salidroside may provide protection against chemotherapy-induced early left ventricular regional systolic dysfunction in patients with breast cancer. Based on preclinical and clinical data, salidroside needs to be investigated further in a larger population for advocating salidroside as an adjuvant to thwart the DOX-induced cardiotoxicity.
2.46. Sesamin
Sesamin is a major lignin obtained from seeds of Sesamum indicum. Sesamin was observed to increase the endogenous antioxidant enzymes and prevent mitochondrial damage via activation of Sirt1 in an acute model of DOX-induced cardiotoxicity [124].
2.47. Sesamol
Sesamol is a phenolic constituent of oil obtained from seeds of Sesamum indicum and is used commonly as an edible oil. The cardioprotective effects of sesamol were confirmed in an in vivo study, wherein sesamol mitigated cumulative DOX-induced cardiomyopathy in rats [125]. Sesamol improved antioxidant defense status, reduced myocyte injury marker enzymes released from cardiomyocytes, and inhibited lipid hydroperoxide. The salvage of tissues evidenced by biochemical and histopathological studies demonstrated cardioprotective effects of sesamol [125]. However, further mechanistic studies should be carried out investigating the effect on DOX efficacy and pharmacokinetic interaction.
2.48. Silibinin
Silibinin, a flavonolignan, is an active component of Silybum marianum (milk thistle), popularly known as silymarin and known to constitute 50–70% of the silymarin extract. The cardioprotective effect exerted by silymarin, silibinin, dehydrosilibinin, and silychristin was comparable to that of dexrazoxane, while silydianin exerted the best protective effect [126].
2.49. Sulforaphane
Sulforaphane is an organosulfur compound found in a significant amount in cruciferous vegetables, especially in broccoli (Brassica oleracea) [127]. The cardioprotective effects of sulforaphane were first demonstrated in H9c2 rat myoblasts as evidenced by reduced number of apoptotic cells along with decreased expression of proapoptotic proteins such as Bax, caspase-3, and cytochrome C [128]. It also reduced ROS generation and restored mitochondrial membrane potential [128]. Moreover, the cardioprotective effects of sulforaphane were found to be mediated by the activation of the Kelch-like ECH-associated protein 1 (Keap1)/NF-E2-related factor-2 (Nrf2)/antioxidant-responsive element (ARE) pathway, which in turn mediates the induction of HO-1 [129].
2.50. Tanshinone IIA and Derivatives
Tanshinones are the group of bioactive compounds isolated from Salvia miltiorrhiza (Danshen), a Chinese medicinal plant reputed for the management of cardiovascular diseases in particular, angina pectoris, atherosclerosis, myocardial infarction, and ischemic-reperfusion injury [130]. Sodium tanshinone IIA sulfonate, a water-soluble derivative of tanshinone IIA, was demonstrated to be beneficial in reducing DOX-induced cardiotoxicity in mice hearts and in cultured cardiomyocytes [131]. Treatment with sodium tanshinone IIA sulfonate prevented decrease in body weight and reduced myocardial lipid peroxidation in mice along with improved activities of endogenous antioxidant enzymes. In addition, the antioxidative mechanism was also supported by in vitro experiments showing that sodium tanshinone IIA sulphonate scavenged DOX semiquinone free radical in heart homogenate and inhibited DOX-induced mitochondrial lipid peroxidation and swelling [131].
Furthermore, another study demonstrated the beneficial effect of tanshinone IIA on decreasing DOX-induced apoptosis in neonatal rat cardiomyocytes and underlying molecular mechanisms [132]. Tanshinone IIA ameliorated apoptosis and ROS generation induced by DOX in a dose-dependent manner. It was further supported by the inhibition of DOX-mediated reduction of the ratio of Bcl-2/Bax [132]. Furthermore, a separate study also recapitulated that tanshinone IIA significantly inhibited DOX-induced toxic effects in H9c2 cells as well as in animal models of cardiotoxicity [133]. In this study, tanshinone IIA was shown to improve cell viability and ameliorate apoptosis of DOX-induced cytotoxicity in H9c2 cells. Furthermore, the cardioprotective effects of tanshinone IIA sodium sulfonate were confirmed by decreased ST interval and QRS interval in ECG; improved histological appearance of myocardium; increased myocardial tensile strength; and decreased fibrosis [133]. Recently, Hong et al. evaluated the protective effect of tanshinone IIA on DOX-induced cardiomyocyte apoptosis and explored its intracellular mechanisms using primary cultured neonatal rat cardiomyocytes. Tanshinone IIA was found to inhibit DOX-induced reactive oxygen species generation, reduce the expression of caspase-3 and cytochrome C, and increase BcL-x(L) expression, resulting in protecting cardiomyocytes from DOX-induced apoptosis. In addition, tanshinone IIA also enhanced Akt phosphorylation in cardiomyocytes and inhibited apoptosis [134].
2.51. Tetrahydroxystilbene Glucoside
Tetrahydroxystilbene glucoside is one of the active components extracted from Polygonum multiflorum (knot grass). For the first time, Zhang et al. demonstrated its protective effect on neonate rat cardiomyocytes and on an acute mouse model of DOX-induced cardiotoxicity [135]. In the mouse model, it was shown to inhibit lipid peroxidation accompanying improved glutathione, reduced animal mortality, preserved histopathological changes, and restored levels of myocyte injury marker enzymes. In the in vitro study, it prevented DOX-induced loss of mitochondrial membrane potential, caspase-3 activation, and upregulation of Bax protein expression along with upregulation of Bcl-2 and inhibited ROS generation. It was also observed to inhibit DOX-induced increases in intracellular Ca2+ and apoptosis of cardiomyocytes in a concentration-dependent manner [135].
2.52. Thymoquinone
Thymoquinone is the main active constituent of the volatile oil of Nigella sativa Linn., popularly known as black seed, used for culinary and medicinal purposes [136]. Thymoquinone suppressed DOX-induced cardiotoxicity in an acute murine model of cardiomyopathy, without compromising antitumor activity of DOX [137]. Furthermore, thymoquinone also circumvented DOX-mediated cardiotoxicity in acute model, wherein the key mechanism was postulated to involve antioxidant pathways [138]. Finally, thymoquinone synergistically increased DOX activity in several cancer cell lines and prevented DOX-induced toxicity in H9c2 cells [139].
2.53. Tetrandrine
Tetrandrine is a bisbenzylisoquinoline alkaloid isolated from the dried root of Stephania tetrandra. In a chronic model of DOX-induced cardiomyopathy, tetrandrine significantly inhibited myocardial apoptosis via quenching of ROS and restoration of mitochondrial capacity. These beneficial effects were corroborated with improved indices of cardiac function [140]. It is pertinent to note that tetrandrine had a negligible effect in DOX pharmacokinetics properties in rodents, suggesting that tetrandrine might be a suitable candidate to be developed as cardioprotective adjuvant [141].
2.54. Z-Guggulsterone
Guggulsterone is a major active component of Commiphora mukul, popularly known as Guggul and reputed for its antihyperlipidemic and cardioprotective effects in Ayurvedic medicine [142]. Wang et al. demonstrated the protective activity of guggulsterone against DOX-induced cytotoxicity in H9c2 cells. It was found to improve cell viability, morphology, and cytotoxicity and cellular antioxidants along with inhibition of apoptosis by altering activity of PARP, caspase-3, cytochrome C release, and Bcl-2 proteins and reducing the activation of NF-κB [143].
2.55. Vincristine
Vincristine is an alkaloidal constituent isolated from Catharanthus roseus (Madagascar periwinkle), also known as Vinca rosea. Recently, its potential to prevent DOX-induced cardiomyocyte death and related mechanisms has been reported in adult mouse cardiac myocytes [144]. Vincristine treatment to cardiomyocytes in the presence of DOX increased the cell viability. This was concordant with decreased PARP and caspase-3 activities and increased activation of prosurvival kinase Akt and diminished MAPK pathways [144]. However, the precise cardioprotective effects in vivo are yet to be demonstrated.
2.56. Visnagin
Visnagin is an active constituent isolated from fruit extracts of Ammi visnaga known as toothpick weed and used in traditional Chinese medicine for cardiovascular diseases [145]. Visnagin was recently shown to be cardioprotective in a zebrafish model of DOX-induced cardiomyopathy that recapitulates the cardiomyocyte apoptosis and contractility similar to those observed in humans [146]. Visnagin was found to rescue the cardiac performance and circulatory defects caused by DOX in zebrafish. It also attenuated DOX-induced apoptosis in cultured cardiomyocytes and in vivo in zebrafish and mouse hearts along with improved cardiac contractility in DOX-treated mice. Additionally, it did not interfere with DOX efficacy in several cultured tumor lines or in zebrafish and mouse xenograft models. Visnagin was observed to bind mitochondrial malate dehydrogenase (MDH2), a key enzyme in the tricarboxylic acid cycle that contributed to cardioprotection [146].
3. Concluding Remarks and Future Perspectives
From the analysis of the literature, it is evident that several phytochemicals exhibited cardioprotective effects in vitro and in vivo against DOX-induced cardiotoxicity. The key pathways modulated by phytochemicals in cardiomyocytes include oxidative stress, inflammation, and cell death pathways, as demonstrated in Figure 1. Majority of the phytochemicals were demonstrated to elicit cardioprotective activity in preclinical studies. However, they have not been translated for clinical utility in human subjects. The major impediment to the development of phytochemical based cardioprotective adjuvants pertains to their negligible pharmacokinetic actions in human subjects. Particularly, the poor or lack of bioavailability in human subjects retards the enthusiasm for further pharmaceutical development [113, 147].
In order to improve the bioavailability of phytochemicals, various synthetic derivatives have been pursued [44]. Although significant strides have been taken in delineating the pathomechanisms for DOX-induced cardiotoxicity, still we do not have bona fide clinical biomarker to predict early changes in the myocardium of patients who received DOX treatment [148]. Therefore, it is of paramount significance to devise a biomarker to predict the DOX-induced cardiotoxicity, because most patients (cancer survivors) exhibit dilated cardiomyopathy several years after exposure to DOX [148]. Furthermore, from Table 1, it is evident that there is discrepancy regarding the employment of appropriate models in studying phytochemicals protective effects in nullifying DOX-induced cardiotoxicity. Therefore, future studies addressing the phytochemicals protective effects against DOX cardiotoxicity should utilize the physiologically relevant cumulative (chronic) dosage regimen in rodents. In addition, future studies should obligatorily investigate the noninterference of phytochemicals against DOX anticancer activities in orthotropic tumor-bearing mouse models.
In sum, to exploit the true potentials of plant-derived compounds for drug development, significant intellectual and financial contributions are warranted from academia and pharmaceutical industry. Unfortunately, the major impediment in this direction is the lack of proper intellectual property rights protection that could protect the financial viability of the drug development projects based on phytochemicals for the treatment of cardiomyopathy. This caveat coupled with other pharmacodynamics and pharmacokinetic lapses pertaining to the phytochemicals precludes the attention of major pharmaceutical companies in their portfolio investments toward the drug development. However, academic research should be directed to develop phytochemicals derived small molecules with significant bioavailability in human subjects. Perhaps this approach could be envisaged for translational application in combating DOX-induced cardiotoxicity.
Acknowledgments
Shreesh Ojha and Mohanraj Rajesh were supported by the intramural program grants sponsored by the College of Medicine and Health Sciences and Office of Research and Graduate Studies of the United Arab Emirates University.
Competing Interests
The authors disclose no competing interests.
Authors' Contributions
Shreesh Ojha, Hasan Al Taee, Sameer Goyal, and Umesh B. Mahajan researched the literature. Shreesh Ojha, Sameer Goyal, and Umesh B. Mahajan drafted the paper. Chandrgouda R. Patil and D. S. Arya edited the paper. Mohanraj Rajesh researched the literature and wrote and edited the paper. All authors read the contents of the paper and approved the same.
References
- 1.Simunek T., Sterba M., Popelova O., Adamcova M., Hrdina R., Gersl V. Anthracycline-induced cardiotoxicity: overview of studies examining the roles of oxidative stress and free cellular iron. Pharmacological Reports. 2009;61(1):154–171. doi: 10.1016/s1734-1140(09)70018-0. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi N., Fujii T., Aoi S., Kozuch P. S., Hortobagyi G. N., Blum R. H. Comparison of cardiac events associated with liposomal doxorubicin, epirubicin and doxorubicin in breast cancer: a Bayesian network meta-analysis. European Journal of Cancer. 2015;51(16):2314–2320. doi: 10.1016/j.ejca.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 3.Jerjian T. V., Glode A. E., Thompson L. A., O'Bryant C. L. Antibody-drug conjugates: a clinical pharmacy perspective on an emerging cancer therapy. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2016;36(1):99–116. doi: 10.1002/phar.1687. [DOI] [PubMed] [Google Scholar]
- 4.Lehenbauer Ludke A. R., Al-Shudiefat A. A.-R. S., Dhingra S., Jassal D. S., Singal P. K. A concise description of cardioprotective strategies in doxorubicin-induced cardiotoxicity. Canadian Journal of Physiology and Pharmacology. 2009;87(10):756–763. doi: 10.1139/y09-059. [DOI] [PubMed] [Google Scholar]
- 5.Asselin B. L., Devidas M., Chen L., et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed t-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-hodgkin lymphoma: a report of the children's oncology group randomized trial pediatric oncology group 9404. Journal of Clinical Oncology. 2016;34(8):854–862. doi: 10.1200/jco.2015.60.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tashakori Beheshti A., Mostafavi Toroghi H., Hosseini G., Zarifian A., Homaei Shandiz F., Fazlinezhad A. Carvedilol administration can prevent doxorubicin-induced cardiotoxicity: a double-blind randomized trial. Cardiology. 2016;134(1):47–53. doi: 10.1159/000442722. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz S. E., Franco V. I., Miller T. L., Colan S. D., Sallan S. E. Cardiovascular disease in adult survivors of childhood cancer. Annual Review of Medicine. 2015;66:161–176. doi: 10.1146/annurev-med-070213-054849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weng C.-J., Yen G.-C. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treatment Reviews. 2012;38(1):76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Wen G., Qu X.-X., Wang D., et al. Recent advances in design, synthesis and bioactivity of paclitaxel-mimics. Fitoterapia. 2016;110:26–37. doi: 10.1016/j.fitote.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Wang W., Liang Y.-S. Artemisinin: a wonder drug from Chinese natural medicines. Chinese Journal of Natural Medicines. 2016;14(1):5–6. doi: 10.3724/SP.J.1009.2016.00005. [DOI] [PubMed] [Google Scholar]
- 11.Angsutararux P., Luanpitpong S., Issaragrisil S. Chemotherapy-induced cardiotoxicity: overview of the roles of oxidative stress. Oxidative Medicine and Cellular Longevity. 2015;2015:13. doi: 10.1155/2015/795602.795602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varga Z. V., Ferdinandy P., Liaudet L., Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. American Journal of Physiology—Heart and Circulatory Physiology. 2015;309(9):H1453–H1467. doi: 10.1152/ajpheart.00554.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh J., Das J., Manna P., Sil P. C. The protective role of arjunolic acid against doxorubicin induced intracellular ROS dependent JNK-p38 and p53-mediated cardiac apoptosis. Biomaterials. 2011;32(21):4857–4866. doi: 10.1016/j.biomaterials.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 14.Choi E. H., Chang H.-J., Cho J. Y., Chun H. S. Cytoprotective effect of anthocyanins against doxorubicin-induced toxicity in H9c2 cardiomyocytes in relation to their antioxidant activities. Food and Chemical Toxicology. 2007;45(10):1873–1881. doi: 10.1016/j.fct.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Saeed M., Kadioglu O., Khalid H., Sugimoto Y., Efferth T. Activity of the dietary flavonoid, apigenin, against multidrug-resistant tumor cells as determined by pharmacogenomics and molecular docking. Journal of Nutritional Biochemistry. 2015;26(1):44–56. doi: 10.1016/j.jnutbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Psotová J., Chlopčíkova Š., Miketová P., Hrbác J., Šimánek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes. Part III. Apigenin, baicalelin, kaempherol, luteolin and quercetin. Phytotherapy Research. 2004;18(7):516–521. doi: 10.1002/ptr.1462. [DOI] [PubMed] [Google Scholar]
- 17.Wang S.-Q., Zhu X.-F., Wang X.-N., Shen T., Xiang F., Lou H.-X. Flavonoids from Malus hupehensis and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Phytochemistry. 2013;87:119–125. doi: 10.1016/j.phytochem.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Li Z., Geng Y.-N., Jiang J.-D., Kong W.-J. Antioxidant and anti-inflammatory activities of berberine in the treatment of diabetes mellitus. Evidence-Based Complementary and Alternative Medicine. 2014;2014:12. doi: 10.1155/2014/289264.289264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv X., Yu X., Wang Y., et al. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047351.e47351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao G., Yu Y., Gu B., Xing Y., Xue M. Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica. 2015;45(11):1024–1029. doi: 10.3109/00498254.2015.1034223. [DOI] [PubMed] [Google Scholar]
- 21.Ciesielska E., Gwardys A., Metodiewa D. Anticancer, antiradical and antioxidative actions of novel Antoksyd S and its major components, baicalin and baicalein. Anticancer Research. 2002;22(5):2885–2892. [PubMed] [Google Scholar]
- 22.Chang W.-T., Li J., Haung H.-H., et al. Baicalein protects against doxorubicin-induced cardiotoxicity by attenuation of mitochondrial oxidant injury and JNK activation. Journal of Cellular Biochemistry. 2011;112(10):2873–2881. doi: 10.1002/jcb.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sahu B. D., Kumar J. M., Kuncha M., Borkar R. M., Srinivas R., Sistla R. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sciences. 2016;144:8–18. doi: 10.1016/j.lfs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P., Tang Y., Li N.-G., Zhu Y., Duan J.-A. Bioactivity and chemical synthesis of caffeic acid phenethyl ester and its derivatives. Molecules. 2014;19(10):16458–16476. doi: 10.3390/molecules191016458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fadillioglu E., Oztas E., Erdogan H., et al. Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. Journal of Applied Toxicology. 2004;24(1):47–52. doi: 10.1002/jat.945. [DOI] [PubMed] [Google Scholar]
- 26.Sena Filho J. G., Nimmo S. L., Xavier H. S., Barbosa-Filho J. M., Cichewicz R. H. Phenylethanoid and lignan glycosides from polar extracts of Lantana, a genus of verbenaceous plants widely used in traditional herbal therapies. Journal of Natural Products. 2009;72(7):1344–1347. doi: 10.1021/np900086y. [DOI] [PubMed] [Google Scholar]
- 27.Kim D.-S., Kim H.-R., Woo E.-R., et al. Protective effect of calceolarioside on adriamycin-induced cardiomyocyte toxicity. European Journal of Pharmacology. 2006;541(1-2):24–32. doi: 10.1016/j.ejphar.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 28.Durst R., Danenberg H., Gallily R., et al. Cannabidiol, a nonpsychoactive Cannabis constituent, protects against myocardial ischemic reperfusion injury. American Journal of Physiology—Heart and Circulatory Physiology. 2007;293(6):H3602–H3607. doi: 10.1152/ajpheart.00098.2007. [DOI] [PubMed] [Google Scholar]
- 29.Fouad A. A., Albuali W. H., Al-Mulhim A. S., Jresat I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environmental Toxicology and Pharmacology. 2013;36(2):347–357. doi: 10.1016/j.etap.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 30.Hao E., Mukhopadhyay P., Cao Z., et al. Cannabidiol protects against doxorubicin-induced cardiomyopathy by modulating mitochondrial function and biogenesis. Molecular Medicine. 2015;21:38–45. doi: 10.2119/molmed.2014.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rajesh M., Mukhopadhyay P., Btkai S., et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. Journal of the American College of Cardiology. 2010;56(25):2115–2125. doi: 10.1016/j.jacc.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horvth B., Mukhopadhyay P., Hask G., Pacher P. The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. American Journal of Pathology. 2012;180(2):432–442. doi: 10.1016/j.ajpath.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pennant M., Steur M., Moore C., Butterworth A., Johnson L. Comparative validity of vitamin C and carotenoids as indicators of fruit and vegetable intake: a systematic review and meta-analysis of randomised controlled trials. British Journal of Nutrition. 2015;114(9):1331–1340. doi: 10.1017/s0007114515003165. [DOI] [PubMed] [Google Scholar]
- 34.Indu R., Azhar T. S., Nair A., Nair C. K. K. Amelioration of doxorubicin induced cardio-and hepato-toxicity by carotenoids. Journal of Cancer Research and Therapeutics. 2014;10(1):62–67. doi: 10.4103/0973-1482.131370. [DOI] [PubMed] [Google Scholar]
- 35.Nabavi S. F., Braidy N., Habtemariam S., et al. Neuroprotective effects of chrysin: from chemistry to medicine. Neurochemistry International. 2015;90:224–231. doi: 10.1016/j.neuint.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Mantawy E. M., El-Bakly W. M., Esmat A., Badr A. M., El-Demerdash E. Chrysin alleviates acute doxorubicin cardiotoxicity in rats via suppression of oxidative stress, inflammation and apoptosis. European Journal of Pharmacology. 2014;728(1):107–118. doi: 10.1016/j.ejphar.2014.01.065. [DOI] [PubMed] [Google Scholar]
- 37.Kim H.-S., Quon M. J., Kim J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biology. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hrelia S., Bordoni A., Angeloni C., et al. Green tea extracts can counteract the modification of fatty acid composition induced by doxorubicin in cultured cardiomyocytes. Prostaglandins Leukotrienes and Essential Fatty Acids. 2002;66(5-6):519–524. doi: 10.1054/plef.2002.0393. [DOI] [PubMed] [Google Scholar]
- 39.Li W., Nie S., Xie M., Chen Y., Li C., Zhang H. A major green tea component, (−)-epigallocatechin-3-gallate, ameliorates doxorubicin-mediated cardiotoxicity in cardiomyocytes of neonatal rats. Journal of Agricultural and Food Chemistry. 2010;58(16):8977–8982. doi: 10.1021/jf101277t. [DOI] [PubMed] [Google Scholar]
- 40.Zheng J., Lee H. C. M., bin Sattar M. M., Huang Y., Bian J.-S. Cardioprotective effects of epigallocatechin-3-gallate against doxorubicin-induced cardiomyocyte injury. European Journal of Pharmacology. 2011;652(1–3):82–88. doi: 10.1016/j.ejphar.2010.10.082. [DOI] [PubMed] [Google Scholar]
- 41.Saeed N. M., El-Naga R. N., El-Bakly W. M., Abdel-Rahman H. M., Salah Eldin R. A., El-Demerdash E. Epigallocatechin-3-gallate pretreatment attenuates doxorubicin-induced cardiotoxicity in rats: a mechanistic study. Biochemical Pharmacology. 2015;95(3):145–155. doi: 10.1016/j.bcp.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 42.Dudka J., Jodynis-Liebert J., Korobowicz E., et al. Activity of NADPH-cytochrome P-450 reductase of the human heart, liver and lungs in the presence of (-)-epigallocatechin gallate, quercetin and resveratrol: an in vitro study. Basic & Clinical Pharmacology & Toxicology. 2005;97(2):74–79. doi: 10.1111/j.1742-7843.2005.pto_98.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu Z., Song X.-D., Xin Y., et al. Protective effect of chrysoeriol against doxorubicin-induced cardiotoxicity in vitro. Chinese Medical Journal. 2009;122(21):2652–2656. doi: 10.3760/cma.j.issn.0366-6999.2009.21.024. [DOI] [PubMed] [Google Scholar]
- 44.Prasad S., Tyagi A. K., Aggarwal B. B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Research and Treatment. 2014;46(1):2–18. doi: 10.4143/crt.2014.46.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Venkatesan N. Curcumin attenuation of acute adriamycin myocardial toxicity in rats. British Journal of Pharmacology. 1998;124(3):425–427. doi: 10.1038/sj.bjp.0701877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dayton A., Selvendiran K., Meduru S., et al. Amelioration of doxorubicin-induced cardiotoxicity by an anticancer-antioxidant dual-function compound, HO-3867. Journal of Pharmacology and Experimental Therapeutics. 2011;339(2):350–357. doi: 10.1124/jpet.111.183681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hosseinzadeh L., Behravan J., Mosaffa F., Bahrami G., Bahrami A., Karimi G. Curcumin potentiates doxorubicin-induced apoptosis in H9c2 cardiac muscle cells through generation of reactive oxygen species. Food and Chemical Toxicology. 2011;49(5):1102–1109. doi: 10.1016/j.fct.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Swamy A. V., Gulliaya S., Thippeswamy A., Koti B. C., Manjula D. V. Cardioprotective effect of curcumin against doxorubicin-induced myocardial toxicity in albino rats. Indian Journal of Pharmacology. 2012;44(1):73–77. doi: 10.4103/0253-7613.91871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pramanik D., Campbell N. R., Das S., et al. A composite polymer nanoparticle overcomes multidrug resistance and ameliorates doxorubicin-associated cardiomyopathy. Oncotarget. 2012;3(6):640–650. doi: 10.18632/oncotarget.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pramod K., Ansari S. H., Ali J. Eugenol: a natural compound with versatile pharmacological actions. Natural Product Communications. 2010;5(12):1999–2006. [PubMed] [Google Scholar]
- 51.Fouad A. A., Yacoubi M. T. Mechanisms underlying the protective effect of eugenol in rats with acute doxorubicin cardiotoxicity. Archives of Pharmacal Research. 2011;34(5):821–828. doi: 10.1007/s12272-011-0516-2. [DOI] [PubMed] [Google Scholar]
- 52.van Acker F. A. A., Boven E., Kramer K., Haenen G. R. M. M., Bast A., van der Vijgh W. J. F. Frederine, a new and promising protector against doxorubicin-induced cardiotoxicity. Clinical Cancer Research. 2001;7(5):1378–1384. [PubMed] [Google Scholar]
- 53.van Acker F. A. A., van Acker S. A. B. E., Kramer K., Haenen G. R. M. M., Bast A., van der Vijgh W. J. F. 7-Monohydroxyethylrutoside protects against chronic doxorubicin-induced cardiotoxicity when administered only once per week. Clinical Cancer Research. 2000;6(4):1337–1341. [PubMed] [Google Scholar]
- 54.van Acker S. A. B. E., Boven E., Kuiper K., et al. Monohydroxyethylrutoside, a dose-dependent cardioprotective agent, does not affect the antitumor activity of doxorubicin. Clinical Cancer Research. 1997;3(10):1747–1754. [PubMed] [Google Scholar]
- 55.Oyagbemi A. A., Saba A. B., Azeez O. I. Molecular targets of [6]-gingerol: its potential roles in cancer chemoprevention. Biofactors. 2010;36(3):169–178. doi: 10.1002/biof.78. [DOI] [PubMed] [Google Scholar]
- 56.El-Bakly W. M., Louka M. L., El-Halawany A. M., Schaalan M. F. 6-Gingerol ameliorated doxorubicin-induced cardiotoxicity: role of nuclear factor kappa B and protein glycation. Cancer Chemotherapy and Pharmacology. 2012;70(6):833–841. doi: 10.1007/s00280-012-1975-y. [DOI] [PubMed] [Google Scholar]
- 57.Nabavi S. F., Sureda A., Habtemariam S., Nabavi S. M. Ginsenoside Rd and ischemic stroke; a short review of literatures. Journal of Ginseng Research. 2015;39(4):299–303. doi: 10.1016/j.jgr.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang H., Yu P., Gou H., et al. Cardioprotective effects of 20(S)-ginsenoside Rh2 against doxorubicin-induced cardiotoxicity in vitro and in vivo . Evidence-Based Complementary and Alternative Medicine. 2012;2012:8. doi: 10.1155/2012/506214.506214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen C.-T., Wang Z.-H., Hsu C.-C., Lin H.-H., Chen J.-H. In vivo protective effects of diosgenin against doxorubicin-induced cardiotoxicity. Nutrients. 2015;7(6):4938–4954. doi: 10.3390/nu7064938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou F., Hao G., Zhang J., et al. Protective effect of 23-hydroxybetulinic acid on doxorubicin-induced cardiotoxicity: a correlation with the inhibition of carbonyl reductase-mediated metabolism. British Journal of Pharmacology. 2015;172(23):5690–5703. doi: 10.1111/bph.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Roohbakhsh A., Parhiz H., Soltani F., Rezaee R., Iranshahi M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sciences. 2015;124:64–74. doi: 10.1016/j.lfs.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 62.Abdel-Raheem I. T., Abdel-Ghany A. A. Hesperidin alleviates doxorubicin-induced cardiotoxicity in rats. Journal of the Egyptian National Cancer Institute. 2009;21(2):175–184. [PubMed] [Google Scholar]
- 63.Khedr N. F., Khalil R. M. Effect of hesperidin on mice bearing Ehrlich solid carcinoma maintained on doxorubicin. Tumor Biology. 2015;36(12):9267–9275. doi: 10.1007/s13277-015-3655-0. [DOI] [PubMed] [Google Scholar]
- 64.Trivedi P. P., Kushwaha S., Tripathi D. N., Jena G. B. Cardioprotective effects of hesperetin against doxorubicin-induced oxidative stress and DNA damage in rat. Cardiovascular Toxicology. 2011;11(3):215–225. doi: 10.1007/s12012-011-9114-2. [DOI] [PubMed] [Google Scholar]
- 65.Granados-Principal S., El-Azem N., Pamplona R., et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochemical Pharmacology. 2014;90(1):25–33. doi: 10.1016/j.bcp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Sun J., Sun G., Meng X., et al. Isorhamnetin protects against doxorubicin-induced cardiotoxicity in vivo and in vitro. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0064526.e64526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adwas A. A., Elkhoely A. A., Kabel A. M., Abdel-Rahman M. N., Eissa A. A. Anti-cancer and cardioprotective effects of indol-3-carbinol in doxorubicin-treated mice. Journal of Infection and Chemotherapy. 2016;22(1):36–43. doi: 10.1016/j.jiac.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Souček P., Kondrová E., Heřmánek J., et al. New model system for testing effects of flavonoids on doxorubicin-related formation of hydroxyl radicals. Anti-Cancer Drugs. 2011;22(2):176–184. doi: 10.1097/CAD.0b013e328341a17b. [DOI] [PubMed] [Google Scholar]
- 69.Xiao J., Sun G.-B., Sun B., et al. Kaempferol protects against doxorubicin-induced cardiotoxicity in vivo and in vitro . Toxicology. 2012;292(1):53–62. doi: 10.1016/j.tox.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 70.Karimi G., Ramezani M., Abdi A. Protective effects of lycopene and tomato extract against doxorubicin-induced cardiotoxicity. Phytotherapy Research. 2005;19(10):912–914. doi: 10.1002/ptr.1746. [DOI] [PubMed] [Google Scholar]
- 71.Yilmaz S., Atessahin A., Sahna E., Karahan I., Ozer S. Protective effect of lycopene on adriamycin-induced cardiotoxicity and nephrotoxicity. Toxicology. 2006;218(2-3):164–171. doi: 10.1016/j.tox.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 72.Ferreira A. L. A., Salvadori D. M. F., Nascimento M. C. M. O., et al. Tomato-oleoresin supplement prevents doxorubicin-induced cardiac myocyte oxidative DNA damage in rats. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2007;631(1):26–35. doi: 10.1016/j.mrgentox.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira A. L. A., Yeum K.-J., Matsubara L. S., et al. Doxorubicin as an antioxidant: maintenance of myocardial levels of lycopene under doxorubicin treatment. Free Radical Biology and Medicine. 2007;43(5):740–751. doi: 10.1016/j.freeradbiomed.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 74.Sahin K., Sahin N., Kucuk O. Lycopene and chemotherapy toxicity. Nutrition and Cancer. 2010;62(7):988–995. doi: 10.1080/01635581.2010.509838. [DOI] [PubMed] [Google Scholar]
- 75.Wang S.-Q., Han X.-Z., Li X., Ren D.-M., Wang X.-N., Lou H.-X. Flavonoids from Dracocephalum tanguticum and their cardioprotective effects against doxorubicin-induced toxicity in H9c2 cells. Bioorganic & Medicinal Chemistry Letters. 2010;20(22):6411–6415. doi: 10.1016/j.bmcl.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 76.Yao H., Shang Z., Wang P., et al. Protection of luteolin-7-O-glucoside against doxorubicin-induced injury through PTEN/Akt and ERK pathway in H9c2 cells. Cardiovascular Toxicology. 2016;16(2):101–110. doi: 10.1007/s12012-015-9317-z. [DOI] [PubMed] [Google Scholar]
- 77.Kok L. D. S., Wong Y. P., Wu T. W., Chan H. C., Kwok T. T., Fung K. P. Morin hydrate: a potential antioxidant in minimizing the free-radicals- mediated damage to cardiovascular cells by anti-tumor drugs. Life Sciences. 2000;67(1):91–99. doi: 10.1016/s0024-3205(00)00605-6. [DOI] [PubMed] [Google Scholar]
- 78.Arozal W., Suyatna F. D., Juniantito V., et al. The effects of mangiferin (Mangifera indica L) in doxorubicin-induced cardiotoxicity in rats. Drug Research. 2015;65(11):574–580. doi: 10.1055/s-0034-1394457. [DOI] [PubMed] [Google Scholar]
- 79.Agustini F. D., Arozal W., Louisa M., et al. Cardioprotection mechanism of mangiferin on doxorubicin-induced rats: focus on intracellular calcium regulation. Pharmaceutical Biology. doi: 10.3109/13880209.2015.1073750. In press. [DOI] [PubMed] [Google Scholar]
- 80.Park H.-S., Oh J. U.-H., Lee J. H., Lee Y.-J. Minor effects of the citrus flavonoids naringin, naringenin and quercetin, on the pharmacokinetics of doxorubicin in rats. Pharmazie. 2011;66(6):424–429. doi: 10.1691/ph.2011.0857. [DOI] [PubMed] [Google Scholar]
- 81.Arafa H. M., Abd-Ellah M. F., Hafez H. F. Abatement by naringenin of doxorubicin-induced cardiac toxicity in rats. Journal of the Egyptian National Cancer Institute. 2005;17(4):291–300. [PubMed] [Google Scholar]
- 82.Shiromwar S. S., Chidrawar V. R. Combined effects of p-coumaric acid and naringenin against doxorubicin-induced cardiotoxicity in rats. Pharmacognosy Research. 2011;3(3):214–219. doi: 10.4103/0974-8490.85012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han X., Pan J., Ren D., Cheng Y., Fan P., Lou H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food and Chemical Toxicology. 2008;46(9):3140–3146. doi: 10.1016/j.fct.2008.06.086. [DOI] [PubMed] [Google Scholar]
- 84.Han X., Ren D., Fan P., Shen T., Lou H. Protective effects of naringenin-7-O-glucoside on doxorubicin-induced apoptosis in H9C2 cells. European Journal of Pharmacology. 2008;581(1-2):47–53. doi: 10.1016/j.ejphar.2007.11.048. [DOI] [PubMed] [Google Scholar]
- 85.Han X., Gao S., Cheng Y., et al. Protective effect of naringenin-7-O-glucoside against oxidative stress induced by doxorubicin in H9c2 cardiomyocytes. BioScience Trends. 2012;6(1):19–25. doi: 10.5582/bst.2012.v6.1.19. [DOI] [PubMed] [Google Scholar]
- 86.Fu X., Kong L., Tang M., et al. Protective effect of ocotillol against doxorubicin-induced acute and chronic cardiac injury. Molecular Medicine Reports. 2014;9(1):360–364. doi: 10.3892/mmr.2013.1791. [DOI] [PubMed] [Google Scholar]
- 87.Andreadou I., Papaefthimiou M., Zira A., et al. Metabonomic identification of novel biomarkers in doxorubicin cardiotoxicity and protective effect of the natural antioxidant oleuropein. NMR in Biomedicine. 2009;22(6):585–592. doi: 10.1002/nbm.1370. [DOI] [PubMed] [Google Scholar]
- 88.Andreadou I., Sigala F., Iliodromitis E. K., et al. Acute doxorubicin cardiotoxicity is successfully treated with the phytochemical oleuropein through suppression of oxidative and nitrosative stress. Journal of Molecular and Cellular Cardiology. 2007;42(3):549–558. doi: 10.1016/j.yjmcc.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 89.Andreadou I., Mikros E., Ioannidis K., et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. Journal of Molecular and Cellular Cardiology. 2014;69:4–16. doi: 10.1016/j.yjmcc.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 90.Xu H., Han Y., Zhang M., Yan M., Gao C. Protective role of Osthole on myocardial cell apoptosis induced by doxorubicin in rats. International Journal of Clinical and Experimental Pathology. 2015;8(9):10816–10823. [PMC free article] [PubMed] [Google Scholar]
- 91.Abdel-Wahab M. H., El-Mahdy M. A., Abd-Ellah M. F., Helal G. K., Khalifa F., Hamada F. M. A. Influence of p-coumaric acid on doxorubicin-induced oxidative stress in rat's heart. Pharmacological Research. 2003;48(5):461–465. doi: 10.1016/s1043-6618(03)00214-7. [DOI] [PubMed] [Google Scholar]
- 92.Panda S., Kar A. Periplogenin-3-O- -D-Glucopyranosyl -(1 → 6)- -D-glucopyaranosyl- -(1 → 4) -D-cymaropyranoside, isolated from Aegle marmelos protects doxorubicin induced cardiovascular problems and hepatotoxicity in rats. Cardiovascular Therapeutics. 2009;27(2):108–116. doi: 10.1111/j.1755-5922.2009.00078.x. [DOI] [PubMed] [Google Scholar]
- 93.Kim D.-S., Woo E.-R., Chae S.-W., et al. Plantainoside D protects adriamycin-induced apoptosis in H9c2 cardiac muscle cells via the inhibition of ROS generation and NF-κB activation. Life Sciences. 2007;80(4):314–323. doi: 10.1016/j.lfs.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Khan M., Varadharaj S., Shobha J. C., et al. C-phycocyanin ameliorates doxorubicin-induced oxidative stress and apoptosis in adult rat cardiomyocytes. Journal of Cardiovascular Pharmacology. 2006;47(1):9–20. doi: 10.1097/01.fjc.0000191520.48404.27. [DOI] [PubMed] [Google Scholar]
- 95.Maimoona A., Naeem I., Saddiqe Z., Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. Journal of Ethnopharmacology. 2011;133(2):261–277. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 96.Boghdady N. A. E. Antioxidant and antiapoptotic effects of proanthocyanidin and ginkgo biloba extract against doxorubicin-induced cardiac injury in rats. Cell Biochemistry and Function. 2013;31(4):344–351. doi: 10.1002/cbf.2907. [DOI] [PubMed] [Google Scholar]
- 97.Ray S. D., Patel D., Wong V., Bagchi D. In vivo protection of DNA damage associated apoptotic and necrotic cell deaths during acetaminophen-induced nephrotoxicity, amiodarone-induced lung toxicity and doxorubicin-induced cardiotoxicity by a novel IH636 grape seed proanthocyanidin extract. Research Communications in Molecular Pathology and Pharmacology. 2000;107(1-2):137–166. [PubMed] [Google Scholar]
- 98.Bagchi D., Ray S. D., Bagchi M., Preuss H. G., Stohs S. J. Mechanistic pathways of antioxidant cytoprotection by a novel IH636 grape seed proanthocyanidin extract. Indian Journal of Experimental Biology. 2002;40(6):717–726. [PubMed] [Google Scholar]
- 99.Zhang X.-Y., Bai D.-C., Wu Y.-J., Li W.-G., Liu N.-F. Proanthocyanidin from grape seeds enhances anti-tumor effect of doxorubicin both in vitro and in vivo. Pharmazie. 2005;60(7):533–538. [PubMed] [Google Scholar]
- 100.Demirkaya E., Avci A., Kesik V., et al. Cardioprotective roles of aged garlic extract, grape seed proanthocyanidin, and hazelnut on doxorubicin-induced cardiotoxicity. Canadian Journal of Physiology and Pharmacology. 2009;87(8):633–640. doi: 10.1139/Y09-051. [DOI] [PubMed] [Google Scholar]
- 101.Cal C., Garban H., Jazirehi A., Yeh C., Mizutani Y., Bonavida B. Resveratrol and cancer: chemoprevention, apoptosis, and chemo-immunosensitizing activities. Current Medicinal Chemistry—Anti-Cancer Agents. 2003;3(2):77–93. doi: 10.2174/1568011033353443. [DOI] [PubMed] [Google Scholar]
- 102.Cao Z., Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. European Journal of Pharmacology. 2004;489(1-2):39–48. doi: 10.1016/j.ejphar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- 103.Tatlidede E., Şehirli Ö., Velioğlu-Öğün A., et al. Resveratrol treatment protects against doxorubicin-induced cardiotoxicity by alleviating oxidative damage. Free Radical Research. 2009;43(3):195–205. doi: 10.1080/10715760802673008. [DOI] [PubMed] [Google Scholar]
- 104.Wang G. Y., Wang Y. M., Zhang L. N., et al. Effect of resveratrol on heart function of rats with adriamycin-induced heart failure. Zhongguo Zhong Yao Za Zhi. 2007;32(15):1563–1565. [PubMed] [Google Scholar]
- 105.Brookins Danz E. D., Skramsted J., Henry N., Bennett J. A., Keller R. S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radical Biology and Medicine. 2009;46(12):1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 106.Zhang C., Feng Y., Qu S., et al. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovascular Research. 2011;90(3):538–545. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- 107.Xu X., Chen K., Kobayashi S., Timm D., Liang Q. Resveratrol attenuates doxorubicin-induced cardiomyocyte death via inhibition of p70 S6 kinase 1-mediated autophagy. Journal of Pharmacology and Experimental Therapeutics. 2012;341(1):183–195. doi: 10.1124/jpet.111.189589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang S., Song P., Zou M.-H. Inhibition of AMP-activated protein kinase α (AMPKα) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: role of p53 and SIRT1. The Journal of Biological Chemistry. 2012;287(11):8001–8012. doi: 10.1074/jbc.m111.315812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen C.-Y., Jang J.-H., Li M.-H., Surh Y.-J. Resveratrol upregulates heme oxygenase-1 expression via activation of NF-E2-related factor 2 in PC12 cells. Biochemical and Biophysical Research Communications. 2005;331(4):993–1000. doi: 10.1016/j.bbrc.2005.03.237. [DOI] [PubMed] [Google Scholar]
- 110.Gu J., Song Z.-P., Gui D.-M., Hu W., Chen Y.-G., Zhang D.-D. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in lymphoma nude mice by heme oxygenase-1 induction. Cardiovascular Toxicology. 2012;12(4):341–349. doi: 10.1007/s12012-012-9178-7. [DOI] [PubMed] [Google Scholar]
- 111.Pinarli F. A., Turan N. N., Pinarli F. G., et al. Resveratrol and adipose-derived mesenchymal stem cells are effective in the prevention and treatment of doxorubicin cardiotoxicity in rats. Pediatric Hematology and Oncology. 2013;30(3):226–238. doi: 10.3109/08880018.2012.762962. [DOI] [PubMed] [Google Scholar]
- 112.Dolinsky V. W., Rogan K. J., Sung M. M., et al. Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. American Journal of Physiology—Endocrinology and Metabolism. 2013;305(2):E243–E253. doi: 10.1152/ajpendo.00044.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Novelle M. G., Wahl D., Diéguez C., Bernier M., de Cabo R. Resveratrol supplementation: where are we now and where should we go? Ageing Research Reviews. 2015;21:15. doi: 10.1016/j.arr.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janeesh P. A., Abraham A. Robinin modulates doxorubicin-induced cardiac apoptosis by TGF-Î21 signaling pathway in Sprague Dawley rats. Biomedicine & Pharmacotherapy. 2014;68(8):989–998. doi: 10.1016/j.biopha.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 115.Kim D.-S., Kim H.-R., Woo E.-R., Hong S.-T., Chae H.-J., Chae S.-W. Inhibitory effects of rosmarinic acid on adriamycin-induced apoptosis in H9c2 cardiac muscle cells by inhibiting reactive oxygen species and the activations of c-Jun N-terminal kinase and extracellular signal-regulated kinase. Biochemical Pharmacology. 2005;70(7):1066–1078. doi: 10.1016/j.bcp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 116.Chlopčíková Š., Psotová J., Miketová P., Soušek J., Lichnovský V., Šimánek V. Chemoprotective effect of plant phenolics against anthracycline-induced toxicity on rat cardiomyocytes part II. Caffeic, chlorogenic and rosmarinic acids. Phytotherapy Research. 2004;18(5):408–413. doi: 10.1002/ptr.1461. [DOI] [PubMed] [Google Scholar]
- 117.Jiang B., Zhang L., Li M., et al. Salvianolic acids prevent acute doxorubicin cardiotoxicity in mice through suppression of oxidative stress. Food and Chemical Toxicology. 2008;46(5):1510–1515. doi: 10.1016/j.fct.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 118.Lin T.-J., Liu G.-T., Liu Y., Xu G.-Z. Protection by salvianolic acid A against adriamycin toxicity on rat heart mitochondria. Free Radical Biology and Medicine. 1992;12(5):347–351. doi: 10.1016/0891-5849(92)90083-S. [DOI] [PubMed] [Google Scholar]
- 119.Li L., Pan Q., Han W., Liu Z., Li L., Hu X. Schisandrin B prevents doxorubicin-induced cardiotoxicity via enhancing glutathione redox cycling. Clinical Cancer Research. 2007;13(22):6753–6760. doi: 10.1158/1078-0432.ccr-07-1579. [DOI] [PubMed] [Google Scholar]
- 120.Che F., Liu Y., Xu C. Prevention and treatment of doxorubicin-induced cardiotoxicity by dexrazoxane and schisandrin B in rabbits. International Journal of Toxicology. 2011;30(6):681–689. doi: 10.1177/1091581811415873. [DOI] [PubMed] [Google Scholar]
- 121.Thandavarayan R. A., Giridharan V. V., Arumugam S., et al. Schisandrin b prevents doxorubicin induced cardiac dysfunction by modulation of DNA damage, oxidative stress and inflammation through inhibition of mapk/p53 signaling. PLoS ONE. 2015;10(3) doi: 10.1371/journal.pone.0119214.e0119214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu Y., Liu Z., Sun J., et al. Schisandrin B prevents doxorubicin-induced chronic cardiotoxicity and enhances its anticancer activity in vivo. PLoS ONE. 2011;6(12) doi: 10.1371/journal.pone.0028335.e28335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang X.-L., Wang X., Xiong L.-L., et al. Salidroside improves doxorubicin-induced cardiac dysfunction by suppression of excessive oxidative stress and cardiomyocyte apoptosis. Journal of Cardiovascular Pharmacology. 2013;62(6):512–523. doi: 10.1097/FJC.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 124.Su S., Li Q., liu Y., et al. Sesamin ameliorates doxorubicin-induced cardiotoxicity: involvement of Sirt1 and Mn-SOD pathway. Toxicology Letters. 2014;224(2):257–263. doi: 10.1016/j.toxlet.2013.10.034. [DOI] [PubMed] [Google Scholar]
- 125.Chennuru A., Saleem M. T. S. Antioxidant, lipid lowering, and membrane stabilization effect of sesamol against doxorubicin-induced cardiomyopathy in experimental rats. BioMed Research International. 2013;2013:5. doi: 10.1155/2013/934239.934239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Raskovic A., Stilinovic N., Kolarovic J., Vasovic V., Vukmirovic S., Mikov M. The protective effects of silymarin against doxorubicin-induced cardiotoxicity and hepatotoxicity in rats. Molecules. 2011;16(10):8601–8613. doi: 10.3390/molecules16108601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Houghton C. A., Fassett R. G., Coombes J. S. Sulforaphane: translational research from laboratory bench to clinic. Nutrition Reviews. 2013;71(11):709–726. doi: 10.1111/nure.12060. [DOI] [PubMed] [Google Scholar]
- 128.Li B., Kim D. S., Yadav R. K., Kim H. R., Chae H. J. Sulforaphane prevents doxorubicin-induced oxidative stress and cell death in rat H9c2 cells. International Journal of Molecular Medicine. 2015;36(1):53–64. doi: 10.3892/ijmm.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Singh P., Sharma R., McElhanon K., Allen C. D., Megyesi J. K., Bene H. Sulforaphane protects the heart from doxorubicin-induced toxicity. Free Radical Biology and Medicine. 2015;86:90–101. doi: 10.1016/j.freeradbiomed.2015.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tian H.-L., Yu T., Xu N.-N., et al. A novel compound modified from tanshinone inhibits tumor growth in vivo via activation of the intrinsic apoptotic pathway. Cancer Letters. 2010;297(1):18–30. doi: 10.1016/j.canlet.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 131.Zhou G.-Y., Zhao B.-L., Hou J.-W., Ma G.-E., Xin W.-J. Protective effects of sodium tanshinone IIA sulphonate against adriamycin-induced lipid peroxidation in mice hearts in vivo and in vitro. Pharmacological Research. 1999;40(6):487–491. doi: 10.1006/phrs.1999.0545. [DOI] [PubMed] [Google Scholar]
- 132.Gao J., Yang G., Pi R., et al. Tanshinone IIA protects neonatal rat cardiomyocytes from adriamycin-induced apoptosis. Translational Research. 2008;151(2):79–87. doi: 10.1016/j.trsl.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 133.Jiang B., Zhang L., Wang Y., et al. Tanshinone IIA sodium sulfonate protects against cardiotoxicity induced by doxorubicin in vitro and in vivo. Food and Chemical Toxicology. 2009;47(7):1538–1544. doi: 10.1016/j.fct.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 134.Hong H.-J., Liu J.-C., Chen P.-Y., Chen J.-J., Chan P., Cheng T.-H. Tanshinone IIA prevents doxorubicin-induced cardiomyocyte apoptosis through Akt-dependent pathway. International Journal of Cardiology. 2012;157(2):174–179. doi: 10.1016/j.ijcard.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 135.Zhang S.-H., Wang W.-Q., Wang J.-L. Protective effect of tetrahydroxystilbene glucoside on cardiotoxicity induced by doxorubicin in vitro and in vivo. Acta Pharmacologica Sinica. 2009;30(11):1479–1487. doi: 10.1038/aps.2009.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woo C. C., Loo S. Y., Gee V., et al. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochemical Pharmacology. 2011;82(5):464–475. doi: 10.1016/j.bcp.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 137.Al-Shabanah O. A., Badary O. A., Nagi M. N., Al-Gharably N. M., Al-Rikabi A. C., Al-Bekairi A. M. Thymoquinone protects against doxorubicin-induced cardiotoxicity without compromising its antitumor activity. Journal of Experimental and Clinical Cancer Research. 1998;17(2):193–198. [PubMed] [Google Scholar]
- 138.Nagi M. N., Mansour M. A. Protective effect of thymoquinone against doxorubicin-induced cardiotoxicity in rats: a possible mechanism of protection. Pharmacological Research. 2000;41(3):283–289. doi: 10.1006/phrs.1999.0585. [DOI] [PubMed] [Google Scholar]
- 139.Brown R. K., Wilson G., Tucci M. A., Benghuzzi H. A. The effects of thymoquinone and Doxorubicin on leukemia and cardiomyocyte cell lines. Biomedical Sciences Instrumentation. 2014;50:391–396. [PubMed] [Google Scholar]
- 140.Xu M., Sheng L., Zhu X., Zeng S., Chi D., Zhang G.-J. Protective effect of tetrandrine on doxorubicin-induced cardiotoxicity in rats. Tumori. 2010;96(3):460–464. doi: 10.1177/030089161009600314. [DOI] [PubMed] [Google Scholar]
- 141.Dai C.-L., Xiong H.-Y., Tang L.-F., et al. Tetrandrine achieved plasma concentrations capable of reversing MDR in vitro and had no apparent effect on doxorubicin pharmacokinetics in mice. Cancer Chemotherapy and Pharmacology. 2007;60(5):741–750. doi: 10.1007/s00280-007-0420-0. [DOI] [PubMed] [Google Scholar]
- 142.Deng R. Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. Cardiovascular Drug Reviews. 2007;25(4):375–390. doi: 10.1111/j.1527-3466.2007.00023.x. [DOI] [PubMed] [Google Scholar]
- 143.Wang W.-C., Uen Y.-H., Chang M.-L., et al. Protective effect of guggulsterone against cardiomyocyte injury induced by doxorubicin in vitro. BMC Complementary and Alternative Medicine. 2012;12(1, article 138):10. doi: 10.1186/1472-6882-12-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chatterjee K., Zhang J., Tao R., Honbo N., Karliner J. S. Vincristine attenuates doxorubicin cardiotoxicity. Biochemical and Biophysical Research Communications. 2008;373(4):555–560. doi: 10.1016/j.bbrc.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xi L. Visnagin—a new protectant against doxorubicin cardiotoxicity? Inhibition of mitochondrial malate dehydrogenase 2 (MDH2) and beyond. Annals of Translational Medicine. 2016;4(4):p. 65. doi: 10.3978/j.issn.2305-5839.2015.10.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu Y., Asnani A., Zou L., et al. Visnagin protects against doxorubicin-induced cardiomyopathy through modulation of mitochondrial malate dehydrogenase. Science Translational Medicine. 2014;6(266) doi: 10.1126/scitranslmed.3010189.266ra170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Anand P., Kunnumakkara A. B., Newman R. A., Aggarwal B. B. Bioavailability of curcumin: problems and promises. Molecular Pharmaceutics. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 148.Airoldi M., Amadori D., Barni S., et al. Clinical activity and cardiac tolerability of non-pegylated liposomal doxorubicin in breast cancer: a synthetic review. Tumori. 2011;97(6):690–692. doi: 10.1700/1018.11082. [DOI] [PubMed] [Google Scholar]