Abstract
The present review summarizes the current advances in the biochemical and physiological aspects in the treatment of type 2 diabetes mellitus (DM2) with thiazolidinediones (TZDs). DM2 is a metabolic disorder characterized by hyperglycemia, triggering the abnormal activation of physiological pathways such as glucose autooxidation, polyol's pathway, formation of advance glycation end (AGE) products, and glycolysis, leading to the overproduction of reactive oxygen species (ROS) and proinflammatory cytokines, which are responsible for the micro- and macrovascular complications of the disease. The treatment of DM2 has been directed toward the reduction of hyperglycemia using different drugs such as insulin sensitizers, as the case of TZDs, which are able to lower blood glucose levels and circulating triglycerides by binding to the nuclear peroxisome proliferator-activated receptor gamma (PPARγ) as full agonists. When TZDs interact with PPARγ, the receptor regulates the transcription of different genes involved in glucose homeostasis, insulin resistance, and adipogenesis. However, TZDs exhibit some adverse effects such as fluid retention, weight gain, hepatotoxicity, plasma-volume expansion, hemodilution, edema, bone fractures, and congestive heart failure, which limits their use in DM2 patients.
1. Introduction
The treatment of type 2 diabetes mellitus (DM2) has been directed toward the reduction of hyperglycemia and glycosylated hemoglobin (HbA1c, ≤7%), in order to prevent cardiovascular and other long term risks [1, 2], specially by the usage of insulin sensitizers such as thiazolidinediones (TZDs) [1–5], an effective type of drugs for lowering blood glucose levels as circulating triglycerides [4, 6–9], with adverse effects such as adipocyte differentiation, fluid retention, weight gain, bone loss, and congestive heart failure [6–8, 10–13].
Clinically, pioglitazone is the only available TZD, even though its commercialization has been restricted to a few countries by the US Food and Drug Administration (FDA) since it may cause urinary bladder cancer. The other TZDs, rosiglitazone and troglitazone, show adverse profiles, so they are no longer available in the worldwide market; for example, rosiglitazone was associated with a significant increase in myocardial infarction, heart failure, and death from cardiovascular diseases, so the European Medicines Agency withdrew the approval for this medication in 2010, and the FDA restricted its prescription in the United States [3, 14–17].
In the present review, we summarize the current advances on the biochemical and physiological aspects involved in the treatment of DM2 with TZDs.
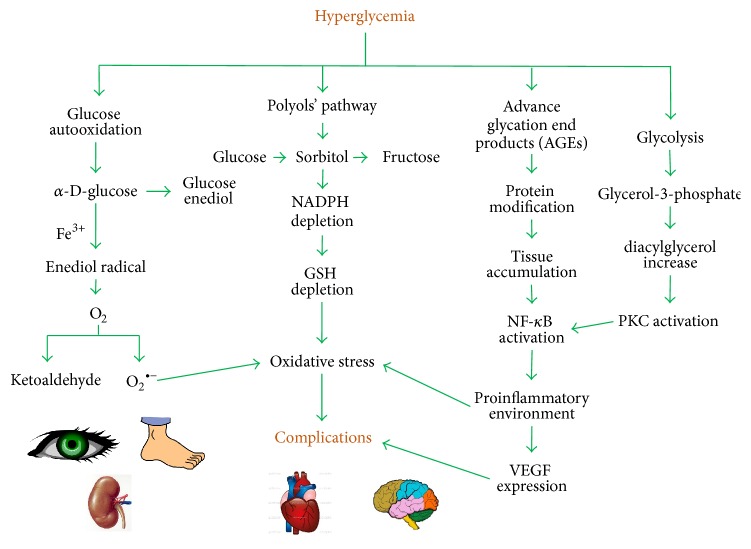
Type 2 Diabetes Mellitus (DM2). DM2 is a metabolic disorder characterized by hyperglycemia, which may be due to a defect in insulin secretion of pancreatic β cells, insulin resistance in peripheral tissues, and/or an excessive accumulation of triglycerides and fatty acid derivatives in skeletal muscles. This pathology remains a leading cause of cardiovascular disorders, such as microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (coronary, cerebrovascular, and peripheral vascular diseases) complications, mainly triggered by the abnormal activation of physiological pathways (Figure 1), and it is also associated with increased risk of cancer, psychiatric illness, cognitive decline, chronic liver disease, and development of arthritis [1–4, 18–24].
Figure 1.
The main pathways triggered by hyperglycemia include glucose autooxidation and constant activation of polyols' pathway and formation of advance glycation end products (AGEs) and excessive glycolysis. With the constant activation of these pathways, living cells and tissues are damaged, mainly by impairment of target protein function, increase in oxidative stress, and activation of signal transduction pathways, leading to the imbalance of normal physiological functions and therefore the development of diabetic complications.
The treatment of DM2 is directed toward the reduction of hyperglycemia and HbA1c (≤7%), in order to prevent cardiovascular and other long term risks (Table 1) [1, 2, 5]; there is a wide range of drugs which can be used in order to reduce glycemia, being notable mechanisms such as improving insulin secretion and reducing insulin resistance of peripheral tissues, as the case of TZDs [1–5], which are drugs targeting the peroxisome proliferator-activated receptor gamma (PPARγ).
Table 1.
Class of drugs used for the treatment of type 2 diabetes mellitus.
| Class | Compounds | Mechanism | Physiological action | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Biguanides | Metformin | Activates AMP kinase | ↓ hepatic glucose production | Low cost, no weight gain, no hypoglycemia, ↓ CVD events |
Gastrointestinal side effects, lactic acidosis, vitamin B12 deficiency |
|
| |||||
| Sulfonylureas | Glyburide Glibenclamide Glipizide Glimepiride | Closes K-ATP channels on β cell plasma membranes | ↑ insulin secretion | Low cost, ↓ microvascular risk |
Hypoglycemia, weight gain |
|
| |||||
| Meglitinides | Repaglinide Nateglinide |
Closes K-ATP channels on β cell plasma membranes | ↑ insulin secretion | ↓ postprandial glucose | High cost, hypoglycemia, weight gain, frequent dosing |
|
| |||||
| α-Glucosidase inhibitors | Acarbose Miglitol | Inhibits intestinal α-glucosidase | Slows intestinal carbohydrate digestion/absorption | Moderate cost, no hypoglycemia, ↓ postprandial glucose ↓ CVD events |
Modest HbA1c efficacy, gastrointestinal side effects, frequent dosing |
|
| |||||
| DPP4 inhibitors | Sitagliptin Vildagliptin Saxagliptin Linagliptin | Inhibits DPP4 activity, increasing postprandial incretin GLP-1 concentration | ↑ insulin secretion ↓ glucagon secretion |
No hypoglycemia | High cost, modest HbA1c efficacy, angioedema, pancreatitis |
|
| |||||
| GLP-1 receptor agonists | Exenatide Liraglutide |
Activates GLP-1 receptors | ↑ insulin secretion ↓ glucagon secretion ↑ satiety slows gastric emptying |
No hypoglycemia, weight loss | High cost, gastrointestinal side effects, acute pancreatitis |
|
| |||||
| Bile acid sequestrants | Colesevelam | Binds bile acids in intestinal tract, increasing hepatic bile acid production | ↓ hepatic glucose production ↑ incretin levels |
No hypoglycemia ↓ LDL |
High cost, modest HbA1c efficacy, constipation ↑ triglycerides |
|
| |||||
| Dopamine 2 agonists | Bromocriptine | Activates dopaminergic receptors | Modulates hypothalamic regulation of metabolism ↑ insulin sensitivity |
No hypoglycemia ↓ CVD events |
High cost, modest HbA1c efficacy, dizziness, syncope, nausea, fatigue |
|
| |||||
| Thiazolidinediones | Pioglitazone Rosiglitazone |
Activates the nuclear transcription factor PPARγ | ↑ insulin sensitivity | No hypoglycemia ↑ HDL ↓ triglycerides ↓ CVD events |
High cost, weight gain, edema/heart failure, bone fractures, bladder cancer (pioglitazone) ↑ LDL |
|
| |||||
| Insulin | Human NPH Human regular Lispro Aspart Glulisine Glargine Detemir Premixed |
Activates insulin receptors | ↑ glucose disposal ↓ hepatic glucose production |
Universally effective ↓ microvascular risk |
Variable cost, hypoglycemia, weight gain |
PPARs have emerged as links between lipids, metabolic diseases, and innate immunity as they regulate energy homeostasis [25, 26], and, specifically talking about PPARγ, this receptor is capable of regulating metabolic genes which will be further discussed and improves insulin sensitivity through glucose and lipid uptake and storage in peripheral tissues such as skeletal muscle, liver, and adipose tissue [26].
The relationship between PPARγ and DM2 has been established using both in vitro and in vivo experimentation, since it has been seen that the inactivation of PPARγ in mature adipocytes leads to insulin resistance, as mice lacking the receptor develop hyperlipidemia, hyperglycemia, and/or hyperinsulinemia [26, 27].
Thiazolidinediones (TZDs). TZDs are compounds used clinically as insulin sensitizers in order to lower blood glucose levels as circulating triglycerides [4, 6–9], but it has also been shown that these also exhibit other biological activities such as anti-inflammatory, antimalarial, antioxidant, cytotoxic, antimicrobial, and aldose reductase inhibitor activities, either in vitro or in animal models [10, 11]. TZDs act as peroxisome proliferator-activated receptors gamma (PPARγ) full agonists, which are also involved in the increase of adipocyte differentiation, fluid retention, weight gain, bone loss, and congestive heart failure. Having such diverse range of pharmacological activities, these molecules have a lot of potential uses, so different strategies have been originated to use them not only for the treatment of DM2 but also for other pathologies [2, 5, 6, 8–11].
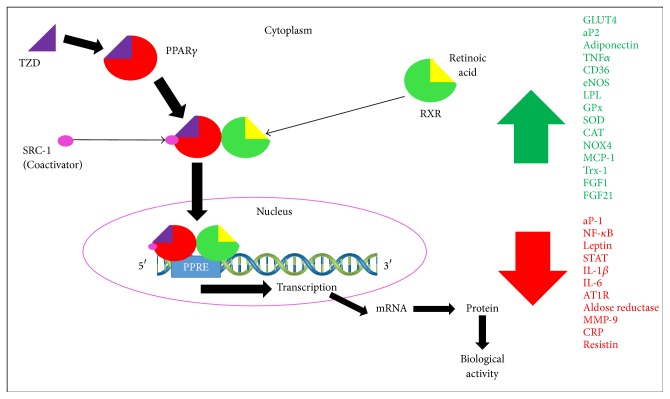
When TZDs interact with PPARγ, the receptor regulates the transcription of different genes, mainly those genes involved in glucose homeostasis and adipogenesis, specifically within white adipose tissue (WAT) by inducing brown adipose tissue- (BAT-) like features in it, a unique characteristic exclusive for PPARγ full agonists, such as rosiglitazone [13, 28].
However, despite their excellent potencies, the incidence of undesirable side effects has been linked to the use of TZDs, such as fluid retention, weight gain, hepatotoxicity (only for troglitazone), plasma-volume expansion, hemodilution, edema, and congestive heart failure; it is unknown if the toxicity is mediated by the activation of PPARγ or if it is due to some other mechanism unique to the TZD drug, since neither rosiglitazone nor pioglitazone has displayed the increased incidence of hepatic adverse events seen with troglitazone, suggesting that hepatotoxicity may not be a class effect of PPARγ agonists [6, 7, 29–32]; it has been proposed that the fluid adverse effects may be due to the regulation of PPARγ through an unknown mechanism involved in the enhancement of urinary vasopressin excretion response [33–35].
Peroxisome Proliferator-Activated Receptors (PPARs). PPARs are nuclear receptors that belong to the thyroid/retinoid nuclear family which act as ligand activated transcription factors. Three isoforms for these receptors have been described, α, β/δ, and γ, regulating tissue specific target genes involved in biological pathways for lipid and glucose homeostasis. PPARα is expressed predominantly in the liver, heart, and BAT, where it expresses genes involved in fatty acid oxidation; its exogenous ligands are the hypolipidemic fibrate drugs. PPAR β/δ is expressed in all kinds of tissues and has a crucial role in fatty acid oxidation, mainly in skeletal muscle, liver, and heart. PPARγ is highly expressed in both WAT and BAT, where it functions as a regulator of adipogenesis and as a modulator of lipid metabolism and insulin sensitivity. Activation of PPARγ is crucial for controlling gene networks involved in glucose homeostasis, including increasing the expression of glucose transporter type 4 (GLUT4), adiponectin, resistin, and tumor necrosis factor α (TNFα), which negatively influence insulin sensitivity. All three isotypes of PPARs, but mainly PPARγ, are ligand activated transcription factors implicated in the physiopathology of various diseases including DM2, obesity, dyslipidemia, atherosclerosis, neoplastic diseases, tumors, inflammatory conditions, and neurodegenerative diseases by forming obligate heterodimers with the retinoid X receptor (RXR), promoting the dissociation of corepressors, recruitment of coactivators, and the subsequent transcription of target genes [3, 6, 12, 13, 36–42].
So far, three isoforms for PPARγ have been described, γ1, γ2, and γ3, which arise as the product of different promoter usage. mRNA of PPARγ1 and PPARγ3 code for the same protein, while PPARγ2 codes for a different protein containing 30 NH2-terminal amino acids due to an alternative promoter usage and mRNA splicing, but no physiologically relevant differences in the function of these two isoforms have been determined [9, 29, 37, 38, 43]. PPARγ is organized in main functional domains. The amino terminal A/B domain contains a ligand dependent transactivation function (AF-1), while the C domain is the central DNA binding domain by containing two zinc finger-like structures and one α helical DNA binding motif; the E/F domain is the ligand binding domain (LBD), which contains a ligand dependent transactivation function (AF-2), which allows the receptors' conformational changes in the presence of the ligand, leading to the recruitment of coactivators, such as the steroid receptor coactivator type 1 (SRC-1), and the release of corepressors (Figure 2) [12, 44–47].
Figure 2.
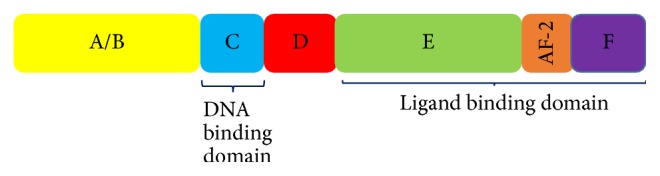
Main functional domains of nuclear PPARs. All three isotypes of PPAR have 4 main functional domains: A/B, which is the activation function 1 (AF-1); C, or DNA binding domain; D, which serves as a hinge between C and E/F; and E/F, which includes AF-2, a ligand binding dimerization transactivation domain.
The PPARγ LBD contains a large binding pocket that allows a wide range of ligands searching for their proper conformations in order to form ligand-receptor complexes. Natural ligands of PPARγ are fatty acids, while synthetic ligands can be classified as either full or partial agonists, such as TZDs, L-tyrosine analogs, and some nonsteroidal anti-inflammatory drugs [7, 8, 12, 30, 31, 39, 44, 48–50]. The structure of the LBD is comprised of 13 helices and 4 β sheets, with a total volume of approximately 1300 to 1400 Å. The cavity is Y shaped, consisting of an entrance which extends from the surface of the protein, and then it branches off into two arms, arm I, which extends toward the AF-2 (helix H12), and arm II, situated between helix H3 and the β sheet (Figure 3) [44, 45, 47, 51]. An important step during the activation process involves ligand-induced alteration of the conformation of H12 to an active position. The main model for ligand dependent activation of nuclear receptors proposes that agonists stabilize a specific conformation of the AF2 (H12) helix, which, along with helices 3 and 4, provides a suitable interface for binding a coactivator, acting as a molecular switch and creating a binding cleft on the receptor for the coactivator [8, 13, 45].
Figure 3.
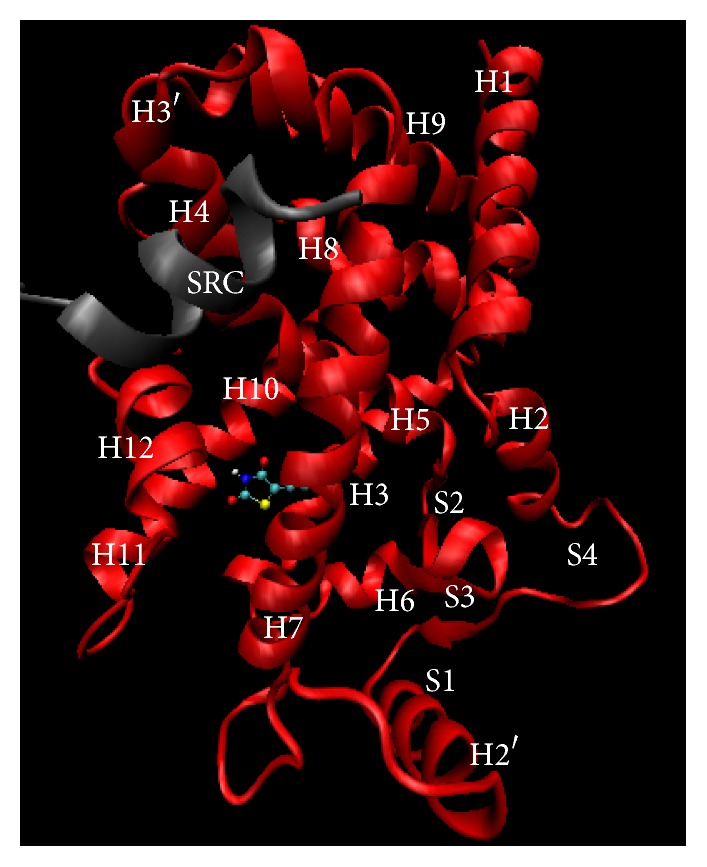
Crystal structure of PPARγ (PDB: 2PRG entry), cocrystalized with rosiglitazone (ligand) and steroid receptor coactivator 1 (SRC-1, coactivator). Figure constructed using Visual Molecular Dynamics (VMD) software.
TZDs are one of the most known PPARγ agonists. They share common features such as a hydrophilic head group, a central hydrophobic body, and a flexible linker to a cyclic tail. The hydrophilic head group can have a hydroxyl, carbonyl, or carboxyl oxygen atoms, allowing it to form H bonds with the key amino acid residues Tyr 473, (AF2, H12), His 449 (H11), His323 (H5), Ser289 (H3), and Gln286 (H3) of the LBD, generating an intermolecular network exclusive for full agonists. These H bond networks stabilize the receptor in the proper conformation; however, the acid head group of commercially available TZDs is prone to racemization under physiological conditions due to its stereogenic center at C5, and it has been demonstrated that only the (S)-enantiomers bind to the receptor, which suggests that approximately 50% of the active substance is inactive. Binding of these ligands results in conformational changes of the receptors that facilitate their interaction with coactivator proteins. The resulting complexes activate the transcription of specific target genes, resulting in the induction of signaling cascades that mediate the physiological effects of the ligands (Figure 4) [7–10, 12, 29–31, 39, 43, 44, 47–52].
Figure 4.
Mechanism of action of PPARγ when it is activated by its exogenous ligands thiazolidinediones (TZDs).
PPARγ and Inflammatory Diseases. Both PPARα and PPARγ isotypes participate in the regulation of inflammation processes. PPARα regulates primarily catabolic and PPARγ regulates primarily anabolic aspects of lipid metabolism [13, 29, 43].
Prostaglandin J2 (PGJ2) activation of PPARγ has been demonstrated to antagonize the activity of activator protein type 1 (aP-1) which enhances the angiogenic response seen in the diabetic microvascular complications [53] and the signal transducer and activator of transcription (STAT) protein which regulates the inflammation cascade [54] and the nuclear factor κB (NF-κB) which also regulates the inflammation cascade mainly in adipocytes [51]; these are known for their positive control on cytokine gene expression [6, 12, 36, 37, 39–41].
Diverse theories propose the molecular mechanisms by which PPARγ exhibits anti-inflammatory effects; among these theories, it can be mentioned that the expression of the receptor is upregulated by oxidized low density lipoproteins (LDL) in macrophages, which will in turn stimulate the expression of the cluster of differentiation 36 (CD36) scavenger receptor gene, resulting in a higher rate of oxidized LDL internalization, which, besides serving as a fatty acid transporter, is a novel biomarker for DM2 [55, 56], but it is also postulated that the expressions of inflammatory mediators such as tumor necrosis factor α (TNFα), interleukin 6 (IL-6), and matrix metallopeptidase 9 (MMP-9) are negatively controlled by PPARγ, which in turn takes importance for the development of atherosclerosis [50, 57]. It is also possible that PPARγ ligands act as anti-inflammatory and antioxidant agents through the inhibition of the transcription factor NF-κB/p65 and the expression of NADPH oxidase 4 (NOX4), thus reducing the levels of IL-6, C-reactive protein (CRP), and monocyte chemoattractant protein 1 (MCP-1), but it has also been postulated that PPARγ activation directly regulates the expression of endogenous antioxidants such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and thioredoxin (Trx-1), therefore playing a crucial role in cardiac redox balance [58–61].
Since diabetic vascular complications are partly mediated by inflammatory processes, the use of TZDs may contribute positively to patients' outcomes since insulin sensitizers suppress the inflammatory processes not only through lowering hyperglycemia but also by modulating the expression of key inflammatory biomarkers as can be seen in Figure 1. These effects may be potentiated when TZDs are used along with other drugs, for example, statins, fibrates, and inhibitors of renin-angiotensin-aldosterone system, which reduce the overall risk for DM2 [62, 63].
It also has been seen that TZDs may exert antiatherogenic effects on vessel wall cells, possibly by downregulating NF-κB inflammatory pathways [62, 63].
PPARγ and Metabolic Disorders. PPARγ is predominately expressed in adipose tissue, but it is also expressed in the lungs, placenta, heart, and leukocytes, where it regulates lipid, glucose, and insulin uptake into adipocytes, as it is responsible for regulating the expression of two markers of terminal adipocyte differentiation, adipocyte protein type 2 (aP-2) and phosphoenolpyruvate carboxykinase. PPARγ is also in charge of regulating the expression of the genes which code for lipoprotein lipase (LPL), increasing triglycerides lipolysis in very low density lipoproteins (VLDLs) and increasing high density lipoproteins (HDLs) [62], the fatty acid transport protein, which regulates fatty acid uptake, and fatty acid translocase, which enhances fatty acid uptake in adipocytes, as the repression of the expression of the ob gene for leptin, which increases the appetite (Figure 4), and this is concordant with the physiological effects of TZDs, such as lowering blood glucose levels and improving insulin sensitivity [3, 12, 13, 29, 32, 42, 57]. However, adipogenesis caused in response to treatment with TZDs has been linked mainly to the identification of two PPARγ-responsive members of the fibroblast growth factor family, fibroblast growth factor 1 (FGF1) and fibroblast growth factor 21 (FGF21), which act locally in visceral adipose tissue, promoting insulin sensitization, so that the activation of the receptor in the brain, rather than in adipose tissue, has a major role in TZD-induced weight gain [13].
Obesity rates and westernization of lifestyle lead to the increase of dysfunctional adipose tissue, which constantly activates NF-κB and delivers inflammatory cytokines such as TNFα, resistin, IL-6, and IL-1β, which, along with the impairment of reactive oxygen species (ROS) and water retention, are mainly present in a wide range of diseases like insulin resistance, DM2, hypertension, hyperlipidemia, and cardiovascular diseases (CVD), therefore maintaining a chronic inflammatory environment [1, 14, 58, 64–67].
Insulin resistance has been identified as a major contributor to the development of DM2 and metabolic syndrome since it increases the delivery of fatty acids (FA) into the circulation, which modulate the ability of the heart to use glucose as a source of energy [59, 66, 68–71] leading to a cellular stress characterized by an excessive ROS production, impaired state of nitric oxide (NO) vasorelaxation, production of inflammatory cytokines, mitochondrial dysfunction, increased advanced glycation end products (AGEs), and dysfunction of endothelial progenitor cells, as the inhibition of the antiatherogenic adipokine adiponectin [15, 59, 64, 66, 68–72].
PPARγ is highly expressed in the vascular system, where it is involved in the repression or expression of certain genes such as angiotensin type 1 receptor (AT1R), which can prevent or ameliorate endothelial dysfunction and atherosclerosis [3, 15, 16, 60, 67, 73, 74]. In accordance with this, it has been seen that, in animal models, repression of the expression of PPARγ promotes cardiomyopathy, lipid deposition, arrhythmias, hypertrophy, and increased expression of cardiac inflammatory markers [61, 66, 70, 71]. It has also been shown that adiponectin increases through PPAR-responsive element in the promoter of adipocytes, playing an essential role for the vascular protective effects of PPARγ agonists, as the case where diabetic db/db mice treated with rosiglitazone stimulated the release of adiponectin, which activated AMP activated protein kinase (AMPK/eNOS) and protein kinase A (cAMP/PKA) pathways in the aorta, consequently leading to the reduction of oxidative stress and the enhancement of NO bioavailability, improving endothelial function [15, 58, 75]. It has also been seen either in clinical practice or in animal models that the continuous treatment with TZDs tends to attenuate the progression of carotid artery intima/media thickness, reducing the onset of restenosis, mainly due to the inhibition of smooth cell migration, the increased apoptosis in vascular smooth cells, and the prevention of insulin driven atherosclerosis by switching myocardial substrate metabolism toward glucose [14, 70, 73, 74, 76].
PPARγ and Cardiovascular Diseases. CVD and DM2 are intimately linked as they share some pathophysiological features [76], for example, the development of atherosclerosis, which may lead to myocardial infarction, coronary heart disease, peripheral artery disease, and critical limb ischemia [77].
It has been previously found that CVD increase the rate of cardiovascular death nearly fivefold in subjects with diabetes, mainly due to myocardial infarction. Also, the relevance of diabetes for the development of atherosclerosis has been made clear through the observation that a majority of patients with coronary heart disease have insulin resistance or have been diagnosed with frank diabetes [77, 78].
The use of TZDs has been controversial in terms of prevention of CVD, since it has been shown that these types of drugs induce and maintain the regression of carotid intima-media thickness in patients with type 2 diabetes, as they have been related to anti-inflammatory and antiproliferative activities in smooth muscle cells, inhibiting the atheromatous plaque progression [13, 77]. Another substantial side effect of TZDs is the fluid retention with associated peripheral edema by the alteration of sodium and water reabsorption in the distal collecting ducts of the kidney, which increases the risk for adverse cardiovascular events, such as congestive heart failure [13].
PPARγ and Bone Fractures. The use of TZDs has been related to bone fractures, especially rosiglitazone, which exhibited an increased risk of fractures in comparison with patients receiving metformin or glyburide [28]. On the other hand, pioglitazone exhibited an increased incidence of distal extremity fractures on the PRO-active trial [3]. These fractures may be due to the expression of PPARγ in bone narrow stromal cells, osteoblasts, and osteoclasts [28], which promotes an alteration on the mesenchymal stem cell maturation, leading to a shift from an osteoblastic lineage to the adipogenic lineage, which turns into an accumulation of ROS and apoptosis of the cells in the osteogenic lineage, thereby decreasing bone formation [13, 28].
2. Conclusions
A great number of studies have shown that the activation of PPARγ is implicated in the development of undesirable adverse effects such as fluid retention, weight gain, hepatotoxicity, plasma-volume expansion, hemodilution, edema, bone fractures, and congestive heart failure, but it is also involved in the prevention of developing atherosclerosis, even though there are certainly another great number of studies which can demonstrate the opposite and also confirm that some agonists of the receptor, specifically rosiglitazone, may increase CVD risk. We believe that the associated risk of CVD during TZDs therapy may be related to different transcription patterns in the PPARγ activation due to different ligands, since troglitazone and pioglitazone do not increase CVD risk, as pioglitazone may cause bladder cancer, or rosiglitazone or troglitazone or hepatotoxicity, which is directly correlated to the use of troglitazone or the other TZDs, as they interact in different ways with the receptor and therefore induce different conformations and different interactions with coactivators/corepressors, as different interactions with the responsive element, therefore triggering the transcription of diverse genes. According to these, it would be important to investigate the different conformations of the receptor in the presence of different ligands, either endogenous or exogenous, so that it would be possible to predict which coactivator or corepressor is more susceptible to be recruited, as the possible biochemical and physiological effects of each one. By doing this prediction, it would be possible to design better ligands derived from TZDs with less adverse effects, and it would also be possible to use them for the treatment of other diseases such as cancer, metabolic syndrome, hypertension, obesity, and even CVD.
Acknowledgments
The authors are grateful to Secretaría de Investigación y Posgrado, IPN (SIP20161383/SIP20160675), CONACyT (I010/0532/2014), SIBE (COFFA), and EDI (SIP)/IPN, México.
Disclosure
The authors alone are responsible for the content and writing of the paper.
Competing Interests
The authors have no conflict of interests in the use of materials or techniques mentioned in this review.
References
- 1.Inzucchi S. E., Bergenstal R. M., Buse J. B., et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35(6):1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qaseem A., Humphrey L. L., Sweet D. E., Starkey M., Shekelle P. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American college of physicians. Annals of Internal Medicine. 2012;156(3):218–231. doi: 10.7326/0003-4819-156-3-201202070-00011. [DOI] [PubMed] [Google Scholar]
- 3.Grygiel-Górniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications—a review. Nutrition Journal. 2014;13, article 17:10. doi: 10.1186/1475-2891-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alex S., Lange K., Amolo T., et al. Short-chain fatty acids stimulate angiopoietin-like 4 synthesis in human colon adenocarcinoma cells by activating peroxisome proliferator-activated receptor γ . Molecular and Cellular Biology. 2013;33(7):1303–1316. doi: 10.1128/mcb.00858-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onitilo A. A., Engel J. M., Glurich I., Stankowski R. V., Williams G. M., Doi S. A. Diabetes and cáncer II: role of diabetes medications and influence of shared risk factors. Cancer Causes & Control. 2012;23(7):991–1008. doi: 10.1007/s10552-012-9971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petersen R. K., Christensen K. B., Assimopoulou A. N., et al. Pharmacophore-driven identification of PPARγ agonists from natural sources. Journal of Computer-Aided Molecular Design. 2011;25(2):107–116. doi: 10.1007/s10822-010-9398-5. [DOI] [PubMed] [Google Scholar]
- 7.Gim H. J., Li H., Lee E., Ryu J.-H., Jeon R. Design and synthesis of alkoxyindolyl-3-acetic acid analogs as peroxisome proliferator-activated receptor-γ/δ agonists. Bioorganic & Medicinal Chemistry Letters. 2013;23(2):513–517. doi: 10.1016/j.bmcl.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 8.Dos Santos J. C., Bernardes A., Giampietro L., et al. Different binding and recognition modes of GL479, a dual agonist of Peroxisome Proliferator-Activated Receptor α/γ . Journal of Structural Biology. 2015;191(3):332–340. doi: 10.1016/j.jsb.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Monsalve F. A., Pyarasani R. D., Delgado-Lopez F., Moore-Carrasco R. Peroxisome proliferator-activated receptor targets for the treatment of metabolic diseases. Mediators of Inflammation. 2013;2013:18. doi: 10.1155/2013/549627.549627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avupati V. R., Yejella R. P., Akula A., et al. Synthesis, characterization and biological evaluation of some novel 2,4-thiazolidinediones as potential cytotoxic, antimicrobial and antihyperglycemic agents. Bioorganic & Medicinal Chemistry Letters. 2012;22(20):6442–6450. doi: 10.1016/j.bmcl.2012.08.052. [DOI] [PubMed] [Google Scholar]
- 11.Mandard S., Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Research. 2013;2013:23. doi: 10.1155/2013/613864.613864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Youssef J. A., Badr M. Z. Peroxisome Proliferator-Activated Receptors. chapter 3. Springer; 2013. Peroxisome proliferator-activated receptors: discovery and recent advances. [DOI] [Google Scholar]
- 13.Ahmadian M., Suh J. M., Hah N., et al. PPARγ signaling and metabolism: the good, the bad and the future. Nature Medicine. 2013;19(5):557–566. doi: 10.1038/nm.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.García-García H. M., Garg S., Brugaletta S., et al. Evaluation of in-stent restenosis in the APPROACH trial (assessment on the prevention of progression by Rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history) The International Journal of Cardiovascular Imaging. 2012;28(3):455–465. doi: 10.1007/s10554-011-9836-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balakumar P., Kathuria S. Submaximal PPARγ activation and endothelial dysfunction: new perspectives for the management of cardiovascular disorders. British Journal of Pharmacology. 2012;166(7):1981–1992. doi: 10.1111/j.1476-5381.2012.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atamer Y., Atamer A., Can A. S., et al. Effects of rosiglitazone on serum paraoxonase activity and metabolic parameters in patients with type 2 diabetes mellitus. Brazilian Journal of Medical and Biological Research. 2013;46(6):528–532. doi: 10.1590/1414-431X20132818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Y., Ma D., Xu W., Shao S., Yu X. Effect and cardiovascular safety of adding rosiglitazone to insulin therapy in type 2 diabetes: a meta-analysis. Journal of Diabetes Investigation. 2015;6(1):78–86. doi: 10.1111/jdi.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmazar M. M., El-Abhar H. S., Schaalan M. F., Farag N. A. Phytol/phytanic acid and insulin resistance: potential role of phytanic acid proven by docking simulation and modulation of biochemical alterations. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0045638.e45638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dixit V. A., Bharatam P. V. SAR and computer-aided drug design approaches in the discovery of peroxisome proliferator-activated receptor γ activators: a perspective. Journal of Computational Medicine. 2013;2013:38. doi: 10.1155/2013/406049.406049 [DOI] [Google Scholar]
- 20.Takahashi M. Glycoscience: Biology and Medicine. Berlin, Germany: Springer; 2015. Glycation of proteins; pp. 1339–1345. [DOI] [Google Scholar]
- 21.Babizhayev M. A., Strokov I. A., Nosikov V. V., et al. The role of oxidative stress in diabetic neuropathy: generation of free radical species in the glycation reaction and gene polymorphisms encoding antioxidant enzymes to genetic susceptibility to diabetic neuropathy in population of type I diabetic patients. Cell Biochemistry and Biophysics. 2015;71(3):1425–1443. doi: 10.1007/s12013-014-0365-y. [DOI] [PubMed] [Google Scholar]
- 22.Soltesova-Prnova M., Ballekova J., Gajdosikova A., Gajdosik A., Stefek M. A novel carboxymethylated mercaptotriazinoindole inhibitor of aldose reductase interferes with the polyol pathway in streptozotocin-induced diabetic rats. Physiological Research. 2015;64(4):587–591. doi: 10.33549/physiolres.933034. [DOI] [PubMed] [Google Scholar]
- 23.Tiwari A. K., Kumar D. A., Sweeya P. S. R., et al. Vegetables' juice influences polyol pathway by multiple mechanisms in favour of reducing development of oxidative stress and resultant diabetic complications. Pharmacognosy Magazine. 2014;10(38):383–391. doi: 10.4103/0973-1296.133290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juskova K. M., Soltesova P. M., Stefek M. A novel zwitterionic inhibitor of aldose reductase interferes with polyol pathway in an ex vivo and in vivo models of diabetic complications. Pharmazie. 2014;69(10):747–751. [PubMed] [Google Scholar]
- 25.Wahli W., Michalik L. PPARs at the crossroads of lipid signaling and inflammation. Trends in Endocrinology & Metabolism. 2012;23(7):351–363. doi: 10.1016/j.tem.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Choi S.-S., Park J., Choi J. H. Revisiting PPARγ as a target for the treatment of metabolic disorders. BMB Reports. 2014;47(11):599–608. doi: 10.5483/bmbrep.2014.47.11.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lamounier R. N., Coimbra C. N., White P., et al. Apoptosis rate and transcriptional response of pancreatic islets exposed to the PPAR gamma agonist pioglitazone. Diabetology & Metabolic Syndrome. 2013;5(1):1–10. doi: 10.1186/1758-5996-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bilezikian J. P., Josse R. G., Eastell R., et al. Rosiglitazone decreases bone mineral density and increases bone turnover in postmenopausal women with type 2 diabetes mellitus. The Journal of Clinical Endocrinology & Metabolism. 2013;98(4):1519–1528. doi: 10.1210/jc.2012-4018. [DOI] [PubMed] [Google Scholar]
- 29.Verma V. A., Halu B., Kulkarni V. R. Understanding the antihyperglycemic agents of thiazolidinediones analogues using quantitative structure activity relationship (QSAR) models. World Journal of Pharmaceutical Sciences. 2014;3(3):2212–2221. [Google Scholar]
- 30.Cho M.-C., Lee D.-H., Kim E. J., et al. Novel PPARγ partial agonists with weak activity and no cytotoxicity, identified by a simple PPARγ ligand screening system. Molecular and Cellular Biochemistry. 2011;358(1-2):75–83. doi: 10.1007/s11010-011-0923-1. [DOI] [PubMed] [Google Scholar]
- 31.Wang L., Waltenberger B., Pferschy-Wenzig E.-M., et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review. Biochemical Pharmacology. 2014;92(1):73–89. doi: 10.1016/j.bcp.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.FCariou B., Hanf R., Lambert-Porcheron S., et al. Dual peroxisome proliferator-activated receptor α/δ agonist GFT505 improves hepatic and peripheral insulin sensitivity in abdominally obese subjects. Diabetes Care. 2013;36(10):2923–2930. doi: 10.2337/dc12-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L., Panasiuk A., Downton M., et al. Systemic PPARγ deletion causes severe disturbance in fluid homeostasis in mice. Physiological Genomics. 2015;47(11):541–547. doi: 10.1152/physiolgenomics.00066.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou L., Liu G., Jia Z., et al. Increased susceptibility of db/db mice to rosiglitazone-induced plasma volume expansion: role of dysregulation of renal water transporters. American Journal of Physiology—Renal Physiology. 2013;305(10):F1491–F1497. doi: 10.1152/ajprenal.00004.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horita S., Nakamura M., Satoh N., Suzuki M., Seki G. Thiazolidinediones and edema: recent advances in the pathogenesis of thiazolidinediones-induced renal sodium retention. PPAR Research. 2015;2015:7. doi: 10.1155/2015/646423.646423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hidalgo-Figueroa S., Ramírez-Espinosa J. J., Estrada-Soto S., et al. Discovery of thiazolidine-2,4-dione/biphenylcarbonitrile hybrid as dual ppar α/γ modulator with antidiabetic effect: in vitro, in silico and in vivo approaches. Chemical Biology & Drug Design. 2013;81(4):474–483. doi: 10.1111/cbdd.12102. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H., Ding C. Z., Lai Z., et al. Synthesis and biological evaluation of novel pyrrolidine acid analogs as potent dual PPARα/γ agonists. Bioorganic & Medicinal Chemistry Letters. 2015;25(6):1196–1205. doi: 10.1016/j.bmcl.2015.01.066. [DOI] [PubMed] [Google Scholar]
- 38.Ye J. Mechanisms of insulin resistance in obesity. Frontiers of Medicine. 2013;7(1):14–24. doi: 10.1007/s11684-013-0262-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carrieri A., Giudici M., Parente M., et al. Molecular determinants for nuclear receptors selectivity: chemometric analysis, dockings and site-directed mutagenesis of dual peroxisome proliferator-activated receptors α/γ agonists. European Journal of Medicinal Chemistry. 2013;63:321–332. doi: 10.1016/j.ejmech.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 40.Laghezza A., Pochetti G., Lavecchia A., et al. New 2-(aryloxy)-3-phenylpropanoic acids as peroxisome proliferator-activated receptor α/γ dual agonists able to upregulate mitochondrial carnitine shuttle system gene expression. Journal of Medicinal Chemistry. 2013;56(1):60–72. doi: 10.1021/jm301018z. [DOI] [PubMed] [Google Scholar]
- 41.García-Rojas P., Antaramian A., González-Dávalos L., et al. Induction of peroxisomal proliferator-activated receptor γ and peroxisomal proliferator-activated receptor γ coactivator 1 by unsaturated fatty acids, retinoic acid, and carotenoids in preadipocytes obtained from bovine white adipose tissue. Journal of Animal Science. 2010;88(5):1801–1808. doi: 10.2527/jas.2009-2579. [DOI] [PubMed] [Google Scholar]
- 42.Lodhi I. J., Yin L., Jensen-Urstad A. P. L., et al. Inhibiting adipose tissue lipogenesis reprograms thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metabolism. 2012;16(2):189–201. doi: 10.1016/j.cmet.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Evans R. M., Mangelsdorf D. J. Nuclear receptors, RXR, and the big bang. Cell. 2014;157(1):255–266. doi: 10.1016/j.cell.2014.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L., Ren X.-M., Wan B., Guo L.-H. Structure-dependent binding and activation of perfluorinated compounds on human peroxisome proliferator-activated receptor γ . Toxicology and Applied Pharmacology. 2014;279(3):275–283. doi: 10.1016/j.taap.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Hughes T. S., Chalmers M. J., Novick S., et al. Ligand and receptor dynamics contribute to the mechanism of graded PPARγ agonism. Structure. 2012;20(1):139–150. doi: 10.1016/j.str.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burston J., Kendall D. Peroxisome proliferator-activated receptors and inflammation. In: Abood M. E., Sorensen R. G., Stella N., editors. endoCANNABINOIDS: Actions at Non-CB1/CB2 Cannabinoid Receptors. Vol. 24. 2012. pp. 221–233. (The Receptors). [DOI] [Google Scholar]
- 47.Kroker A. J., Bruning J. B. Review of the structural and dynamic mechanisms of PPARγ partial agonism. PPAR Research. 2015;2015:15. doi: 10.1155/2015/816856.816856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao B., Yin J., Park M., et al. Design and synthesis of marine fungal phthalide derivatives as PPAR-γ agonists. Bioorganic and Medicinal Chemistry. 2012;20(16):4954–4961. doi: 10.1016/j.bmc.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 49.Georgiadi A., Kersten S. Mechanisms of gene regulation by fatty acids. Advances in Nutrition. 2012;3(2):127–134. doi: 10.3945/an.111.001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gim H. J., Cheon Y.-J., Ryu J.-H., Jeon R. Design and synthesis of benzoxazole containing indole analogs as peroxisome proliferator-activated receptor-γ/δ dual agonists. Bioorganic & Medicinal Chemistry Letters. 2011;21(10):3057–3061. doi: 10.1016/j.bmcl.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 51.Moras D., Billas I. M. L., Rochel N., Klaholz B. P. Structure-function relationships in nuclear receptors: the facts. Trends in Biochemical Sciences. 2015;40(6):287–290. doi: 10.1016/j.tibs.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 52.Ketsawatsomkron P., Sigmund C. D. Molecular mechanisms regulating vascular tone by peroxisome proliferator activated receptor gamma. Current Opinion in Nephrology and Hypertension. 2015;24(2):123–130. doi: 10.1097/MNH.0000000000000103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jia J., Ye T., Cui P., Hua Q., Zeng H., Zhao D. AP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation. Microvascular Research. 2016;105:103–108. doi: 10.1016/j.mvr.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Y., Ge X., Dou X., et al. Protein Inhibitor of Activated STAT 1 (PIAS1) protects against obesity-induced insulin resistance by inhibiting inflammation cascade in adipose tissue. Diabetes. 2015;64(12):4061–4074. doi: 10.2337/db15-0278. [DOI] [PubMed] [Google Scholar]
- 55.Alkhatatbeh M. J., Enjeti A. K., Acharya S., Thorne R. F., Lincz L. F. The origin of circulating CD36 in type 2 diabetes. Nutrition & Diabetes. 2013;3, article e59 doi: 10.1038/nutd.2013.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gautam S., Gupta A. C., Monisha B. CD36 gene variants in early prediction of type 2 diabetes mellitus. Genetic Testing and Molecular Biomarkers. 2015;19(3):144–149. doi: 10.1089/gtmb.2014.0265. [DOI] [PubMed] [Google Scholar]
- 57.Bozaykut P., Karademir B., Yazgan B., et al. Effects of vitamin e on peroxisome proliferator-activated receptor γ and nuclear factor-erythroid 2-related factor 2 in hypercholesterolemia-induced atherosclerosis. Free Radical Biology and Medicine. 2014;70:174–181. doi: 10.1016/j.freeradbiomed.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 58.Kim T., Yang Q. Peroxisome-proliferator-activated receptors regulate redox signaling in the cardiovascular system. World Journal of Cardiology. 2013;5(6):164–174. doi: 10.4330/wjc.v5.i6.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ali K. M., Wonnerth A., Huber K., Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol—current therapies and future opportunities. British Journal of Pharmacology. 2012;167(6):1177–1194. doi: 10.1111/j.1476-5381.2012.02081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Auclair M., Vigouroux C., Boccara F., et al. Peroxisome proliferator-activated receptor-γ mutations responsible for lipodystrophy with severe hypertension activate the cellular renin-angiotensin system. Arteriosclerosis, Thrombosis & Vascular Biology. 2013;33(4):829–838. doi: 10.1161/atvbaha.112.300962. [DOI] [PubMed] [Google Scholar]
- 61.He H., Tao H., Xiong H., et al. Rosiglitazone causes cardiotoxicity via peroxisome proliferator-activated receptor &-independent mitochondrial oxidative stress in mouse hearts. Toxicological Sciences. 2014;138(2):468–481. doi: 10.1093/toxsci/kfu015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scheen A. J., Esser N., Paquot N. Antidiabetic agents: potential anti-inflammatory activity beyond glucose control. Diabetes & Metabolism. 2015;41(3):183–194. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Esser N., Paquot N., Scheen A. J. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opinion on Investigational Drugs. 2015;24(3):283–307. doi: 10.1517/13543784.2015.974804. [DOI] [PubMed] [Google Scholar]
- 64.Fuentes E., Guzmán-Jofre L., Moore-Carrasco R., Palomo I. Role of PPARs in inflammatory processes associated with metabolic syndrome (Review) Molecular Medicine Reports. 2013;8(6):1611–1616. doi: 10.3892/mmr.2013.17143. [DOI] [PubMed] [Google Scholar]
- 65.Bays H. E. Adiposopathy: is ‘sick fat’ a cardiovascular disease? Journal of the American College of Cardiology. 2011;57(25):2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 66.Abel E. D., O'Shea K. M., Ramasamy R. Insulin resistance: metabolic mechanisms and consequences in the heart. Arteriosclerosis, Thrombosis & Vascular Biology. 2012;32(9):2068–2076. doi: 10.1161/atvbaha.111.241984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheung B. M. Y., Li C. Diabetes and hypertension: is there a common metabolic pathway? Current Atherosclerosis Reports. 2012;14(2):160–166. doi: 10.1007/s11883-012-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung U. J., Choi M.-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. International Journal of Molecular Sciences. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang J. V., Greyson C. R., Schwartz G. G. PPAR-& as a therapeutic target in cardiovascular disease: evidence and uncertainty. Journal of Lipid Research. 2012;53(9):1738–1754. doi: 10.1194/jlr.r024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang T. H.-W., Roufogalis B. D. Healing the diabetic heart: modulation of cardiometabolic syndrome through peroxisome proliferator activated receptors (PPARs) Current Molecular Pharmacology. 2012;5(2):241–247. doi: 10.2174/1874467211205020241. [DOI] [PubMed] [Google Scholar]
- 71.Drosatos K., Khan R. S., Trent C. M., et al. Peroxisome proliferator-activated receptor-γ activation prevents sepsis-related cardiac dysfunction and mortality in mice. Circulation: Heart Failure. 2013;6(3):550–562. doi: 10.1161/circheartfailure.112.000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Balakumar P., Mahadevan N. Interplay between statins and PPARs in improving cardiovascular outcomes: a double-edged sword? British Journal of Pharmacology. 2012;165(2):373–379. doi: 10.1111/j.1476-5381.2011.01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giaginis C., Klonaris C., Katsargyris A., Kouraklis G., Spiliopoulou C., Theocharis S. Correlation of peroxisome proliferator-activated receptor-γ (PPAR-γ) and retinoid x receptor-α (RXR-α) expression with clinical risk factors in patients with advanced carotid atherosclerosis. Medical Science Monitor. 2011;17(7):CR381–CR391. doi: 10.12659/MSM.881849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung U. J., Torrejon C., Chang C. L., Hamai H., Worgall T. S., Deckelbaum R. J. Fatty acids regulate endothelial lipase and inflammatory markers in macrophages and in mouse aorta: a role for PPARγ . Arteriosclerosis, Thrombosis & Vascular Biology. 2012;32(12):2929–2937. doi: 10.1161/atvbaha.112.300188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hulsmans M., Geeraert B., Arnould T., Tsatsanis C., Holvoet P. PPAR agonist-induced reduction of Mcp1 in atherosclerotic plaques of obese, insulin-resistant mice depends on adiponectin-induced Irak3 expression. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0062253.e62253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y., Di Lorenzo A., Jiang W., Cantalupo A., Sessa W. C., Giordano F. J. Hypoxia-inducible factor-1α in vascular smooth muscle regulates blood pressure homeostasis through a peroxisome proliferator-activated receptor-γ-angiotensin II receptor type 1 axis. Hypertension. 2013;62(3):634–640. doi: 10.1161/hypertensionaha.111.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beckman J. A., Paneni F., Cosentino F., Creager M. A. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. European Heart Journal. 2013;34(31):2444–2456. doi: 10.1093/eurheartj/eht142. [DOI] [PubMed] [Google Scholar]
- 78.Araújo C. V., Estato V., Tibiriçá E., Bozza P. T., Castro-Faria-Neto H. C., Silva A. R. PPAR gamma activation protects the brain against microvascular dysfunction in sepsis. Microvascular Research. 2012;84(2):218–221. doi: 10.1016/j.mvr.2012.05.006. [DOI] [PubMed] [Google Scholar]