Abstract
Pioneer factors such as FoxA target nucleosomal DNA and initiate cooperative interactions at silent genes during development, cellular reprogramming, and steroid hormone induction. Biophysical studies previously showed that the nuclear mobility of FoxA1 is slower than many other transcription factors, whereas a new single molecule study (Swinstead et al. 2016 Cell) shows comparable chromatin residence times for FoxA1 and steroid receptors. Despite that steroid receptors engage nucleosome remodeling complexes, the vast majority of co-bound sites with FoxA are dependent upon FoxA, not vice-versa. Taken together, the distinguishing feature of pioneer factors remains nucleosomal access rather than an exceptional residence time in chromatin.
Pioneer transcription factors (TFs) were discovered by dimethyl sulfate (DMS)-based in vivo footprinting on embryonic endoderm tissue. Binding sites for FoxA and GATA factors were occupied at a liver-specific enhancer prior to hepatic induction (Bossard and Zaret, 1998; Gualdi et al., 1996). Flanking TF sites became occupied as the enhancer’s target gene turned on and the cells were induced to become liver. Further studies showed that FoxA1, and to a lesser extent GATA4, had the intrinsic ability to target DNA sites on isolated nucleosomes (Cirillo et al., 1998; Cirillo and Zaret, 1999) and on a specific nucleosome embedded in linker histone-compacted chromatin in vitro (Cirillo et al., 2002). By contrast, the liver transcription factors that footprinted the DNA later in development did not exhibit independent nucleosome binding. Together, these studies engendered the concept that certain transcription factors are pioneer factors, having the ability to target DNA in nucleosomes, i.e. closed chromatin, and elicit chromatin opening sufficiently to allow other factors to bind (Cirillo et al., 2002) (Figure 1). “Pioneer” activity has been found for diverse transcription factors that function in embryonic development, cellular reprogramming, and hormonal induction (Jozwik and Carroll, 2012; Sherwood et al., 2014; Zaret and Mango, 2016). Dual gene inactivation in the adult mouse liver demonstrated that FoxA1 and FoxA2 also maintain exposed chromatin by displacing linker histone, thereby enabling other factors to bind (Iwafuchi-Doi et al., 2016). Pioneer factors solve the “chicken and egg” problem of how unprogrammed regions of nucleosomal DNA in chromatin become functional regulatory sequences, either for activation or repression. But how do pioneer factors find their targets and are there instances where they are subordinate to other transcription factors?
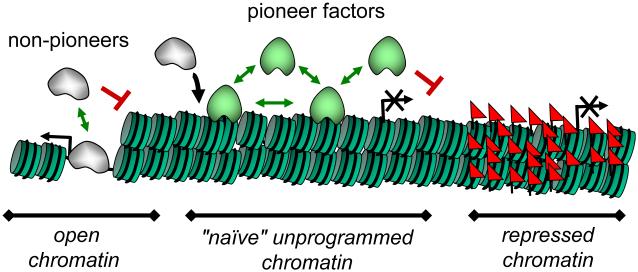
Figure. Chromatin scanning by pioneer factors.
Chromatin across the bottom of the figure is depicted as consisting of open domains, naïve/unprogrammed domains, and repressed or heterochromatic domains. Pioneer factors (green blobs) are able to target the naïve domains, whereas non-pioneer factors (grey blobs) are typically dependent upon pioneers for binding there (black arrow). The green arrows depict transient, on-and-off association with chromatin as the factors scan the different domains; the red bars indicate the factors being impeded from scanning certain domains.
It has become clear that all transcription factors, including pioneer factors, do not occupy all of their potential DNA motifs in the genome. Indeed, heterochromatin can block transcription factors from binding, including pioneer factors at the onset of cell reprogramming (Soufi et al., 2012) (Figure 1). In a comparison of FoxA1 binding in three breast cancer cell lines, among the 43,000-80,000 binding events in each line, about 20,000 overlapped, exemplifying cell specificity (Hurtado et al., 2011). Early studies had shown that FoxA1 acts as a pioneer factor for the binding of estrogen receptor in MCF-7 breast cancer cells (Carroll et al., 2005; Laganiere et al., 2005). Of ~14,000 estrogen receptor (ER) binding events in hormone-depleted MCF-7 cells, over 7000 sites overlapped with FoxA1 binding events and 90% of the sites exhibited at least a two-fold loss of ER when FoxA1 was knocked down (Hurtado et al., 2011). And while FoxA1 silencing also inhibited hormone-dependent binding of ER, the study reported no sites where FoxA1 binding was dependent upon ER.
By contrast, an independent study of unliganded ER found about 10 sites in the MCF-7 genome where FoxA1 binding was dependent upon ER (Caizzi et al., 2014). In a new paper by Swinstead et al. in Cell (Swinstead et al., 2016), the authors report that among ~19,000 FoxA1 binding events in MCF-7 cells treated with estrogen (E2) or glucocorticoid receptor (GR) ligand (dex), about 5% of the binding events were gained upon hormone treatment and closely spaced to ER or GR binding events. Another recent study found a subset of sites where TNFα signaling allowed FoxA1 binding, which was in turn necessary for estrogen receptor binding (Franco et al., 2015). In conclusion, while FoxA1 predominantly enables other factors to engage chromatin in the above cell line studies, as well as in vivo (Li et al., 2012), cooperative interactions can facilitate FoxA1 binding at a subset of sites.
Swinstead and colleagues investigated the interesting subset of non-pioneering FoxA1 binding events that are enabled by ER or GR. How do these sites differ from the ER or GR independent sites? FoxA1 ChIP signals are weaker at the hormone-induced FoxA1 binding events and the target sites are poorly enriched for the canonical FoxA1 DNA motif, compared to the hormone-independent FoxA1 binding sites. There is no information about whether the weak, non-canonical FoxA1 sites that are dependent upon receptors are relevant to a specific hormone response network. Genome editing or other assays may determine the biological function of these sites, in relation to canonical and pioneering FoxA1 binding events. Swinstead et al. consider ER and GR enhancing the weak FoxA1 binding events as “assisted loading” (Voss and Hager, 2014) and it yields local DNase sensitivity, as for canonical FoxA1 binding events.
Assisted loading appears to involve ATP-dependent, nucleosome remodeling complexes (Engel and Yamamoto, 2011; Voss and Hager, 2014; Voss et al., 2011) that enable the steroid receptors to enhance FoxA1 binding at non-canonical targets. Diverse nucleosome remodelers are prevalent at open sites in chromatin (de Dieuleveult et al., 2016) and the BAF remodeler promotes cell differentiation in conjunction with developmental transcription factors (Ninkovic et al., 2013). Yet as noted above, the vast majority of FoxA sites co-bound with steroid receptor are dependent upon FoxA, not vice versa. Thus, despite their association with remodeling complexes, steroid receptors are not the dominant chromatin-engaging partner at most sites co-bound with FoxA. This raises the central issue about pioneer factors: their ability to target novel sites in chromatin, e.g. in development or reprogramming, that have not yet been subjected to cycles of activation and silencing within a given cell lineage.
How does FoxA1 scan chromatin, and what is the role of other factors in eliciting stable binding? A systematic comparison of 19 different transcription factors, including many different types, by fluorescence recovery after photobleaching (FRAP) revealed that while most of the factors were bound to chromatin at steady state, all transcription factors had a bleaching recovery time on the order of seconds, reflecting mobility in the nucleus (Phair et al., 2004). Similar results were observed for the glucocorticoid receptor (Nagaich et al., 2004) and for FoxA1 (Sekiya et al., 2009). Yet within a cohort of liver transcription factors tested side-by-side, FRAP studies showed that FoxA1 had the slowest nuclear mobility, approaching that of linker histone (Sekiya et al., 2009). In addition, the chromatin mobility of FoxA1 is imparted by both specific and nonspecific DNA interactions (Sekiya et al., 2009). Further studies showed that, like linker histone and unlike various other transcription factors tested, FoxA1 is quantitatively retained on mitotic chromosomes (Caravaca et al., 2013). Binding to the compacted mitotic chromatin was largely, but not completely, due to nonspecific binding, with a faster overall rate of FRAP than in interphase cells. Thus it was established that FoxA1 is mobile in chromatin, with its ability to bind nucleosomal DNA via nonspecific binding associated with its potential to scan closed chromatin, even in mitosis (Figure 1). But at the single molecule level, how does FoxA1 behave?
Swinstead and colleagues used the Single Molecule Tracking (SMT) approach, based on Highly Inclined Laminated Optical sheet (HILO) microscopy (Swinstead et al., 2016), to assay the movement FoxA1 molecules in two dimensions in the living nucleus. The HILO approach is more precise and direct than FRAP and allowed the calculation of a “slow” component residence time of 10.8 seconds for FoxA1 in hormone-untreated cells. In presence of their ligands, ER and GR exhibited residence times of 11.7 and 7.37 seconds, respectively. Thus the mobility of FoxA1, in this system, was on average comparable to that of ER and GR, despite that FoxA1 enables a vastly greater number of ER and GR binding events than vice-versa. At present, it is unclear whether these events, or those in other SMT studies, represent specific or nonspecific chromatin binding. Tests of variant proteins that are deficient in specific DNA binding, while preserving nonspecific DNA binding, will be informative in this regard (Chen et al., 2014; Sekiya et al., 2009). In conclusion, the residence time of the pioneer factor FoxA1 in chromatin is unrelated to its ability to act as a pioneer for steroid receptors.
Given the observation that a deep DNaseI footprint correlates with a relatively long DNA residence time for the CTCF factor (Sung et al., 2014), Swinstead et al. conducted a high concentration-DNaseI analysis for FoxA, ER, and GR. They failed to detect footprints for any of the factors, with the SMT results implying that the binding of the factors is too dynamic for footprinting. Yet with the high concentration of DNase used, the group reported weak DNase footprints for C/EBPβ (Sung et al., 2014); while when using lower concentrations of DNase (Siersbaek et al., 2011), the same group observed robust footprints for C/EBPβ (Siersbaek et al., 2014). Thus the ability to see a clear footprint in vivo is related to the extent of overall chromatin digestion at the endpoint of the assay, with some footprints evident earlier in the DNase digests and some evident later. A direct comparison of the local chromatin around FoxA and CTCF bound sites shows marked differences in nucleosomal organization (Iwafuchi-Doi et al., 2016), possibly affecting DNase access. It will be interesting to assess footprints for FoxA and other transcription factors tested at high concentrations of DNase (Sung et al., 2014; Swinstead et al., 2016) for their robustness at lower concentrations of enzyme or earlier in the digest.
An important question to be addressed relates to the potential correlation between residence time and footprinting. For example, CTCF moves faster in chromatin than FoxA1, based on FRAP analysis (compare Nakahashi et al, 2013 and Sekiya et al, 2009). SMT should be performed for CTCF along with FoxA, to directly assess the correlation between residence time and depth of footprinting.
Taking together the results with SMT, it appears that the off rate of FoxA1 for chromatin is faster than that discerned at high DNase concentrations, but slower than the rate at which DMS modifies DNA in vivo, in the original DMS footprinting studies on embryos. Swinstead et al. point out that the mobility of FoxA1 in chromatin, and that FoxA1’s weak binding sites require assist from steroid receptors, means that FoxA is no more or less of a pioneer factor than the receptors at such weak sites. Yet the observations remain that FoxA1 and other pioneer factors are far more efficient than other transcription factors at targeting silent, nucleosomal DNA and enabling other factors to bind chromatin (Soufi et al., 2015). A synthesis of the two points of view, from the advances of Swinstead et al., would be that targeting chromatin is a constant battle, with cycles of binding, release, and binding again. And some factors win that battle at closed chromatin much better than others.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bossard P, Zaret KS. GATA transcription factors as potentiators of gut endoderm differentiation. Development. 1998;125:4909–4917. doi: 10.1242/dev.125.24.4909. [DOI] [PubMed] [Google Scholar]
- Caizzi L, Ferrero G, Cutrupi S, Cordero F, Ballare C, Miano V, Reineri S, Ricci L, Friard O, Testori A, et al. Genome-wide activity of unliganded estrogen receptor-alpha in breast cancer cells. Proc Natl Acad Sci U S A. 2014;111:4892–4897. doi: 10.1073/pnas.1315445111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caravaca JM, Donahue G, Becker JS, He X, Vinson C, Zaret KS. Bookmarking by specific and nonspecific binding of FoxA1 pioneer factor to mitotic chromosomes. Genes Dev. 2013;27:251–260. doi: 10.1101/gad.206458.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JS, Liu XS, Brodsky AS, Li W, Meyer CA, Szary AJ, Eeckhoute J, Shao W, Hestermann EV, Geistlinger TR, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122:33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang Z, Li L, Chen BC, Revyakin A, Hajj B, Legant W, Dahan M, Lionnet T, Betzig E, et al. Single-molecule dynamics of enhanceosome assembly in embryonic stem cells. Cell. 2014;156:1274–1285. doi: 10.1016/j.cell.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo L, Lin FR, Cuesta I, Jarnik M, Friedman D, Zaret K. Opening of compacted chromatin by early developmental transcription factors HNF3 (FOXA) and GATA-4. Mol. Cell. 2002;9:279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- Cirillo LA, McPherson CE, Bossard P, Stevens K, Cherian S, Shim E-Y, Clark EA, Burley SK, Zaret KS. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 1998;17:244–254. doi: 10.1093/emboj/17.1.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo LA, Zaret KS. An early developmental transcription factor complex that is more stable on nucleosome core particles than on free DNA. Mol. Cell. 1999;4:961–969. doi: 10.1016/s1097-2765(00)80225-7. [DOI] [PubMed] [Google Scholar]
- de Dieuleveult M, Yen K, Hmitou I, Depaux A, Boussouar F, Bou Dargham D, Jounier S, Humbertclaude H, Ribierre F, Baulard C, et al. Genome-wide nucleosome specificity and function of chromatin remodellers in ES cells. Nature. 2016;530:113–116. doi: 10.1038/nature16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel KB, Yamamoto KR. The glucocorticoid receptor and the coregulator Brm selectively modulate each other's occupancy and activity in a gene-specific manner. Mol Cell Biol. 2011;31:3267–3276. doi: 10.1128/MCB.05351-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Nagari A, Kraus WL. TNFalpha signaling exposes latent estrogen receptor binding sites to alter the breast cancer cell transcriptome. Mol Cell. 2015;58:21–34. doi: 10.1016/j.molcel.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS. Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev. 1996;10:1670–1682. doi: 10.1101/gad.10.13.1670. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 2011;43:27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwafuchi-Doi M, Donahue G, Kakumanu A, Watts JA, Mahony S, Pugh BF, Lee D, Kaestner KH, Zaret KS. The pioneer transcription factor FoxA maintains an accessible nucleosome configuration at enhancers for tissue-specific gene activation. Mol. Cell. 2016 doi: 10.1016/j.molcel.2016.03.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nat Rev Cancer. 2012;12:381–385. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- Laganiere J, Deblois G, Lefebvre C, Bataille AR, Robert F, Giguere V. Location analysis of estrogen receptor alpha target promoters reveals that FOXA1 defines a domain of the estrogen response. Proc Natl Acad Sci U S A. 2005;102:11651–11656. doi: 10.1073/pnas.0505575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Tuteja G, Schug J, Kaestner KH. Foxa1 and Foxa2 are essential for sexual dimorphism in liver cancer. Cell. 2012;148:72–83. doi: 10.1016/j.cell.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaich AK, Walker DA, Wolford R, Hager GL. Rapid periodic binding and displacement of the glucocorticoid receptor during chromatin remodeling. Mol Cell. 2004;14:163–174. doi: 10.1016/s1097-2765(04)00178-9. [DOI] [PubMed] [Google Scholar]
- Ninkovic J, Steiner-Mezzadri A, Jawerka M, Akinci U, Masserdotti G, Petricca S, Fischer J, von Holst A, Beckers J, Lie CD, et al. The BAF complex interacts with Pax6 in adult neural progenitors to establish a neurogenic cross-regulatory transcriptional network. Cell Stem Cell. 2013;13:403–418. doi: 10.1016/j.stem.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phair RD, Scaffidi P, Elbi C, Vecerova J, Dey A, Ozato K, Brown DT, Hager G, Bustin M, Misteli T. Global nature of dynamic protein-chromatin interactions in vivo: three-dimensional genome scanning and dynamic interaction networks of chromatin proteins. Mol Cell Biol. 2004;24:6393–6402. doi: 10.1128/MCB.24.14.6393-6402.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Muthurajan UM, Luger K, Tulin AV, Zaret KS. Nucleosome-binding affinity as a primary determinant of the nuclear mobility of the pioneer transcription factor FoxA. Genes Dev. 2009;23:804–809. doi: 10.1101/gad.1775509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood RI, Hashimoto T, O'Donnell CW, Lewis S, Barkal AA, van Hoff JP, Karun V, Jaakkola T, Gifford DK. Discovery of directional and nondirectional pioneer transcription factors by modeling DNase profile magnitude and shape. Nat Biotechnol. 2014;32:171–178. doi: 10.1038/nbt.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siersbaek R, Baek S, Rabiee A, Nielsen R, Traynor S, Clark N, Sandelin A, Jensen ON, Sung MH, Hager GL, et al. Molecular architecture of transcription factor hotspots in early adipogenesis. Cell Rep. 2014;7:1434–1442. doi: 10.1016/j.celrep.2014.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siersbaek R, Nielsen R, John S, Sung MH, Baek S, Loft A, Hager GL, Mandrup S. Extensive chromatin remodelling and establishment of transcription factor 'hotspots' during early adipogenesis. EMBO J. 2011;30:1459–1472. doi: 10.1038/emboj.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Donahue G, Zaret KS. Facilitators and impediments of the pluripotency reprogramming factors' initial engagement with the genome. Cell. 2012;151:994–1004. doi: 10.1016/j.cell.2012.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A, Garcia MF, Jaroszewicz A, Osman N, Pellegrini M, Zaret KS. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–568. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung MH, Guertin MJ, Baek S, Hager GL. DNase footprint signatures are dictated by factor dynamics and DNA sequence. Mol Cell. 2014;56:275–285. doi: 10.1016/j.molcel.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinstead EE, Miranda TB, Paakinaho V, Baek S, Goldstein I, Hawkins M, Karpova TS, Ball D, Mazza D, Lavis LD, et al. Steroid Receptors Reprogram FoxA1 Occupancy through Dynamic Chromatin Transitions. Cell. 2016;165:593–605. doi: 10.1016/j.cell.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss TC, Hager GL. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat Rev Genet. 2014;15:69–81. doi: 10.1038/nrg3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss TC, Schiltz RL, Sung MH, Yen PM, Stamatoyannopoulos JA, Biddie SC, Johnson TA, Miranda TB, John S, Hager GL. Dynamic exchange at regulatory elements during chromatin remodeling underlies assisted loading mechanism. Cell. 2011;146:544–554. doi: 10.1016/j.cell.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaret KS, Mango SE. Pioneer transcription factors, chromatin dynamics, and cell fate control. Curr Opin Genet Dev. 2016;37:76–81. doi: 10.1016/j.gde.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]