Abstract
Objective
Previous research suggested that panic disorder with agoraphobia is associated with abnormalities on vestibular and balance function tests. The purpose of this study was to further examine psychiatric correlates of vestibular/balance dysfunction in patients with anxiety disorders and the specific nature of the correlated vestibular abnormalities. The psychiatric variables considered included anxiety disorder versus normal control status, panic disorder versus non-panic anxiety disorder diagnosis, presence or absence of comorbid fear of heights, and degree of space and motion discomfort (SMD). The role of anxiety responses to vestibular testing was also re-examined.
Methods
104 subjects were recruited: 29 psychiatrically normal individuals and 75 psychiatric patients with anxiety disorders. Anxiety patients were assigned to four subgroups depending on whether or not they had panic disorder and comorbid fear of heights. SMD and anxiety responses were measured by questionnaires. Subjects were examined for abnormal unilateral vestibular hypofunction on caloric testing indicative of peripheral vestibular dysfunction, asymmetric responses on rotational testing as an indicator of an ongoing vestibular imbalance and balance function using Equitest dynamic posturography as an indicator of balance control. Logistic regression was used to establish the association between the psychiatric variables and vestibular or balance test abnormalities.
Results
Rotational test results were not significantly related to any of the psychiatric variables. The presence of either panic attacks or fear of heights increased the probability of having caloric hypofunction in a non-additive fashion. SMD and anxiety responses were independently associated with abnormal balance. Among specific posturography conditions, the association with SMD was significant for a condition that involved the balance platform tilting codirectionally with body sway, suggesting an abnormal dependence on somatosensory cues in the control of balance.
Conclusion
In patients with anxiety disorders, higher SMD is indicative of somatosensory dependence in the control of balance. The absence of both panic and fear of heights reduces the probability of having peripheral vestibular dysfunction. Future research should examine if vestibular rehabilitation can be of value for patients with anxiety disorders complicated by SMD.
Patients with vestibular or balance disorders often have symptoms of anxiety, and conversely, patients with anxiety disorders often have abnormalities on clinical balance or vestibular function tests.1,2 The vestibular abnormalities that have most consistently been found are those reflecting altered vestibulospinal or balance function,3–5 but caloric abnormalities, indicative of vestibulo-ocular and peripheral vestibular dysfunction, have also been observed.6–8 Furthermore, vestibular or balance abnormalities appear to be particularly common in panic disorder with agoraphobia.3–6 However, it is not known if these abnormalities are linked to specific psychiatric diagnoses, for example panic disorder, or to symptom phenotypes independent of diagnosis. The present study aimed to determine which aspects of anxiety disorder phenomenology would be predictive of vestibular or balance dysfunction, and which specific aspects of vestibular or balance function would be affected.
An aspect of anxiety phenomenology of interest for balance control is fear of heights. Vestibular abnormalities have long been suspected in height phobia,9 and patients with fear of heights are often dizzy.10,11 Among patients with primary vestibular disorders, fear of heights is common.12,13 In a study conducted in a dizziness clinic, it was found that patients whose vestibular symptoms preceded their anxiety symptoms had situational phobias, including phobia of heights.14 The situation of heights is also of theoretical interest. Exposure to heights reflexively increases body sway in normal subjects.15,16 This is due to the prevailing long visual distances that for geometric reasons reduce visual feedback to body sway,15 and the associated absence of motion parallax.17 Height phobics at height sway even more than normal subjects.18 One hypothesis for the present study was that fear of heights and vestibular/balance abnormalities would be associated regardless of a concomitant diagnosis of panic disorder.
The situational phobias in balance disorders may be part of a situational profile called space and motion discomfort (SMD).19,20 SMD is elicited by a multitude of situational stimuli, including moving crowds, supermarkets, repetitive geometric wall patterns, spiral staircases, heights, or vibrating or moving floors.19–22 SMD may be related to specific modes of sensory integration that help maintain balance. This integration involves three sensory channels: the vestibular, somatosensory and visual. SMD may be related to strategies fixated on vision or somatosensation called “visual dependence” and “somatosensory dependence,” respectively. The visual component of SMD also has been called “visual vertigo,”23 a construct that is measured similarly to SMD.24,25
Somatosensory dependence with respect to balance control can be assessed as increased sway when somatosensory feedback to body sway is reduced, for example by vibratory stimulation of the calf muscles,26 or “sway-referencing” the support surface.27 Sway-referencing is accomplished by having the platform on which the subject is standing tilt forward when the subject sways in the forward direction and vice versa. Somatosensory dependence has been observed in vestibular disorders,28,29 and panic disorder and agoraphobia, particularly in the presence of fear of heights or high SMD.3
Visual dependence with respect to balance control can be assessed by looking for increased sway when the visual surround moves in full-field motion (“optic flow”).30 In this case, visual cues are erroneous and lead to increased sway, especially in visually dependent persons. This has been observed in patients with primary vestibular disorders,31 and in patients with anxiety disorders and excessive SMD.32 Visual dependence can also be assessed by visual sway referencing, that is, by having the visual surround move in phase with the patient’s anterior–posterior body sway.
The Equitest protocol of computerised dynamic posturography used in the present study incorporates both a sway-referenced support surface to assess somatosensory dependence and a sway-referenced visual surround to assess visual dependence, in isolation or in combination.33,34 We hypothesised that SMD would show specific associations with Equitest conditions reflecting surface dependence and visual dependence.
The present study addresses the association between indices of vestibular/balance dysfunction and anxiety or panic disorder diagnosis, fear of heights and SMD. Indicators of vestibular/balance dysfunction include unilateral hypofunction on the caloric test, to identify peripheral vestibular lesions, rotational testing as an indicator of ongoing vestibular imbalance, and increased sway on computerised dynamic posturography, to identify abnormal balance control. Anxiety or arousal increases the gain of the vestibulo-ocular reflex,35,36 changes the direction of the beating field of nystagmus37 and induces a postural “stiffening up” response.18,38,39 Therefore, vestibular test results might be affected by anxiety responses to the testing itself. Such effects were deemed unlikely in a previous study6 but were nonetheless reconsidered in this study.
CLINICAL MATERIALS AND METHODS
Subject characteristics
One hundred and thirty-nine subjects were recruited over 4 years. Of these, 104 subjects received at least one vestibular test and are the focus of the study. These included 29 psychiatrically normal control subjects and 75 patients with an anxiety disorder. Of the anxiety patients, 25 had panic disorder; 19 with fear of heights (PH+) and six without (PH−). The remaining 50 anxiety patients had a non-panic anxiety disorder (NPA, typically Generalised Anxiety Disorder or Social Phobia); 28 with fear of heights (NPAH+) and 22 without (NPAH−). Table A1 (Appendix 1) summarises the subject characteristics. The gender distribution was 80% female, which is higher than the known female predominance of anxiety disorders in population surveys (eg, 62%),40 perhaps reflecting the higher propensity for females to seek psychiatric treatment.41 The average age was 31.6 years (range 18–55). Thirty-one per cent of the patients were using psychiatric medications, particularly antidepressants (25% of the patients), mostly selective serotonin-reuptake inhibitors (21% of the patients).
Psychiatric and questionnaire assessments
Patients were assessed in a 2 h structured interview, the Anxiety Disorders Interview Schedule for DSM-IV (ADIS),42,43 which established their DSM-IV diagnosis, and completed several questionnaires. SMD was measured with the “SMD-1” subscale of the Situational Characteristics Questionnaire Woody (Appendix 2).20 Although SMD was a continuous measure, to further illustrate some of the results, “high SMD” was defined as an SMD score of 6 or higher. This cut-off was chosen a priori based on an inspection of the distribution of SMD scores among fear of heights patients, which appeared to be bimodal with a trough at 6.0 (see fig 1).
Figure 1.
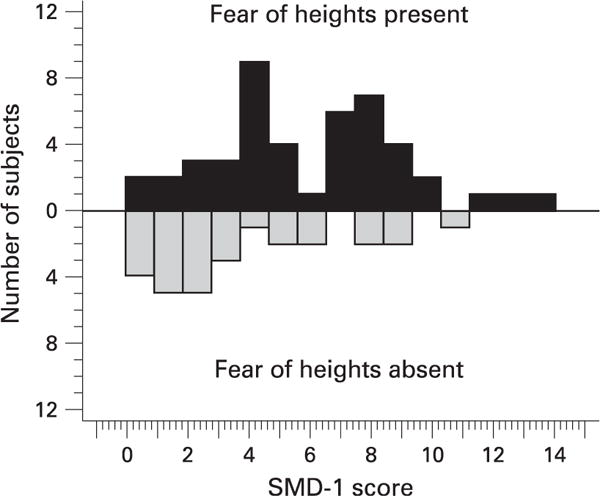
Frequency distribution of SMD-1 scores for anxiety patients with fear of heights (black bars) and without fear of heights (gery bars). The bimodal distribution suggests two fear-of-height subgroups, those with and without SMD.
The Spielberger State Anxiety Inventory (Form X)44 was completed upon arrival to the vestibular laboratory, after each vestibular/balance test, and before leaving the laboratory. The anxiety response (R) to a vestibular test was defined as the difference between the subject’s baseline score and that following the vestibular test, the baseline being calculated as the average of the anxiety levels recorded upon arrival on the second vestibular test day and just prior to leaving the laboratory on that day.
Vestibular and balance tests
The clinical vestibular tests were those performed routinely in the clinical assessment of patients with dizziness or balance disorders and included rotational testing, caloric testing, and Equitest computerised dynamic posturography.33 Unilateral caloric hypofunction was diagnosed as a side difference exceeding 24% according to the Jongkees formula (see Appendix 1 for further details).
Statistical analysis
Correlation coefficients (Phi, point biserial and Pearson product moment)45,46 were used as indicators of the effect size of an association. Confirmatory analyses involved logistic regression modelling47–49 of associations between a binary “dependent” variable, that is, abnormal versus normal vestibular/balance test result, and one or more categorical or continuous “independent” variables. Because of covariation among the independent variables and because of missing data, not all of the independent variables could be included simultaneously in one model. Therefore, a core set of four models was run for each vestibular test. An initial model (Model 1) simply examined the effect of having an anxiety disorder (A), that is, patient versus control status. Model 2, “the Panic/Height model,” was built on Model 1 by adding the predictor variables of Panic Disorder Diagnosis (P), Fear of Height (H), and their interaction (P×H). Model 3, the “SMD model,” assessed the effect of adding SMD to Model 1. Model 4, the “Anxiety Response model,” tested the effect adding anxiety response (R) and its interaction with patient status (R×A) to Model 1. Follow-on models, reported in the Results section and designated as “exploratory,” were performed as suggested by significant findings on these core models. Target significance level was estimated according to Curran-Everett50 at p<0.022, corresponding to an overall “false discovery rate” of 5% among 30 statistical tests.
RESULTS
There were no effects of race, gender, age or use of antidepressant medication on the vestibular/balance test results. Table 1 shows the overall abnormality rate for rotational testing, caloric testing and posturography. For rotational test abnormalities, none of the relations with the psychiatric variables (Models 1–4) was significant. The rotational test was also used to explore evidence for central vestibular compensation; these results are presented in Appendix 1.
Table 1.
Prevalence of abnormal test findings by panic and fear-of-height subgroups and by space and motion discomfort (SMD) status
| Normal subjects compared with anxiety disorder patients
|
Contrasting subgroups of patients with anxiety disorders
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Normal subjects N=29 |
Anxiety disorder patients N=75 |
PH+ N=19 |
PH− N=6 |
NPAH+ N=28 |
NPAH− N=22 |
SMD-1⩾6 N=30 |
SMD-1<6 N=43 |
|
| N (%) | N (%) | N (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Rotational test asymmetry | 7 (25) | 26 (36) | 5 (28) | 1 (17) | 13 (48) | 7 (32) | 12 (41) | 14 (33) |
| Caloric test, unilateral reduced response | 4 (14) | 19 (27) | 6 (33) | 3 (60) | 9 (33) | 1 (5) | 7 (25) | 11 (28) |
| Dynamic posturography (any of conditions I–VI abnormal) | 3 (11) | 22 (31) | 8 (42) | 0 | 7 (28) | 7 (32) | 14 (52) | 8 (20) |
NPAH+, non-panic anxiety disorder with fear of heights; NPAH−, non-panic anxiety disorder without fear of heights; PH+, panic disorder with fear of heights; PH−, panic disorder without fear of heights
Caloric test: unilateral reduced response
Model 2, testing the effects of adding panic (P), fear of height (H) and their interaction (P×H) as predictors was statistically significant (table 2; p = 0.019). Models 1, 3 and 4 were not statistically significant. Follow-up testing of the findings for panic and fear of heights in Model 2 indicated that, of its three components, only the P×H interaction was independently significant (p = 0.017). There was no significant difference in the prevalence of abnormalities among the PH+, PH− and NPAH+ groups (average of 36%; χ2 = 1.39, df = 2, p = 0.50); however, the 5% rate in the group with neither panic nor fear of heights, NPAH–, was significantly lower (Fisher exact probability test, p = 0.008 two-tailed). Thus, when neither panic nor fear of heights was present, caloric test results were more likely to be normal than when either P or H or both were present. An exploratory model focusing on this “P or H effect” was associated with p = 0.003 (table 2).
Table 2.
Summary of main findings for unilateral hypofunction on caloric testing
| Model designation | Variables of interest | Variable(s) being controlled | χ2 | df | p Value |
|---|---|---|---|---|---|
| Model 1 | Anxiety disorder diagnosis (A) (ie, normals vs patients) | …. | 1.98 | 1 | 0.16 |
| Model 2 | Panic, fear of height and their interaction | A | 9.90 | 3 | 0.019* |
| Exploratory model | Panic and/or fear of height versus neither panic nor fear of height | A | 8.52 | 1 | 0.003* |
| Model 3 | Space and motion discomfort | A | 2.10 | 1 | 0.15 |
| Model 4 | Anxiety response and its interaction with A | A | 1.28 | 2 | 0.53 |
Statistically significant, assuming an overall false discovery rate of 5%.
Dynamic posturography
In Model 1, testing the effect of patient-versus-control status, there was a trend for anxiety patients to have an increased prevalence of abnormalities (table 3: p = 0.033>target level of p = 0.022), reflecting the prevalence rates of 31% compared with 11% in table 1. In Model 2, there were no significant independent effects of panic, fear of heights, or their interaction (p = 0.18). Model 3 showed a significant effect of SMD independent of patient-versus-control status (p = 0.00133). Patients with SMD⩾6 had an abnormality rate of 52%, whereas those with SMD<6 had an abnormality rate of 20% (table 1).
Table 3.
Summary of main findings for dynamic posturography (all conditions combined)
| Statistical model designation | Predictor variables of interest | Variable(s) being controlled | χ2 | df | p Value |
|---|---|---|---|---|---|
| Model 1 | Anxiety disorder diagnosis (A) (ie, “normals” vs patients) | …. | 4.57 | 1 | 0.033 |
| Model 2 | Panic, fear of heights and their interaction | A | 4.86 | 3 | 0.18 |
| Model 3 | Space and motion discomfort (SMD) | A | 10.30 | 1 | 0.00133* |
| Model 4 | Anxiety response (R) and A×R, its interaction with A | A | 10.37 | 2 | 0.006* |
| Exploratory (model 3 and 4 combined) | SMD, A×R | A, R | 18.9 | 2 | 0.00008* |
| Component of above | A×R | SMD, A, R | 7.64 | 1 | 0.0057* |
| Component of above | SMD | A, R, A×R, | 10.29 | 1 | 0.00134* |
Statistically significant (p,0.022), assuming an overall false discovery rate of 5%.
The average anxiety response (R) during posturography was negligible (mean = 0.0, SD = 7.0 scale units for the patients, mean decrease = 0.7, SD = 5 units for normal subjects). However, Model 4, assessing anxiety response during testing (R) and its interaction with patient status (A×R), was statistically significant (p = 0.006). The A×R interaction was such that, unlike the normal controls, the patients who experienced a higher anxiety response were more likely to have abnormal posturography. To see if the effects of SMD and A×R were independent from each other, an exploratory model was tested that included SMD and the A×R interaction while controlling for A and R. It showed that both SMD and A×R were independently associated with posturographic abnormalities (for SMD, p = 0.0013; for A×R, p = 0.0057; table 3).
Individual posturography conditions
To simplify the statistics, these analyses were conducted for the anxiety patients only. On each of the individual posturography conditions, abnormalities tended to be more common in patients who had become more anxious and in patients with higher levels of SMD (table 4). The correlation with SMD was the highest for Condition IV (sway referenced platform testing somatosensory dependence), whereas that for Anxiety Response (R) was highest for Condition VI (both visual surround and platform sway referenced). An exploratory model predicting abnormal results from R and SMD was run for each of Conditions III–IV (table 5). It was statistically significant (p<0.022) for Conditions III, IV and VI. Anxiety response (R) independently predicted abnormal findings on Condition VI (p = 0.011). SMD was independently associated with abnormalities on Conditions IV (p = 0.0001) and showed a trend for Condition III (p = 0.035).
Table 4.
Descriptive results for individual sensory organisation tests of dynamic posturography
| Sensory condition | Change in anxiety during posturography (N=65)
|
SMD (N=69)
|
||||
|---|---|---|---|---|---|---|
| Correlation with continuous variable
|
Abnormality rate by anxiety response category
|
Correlation with continuous variable
|
Abnormality rate by SMD category
|
|||
| Decrease in anxiety
|
Increase in anxiety
|
SMD-1<6,
|
SMD-1⩾6
|
|||
| r* | n (%) | n (%) | r* | n (%) | n (%) | |
| Condition I | … | 0 (0.0) | 1 (3.3) | … | 0 (0.0) | 1 (3.6) |
| Condition II | … | 0 (0.0) | 2 (6.7) | … | 0 (0.0) | 2 (7.1) |
| Condition III | 0.30 | 1 (2.2) | 5 (16.7) | 0.31 | 1 (2.4) | 5 (17.9) |
| Condition IV | 0.24 | 4 (11.4) | 9 (30.0) | 0.48 | 3 (7.3) | 10 (35.7) |
| Condition V | 0.23 | 2 (5.7) | 7 (23.3) | 0.11 | 4 (9.8) | 6 (21.4) |
| Condition VI | 0.40 | 2 (5.7) | 8 (26.7) | 0.10 | 5 (12.2) | 6 (21.4) |
Point biserial correlation between continuous variables (ie, anxiety response and SMD), and binary “dependent” variable (ie, normal vs abnormal posturography result). SMD, space and motion discomfort.
Table 5.
Statistical results for individual sensory organisation tests (conditions III–VI) of dynamic posturography
| Sensory condition | Surface on which subject is standing | Vision or visual environment | Full model
|
Component
|
||||
|---|---|---|---|---|---|---|---|---|
| SMD+R
|
R
|
SMD
|
||||||
| χ2 (df=2) | p Value | χ2 (df=1) | p Value | χ2 (df=1) | p Value | |||
| Condition III | Stable | Sway-referenced† | 10.2 | 0.006* | 3.12 | 0.08 | 4.445 | 0.035 |
| Condition IV | Sway-referenced | Stable | 18.8 | 0.00002* | 0.77 | 0.38 | 14.91 | 0.0001* |
| Condition V | Sway-referenced | Eyes closed | 3.8 | 0.15 | 2.29 | 0.13 | 0.51 | 0.48 |
| Condition VI | Sway-referenced | Sway-referenced | 11.2 | 0.0008* | 9.99 | 0.011* | 0.012 | 0.9 |
Condition I (stable support, visual environment stable) and Condition II (stable support, eyes closed) are not reported, due to low abnormality rates.
Statistically significant, assuming an overall false discovery rate of 5%.
Sway referenced: visual surround or support surface is moving in phase with anterior–posterior body sway so as to reduce visual or somatosensory input for control of balance.
R, anxiety response; SMD, space and motion discomfort.
DISCUSSION
This study yielded both negative and positive findings that were noteworthy. One noteworthy negative finding concerns the lack of a relationship between SMD and unilateral caloric hypofunction. This, along with further analyses including the rotational test (Appendix 1), suggests that SMD is not related to peripheral vestibular dysfunction, compensated or uncompensated. Rotational test abnormalities by themselves were unrelated to the psychiatric predictor variables. Elsewhere, we report similar negative results for off-vertical axis rotational testing, the latter as a test of otolith function.51
Another negative finding was the absence of main effects of panic disorder diagnosis or fear of height. Rather, the absence of both of these was especially indicative of normal peripheral vestibulo-ocular function. This pattern seems inconsistent with a specific linkage between peripheral vestibular lesions and panic disorder diagnosis or fear of height but does suggest that peripheral vestibular lesions complicate the phenomenology of anxiety disorders.
The main positive result of the study concerned the relationship between SMD and abnormal balance. This type of result seems to be the most robust finding across studies from our laboratory.6,32 The association between SMD and abnormal balance was specific for somatosensory dependence (Condition IV). The evidence for visual dependence (Condition III) was inconclusive; however, in a test for visual dependence that used an independently moving visual surround rather than just sway referencing, we found increased sway responses in the anxiety patients, particularly if high in SMD.52
The posturography pattern associated with SMD was different from the typical “vestibular pattern” observed in patients with vestibular disorders, in which Conditions V (sway-referenced surface and eyes closed) and VI (both vision and support sway-referenced) are primarily affected.28 The pattern observed indicates that SMD identifies an abnormal intersensory integration pattern that is different from that typical for vestibular disorders. The absence of association with peripheral vestibular dysfunction suggests that SMD expresses a centrally medicated process. At the cortical level, the visual and vestibular sensory modalities inhibit one another;53 vestibular stimulation also inhibits somatosensory cortical areas.54 Normally, information from a channel yielding erroneous information is quickly downweighted.55 High SMD could result from a lack of flexibility in selecting the appropriate sensory cues for the situation at hand.
SMD is related to fear of heights in a way that suggests the existence of a subgroup of height phobia characterised by high SMD (fig 1) that may be particularly prone to imbalance at high elevations.56 A reflexive increase in sway at height could be experienced as urges to jump or fly, a common cognition in height phobia.57 Sensitivity and specificity analysis of the relationship between posturography and SMD (Appendix 1) suggest that measures of SMD could be used to identify anxiety patients likely to have altered balance control.
The presence of altered balance control in patients with anxiety disorders and SMD may have implications for treatment. Specific vestibular rehabilitation techniques have been developed for SMD in patients with balance disorders.58 Such techniques may also find a clinical role in the treatment of patients with anxiety disorders complicated by SMD.59
Supplementary Material
Footnotes
Presented in part at the 29th Midwinter Research Meeting of the Association for Research in Otolaryngology (ARO) Baltimore, Maryland, 6 February 2006, and at 41ème Symposium de la Société Internationale d’Otoneurologie, Paris, 18 May 2007.
Competing interests: None.
References
- 1.Jacob RG, Brandt T, Furman JM. Psychiatric aspects of dizziness and imbalance. In: Bronstein AM, Brandt T, Woollacott MH, editors. Clinical disorders of balance posture and gait. 2nd. London: Arnold; 2004. [Google Scholar]
- 2.Furman JM, Balaban CD, Jacob RG, et al. Migraine-anxiety related dizziness (MARD): a new disorder? J Neurol Neurosurg Psychiatry. 2005;76:1–8. doi: 10.1136/jnnp.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacob RG, Furman JM, Durrant JD, et al. Surface dependence: a balance control strategy in panic disorder with agoraphobia. Psychosom Med. 1997;59:323–30. doi: 10.1097/00006842-199705000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Yardley L, Britton J, Lear S, Bird J, Luxon LM. Relationship between balance system function and agoraphobic avoidance. Behav Res Ther. 1995;33:435–9. doi: 10.1016/0005-7967(94)00060-w. [DOI] [PubMed] [Google Scholar]
- 5.Perna G, Dario A, Caldirola D, et al. Panic disorder: the role of the balance system. J Psychiatr Res. 2001;35:279–86. doi: 10.1016/s0022-3956(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 6.Jacob RG, Furman JM, Durrant JD, et al. Panic, agoraphobia, and vestibular dysfunction. Am J Psychiatry. 1996;153:503–12. doi: 10.1176/ajp.153.4.503. [DOI] [PubMed] [Google Scholar]
- 7.Tecer A, Tukel R, Erdamar B, et al. Audiovestibular functioning in patients with panic disorder. J Psychosom Res. 2004;57:177–82. doi: 10.1016/s0022-3999(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 8.Yardley L, Luxon L, Lear S, et al. Vestibular and posturographic test results in people with symptoms of panic and agoraphobia. J Audiol Med. 1994;3:48–65. [Google Scholar]
- 9.Pogany E. Height vertigo. Monatsschr Ohrenheilkd Laryngorhinol. 1958;92:209–13. [PubMed] [Google Scholar]
- 10.Yardley L, Owen N, Nazareth I, et al. Panic disorder with agoraphobia associated with dizziness: characteristic symptoms and psychosocial sequelae. J Nerv Ment Dis. 2001;189:321–7. doi: 10.1097/00005053-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Davey GCL, Menzies R, Gallardo B. Height phobia and biases in the interpretation of bodily sensations: Some links between acrophobia and agoraphobia. Behav Res Ther. 1997;35:997–1001. doi: 10.1016/s0005-7967(97)10004-3. [DOI] [PubMed] [Google Scholar]
- 12.Clark DB, Leslie MI, Jacob RG. Balance complaints and panic disorder—a clinical-study of panic symptoms in members of a self-help group for balance disorders. J Anxiety Disord. 1992;6:47–53. [Google Scholar]
- 13.Rahko T. The test and treatment methods of benign paroxysmal positional vertigo and an addition to the management of vertigo due to the superior vestibular canal (BPPV-SC) Clin Otolaryngol Allied Sci. 2002;27:392–5. doi: 10.1046/j.1365-2273.2002.00602.x. [DOI] [PubMed] [Google Scholar]
- 14.Staab JP, Ruckenstein MJ. Which comes first? Psychogenic dizziness versus otogenic anxiety. Laryngoscope. 2003;113:1714–8. doi: 10.1097/00005537-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Bles W, Kapteyn TS, Brandt T, et al. The mechanism of physiological height vertigo. II. Posturography. Acta Otolaryngol. 1980;89:534–40. doi: 10.3109/00016488009127171. [DOI] [PubMed] [Google Scholar]
- 16.Simeonov P, Hsiao H. Height, surface firmness, and visual reference effects on balance control. Inj Prev. 2001;7(1 Suppl):i50–3S. doi: 10.1136/ip.7.suppl_1.i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guerraz M, Sakellari V, Burchill P, et al. Influence of motion parallax in the control of spontaneous body sway. Exp Brain Res. 2000;131:244–52. doi: 10.1007/s002219900307. [DOI] [PubMed] [Google Scholar]
- 18.Nakahara H, Takemori S, Tsuruoka H. Influence of height on the spatial orientation and equilibrium of the body. Otolaryngol Head Neck Surg. 2000;123:501–4. doi: 10.1067/mhn.2000.107316. [DOI] [PubMed] [Google Scholar]
- 19.Jacob RG, Furman JM, Cass SP. Psychiatric consequences of vestibular dysfunction. In: Luxon LMFJ, Martini A, Stephens D, editors. Audiological medicine. London: ISIS Press; 2003. [Google Scholar]
- 20.Jacob RG, Woody SR, Clark DB, et al. Discomfort with space and motion—a possible marker of vestibular dysfunction assessed by the Situational Characteristics Questionnnaire. J Psychopathol Behav Assess. 1993;15:299–324. [Google Scholar]
- 21.Balaban CD, Jacob RG. Background and history of the interface between anxiety and vertigo. J Anxiety Disord. 2001;15:27–51. doi: 10.1016/s0887-6185(00)00041-4. [DOI] [PubMed] [Google Scholar]
- 22.Lilienfeld SO, Jacob RG, Furman JM. Vestibular dysfunction followed by panic disorder with agoraphobia. J Nerv Ment Dis. 1989;177:700–1. doi: 10.1097/00005053-198911000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Bronstein AM. The visual vertigo syndrome. Acta Otolaryngol Suppl. 1995;520(1 Pt):45–8. doi: 10.3109/00016489509125186. [DOI] [PubMed] [Google Scholar]
- 24.Guerraz M, Yardley L, Bertholon P, et al. Visual vertigo: symptom assessment, spatial orientation and postural control. Brain. 2001;124(8 Pt):1646–56. doi: 10.1093/brain/124.8.1646. [DOI] [PubMed] [Google Scholar]
- 25.Pavlou M, Davies RA, Bronstein AM. The assessment of increased sensitivity to visual stimuli in patients with chronic dizziness. J Vestib Res. 2006;16:223–31. [PubMed] [Google Scholar]
- 26.Holmberg J, Karlberg M, Fransson PA, et al. Phobic postural vertigo: body sway during vibratory proprioceptive stimulation. Neuroreport. 2003;14:1007–11. doi: 10.1097/01.wnr.0000070191.28954.a5. [DOI] [PubMed] [Google Scholar]
- 27.Clark S, Riley MA. Multisensory information for postural control: sway-referencing gain shapes center of pressure variability and temporal dynamics. Exp Brain Res. 2007;176:299–310. doi: 10.1007/s00221-006-0620-6. [DOI] [PubMed] [Google Scholar]
- 28.Furman JM. Role of posturography in the management of vestibular patients. Otolaryngol Head Neck Surg. 1995;112:8–15. doi: 10.1016/S0194-59989570300-4. [DOI] [PubMed] [Google Scholar]
- 29.Furman JM, Sparto PJ, Soso M, et al. Vestibular function in migraine-related dizziness: a pilot study. J Vestib Res. 2005;15:327–32. [PubMed] [Google Scholar]
- 30.Tossavainen T, Toppila E, Pyykko I, et al. Virtual reality in posturography. IEEE Trans Inf Technol Biomed. 2006;10:282–92. doi: 10.1109/titb.2005.859874. [DOI] [PubMed] [Google Scholar]
- 31.Redfern MS, Furman JM. Postural sway of patients with vestibular disorders during optic flow. J Vestib Res Equil Orient. 1994;4:221–30. [PubMed] [Google Scholar]
- 32.Jacob RG, Redfern MS, Furman JM. Optic flow-induced sway in anxiety disorders associated with space and motion discomfort. J Anxiety Disord. 1995;9:411–25. [Google Scholar]
- 33.Nashner LM, Black FO, Wall C., 3rd Adaptation to altered support and visual conditions during stance: patients with vestibular deficits. J Neurosci. 1982;2:536–44. doi: 10.1523/JNEUROSCI.02-05-00536.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black FO. What can posturography tell us about vestibular function? Ann N Y Acad Sci. 2001;942:446–64. doi: 10.1111/j.1749-6632.2001.tb03765.x. [DOI] [PubMed] [Google Scholar]
- 35.Collins WE. Arousal and vestibular habituation. In: Kornhuber HH, editor. Vestibular system. Part 2: Psychophysics, applied aspects and general interpretations. Berlin: Springer Verlag; 1974. pp. 361–68. [Google Scholar]
- 36.Yardley L, Watson S, Britton J, et al. Effects of anxiety arousal and mental stress on the vestibuloocular reflex. Acta Oto-Laryngol. 1995;115:597–602. doi: 10.3109/00016489509139373. [DOI] [PubMed] [Google Scholar]
- 37.Viaud-Delmon I, Siegler I, Israel I, et al. Eye deviation during rotation in darkness in trait anxiety: an early expression of perceptual avoidance? Biol Psychiatry. 2000;47:112–8. doi: 10.1016/s0006-3223(99)00111-0. [DOI] [PubMed] [Google Scholar]
- 38.Adkin AL, Frank JS, Carpenter MG, et al. Postural control is scaled to level of postural threat. Gait Posture. 2000;12:87–93. doi: 10.1016/s0966-6362(00)00057-6. [DOI] [PubMed] [Google Scholar]
- 39.Carpenter MG, Frank JS, Silcher CP. Surface height effects on postural control: a hypothesis for a stiffness strategy for stance. J Vestib Res. 1999;9:277–86. [PubMed] [Google Scholar]
- 40.Gater R, Tansella M, Korten A, et al. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings—Report from the World Health Organization collaborative study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 41.Rhodes AE, Goering PN, To T, et al. Gender and outpatient mental health service use. Soc Sci Med. 2002;54:1–10. doi: 10.1016/s0277-9536(01)00002-8. [DOI] [PubMed] [Google Scholar]
- 42.Brown TA, DiNardo PA, Barlow HH. Anxiety disorders interview schedule for DSM-IV, adult version. San Antonio: Graywind Publications; 1994. [Google Scholar]
- 43.Brown TA, Di Nardo PA, Lehman CL, et al. Reliability of DSM-IV anxiety and mood disorders: implications for the classification of emotional disorders. J Abnorm Psychol. 2001;110:49–58. doi: 10.1037//0021-843x.110.1.49. [DOI] [PubMed] [Google Scholar]
- 44.Spielberger CD, Gorsuch RL, Luschene RE. Manual for the state-trait anxiety inventory. Palo Alto: Consulting Psychologists Press; 1974. [Google Scholar]
- 45.Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 46.Kraemer HC. Correlation coefficients in medical research: from product moment correlation to the odds ratio. Stat Methods Med Res. 2006;15:525–45. doi: 10.1177/0962280206070650. [DOI] [PubMed] [Google Scholar]
- 47.Hosmer DW, Lemesow S. Applied logistic regression. New York: Wiley; 2000. [Google Scholar]
- 48.Collett D. Modelling binary data. Boca Raton: Chapman & Hall/CRC; 2003. [Google Scholar]
- 49.Statgraphics Plus [computer program] 5.0 version, 2000
- 50.Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol. 2000;279:1–8R. doi: 10.1152/ajpregu.2000.279.1.R1. [DOI] [PubMed] [Google Scholar]
- 51.Furman JM, Redfern MS, Jacob RG. Vestibulo-ocular function in anxiety disorders. J Vestib Res. 2006;16:209–15. [PubMed] [Google Scholar]
- 52.Redfern MS, Furman JM, Jacob RG. Visually induced postural sway in anxiety disorders. J Anxiety Disord. 2007;21:704–16. doi: 10.1016/j.janxdis.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Deutschlander A, Bense S, Stephan T, et al. Sensory system interactions during simultaneous vestibular and visual stimulation in PET. Hum Brain Mapp. 2002;16:92–103. doi: 10.1002/hbm.10030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schlindwein P, Mueller M, Bauermann T, et al. Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. Neuroimage. 2008;39:19–31. doi: 10.1016/j.neuroimage.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Mahboobin A, Loughlin PJ, Redfern MS, et al. Sensory re-weighting in human postural control during moving-scene perturbations. Exp Brain Res. 2005;167:260–7. doi: 10.1007/s00221-005-0053-7. [DOI] [PubMed] [Google Scholar]
- 56.Hsiao H, Simeonov P. Preventing falls from roofs: a critical review. Ergonomics. 2001;44:537–61. doi: 10.1080/00140130110034480. [DOI] [PubMed] [Google Scholar]
- 57.Hall S. A study of fears. Am J Psychol. 1897;8:147–249. [Google Scholar]
- 58.Pavlou M, Lingeswaran A, Davies RA, et al. Simulator based rehabilitation in refractory dizziness. J Neurol. 2004;251:983–95. doi: 10.1007/s00415-004-0476-2. [DOI] [PubMed] [Google Scholar]
- 59.Whitney SL, Jacob RG, Sparto PJ, et al. Acrophobia and pathological height vertigo: indications for vestibular physical therapy? Phys Ther. 2005;85:443–58. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


