Abstract
Studies have causally linked external thyroid radiation exposure in childhood with thyroid cancer. In 1995, investigators conducted relative risk analyses of pooled data from seven epidemiologic studies. Doses were mostly <10 Gy, although childhood cancer therapies can result in thyroid doses >50 Gy. We pooled data from 12 studies of thyroid cancer patients who were exposed to radiation in childhood (ages <20 years), more than doubling the data, including 1,070 (927 exposed) thyroid cancers and 5.3 million (3.4 million exposed) person-years. Relative risks increased supralinearly through 2–4 Gy, leveled off between 10–30 Gy and declined thereafter, remaining significantly elevated above 50 Gy. There was a significant relative risk trend for doses <0.10 Gy (P < 0.01), with no departure from linearity (P = 0.36). We observed radiogenic effects for both papillary and nonpapillary tumors. Estimates of excess relative risk per Gy (ERR/Gy) were homogeneous by sex (P = 0.35) and number of radiation treatments (P = 0.84) and increased with decreasing age at the time of exposure. The ERR/Gy estimate was significant within ten years of radiation exposure, 2.76 (95% CI, 0.94–4.98), based on 42 exposed cases, and remained elevated 50 years and more after exposure. Finally, exposure to chemotherapy was significantly associated with thyroid cancer, with results supporting a nonsynergistic (additive) association with radiation.
INTRODUCTION
Epidemiologic studies strongly support a causal association between external radiation exposure and thyroid cancer, with the young thyroid gland being particularly radiosensitive (1–3). In 1995, a pooled analysis of seven studies of external radiation exposure and thyroid cancer was performed to evaluate the strength of the association between radiation and thyroid cancer in terms of excess relative risk per Gy (ERR/Gy) (4). The ERR was consistent with linearity through nearly 10 Gy, although there is evidence to suggest curvilinearity at higher doses. The analysis also demonstrated that the strength of association decreased with increasing age at exposure and that an excess risk persisted for 30 years and more after radiation exposure (4).
Except for a single case-control study of 22 incident thyroid cancers, these studies involved mainly lower radiation doses with study-specific mean doses under 1.5 Gy and with nearly all cases exposed to under 10 Gy (4). Because radiation therapy in pediatric cancers can result in a dose of 50 Gy or more to the thyroid, the 1995 pooling did not permit a characterization of the dose response across the full range of possible thyroid radiation doses, in particular, the shape and magnitude of the dose response at high doses and the dose-response relationship at lower doses.
Since 1995, the amount of information on thyroid cancer and childhood radiation exposure has increased substantially. Many of the original cohorts have extended their follow-ups and investigators have reported several new studies, resulting in a commensurate increase in the number of reported thyroid cancer cases. For several of the new studies the purpose was to investigate thyroid cancer after radiation therapy for childhood cancer and involved thyroid radiation doses in excess of 50 Gy. However, the merging of these higher dose studies with lower dose studies of thyroid cancer after radiotherapy for benign diseases or environmental exposure introduced an added complexity, namely, the role of chemotherapeutic agents to treat many childhood cancers as potential thyroid carcinogens (5–8).
We assembled and harmonized original data from 12 studies of external radiation exposure in childhood and incident thyroid cancer and conducted a new pooled analysis of individuals who were under age 20 years at the time of their first radiation exposure (6, 7, 9–27). The current analysis includes 1,070 (927 exposed) thyroid cancer cases, compared with 495 (458 exposed) in the earlier report (4).
We previously reported results of a pooled analysis of the four childhood cancer survivor studies (CCSS) of second primary thyroid cancer in patients with information on individualized thyroid radiation dose estimates and on chemotherapeutic agents (8). Mean doses ranged from 6 to 14 Gy, with doses in some patients exceeding 70 Gy. Relative risks with ever exposure to chemotherapy or ever exposure to the subgroup of alkylating agents were elevated, but declined with increasing thyroid radiation dose (8), suggesting a sub-multiplicative association. The current pooling included these data to enable a comprehensive characterization of risk patterns of thyroid cancer across the full range of childhood radiation exposures and allow a more formal evaluation of the synergistic/nonsynergistic relationship for thyroid radiation dose and chemotherapy.
The goals of the current analysis were: 1. To evaluate thyroid radiation dose-response patterns at low doses; 2. To evaluate dose-response patterns across the full range of doses while accounting for chemotherapy; and 3. To examine factors that influence the ERR/Gy, such as sex, age at exposure, number of radiation treatments, attained age and time since exposure.
METHODS
Study Populations
We included all epidemiologic studies of childhood external radiation exposure and thyroid cancer that included individual thyroid dose estimates and a minimum of 1,000 irradiated subjects or 10 thyroid cancer cases. Based on a MEDLINE search, there were 12 eligible studies (10 cohort and 2 case-control studies). We distinguished studies by the source of radiation, i.e., cancer treatment, treatment of benign diseases and environmental exposure (see Table 1 and Supplementary Material Part A). For the cohort studies, we included only individuals who were free of thyroid cancer at start of follow-up. For studies of benign diseases and of environmental exposure cases were comprised of patients who developed thyroid cancer as the first primary cancer or second primary cancer if the first primary malignancy was non-melanoma skin cancer. The CCSS cohorts were comprised of patients who developed thyroid cancer as the second primary cancer or third primary cancer if the second primary cancer was non-melanoma skin cancer. For comparability across studies, we did not include autopsy identified thyroid cancers as cases, but censored them at date of death. The appropriate institutional review boards approved each participating study and the data pooling.
TABLE 1.
Summary of Studies of Thyroid Cancer and External Radiation Exposure to the Thyroid Gland Included in the Pooled Analysis
| Name of studya: location (ref.) |
Statusb | Calendar years of: | Females (%) |
Nonexposed | Exposed | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Cs accrual | Cs | Non-csc | Cs | Non-csc | Dose (Gy)d |
Age at exposured |
Age at dxd |
|||
| Medical radiation: cancer treatment |
|||||||||||
| CCSS-LESG: (International) (10) |
No change |
1936–1979 | 1938–1984 | 45 | 0 | 11 | 22 | 71 | 14.2 (0.1–76.0) | 5 (0–18) | 19 (3–29) |
| CCSS-Nordic: Nordic countries (9) |
New | 1960–1981 | 1960–1991 | 78 | 1 | 20 | 12 | 16 | 10.1 (<0.1–55.2) | 9 (2–19) | 25 (11–40) |
| CCSS-Fr/UK: France, UK (11) |
New | 1942–2000 | 1946–2009 | 44 | 3 | 22,749 | 42 | 59,085 | 5.6 (<0.1–72.0) | 6 (0–19) | 30 (13–56) |
| CCSS-US: U.S. and Canada (7) |
New | 1970–1986 | 1975–2006 | 47 | 9 | 61,707 | 96 | 127,598 | 10.8 (<0.1– 63.4) | 8 (0–19) | 26 (11–46) |
| Medical radiation: benign disease treatment |
|||||||||||
| Thymus: Rochester, NY (12) |
Updated | 1926–1957 | 1935–2008 | 46 | 7 | 208,347 | 42 | 116,146 | 1.3 (<0.1–18.6) | 0.2 (0–6) | 25 (7–63) |
| Tinea Capitis: Israel (13) |
Updated | 1943–1960 | 1944–2007 | 50 | 54 | 733,027 | 137 | 602,887 | 0.09 (<0.1–0.5) | 7 (0–19) | 35 (11–61) |
| Tonsils (MRH): Chicago, IL (17) |
Updated | 1939–1962 | 1939–2007 | 40 | 0 | 0 | 359 | 115,258 | 0.59 (<0.1–5.8) | 4 (0–15) | 28 (8–65) |
| Tonsils (CHMC): Boston, MA (18) |
No change |
1938–1969 | 1938–1981 | 40 | 0 | 31,438 | 10 | 34,546 | 0.24 (<0.1–0.55) | 6 (0–17) | 29 (10–42) |
| Hemangioma: Stockholm, Sweden (19) |
New | 1920–1959 | 1958–2005 | 67 | 0 | 0 | 35 | 623,515 | 0.25 (<0.1–28.5) | 0.5 (0–1) | 41 (21–77) |
| Hemangioma: Göteborg, Sweden (22) |
New | 1930–1965 | 1958–2005 | 66 | 0 | 0 | 24 | 499,171 | 0.15 (0–10.2) | 0.5 (0–1) | 32 (12–61) |
| Hemangioma (IGR): Francee (24) |
New | 1940–1973 | 1942–2007 | 71 | 2 | 33,477 | 9 | 120,750 | 0.04 (<0.1–5.4) | 1.0 (0–19)) | 29 (6–36) |
| Environmental exposure |
|||||||||||
| Atomic bomb survivorse: Japan (26, 27) |
Updated | 1945 | 1958–2002 | 51 | 67 | 806,648 | 139 | 1,093,420 | 0.18 (<0.1–4.2) | 9 (0–19) | 53 (17–73) |
Notes. Cs = cases; Dx = diagnosis of thyroid cancer; CCSS = Childhood Cancer Survivors Study; LESG = Late Effect Study Group; MRH = Michael Reese Hospital; CHMC = Children’s Hospital Medical Center; IRG = Institut Gustave-Roussy.
Study design: CCSS-LESG and CCSS-Nordic studies were case-control studies and others were cohort studies.
Studies from Ron et al. (4) included without change, denoted “no change”, or with additional follow-up, denoted “updated”, or new to current pooling, denoted “new”.
Number of control subjects from case-control studies or number of exposed person-years from cohort studies.
Person-years weighted mean for cohort studies or control mean for case-control studies.
Subjects with ages at exposure <20 years old. Exposure is defined as ≥0.01 Gy to the thyroid.
Organization of Data
Due to different inclusion criteria and ongoing follow-up, numbers of patients and thyroid cancer cases for individual studies may differ slightly from original publications. Investigators assigned zero dose to patients known to be exposed to radiation but with measurements below the detection limit (DL) (5 mGy in CCSS-US, 1 mGy in CCSS-Fr/UK and Hemangioma-France and 10 mGy in Hemangioma-Sweden). We inserted as the thyroid dose (28). This affected 7,568 patients, including 33 (0.4%) in the CCSS-US, 293 (9%) in the CCSS-Fr/UK, 1,254 (11%) in the Hemangioma-Göteborg, 3,568 (25%) in the Hemangioma-Stockholm and 2,438 (70%) in the Hemangioma-France cohort studies. Since relative risk (RR) trends are only minimally influenced by low doses (29, 30), this simplified imputation should have little impact on results (28).
For most cohort studies, person-time accrual started at first radiation exposure or at enrollment for nonexposed and continued until the earliest date of death, loss to follow-up, incident cancer or end of study. For the Atomic Bomb Survivors study, follow-up and case accrual started in 1958, 13 years after exposure. For the CCSS cohorts, follow-up started five years (CCSS-US) or three years (CCSS-Fr/UK) after first cancer and ended at the earliest date of death, loss to follow-up, occurrence of a second primary cancer or end of study.
For the cohort studies, we cross-tabulated person-years by the following: sex; age at exposure (<1, 1–4, 5–9, 10–14, >15 years); calendar year of follow-up (<1935, 1935–1940, …, 1995–1999, ≥2000); time since exposure (<5, 5–9, 10–14, …, 45–49, ≥50 years); attained age (<10, 10–14, …, 65–69, ≥70 years); any exposure to chemotherapy (yes/no); any exposure to the subgroup of alkylating agents (yes/no); thyroid radiation dose (0, >0–<0.05, 0.05–<0.1, 0.1–<0.2, …, 1–<2, 2–<5, 5–<10, 10–<15, 15–<20, 20–<25, 25–<30, 30–<35, 35<40, ≥40 Gy); number of radiation treatments (0, 1, ≥2), where one treatment represented all doses within six months; and study. Analyses adjusted for additional variables for selected individual studies. For the Israel Tinea Capitis study, variables included country of origin (North Africa/Others) and comparison group (sibling/population). For the Rochester Thymus study, variables included the presence of goiter (yes/no) and Jewish religion (yes/no). For the Atomic Bomb Survivors study, variables included city of exposure (Hiroshima/Nagasaki), not in city at the time of the bombing (yes/no) and enrollment in the Adult Health Study (AHS) (yes/no). We included the latter variable to account for possible surveillance-related differences in background thyroid cancer rates (4). For the CCSS cohorts, variables included type of first cancer (Hodgkin or other) and chemotherapy (yes/no) or treatment with alkylating agents (yes/no). As part of the inclusion criteria, radiation dose estimates were required for all participants. We assumed that the 14 patients (all noncases) from the CCSS-U.S. who were exposed to chemotherapy but had missing information for use of alkylating agents were not exposed to alkylating agents. Also, since most radiation-exposed cases had only one treatment (84%), we assumed exposed participants with missing number of treatments received one treatment. We also computed person-years weighted means within each cell of the cross-tabulation for continuous variables. We used Poisson regression to model disease risk.
For the case-control studies, data were limited and we enlarged categories for study-specific analysis: age at exposure (<1, 1–4, 5–9, ≥10 years), time since exposure (<10, ≥10 years), attained age (<20, ≥20 years) and thyroid radiation dose (0–1, 2–9, 10–29 and ≥30 Gy), with the lowest dose category as the referent for dose-response analysis. We used unconditional likelihood regression for binary (Bernoulli) outcomes for analysis.
For pooled analyses, we used a likelihood that combined binomial and Bernoulli probabilities, since for rare diseases a binomial distribution closely approximates a Poisson distribution. We assumed that each cell of the cross-tabulation was binomially distributed, where the number of cases represented “successes” from person-time “trials”. The likelihood was the product of cell-specific binomial and subject-specific Bernoulli probabilities.
The pooled and case-control analyses estimated odds ratios, while the Poisson regression of cohort data estimated RRs. Since thyroid cancer is rare, these measures are similar and we used RRs throughout for simplicity.
Models for Thyroid Cancer Risk
We modeled thyroid cancer incidence rate, r(x, z, d), in terms of a vector of explanatory variables, x, which varied with analysis but generally included study, sex, attained age, year of birth and other factors, thyroid radiation dose, d, and a vector of potential modifying factors, z. With CCSS data, explanatory variables included type of first cancer and treatment with chemotherapy or alkylating agents. Models had the form:
| (1) |
where r0(x) = exp(θx) described the thyroid cancer incidence rate among non-radiation exposed with θ a vector of parameters and where ERR(d, z) represented the ERR in radiation dose and other factors. We applied the following general linear-exponential form for the ERR:
| (2) |
This formulation incorporated the linear model, γ1 = γ2 = γ3 = γ4 = 0, as well as various forms for the dose-related curvature, involving dose and its natural logarithm. The best-fitting model across the full dose range for all data included a linear component (β) and an exponential curvature factor in dose (γ1), dose squared (γ2) and the logarithm of dose (γ3). We used a likelihood ratio statistic to test hypotheses and a likelihood-based 95% confidence interval (CI) for dose-response parameters.
Using model (2), we evaluated categorical effect modifiers for the linear component using likelihood ratio tests. For an effect modifier z with J levels, we replaced β with (∑j βj zj) where zj was a zero/one indicator variable and βj was the ERR/Gy for the jth category. The likelihood ratio of no effect modification, i.e., β1 = … = βJ, had J − 1 degrees of freedom (df). We evaluated sex, age at exposure, attained age, time since exposure, number of radiation treatments and chemotherapy or treatment with alkylating agents as potential modifiers of the ERR. Data were insufficient to evaluate changes in curvature across effect modifiers. Inference was unchanged when we fitted models with continuous variables for the effect modifiers (see Supplementary Material Part C).
We considered various forms for the joint association of radiation dose and chemotherapy or treatment with alkylating agents. For a binary variable denoting treatment with chemotherapy or alkylating agents (xt = 1) or otherwise (xt = 0), we fitted a multiplicative model:
| (3) |
and an additive model:
| (4) |
where θt represented the ERR for treatment. Equations (3) and (4) were not nested and we used two approaches to embed each model in a larger class to evaluate the fit of each form.
The first approach used a “full” model, in which we replaced the linear dose component β in Eq. (3) with treatment-specific effects, β1 and β0. For chemotherapy and for treatment with alkylating agents, the multiplicative relationship was rejected (P < 0.01 for both), while the additive was not (P = 0.36 and P = 0.91, respectively). We extended this “full” model to allow the curvature components, dose (γ1), its quadratic (γ2) or its logarithm (γ3), to also vary by treatment status, singly and jointly, and found that the additive form remained preferred (not shown). A second approach fitted a geometric mixture, namely,
| (5) |
where λ was the mixing parameter with λ = 1 identifying the multiplicative model and λ = 0 identifying the additive model. This model rejected the multiplicative form (P < 0.01 for both) but not the additive (P = 0.44 for chemotherapy and P = 0.99 for alkylating agents). With both approaches, the additive model, Eq. (4), provided a consistently better characterization of the joint association (Supplementary Material Part C). The following analyses used Eq. (4).
We also analyzed data restricted to ≤2 Gy and additionally fitted power, r(x, z, d) = r0(x) {dδ + θtxt} and exponential, r(x, z, d) = r0(x) {exp(δd) + θtxt}, models.
Finally, for the cohort studies, we fitted an excess absolute risk (EAR) model, r(x, z, d) = r0(x) + EAR(d, z). However, as in previous poolings, the EAR form provided a substantially poorer fit (4, 8) and therefore we omitted these results from the current analyses. We used the Epicure software program with the AMFIT module for Poisson regression and the GMBO module for the case-control analyses and for the Bernoulli/binomial regression of pooled data (31).
RESULTS
Analysis of Individual Studies
A linear model described RR trends for all studies of medical radiation for benign diseases, whereas nonlinear models best fitted the data from the CCSS and the Atomic Bomb Survivors study. For radiation doses <3 Gy, the Atomic Bomb Survivors study exhibited no evidence of departure from linearity Gy (P = 0.45) (Supplementary Table S1). The fitted RR at 1 Gy varied across the studies, ranging from 2.0 (95%CI, 1.3–4.3) in the CCSS-Fr/UK to 22.0 (95%CI, 14.1–34.7) in the Tinea Capitis study. The result from the Tinea Capitis study markedly declined when model (1) included an indicator variable for radiation exposure status as an adjustment factor within x with the fitted RR at 1 Gy reduced from 22.0 (95% CI, 14.1–34.7) to 5.2 (95%CI, 1.5–35.7) (see Supplementary Table S1, footnote “i”). However, the indicator variable was not statistically significant (P = 0.72) and an indicator was not included in the pooled analyses.
For each study, we evaluated effect modification of the radiation risk by sex, age at exposure, attained age, number of treatments and time since exposure (Supplementary Table S2). The fitted RRs did not differ significantly for males and females. There were significantly decreasing risks with increasing age at exposure only for the CCSS-U. S. and Tonsils studies, although several studies showed a similar pattern, albeit nonsignificant. No studies exhibited significant variation in risk by attained age, although the Thymus study had a significantly decreasing trend using attained age as a continuous variable. The ERR/Gy estimates varied significantly with time since exposure, with estimates decreasing in the Thymus study, but increasing in the Tonsils study.
Pooled Analysis: Radiation Dose Response
Mean thyroid radiation dose ranged from 0.04 Gy in the Hemangioma-France study to 14.2 Gy in the Late Effect Study Group (LESG), with individual doses ranging to 76 Gy (Table 1). The pooled data included 1,070 thyroid cancer cases, with cohorts contributing 5.3 million person-years of follow-up (Table 2).
TABLE 2.
Characteristics of Subjects Included in the Pooled Dataa
| Characteristics | Pooled data |
|---|---|
| Thyroid cancer cases | 1,070 |
| Papillary tumors (%) | 79 |
| Females (%) | 66 |
| Controls (c-c studies) | 118 |
| Females (%) | 68 |
| Person-years (cohort studies) | 5,289,800 |
| Females (%) | 55 |
| Follow-up periodb | 1935–2009 |
| Year of first exposure | 1920–2000 |
| Mean age at exposurec (range) | 5 (0–19) |
| Mean time since exposurec (range) | 28.9 (>l0–78.9) |
| Mean age at thyroid cancer diagnosisc (range) | 41 (3–77) |
| Thyroid dose (Gy)c | |
| Mean | 0.71 |
| Median | 0.07 |
| Range | >0–76.0 |
Subjects aged <20 years at exposure.
Follow-up period for cohort studies or case ascertainment period for case-control studies.
Mean-weighted by trials among exposed subjects (see text).
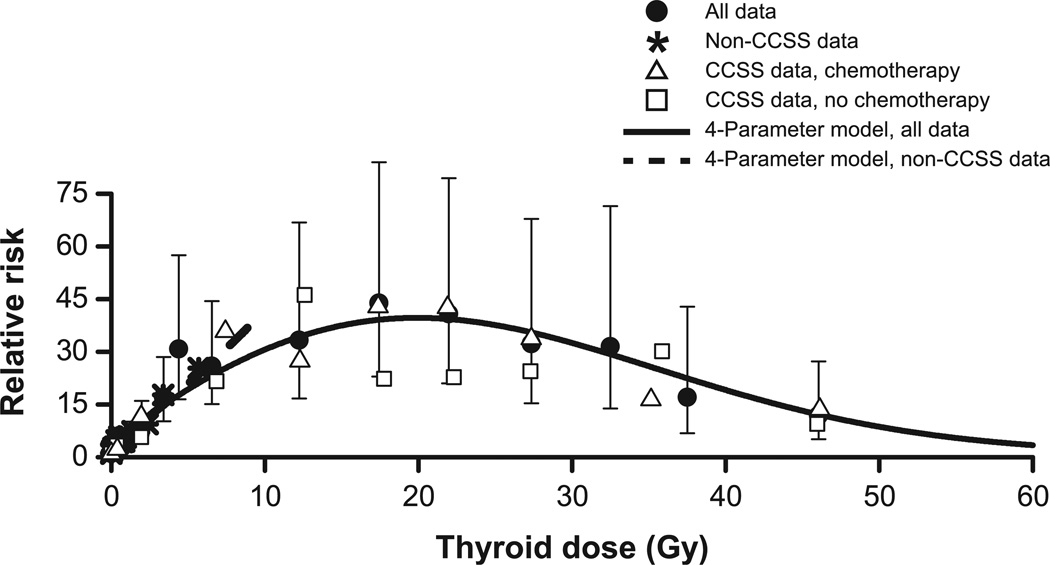
We plotted RRs by radiation dose categories for all data (solid circle) and separately for non-CCSS data (star) and CCSS data for those with (triangle) and without (square) treatment with chemotherapy (Fig. 1; RRs displayed in Supplementary Table S3). For clarity, the Fig. 1 shows only selected CIs for RRs for all data. Relative risks increased at low doses, leveled off between 10 and 30 Gy with RRs of 30–40 and declined above 40 Gy. The model with an exponential curvature function in dose, dose squared and the logarithm of dose was preferred, although a curvature function in dose, the logarithm of dose and its square also fitted well (Table 3). The fitted model closely tracked the category-specific RRs (solid line). The estimate of β was 5.5 (95% CI, 3.9–7.5) and the RR for chemotherapy treatment was 3.6 (95% CI, 1.7–7.2). [Addition of an exposure indicator variable for the Tinea Capitis study did not significantly change the pooled parameter estimates, P = 0.77. Fitted RRs at 1.0 Gy without and with an exposure indicator were 6.5 (95% CI, 5.1–8.5) and 5.6 (95% CI, 4.3, 7.5), respectively.] Results with an additive adjustment for use of alkylating agents were similar (Supplementary Table S4 and Fig. S2).
FIG. 1.
Category-specific relative risks for thyroid radiation dose for all data (solid circle) with selected 95% CIs, for non-CCSS data (star) and CCSS data for those who were (open triangle) or were not (open square) treated with chemotherapy, and the 4-parameter fitted dose-response model to all data (solid line) and to non-CCSS data only (dash line). Display includes full range of doses.
TABLE 3.
Assessment of the Relative Risk (RR) Model with Radiation Dose Under an Additive Joint RR Association for Treatment with Chemotherapya
| Model for R(d, xt) | Deviationb | Parametersc | P valued | AICe | β | γ1 | γ2 | γ3 | γ4 | θ |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 + βd exp(γ1d) + θtxt | 0.0 | 3 | – | 0.0 | 5.67 | −0.064 | 2.22 | |||
| 1 + βd exp(γ1d + γ2d2) + θtxt | 0.0 | 4 | 0.992 | 2.0 | 5.67 | −0.064 | 0.7 × 10−5 | 2.22 | ||
| 1 + βd exp{γ1d + γ3ln(d)} + θtxt | 5.8 | 4 | 0.016 | −3.8 | 6.11 | −0.047 | −0.176 | 1.95 | ||
| 1 + βd exp{γ1d + γ3ln(d) + γ4ln(d)2} θtxt | 9.0 | 5 | 0.011 | −5.0 | 5.98 | −0.070 | −0.115 | 0.053 | 2.24 | |
| 1 + βd exp{γ1d + γ2d2 + γ3ln(d)} + θtxt | 9.7 | 5 | 0.008 | −5.7 | 5.45 | 0.016 | −0.001 | −0.281 | 2.55 |
Note. Analysis includes all data combined.
Model for the excess relative risk (ERR) with continuous radiation dose: ERR(d) = βd exp{γ1d + γ2d2 + γ3ln(d) + γ4ln(d)2}, with linear parameter β and the γ’s parameters defining curvature. Models fitted with an additive adjustment for treatment with chemotherapy, xt, RR(d, xt) = 1 + ERR(d) θxt, with multiplicative adjustment for study, sex, age and other study-specific factors (see text).
Change in model deviation in relationship to the 3-parameter linear-exponential (linear) model.
Number of parameters in ERR for dose and chemotherapy variables.
P value for no improvement in model fit relative to linear-exponential(linear) model.
AIC (Akaike information criterion) adjusted to linear-exponential (linear) model. A smaller value denotes a more preferred model.
Pooled Analysis: Dose Response at Lower Doses
We evaluated RRs at lower doses by testing homogeneity of the β estimates by study type (non-CCSS and CCSS), where study type represented a crude surrogate for lower and higher dose ranges, and found no effect modification (Table 4, P = 0.68). This suggested general model consistency across the dose range. Within the non-CCSS dose range, the 4-parameter model fitted to non-CCSS data only (Fig. 1, dash line) closely followed the category-specific RRs.
TABLE 4.
Number of Radiation Exposed Cases and Parameter Estimates for Modifiers of the Linear Component (β) of the Excess Relative Risk (ERR) Radiation Dose Model with Additive Adjustment for Treatment with Chemotherapya
| Modifier | Cases | β | γ1 | γ2 | γ3 | RR0.2 Gyb | RR10 Gyb | θ |
|---|---|---|---|---|---|---|---|---|
| None | 927 | 5.45 | 0.016 | −0.001 | −0.281 | 2.7 | 30.4 | 2.55 |
| Gender | ||||||||
| Male | 345 | 6.79 | 0.0145 | −0.001 | −0.280 | 3.1 | 37.3 | 2.60 |
| Female | 582 | 5.12 | 2.6 | 28.3 | ||||
| P valuec | 0.35 | |||||||
| Chemotherapy | ||||||||
| No | 797 | 5.47 | 0.009 | −0.001 | −0.277 | 2.7 | 29.1 | 2.67 |
| Yes | 130 | 6.66 | 3.1 | 35.3 | ||||
| P value | 0.36 | |||||||
| Alkylating agentd | ||||||||
| No | 831 | 5.42 | 0.016 | −0.001 | −0.282 | 2.7 | 30.2 | 3.81 |
| Yes | 96 | 5.31 | 2.7 | 29.5 | ||||
| P value | 0.91 | |||||||
| Age at exposure (years) | ||||||||
| <1 | 146 | 9.17 | 0.019 | −0.001 | −0.290 | 3.9 | 51.9 | 2.69 |
| 1–4 | 380 | 7.80 | 3.5 | 44.3 | ||||
| 5–9 | 214 | 4.18 | 2.3 | 24.2 | ||||
| 10–14 | 128 | 3.88 | 2.2 | 22.6 | ||||
| 15–19 | 59 | 1.51 | 1.5 | 9.4 | ||||
| P value | <0.001 | |||||||
| Attained age (years) | ||||||||
| <20 | 109 | 11.54 | –0.021 | −0.001 | −0.162 | 4.0 | 61.4 | 5.89 |
| 20–29 | 248 | 12.23 | 4.2 | 65.0 | ||||
| 30–39 | 284 | 11.41 | 4.0 | 60.7 | ||||
| 40–49 | 141 | 3.59 | 1.9 | 19.8 | ||||
| 50–59 | 101 | 2.06 | 1.5 | 11.8 | ||||
| 60+ | 44 | 1.40 | 1.4 | 8.3 | ||||
| P value | <0.001 | |||||||
| Time since exposure (years) | ||||||||
| <10 | 42 | 2.76 | 0.005 | −0.001 | −0.204 | 1.8 | 17.1 | 3.35 |
| 10–14 | 65 | 3.39 | 1.9 | 20.8 | ||||
| 15–19 | 128 | 8.28 | 3.3 | 49.3 | ||||
| 20–24 | 119 | 7.64 | 3.1 | 45.5 | ||||
| 25–29 | 183 | 14.01 | 4.9 | 82.6 | ||||
| 30–34 | 118 | 9.06 | 3.5 | 53.8 | ||||
| 35–39 | 77 | 4.91 | 2.4 | 29.6 | ||||
| 40–44 | 70 | 3.65 | 2.0 | 22.3 | ||||
| 45–49 | 64 | 3.16 | 1.9 | 19.4 | ||||
| 50+ | 61 | 1.54 | 1.4 | 10.0 | ||||
| P value | <0.001 | |||||||
| Study group | ||||||||
| Non-CCSS | 755 | 5.47 | 0.022 | −0.001 | −0.284 | 2.7 | 31.6 | 1.96 |
| CCSS | 172 | 4.21 | 2.3 | 24.6 | ||||
| P value | 0.68 | |||||||
| Number of treatments (all data)e | ||||||||
| 1 | 783 | 5.51 | 0.016 | −0.001 | −0.276 | 2.7 | 30.9 | 2.61 |
| ≥2 | 144 | 5.36 | 2.9 | 30.1 | ||||
| P valuec | 0.84 | |||||||
| Number of treatments (Non-CCSS data)e,f | ||||||||
| 1 | 636 | 5.55 | –0.301 | 2.0 | –g | |||
| ≥2 | 119 | 5.83 | 2.1 | –g | ||||
| P valuec | 0.73 | |||||||
| Number of treatments (CCSS data)e,f | ||||||||
| 1 | 147 | 5.79 | –0.062 | –g | 32.1 | 2.36 | ||
| ≥2 | 25 | 4.50 | –g | 25.2 | ||||
| P valuec | 0.38 | |||||||
Model for the ERR with continuous radiation dose: ERR(d) = βd exp{γ1d + γ2d2 + γ3ln(d)} with linear parameter β and the γ parameters defining the curvature. Models fitted with an additive adjustment for treatment with chemotherapy, xt, RR(d, xt) = 1 + ERR(d) + θxt. Models adjusted for study, sex, age and other study-specific factors (see text). For modifiers, βd was replaced by (∑j βj zj d), where zj was a zero/one indicator variable for the jth category and βj represented the linear parameter within the jth category. Number of cases reflect radiation exposed only, with nonexposed cases numbering 143.
Fitted RR at 0.2 and 10 Gy, the approximate trials-weighted mean doses in exposed participants in non-CCSS (0.22 Gy) and CCSS (9.3 Gy) studies.
P value for J − 1 degrees of freedom likelihood ratio test of homogeneity, β1 = … = βJ.
Treatment with an alkylating agent replaces chemotherapy as an adjustment variable.
There was no single definition for fractionation across studies. Length of time between fractions, dose per fraction and reason for fractionation differed in each study. In general, one treatment course included all fractions of radiation dose received within six months in most studies and at least a one year interval for tinea capitis.
Evaluation used AIC-preferred models: the linear-exponential (log) model, βj, γ3, for non-CCSS data and the linear-exponential (linear) model, βj, γ1, for the CCSS data.
Not in applicable dose range and estimate omitted.
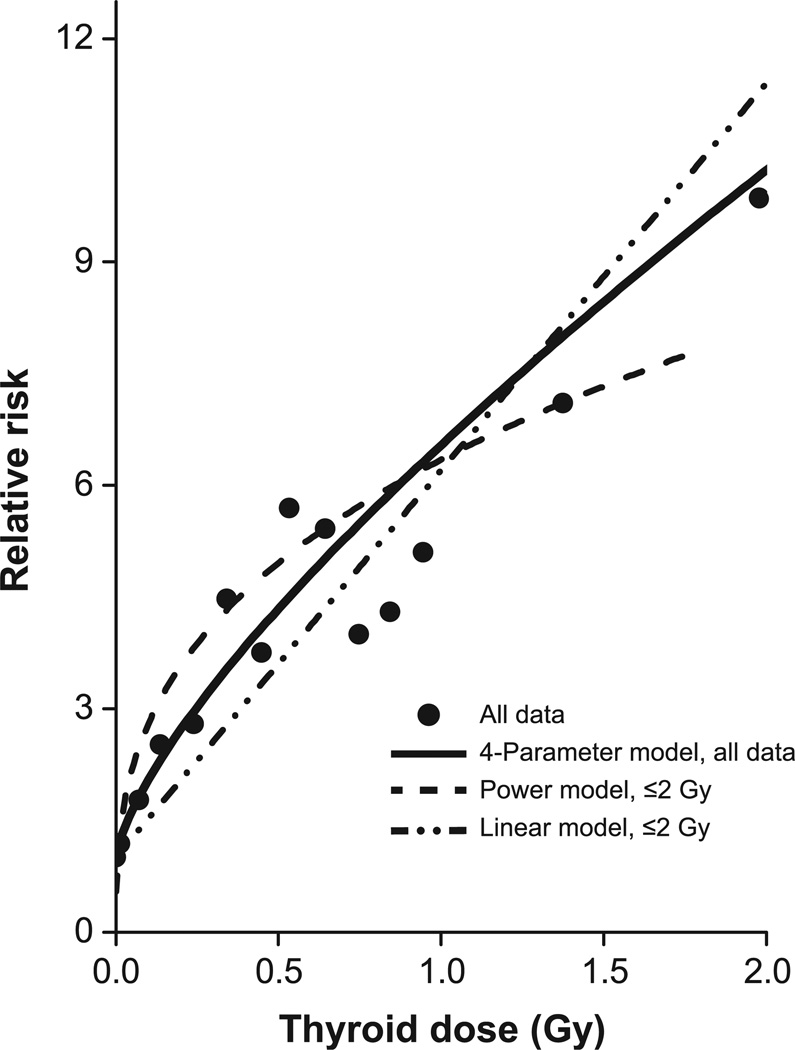
We further evaluated risk at low doses by limiting data to doses ≤2 Gy and fitting several models, including the power (δ), exponential (δ), linear (β), linear-exponential-linear (β and γ1), linear-exponential-log (β and γ3) and full data (β, γ1, γ2 and γ3) models. All models, except the linear and exponential, had similar values for the Akaike information criterion (AIC), with the power model having the smallest AIC (Supplementary Table S5). As shown in Fig. 2, the model fitted to all data (solid line) and the power model fitted to ≤2 Gy (dash line) closely tracked the category-specific RRs (solid symbols), with confidence limits omitted for clarity. For the power model, we assigned 0.01 Gy to zero dose, which minimized model deviation, and centered the plot, using RR = 2.82, the exponential of the case-weighted category-specific ln(RR) and the mean dose of 0.10 Gy. For comparison, we fitted a linear model (dash-dot-dot line), which underestimated RRs below 0.8 Gy. Comparable results occurred with adjustment for use of alkylating agents (Supplementary Table S5 and Fig. S3).
FIG. 2.
Category-specific relative risks for thyroid radiation dose for all data (solid circle) and the 4-parameter fitted dose-response model to all data (solid line) and a power (dash line) and linear (dash-dot-dot line) model to doses ≤2 Gy, with an additive adjustment for use of chemotherapy. For the power model, 0.01 Gy replaced zero doses and the plot is adjusted to reflect the case-weighted category-specific relative risk of 2.82 at a mean dose of 0.10 Gy. Plot uses the same aspect ratio as Fig. 1.
We fitted the full model (β, γ1, γ2 and γ3) with parameters fixed at their full data values (Table 3). The deviation from this model differed only slightly from the 4-parameter model with parameters freely estimated (Supplementary Table S5). Note that the AIC for the fixed 4-parameter model cannot be properly computed since the “fixed” parameter values were in part determined from the restricted data.
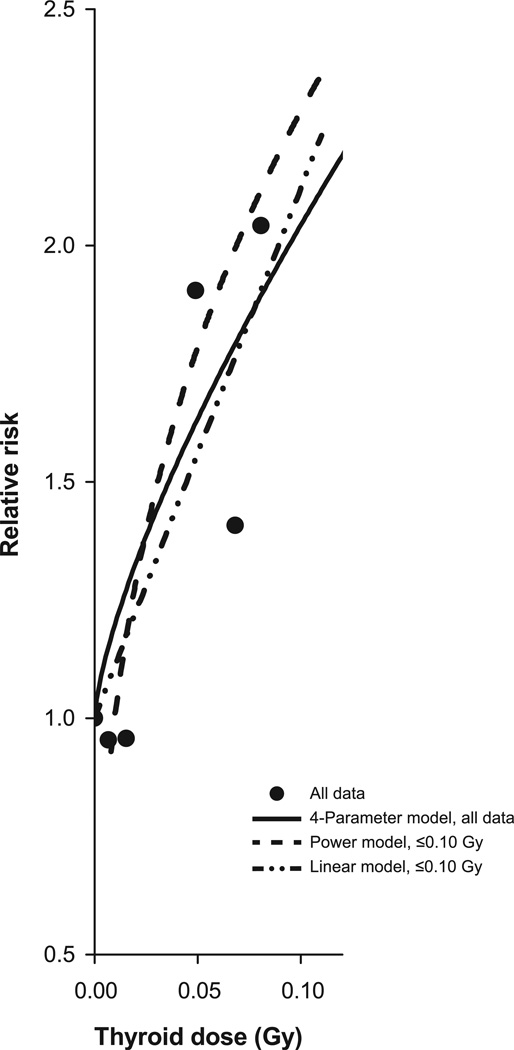
Finally, the trend in RRs continued at lower doses (Fig. 2). We further limited the data to ≤0.10 Gy, including 184 exposed thyroid cancer cases, and found a statistically significant linear trend (P < 0.01), with no evidence of a departure from linearity (P = 0.36) (Fig. 3). The estimate of the ERR/Gy was 11.21 (95% CI, 4.8–19.7). The linear model (dash-dot-dot line), the full data model (solid line) and the power model for doses ≤2.0 Gy (dash line, with plot adjusted to the case-weighted RR of 1.22 and mean dose 0.02 Gy), conformed closely to the category-specific RRs, where quintiles of exposed cases defined the cut points. The plot retained the aspect ratio of Figs. 1 and 2. Fitted RRs at 0.05 Gy were 1.6 (95% CI, 1.2–2.0) for the linear model and 1.6 (95% CI, 1.4–2.1) for the full data model.
FIG. 3.
Category-specific relative risks for thyroid radiation dose for all data (solid circle) and the 4-parameter fitted dose-response model to all data (solid line) and a power (dash line) and linear (dash-dot-dot line) model to doses ≤0.10 Gy, with an additive adjustment for use of chemotherapy. For the power model, 0.01 Gy replaced zero doses and the plot is adjusted to reflect the case-weighted category-specific relative risk of 1.22 at a mean dose of 0.02 Gy. Plot uses the same aspect ratio as Fig. 1.
Pooled Analysis: Modification of the Radiation Dose Response
With adjustment for study, age and other factors, there was no modification of the radiation dose response by sex (P = 0.35) or with use of chemotherapy (P = 0.36) or alkylating agents (P = 0.91) (Table 4). The latter results reflected consistency of the additive adjustment.
The ERR/Gy decreased with attained age (P < 0.001) and varied significantly with age at exposure (P < 0.001) (Table 4). The ERR/Gy was elevated within 10 years of radiation exposure, increased through 20–30 years after exposure, before declining, with the ERR/Gy remaining elevated 50 and more years after exposure. These patterns are shown in Supplementary Fig. S4 along with fitted models using continuous variables for the modifiers.
Finally, there were no differential effects of number of radiation treatments in the full data (P = 0.84) or the subgroups of non-CCSS (P = 0.73) and CCSS (P = 0.38) data.
Histology Specific Results
Among the 1,070 microscopically confirmed thyroid cancer cases, 841 (79%) were papillary cancers, 143 (13%) were nonpapillary cancers (95 follicular, 8 medullary, 5 anaplastic, 17 other histologies and 18 unspecified) and 86 (8%) lacked histologic information. A subset of the follicular cancers occurred before the recognition of the follicular variant of papillary cancer and may, in fact, be papillary cancers. We fitted model (4) to all thyroid cancer cases, to papillary and to non-papillary tumors (Table 5). Results indicated that radiation dose risk patterns with cases restricted to papillary tumors were similar to patterns for all thyroid cancers and that non-papillary tumors were also radiogenic (P < 0.001). Fitted RRs at 0.2 and 10 Gy were higher for non-papillary tumors (Table 5); however, radiation risk patterns for papillary and non-papillary tumors were statistically homogeneous, P = 0.30 (see Supplementary Fig. S5). Exposure to chemotherapy increased thyroid cancer risk for papillary tumors, RR = 2.97 (95% CI, 1.3, 6.6) and non-papillary tumors, RR = 3.72 (95% CI, 0.6, 22.2).
TABLE 5.
Parameter Estimates for the Best Fitting Modela for the Excess Relative Risk (ERR) for All Thyroid Cancers, Papillary Tumors, and Non-Papillary Tumors
| Case groupb | β | γ1 | γ2 | γ3 | θ |
P valuec |
P valued |
RR0.2 Gye (95% CI) |
RR10 Gye (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All thyroid cancers | 5.45 | 0.016 | −0.001 | −0.281 | 2.58 | <0.001 | 0.001 | 2.7 (2.2–3.5) | 30.4 (20.9–44.4) |
| Papillary tumors | 3.68 | 0.055 | −0.002 | −0.377 | 1.97 | <0.001 | 0.009 | 2.4 (1.9–3.1) | 22.4 (14.4–35.3) |
| Non-papillary tumors | 10.78 | −0.009 | −0.0009 | −0.220 | 2.72 | <0.001 | 0.234 | 4.1 (2.4–7.7) | 55.7 (21.2–148.5) |
Model for the ERR with continuous radiation dose: ERR(d) = βd exp{γ1d + γ2d2 + γ3ln(d)}, with linear parameter β and the γ’s parameters defining the curvature. Models fitted with an additive adjustment for treatment with chemotherapy, xt, RR(d, xt) = 1 + ERR(d) + θxt. Models also adjusted for study, sex, age and other study-specific factors (see text).
Among the 1,070 thyroid cancer cases, 841 were papillary tumors, 143 cases were classified as non-papillary tumors (with 95 follicular, 8 medullary, 5 anaplastic, 17 other and 18 unspecified) and 86 cases had no information on histology.
P value for test of no departure from linearity, γ1 = γ2 = γ3 = 0.
P value for test of no effect of chemotherapy (θ = 0).
Fitted RR at 0.2 and 10 Gy, approximately the trials-weighted mean doses in exposed subjects in non-CCSS (0.22 Gy) and CCSS (9.3 Gy) studies.
For doses ≤2 Gy, there were 599 radiation-exposed and 118 nonexposed papillary tumors and 96 exposed and 21 nonexposed non-papillary tumors. Relative risks increased for both papillary and non-papillary tumors in the restricted range. Again, while patterns were statistically homogeneous (P = 0.27), radiogenic risks for non-papillary tumors appeared at least as great as for papillary tumors (Supplementary Fig. S5).
Variations of Dose Effects Across Studies and Influence Analysis
With an additive adjustment for either chemotherapy or alkylating agents, the test of homogeneity of the linear component of the model across studies, i.e., the comparison of Eq. (2) with β and with β1, …, β12 was rejected (P < 0.01). However, participants varied within and between studies. With additional adjustment for age at exposure, tests for homogeneity of the dose effects were still rejected (P = 0.03 and P = 0.05 with additive adjustment for chemotherapy and alkylating agents, respectively). These were fixed effects models, i.e., there was one ERR/Gy for all populations. Assuming random effects for ERR/Gy (β) due to population differences and adjusting for age at exposure, Cochran’s Q statistics resulted in P = 0.11 and P = 0.15 with adjustment for use of chemotherapy or alkylating agents, respectively, which suggested data were compatible with a common ERR/Gy (32–34). We also found no departure from homogeneity across studies grouped as CCSS and non-CCSS (P = 0.68) or by study type, i.e., medical cancer treatment, medical benign disease treatment and environmental exposure studies (P = 0.20).
For doses ≤2 Gy and with a random effects assumption, Cochran’s Q statistics again suggested no departure from homogeneity of the ERR/Gy estimates (P = 0.90 and P = 0.93 with an additive adjustment for chemotherapy or alkylating agents, respectively).
With the model for all data and with modification by age at exposure (Table 4), we omitted each study sequentially to evaluate its influence (Supplementary Fig. S6). The fitted RRs at 0.2 and 10 Gy revealed that no study had an excessive influence on summary results.
DISCUSSION
The radiosensitivity of the thyroid gland is well established, particularly with childhood exposure when cell proliferation is greatest (1–3). The current pooled analysis combined all available studies of environmental and medical exposures, thereby enabling dose-response modeling across the full range of radiation doses to the thyroid. Across the full dose range, RRs increased supralinearly through about 2–4 Gy, leveled at doses between 10 to 30 Gy and thereafter declined. For doses ≤0.10 Gy, including 184 exposed cases, RRs increased significantly (P < 0.01) with no significant departure from linearity (P = 0.36).
The leveling and downturn of RRs at high doses may have reflected a cell killing effect, although RRs remained substantially elevated at high doses, with, for example, a fitted RR estimate at 60 Gy of 3.4 (95% CI, 1.4, 13.4). In their published work, Sachs and Brenner suggested that stem cell repopulation may compensate for cell killing and that accelerated repopulation may explain why risks for solid cancers after radiotherapy remain substantially greater than predictions based solely on initiation and cell-killing mechanisms (35).
The fitted RRs at 1 Gy were 6.5 (95% CI, 5.1–8.5) with an additive adjustment for chemotherapy, which was similar to 6.2 (95% CI, 2.4–19.7) under the preferred linear-exponential-linear model from the previous pooling of CCSS data only (8), although with additive adjustments for chemotherapy. While more precise, our estimate was only slightly lower than the estimated 8.7 at 1 Gy (95% CI, 3.1, 29.7) from the 1995 pooled analysis of populations irradiated primarily at low to moderate doses (4).
One goal of the pooling was to evaluate RRs across the full dose range. We found that the downward extrapolation from high to low doses using the full dose model was consistent both with the category-specific RRs at low doses and with a model based solely on low-dose data. The figures visually confirmed the close correspondence and we found no significant difference in the dose response for non-CCSS and CCSS data (P = 0.68). Nonetheless, a test of homogeneity of the study-specific linear radiation effects, i.e., β1 = …, = β12 was rejected (P < 0.01). This perhaps was unsurprising since, while all studies involved childhood radiation exposures, there were marked differences in ages at exposure, times since exposure and ages at event, all modifiers of risk. However, study populations also differed in calendar years of enrollment, countries of origin and reasons for exposure, suggesting the need to allow for a random radiation effect. Accounting for age at exposure and using a random effects approach (32, 33), we found that linear dose responses were compatible with a common value across populations. This result highlights the importance of accounting for factors such as age at exposure and use of chemotherapy when comparing thyroid radiation-associated risks among diverse studies and when estimating individualized risk (5).
Studies have shown that ERR/Gy estimates generally remained elevated, even with extended time after exposure. Although the ERR/Gy estimate in the pooled data appeared to peak 20–30 years after exposure, it remained elevated throughout the follow-up period, in particular 50 and more years after exposure. This highlights the potential need for continued clinical monitoring of those who were exposed to radiation as children.
The Ron et al. pooling of data suggested a greater ERR/Gy in females compared to males (P = 0.07), although study-specific results were inconsistent (4). Our analysis with more than twice the number of thyroid cancers failed to support a difference. We found similar ERR/Gy estimates by gender (P = 0.35), a finding which agreed with results from the more limited four CCSS pooling (8).
Our analysis provided no consistent support for a differential effect by number of radiation treatments, overall or for data limited to non-CCSS or CCSS studies. However, the null findings may be due to limitations in the data. Harmonization of numbers of treatments proved problematic due to the diverse medical settings and treatment regimens for malignant and benign diseases, the lack of treatment-specific dose estimates and the lack of a consistent definition of treatment dose and dose fraction within treatment and time between periods of treatment. These factors suggested a potential for substantial misclassification of information on fractionation. Alternatively, dose fractionation may be important only at very low doses, <0.10 Gy, a range at which the pooled data have little power to examine this effect (36). Nonetheless, our findings were consistent with dose rate having no effect or at best a weak effect on thyroid cell line survival (37) and largely compatible with results from studies of persons receiving 131I radiation during childhood from the Chernobyl accident (38).
Ron et al. reported two thyroid cancer cases within five years of radiation exposure and no excess risk (4). In the current pooling, there was a statistically significant ERR/Gy estimate of 2.76 (95% CI, 1.3–5.7) within <10 years since exposure, based on 42 exposed cases. We subdivided the category and found 3 and 39 exposed cases <5 years and 5–9 years since exposure, respectively. Like the earlier pooling, we found that the ERR/Gy was not estimable in the former category and was a significant 3.51 (95% CI, 1.6–6.2) in the latter category. This result supports a minimum latency between 5 and 10 years but the few cases do not further inform a lesser latency in relationship to the observed increase in thyroid cancer cases in children in Belarus within three years of the Chernobyl accident (39–41). While screening may have impacted the Belarus findings, the primary concern is the extent that screening acted as a confounding variable to affect the latency results. In our data, nine different studies contributed radiation-exposed thyroid cancer cases that occurred <10 years since irradiation. While we cannot be certain, there is little reason to suppose that thyroid cancer screening was radiation dose dependent, suggesting that the elevated ERR/Gy for <10 years since exposure was not confounded by screening. Note that the CCSS-Fr/UK, CCSS-US and Atomic Bomb Survivor studies made limited or no contribution to this result, since follow-up started three, five and 13 years after exposure, respectively.
Chemotherapeutic agents may themselves be carcinogenic to the thyroid (5–8, 10, 42). After adjustment for radiation exposure, our analysis found a fourfold risk of thyroid cancer with chemotherapy exposure. This created the analytic challenge of evaluating the form of the joint association with radiation. In CCSS-US data, RRs for thyroid cancer were elevated with use of alkylating agents, but mainly among patients with radiation doses <20 Gy (6), while our previous pooling of four CCSS datasets suggested a sub-multiplicative pattern (8). We conducted a more formal evaluation of the synergistic/nonsynergistic relationship for thyroid radiation dose and chemotherapy and demonstrated that an additive, i.e., a nonsynergistic, joint association was consistent with the data, while a multiplicative association was rejected. Under a geometric mixture model, the estimated mixing parameter for radiation and use of chemotherapy or alkylating agents was λ = 0.1 (95% CI, −0.2, 0.6) or λ = 0.0 (95% CI, −0.3, 0.3), respectively, where λ = 0.0 denoted an additive model and λ = 1.0 denoted a multiplicative model. The preference for additivity of RRs for radiation and chemotherapy suggests that the causal effect on thyroid cancer occurrence from either thyroid radiation dose or chemotherapy is not influenced by the presence of the other factor.
A precise evaluation of the joint association of radiation and chemotherapy was problematic and cautious interpretation is warranted. In particular, chemotherapy is a general term that encompasses a variety of compounds, including alkylating agents, anthracyclines and bleomycin and often involves diverse treatment regimens. This diversity prevented a quantitative harmonization across studies and development of a quantitative metric. In addition, combination therapies were common and, except for alkylating agents, data were insufficient to disentangle effects of individual classes of agents or potentially distinct patterns of interaction. Furthermore, if carcinogenic effects were agent specific, then our use of general groupings increased misclassification, which may have biased the association towards additivity.
Papillary and non-papillary cancers comprised 79% and 13% of thyroid cancers in the pooled data, respectively. An early meta-analysis reported a radiation-related excess risk for follicular thyroid cancer, but lower than for papillary cancers (43). In our pooled analysis, non-papillary cancers were radiogenic, although data were insufficient to compare levels of radiation-related risks. This agrees with our pooling of four CCSS datasets, which included a smaller number of non-papillary cancers (n = 32). Further follow-up of the cohort studies may allow a more detailed evaluation of histologic-specific radiogenic effects.
Our analysis did not specifically account for uncertainties in dosimetry, which may affect standard errors and dose response estimates. In the Atomic Bomb Survivors study, we used adjusted estimates of dose that take into account dose measurement error (27). In the Tinea Capitis study, accounting for various measurement uncertainties had little apparent influence on the estimated dose response or the evaluation of effect modifiers (44, 45). The investigators suggested that the similarity of unadjusted and adjusted estimates resulted from the linearity of dose response and a predominance of Berkson-type error. The extent that these conditions may have occurred in the other datasets of the pooling suggested that dose uncertainties may not have greatly influenced the modeling of thyroid cancer risk in the pooled data.
Our analyses identified a consistent risk model across the full range of external radiation doses to the thyroid, with RRs increasing approximately supralinearly through 2–4 Gy, then leveling and declining above approximately 30 Gy, although RRs remained elevated. Radiogenic effects occurred for both papillary and non-papillary tumors. For doses ≤0.10 Gy, RRs increased significantly with dose, with no significant departure from linearity. Data supported a nonsynergistic (additive) association for thyroid radiation dose and use of chemotherapy for a first primary cancer in childhood. Importantly, significant thyroid cancer risks appeared within five to ten years of radiation exposure and remained elevated for potentially many decades.
Supplementary Material
Acknowledgments
This study was supported by the Intramural Research Program of the U.S. National Institutes of Health (NIH), National Cancer Institute, Division of Cancer Epidemiology and Genetics. The Childhood Cancer Survivor Study was funded by the National Cancer Institute (grant no. U24 CA55727), the Children’s Cancer Research Fund, the Lance Armstrong Foundation (grant no. 147149) and the Intramural Research Program of the NIH, National Cancer Institute, Division of Cancer Epidemiology and Genetics. We would also like to acknowledge members of the Nordic Countries Childhood Cancer Survivor Study Group, who have contributed to the Nordic Countries Childhood Cancer Survivor Study, including: H. Hertz, J. Olsen (Denmark); G. Jonmundsson, H. Tulinius (Iceland); M. Lanning, R. Sankila (Finland); H. Døllner, F. Langmark (Norlway) and H. Anderson, S. Garwicz, T. Möller and G. Svahn-Tapper (Sweden). We particularly wish to recognize the late Dr. Elaine Ron, who initiated and guided this pooling project.
Footnotes
Supplementary materials are available online at: http://dx.doi.org/10.1667/RR14213.1.S1.
REFERENCES
- 1.Ron E, Schneider AB. Thyroid Cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. 3rd. New York: Oxford University Press, Inc.; 2006. pp. 975–994. [Google Scholar]
- 2.UNSCEAR. Sources and Effects of Ionizing Radiation 2013 Report to the General Assembly, with Scientific Annexes. New York: United Nations Publications; 2013. [Google Scholar]
- 3.Saad AG, Kumar S, Ron E, Lubin JH, Stanek J, Bove KE, et al. Proliferative activity of human thyroid cells in various age groups and its correlation with the risk of thyroid cancer after radiation exposure. J Clin Endocrinol Metab. 2006;91:2672–2677. doi: 10.1210/jc.2006-0417. [DOI] [PubMed] [Google Scholar]
- 4.Ron E, Lubin JH, Shore R, Mabuchi K, Modan B, Pottern LM, et al. Thyroid cancer after exposure to external radiation - a pooled analysis of 7 studies. Radiat Res. 1995;141:259–277. [PubMed] [Google Scholar]
- 5.Kovalchik SA, Ronckers CM, Veiga LH, Sigurdson AJ, Inskip PD, de Vathaire F, et al. Absolute risk prediction of second primary thyroid cancer among 5-year survivors of childhood cancer. J Clin Oncol. 2013;31:119–127. doi: 10.1200/JCO.2012.41.8996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veiga LHS, Bhatti P, Ronckers CM, Sigurdson AJ, Stoval M, Smith SA, et al. Chemotherapy and thyroid cancer risk: a report from the Childhood Cancer Survivor Study. Cancer Epidemiol Biomarkers Prev. 2012;21:92–101. doi: 10.1158/1055-9965.EPI-11-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatti P, Veiga LHS, Ronckers CM, Sigurdson AJ, Stovall M, Smith SA, et al. Risk of second primary thyroid cancer after radiotherapy for a childhood cancer in a large cohort study: an update from the childhood cancer survivor study. Radiat Res 174. 2010:741–752. doi: 10.1667/RR2240.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veiga LH, Lubin JH, Anderson H, de Vathaire F, Tucker M, Bhatti P, et al. A pooled analysis of thyroid cancer incidence following radiotherapy for childhood cancer. Radiat Res. 2012;178:365–376. doi: 10.1667/rr2889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svahn-Tapper G, Garwicz S, Anderson H, Shamsaldin A, de Vathaire F, Olsen JH, et al. Radiation dose and relapse are predictors for development of second malignant solid tumors after cancer in childhood and adolescence: A population-based case-control study in the five Nordic countries. Acta Oncol. 2006;45:438–448. doi: 10.1080/02841860600658633. [DOI] [PubMed] [Google Scholar]
- 10.Tucker MA, Jones PHM, Boice JD, Robison LL, Stone BJ, Stovall M, et al. Therapeutic radiation at a young age is linked to secondary thyroid cancer. Cancer Res. 1991;51:2885–2888. [PubMed] [Google Scholar]
- 11.de Vathaire F, Hardiman C, Shamsaldin A, Campbell S, Grimaud E, Hawkins M, et al. Thyroid carcinomas after irradiation for a first cancer during childhood. Arch Intern Med. 1999;159:2713–2719. doi: 10.1001/archinte.159.22.2713. [DOI] [PubMed] [Google Scholar]
- 12.Adams MJ, Shore RE, Dozier A, Lipshultz SE, Schwartz RG, Constine LS, et al. Thyroid cancer risk 40+ years after irradiation for an enlarged thymus: an update of the Hempelmann Cohort. Radiat Res. 2010;174:753–762. doi: 10.1667/RR2181.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadetzki S, Chetrit A, Lubina A, Stovall M, Novikov I. Risk of thyroid cancer after childhood exposure to ionizing radiation for tinea capitis. J Clin Endocrinol Metab. 2006;91:4798–4804. doi: 10.1210/jc.2006-0743. [DOI] [PubMed] [Google Scholar]
- 14.Ron E, Modan B, Preston D, Alfandary E, Stovall M, Boice JD. Thyroid neoplasia following low-dose radiation in childhood. Radiat Res. 1989;120:516–531. [PubMed] [Google Scholar]
- 15.Ron E, Modan B. Benign and malignant thyroid neoplasms after childhood irradiation for tinea capitis. J Natl Cancer Inst. 1980;65:7–11. [PubMed] [Google Scholar]
- 16.Schneider AB, Ron E, Lubin J, Stovall M, Gierlowski TC. Dose-response relationships for radiation-induced thyroid cancer and thyroid nodules: evidence for the prolonged effects of radiation on the thyroid. J Clin Endocrinol Metab. 1993;77:362–369. doi: 10.1210/jcem.77.2.8345040. [DOI] [PubMed] [Google Scholar]
- 17.Mihailescu D, Shore-Freedman E, Mukani S, Lubin J, Ron E, Schneider AB. Multiple neoplasms in an irradiated cohort: pattern of occurrence and relationship to thyroid cancer outcome. J Clin Endocrinol Metab. 2002;87:3236–3241. doi: 10.1210/jcem.87.7.8701. [DOI] [PubMed] [Google Scholar]
- 18.Pottern LM, Kaplan MM, Larsen PR, Silva JE, Koenig RJ, Lubin JH, et al. Thyroid nodularity after childhood irradiation for lymphoid hyperplasia - a comparison of questionnaire and clinical findings. J Clin Epidemiol. 1990;43:449–460. doi: 10.1016/0895-4356(90)90133-a. [DOI] [PubMed] [Google Scholar]
- 19.Eidemueller M, Holmberg E, Jacob P, Lundell M, Karlsson P. Breast cancer risk after radiation treatment at infancy: potential consequences of radiation-induced genomic instability. Radiat Prot Dosimetry. 2011;143:375–379. doi: 10.1093/rpd/ncq473. [DOI] [PubMed] [Google Scholar]
- 20.Furst CJ, Lundell M, Holm LE, Silfversward C. Cancer incidence after radiotherapy for skin hemangioma - a retrospective cohort study in Sweden. J Natl Cancer Inst. 1988;80:1387–1392. doi: 10.1093/jnci/80.17.1387. [DOI] [PubMed] [Google Scholar]
- 21.Lundell M, Hakulinen T, Holm LE. Thyroid cancer after radiotherapy for skin hemangioma in infancy. Radiat Res. 1994;140:334–339. [PubMed] [Google Scholar]
- 22.Lindberg S. Radiotherapy of childhood haemangiomas: From active treatment to radiation risk estimates. Radiat Environ Biophys. 2001;40:179–189. doi: 10.1007/s004110100103. [DOI] [PubMed] [Google Scholar]
- 23.Lindberg S, Karlsson P, Arvidsson B, Holmberg E, Lundberg LM, Wallgren A. Cancer incidence after radiotherapy for skin hemangioma during infancy. Acta Oncologica. 1995;34:735–740. doi: 10.3109/02841869509127180. [DOI] [PubMed] [Google Scholar]
- 24.Haddy N, Andriamboavonjy T, Paoletti C, Dondon MG, Mousannif A, Shamsaldin A, et al. Thyroid adenomas and carcinomas following radiotherapy for a hemangioma during infancy. Radiother Oncol. 2009;93:377–382. doi: 10.1016/j.radonc.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Shamsaldin A, Lundell M, Diallo I, Ligot L, Chavaudra J, de Vathaire F. Estimation of the radiation dose from radiotherapy for skin haemangiomas in childhood: the ICTA software for epidemiology. Phys Med Biol. 2000;45:3589–3599. doi: 10.1088/0031-9155/45/12/306. [DOI] [PubMed] [Google Scholar]
- 26.Preston DL, Ron E, Tokuoka S, Funamoto S, Nishi N, Soda M. Solid cancer incidence in atomic bomb survivors: 1958–1998. Radiat Res. 2007;168:1–64. doi: 10.1667/RR0763.1. [DOI] [PubMed] [Google Scholar]
- 27.Furukawa K, Preston D, Funamoto S, Yonehara S, Ito M, Tokuoka S, et al. Long-term trend of thyroid cancer risk among Japanese atomic-bomb survivors: 60 years after exposure. Int J Cancer. 2013;132:1222–1226. doi: 10.1002/ijc.27749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 29.Schisterman EF, Vexler A, Whitcomb BW, Liu AY. The limitations due to exposure detection limits for regression models. Am J Epidemiol. 2006;163:374–383. doi: 10.1093/aje/kwj039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston DL, Lubin JH, Pierce DA, McConney ME. Epicure users guide. Seattle: HiroSoft International Corporation; 2008. [Google Scholar]
- 32.Dersimonian R, Laird N. Metaanalysis in clinical-trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.DerSimonian R, Kacker R. Random-effects model for metaanalysis of clinical trials: An update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 35.Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci U S A. 2005;102:13040–13045. doi: 10.1073/pnas.0506648102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.UNSCEAR. Effects of Ionizing Radiation, 2006 Report to the General Assembly, with Scientific Annexes. New York: United Nations Publications; 2006. [Google Scholar]
- 37.Challeton C, Branea F, Schlumberger M, Gaillard N, DeVathaire F, Badie C, et al. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys. 1997;37:163–169. doi: 10.1016/s0360-3016(96)00449-x. [DOI] [PubMed] [Google Scholar]
- 38.Ron E. Thyroid cancer incidence among people living in areas contaminated by radiation from the Chernobyl accident. Health Phys. 2007;93:502–511. doi: 10.1097/01.HP.0000279018.93081.29. [DOI] [PubMed] [Google Scholar]
- 39.Baverstock K, Egloff B, Pinchera A, Ruchti C, Williams D. Thyroid cancer after Chernobyl. Nature. 1992;359:21–22. doi: 10.1038/359021b0. (Scientific Correspondence) [DOI] [PubMed] [Google Scholar]
- 40.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:21–21. doi: 10.1038/359021a0. (Scientific Correspondence) [DOI] [PubMed] [Google Scholar]
- 41.Heidenreich WF, Bogdanova TI, Biryukov AG, Tronko ND. Time trends of thyroid cancer incidence in Ukraine after the Chernobyl accident. J Radiol Prot. 2004;24:283–293. doi: 10.1088/0952-4746/24/3/007. [DOI] [PubMed] [Google Scholar]
- 42.Sigurdson AJ, Ronckers CM, Mertens AC, Stovall M, Smith SA, Liu Y, et al. Primary thyroid cancer after a first tumour in childhood (the Childhood Cancer Survivor Study): a nested case-control study. Lancet. 2005;365:2014–2023. doi: 10.1016/S0140-6736(05)66695-0. [DOI] [PubMed] [Google Scholar]
- 43.Shore RE. Issues and epidemiologic evidence regarding radiation-induced thyroid cancer. Radiat Res. 1992;131:98–111. [PubMed] [Google Scholar]
- 44.Lubin JH, Schafer DW, Ron E, Stovall M, Carroll RJ. A reanalysis of thyroid neoplasms in the Israeli Tinea Capitis Study accounting for dose uncertainties. Radiat Res. 2004;161:359–368. doi: 10.1667/rr3135. [DOI] [PubMed] [Google Scholar]
- 45.Schafer DW, Lubin JH, Ron E, Stovall M, Carroll RJ. Thyroid cancer following scalp irradiation: A reanalysis accounting for uncertainty in dosimetry. Biometrics. 2001;57:689–697. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.