Abstract
Research in the area of stem/progenitor cells has led to the identification of multiple stem-like cell populations implicated in prostate homeostasis and cancer initiation. Given that there are multiple cells that can regenerate prostatic tissue and give rise to prostate cancer, our focus should shift to defining the signaling mechanisms that drive differentiation and progenitor self-renewal. In this article, we will review the literature, present the evidence and raise important unanswered questions that will help guide the field forward in dissecting critical mechanisms regulating stem-cell differentiation and tumor initiation.
Keywords: prostate, carcinoma
Introduction
The study of the control of epithelial differentiation began with the so-called ‘Hen’s tooth’ experiment. Primitive epithelium from chicken beak could be instructed to develop into teeth when recombined with mouse embryonic dermal papilla, even though the ancestor of modern birds lost the ability to grow teeth ~100–80 million years ago (Kollar & Fisher 1980). This ingenious experiment demonstrated that epithelial differentiation was not simply a cell-autonomous event, and it was subsequently demonstrated across multiple organs that tissue interactions maintain the stem cell niche and dictate epithelial cell fate. Multiple lines of evidence have now demonstrated that organs harbor tissue-restricted multipotent progenitors into adulthood. The regulation of these progenitors has been the subject of intense research and debate with implications for a wide range of diseases, including those of the prostate.
Because of the resistance of prostate progenitor cells to current anti-androgen therapies, research has focused on the identification of cells of origin for benign and malignant prostatic growth. Striking similarities in the regulation of epithelial stem cell niches have been recognized across various tissues (Blanpain et al. 2007), which may provide clues to the regulation of epithelial differentiation and androgen-independent growth of initiated stem cells in the prostate. There is ample evidence that multiple cell types can act as progenitors for fully differentiated secretory luminal cells but also as cells of origin for prostate tumors (Fig. 1), which may relate to the genotypic heterogeneity in prostate cancer. We suggest that a deeper understanding of the mechanisms that govern cell fate decisions in prostate development, homeostasis and disease may provide new avenues for patient-specific treatments.
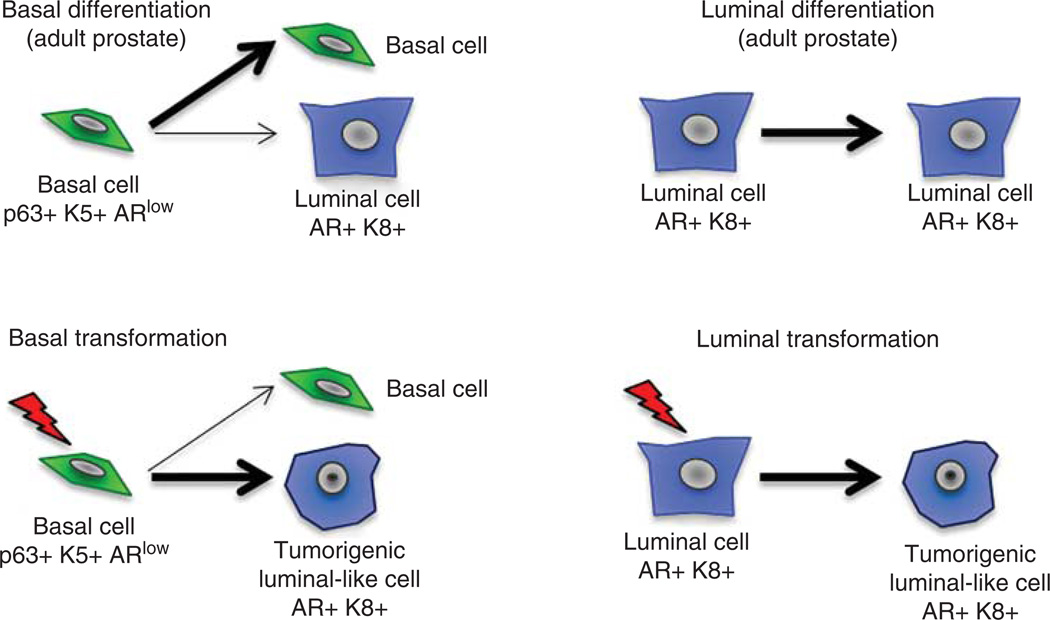
Figure 1.
Model of prostate epithelial homeostasis and cancer initiation. In the benign prostate, basal cells and luminal cells predominantly self-renew to make more of themselves, while rare basal cells differentiate into the luminal lineage. Upon basal cell transformation, basal to luminal differentiation is enhanced and cancers become driven by tumorigenic luminal cells in the absence of the initiating basal cells. Luminal transformation rapidly gives rise to tumorigenic luminal cells.
Prostate diseases
The normal prostate is around 20 g in men between 21 and 30 years old. Benign prostate hyperplasia (BPH) of the transition zone is a common age-related disorder, observed histologically in 50% of men over 50 with doubling time of 4.5 years in men between 51 and 70 years old (Berry et al. 1984). Lower urinary tract symptoms (LUTS) due to an enlarged prostate are predominantly treated with 5 alpha reductase inhibitors (5ARI), which reduce prostate volume ~25%, predominantly through apoptosis of androgen-dependent epithelia (The Finasteride Study Group 1993). Baseline prostate volume is the most reliable indicator of future resistance to 5ARI therapy (Roehrborn 2006), making the understanding of androgen-independent prostate growth crucial to slowing symptomatic progression.
Prostate cancer is the most common non-cutaneous malignancy and second leading cause of cancer mortality in Western men, and has been treated with surgical and chemical androgen deprivation therapy for 60 years (Huggins & Hodges 2002). Because of the inability to predict which tumors will progress to cause lethality, biopsy and surgical intervention are necessarily overused (Vickers et al. 2011, Schroder et al. 2012). Although most men initially respond to androgen deprivation, castration-resistant cancer almost universally recurs (The Leuprolide Study Group 1984). Furthermore, our continued effort at better targeting androgen signaling has not drastically improved survival, due to tumor cell compensatory mechanisms (Antonarakis et al. 2014).
The design of new treatments for androgen-independent progression of BPH and prostate cancer relies on a deeper understanding of the extrinsic and intrinsic regulators of prostate epithelial differentiation. Persistent growth in both benign and malignant disease has led many to suggest that progression is driven either by clonal evolution (Gundem et al. 2015, Hong et al. 2015) or by androgen-independent progenitors giving rise to androgen-dependent progeny. The search for these progenitors has revealed a deeper understanding of the lineage hierarchy of prostate epithelium, but many questions remain as to the triggers that regulate self-renewal and differentiation. The answers to these questions may provide a way to therapeutically target the tumor-propagating cell types.
Prostate glandular composition and the origins of disease
Prostate glands are composed of a pseudostratified bilayer of basal and luminal epithelium, which are positional terms that do not fully reflect the cellular subtypes within each layer (Abate-Shen & Shen 2000). The basal epithelial layer is believed to contain a small (<5%) population of multipotent stem cells, which are thought to give rise to committed basal, transit amplifying, intermediate cell phenotypes and the luminal/secretory layer, which also contains a small population (<1%) of progenitors (Uzgare et al. 2004, Xin et al. 2007, Wang et al. 2009, Rane et al. 2014). The basal and luminal epithelial layers are thought to be important in the pathogenesis of both benign and malignant prostate disease, albeit for different reasons (De Marzo et al. 1998). Proliferation of basal/stem cells is thought to contribute to benign prostatic enlargement, although direct evidence for this is still lacking (Dermer 1978, Isaacs & Coffey 1989). Alternatively, primary prostate cancers are characterized by the loss of basal epithelium (Brawer et al. 1985) and the multiclonal expansion of luminal epithelial foci (Grisanzio & Signoretti 2008, Lindberg et al. 2013); however, after androgen deprivation therapy, residual tumor-propagating cells repopulate the de-bulked tumor (Germann et al. 2012) and lead to lethal monoclonal metastases (Liu et al. 2009). BPH nodules also appear to be clonal (Blackwood et al. 2011, Gaisa et al. 2011) and many men are, or become resistant to 5ARI therapy (McConnell et al. 2003), which may be due at least in part to androgen-independent growth mechanisms in basal cells (Isaacs 2008, Bauman et al. 2014, Lin-Tsai et al. 2014).
Early evidence that basal cells could give rise to luminal cells in the adult was shown by [3H]-thymidine uptake and Ki67 immunoreactivity in BPH tissue, which showed that actively dividing cells were predominantly localized to the basal compartment (Dermer 1978, Bonkhoff et al. 1994). This observation led to the hypothesis that a resident stem cell within the basal compartment could give rise to a luminal cell since the ratio of basal to luminal cells was unchanged (Isaacs & Coffey 1989). Many have since demonstrated that an indigenous, androgen-independent prostate progenitor cell survives castration and can repopulate the luminal layer upon re-administration of androgen (English et al. 1987, Verhagen et al. 1988, Wang et al. 2009, Germann et al. 2012, Shi et al. 2014). The development of cell lineage tracing in mouse models coupled with antibody-based cell sorting and ex vivo culturing have more specifically identified both basal and luminal progenitors that contribute to glandular development, adult homeostasis and post-castration regeneration (Collins et al. 2001, Wang et al. 2009, Ousset et al. 2012, Shi et al. 2014), and these same markers can be used to identify and study progenitors in human prostate (Goldstein et al. 2008, Karthaus et al. 2014).
Developmental signaling in the urogenital mesenchyme directs solid cords of p63-positive epithelial progenitors to bud from the urogenital sinus epithelium and give rise to basal, luminal and neuroendocrine cell types (Signoretti et al. 2005). Cell lineage tracing has confirmed that these p63+, ck14+ progenitors give rise to all epithelial lineages through asymmetrical divisions during development, but that fully differentiated luminal cells are derived mainly from symmetrical division of luminal progenitors in the homeostatic adult (Choi et al. 2012, Ousset et al. 2012, Wang et al. 2014a). However, stress conditions during adulthood such as androgen deprivation and inflammation drive luminal cell differentiation from both basal and luminal progenitors (Wang et al. 2013, Kwon et al. 2014).
In adulthood, a subset of basal epithelial cells are multipotent and have been reported to be enriched or isolated based on either expression of various cell surface markers, including Sca-1, CD44, CD49f, α2β1 integrin, Trop2, CD117 and CD133, and the capacity for self-renewal in serially passaged 3D cultures (Collins et al. 2001, Bhatt et al. 2003, Hudson 2004, Richardson et al. 2004, Xin et al. 2005, Goldstein et al. 2008, Leong et al. 2008, Garraway et al. 2010, Ousset et al. 2012). The luminal layer also contains a small population of CK18+, Nkx3.1+ progenitors that are resistant to castration and can contribute to the repopulation of luminal secretory cells after re-administration of androgen (English et al. 1987, Leong et al. 2008, Wang et al. 2009, Chua et al. 2014, Shi et al. 2014). Finally, recent evidence in mice suggests that a label-retaining progenitor population that expands following castration and regeneration is CD133+, Sca-1+, CD44+, CD49f+ and CD117+, but also AR+ (Shi et al. 2014). A similar α2β1Hi, CD133+ progenitor enriched by FACS from human prostate was also shown to express functional androgen receptor (AR) (Williamson et al. 2012), but it’s still unclear whether androgen deprivation therapy may inadvertently expand a progenitor population capable of tumor propagation or 5ARI-resistant BPH.
Models for the characterization of progenitors
There is still considerable controversy over the identity of the tumor-propagating cell in prostate cancer, predominantly because of the various techniques used to define progenitor cell properties. Flow cytometry, serial passaging of ex vivo 3D spheres, tissue regeneration with inductive mesenchyme and genetic lineage tracing are each used to characterize whether a cell displays the ability to self-renew or differentiate. Prostate stem cells were originally isolated by flow cytometry using cell surface markers that were enriched either in functionally analogous epidermal stem cells (Collins et al. 2001) or after castration (Lawson et al. 2007, Goldstein et al. 2008).
Functional characterization of the self-renewal and differentiation capacity of these putative prostate stem cell populations is accomplished using tissue regeneration with inductive mesenchyme followed by kidney capsule xenografting (Xin et al. 2003) as well as serial passaging as 3D spheres (Collins et al. 2001, Xin et al. 2007). These studies clarify that only a subpopulation of basal cells has the capacity to self-renew or give rise to differentiated progeny in vivo. However, lineage tracing studies of basal cell self-renewal and differentiation in the homeostatic adult indicate that, when removed from their endogenous tissue microenvironment, the plasticity of basal cells in 3D cultures or tissue regeneration, xenografting causes an overestimation of the physiological relevance of their contribution as progenitors of luminal epithelium, mimicking non-homeostatic conditions such as castration, inflammation or initiation (Wang et al. 2013, 2014b). We will look at the meaningful differences and overlapping contributions of each model system.
Mouse models
Due to the emergence of castration-resistant lethal cell types after hormonal therapy, there has been an intense search for the tumor cell of origin in order to therapeutically target the tumor-propagating cell type. In contrast to reductionist cell culture models, mouse models provide an experimental system to test the effects of genetic manipulation in a cell’s native environment. For many years, oncogenes and tumor suppressor genes were driven or knocked out in mice using the probasin promoter, which is predominantly expressed in luminal epithelium (Grabowska et al. 2014). The realization that the basal epithelium contains a stem cell population and many of the tumor suppressors (e.g., PTEN, Notch, p53, p63, Bcl-2) and oncogenes (e.g., c-MYC, β-catenin) altered in human prostate cancer progression prompted the generation of mouse models using promoters from genes expressed in basal cells (CK5, CK14) in order to determine whether a basal cell could be a tumor cell of origin (Abate-Shen & Shen 2000). Direct comparisons of genes using basal and luminal promoters suggests that basal and luminal cells can each serve as targets of prostate cancer initiation (Wang et al. 2006, Korsten et al. 2009, Choi et al. 2012), but that tumors propagated by transformed luminal cells more closely resemble human prostate carcinomas (Wang et al. 2014b).
Tissue regeneration
Early work by Cunha & Lung (1978) identified presumptive stroma, or mesenchyme, as essential for prostate-specific epithelial differentiation. Cunha et al. (1983a,b) went on to show, using heterotypic tissue recombination, that mesenchyme from any species could instruct epithelium from other endodermal lineages to differentiate into a new cell type. Various androgen-regulated stromal factors were shown as paracrine factors (insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), vascular endothelial growth factor (VEGF), Wnt) that could regulate epithelial differentiation, though these ‘andromedins’ are still poorly characterized (Thomson 2008). These studies demonstrated the powerful extrinsic control of tissue interactions on epithelial differentiation during development and paved the way for the study of stromal alterations that contribute to prostate cancer progression (Strand et al. 2010).
Lineage tracing in mouse models demonstrates that initiation of basal cells causes both progenitor cell enrichment (Mulholland et al. 2009) and basal-to-luminal differentiation (Lu et al. 2013, Wang et al. 2013). Concordant results are seen when murine or human basal epithelium isolated by FACS and initiated with oncogenes ex vivo recapitulate the histological and molecular features of human prostate cancer upon tissue regeneration with inductive fetal mesenchyme (Goldstein et al. 2010, Lawson et al. 2010). Furthermore, luminal tumors can be serially propagated in the absence of basal cells (Stoyanova et al. 2013). What is still unclear is whether the serially passaged tumor is propagated by an emergent intermediate or luminal progenitor derived from the transformed basal cell.
While the majority of differentiation events occur from basal to luminal cells, luminal to basal cell differentiation has also been observed in both lineage-traced mice and in ex vivo organoid cultures (Karthaus et al. 2014). Regardless of the representative numbers of specific progenitor populations in vivo, the functional capacity of distinct basal and luminal progenitors to give rise to fully differentiated luminal progeny, especially under inflamed or castrate conditions, is of utmost importance for developing therapeutic strategies to target androgen-independent progenitors.
Cell culture
Because of the limited access to patient samples and the limited amount of starting material to work with, one of the most promising advances in prostate stem cell research is the optimization of culture conditions for expanding and differentiating stem cells in culture (Sato et al. 2011, Clevers et al. 2014). The inductive power of fetal mesenchyme in driving epithelial differentiation is still poorly understood (Cunha & Lung 1978), so optimizing the media conditions necessary for feeder-free cultures will provide the reductionist systems necessary for dissecting the mechanisms responsible for cell fate decisions.
The cancer stem cell hypothesis states that a genetically unstable progenitor retains unlimited self-renewal while a subset of its progeny still matures to a luminal-like secretory cell phenotype (Visvader & Lindeman 2012). Prostate carcinomas display luminal exocrine, neuroendocrine and intermediate cell phenotypes. The transit amplifying or intermediate cells are proposed to be the progenitors within the tumor and consequently targets for androgen-independent progression (van Leenders & Schalken 2003).
In order to understand the underlying mechanisms of self-renewal and differentiation, a variety of different culture models have been attempted for the propagation and differentiation of prostate epithelial progenitors. Litvinov et al. show that low-calcium, serum-free media can select for CD133+ /ABCG2+ /α2β1Hi /p63+ /PSCA− /AR− /PSA− stem cells from primary human prostate as well as immortalized human prostate epithelial cultures, and that the selected stem cells could give rise to both neuroendocrine and CD133− /p63+ /PSCA+ intermediate cell lineages. However, the intermediate cell lineage could not be fully differentiated into CD133−/p63−/PSCA− /AR+/PSA+ secretory luminal epithelium even after the addition of dihydrotestosterone (DHT) (Litvinov et al. 2006).
Taking this work one step forward, Heer et al. (2006) used flow cytometry to sort human prostate CD133−/α2β1Hi transit amplifying cells and CD133+/α2β1Hi stem cells to determine the mechanisms that cause terminal differentiation into luminal secretory cells. They demonstrate that keratinocyte growth factor (KGF) can drive transit amplifying progenitor (TAP) differentiation by downregulating β1 integrin through p38 activation. In addition, they demonstrate that CD133− /α2β1Hi transit amplifying cells express AR mRNA, but AR protein is under constant proteasomal degradation. The CD133+ /α2β1Hi stem cell population did not express AR mRNA or protein (Heer et al. 2007).
Lamb et al. (2010) developed a primary human prostate epithelial cell stratification model where a confluent population of K5+ /K14+ /Bcl− 2+ /EGFR+ /AR− basal cells would give rise to overlaying patches of fully differentiated K18+ /K19+ /AR+ /Nkx3.1+ /TMPRSS2+ secretory luminal cells after 14 days in culture with DHT and the stromal derived factors FGF7 or FGF10. Although the nuclear localization of AR was limited in this model, it still represented an advance over previous attempts to culture fully differentiated luminal epithelium using retinoic acid, insulin or FGFs (Peehl et al. 1996, Gustafson et al. 2006). Another group using primary cultured basal (K14+ /p63+) and transit amplifying (K18+ /AR−) epithelial cells in culture showed that the addition of 1,25-diydroxyvitamin D3, all-trans retinoic acid, and TGF-β1 could induce low levels of AR transcription while the added inhibition of the mitochondrial protein MAO-A with clorgyline increased AR protein levels (Zhao et al. 2008). This is consistent with the observation that basal cells are more densely populated with mitochondria than luminal cells (El-Alfy et al. 2000) and may suggest that a metabolic rewiring is partially necessary for cellular differentiation.
One of the key difficulties with these culture models is that primary cells eventually undergo senescence after a few passages (Litvinov et al. 2006), requiring a constant supply of fresh tissue from patients with inherently variable genetic and clinical backgrounds. A further complication is the limited number of stem cells within normal tissue (1–5% of total cells) and the limited amount of starting tissue. Most immortalized cell lines do not fully recapitulate normal glandular architecture, and while spontaneously immortalized human prostate epithelial cell lines have been developed for the serial study of normal differentiation, their self-renewal properties are difficult to assess given the random duplications and deletions acquired during the immortalization process (Jiang et al. 2010). However, it has recently been demonstrated that both basal (Xin et al. 2007, Lamb et al. 2010, Lukacs et al. 2010, Goldstein et al. 2011, Hofner et al. 2015) and luminal (Wang et al. 2009, Karthaus et al. 2014) progenitors can be propagated and differentiated ex vivo under optimized culture conditions, enabling their molecular, cellular and pharmacological assessment. Using an R-spondin-based organoid technology developed for the culture of a variety of epithelial tissues including the intestine (Sato et al. 2009), several groups have identified factors that promote both multi-lineage differentiation and long-term expansion of prostate tissue (Chua et al. 2014, Gao et al. 2014, Karthaus et al. 2014). In this assay, both basal and luminal cells appear multipotent, capable of generating organoids containing markers of both the basal and luminal lineages (Karthaus et al. 2014). The organoid system may enable a new approach to investigate lineage hierarchy, transformation and mechanisms of self-renewal and differentiation without the use of animal models.
Molecular regulation of prostate stem cell self-renewal and differentiation
The optimization of culture conditions for the propagation of stem cells was based on the discovery of common mechanisms of stem cell self-renewal and differentiation (Blanpain et al. 2007, Karthaus et al. 2014). In particular, the addition of the Wnt pathway agonist R-spondin1 is necessary to maintain human and mouse organoids (Karthaus et al. 2014). The R-spondin receptor Lgr4 is strongly expressed in Sca-1+ /CD49f+ prostate progenitor cells and is required for proper luminal differentiation as shown in Lgr4 knockout mice (Luo et al. 2013). The authors went on to show that Wnt3a plus R-spondin3 co-treatment promotes β-catenin-mediated p63high cell proliferation and differentiation. It may seem counterintuitive to characterize basal cells as ‘differentiated,’ but it has been recognized by some groups that the 95–99% of basal cells that do not display self-renewing progenitor characteristics are an independent lineage termed ‘committed’ basal cells (Maitland et al. 2011, Rane et al. 2014).
Paracrine regulation of epithelial differentiation and tumorigenesis
The Wnt/β-catenin pathway is a prime example of the control of stem cell self-renewal and differentiation control by paracrine interactions with stroma. The ability of stroma to drive epithelial transformation in the adult has been demonstrated by tissue recombination using both human and mouse tissues. Hayward et al. (2001) showed that freshly isolated human carcinoma associated fibroblasts could drive transformation of a non-tumorigenic human prostate epithelial cell line. At around the same time, the stromal reaction adjacent to sites of prostatic intraepithelial neoplasia, a precursor to prostate cancer, was characterized (Tuxhorn et al. 2002), and this stromal reaction could be used to independently predict prostate cancer recurrence (Ayala et al. 2003).
Among others, alterations to the Wnt/TGF-β pathway have been shown to be critical to the protumorigenic activity of the stroma (Li et al. 2008, Placencio et al. 2008, Franco et al. 2011, Carstens et al. 2014). The abrogation of TGF-β signaling in a subpopulation of stromal cells alone is sufficient to drive epithelial carcinogenesis in either mouse or human experimental systems (Bhowmick et al. 2004, Franco et al. 2011), and this is at least partially due to increased stromal Wnt production (Li et al. 2008, Placencio et al. 2008).
Regional differences in the human prostate stem cell niche
The development of benign and malignant prostate disease in humans is largely restricted to anatomical zones (transition and peripheral respectively), suggesting intrinsic differences in control of cytodifferentiation (McNeal et al. 1988). Given the concentration of progenitor cells in the proximal prostate in mouse (Tsujimura et al. 2002, Goldstein et al. 2008), it would be intriguing to determine whether the same anatomical concentration of progenitors in the basal layer could be detected in human prostate transition vs peripheral zones. Since basal cells are more resistant to transformation (Choi et al. 2012) and are likely cytoprotective due to their loss in cancer, it would also be informative to determine whether there are molecular differences between the stem cell niches in the transition vs peripheral zones.
Role of progenitor cells in BPH
It has been postulated that stem cell expansion is responsible for the nodular growth of the transition zone in BPH, but direct evidence for this is still lacking. Although there are a variety of histological phenotypes associated with lower urinary tract symptoms, the treatment of an enlarged prostate is still the most difficult, with only a third of patients responding to 5ARIs (McConnell et al. 2003, Roehrborn 2006). Molecular signatures of men who undergo surgery for lower urinary tract symptoms related to benign prostatic hyperplasia/lower urinary tract symptoms (BPH/LUTS) have been generated and are correlated with AP-1 transcription factor expression (Descazeaud et al. 2008, Lin-Tsai et al. 2014), but analyses of progenitor populations have not been performed. However, recent histological evidence does suggest that activation of the Wnt/β-catenin pathway in hyperplastic basal cells is associated with surgical intervention for BPH/LUTS (Bauman et al. 2014), suggesting potential activation of a progenitor pathway.
Transformation of basal cells results in a luminal phenotype
Although prostate tumors share the genetic variability observed in other organs (Watson et al. 2013), 95% of human prostate tumors are luminal-like adenocarcinomas (Grisanzio & Signoretti 2008, Wang et al. 2014b). Even when basal cells are experimentally transformed for tissue regeneration, luminal adenocarcinomas are mostly propagated rather than basal cell carcinomas, suggesting a differentiation event occurs before full transformation (Goldstein et al. 2010, Lawson et al. 2010, Stoyanova et al. 2013, Wang et al. 2013). However, if a basal cell is a tumor cell of origin, as has been shown in experimental animal models, one has to wonder why basal or squamous cell carcinomas represent such a small percentage of prostate tumor phenotypes (and usually occur in the transition zone) (Ali & Epstein 2007). In both animal models and tissue recombination xenografting experiments, the transformation of the basal epithelium can lead to both basal- and luminal-like tumors, with luminal phenotypes capable of repeated propagation in the absence of the initiating transformed basal cell (Choi et al. 2012, Stoyanova et al. 2013). This has led many to posit that transformation of basal epithelium simply leads to a differentiation event (Hudson et al. 2001, Choi et al. 2012, Wang et al. 2013). Therefore, the question of whether prostate cancer is a basal or luminal phenotype (Wang et al. 2009, Maitland et al. 2011, Choi et al. 2012) may not be as important as understanding the mechanisms that regulate self-renewal vs differentiation in normal and transformed progenitor cells. In fact, the use of differentiation-promoting histone deacetylase inhibitors (Gottlicher et al. 2001) can sensitize stem-like prostate cancer cells to radiation (Frame et al. 2013), suggesting that further understanding of the mechanisms promoting differentiation may be useful to enhancing current therapies.
Neuroendocrine differentiation
Studies in mouse models demonstrate that basal cells and some luminal cells can give rise to neuroendocrine cells in the normal prostate (Goldstein et al. 2008, Wang et al. 2009). It has yet to be shown whether primary human prostate stem/progenitor cells can generate neuroendocrine cells in vivo. Other data indicate that prostate cancer cell lines with a luminal-like adenocarcinoma phenotype can take on neuroendocrine features following androgen deprivation (Burchardt et al. 1999). These data suggest that neuroendocrine cells can be derived from neighboring epithelial cells and tumor cells. Given the emergence of castration-resistant tumors with small cell or neuroendocrine features in response to newer therapies capable of suppressing the androgen-signaling axis (Beltran et al. 2014), understanding the role of neuroendocrine differentiation from normal and malignant prostate epithelial cells is critical for treating aggressive treatment-resistant disease.
Difficulty in recapitulating native tissue interactions using human models
Ex vivo 3D culturing systems of freshly isolated mouse and human prostate epithelia are being developed to study differentiation in the absence of interaction with stroma (Karthaus et al. 2014). While these reductionist culture conditions will be highly valuable in answering specific questions about the intrinsic control of cellular differentiation, the study of the role of the microenvironment in controlling epithelial differentiation and possibly even tumor genotype still requires an experimental system capable of tissue interactions (Goldstein & Witte 2013). This dichotomy is particularly evident when comparing lineage tracing of progenitors in mouse models to either serial passaging of progenitors in a non-native matrix ex vivo or in further tissue regeneration experiments with inductive (reprogramming) mesenchyme. Given that tissue regeneration with human cells requires the use of immunocompromised mice, transgenic mouse models are particularly useful for studying the role of both the stroma and inflammation as paracrine regulators of epithelial differentiation. More work is necessary to improve human models to account for epithelial–epithelial and epithelial–stromal interactions.
Summary
Using a number of model systems, researchers have demonstrated that a range of cell-types can generate luminal cells in vitro or in vivo. We hypothesize that any progenitor cell that can give rise to the luminal lineage under experimental conditions can respond to oncogenic transformation by generating malignant luminal progeny. It is now critical to determine whether the cell of origin influences the fate of the tumor (aggressive vs indolent) or whether this is determined by genetic alterations or the tumor microenvironment. This is a difficult question to model in mice given the huge differences between mouse and human prostate epithelial basal-to-luminal ratio and basal cell phenotype. It will also be critical to determine whether there are common mechanisms required to make a luminal cell (or prostate cancer cell) regardless of the starting cell and whether that information can be used to detect, prevent or treat prostate cancer.
Acknowledgments
Funding
This review did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
References
- Abate-Shen C, Shen MM. Molecular genetics of prostate cancer. Genes and Development. 2000;14:2410–2434. doi: 10.1101/gad.819500. [DOI] [PubMed] [Google Scholar]
- Ali TZ, Epstein JI. Basal cell carcinoma of the prostate: a clinicopathologic study of 29 cases. American Journal of Surgical Pathology. 2007;31:697–705. doi: 10.1097/01.pas.0000213395.42075.86. [DOI] [PubMed] [Google Scholar]
- Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. New England Journal of Medicine. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, Wheeler M, Spitler J, Rowley DR. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clinical Cancer Research. 2003;9:4792–4801. [PubMed] [Google Scholar]
- Bauman TM, Vezina CM, Huang W, Marker PC, Peterson RE, Ricke WA. Beta-catenin is elevated in human benign prostatic hyperplasia specimens compared to histologically normal prostate tissue. American Journal of Clinical and Experimental Urology. 2014;2:313–322. [PMC free article] [PubMed] [Google Scholar]
- Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, Huang J, True L, Gleave ME, Soule H, et al. Aggressive variants of castration-resistant prostate cancer. Clinical Cancer Research. 2014;20:2846–2850. doi: 10.1158/1078-0432.CCR-13-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. Journal of Urology. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- Bhatt RI, Brown MD, Hart CA, Gilmore P, Ramani VA, George NJ, Clarke NW. Novel method for the isolation and characterisation of the putative prostatic stem cell. Cytometry. Part A. 2003;54:89–99. doi: 10.1002/cyto.a.10058. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- Blackwood JK, Williamson SC, Greaves LC, Wilson L, Rigas AC, Sandher R, Pickard RS, Robson CN, Turnbull DM, Taylor RW, et al. In situ lineage tracking of human prostatic epithelial stem cell fate reveals a common clonal origin for basal and luminal cells. Journal of Pathology. 2011;225:181–188. doi: 10.1002/path.2965. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Horsley V, Fuchs E. Epithelial stem cells: turning over new leaves. Cell. 2007;128:445–458. doi: 10.1016/j.cell.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K. The proliferative function of basal cells in the normal and hyperplastic human prostate. Prostate. 1994;24:114–118. doi: 10.1002/pros.2990240303. [DOI] [PubMed] [Google Scholar]
- Brawer MK, Peehl DM, Stamey TA, Bostwick DG. Keratin immunoreactivity in the benign and neoplastic human prostate. Cancer Research. 1985;45:3663–3667. [PubMed] [Google Scholar]
- Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. Journal of Urology. 1999;162:1800–1805. [PubMed] [Google Scholar]
- Carstens JL, Shahi P, Van Tsang S, Smith B, Creighton CJ, Zhang Y, Seamans A, Seethammagari M, Vedula I, Levitt JM, et al. FGFR1-WNT-TGF-β signaling in prostate cancer mouse models recapitulates human reactive stroma. Cancer Research. 2014;74:609–620. doi: 10.1158/0008-5472.CAN-13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N, Zhang B, Zhang L, Ittmann M, Xin L. Adult murine prostate basal and luminal cells are self-sustained lineages that can both serve as targets for prostate cancer initiation. Cancer Cell. 2012;21:253–265. doi: 10.1016/j.ccr.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, Badani KK, McKiernan JM, Benson MC, Hibshoosh H, et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nature Cell Biology. 2014;16:951–961. 1–4. doi: 10.1038/ncb3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Collins AT, Habib FK, Maitland NJ, Neal DE. Identification and isolation of human prostate epithelial stem cells based on α(2)β(1)-integrin expression. Journal of Cell Science. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. Journal of Experimental Zoology. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Sekkingstad M, Meloy BA. Heterospecific induction of prostatic development in tissue recombinants prepared with mouse, rat, rabbit and human tissues. Differentiation; Research in Biological Diversity. 1983a;24:174–180. doi: 10.1111/j.1432-0436.1983.tb01317.x. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Fujii H, Neubauer BL, Shannon JM, Sawyer L, Reese BA. Epithelial-mesenchymal interactions in prostatic development. I. morphological observations of prostatic induction by urogenital sinus mesenchyme in epithelium of the adult rodent urinary bladder. Journal of Cell Biology. 1983b;96:1662–1670. doi: 10.1083/jcb.96.6.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzo AM, Nelson WG, Meeker AK, Coffey DS. Stem cell features of benign and malignant prostate epithelial cells. Journal of Urology. 1998;160:2381–2392. doi: 10.1097/00005392-199812020-00004. [DOI] [PubMed] [Google Scholar]
- Dermer GB. Basal cell proliferation in benign prostatic hyperplasia. Cancer. 1978;41:1857–1862. doi: 10.1002/1097-0142(197805)41:5<1857::aid-cncr2820410529>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Descazeaud A, Rubin MA, Hofer M, Setlur S, Nikolaief N, Vacherot F, Soyeux P, Kheuang L, Abbou CC, Allory Y, et al. BPH gene expression profile associated to prostate gland volume. Diagnostic Molecular Pathology: the American Journal of Surgical Pathology, Part B. 2008;17:207–213. doi: 10.1097/PDM.0b013e31816f6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Alfy M, Pelletier G, Hermo LS, Labrie F. Unique features of the basal cells of human prostate epithelium. Microscopy Research and Technique. 2000;51:436–446. doi: 10.1002/1097-0029(20001201)51:5<436::AID-JEMT6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- English HF, Santen RJ, Isaacs JT. Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement. Prostate. 1987;11:229–242. doi: 10.1002/pros.2990110304. [DOI] [PubMed] [Google Scholar]
- The Finasteride Study Group. Finasteride (MK-906) in the treatment of benign prostatic hyperplasia. The Finasteride Study Group. Prostate. 1993;22:291–299. doi: 10.1002/pros.2990220403. [DOI] [PubMed] [Google Scholar]
- Frame FM, Pellacani D, Collins AT, Simms MS, Mann VM, Jones GD, Meuth M, Bristow RG, Maitland NJ. HDAC inhibitor confers radiosensitivity to prostate stem-like cells. British Journal of Cancer. 2013;109:3023–3033. doi: 10.1038/bjc.2013.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, II, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Research. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisa NT, Graham TA, McDonald SA, Poulsom R, Heidenreich A, Jakse G, Knuechel R, Wright NA. Clonal architecture of human prostatic epithelium in benign and malignant conditions. Journal of Pathology. 2011;225:172–180. doi: 10.1002/path.2959. [DOI] [PubMed] [Google Scholar]
- Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, Dowling C, Wanjala JN, Undvall EA, Arora VK, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell. 2014;159:176–187. doi: 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraway IP, Sun W, Tran CP, Perner S, Zhang B, Goldstein AS, Hahm SA, Haider M, Head CS, Reiter RE, et al. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate. 2010;70:491–501. doi: 10.1002/pros.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann M, Wetterwald A, Guzmán-Ramirez N, van der Pluijm G, Culig Z, Cecchini MG, Williams ED, Thalmann GN. Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer. Stem Cells. 2012;30:1076–1086. doi: 10.1002/stem.1087. [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Witte ON. Does the microenvironment influence the cell types of origin for prostate cancer? Genes and Development. 2013;27:1539–1544. doi: 10.1101/gad.222380.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON. Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics. PNAS. 2008;105:20882–20887. doi: 10.1073/pnas.0811411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON. Identification of a cell of origin for human prostate cancer. Science. 2010;329:568–571. doi: 10.1126/science.1189992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein AS, Drake JM, Burnes DL, Finley DS, Zhang H, Reiter RE, Huang J, Witte ON. Purification and direct transformation of epithelial progenitor cells from primary human prostate. Nature Protocols. 2011;6:656–667. doi: 10.1038/nprot.2011.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlicher M, Minucci S, Zhu P, Krämer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO Journal. 2001;20:6969–6978. doi: 10.1093/emboj/20.24.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska MM, DeGraff DJ, Yu X, Jin RJ, Chen Z, Borowsky AD, Matusik RJ. Mouse models of prostate cancer: picking the best model for the question. Cancer Metastasis Reviews. 2014;33:377–397. doi: 10.1007/s10555-013-9487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisanzio C, Signoretti S. p63 in prostate biology and pathology. Journal of Cellular Biochemistry. 2008;103:1354–1368. doi: 10.1002/jcb.21555. [DOI] [PubMed] [Google Scholar]
- Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM, Högnäs G, Annala M, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–357. doi: 10.1038/nature14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson MP, Xu C, Grim JE, Clurman BE, Knudsen BS. Regulation of cell proliferation in a stratified culture system of epithelial cells from prostate tissue. Cell and Tissue Research. 2006;325:263–276. doi: 10.1007/s00441-005-0093-0. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Wang Y, Cao M, Hom YK, Zhang B, Grossfeld GD, Sudilovsky D, Cunha GR. Malignant transformation in a nontumorigenic human prostatic epithelial cell line. Cancer Research. 2001;61:8135–8142. [PubMed] [Google Scholar]
- Heer R, Collins AT, Robson CN, Shenton BK, Leung HY. KGF suppresses α2β1 integrin function and promotes differentiation of the transient amplifying population in human prostatic epithelium. Journal of Cell Science. 2006;119:1416–1424. doi: 10.1242/jcs.02802. [DOI] [PubMed] [Google Scholar]
- Heer R, Robson CN, Shenton BK, Leung HY. The role of androgen in determining differentiation and regulation of androgen receptor expression in the human prostatic epithelium transient amplifying population. Journal of Cellular Physiology. 2007;212:572–578. doi: 10.1002/jcp.21154. [DOI] [PubMed] [Google Scholar]
- Hofner T, Eisen C, Klein C, Rigo-Watermeier T, Goeppinger SM, Jauch A, Schoell B, Vogel V, Noll E, Weichert W, et al. Defined conditions for the isolation and expansion of basal prostate progenitor cells of mouse and human origin. Stem Cell Reports. 2015;4:503–518. doi: 10.1016/j.stemcr.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong MK, Macintyre G, Wedge DC, Van Loo P, Patel K, Lunke S, Alexandrov LB, Sloggett C, Cmero M, Marass F, et al. Tracking the origins and drivers of subclonal metastatic expansion in prostate cancer. Nature Communications. 2015;6:6605. doi: 10.1038/ncomms7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson DL. Epithelial stem cells in human prostate growth and disease. Prostate Cancer and Prostatic Diseases. 2004;7:188–194. doi: 10.1038/sj.pcan.4500745. [DOI] [PubMed] [Google Scholar]
- Hudson DL, Guy AT, Fry P, O’Hare MJ, Watt FM, Masters JR. Epithelial cell differentiation pathways in the human prostate: identification of intermediate phenotypes by keratin expression. Journal of Histochemistry and Cytochemistry. 2001;49:271–278. doi: 10.1177/002215540104900214. [DOI] [PubMed] [Google Scholar]
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. Journal of Urology. 2002;167:948–951. discussion 952. [PubMed] [Google Scholar]
- Isaacs JT. Prostate stem cells and benign prostatic hyperplasia. Prostate. 2008;68:1025–1034. doi: 10.1002/pros.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JT, Coffey DS. Etiology and disease process of benign prostatic hyperplasia. Prostate. Supplement. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- Jiang M, Strand DW, Fernandez S, He Y, Yi Y, Birbach A, Qiu Q, Schmid J, Tang DG, Hayward SW. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 2010;28:344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159:163–175. doi: 10.1016/j.cell.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- Korsten H, Ziel-van der Made A, Ma X, van der Kwast T, Trapman J. Accumulating progenitor cells in the luminal epithelial cell layer are candidate tumor initiating cells in a Pten knockout mouse prostate cancer model. PLoS ONE. 2009;4:e5662. doi: 10.1371/journal.pone.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OJ, Zhang L, Ittmann MM, Xin L. Prostatic inflammation enhances basal-to-luminal differentiation and accelerates initiation of prostate cancer with a basal cell origin. PNAS. 2014;111:E592–E600. doi: 10.1073/pnas.1318157111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb LE, Knudsen BS, Miranti CK. E-cadherin-mediated survival of androgen-receptor-expressing secretory prostate epithelial cells derived from a stratified in vitro differentiation model. Journal of Cell Science. 2010;123:266–276. doi: 10.1242/jcs.054502. [DOI] [PubMed] [Google Scholar]
- Lawson DA, Xin L, Lukacs RU, Cheng D, Witte ON. Isolation and functional characterization of murine prostate stem cells. PNAS. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson DA, Zong Y, Memarzadeh S, Xin L, Huang J, Witte ON. Basal epithelial stem cells are efficient targets for prostate cancer initiation. PNAS. 2010;107:2610–2615. doi: 10.1073/pnas.0913873107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leenders GJ, Schalken JA. Epithelial cell differentiation in the human prostate epithelium: implications for the pathogenesis and therapy of prostate cancer. Critical Reviews in Oncology/Hematology. 2003;46:S3–S10. doi: 10.1016/s1040-8428(03)00059-3. [DOI] [PubMed] [Google Scholar]
- Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- The Leuprolide Study Group. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. The Leuprolide Study Group. New England Journal of Medicine. 1984;311:1281–1286. doi: 10.1056/NEJM198411153112004. [DOI] [PubMed] [Google Scholar]
- Li X, Placencio V, Iturregui JM, Uwamariya C, Sharif-Afshar AR, Koyama T, Hayward SW, Bhowmick NA. Prostate tumor progression is mediated by a paracrine TGF-β/Wnt3a signaling axis. Oncogene. 2008;27:7118–7130. doi: 10.1038/onc.2008.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg J, Klevebring D, Liu W, Neiman M, Xu J, Wiklund P, Wiklund F, Mills IG, Egevad L, Grönberg H. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. European Urology. 2013;63:347–353. doi: 10.1016/j.eururo.2012.03.050. [DOI] [PubMed] [Google Scholar]
- Lin-Tsai O, Clark PE, Miller NL, Fowke JH, Hameed O, Hayward SW, Strand DW. Surgical intervention for symptomatic benign prostatic hyperplasia is correlated with expression of the AP-1 transcription factor network. Prostate. 2014;74:669–679. doi: 10.1002/pros.22785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov V, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free defined medium selects for growth of normal prostatic epithelial stem cells. Cancer Research. 2006;66:8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Laitinen S, Khan S, Vihinen M, Kowalski J, Yu G, Chen L, Ewing CM, Eisenberger MA, Carducci MA, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nature Medicine. 2009;15:559–565. doi: 10.1038/nm.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TL, Huang YF, You LR, Chao NC, Su FY, Chang JL, Chen CM. Conditionally ablated Pten in prostate basal cells promotes basal-to-luminal differentiation and causes invasive prostate cancer in mice. American Journal of Pathology. 2013;182:975–991. doi: 10.1016/j.ajpath.2012.11.025. [DOI] [PubMed] [Google Scholar]
- Lukacs RU, Goldstein AS, Lawson DA, Cheng D &Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nature Protocols. 2010;5:702–713. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Rodriguez M, Valdez JM, Zhu X, Tan K, Li D, Siwko S, Xin L, Liu M. Lgr4 is a key regulator of prostate development and prostate stem cell differentiation. Stem Cells. 2013;31:2492–2505. doi: 10.1002/stem.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland NJ, Frame FM, Polson ES, Lewis JL, Collins AT. Prostate cancer stem cells: do they have a basal or luminal phenotype? Hormones & Cancer. 2011;2:47–61. doi: 10.1007/s12672-010-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell JD, Roehrborn CG, Bautista OM, Andriole GL, Jr, Dixon CM, Kusek JW, Lepor H, McVary KT, Nyberg LM, Jr, Clarke HS, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. New England Journal of Medicine. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- McNeal JE, Redwine EA, Freiha FS, Stamey TA. Zonal distribution of prostatic adenocarcinoma. Correlation with histologic pattern and direction of spread. American Journal of Surgical Pathology. 1988;12:897–906. doi: 10.1097/00000478-198812000-00001. [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H. Lin-Sca-1CCD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer Research. 2009;69:8555–8562. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C. Multipotent and unipotent progenitors contribute to prostate postnatal development. Nature Cell Biology. 2012;14:1131–1138. doi: 10.1038/ncb2600. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Wong ST, Rubin JS. KGF and EGF differentially regulate the phenotype of prostatic epithelial cells. Growth Regulation. 1996;6:22–31. [PubMed] [Google Scholar]
- Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, Neilson EG, Shen MM, Matusik RJ, Hayward SW, Bhowmick NA. Stromal transforming growth factor-β signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Research. 2008;68:4709–4718. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rane JK, Droop AP, Pellacani D, Polson ES, Simms MS, Collins AT, Caves LS, Maitland NJ. Conserved two-step regulatory mechanism of human epithelial differentiation. Stem Cell Reports. 2014;2:180–188. doi: 10.1016/j.stemcr.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson GD, Robson CN, Lang SH, Neal DE, Maitland NJ, Collins AT. CD133, a novel marker for human prostatic epithelial stem cells. Journal of Cell Science. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Roehrborn CG. Definition of at-risk patients: baseline variables. BJU International. 2006;97(Suppl 2):7–11. doi: 10.1111/j.1464-410X.2006.06098.x. discussion 21-2. [DOI] [PubMed] [Google Scholar]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, et al. Prostate-cancer mortality at 11 years of follow-up. New England Journal of Medicine. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Gipp J, Dries M, Bushman W. Prostate progenitor cells proliferate in response to castration. Stem Cell Research. 2014;13:154–163. doi: 10.1016/j.scr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signoretti S, Pires MM, Lindauer M, Horner JW, Grisanzio C, Dhar S, Majumder P, McKeon F, Kantoff PW, Sellers WR, et al. p63 regulates commitment to the prostate cell lineage. PNAS. 2005;102:11355–11360. doi: 10.1073/pnas.0500165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoyanova T, Cooper AR, Drake JM, Liu X, Armstrong AJ, Pienta KJ, Zhang H, Kohn DB, Huang J, Witte ON, et al. Prostate cancer originating in basal cells progresses to adenocarcinoma propagated by luminal-like cells. PNAS. 2013;110:20111–20116. doi: 10.1073/pnas.1320565110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand DW, Franco OE, Basanta D, Anderson AR, Hayward SW. Perspectives on tissue interactions in development and disease. Current Molecular Medicine. 2010;10:95–112. doi: 10.2174/156652410791065363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AA. Mesenchymal mechanisms in prostate organogenesis. Differentiation; Research in Biological Diversity. 2008;76:587–598. doi: 10.1111/j.1432-0436.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. Journal of Cell Biology. 2002;157:1257–1265. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clinical Cancer Research. 2002;8:2912–2923. [PubMed] [Google Scholar]
- Uzgare AR, Xu Y, Isaacs JT. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. Journal of Cellular Biochemistry. 2004;91:196–205. doi: 10.1002/jcb.10764. [DOI] [PubMed] [Google Scholar]
- Verhagen AP, Aalders TW, Ramaekers FC, Debruyne FM, Schalken JA. Differential expression of keratins in the basal and luminal compartments of rat prostatic epithelium during degeneration and regeneration. Prostate. 1988;13:25–38. doi: 10.1002/pros.2990130104. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Till C, Tangen CM, Lilja H, Thompson IM. An empirical evaluation of guidelines on prostate-specific antigen velocity in prostate cancer detection. Journal of the National Cancer Institute. 2011;103:462–469. doi: 10.1093/jnci/djr028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wang S, Garcia AJ, Wu M, Lawson DA, Witte ON, Wu H. Pten deletion leads to the expansion of a prostatic stem/progenitor cell subpopulation and tumor initiation. PNAS. 2006;103:1480–1485. doi: 10.1073/pnas.0510652103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kruithof-de Julio M, Economides KD, Walker D, Yu H, Halili MV, Hu YP, Price SM, Abate-Shen C, Shen MM. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZA, Mitrofanova A, Bergren SK, Abate-Shen C, Cardiff RD, Califano A, Shen MM. Lineage analysis of basal epithelial cells reveals their unexpected plasticity and supports a cell-of-origin model for prostate cancer heterogeneity. Nature Cell Biology. 2013;15:274–283. doi: 10.1038/ncb2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhu HH, Chu M, Liu Y, Zhang C, Liu G, Yang X, Yang R, Gao WQ. Symmetrical and asymmetrical division analysis provides evidence for a hierarchy of prostate epithelial cell lineages. Nature Communications. 2014a;5:4758. doi: 10.1038/ncomms5758. [DOI] [PubMed] [Google Scholar]
- Wang ZA, Toivanen R, Bergren SK, Chambon P, Shen MM. Luminal cells are favored as the cell of origin for prostate cancer. Cell Reports. 2014b;8:1339–1346. doi: 10.1016/j.celrep.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson IR, Takahashi K, Futreal PA, Chin L. Emerging patterns of somatic mutations in cancer. Nature Reviews. Genetics. 2013;14:703–718. doi: 10.1038/nrg3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SC, Hepburn AC, Wilson L, Coffey K, Ryan-Munden CA, Pal D, Leung HY, Robson CN, Heer R. Human α(2)β(1)(HI). CD133(CVE) epithelial prostate stem cells express low levels of active androgen receptor. PLoS ONE. 2012;7:e48944. doi: 10.1371/journal.pone.0048944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Ide H, Kim Y, Dubey P, Witte ON. In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. PNAS. 2003;100(Suppl 1):11896–11903. doi: 10.1073/pnas.1734139100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Lawson DA, Witte ON. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. PNAS. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L, Lukacs RU, Lawson DA, Cheng D, Witte ON. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- Zhao H, Nolley R, Chen Z, Reese SW, Peehl DM. Inhibition of monoamine oxidase A promotes secretory differentiation in basal prostatic epithelial cells. Differentiation; Research in Biological Diversity. 2008;76:820–830. doi: 10.1111/j.1432-0436.2007.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]