Summary
Intrahepatic cholestasis of pregnancy poses a great risk to both maternal and fetal health. Despite extensive research, much of the pathogenesis of this disorder is unknown. The increase in bile acids observed in patients with intrahepatic cholestasis of pregnancy has been noted to cause a change in the immune system from the normally mediated TH2 response to one that is more oriented towards TH1. In this literature review, we have critically reviewed the current literature regarding the changes in the immune system and the potential effects of immunological changes in the management of the patient. The current treatment, ursodeoxycholic acid, is also discussed along with potential combination therapies and future directions for research.
Keywords: Intrahepatic cholestasis of pregnancy, Bile acids, S-adenosylmethionine, T-lymphocytes, Ursodeoxycholic acid
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a disorder that can occur during second and the third trimester of pregnancy. It is characterized by maternal pruritus, altered liver function, as can be seen by significantly increased levels of the enzymes aspartate aminotransferase (AST) and alanine transaminase (ALT), and excess bile acids in the blood (>10 µmol/L) [1, 2]. Due to a decrease in bile flow through the liver and subsequent excretion, serum bile acid levels in both the mother and fetus become significantly increased, specifically due to cholic and chenodeoxycholic acid [3]. After the delivery of the placenta, signs and symptoms of ICP disappear [4]. Currently the etiology of ICP is not fully understood. Research is ongoing with regards to genetics, hormones and immunology in attempt to find the cause of ICP. One study investigated gene expression in patients with ICP and found that the expression of 392 genes were significantly altered; these genes have roles in many processes ranging from metabolic processes to the immune system to reproduction [4]. The goal of this article is to critically review the current literature on the immunological basis of ICP. In addition, the current treatments and their effect on the immune system are examined along with combination therapies.
For this article, we conducted a literature search via MEDLINE COMPLETE using terms related to ICP and its treatments. Such terms included ICP, ICP immunology, ICP pathogenesis, ICP treatment, ursodeoxycholic acid ICP, rifampicin ICP, S-adenosylmethionine ICP, and polyunsaturated phosphatidylcholine ICP. Articles published from 1989 to 2015 were included, with the majority of literature being published after 2010.
Risk Factors and effects of ICP
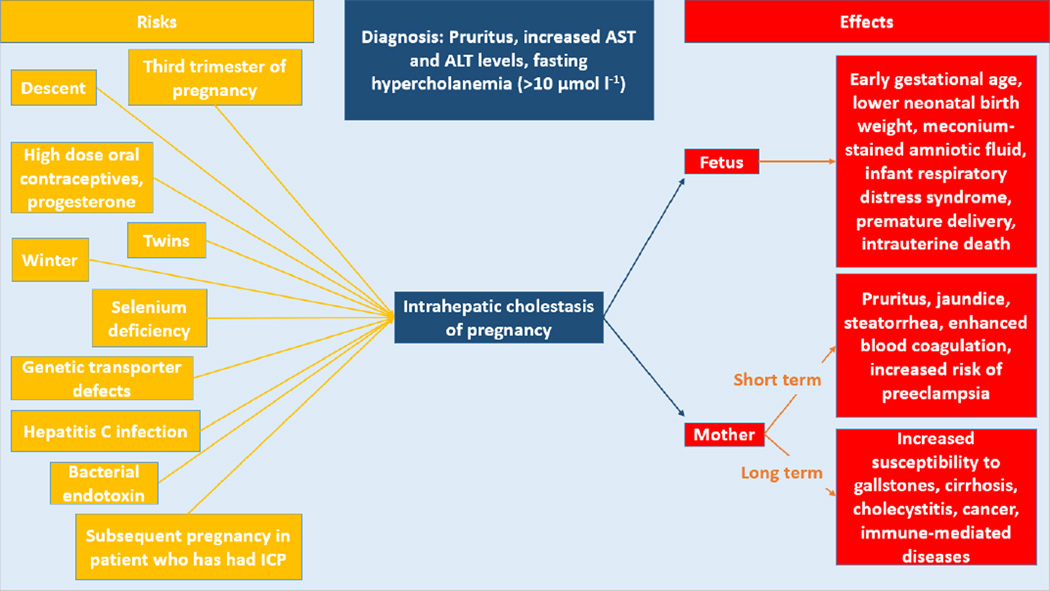
In patients with ICP, many factors have been noted to increase the risk of having this disorder (Figure 1). Epidemiologically, the prevalence worldwide has been found to be 0.7% overall [5]. One study found a prevalence as high as 27.6% among the Araucanian Indians of Chile [6]. This is followed by the Aimara Indians of Chile (11.8%) or Bolivia (13.8%), Chinese (5.2%) [7] and Scandinavian patients (1–1.5%) [6]. Other factors known to increase the risk involve hormones. For instance, taking a high dose of oral contraceptives and progesterone has been shown to trigger ICP [8]. Also, having twins increases the rate of ICP [9]. Environmental risk factors have also been noted. There is an increased incidence of ICP in the winter months [10]. Selenium deficiency increases the risk because selenium is a cofactor for enzymes in the oxidative metabolism of the liver [11]. Genetic transporter defects are thought to cause a minority of cases, and hepatitis C and bacterial endotoxin increase the risk of ICP [12]. As the recurrence rate of ICP in multiparous women is less than 70%, the risk of ICP is increased with subsequent pregnancies thus suggesting a role of hormonal factors in the onset of ICP [11].
Figure 1. The risks and effects of intrahepatic cholestasis of pregnancy.
Risk factors, diagnostic criteria and effects of intrahepatic cholestasis of pregnancy on the fetus and mother are shown above. Short term effects of ICP on the mother occur from the onset of intrahepatic cholestasis of pregnancy until the delivery of the placenta while long term effects are those that occur a significant time later.
AST: Aspartate aminotransferase; ALT: Alanine transaminase
ICP has effects on both maternal and fetal health, with the effects on the fetus being the most concerning. One study reported that fetal complications do not occur until bile acids are greater than or equal to 40 µmol/L, at which point the incidence of complications increases by 1–2% with each additional µmol/L increase [13]. These complications range from earlier gestational age and lower neonatal birth weight [4] to meconium-stained amniotic fluid, premature delivery and intrauterine death [2]. Death could be occurring through the action of bile acid receptors in cardiomyocytes. As the level of bile acids increase, it can lead to death by causing arrhythmias [14]. ICP has also been implicated in damaging the fetal lungs and causing infant respiratory distress syndrome [15]. Studies have not yet examined potential long term effects of ICP on the fetus.
In the mother, ICP has both short and long term effects. While pregnant, women can have jaundice, steatorrhea [10] and increased risk of preeclampsia [16]. Enhanced blood coagulation has been noted through increases in mean platelet volume [17] along with significant increases in factor VIII, von Willebrand factor and fibrinogen levels [18]. Much later in life, women who have had ICP are more prone to a variety of health complications. These include cholesterol gallstone formation, hepatitis C, nonalcoholic liver cirrhosis, cholecystitis, nonalcoholic pancreatitis [19], liver cancer, immune-mediated and cardiovascular diseases [20]. Clearly, the risks associated with ICP warrant further research into the pathogenesis of this disorder.
Bile Acid Transporters and Hormones
Studies have explored the role of genetic transporter mutations in ICP due to the finding of such mutations in other forms of cholestasis including progressive familial intrahepatic cholestasis (PFIC) and benign recurrent intrahepatic cholestasis (BRIC). In both PFIC type 1 and BRIC, homozygous mutations have been identified in the ATP8B1 gene, which is responsible for the production of familial intrahepatic cholestasis protein 1 (FIC1) [21]. While the exact function of FIC1 is unknown, it is proposed to be an amino-phospholipid translocase. If true, it acts to maintain the distribution of phospholipids and thus the function of bile acid transporters [21]. Sequencing patients with ICP, two mutations were found in ATP8B1 that are distinct from those of the other aforementioned forms of cholestasis [21].
Mutations in the ABCB4 gene have been implicated in ICP. In heterozygous mothers of children with PFIC, recurrent episodes of ICP have been observed [22, 23]. This gene leads to the production of the canalicular transporter named multidrug resistance protein 3 (MDR3), an exporter of phospholipids [23]. When studied in women with ICP but no history of PFIC, a missense mutation was found resulting in less functional protein [22]. Other studies found slicing mutations in the ABCB4 gene [24] and a single-nucleotide deletion of MDR3 causing truncated, inactive protein [23]. Additionally, mutations in the gene ABCB11 encoding the bile salt export pump (BSEP) [25] are proposed to increase the susceptibility to ICP [26]. One study reports that patients who are heterozygous for ABCB11 mutations account for one percent of European ICP cases [27]. The mutations identified are thought to decrease protein levels by reducing the folding efficiency of BSEP [27].
Aside from genetic transporter mutations, there is the issue of γ-glutamyl transpeptidase (γ-GT). Bile acids assist in the detachment of this enzyme from the canalicular membrane. If there is a failure to produce or secrete bile acids, γ-GT will not be elevated [28]. In ICP, patients are found to have both normal and increased levels of γ-GT. In ICP patients with MDR3 mutations, there is an increase in γ-GT levels as seen in PFIC type 3 due to the bile acids high detergent power [22, 23]. However, splicing mutations in MDR3 have been identified in ICP patients with normal levels of γ-GT as well [29]. Similarly, mutations in the ABCB4 gene have been implicated with normal [27] and high γ-GT levels [24]. These mutations increase the susceptibility to ICP and as such require further research.
In regards to hormones, levels of both estrogen and progesterone are the highest in the late stages of pregnancy when ICP occurs [12]. Both serve to inhibit bile acid flow through the liver. Estrogen-glucuronides are sent out of the liver by multidrug resistance associated protein 2 (MRP2) and cause internalization of BSEP into endocytic vesicles thus reducing the amount of bile excreted from the liver [1, 11, 30]. Specifically, 17β-estradiol represses BSEP through reduction in the coactivator peroxisome proliferator-activated receptor-γ coactivator-1 recruitment while also increasing corepressor recruitment to the BSEP promoter [31]. Estrogen has also been shown to reduce expression of Na+/taurocholate co-transporter (NTCP) and the organic anion transporting polypeptides (OATP) in rats [30]. Both NTCP and OATP are involved in the transport of bile acids into the liver from the blood, with OATP1B3 being specifically expressed in the liver and placenta [32].
Sulfated progesterone metabolites are increased in ICP and act on bile acid metabolism and transport pathways. Similar to estrogen, sulfated progesterone can cause trans-inhibition of BSEP [33, 34]. It also inhibits NTCP uptake of bile acid in a competitive manner, as shown in a Xenopus oocyte experiment [33, 35]. The sulfated metabolites also act on a nuclear receptor called the farnesoid X receptor (FXR). FXR heterodimerizes with the retinoid X receptor in the presence of high bile acids and upregulates efflux transporters of bile acids like BSEP [36]. FXR also inhibits the short heterodimer partner (SHP) which blocks expression of NTCP and various enzymes in the bile acid pathway [36]. Sulfated progesterone metabolites increase bile acid levels by inhibiting the action of FXR [33, 36]. The reduced bile metabolism and excretion leads to changes in immunological factors that are vital in understanding ICP.
Immunology
Cytokines
In normal pregnancy, patients experience elevations in both pro- and anti-inflammatory cytokines [37]. There is decrease in type 1 cytokines along with an increase in type 2 cytokines in order to protect the fetus from potentially lethal TH1 and cytotoxic T cell responses [30, 37]. In ICP however, patients experience a shift in the differentiation of TH0 cells to TH1 development as seen by changes in cytokines. Significant increases have been found in the levels of TH1 and TH17-associated cytokines, TNF-α [1, 37, 38], IL-6 [37], IL-12 [1, 37], IL-17A [39], IL-18 [1] and IFN-γ [40–42] while TH2-associated cytokines, IL-4 [37, 38, 42], IL-10 [41], TGF-β1 [30] and TGF- β2 [30] and suppressor of cytokine signaling-3 (SOCS3) [41] are reduced. Increases in NF-kB further shift to a TH1 response. Figure 2 demonstrates some of these immunologic changes along with associated implications in the pathology of ICP.
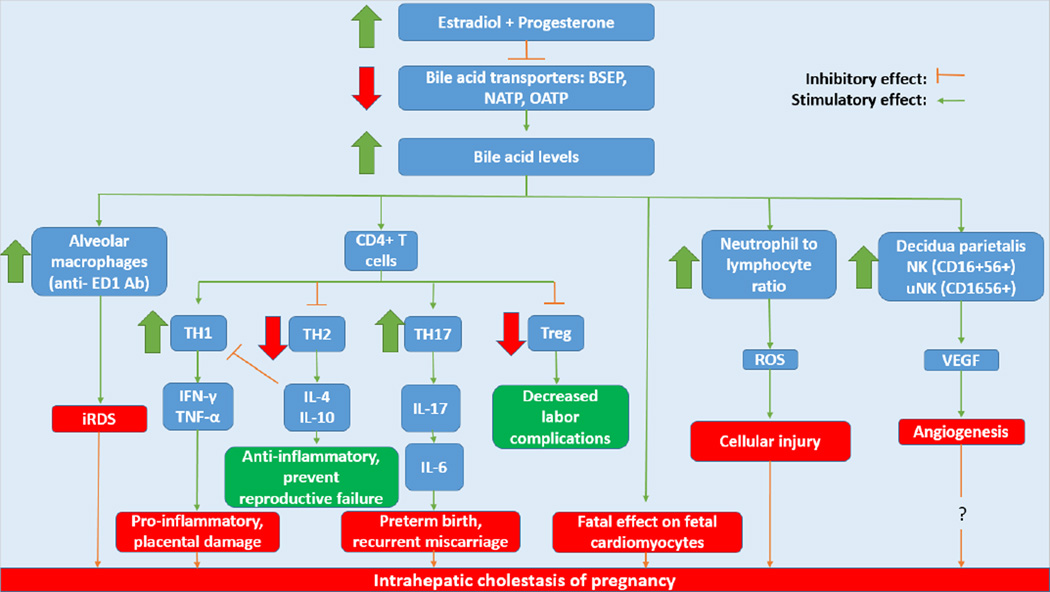
Figure 2. Immunologic changes occurring in intrahepatic cholestasis of pregnancy.
The rise in estradiol and progesterone in the third trimester of pregnancy modulates immune cells including macrophages, CD4+ T cells, neutrophils and NK cells via increases in bile acids. The increase in bile acid levels lead to increased activation of alveolar macrophages which have been implicated in infant respiratory distress syndrome, one of the fetal risks involved this disorder. Changes occur in the differentiation of CD4+ T cells. TH1 cell proliferation becomes increased and secrete pro-inflammatory cytokines such as IFN-γ and TNF-α that cause placental damage. TH2 cells which secrete anti-inflammatory IL-4 and IL-10 are decreased along with T-regulatory cells which have been shown to decrease the incidence of labor complications. Meanwhile TH17 cells produce IL-17 which induces the production of IL-6, a cytokine shown to cause preterm birth and recurrent miscarriage when at increased levels. Neutrophils, which are increased, create ROS that cause cellular injury. Finally, NK and uNK cells secrete VEGF, a factor that causes angiogenesis. While VEGF is increased in ICP, other angiogenic factors are decreased thus questioning the role of angiogenesis in intrahepatic cholestasis of pregnancy.
BSEP: Bile Salt Export Pump; NATP: Na+/taurocholate co-transporter; OATP: Organic anion transporting polypeptides; iRDS: Infant respiratory distress syndrome; Treg: T-regulatory cells; ROS: Reactive oxygen species; NK: Natural killer cells; uNK: Uterine natural killer cells; VEGF: Vascular endothelial growth factor.
TNF-α is important in the growth of the fetus and placenta but can be lethal when expressed at very high levels. In ICP, the significant increase comes from production by the placenta [37]. In obstructive cholestasis in rats, TNF-α downregulates BSEP along with IL-1 β [30]. TNF-α and IFN-γ are pro-inflammatory cytokines synthesized and secreted by TH1 cells. These cytokines are responsible for decreased bile acid secretion, trophoblast apoptosis and damage to the placenta [37] along with increased abortion rates in mice [43].
Along with the effects of TNF-α, it can induce production of another cytokine, IL-6, in states of inflammation, trauma or stress [44]. IL-6 is made by TH2 cells but is considered a pro-inflammatory cytokine [37]. It is a major cytokine in inflammation and can be induced by IL-17 [45]. In CD4+ T cells, IL-6 promotes differentiation to TH17 cells and inhibits Foxp3+ T-regulatory cell generation [44]. Elevated levels of IL-6 have been associated with aspects of ICP, including preterm birth and recurrent miscarriage [44].
IL-12, a pro-inflammatory cytokine, is a dominant factor in driving the development of TH1 cells [46]. The commitment to TH1 is further enhanced by IFN-γ which increases the number of IL-12 receptors available while inhibiting the growth of TH2 cells [46]. IL-12 production can be induced in macrophages along with IL-6, IL-18 and TNF [47]. IL-18 synergizes with IL-12 to cause production of IFN- γ by TH1 cells [47]. IL-18 can also activate NF-κB by causing auto-phosphorylation of interleukin receptor-associated kinase (IRAK) and natural killer cells [46, 47]. Hence, both IL-12 and IL-18 act to perpetuate the TH1 response.
The final cytokine increased is IL-17A, which was originally identified as CTL-associated antigen 8, and the increase is only significant in patients with severe ICP or who had total bile acid concentrations greater than or equal to 40µmol/L [45]. IL-17 can induce production of IL-6 and IL-8, a chemokine ligand that recruits neutrophils [45]. It is secreted by TH17 cells and has been associated with a number of diseases involving chronic inflammation [45].
While IL-4 production directs the development of a TH2 response, it is decreased in ICP [37]. This cytokine is important in the development and maintenance of T-regulatory cell development and can inhibit TH1 immunity [48]. IL-10, which is also decreased in ICP, inhibits the proliferation of a TH1 response by causing less production of IL-12 and IL-18 from stimulated macrophages [46]. Both IL-4 and IL-10 have an anti-inflammatory effect and can prevent reproductive failure [48]; increases, instead of decreases, in both IL-4 and IL-10 could be beneficial in patients with ICP.
TGF-β and TGF- β2 are also found in low levels but can inhibit the TH1 response [30]. TGF-β, along with TNF-α and IFN-γ, can be made by uterine specific natural killer cells (uNK cells) [30]. The suppressor of cytokine signaling 3 levels are significantly lower in placenta cells of ICP as well [41]. The levels of these cytokines provide further evidence to the pro-inflammatory nature of this disorder.
Finally, the nuclear factor, NF- κB, plays a role in the potentiation of the TH1 response [49]. Once activated by pro-inflammatory cytokines like TNF-α, it goes to the nucleus to promote transcription of pro-inflammatory cytokines in the placenta such as TNF-α, IL-6 and IL-8 [37]. As the severity of ICP increases, there is a corresponding increase in the levels of NF-κB [37]. Both peroxisome proliferator-activated receptors α (PPARα) and γ (PPARγ) have anti-inflammatory effects by inhibiting the signaling pathway of NF-κB [37]. High levels of estrogen, as observed in patients with ICP, have been found to inhibit the mRNA expression of PPARα [10]. In regards to PPARγ, one study found ICP patients to have increased levels of PPARγ but with no anti-inflammatory effect [37]. This finding suggests that further research is needed into the role of PPARγ in patients with ICP.
Chemokines
These secondary pro-inflammatory mediators are induced by cytokines such as IL-1 or TNF [50]. In ICP patients, CXCL6, CXCL14, IL-7R, CCL3 and CCL25 are upregulated [51] along with CXCL1, CXCL4 and CXCL7 [4]. Increases in CXCL cytokines stimulate neutrophil chemotaxis while the CCL chemokines stimulate monocyte migration along with lymphocytes [50]. This recruitment of lymphocytes leads to cellular damage as will be seen in the future discussion concerning neutrophils.
Lymphocytes
In ICP, there is mixed data on the changes in the T cell population. Researchers found that high serum estradiol interacts with receptors on the surface of cytotoxic T lymphocytes and decreases their activity in ICP; thus, there is an increase in the ratio of CD4+/CD8+ cells [30, 52]. Another study found that ICP was associated with decreased amounts of CD3+CD4+ cells and no change in the amount of CD3+CD8+ cells in the decidua parietalis [40]. This evidence requires future research into the activity of CD4+ and CD8+ T cell populations. Regarding regulatory T cells in ICP, there is not conflicting evidence. The CD4+CD25+ cells in both peripheral blood and decidua were found to be significantly decreased [53]. T-regulatory cells normally maintain immune tolerance by suppressing the inflammatory state, natural killer cells and T cell responses involved in labor complications [54]. This decrease in regulatory T cells contributes to the pathogenesis of ICP.
Significant increases in CD19+ B cells have been found in the placenta of patients with ICP [51]. NK cell populations have also been found to be increased. Specifically, there are increases in CD3−CD56+ (NK cells), CD3+CD56+ (NKT cells), CD56+CD16+, CD56+CD16− (uNK cells) and CD56+NKG2D+ cells, with no change in CD56+NKG2A+ cells [40]. CD56+CD16+ cells are NK cells that have high cytolytic activity through IgG Fc receptors (CD16). CD56+NKG2D+ cells have activating receptors that induce NK cells to kill while CD56+NKG2A+ cells have inhibitory receptors. These cells can also secrete angiogenic factors such as vascular endothelial growth factor (VEGF) [55, 56]. ICP has been associated with an increase in the expression of VEGFC gene that encodes a ligand for VEGF receptor-3 (VEGFR3), also known as receptor tyrosine kinase FLT4, but also with decreases in other factors involved in angiogenesis [51]. Thus, the role of angiogenesis in ICP is uncertain. Since there are significant changes in the expression of both B lymphocytes and NK cells, further research is warranted to elucidate their role in the pathogenesis of ICP.
Adhesion Molecules
The expression of both intracellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are found to be increased in ICP [30, 57], which is supported by bile duct ligation experiments in mice [58]. Bile acids increase the concentration of these adhesion molecules through the NF-κB pathway [57]. These increases are important to note because they enable neutrophils and monocytes to transmigrate to the liver and become active.
Macrophages
Neopterin, a marker for the activation of macrophages, is increased in ICP [4, 30, 59]. In rat experiments focused on obstructive cholestasis, a large amount of macrophages were observed which expressed phospholipase A2 and the bile acid receptor TGR5 [15]. The effect of ICP on TGR5 appears to be significant. For example, activation of this receptor results in an increase in cAMP levels and inhibition of pro-inflammatory secretion [60]. TGR5 also can increase the production of reactive oxygen species (ROS) as seen in astrocytes [60]. The significantly lower levels of TGR5 that is found in placentas of patients with ICP due to progesterone metabolites [60] contributes to the pro-inflammatory cytokine environment while also potentially protecting the fetus from ROS. In addition to promoting the pro-inflammatory environment, alveolar macrophages marked via anti-ED1 antibodies have been implicated in the development of infant respiratory distress syndrome, which is seen in ICP [15].
Neutrophils
The neutrophil-to-lymphocyte ratio is significantly higher in women with ICP [17, 61]. In fact, as the severity of ICP increases based on levels of bile acids, there is an increase in the ratio of neutrophils-to-lymphocytes [61]. In an experiment on obstructive cholestasis in bile duct-ligated mice, the extravasation of neutrophils and production of ROS, such as superoxide and hypochlorous acid [62], were responsible for the inflammatory injury [63]. Neutrophils contribute to the pathogenesis of ICP by systemic inflammation.
Human Leukocyte Antigen
Levels of both non-classical molecules HLA-E [30] and HLA-G [38, 64] are decreased in ICP. The decrease in HLA-E most likely provides protection for the fetus from the maternal immune system by interacting with the CD94/NKG2a NK-cell inhibitory receptor [30]. With regards to HLA-G expression in the placenta, it is downregulated by MiR-148a, which is at elevated levels in ICP and is positively correlated with increases in total bile acid levels [64]. MiR-148a binds to the 3’-UTR of HLA-G mRNA thus causing degradation and inhibition of translation. The low HLA-G expression in the placenta contributes to the induction of the TH1 cytokine response seen in ICP [64] while also protecting the fetus by regulating maternal CD4+ T-cell activity [30].
Apoptosis
Cell apoptosis and intracellular edema are increased by the placenta in ICP [65]. This can be seen through the expression of Fas and FasL. FasL is able to induce apoptosis of maternal lymphocytes in order to protect the fetus. In ICP, expression of FasL is decreased while Fas is increased in syncytiotrophoblasts [30]. This increase in Fas comes from the induction by TNF-α and IFN-γ [30]. Bile acids also activate Fas receptors in hepatocytes resulting in injury and cell death [66]. These changes ultimately result in the apoptosis of placenta cells with a decrease in the inactivation of maternal lymphocytes.
Higher levels of bax and p53, both pro-apoptotic proteins, and lower levels of bcl-2, an anti-apoptotic protein, have been noted in ICP [30]. Bax expression can be induced by bile acids in the mitochondria and will cause dysfunction then death [67]. Other pro-apoptotic proteins noted to be upregulated in ICP are deoxyribonuclease 1-like 3 (DNASE1L3), tumor protein p73-like (TP73L), ring finger protein 36 (RNF36) and KIAA0367 [4]. Endoplasmic reticulum protein 29 (ERp29) has also been found to be increased in ICP [68] and may induce apoptosis due to bile acids by activating p38 and caspase-3 [69].
Finally, M30 antibody or caspase-cleaved cytokeratin-18 is thought to be specific to liver apoptosis. In fact, cytokeratin-18 is the main protein filament in the liver and is cleaved during apoptosis by caspases [70]. This M30 antibody is found to be significantly higher in both maternal and umbilical cord serum signifying liver damage [70]. The increased levels of many apoptotic proteins signify that cell death is occurring more rapidly and at multiple sites in patients with ICP; a well-designed treatment is needed to restore the immune system for these patients.
Treatment
Ursodeoxycholic acid (UDCA) is the commonly used treatment for ICP [71]. It has been shown to improve both the outcomes for the mother and fetus by decreasing pruritus, improving liver function, increasing the birth weight and being associated with less meconium staining [72]. Currently, the mechanisms by which UDCA act are not completely revealed. One of the beneficial effects of this treatment is its ability to decrease bile acid accumulation [2, 3, 10]. Studies found that UDCA has this effect by increasing the activity of the canalicular BSEP and MDR3, used for exporting phospholipids, along with basolateral MRP4 which exports bile salt conjugates and placental ABCG2 protein, an export of bile acids and sulfated progesterone in the placenta [2, 73, 74]. With BSEP, UDCA seems to be doubling the half-life of the protein by slowing down the endocytosis of the pump [73]. This decreased degradation is most likely occurring with ABCG2 as well because its mRNA was not found to be increased with UDCA treatment [2].
Along with increasing the activity of these pumps, UDCA has many other effects. It decreases the toxicity of the bile pool [65, 75], prevents oxidative stress and apoptosis [76], and upregulates serum expression of the vasodilator, corticotropin-releasing hormone, which is down regulated in ICP [77]. UDCA has also been shown to prevent arrhythmic events in a rat model of the fetal heart [78] and may have an effect on progesterone levels and pruritus. The results are mixed on the changes in progesterone with UDCA treatment. One study stated that it has no effect on reducing the level of sulfated progesterone metabolites in maternal serum [2]. However, another study found stimulation of disulphate progesterone metabolites excretion in the urine to be associated with improvements in pruritus [79]. In regards to pruritus, it is mediated by lysophosphatidic acid or LPA, a phospholipid derivate that is increased in ICP [80]. LPA is created by autotaxin, which is also increased in ICP, from lysophosphatidylcholine [80]. Unfortunately, not all women experience relief from pruritus with UDCA [81].
Combination therapies have been used to improve the treatment for ICP. This is due to the fact some studies have not found UDCA to be effective [65]. These therapies include combinations of UDCA with rifampicin, S-adenosylmethionine (SAMe) or polyunsaturated phosphatidylcholine (PPC), all of whose mechanisms are represented in Figure 3. Rifampicin acts by enhancing the elimination of bilirubin and bile acids from hepatocytes. Specifically, it increases expression of the UDP glucuronosyltransferase 1 family, polypeptide A1 (UGT1A1), a bilirubin-conjugating enzyme, and MRP2, which excretes bilirubin at the canalicular membrane [74]. Furthermore, it reduces bile acid levels by increasing the expression of cytochrome P450 3A4 (CYP3A4) via the binding of the pregnane nuclear receptor (PXR) to the 9-cis retinoic acid receptor (RXR), which is at reduced levels with rifampicin treatment [82]. CYP3A4 converts bile acids by 6α-hydroxylation; the bile acids are then glucuronidated and excreted in the urine [74]. In a retrospective study, rifampicin was shown to decrease bile acid levels in combination with UDCA for patients who did not respond to UDCA monotherapy [81]. More studies need to be done on Rifampicin treatment with UDCA as the current research available is limited due to the number of patients studied.
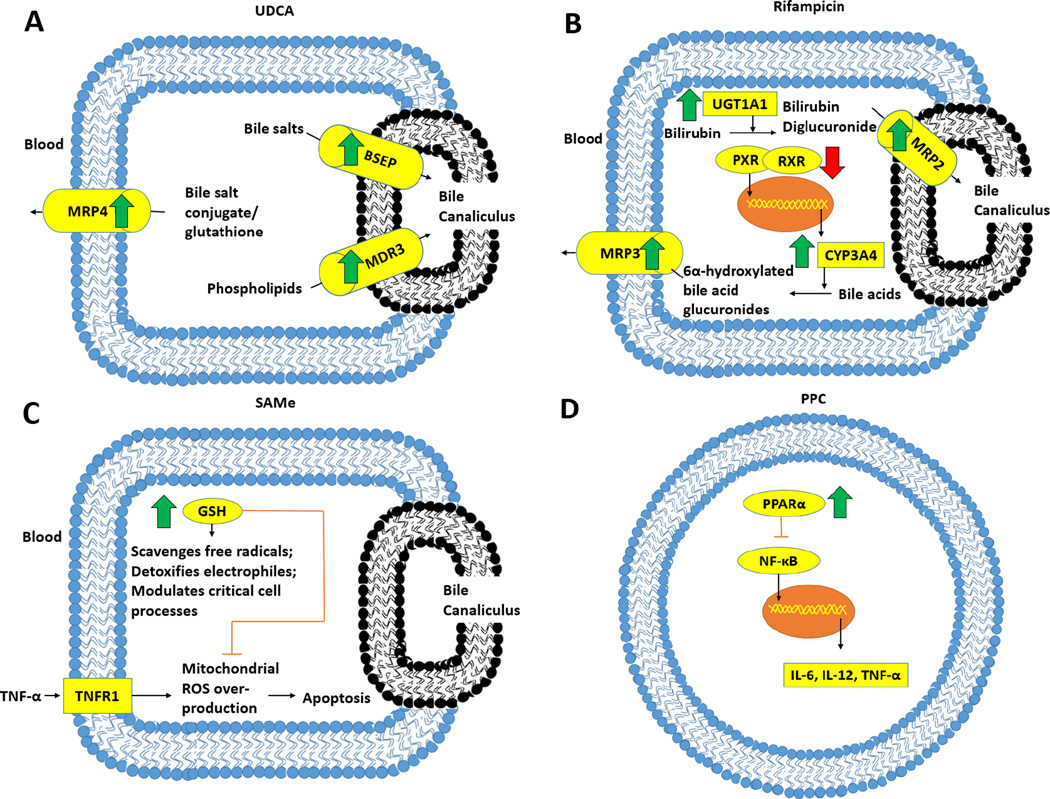
Figure 3. Mechanisms of treatment for intrahepatic cholestasis of pregnancy.
In parts A, B and C, the effects of treatments are shown in hepatocytes; D shows PPC’s effects occurring in immune cells such as leukocytes. UDCA, as shown in A, treats intrahepatic cholestasis of pregnancy in part by decreasing bile acid via higher protein levels of the canalicular transporters BSEP and MDR3 and the basolateral MRP4 protein. In B, Rifampicin increases expression of CYP3A4 through binding of PXR to RXR which activates its transcription. CYP3A4 converts bile acids to 6α-hydroxylated bile acids that are later glucuronidated and exported into the blood via MRP3. The elimination of bilirubin is also increased with rifampicin through increased UGT1A1 which converts bilirubin to bilirubin diglucuronide that is then excreted through MRP2, a canalicular exporter. In C, SAMe’s effect on GSH is shown. It increases levels of GSH, which protects hepatocytes by scavenging free radicals, detoxifying electrophiles and modulating critical cell processes such as DNA synthesis. GSH also controls the generation of ROS in mitochondria. This production can be induced by the binding of TNF-α to its receptor TNFR1 to cause apoptosis. Finally, PPC in D acts by increasing activation of PPARα which inhibits the NF-κB and thus the production of pro-inflammatory cytokines in cells such as macrophages and lymphocytes.
UDCA: ursodeoxycholic acid; BSEP: Bile salt export pump; MDR3: multidrug resistance protein 3; MRP2, 3, 4: Multidrug resistance associated protein 2, 3, 4; UGT1A1: UDP glucuronosyltransferase 1A1; PXR: Pregnane X receptor; RXR: 9-cis retinoic acid receptor; CYP3A4: Cytochrome P450 3A4; SAMe: S-adenosylmethionine; GSH: Glutathione; ROS: Reactive oxygen species; TNFR1: Tumor necrosis factor receptor type 1; PPC: Polyunsaturated phosphatidylcholine; PPARα: Peroxisome proliferator-activated receptor α.
The next therapy, SAMe, is endogenously produced in the liver and is an important methyl group donor [83, 84]. Its main function is to raise levels of glutathione (GSH) which protects the liver by detoxifying electrophiles, scavenging free radicals and affecting cell processes such as DNA synthesis and the immune system [85]. In addition, GSH can save hepatocytes from apoptosis by defending against the generation of ROS in mitochondria that results from the binding of TNF-α to its associated type 1 receptor TNFR1 [86]. A meta-analysis comparing the glutathione precursor SAMe, UDCA and the combination of the two showed that SAMe and UDCA together further reduced Cesarean sections, preterm birth and fetal asphyxia [87].
Finally, PPC is a transcriptional activator that increases expression of the mRNA of PPARα [10]. PPARα has been shown to antagonize signaling through NF-κB and thus the expression of IL-6, IL-12 and TNF-α [88]. Hence, PPC reduces the production of pro-inflammatory cytokines seen in ICP. The combination of PPC and UDCA led to less pruritus for patients and improved liver function [89]. This therapy is suggested in ICP cases that are severe or have an early onset [89]. This and the other combination therapies are promising, but their mechanisms need to be examined more closely to understand how patients are benefiting and what can be done to further enhance the treatments.
Ideas for future treatments may further help ameliorate ICP. For example, drugs that block autotaxin or LPA receptors may be better at improving pruritus than current options [80]. One study supporting this idea found that a variant of adipoturin, which metabolizes LPA, decreased itch severity in ICP through increased metabolism [90]. Another potential treatment is FXR agonists. These agonists, such as W450 or WAY-362450 on the placenta, have been effective in reducing bile acid levels in maternal serum, liver and amniotic fluid in mice [65]. Furthermore, HLA-G is a potential target as it has the ability to alter the immune response to be more TH1 or TH2 oriented [64]. High levels of HLA-G suppress the T-lymphocyte response and induces TH2 development [64]. Future, targeted treatments need to be explored in order to reduce this disorder that affects both the mother and fetus.
Expert commentary & five-year view
While the exact mechanism behind ICP is unknown, many factors have been shown to contribute to this disorder. Increases in estrogen and progesterone result in reduced bile acid uptake and excretion by the liver. The increase in serum bile acid then causes a change in the immune system from a TH2 mediated response to TH1. As pregnancies that have a shift to a TH1 immune response have more adverse outcomes, many of the risks of ICP are most likely mediated by the immune system. The pathways by which the immune cells take this effect and how exactly they are involved requires further research.
The current treatment, UDCA, is shown to be beneficial in some studies but the mechanism of its action is not fully understood either. It has been shown to act on bile acid accumulation but no studies have explored how it acts on the immune system. As not all patients respond to this treatment, research needs to delve more into UDCA, combination treatments with UDCA and potential future treatments in order to achieve better outcomes for patients with ICP.
Key Issues.
Intrahepatic cholestasis of pregnancy can have adverse effects for both the fetus and mother
Increases in bile acids result in a change to TH1 mediated immunity; however, the mechanisms behind this is unknown.
Further investigation into the alterations of the immune system, pathways involved and implications of these changes is needed to better understand intrahepatic cholestasis of pregnancy.
Markers for apoptosis signify the occurrence of intracellular edema and cell death in the liver and placenta
UDCA has shown to be effective at treating some patients but not all; further studies should assess the factors behind the ineffectiveness of UDCA.
Combination therapies with UDCA including rifampicin, SAMe and PPC merit more examination as these lead to improvements over UDCA monotherapy.
Potential future treatments that have specific targets known to play a role in ICP need to be investigated to improve the care of patients.
Acknowledgments
This work was supported by research grants R01 HL116042, R01 HL112597, and R01 HL120659 to DK Agrawal from the Office of the Director, National Institutes of Health, and National Heart, Lung and Blood Institute, NIH USA. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. No writing assistance was utilized in the production of this manuscript.
Footnotes
Financial and competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Jin P, Shao Y. Expression and significance of interleukin-18, 12 and tumor necrosis factor-α in intrahepatic cholestasis of pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2011;46(5):329–332. [PubMed] [Google Scholar]
- 2.Estiu MC, Monte MJ, Rivas L, et al. Effect of ursodeoxycholic acid treatment on the altered progesterone and bile acid homeostasis in the mother-placenta-foetus trio during cholestasis of pregnancy. Br J Clin Phramacol. 2015;79(2):316–329. doi: 10.1111/bcp.12480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geenes V, Lovgren-Sandblom A, Benthin L, et al. The reversed feto-maternal bile acid gradient in intrahepatic cholestasis of pregnancy is corrected by ursodeoxycholic acid. PLoS One. 2014;9(1):e83828. doi: 10.1371/journal.pone.0083828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wei J, Wang H, Yang X, et al. Altered gene profile of placenta from women with intrahepatic cholestasis of pregnancy. Arch Gynecol Obstet. 2010;281(5):801–810. doi: 10.1007/s00404-009-1156-3. • This study investigated alterations in the gene profiles of placentas from patients with ICP. Among many changes, it found specific chemokines to be upregulated.
- 5.Abedin P, Weaver JB, Egginton E. Intrahepatic cholestasis of pregnancy: prevalence and ethnic distribution. Ethn Health. 1999;4(1–2):35–37. doi: 10.1080/13557859998173. [DOI] [PubMed] [Google Scholar]
- 6.Riely CA, Bacq Y. Intrahepatic cholestasis of pregnancy. Clin Liver Dis. 2004;8(1):167–176. doi: 10.1016/S1089-3261(03)00131-4. [DOI] [PubMed] [Google Scholar]
- 7.Wang XD, Yao Q, Peng B, et al. A clinical analysis of intrahepatic cholestasis of pregnancy in 1241 cases. Zhonghua Gan Zang Bing Za Zhi. 2007;15(4):291–293. [PubMed] [Google Scholar]
- 8.Nguyen KD, Sundaram V, Ayoub WS. Atypical causes of cholestasis. World J Gastroenterol. 2014;20(28):9418–9426. doi: 10.3748/wjg.v20.i28.9418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalez MC, Reyes H, Arrese M, et al. Intrahepatic cholestasis of pregnancy in twin pregnancies. J Hepatol. 1989;9(1):84–90. doi: 10.1016/0168-8278(89)90079-2. [DOI] [PubMed] [Google Scholar]
- 10.Pradhan M, Shao Y. Clinical grading of intrahepatic cholestasis pregnancy. JNMA J Nepal Med Assoc. 2013;52(190):413–419. [PubMed] [Google Scholar]
- 11.Lammert F, Marschall HU, Glantz A, et al. Intrahepatic cholestasis of pregnancy: molecular pathogenesis, diagnosis and management. J Hepatol. 2000;33(6):1012–1021. doi: 10.1016/s0168-8278(00)80139-7. [DOI] [PubMed] [Google Scholar]
- 12.Zollner G, Trauner M. Mechanisms of Cholestasis. Clinics in Liver Disease. 2008;12(1):1–26. doi: 10.1016/j.cld.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 13.Glantz A, Marschall HU, Mattsson LA. Intrahepatic cholestasis of pregnancy: Relationships between bile acid levels and fetal complication rates. Hepatology. 2004;40(2):464–474. doi: 10.1002/hep.20336. [DOI] [PubMed] [Google Scholar]
- 14.Kirbas O, Biberoglu EH, Kirbas A, et al. Evaluation of ventricular repolarization in pregnant women with intrahepatic cholestasis. Int J Cardiol. 2015;189:25–29. doi: 10.1016/j.ijcard.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Herraez E, Lozano E, Poli E, et al. Role of macrophages in bile acid-induced inflammatory response of fetal lung during maternal cholestasis. Journal of Molecular Medicine. 2014;92(4):359–372. doi: 10.1007/s00109-013-1106-1. [DOI] [PubMed] [Google Scholar]
- 16.Raz Y, Lavie A, Vered Y, et al. Severe intrahepatic cholestasis of pregnancy is a risk factor for preeclampsia in singleton and twin pregnancies. Am J Obstet Gynecol. 2015 doi: 10.1016/j.ajog.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 17.Oztas E, Erkenekli K, Ozler S, et al. Can routine laboratory parameters predict adverse pregnancy outcomes in intrahepatic cholestasis of pregnancy? J Perinat Med. 2014 doi: 10.1515/jpm-2014-0207. [DOI] [PubMed] [Google Scholar]
- 18.Binder T, Salaj P, Dyr EJ, et al. Hematological aspects of gestational cholestatic hepatosis. Ceska Gynekol. 2006;71(2):99–102. [PubMed] [Google Scholar]
- 19.Ropponen A, Sund R, Riikonen S, et al. Intrahepatic cholestasis of pregnancy as an indicator of liver and biliary diseases: a population-based study. Hepatology. 2006;43(4):723–728. doi: 10.1002/hep.21111. [DOI] [PubMed] [Google Scholar]
- 20.Wikstrom Shemer EA, Stephansson O, Thuresson M, et al. Intrahepatic cholestasis of pregnancy and cancer, immune-mediated and cardiovascular diseases: A population-based cohort study. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Müllenbach R, Bennett A, Tetlow N, et al. ATP8B1 mutations in British cases with intrahepatic cholestasis of pregnancy. Gut. 2005;54(6):829–834. doi: 10.1136/gut.2004.058115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dixon PH, Weerasekera N, Linton KJ, et al. Heterozygous MDR3 missense mutation associated with intrahepatic cholestasis of pregnancy: evidence for a defect in protein trafficking. Hum Mol Genet. 2000;9(8):1209–1217. doi: 10.1093/hmg/9.8.1209. [DOI] [PubMed] [Google Scholar]
- 23.Jacquemin E, Cresteil D, Menouvrier S, et al. Heterozygous non-sense mutation of the MDR3 gene in familial intrahepatic cholestasis of pregnancy. Lancet. 1999;353(9148):210–211. doi: 10.1016/S0140-6736(05)77221-4. [DOI] [PubMed] [Google Scholar]
- 24.Tavian D, Degiorgio D, Roncaglia N, et al. A new splicing site mutation of the ABCB4 gene in intrahepatic cholestasis of pregnancy with raised serum gamma-GT. Dig Liver Dis. 2009;41(9):671–675. doi: 10.1016/j.dld.2008.12.101. [DOI] [PubMed] [Google Scholar]
- 25.Kamimura K, Abe H, Kamimura N, et al. Successful management of severe intrahepatic cholestasis of pregnancy: report of a first Japanese case. BMC Gastroenterology. 2014;14:160. doi: 10.1186/1471-230X-14-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anzivino C, Odoardi M, Meschiari E, et al. ABCB4 and ABCB11 mutations in intrahepatic cholestasis of pregnancy in an Italian population. Digestive and Liver Disease. 2013;45(3):226–232. doi: 10.1016/j.dld.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Dixon PH, van Mil SW, Chambers J, et al. Contribution of variant alleles of ABCB11 to susceptibility to intrahepatic cholestasis of pregnancy. Gut. 2009;58(4):537–544. doi: 10.1136/gut.2008.159541. [DOI] [PubMed] [Google Scholar]
- 28.Clayton PT. Disorders of bile acid synthesis. J Inherit Metab Dis. 2011;34(3):593–604. doi: 10.1007/s10545-010-9259-3. [DOI] [PubMed] [Google Scholar]
- 29.Schneider G, Paus TC, Kullak-Ublick GA, et al. Linkage between a new splicing site mutation in the MDR3 alias ABCB4 gene and intrahepatic cholestasis of pregnancy. Hepatology. 2007;45(1):150–158. doi: 10.1002/hep.21500. [DOI] [PubMed] [Google Scholar]
- 30.Yayi H, Danqing W, Shuyun L, et al. Immunologic abnormality of intrahepatic cholestasis of pregnancy. Am J Reprod Immunol. 2010;63(4):267–273. doi: 10.1111/j.1600-0897.2009.00798.x. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Vasilenko A, Song X, et al. Estrogen and Estrogen Receptor-α-Medicated Transrepression of Bile Salt Export Pump. Mol Endocrinol. 2015;29(4):613–626. doi: 10.1210/me.2015-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yan Z, Li E, He L, et al. Role of OATP1B3 in the transport of bile acids assessed using first-trimester trophoblasts. J Obstet Gynaecol Res. 2015;41(3):392–401. doi: 10.1111/jog.12549. [DOI] [PubMed] [Google Scholar]
- 33. Abu-Hayyeh S, Williamson C. Estradiol, farnesiod X receptor, and altered metabolism in pregnancy. Hepatology. 2014;60(6):1815–1817. doi: 10.1002/hep.27280. • In this article, the roles of estradiol, progesterone metabolites and BSEP are discussed in regards to the etiology of ICP.
- 34.Vallejo M, Briz O, Serrano MA, et al. Potential role of trans-inhibition of the bile salt export pump by progesterone metabolites in the etiopathogenesis of intrahepatic cholestasis of pregnancy. J Hepatol. 2006;44(6):1150–1157. doi: 10.1016/j.jhep.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 35.Abu-Hayyeh S, Martinez-Becerra P, Sheikh Abdul Kadir SH, et al. Inhibition of Na+-taurocholate Co-transporting polypeptide-mediated bile acid transport by cholestatic sulfated progesterone metabolites. J Biol Chem. 2010;285(22):16504–16512. doi: 10.1074/jbc.M109.072140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abu-Hayyeh S, Papacleovoulou G, Lovgren-Sandblom A, et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology. 2013;57(2):716–726. doi: 10.1002/hep.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Lingqing H, Cui Y, et al. Roles of PPARγ/NF-κB signaling Pathway in the Pathogenesis of Intrahepatic Cholestasis of Pregnancy. PLOS ONE. 2014;9(1):e87343. doi: 10.1371/journal.pone.0087343. • • Study examining the maternal serum expression of IL-4, IL-6, IL-12 and TNF-α along with the expression of NF-κB and PPARγ in ICP.
- 38.Yi J, Ding Y. Expression of HLA-G protein in placental tissues and its influence on Th1/Th2 cytokines in peripheral blood in patients with intrahepatic cholestasis of pregnancy. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2010;35(3):241–246. doi: 10.3969/j.issn.1672-7347.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 39. Kirbas A, Biberoglu E, Ersoy AO, et al. The role of interleukin-17 in intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2015:1–5. doi: 10.3109/14767058.2015.1028354. • This research is the first to investigate changes in IL-17 levels of ICP. They found IL-17 to be significantly increased in patients with severe ICP, or who had total bile acid levels ≥ 40µmol/L.
- 40. Ling B, Yao F, Zhou Y, et al. Cell-mediated immunity imbalance in patients with intrahepatic cholestasis of pregnancy. Cell Mol Immunol. 2007;4(1):71–75. • This report explored the changes in, T, NK and NKT cell populations in patients with ICP. They found that NK and NKT cells are increased in the decidua parietalis while CD3+ T cells are decreased.
- 41.Zhang LJ, Li MY. Expression of suppressor of cytokine signaling 3 and its significance in human placenta with pregnant intrahepatic cholestasis. Zhonghua Fu Chan Ke Za Zhi. 2010;45(6):406–410. [PubMed] [Google Scholar]
- 42.Peng B, Liu S. Study of relationship between T helper cell type-1 and type-2 cytokines and intrahepatic cholestasis of pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2002;37(9):516–518. [PubMed] [Google Scholar]
- 43.Piccinni MP, Lombardelli L, Logiodice F, et al. T helper cell mediated-tolerance towards fetal allograft in successful pregnancy. Clin Mol Allergy. 2015;13(1):9. doi: 10.1186/s12948-015-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prins J, Gomez-Lopez N, Robertson SA. Interleukin-6 in pregnancy and gestational disorders. J Reprod Immunol. 2012;95(1–2):1–14. doi: 10.1016/j.jri.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Miossec P, Kolls JK. Targeting IL-17 and TH17 cell in chronic inflammation. Nat Rev Drug Discov. 2012;11(10):763–776. doi: 10.1038/nrd3794. [DOI] [PubMed] [Google Scholar]
- 46.O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends in Cell Biology. 2000;10(12):542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 47.Okamura H, Kashiwamura S, Tsutsui H, et al. Regulation of interferon- γ production by IL-12 and IL-18. Curr. Opin Immunol. 1998;10(3):259–264. doi: 10.1016/s0952-7915(98)80163-5. [DOI] [PubMed] [Google Scholar]
- 48.Chatterjee P, Chiasson VL, Bounds KR, et al. Regulation of the Anti-Inflammatory Cytokines Interleukin-4 and Interleukin-10 during Pregnancy. Front Immunol. 2014;5:253. doi: 10.3389/fimmu.2014.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hadfield KA, McCracken SA, Ashton AW, et al. Regulated suppression of NF-κB throughout pregnancy maintains a favourable cytokine environment necessary for pregnancy success. J Reprod Immunol. 2011;89(1):1–9. doi: 10.1016/j.jri.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Graves DT, Jiang Y. Chemokines, a family of chemotactic cytokines. Crit Rev Oral Biol Med. 1995;6(2):109–118. doi: 10.1177/10454411950060020101. [DOI] [PubMed] [Google Scholar]
- 51.Du Q, Pan Y, Zhang Y, et al. Placental gene-expression profiles of intrahepatic cholestasis of pregnancy reveal involvement of multiple molecular pathways in blood vessel formation and inflammation. BMC Med Genomics. 2014;7:42. doi: 10.1186/1755-8794-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Q, Liu S, Xiong Q. The changes of serum estrogen, progesterone and the function of immune system in intrahepatic cholestasis of pregnancy. Zhonghua Fu Chan Ke Za Zhi. 1998;33(12):724–726. [PubMed] [Google Scholar]
- 53.Lin L, Zhang LJ. Role of CD4+ CD25 high regulatory T cells in the pathogenesis of intrahepatic cholestasis of pregnancy. Zhonghua Fu Chan Ke Za Zhi. 2008;43(12):900–903. [PubMed] [Google Scholar]
- 54.Moreno-Eutimio MA, Tover-Rodriguez JM, Vargas-Avila K, et al. Increased serum levels of inflammatory mediators and low frequency of regulatory T cells in the peripheral blood of preeclamptic Mexican women. Biomed Res Int. 2014;2014:413249. doi: 10.1155/2014/413249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matson BC, Caron KM. Uterine natural killer cells as modulators of the maternal-fetal vasculature. Int J Dev Biol. 2014;58(2–4):199–204. doi: 10.1387/ijdb.140032kc. [DOI] [PubMed] [Google Scholar]
- 56.Li LP, Fang YC, Dong GF, et al. Depletion of invariant NKT cells reduces inflammation-induced pre-term delivery in mice. J Immunol. 2012;188(9):4681–4689. doi: 10.4049/jimmunol.1102628. [DOI] [PubMed] [Google Scholar]
- 57.Du Q, Zhou L, Hao K, et al. Study on the regulation of cell adhesion molecule expression and function in placenta from women with intrahepatic cholestasis of pregnancy. Med Hypotheses. 2013;81(3):374–375. doi: 10.1016/j.mehy.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 58.Kim ND, Moon JO, Slitt AL, et al. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90(2):586–595. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 59.Wang Z, Dong M, Chu H, et al. Increased serum levels of neopterin and soluble interleukin-2 receptor in intrahepatic cholestasis of pregnancy. Acta Obstet Gynecol Scand. 2004;83(11):1067–1070. doi: 10.1111/j.0001-6349.2004.00601.x. [DOI] [PubMed] [Google Scholar]
- 60.Keitel V, Spomer L, Marin J, et al. Effect of maternal cholestasis on TGR5 expression in human and rat placenta at term. Placenta. 2013;34(9):810–816. doi: 10.1016/j.placenta.2013.06.302. [DOI] [PubMed] [Google Scholar]
- 61.Kirbas A, Biberoglu E, Daglar K, et al. Neutrophil-to-lymphocyte ratio as a diagnostic marker of intrahepatic cholestasis of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2014;180:12–15. doi: 10.1016/j.ejogrb.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 62.Woolbright BL, Jaeschke H. Novel insight into mechanisms of cholestatic liver injury. World J Gastroenterol. 2012;18(36):4985–4993. doi: 10.3748/wjg.v18.i36.4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gujral JS, Farhood A, Bajt M, et al. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38(2):355–363. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 64.Zhang X, Yu L, Ding Y. Human leukocyte antigen G and miR-148a are associated with the pathogenesis of intrahepatic cholestasis of pregnancy. Exp Ther Med. 2014;8(6):1701–1706. doi: 10.3892/etm.2014.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wu WB, Xu TT, Cheng WW, et al. Agonist of farnesoid X receptor protects against bile acid induced damage and oxidative stress in mouse placenta--a study on maternal cholestasis model. Placenta. 2015;36(5):545–551. doi: 10.1016/j.placenta.2015.02.005. • This study explored a future treatment for ICP by using a FXR agonist in a mouse model. They found that the FXR agonist attenuated cell apoptosis and edema accumulation in the placenta.
- 66.Faubion WA, Guicciardi ME, Miyoshi H, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103(1):137–145. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oh SH, Yun KJ, Nan JX, et al. Changes in expression and immunolocalization of protein associated with toxic bile salts-induced apoptosis in rat hepatocytes. Arch Toxicol. 2003;77(2):110–115. doi: 10.1007/s00204-002-0415-x. [DOI] [PubMed] [Google Scholar]
- 68.Zhang T, Guo Y, Guo X, et al. Comparative proteomics analysis of placenta from pregnant women with intrahepatic cholestasis of pregnancy. PLoS ONE. 2013;8(12):e83281. doi: 10.1371/journal.pone.0083281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang T, Zhao C, Luo L, et al. High concentraction of taurocholic acid induced apoptosis in HTR-8/SVneo cells via overexpression of ERp29 and activation of p38. Placenta. 2014;35(7):496–500. doi: 10.1016/j.placenta.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 70.Ersoy AO, Kirbas A, Ozler S, et al. Maternal and fetal serum levels of caspase-cleaved fragments of cytokeratin-18 in intrahepatic cholestasis of pregnancy. J Matern Fetal Neonatal Med. 2015;10:1–5. doi: 10.3109/14767058.2015.1011116. [DOI] [PubMed] [Google Scholar]
- 71.Mays JK. The active management of intrahepatic cholestasis of pregnancy. Curr Opin Obstet Gynecol. 2010;22(2):100–103. doi: 10.1097/GCO.0b013e328337238d. [DOI] [PubMed] [Google Scholar]
- 72.Grand’Maison S, Durand M, Mahone M. The effects of ursodeoxycholic acid treatment for intrahepatic cholestasis of pregnancy on maternal and fetal outcomes: a meta-analysis including non-randomized studies. J Obstet Gynaecol Can. 2014;36(7):632–641. doi: 10.1016/S1701-2163(15)30544-2. [DOI] [PubMed] [Google Scholar]
- 73.Kagawa T, Orii R, Hirose S, et al. Ursodeoxycholic acid stabilizes the bile salt export pump in the apical membrane in MDCK II cells. J Gastroenterol. 2014;49(5):890–899. doi: 10.1007/s00535-013-0833-y. [DOI] [PubMed] [Google Scholar]
- 74.Marschall HU, Wagner M, Zollner G, et al. Complementary Stimulation of Hepatobiliary Transport and Detoxification Systems by Rifampicin and Ursodeoxycholic Acid in Humans. Gastroenterol. 2005;129:476–485. doi: 10.1016/j.gastro.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 75.Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36(3):525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 76.Perez MJ, Macias RI, Marin JJ. Maternal cholestasis induces placental oxidative stress and apoptosis. Protective effect of ursodeoxycholic acid. Placenta. 2006;27(1):34–41. doi: 10.1016/j.placenta.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 77.Zhuo F, Zhang L, He MM, et al. Corticotropin-relasing hormone expression in patients with intrahepatic cholestasis of pregnancy after ursodeoxycholic acid treatment: an initial experience. Curr Med Res Opin. 2014;30(8):1529–1535. doi: 10.1185/03007995.2014.907560. [DOI] [PubMed] [Google Scholar]
- 78.Miragoli M, Kadir SH, Sheppard MN, et al. A protective antiarrhythmic role of ursodeoxycholic acid in an in vitro rat model of the cholestatic fetal heart. Hepatology. 2011;54(4):1282–1292. doi: 10.1002/hep.24492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Glantz A, Reilly SJ, Benthin L, et al. Intrahepatic cholestasis of pregnancy: Amelioration of pruritus by UDCA is associated with decreased progesterone disulphates in urine. Hepatology. 2008;47(2):544–551. doi: 10.1002/hep.21987. [DOI] [PubMed] [Google Scholar]
- 80.Kremer AE, Martens JJ, Kulik W, et al. Lysophosphatidic Acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139(3):1008–1018. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 81.Geenes V, Chambers J, Khurana R, et al. Rifampicin in the treatment of severe intrahepatic cholestasis of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2015;189:59–63. doi: 10.1016/j.ejogrb.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 82.Kliewer SA, Willson TM. Regulation of xenobiotic and bile acid metabolism by the nuclear pregnane X receptor. J Lipid Res. 2002;43(3):359–364. [PubMed] [Google Scholar]
- 83.Gurung V, Middleton P, Milan SJ, et al. Interventions for treating cholestasis in pregnancy. Cochrane Database Syst Rev. 2013;6:CD000493. doi: 10.1002/14651858.CD000493.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu S, Mato JM. S-adenosylmethionine in liver health, injury, and cancer. Physiol Rev. 2012;92(4):1515–1542. doi: 10.1152/physrev.00047.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lu S. Regulation of glutathione synthesis. Mol Aspects Med. 2009;30(1–2):42–59. doi: 10.1016/j.mam.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Garcia-Ruiz C, Fernandez-Checa J. Redox regulation of hepatocyte apoptosis. J Gastroenterol Hepatol. 2002;22:S38–S42. doi: 10.1111/j.1440-1746.2006.04644.x. [DOI] [PubMed] [Google Scholar]
- 87.Zhuo F, Gao B, Wang X, et al. Meta-analysis of ursodeoxycholic acid and S-adenosylmethionine for improving the outcomes of intrahepatic cholestasis of pregnancy. Zhonghua Gan Zang Bing Za Zhi. 2014;22(4):299–304. doi: 10.3760/cma.j.issn.1007-3418.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 88.Poynter M, Daynes R. Peroxisome Proliferator-activated Receptor α Activation Modulates Cellular Redox Status, Represses Nuclear Factor-κB Signaling, and Reduces Inflammatory Cytokine Production in Aging. J Biol Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 89.Marciniak B, Kimber-Trojnar Z, Leszczynska-Gorzelak B, et al. Treatment of obstetric cholestasis with polyunsaturated phosphatidylcholine and ursodeoxycholic acid. Ginekol Pol. 2011;82(1):26–31. [PubMed] [Google Scholar]
- 90.Krawczyk M, Milkiewicz M, Marschall HU, et al. Variant adiponutrin confers genetic protection against cholestatic itch. Sci Rep. 2014;4:6374. doi: 10.1038/srep06374. [DOI] [PMC free article] [PubMed] [Google Scholar]