Abstract
Effective drug delivery to the eye is an ongoing challenge due to poor patient compliance coupled with numerous physiological barriers. Eye drops for the front of the eye and ocular injections for the back of the eye are the most prevalent delivery methods, both of which require relatively frequent administration and are burdensome to the patient. Novel drug delivery techniques stand to drastically improve safety, efficacy and patient compliance for ocular therapeutics. Remarkable advances in nanofabrication technologies make the application of nanostructured materials to ocular drug delivery possible. This article focuses on the use of nanostructured materials with nanoporosity or nanotopography for ocular delivery. Specifically, we discuss nanotopography for enhanced bioadhesion and permeation and nanoporous materials for controlled release drug delivery. As examples, application of polymeric nanostructures for greater transepithelial permeability, nanostructured microparticles for enhanced preocular retention time and nanoporous membranes for tuning drug release profile are covered.
Improving therapeutic delivery to the eye is both a challenging and lucrative endeavor. The size of the patient population and market are astounding. In 2010, 733 million people globally were affected by vision loss or blindness [1]. Fortunately, for many of the leading causes of vision loss there are treatments available to at least slow or halt disease progression, if not improve sight. In 2014, the market for such ophthalmic therapeutics and devices reached US$36 billion [1] and it is only expected to grow.
There are two main factors affecting the projected growth of this market. The first is the anticipated increase in the patient population due to an aging population and increasing incidence of diabetes, both of which are prominent risk factors for diseases associated with vision loss [2]. The second is improvement in ophthalmic therapeutics and ocular drug delivery leading to improved patient compliance and new treatment options. In 2001, 95% of the market share in global ophthalmic drugs and devices came from therapeutics delivered to the front of the eye [2]. This is not because there is greater incidence of anterior eye disease (only 45% of vision loss cases are anterior disease) [2] but because delivering therapeutics to the back of the eye is immensely challenging. With new technologies emerging both in therapeutics and delivery, this is expected to change.
Even though the front of the eye is easier to treat than the back, there are still many challenges associated with effective treatment. To start with, it is difficult to get drugs into the eye. The most common application technique for front of the eye delivery uses eye drops but even in the best of cases a large portion of the drop either never makes it into the eye or is rapidly cleared by nasolacrimal drainage before it can permeate through the cornea. On average only 5% of drug applied as an eye drop actually makes it past the cornea [2]. Patients typically have to dose at least once a day if not more frequently and due to the high systemic absorption, there can be significant side effects. Increasing corneal residence time and/or corneal permeability would improve this scenario and increase efficacy. Ocular drug delivery systems have the potential to do both.
When delivering therapeutics to the back of the eye, there are few options available. While small molecules can potentially be applied to the front of the eye with hopes of permeation through the eye's multiple barriers, very little drug is expected to actually reach to the site of action at the back of the eye. Biologics typically do not permeate at all through the corneal epithelium. Therefore, most back of the eye therapeutics rely on direct injection. While this gets the drug where it needs to go in effective doses, it is undeniably unpleasant to receive an ocular injection and many treatments require patients to undergo this once or twice a month. As a result, patient compliance can be a major hurdle, leading to undertreatment in many cases. Additionally, ocular injections pose their own risk of complications resulting from the procedure itself so minimizing the number of injections into the eye is critical for improving therapeutic delivery. Therefore, there is strong interest in long-acting drug delivery systems to improve patient compliance, safety and efficacy of posterior ocular therapeutics.
By now you should be convinced that drug delivery systems are an integral part of the future of ocular therapeutics. Drug delivery systems can increase bioavailability and efficacy, improve patient compliance and reduce the risk of complications by reducing frequency of administration. But how does nanotechnology fit into all of this? Nanotechnology is on the rise in biomedical applications. As fabrication techniques advance, the unique properties of features on the nanoscale are being leveraged for many applications in drug delivery, ranging from enhanced adhesion to controlled drug release. In this review we will explore nanofeatures relevant to improving ocular drug delivery.
Use of nanostructured materials in overcoming physiological barriers
Physiological barriers in ocular drug delivery
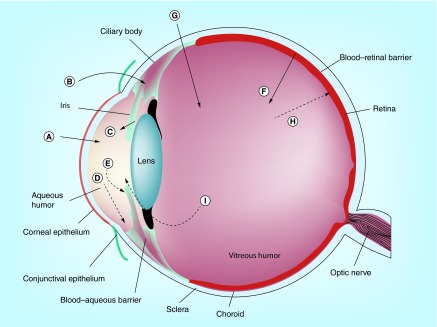
As mentioned earlier, while ocular drug delivery heavily relies on the use of eyedroppers due to their ease of use, topical administration typically achieves very low ocular bioavailability (<5%). This is because topically administered ocular agents must penetrate physiological barriers (Figure 1), such as the tear film and the cornea, to reach the target site. More specifically, a topically administered agent first meets the tear film, which drains a large portion of the drug to the systemic route before it reaches the ocular tissues. Then, the remaining drug still needs to penetrate the cornea, which consists of three main layers: epithelium, stroma and endothelium. The superficial layer of the corneal epithelium, which lines the outer surface of the cornea, is joined by tight junction complexes that highly retard paracellular drug permeation [3]. In addition, the corneal epithelium is known to express several efflux transporters, such as MRP1, MRP5 and breast cancer resistance protein [4], that actively pump drugs out of the cell and may further lower ocular bioavailability. Therefore, the corneal epithelium is often considered a major barrier to topical ocular drug delivery. Since these barriers greatly limit the amount of drug that can reach the back of the eye, agents that target the posterior eye are often administered systemically or via intravitreal injection.
Figure 1. . Ocular structures and possible drug permeation/elimination routes.
(A) Transcorneal permeation, (B) noncorneal drug permeation, (C) drug from the blood to the anterior chamber, (D) drug elimination by aqueous humor turnover, (E) drug elimination to systemic circulation, (F) drug permeation across the blood–retinal barrier, (G) intravitreal drug administration, (H) drug elimination via blood–retinal barrier and (I) drug elimination via anterior route.
Adapted with permission from [9] © Elsevier (2006).
However, when administered systemically, delivery of large molecule drugs, such as biologics, to the posterior eye is still hindered by retinal barriers such as the retinal pigment epithelium, and high dose is typically required to reach therapeutically meaningful concentration, which may lead to more pronounced systemic side effects. The blood–retinal barrier, which consists of outer (retinal pigment epithelium) and inner (retinal endothelial layer) barriers, effectively blocks introduction of foreign agents from the bloodstream to the retina. Furthermore, efflux transporters, such as MRP1, 4 and 5, are also expressed in the posterior segment of the eye [5] and may affect the degree to which drugs reach the target site. Therefore, many large molecule therapeutics for the back of the eye, such as Lucentis® (Genentech) or Eylea (Regeneron) for treatment of wet age-related macular degeneration are injected intravitreally, which is a highly invasive procedure, accompanied with various side effects including endophthalmitis [6].
In addition, it is important to note the presence of flow and shear stress in the eye. Preocular retention time for topically administered agents is often limited due to tear turnover and frequent blinking that results in a very short ocular contact half time (<10 min) [7]. Further reducing preocular retention time is reflex lacrimal secretion and variability in tear production caused by the physical stimuli during topical administration [8]. Further information on ocular drug delivery barriers is well outlined in the review by Urtti [9]. While discussions of nanomaterials often include various nanocarriers, such as nanoparticles and liposomes, this review will focus specifically on the effect of nanotopography on adhesion and transepithelial penetration and the use of nanoporous materials for sustained or controlled drug delivery to the eye. For information on the use of nanocarriers in ocular drug delivery, we refer the readers to reviews written by Sahoo et al. [10] and Nagarwal et al. [11].
Bioadhesive nanostructures
The importance of bioadhesion has been well acknowledged in the field of ocular drug delivery. For instance, various mucoadhesive materials, such as polyacrylic acid [12], chitosan [13], and hyaluronan [14], have been widely explored to increase preocular retention time of drug carriers. These approaches have been well summarized in reviews by Ludwig [15] and Kaur et al. [16].
On the other hand, drug delivery studies that focus on other target sites, such as those for oral drug administration, have also explored an interesting aspect of nanotopography to achieve enhanced bioadhesion. The idea of using nanotopography for adhesion was originally derived from gecko feet [17,18]. Geckos have been noted for their ability to climb smooth surfaces thanks to the hierarchical micro- and nanostructures on the surface of their feet. Mechanisms of bioadhesion between solid interfaces have been generally well studied in the field and include electronic, adsorption and interpenetration/diffusion theories [19,20]. To briefly discuss, electronic theory finds electrostatic attraction at the interface between tissue and biomaterial responsible for bioadhesion. Adsorption theory says secondary forces, such as hydrogen bonding and van der Waals force, cause bioadhesion. On the other hand, interpenetration/diffusion theory claims that interpenetration of polymer chains at the bioadhesion interface is responsible for bioadhesion [19,20]. In the case of bioadhesion in gecko feet, the mechanism behind this phenomenon is thought to rely on van der Waals force and not on the surface properties of gecko feet [18]. In addition, both intestinal epithelium [21] and the corneal epithelium [22,23], which line the surface of the intestine and the cornea respectively, are known to have microscale projections on the surface that can interact with nanostructures, creating better bioadhesion. Using this approach, researchers have used nanostructured microparticles for increased retention time in oral drug delivery applications. For example, in vitro studies have shown that nanoengineered microparticles, fabricated by growth of silicon nanowires on the surface of glass microbeads, are able to achieve significantly enhanced bioadhesion to a layer of intestinal epithelial (Caco-2) cells in the presence of flow compared with bare microparticles [21,24,25].
While this approach is highly promising and amenable to ocular drug delivery, a more biocompatible and biodegradable platform is desired to alleviate concerns with permanent device retention. In addition, it is important to note that drug loading was achieved by localization of drug solution at the base of nanostructures due to capillary force. Because of this, the devices rapidly eluted model drugs such as bovine serum albumin within 2 h [24]. While this relatively short drug release profile may be utilized without problems for oral drug delivery through utilization of enteric coating, a drug loading method that is able to achieve longer (>2 weeks) release profile is desirable for sustained ocular drug delivery.
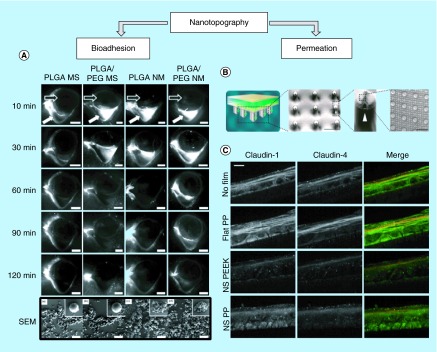
With these concerns in mind, the idea of geometry-based bioadhesion has been applied to enhance ocular drug delivery, as noted in studies by Choy et al. [26] and Park et al. [27]. These studies addressed concerns with material biocompatibility by using biodegradable materials, such as poly(lactic-co-glycolic acid) (PLGA), PEG and polyvinyl alcohol (PVA). In this study, nanostructured microparticles were fabricated by preparing PLGA/PEG nanofibrous sheets via electrospinning and freeze-milling the resulting sheet. The nanostructured microparticles were further embedded into a polyvinyl alcohol tablet to ensure ease of administration and patient compliance. In addition to utilizing nanostructures for bioadhesion, this study incorporated mucoadhesive materials, such as PEG, to achieve further prolonged preocular retention time in rabbit eye up to 90 min after administration (Figure 2A). Figure 2 shows fluorescence images of the rabbit preocular surface at several time points after administration of bare or nanostructured microparticles. The particles were tagged with Nile Red, a fluorescent dye, for visualization. As shown in the fourth row of Figure 2A, PLGA/PEG particles with nanotopography (NM) could still be seen on the rabbit eye 90 min after administration while PLGA/PEG bare microparticles were mostly cleared from the eye. Moreover, while this study did not fully explore the drug elution potential of nanoengineered PLGA/PEG microparticles, their potential as controlled release system has been noted by the versatility of the fabrication method, which allows modification of the encapsulating wall material that may achieve controlled drug release.
Figure 2. . Nanotopography-inspired approaches to enhance bioadhesion and permeation.
(A) Fluorescence images of the preocular surface of the rabbit eye after administration of particles tagged with Nile Red and scanning electron microscope (SEM) images of particles. Compared with bare PLGA or PLGA/PEG microspheres, microparticles with nanotopography achieved greater preocular retention time in vivo. White arrow indicates lower fornix of the rabbit eye and black arrow indicates the location of the eyeball. The bottom row shows SEM images of the particles. Scale bar = 5 mm for the top five rows and 20 µm for bottom row of SEM images [27]. (B) Nanostructured microneedles were fabricated by draping polymeric nanostructured film on a microneedle array. Scale bars = 300 µm and 3 µm in order [28]. (C) Nanostructures made with polypropylene or polyether ether ketone downregulate expression of tight junction proteins, such as Claudin-1 and 4, in cultured human keratinocytes as demonstrated by immunohistochemical staining. Scale bar = 10 µm [28].
PEG: Polyethylene glycol; PLGA: Poly(lactic-co-glycolic acid); PP: Polypropylene; NS: Nanostructured, PEEK: Polyether ether ketone.
Adapted with permission from [27,28] © Elsevier (2014) and © American Chemical Society (2015).
Nanostructure-mediated permeation enhancemen
In addition to the need for enhanced bioadhesion, topical administration of drug to the eye is limited by the tight junctional complexes of the corneal epithelium [29], which line the surface of the cornea. The presence of several efflux transporters at the apical side of the corneal epithelium can also limit penetration of ocular therapeutics [4,5,30]. To address this challenge, various penetration enhancers, such as surfactants, calcium chelators and preservatives [16], have been utilized in ocular drug delivery. These agents are thought to enhance permeation by modulation of transcellular and/or paracellular transport routes. However, several studies have reported potential ocular toxicity of these enhancers, including changes in corneal hydration [31] and electrophysical properties [32], reduction in corneal epithelial cell viability [33] and structural changes of the corneal epithelium [34]. Therefore, a new mode of permeation enhancement that can augment existing permeation enhancers may help provide more effective and safe ocular drug delivery.
In a series of studies on nanoengineered microparticles mentioned above, researchers noted an increase in transepithelial transport of large molecules, such as insulin, across Caco-2 cells [25] in addition to an increase in bioadhesion [24]. Furthermore, more recent studies by Kam et al. have demonstrated a significant increase in large molecule (150 kDa) transport across a Caco-2 monolayer when the cells were placed in contact with polypropylene nanostructured films [35]. According to their study, the increase in transport is thought to be governed by changes in paracellular transport due to the reorganization of tight junctional proteins. Interestingly, the study also noted that the increase in permeation enhancement was reversible upon removal of the nanostructured film.
While these studies are highly encouraging, it is important to note the difference between intestinal and corneal epithelial cells. Unlike intestinal epithelial cells, corneal epithelium consists of multiple cell layers. While tight junctional complexes are known to be primarily expressed in the most apical layer of the corneal epithelium [29], permeability of large molecules in corneal epithelial cells is typically lower than that in intestinal epithelial cells. For example, while the apparent permeability (Papp) of PEG above molecular weight of 1051 (PEG1051) could not be measured in the cornea [36], Papp of PEG4000 in Caco-2 cells have been reported to be approximately 0.78 × 10-6 cm/s [37]. This value is higher than Papp of PEG942 in corneal epithelial models, which is 0.50 × 10-6 cm/s [38]. Furthermore, intestinal and corneal epitheliums are known to express different subset of tight junction proteins [29,38,39], which may be modulated differently upon nanostructure contact. Therefore, application of nanostructured films on corneal epithelial cells will most likely involve additional optimization to properly address these differences in epithelial physiology.
With this information in mind, we would like to note more recent studies by Walsh et al. [28]. In this new set of studies, they expanded the application of nanostructured films to transdermal drug delivery applications by draping polymeric nanostructured film on a microneedle array (Figure 2B), creating a nanostructured microneedle array. The epithelial layer of the skin is more similar to the corneal epithelium as it is also multilayered with its outer surface exposed to open air. By combining microneedle technology with polymeric nanostructures, Walsh et al. were able to achieve remarkable increase in delivery of etanercept, a large molecule therapeutic (150 kDa) used for severe psoriasis, via transdermal administration in vivo. More specifically, AUC of serum etanercept concentration over 72 h was 10.4-times greater with nanostructured microneedles compared with that of bare microneedles. In addition, they showed that polymeric nanostructures significantly downregulate major tight junction proteins, such as claudin-1 and -4 (Figure 2C), all of which are also expressed in the corneal epithelium [29].
Considering numerous studies on using microneedles for topical ocular drug delivery applications [40–42] and the ability of nanostructures to regulate tight junctions that are present in the corneal epithelium, the approach by Walsh et al. may also be applicable to topical ocular applications. However, it is important to note that unlike the skin, the cornea requires maintenance of its optical properties for clear vision and the effect of tight junctional reorganization on optical properties of corneal tissue has not been well characterized. While previous studies on corneal microneedles showed no noticeable safety issues [40], the effect of nanostructured microneedles on the cornea remains to be investigated. Nonetheless, with these concerns in mind, the use of nanotopography for permeation enhancement in conjunction with microneedle technology may be able to provide localized permeation enhancement, allowing reduction in side effects that arise from nonspecific enhancement.
Furthermore, as briefly mentioned earlier, delivery of large molecules to the back of the eye is greatly hindered by the retinal barrier. Applying the aforementioned geometry-based approach, Wade et al. fabricated planar SU-8/poly(ethyleneglycol)dimethacrylate microdevices for drug delivery across the retinal barrier [43]. In this study, Wade et al. noted an increase in transport of fluorescein isothiocyate (FITC)-conjugated dextrans across an in vitro retinal epithelial model with the planar microdevices compared with that of a bolus dose. Similar to previous studies noted above [28,35], this study also suggested that the mechanism of enhanced transport lies on modulation of the paracellular pathway. Wade et al. noted downregulation of tight junctional proteins, such as zonula occludens-1 and occludin, upon contact with planar microdevices and observed no statistically significant difference in expression of MRP-1, an efflux transporter that plays an important role in transcellular transport.
To summarize, we provided an overview of how nanostructures can be applied to provide enhanced bioadhesion to ocular surfaces as well as to increase transepithelial permeability. Next we will outline the use of nanoporous materials for sustained drug delivery to the eye.
Nanoporous materials in sustained-release drug delivery systems
Sustained release in ocular drug delivery
As already discussed, delivery of ocular therapeutics is challenging. Sustained release drug delivery systems aim to improve ocular delivery by reducing the frequency of administration, increasing ocular bioavailability and improving patient compliance. There are many commercial examples of how sustained release systems improve ocular therapeutics and even incremental improvements such as the gel-forming solution Timoptic-XE (Merck), which reduces timolol administration from twice to once daily eye drops, are relevant. In addition to extended release topical solutions, there are a variety of ocular implants lasting on the order of weeks to years, some of which are biodegradable and others that require retrieval [44]. Four intravitreal implants are commercially available, Vitrasert® (Bausch & Lomb, Inc., NY, USA) to treat cytomegalovirus (CMV) retinitis, Retisert® (Bausch & Lomb, Inc., Rochester, NY, USA) for noninfectious posterior uveitis, Iluvien® (Alimera Sciences, Inc., GA, USA) for diabetic macular edema and Ozurdex® (Allergan, Inc., CA, USA) for diabetic macular edema, retinal vein occlusion and posterior uveitis. While none of these implants utilize nanostructured materials in their design for the delivery of small molecules, they pave the way for more advanced technologies utilizing nanostructured materials, particularly in the area of delivering protein therapeutics. The market success of improved delivery systems for ocular therapeutics combined with the prevalence and diversity of such systems in preclinical and clinical development indicates both the need and the interest in improved ocular delivery through sustained release drug delivery systems.
Controlling release with nanopores
As novel fabrication techniques emerge and process control and precision of existing techniques are honed, nanoporous materials show increasing promise for sustained release drug delivery. In recent years, researchers have demonstrated the use of nanoporous membranes from a variety of inorganic and polymeric materials to deliver both small molecule therapeutics and biologics. Nanoporous materials are used in three primary ways for drug delivery: as porous microparticles, as rate-limiting membranes, and as films or coatings that facilitate loading in addition to controlling release. As nanoparticles are outside the scope of this review, we will focus only on nanoporous membranes and coatings.
Nanoporous materials have been used to sustain the release of small molecules as well as biologics from days to months [45,46]. In delivering small molecules, nanoporous membranes can act as a membrane barrier, slowing release of therapeutics from a reservoir to achieve sustained release. While release in these systems is still described by Fickian diffusion, a zero-order release profile can be achieved by loading the therapeutic above the limit of solubility in the volume of the reservoir or nanopore coating. As long as solid drug remains, a saturated solution is contained in the reservoir or pores, creating a constant activity driving force for diffusion. In such systems, the rate of release depends on the physicochemical properties of the drug and is controlled by the pore diameter, length, tortuosity, interconnectivity and density. As coatings, nanoporous materials not only restrict diffusion of loaded drug from the surface, but also provide a mechanism of drug loading. With high surface-to-volume ratios, nanoporous coatings provide an increased capacity for drug adsorption [47]. Additionally, surface pores serve as ‘nano-reservoirs’ and can be loaded with drug through capillary action by either applying a drug solution directly to the surface of the coating or by submerging the nanoporous-coated structure into a concentrated drug solution, allowing drug to sequester into the pores [48,49].
Nanoporous materials to deliver small molecules
There are many examples of nanoporous membranes that can deliver small molecules, demonstrating the utility of this mechanism for drug loading and controlled release. Yan et al. demonstrated that introducing 20–40 nm pores into a polymer thin film increases the loading capacity and release rates of Rhodamine B, a small molecule often used as a model for pharmaceutics [50]. Gultepe et al. [46] provided an excellent review of inorganic nanoporous membranes in drug delivery, including the sustained release of molecules such as FITC [51] and glucose [52] from a porous alumina reservoir system and antibiotics from titania nanotube coating [53]. However, few examples of the use of such systems to deliver small molecule ophthalmic therapeutics are found in the literature.
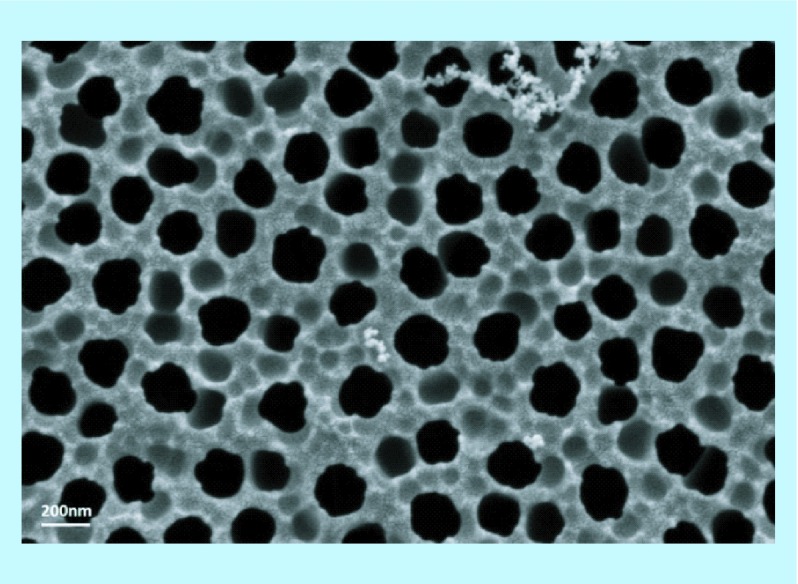
One of the most recent reports of a novel ocular delivery system leveraging nanopore technology describes a biodegradable polyvinylacetate ‘nanowafer’ with arrays of drug-filled nanopores for front of the eye delivery [54]. The nanowafer utilizes the nanopore array to increase loading capacity and sustain the delivery of drug relative to eye drops. The nanowafer is a translucent thin film, similar to a contact lens that can be applied to the front of the eye and is designed to adhere to the wet surface of the eye and is flexible enough to conform to the eye's curvature. The nanowafer, made of a water soluble polymer, begins to dissolve upon application, releasing its payload. Yuan et al. demonstrated sustained release and enhanced corneal permeability of doxycycline delivered with the nanowafer compared with topical eye drops, increasing corneal residence time from mere minutes to up to 24 h in mice. They went on to evaluate the efficacy of several tyrosine kinase receptor inhibitor drugs delivered from a nanowafer in inhibiting corneal neovascularization. This work demonstrates the vast potential for utilizing nanoporous materials to overcome barriers in ocular drug delivery. In a different approach using a nanoporous membrane as a filter to slow diffusion, Orosz et al. demonstrated in vitro the use of commercially available porous alumina membranes (GE Healthcare, NJ, USA) (Figure 3) to deliver ophthalmic therapeutics including ascorbic acid [55]. The authors suggest the use of these inorganic membranes in the form of implantable capsules for the treatment of diseases such as age-related macular degeneration or proliferative diabetic retinopathy.
Figure 3. . SEM image of nanoporous anodized aluminum oxide membrane with pore size of 200 nm (GE Healthcare, NJ, USA).
SEM image provided by C Fox.
The study demonstrated in vitro that ascorbic acid delivered through the nanoporous alumina membrane can inhibit sprouting in human retinal endothelial cells (HREC), indicative of antiangiogenesis activity, over a 7-day period. However, much additional work is needed to translate this proof-of-concept work into an applicable ocular drug delivery system.
While both these studies suggest that nanoporous materials could be effectively implemented to deliver relevant small molecule ophthalmic therapeutics, a more thorough analysis of the diffusion kinetics describing drug release from the system is desired. In addition, an investigation of parameters controlling release that may provide a mechanism to tune release rates is needed.
Nanoporous materials to deliver biologics
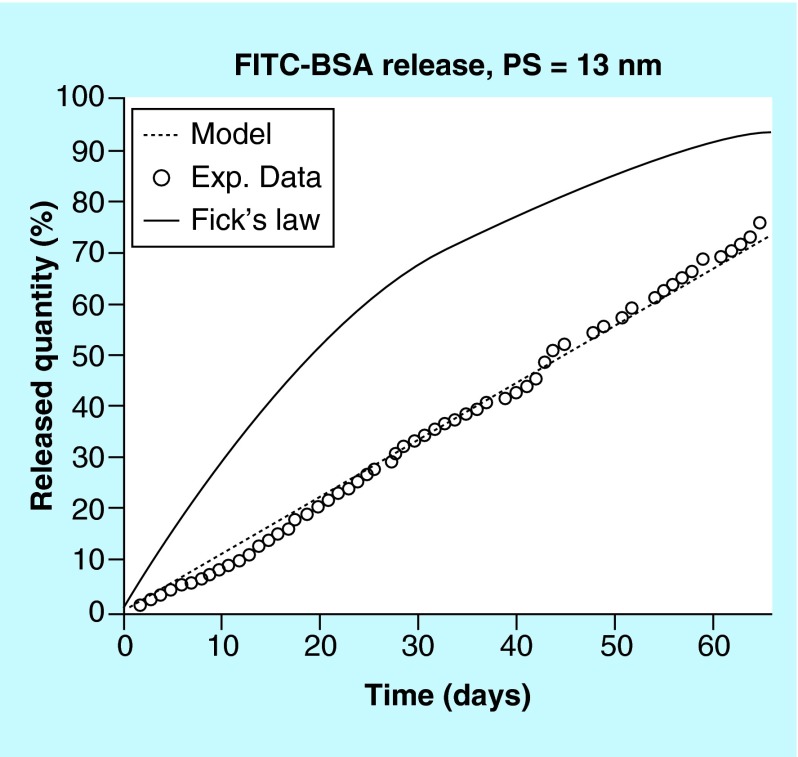
In delivering biologics, nanoporous materials are of particular interest in controlling release. Based on the theory of ‘single-file diffusion’, nanoporous membranes can be tuned to achieve zero-order release of protein therapeutics [56]. Single-file diffusion occurs when solute diameter is comparable in size to the pore through which it diffuses. Constrained by the pore diameter, solute molecules are unable to pass by one another, restricting diffusion through the pore to a single molecule at a time regardless of the concentration gradient driving force [57–59]. Martin et al. clearly illustrated this effect by comparing FITC-labeled bovine serum albumin (FITC-BSA) diffusion through 13 nm pores to the predicted release profile based on Fickian diffusion [60]. In Fickian diffusion, release is directly proportional to the concentration gradient, resulting in a first-order release profile from a drug delivery system under sink conditions. Whereas Fickian diffusion, based on a the concentration-dependent driving force, predicts a release rate that slows as the device depletes, diffusion through 13 nm pores continues at a constant, concentration-independent, release rate up to at least 70% release (Figure 4). While the pore diameter constrains diffusion and determines the release profile shape, pore length and structure, density, effective surface area and membrane material impact release rate.
Figure 4. . In vitro diffusion kinetics of fluorescein isothiocyanate-labeled bovine serum albumin through 13 nm pore size membrane under sink conditions: experimental data (o), Fick's law prediction (solid line) and model based simulation (dashed line).
FITC-BSA: Fluorescein isothiocyanate-labeled bovine serum albumin.
Reproduced with permission from [60] © American Chemical Society (2004).
Over the past 20 years, numerous examples of drug delivery systems achieving zero-order release of biologics have surfaced. As fabrication technologies advance, so does our ability to precisely control pore sizes on the nanometer scale in a range of materials. While much of the early work in using nanoporous membranes for drug delivery utilized silicon or anodized aluminum oxide based membranes, polymeric systems are becoming increasingly popular due to their biocompatibility and/or biodegradability. For example, Yang et al. reported the fabrication of PMMA-based membranes featuring cylindrical nanopores with diameters tunable from 6 to 15 nm based on Au surface deposition. Similar to the work done by Martin et al. with silicon-based nanoporous membranes, Yang et al. demonstrated zero-order release of two biologics, BSA and human growth hormone, for up to 60 days and compared the long-acting in vivo performance and pharmacokinetic benefit of such implants to subcutaneous injection [61].
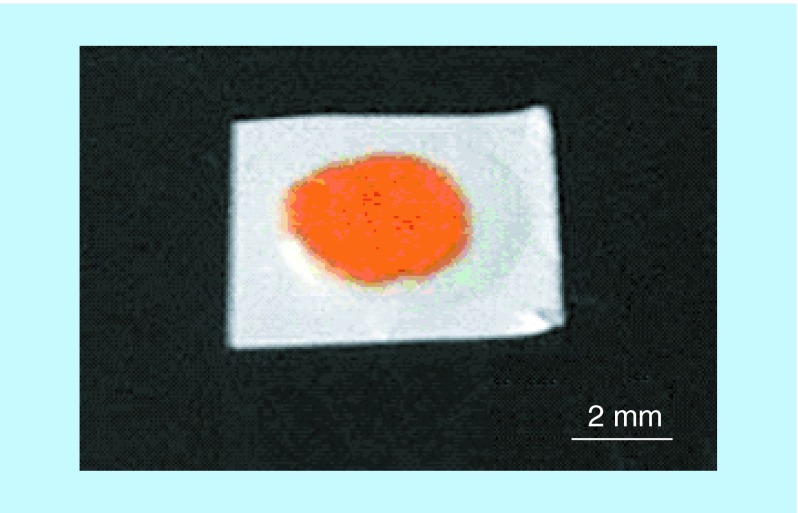
With an increasing number of biologics emerging as ocular therapeutics and an ever-growing number of patients suffering from vision loss due to an aging population and diabetes co-morbidities, the need for long-acting ocular delivery systems for biologics is high [2]. As we saw with nanoporous drug delivery systems for small molecule therapeutics, there are few examples of nanopore controlled ophthalmic delivery of biologics despite the use of such systems in other indications. However, this technology is well suited for long-acting ophthalmic implants that can potentially take the place of more frequent intraocular injections. In 2012, Bernards et al. introduced a biodegradable reservoir device with a nanoporous polycaprolactone membrane for long-acting and constant-rate delivery of proteins [45]. The devices presented in this work were made from a flexible membrane and were small in size (Figure 5), suitable for ocular delivery. While in vivo ocular release of relevant protein therapeutics from these systems has not yet been reported, Bernards et al. did demonstrate in rabbits the structural integrity and ocular biocompatibility of the polycaprolactone devices out to 6 months [62]. In another approach leveraging nanopores for protein delivery, the company pSivida has demonstrated in preclinical in vitro studies the use of nanoporous silicon for sustained release of bevacizumab for up to 20 days. In this system, protein is loaded via adsorption onto the walls of the silicon nanopores. Furthermore, they report that release can be tuned by altering nanopore diameter and surface area [63].
Figure 5. . Example of prototypical nanoporous polycaprolactone thin film reservoir device loaded with fluorescein isothiocyanate-labeled bovine serum albumin.
Reproduced with permission from [45] © American Chemical Society (2012).
There is a great need for long-acting ophthalmic drug delivery systems for both small molecule and protein therapeutics. With less frequent administration, patient compliance increases and the risk of infection or complications during ocular injections decreases. Whether for front or back of the eye delivery, nanoporous materials could be integrated into drug delivery systems to achieve sustained release. Nanopores could serve to increase loading capacity for ocular delivery systems, an important feature when the delivery system size is restricted by the small size of the eye. Nanopores in polymeric devices could also be implemented for the controlled release of protein therapeutics from long-acting ocular implants. These are just some examples of how nanopore technology is currently being applied to ocular therapeutics, but as nanopore technology advances in preclinical development across indications, there are likely to be additional opportunities and applications for these approaches in ocular drug delivery.
There are not currently any commercially available ophthalmic products utilizing nanopore technology, and the bulk of these promising technologies are still being developed in vitro. Early development of these systems, prior to even the first in vivo evaluations, requires in depth development and understanding of system design and function. While nanotechnology provides certain powerful benefits, it also poses challenges in characterization and reproducibility or quality control. However, as the field of nanotechnology advances in fabrication and characterization techniques, these hurdles are being overcome. This combined with an increased knowledge base of novel routes of administration and delivery systems in ophthalmology paves the way for the translation and application of these nanostructured materials for improved ocular drug delivery systems.
While there are not currently any commercially available ophthalmic products utilizing nanopore technology, preclinical evaluations of these technologies demonstrate their potential. Many of the nanoporous materials in development are discussed in this section on nanoporous materials to deliver small molecules.
Conclusion & future perspective
To date, advances in micro and nanofabrication have yielded many highly promising platforms that can be applied to ocular drug delivery to achieve enhanced bioadhesion and tissue penetration as well as controlled drug elution profiles in long term. While nanotopography and nanoporous materials have been discussed as separate platforms in this review, there is great benefit to combining the two, creating a single drug delivery device that can achieve all advantages associated with nanostructures. For instance, an implantable device that can achieve zero-order release by single-file diffusion of large molecules could be partially modified with a nanowired surface to allow greater tissue penetration of the therapeutics of interest.
As promising as these nanotechnology systems are in early preclinical development, additional work is needed to fully understand their mechanisms and performance. A translation gap remains in the in vitro or even preclinical evaluation of these nanostructured systems and ophthalmic products. In many cases, fundamentals such as a more thorough investigation of mechanisms controlling drug release and system tunability are needed. In the novel area of utilizing nanostructures to enhance epithelial permeability, the effect of nanostructure-mediated tight junction reorganization on physical and chemical properties of ocular tissues, such as the cornea, needs to be explored further to ensure safe application of nanotechnology to ophthalmology. And in all cases, the application of these novel nanostructured materials relies on demonstration of ocular biocompatibility of the bulk material as well as of the shape, form and nanostructured components of the system. In nanostructured systems, particular attention should be paid to the fouling or damage of the nanostructured features that could lead to a loss of function. Furthermore, for degradable systems the ocular compatibility must be considered of the degradation products with careful consideration being paid to the generation of particulates that could potentially lead to an inflammatory response. Because the volume of the eye is small and patient compliance relies on comfort and ease of use, the form and the method of application of the delivery system must also be considered in translation. For example, a flexible, soft and low-density polymeric intravitreal implant may have more translation success than an inorganic, rigid and more dense implant that could potentially sink in the vitreous and cause unwanted side effects such as retinal detachment. And finally, any delivery system must also demonstrate physical and chemical compatibility with the therapeutic agent being delivered. While there are many requirements for the successful translation of an ocular drug delivery system, particularly when considering the implementation of novel features such as nanostructures, the need for these solutions will continue to drive the preclinical development and eventual translation of these systems into valuable products.
Key terms.
Tight junction: Tight junction refers to the connection between cells that act as a barrier to paracellular transport. Tight junction proteins, such as zonula occludens and claudins, are thought to influence the degree to which paracellular transport takes place.
Bioadhesion: Bioadhesion refers to adhesive properties between a synthetic material and a biological surface. Sufficient bioadhesion of drug delivery devices to the target tissue is critical to ensure proper delivery of drug payload. This is especially true when the target tissue is subject to flow conditions or physical perturbations that may remove the device from the surface.
Transepithelial permeability: Transepithelial permeability refers to drug penetration through the epithelial barrier, which is generally considered a major barrier to drug delivery. In case of ocular drug delivery, transepithelial permeation is relevant for both front (corneal epithelium) and back-of-the-eye (retinal pigment epithelium) applications. Transepithelial permeation can be passive (e.g., paracellular transport through the tight junctional complex) or active (e.g., transporter-mediated transport).
Fickian diffusion: Described by Fick's laws of diffusion, Fickian diffusion is a concentration-dependent diffusion in which flux is directly proportional to the concentration gradient. Fickian diffusion in drug delivery systems results in a first order release profile, with release rate decreasing as the drug source depletes.
Single file diffusion: Single file diffusion occurs when solute diameter is comparable in size to the pore through which it diffuses. Constrained by the pore diameter, solute molecules are unable to pass by one another, restricting diffusion through the pore to a single molecule at a time regardless of the concentration gradient driving force.
Executive summary.
Recent advances in nanofabrication provided various platforms that can be applied to enhance ocular drug delivery for both front and back of the eye applications.
Nanostructures can enhance ocular bioadhesion, increasing preocular retention time.
Through reorganization of tight junctions, nanostructures can increase permeation of large molecule drugs across epithelial barriers, as demonstrated with a retinal epithelial model.
Nanoporous materials can be used to provide sustained or zero-order release of small molecules and biologics.
Combination of nanotopographical cues and nanoporous membranes may be able to achieve several advantages in a single device.
Ocular biocompatibility and degradability of materials used for nanofabrication should be further investigated.
Footnotes
Financial & competing interests disclosure
This work was supported by the NIH R01-EY021574 and R01-EB018842. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.O'Roruke M. Next generation ocular drug delivery platforms. OIS. 2014;1:7. [Google Scholar]
- 2.Rupenthal I. Sector overview: ocular drug delivery technologies: exciting times ahead. 2015. www.ondrugdelivery.com
- 3.Jarvinen K, Jarvinen T, Urtti A. Ocular absorption following topical delivery. Adv. Drug Deliv. Rev. 1995;16:3–19. [Google Scholar]
- 4.Vellonen KS, Mannermaa E, Turner H. Effluxing ABC transporter in human corneal epithelium. J. Pharm. Sci. 2010;99(2):1087–1098. doi: 10.1002/jps.21878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannermaa E, Vellonen KS, Ryhanen T, et al. Efflux protein expression in human retinal pigment epithelium. Pharm. Res. 2009;26(7):1785–1791. doi: 10.1007/s11095-009-9890-6. [DOI] [PubMed] [Google Scholar]
- 6.Shah CP, Garg SJ, Vander JF, et al. Outcomes and risk factors associated with endophthalmitis after intravitreal injection of anti-vascular endothelial growth factor agents. Ophthalmology. 2011;118:2028–2034. doi: 10.1016/j.ophtha.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 7.Hardberger RE, Hanna C, Goodart R. Effects of drug vehicles on ocular uptake of tetracycline. Am. J. Ophthalmol. 1975;80:133–138. doi: 10.1016/0002-9394(75)90883-1. [DOI] [PubMed] [Google Scholar]
- 8.Fraunfelder FT. Extraocular fluid dynamics: how best to apply topical ocular medication. Trans. Am. Ophthalmol. Soc. 1976;74:457–487. [PMC free article] [PubMed] [Google Scholar]
- 9.Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006;58:1131–1135. doi: 10.1016/j.addr.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 10.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov. Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 11.Nagarwal RC, Kant S, Singh PN, Maiti P, Pandit JK. Polymeric nanoparticulate system: a potential approach for ocular drug delivery. J. Control. Release. 2009;136:2–13. doi: 10.1016/j.jconrel.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Thermes F, Rozier A, Plazonnet B, et al. Bioadhesion: the effect of polyacrylic acid on the ocular bioavailability of timolol. Int. J. Pharm. 1992;81(1):59–65. [Google Scholar]
- 13.de la Fuente M, Ravina M, Paolicelli P, et al. Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv. Drug Deliv. Rev. 2010;62:100–117. doi: 10.1016/j.addr.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 14.Barbault-Foucher S, Gref R, Russo P, Guechot J, Bochot A. Design of poly-epsilon-caprolactone nanospheres coated with bioadhesive hyaluronic acid for ocular delivery. J. Control. Release. 2002;83:365–375. doi: 10.1016/s0168-3659(02)00207-9. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv. Drug Deliv. Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev. Ind. Pharm. 2002;28:353–369. doi: 10.1081/ddc-120002997. [DOI] [PubMed] [Google Scholar]
- 17.Autumn K, Liang Y, Hsieh S, et al. Adhesive force of a single gecko foot-hair. Nature. 2000;405:681–685. doi: 10.1038/35015073. [DOI] [PubMed] [Google Scholar]
- 18.Autumn K, Sitti M, Liang Y, et al. Evidence for van der Waals adhesion in gecko setae. Proc. Natl Acad. Sci. USA. 2002;99:12252–12256. doi: 10.1073/pnas.192252799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peppas NA. Surface, interfacial and molecular aspects of polymer bioadhesion on soft tissues. J. Control. Release. 1985;2:257–275. [Google Scholar]
- 20.Huang Y, Leobandung W, Foss A, Peppas NA. Molecular aspects of muco- and bioadhesion: tethered structures and site-specific surfaces. J. Control. Release. 2000;65(1–2):63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 21.Fischer KE, Aleman BJ, Tao SL, et al. Biomimetic nanowire coatings for next generation adhesive drug delivery systems. Nano Lett. 2009;9:716–720. doi: 10.1021/nl803219f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols B, Dawson CR, Togni B. Surface features of the conjunctiva and cornea. Invest. Ophthalmol. Vis. Sci. 1983;24:570–576. [PubMed] [Google Scholar]
- 23.Koizumi N, Copper LJ, Fullwood NJ, et al. An evaluation of cultivated corneal limbal epithelial cells, using cell-suspension culture. Invest. Ophthalmol. Vis. Sci. 2002;43:2114–2121. [PubMed] [Google Scholar]
- 24.Fischer KE, Jayagopal A, Nagaraj G, et al. Nanoengineered surfaces enhance drug loading and adhesion. Nano Lett. 2011;11:1076–1081. doi: 10.1021/nl103951e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uskokovic V, Lee K, Lee PP, Fischer KE, Desai TA. Shape effect in the design of nanowire-coated microparticles as transepithelial drug delivery devices. ACS Nano. 2012;6:7832–7841. doi: 10.1021/nn3019865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choy YB, Park JH, McCarey BE, Edelhauser HF, Prausnitz MR. Mucoadhesive microdiscs engineered for ophthalmic drug delivery: effect of particle geometry and formulation on preocular residence time. Invest. Ophthalmol. Vis. Sci. 2008;49:4808–4815. doi: 10.1167/iovs.08-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CG, Kim M, Park M, et al. Nanostructured mucoadhesive microparticles for enhanced preocular retention. Acta Biomater. 2014;10:77–86. doi: 10.1016/j.actbio.2013.08.026. [DOI] [PubMed] [Google Scholar]; •• Prime example of how nanostructures can be used to increase preocular retention time in ocular drug delivery.
- 28.Walsh L, Ryu J, Bock S, et al. Nanotopography facilitates in vivo transdermal delivery of high molecular weight therapeutics through an integrin-dependent mechanism. Nano Lett. 2015;15:2434–2441. doi: 10.1021/nl504829f. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Demonstrates the use of nanostructured microneedles for drug delivery across multilayered epithelium of the skin and tight junction regulation, which also show potential in ocular drug delivery applications.
- 29.Ban Y, Dota A, Cooper L, et al. Tight junction-related protein expression and distribution in human corneal epithelium. Exp. Eye Res. 2003;76:663–669. doi: 10.1016/s0014-4835(03)00054-x. [DOI] [PubMed] [Google Scholar]
- 30.Mannermaa E, Vellonen KS, Urtti A. Drug transport in corneal epithelium and blood–retina barrier: emerging role of transporters in ocular pharmacokinetics. Adv. Drug Deliv. Rev. 2006;58:1136–1163. doi: 10.1016/j.addr.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 31.Monti D, Chetoni P, Burgalassi S, Najarro M, Saettone MF. Increased corneal hydration induced by potential ocular penetration enhancers: assessment by differential scanning calorimetry (DSC) and by desiccation. Int. J. Pharm. 2002;232:139–147. doi: 10.1016/s0378-5173(01)00907-3. [DOI] [PubMed] [Google Scholar]
- 32.Chetoni P, Burgalassi S, Monti D, Saettone MF. Ocular toxicity of some corneal penetration enhancers evaluated by electrophysiology measurements on isolated rabbit corneas. Toxicol. In Vitro. 2003;17:497–504. doi: 10.1016/s0887-2333(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 33.Burgalassi S, Chetoni P, Monti D, Saettone MF. Cytotoxicity of potential ocular permeation enhancers evaluated on rabbit and human corneal epithelial cell lines. Toxicol Lett. 2001;122:1–8. doi: 10.1016/s0378-4274(01)00261-2. [DOI] [PubMed] [Google Scholar]
- 34.Furrer P, Mayer JM, Plazonnet B, Gurny R. Ocular tolerance of absorption enhancers in ophthalmic preparations. AAPS PharmSci. 2002;4(1):1–5. doi: 10.1208/ps040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kam K, Walsh L, Bock S, et al. Nanostructure-mediated transport of biologics across epithelial tissue: enhancing permeability via nanotopography. Nano Lett. 2013;13:164–171. doi: 10.1021/nl3037799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liaw J, Robinson J. The effect of polyethylene glycol molecular weight on corneal transport and the related influence of penetration enhancers. Int. J. Pharm. 1992;88(1–3):125–140. [Google Scholar]
- 37.Yee S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man – fact or myth. Pharm. Res. 1997;14:763–766. doi: 10.1023/a:1012102522787. [DOI] [PubMed] [Google Scholar]
- 38.Toropainen E, Ranta V, Vellonen K, et al. Paracellular and passive transcellular permeability in immortalized human corneal epithelial cell culture model. Eur. J. Pharm. Sci. 2003;20:99–106. doi: 10.1016/s0928-0987(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 39.Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J. Comp. Physiol. B. 2010;180:591–598. doi: 10.1007/s00360-009-0440-7. [DOI] [PubMed] [Google Scholar]
- 40.Jiang J, Gill H, Ghate D, et al. Coated microneedles for drug delivery to the eye. Invest. Ophthalmol. Vis. Sci. 2007;48:4038–4043. doi: 10.1167/iovs.07-0066. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J, Moore JS, Edelhauser HF, Prausnitz MR. Intrascleral drug delivery to the eye using hollow microneedles. Pharm. Res. 2009;26:395–403. doi: 10.1007/s11095-008-9756-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palakurthi NK, Correa ZM, Augsburger JJ, Banerjee RK. Toxicity of a biodegradable microneedle implant loaded with methotrexate as a sustained release device in normal rabbit eye: a pilot study. J. Ocul. Pharmacol. Ther. 2011;27:151–156. doi: 10.1089/jop.2010.0037. [DOI] [PubMed] [Google Scholar]
- 43.Wade JS, Desai TA. Planar microdevices enhance transport of large molecular weight molecules across retinal pigment epithelial cells. Biomed. Microdevices. 2014;16:629–638. doi: 10.1007/s10544-014-9865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasukawa T, Ogura Y, Kimura H, Sakurai E, Tabata Y. Drug delivery from ocular implants. Expert Opin. Drug Deliv. 2006;3:261–273. doi: 10.1517/17425247.3.2.261. [DOI] [PubMed] [Google Scholar]
- 45.Bernards DA, Lance KD, Ciaccio NA, Desai TA. Nanostructured thin film polymer devices for constant-rate protein delivery. Nano Lett. 2012;12:5355–5361. doi: 10.1021/nl302747y. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Utilizes polycaprolactone, a biocompatible polymer, for controlled delivery of model large molecule drugs, which can be used in the eye in the form of implantable ocular drug delivery device.
- 46.Gultepe E, Nagesha D, Sridhar S, Amiji M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Deliv. Rev. 2010;62:305–315. doi: 10.1016/j.addr.2009.11.003. [DOI] [PubMed] [Google Scholar]; • Provides an excellent review of inorganic nanoporous membranes in drug delivery.
- 47.Ozin GA. Nanochemistry: synthesis in diminishing dimensions. Adv. Mater. 1992;4(10):612–649. [Google Scholar]
- 48.Ayon AA, Cantu M, Chava K, et al. Drug loading of nanoporous TiO2 films. Biomed. Mater. 2006;1:L11–15. doi: 10.1088/1748-6041/1/4/L01. [DOI] [PubMed] [Google Scholar]
- 49.Foraker AB, Walczak RJ, Cohen MH, Boiarski TA, Grove CF, Swaan PW. Microfabricated porous silicon particles enhance paracellular delivery of insulin across intestinal Caco-2 cell monolayers. Pharm. Res. 2003;20:110–116. doi: 10.1023/a:1022211127890. [DOI] [PubMed] [Google Scholar]
- 50.Yan W, Hsiao VKS, Zheng YB, Shariff YM, Gao T, Huang TJ. Towards nanoporous polymer thin film-based drug delivery systems. Thin Solid Films. 2009;517(5):1794–1798. [Google Scholar]
- 51.Gong D, Yadavill V, Paulose M, Pishko M, Grimes CA. Controlled molecular release using nanoporous alumina capsules. Biomed. Microdevices. 2003;5(1):75–80. [Google Scholar]
- 52.La Flamme KE, Mor G, Gong D, et al. Nanoporous alumina capsules for cellular macroencapsulation: transport and biocompatibility. Diabetes Technol. Ther. 2005;7:684–694. doi: 10.1089/dia.2005.7.684. [DOI] [PubMed] [Google Scholar]
- 53.Popat KC, Eltgroth M, LaTempa TJ, Grimes CA, Desai TA. Titania nanotubes: a novel platform for drug-eluting coatings for medical implants? Small. 2007;3:1878–1881. doi: 10.1002/smll.200700412. [DOI] [PubMed] [Google Scholar]
- 54.Yuan X, Marcano DC, Shin CS, et al. Ocular drug delivery nanowafer with enhanced therapeutic efficacy. ACS Nano. 2015;9:1749–1758. doi: 10.1021/nn506599f. [DOI] [PubMed] [Google Scholar]; •• Introduces a novel biodegradable polymeric nanowafer with arrays of drug-filled nanopores for front of the eye delivery. Compared with topical eye drops, the sustained release nanowafer increases corneal residence time from mere minutes to up to 24 h.
- 55.Orosz KE, Gupta S, Hassink M, et al. Delivery of antiangiogenic and antioxidant drugs of ophthalmic interest through a nanoporous inorganic filter. Mol. Vis. 2004;10:555–565. [PubMed] [Google Scholar]
- 56.Desai TA, Hansford DJ, Kulinsky L, et al. Nanopore technology for biomedical applications. Biomed Microdevices. 1999;2(1):11–40. [Google Scholar]; • Introduces the concept of single-file diffusion through nanoporous membranes, a key principle being applied in the controlled release delivery of protein therapeutics.
- 57.Hahn K, Karger J, Kukla VV. Single-file diffusion observation. Phys. Rev. Lett. 1996;76:2762–2765. doi: 10.1103/PhysRevLett.76.2762. [DOI] [PubMed] [Google Scholar]
- 58.Clark LA, Ye GT, Snurr RQ. Molecular traffic control in a nanoscale system. Phys. Rev. Lett. 2000;84:2893–2896. doi: 10.1103/PhysRevLett.84.2893. [DOI] [PubMed] [Google Scholar]
- 59.Levitt DG. Dynamics of a single-file pore: non-fickian behavior. Phys. Rev. A. 1973;8:3050. [Google Scholar]
- 60.Martin F, Walczak R, Boiarski A, et al. Tailoring width of microfabricated nanochannels to solute size can be used to control diffusion kinetics. J. Control. Release. 2005;102:123–133. doi: 10.1016/j.jconrel.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 61.Yang SY, Yang JA, Kim ES, et al. Single-file diffusion of protein drugs through cylindrical nanochannels. ACS Nano. 2010;4:3817–3822. doi: 10.1021/nn100464u. [DOI] [PubMed] [Google Scholar]
- 62.Bernards DA, Bhisitkul RB, Wynn P, et al. Ocular biocompatibility and structural integrity of micro- and nanostructured poly(caprolactone) films. J. Ocul. Pharmacol. Ther. 2013;29:249–257. doi: 10.1089/jop.2012.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nadarassan DK. ARVO Annual Meeting. Orlando, FL, USA: 4–8 May 2014. Sustained release of bevacizumab (Avastin) from BioSilicon. Presented at. [Google Scholar]