ABSTRACT
Sustained endoplasmic reticulum (ER) stress disrupts normal cellular homeostasis and leads to the development of many types of human diseases, including metabolic disorders. TAK1 (also known as MAP3K7) is a member of the mitogen-activated protein kinase kinase kinase (MAP3K) family and is activated by a diverse set of inflammatory stimuli. Here, we demonstrate that TAK1 regulates ER stress and metabolic signaling through modulation of lipid biogenesis. We found that deletion of Tak1 increased ER volume and facilitated ER-stress tolerance in cultured cells, which was mediated by upregulation of sterol-regulatory-element-binding protein (SREBP)-dependent lipogenesis. In the in vivo setting, central nervous system (CNS)-specific Tak1 deletion upregulated SREBP-target lipogenic genes and blocked ER stress in the hypothalamus. Furthermore, CNS-specific Tak1 deletion prevented ER-stress-induced hypothalamic leptin resistance and hyperphagic obesity under a high-fat diet (HFD). Thus, TAK1 is a crucial regulator of ER stress in vivo, which could be a target for alleviation of ER stress and its associated disease conditions.
KEY WORDS: TAK1, Endoplasmic reticulum stress, Leptin, Hypothalamus, Obesity
Highlighted Article: Deficiency of the protein kinase Tak1 protects cells against endoplasmic reticulum stress through activation of de novo lipogenesis, which prevents leptin resistance in the hypothalamus and hyperphagic obesity.
INTRODUCTION
The endoplasmic reticulum (ER) is a large organelle that forms an interconnected network in the cytoplasm. Newly synthesized secreted and membrane proteins are translated by ER-bound ribosomes, and translocate into the ER lumen where they are subjected to covalent post-translational modifications, chaperone-mediated folding and transport toward their final destinations (Sitia and Braakman, 2003; Voeltz et al., 2002). Disruption of these processes results in accumulation of unfolded and misfolded proteins in the ER lumen, leading to a condition known as ER stress. ER stress activates intracellular signaling pathways, collectively referred to as the unfolded protein response (UPR), which is initiated by three sensor (mediator) proteins that are localized to the ER membrane; protein kinase RNA-like endoplasmic reticulum kinase (PERK; also known as EIF2AK3), inositol-requiring enzyme 1α (IRE1α; also known as ERN1) and ATF6. The UPR activates expression of protein chaperone genes, pauses canonical protein synthesis and increases biogenesis of lipid membrane, which upregulate ER functional capacity and accelerate the processing of accumulated proteins in the ER (Walter and Ron, 2011). However, if ER stress is unresolved and sustained, the UPR also activates caspase-dependent apoptosis (Lu et al., 2014; Tabas and Ron, 2011).
Emerging evidence suggests that ER stress contributes to the development of a diverse set of human diseases, including neurodegenerative and inflammatory diseases (Hetz and Mollereau, 2014; Hotamisligil, 2010). Specifically, ER stress is known to be causally associated with the development of metabolic disorders, such as obesity and type-II diabetes, which can be initiated by chronic ER stress in multiple tissues, including the liver, adipose tissues and the hypothalamus (Ozcan et al., 2006; Schneeberger et al., 2013; Zhang et al., 2008). High-fat diet (HFD)-induced obesity is a well-characterized ER-stress-associated disorder in mouse models. Under normal conditions, feeding stimulates secretion of the satiety hormone leptin from adipose tissues. Circulating leptin binds to its receptors, activating neurons of the arcuate nucleus within the hypothalamus [specifically pro-opiomelanocortin (POMC) and agouti-related peptide (AgRP) neurons], leading to appetite suppression (Myers et al., 2008; Woods et al., 1998). Chronic HFD is known to induce ER stress and impair leptin signaling in the hypothalamus, which results in the conditions known as leptin resistance and hyperphagic obesity (Ozcan et al., 2009). The causal relationship between ER stress and hypothalamic leptin resistance has been demonstrated by a series of mouse studies (Engin and Hotamisligil, 2010; Ozcan et al., 2009; Won et al., 2009).
Transforming growth factor β-activated kinase 1 (TAK1; also known as MAP3K7) is a member of the mitogen activated kinase kinase kinase (MAP3K) family that is a key signaling intermediate of proinflammatory signaling pathways (Mihaly et al., 2014; Sakurai, 2012). TAK1 is activated by a diverse set of inflammatory stimuli, such as TNF and IL-1. Transcription factor NF-κB and the MAPK cascades are well-documented downstream targets of TAK1, which activate a number of genes promoting cell survival and inflammatory responses. Tak1 deficiency is associated with hypersensitivity to TNF, Toll-like receptor (TLR)-ligand- and reactive oxygen species (ROS)-induced cell death in fibroblasts, keratinocytes, macrophages, hepatocytes, intestinal epithelial cells and endothelial cells (Mihaly et al., 2014; Sato et al., 2005). Tissue-specific deletion of Tak1 results in profound cell death and tissue injury in the epidermis, intestinal epithelium, liver and endothelium (Bettermann et al., 2010; Inokuchi et al., 2010; Morioka et al., 2012; Omori et al., 2006). Thus, TAK1 has been recognized as a pro-survival factor. However, we found that Tak1-deficient cells are not always hypersensitive to cell-killing stimuli, but they are rather resistant to other certain types of stimuli, such as ER stressors. In the present study, we have investigated this previously unrecognized role of TAK1 in ER-stress regulation.
RESULTS
Tak1-deficient cells are resistant to ER stress-induced cell death
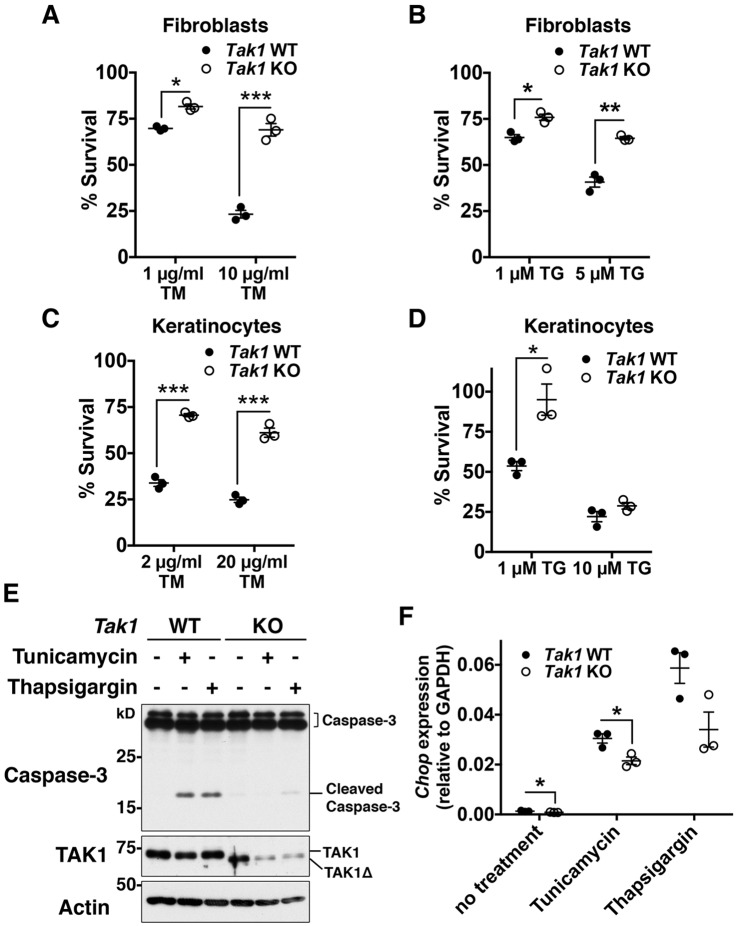
Tak1 deficiency induces cell death in multiple tissues, and TNF has been identified as a primary inducer of cell death in the epidermis, liver and endothelium (Inokuchi et al., 2010; Morioka et al., 2012; Omori et al., 2006). However, ablation of TNF signaling does not always block cell death in some tissues, including the adult intestinal epithelium (Kajino-Sakamoto et al., 2010). To determine additional inducers of Tak1-deficiency-dependent cell death, we initially tested several cell-death-associated stimuli, including ER stress inducers. We treated Tak1-deficient mouse fibroblasts with ER-stress inducers, tunicamycin and thapsigargin, and monitored the viability of control and Tak1-deficient cells at 18 h post ER-stress induction. Unexpectedly, we found that Tak1 deficiency did not decrease, but rather increased, cell survival during ER stress compared to that in wild-type fibroblasts (Fig. 1A,B). ER-stress resistance was also observed in Tak1-deficient keratinocytes (Fig. 1C,D). Sustained ER stress is known to induce apoptosis through activation of caspases (Lu et al., 2014). We found that proteolytic cleavage of caspase-3 upon ER stress was markedly attenuated in Tak1-deficient fibroblasts compared to that in control fibroblasts (Fig. 1E). Resistance to ER-stress-induced cell death can be due to alleviation of ER stress or alternative defects in apoptotic cell death pathways. However, as TNF stimulation induces caspase-dependent apoptosis in Tak1-deficient fibroblasts and keratinocytes (Morioka et al., 2014; Omori et al., 2008), Tak1-deficiency is likely to alleviate ER stress. Indeed, induction of Chop (also known as Ddit3) expression by ER stressors, an indicator of prolonged or unresolved ER stress (Oyadomari and Mori, 2004), was reduced in Tak1-deficient cells (Fig. 1F), supporting the idea that Tak1 deficiency alleviates ER stress.
Fig. 1.
Tak1-deficiency protects cells from ER-stress-induced cell death. (A–D) Tak1 wild-type (Tak1 WT) and Tak1-deficient (Tak1 KO) fibroblasts (A,B) or keratinocytes (C,D) were seeded on 24-well plates and treated with vehicle (DMSO) alone or the indicated concentrations of tunicamycin (TM) or thapsigargin (TG) for 18 h. Cell viability was measured by Crystal Violet staining. n=3 per treatment; mean±s.e.m.; *P<0.05; **P<0.01; ***P<0.001. (E) Tak1 WT and Tak1 KO fibroblasts were treated with vehicle alone, 10 μg/ml tunicamycin or 5 μM thapsigargin for 24 h. Caspase-3 and TAK1 were analyzed by immunoblotting. Cleaved caspase-3 and a truncated form of TAK1 (TAK1Δ) are indicated. In our Tak1-floxed system, TAK1Δ that lacks a small deletion of the ATP-binding site is generated, but TAK1Δ protein is unstable and nonfunctional (Sato et al., 2006). β-actin is shown as a loading control. (F) Tak1 WT and Tak1 KO fibroblasts were treated with 0.5 μg/ml of tunicamycin or 0.1 μM thapsigargin for 18 h. The mRNA level of Chop was analyzed by performing quantitative real-time PCR. The mRNA level of Chop was quantified relative to Gapdh gene expression. n=3 per genotype; mean±s.e.m.; *P<0.05.
Tak1 deficiency increases ER volume
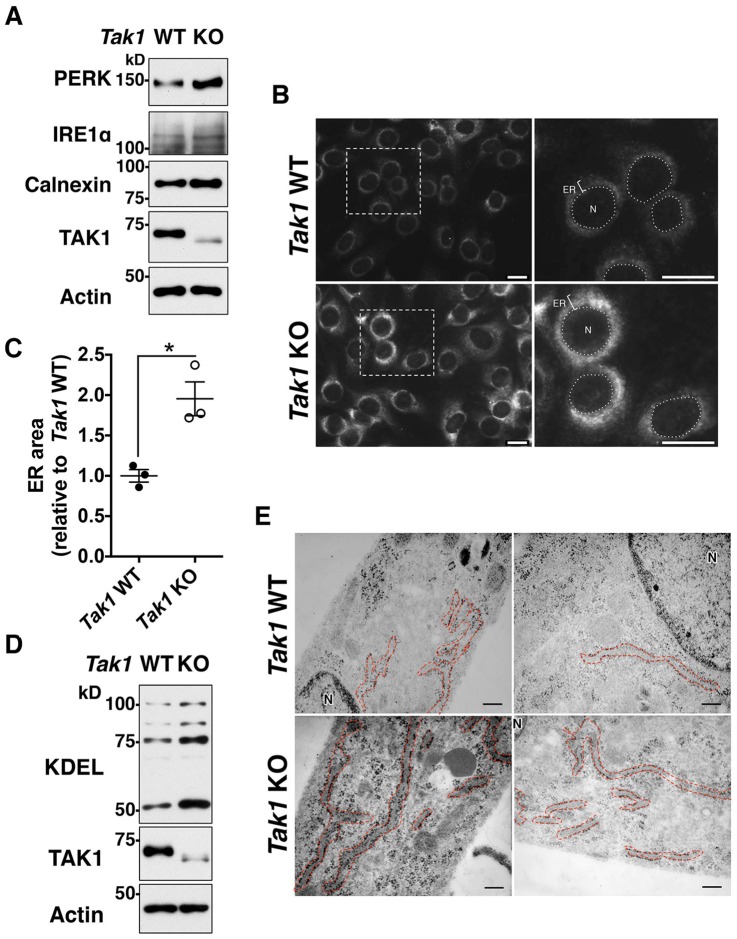
To determine how TAK1 regulates ER stress, we first tested the possibility that TAK1 directly modulates signal transduction of the UPR. Because effective induction of the UPR can prevent ER-stress-induced cell death (Ron and Walter, 2007), Tak1 deficiency might upregulate the UPR. However, quantification of the levels of UPR-induced mRNAs [Atf4, spliced Xbp1 mRNA (Xbp1S) and Grp78 (also known as Hspa5 or Bip)] upon treatment with ER stressors did not reveal any clear trends in the upregulation of the UPR in Tak1-deficient cells compared to that in wild-type cells (Fig. S1A–F). To further investigate the UPR in Tak1-deficient cells, Xbp1 splicing and phosphorylation of PERK were examined. For Xbp1 splicing, unspliced Xbp1 (Xbp1U) and the Xbp1S forms of mRNA were analyzed by performing reverse-transcription PCR (Fig. S1G). Xbp1 splicing effectively occurred both in wild-type and Tak1-deficient cells upon treatment with ER stressors, which generated Xbp1S at similar levels, consistent with the results in real-time PCR analyses of Xbp1S shown in Fig. S1B and E. PERK phosphorylation was also found to be induced both in wild-type and Tak1-deficient cells, and the levels of phosphorylation did not show increasing trends with Tak1 deficiency (Fig. S1H). However, we found that the protein level of PERK in untreated Tak1-deficient cells was higher compared to that of wild-type cells (Fig. S1H; discussed later in this section). These results suggest that the ER-stress resistance of Tak1-deficient cells is not due to upregulation of UPR signaling pathways. Intriguingly, in the course of UPR signaling analysis, we realized that the amounts of ER-resident proteins, including PERK, IRE1α and calnexin were basally elevated in Tak1-deficient fibroblasts (Fig. 2A; Fig. S1H). Because expression levels of ER-resident proteins are coordinately regulated with ER volume (Wiest et al., 1990), this prompted us to test the possibility that ER volume is increased in Tak1-deficient cells. To visualize the ER, we utilized the ER-retention signal motif KDEL, which is included in multiple ER-resident proteins and widely used to assess ER distribution (Jackson et al., 1990). Immunofluorescence staining of KDEL-motif-containing proteins revealed that ER volume was indeed markedly increased in Tak1-deficient fibroblasts compared to that in wild-type fibroblasts (Fig. 2B and C), which was confirmed by immunoblotting of KDEL-motif-containing proteins (Fig. 2D). Consistent with elevated levels of KDEL proteins, electron microscopy analysis demonstrated that the membranous structure of the rough ER, determined by membrane-bound ribosomes, was pronouncedly elongated and distributed throughout the cytoplasm of Tak1-deficient fibroblasts (Fig. 2E). ER volume is primarily regulated by ER membrane biogenesis (Sriburi et al., 2004), and proliferation of ER membrane is known to upregulate the functional capacity of the ER, resulting in ER-stress resistance (Schuck et al., 2009). Thus, Tak1 deficiency alleviates ER stress and protects cells, potentially by increasing ER membrane biogenesis.
Fig. 2.
Tak1-deficiency increases ER volume. (A,D) Tak1 wild-type (Tak1 WT) and Tak1-deficient (Tak1 KO) fibroblasts were analyzed by immunoblotting lysates for the indicated proteins. (B,C) Tak1 WT and Tak1 KO fibroblasts were analyzed by immunofluorescence staining with an anti-KDEL antibody (B). The average ER area (relative to that of Tak1 WT fibroblasts) is shown in (C). Cell size was comparable between Tak1 WT and KO fibroblasts, as shown in Fig. S2A. N, nucleus; ER, endoplasmic reticulum. Scale bars: 20 μm. (E) Tak1 WT and KO fibroblasts were analyzed by using a transmission electron microscope. The rough ER is indicated by red dashed line. Two representative pictures for each genotype are shown. N, nucleus. Scale bars: 20 nm.
Tak1 deficiency increases ER volume and ER-stress tolerance by upregulating SREBPs
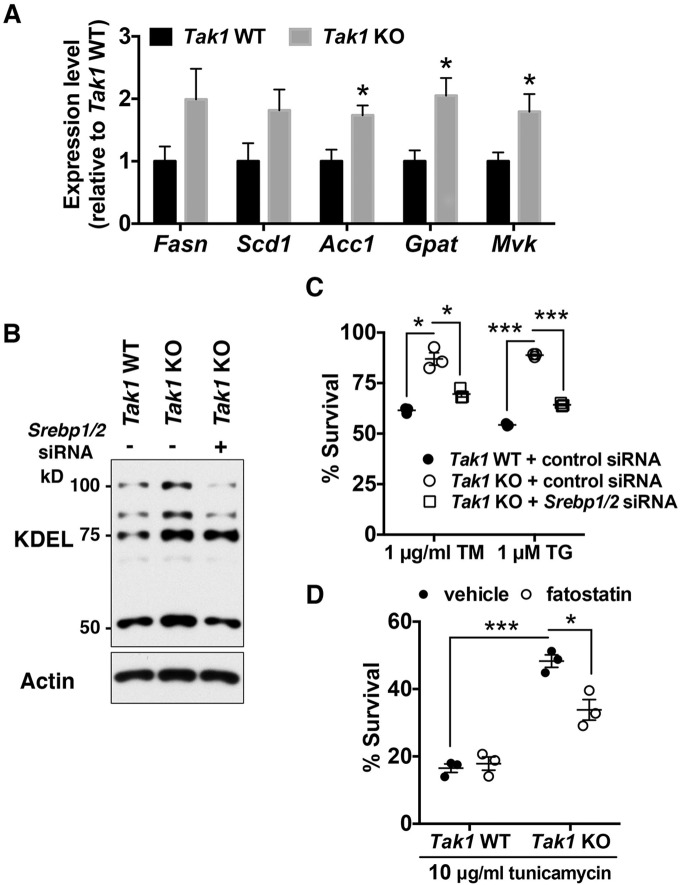
Next, we examined whether Tak1 deletion increases ER volume by upregulating ER membrane biogenesis. Membrane lipids are generated through lipogenesis, in which the sterol-regulatory-element-binding proteins (SREBPs) SREBP1 and SREBP2 transcriptionally regulate the expression of lipogenic enzymes (Shimano, 2001). We have recently found that TAK1 directly binds to and inhibits SREBPs, and that deletion of Tak1 upregulates lipogenic enzymes in the mouse liver (Morioka et al., 2016). This raises the possibility that TAK1 regulates ER membrane biogenesis and ER-stress tolerance through SREBPs. We observed that the expression of SREBP-target lipogenic enzymes acetyl-CoA carboxylase1 (Acc1; also known as Acaca), glycerol-3-phosphate acyltransferase (Gpat; also known as Gpam) and mevalonate kinase (Mvk) were significantly upregulated in Tak1-deficient cells. Fatty acid synthase (Fasn) and stearyl coenzyme-A desaturase1 (Scd1) also showed higher expression in Tak1-deficient cells, although the differences did not reach statistical significance (Fig. 3A). To investigate whether deletion of Tak1 increases lipid synthesis, we conducted a lipid fluxomics analysis. We observed little incorporation of 13C into palmitic acid (Fig. S2B) and stearic acid (Fig. S2C) in wild-type cells during a 12-h incubation with [1,2-13C]acetate, whereas we detected significantly increased incorporation of 13C into those fatty acids in Tak1-deficient cells, although the percentages of the newly synthesized lipids were still less than 10% of the unlabeled lipids. These results suggest that, although the de novo lipid synthesis pathway is normally very low in cultured fibroblasts, Tak1 deficiency indeed enhances de novo fatty acid synthesis. To determine the causal relationship between upregulation of SREBPs and ER-stress resistance in Tak1-deficient fibroblasts, we introduced small interfering (si)RNAs that targeted Srebp1 and Srebp2 into Tak1-deficient fibroblasts. mRNA levels of Srebp1 and Srebp2 were effectively reduced by the siRNA transfection, which resulted in attenuation of Fasn, Scd1, Acc1, Gpat and Mvk expression levels (Fig. S2D). Immunoblotting for KDEL-containing proteins demonstrated that depletion of SREBPs in Tak1-deficient cells reduced the amount of ER-resident proteins to levels similar to those in wild-type cells (Fig. 3B). Under this condition, tolerance to ER stress of Tak1-deficient cells was restored to a level comparable to that of wild-type cells (Fig. 3C). To further confirm that SREBPs are important for Tak1-deficiency-induced ER-stress resistance, we treated Tak1-deficient cells with fatostatin, a pharmacological inhibitor of SREBPs (Kamisuki et al., 2009). Consistently, fatostatin reduced the ER-stress tolerance in Tak1-deficient fibroblasts (Fig. 3D). Taken together, these results demonstrate that TAK1 negatively regulates ER membrane biogenesis and ER-stress tolerance by inhibiting SREBPs, and that deletion of Tak1 alleviates ER stress by upregulating SREBP-dependent ER membrane biogenesis.
Fig. 3.
Tak1-deficiency enhances ER-stress tolerance through SREBPs. (A) mRNA levels of SREBP-target genes in Tak1 wild-type (Tak1 WT) and Tak1-deficient (Tak1 KO) fibroblasts were analyzed by performing quantitative real-time PCR. All mRNA levels were quantified relative to Gapdh gene expression and presented as the fold change relative to levels in Tak1 WT fibroblasts. Fasn, fatty acid synthase; Scd1, stearyl coenzyme-A desaturase1; Acc1, acetyl-CoA carboxylase1; Gpat, glycerol-3-phosphate acyltransferase; Mvk, mevalonate kinase; n=6 per genotype; mean±s.e.m.; *P<0.05; **P<0.01; ***P<0.001. (B,C) Tak1 WT and Tak1 KO fibroblasts were transfected with non-targeting control siRNA or a mixture of siRNAs against Srebp1 and Srebp2 (Srebp1/2). At 72 h post transfection, cell lysates were analyzed by immunoblotting with anti-KDEL antibody (B). Alternatively, at 72 h post transfection, cells were treated with vehicle alone, 1 μg/ml tunicamycin (TM) or 1 μM thapsigargin (TG) for 18 h. Cell viability was measured by Crystal Violet staining (C). Expression levels of Srebp1 and Srebp2 were effectively downregulated by the transfection of siRNAs (Fig. S2D). n=3 per treatment; mean±s.e.m.; *P<0.05; ***P<0.001. (D) Tak1 WT and Tak1 KO fibroblasts were pre-treated with vehicle alone or 10 μg/ml of fatostatin for 24 h. Cells were then treated with either vehicle or 10 μg/ml tunicamycin for 18 h. Cell viability was measured by Crystal Violet staining. n=3 per treatment; mean±s.e.m.; *P<0.05; ***P<0.001.
Tak1 deficiency is protective against ER-stress-induced leptin resistance
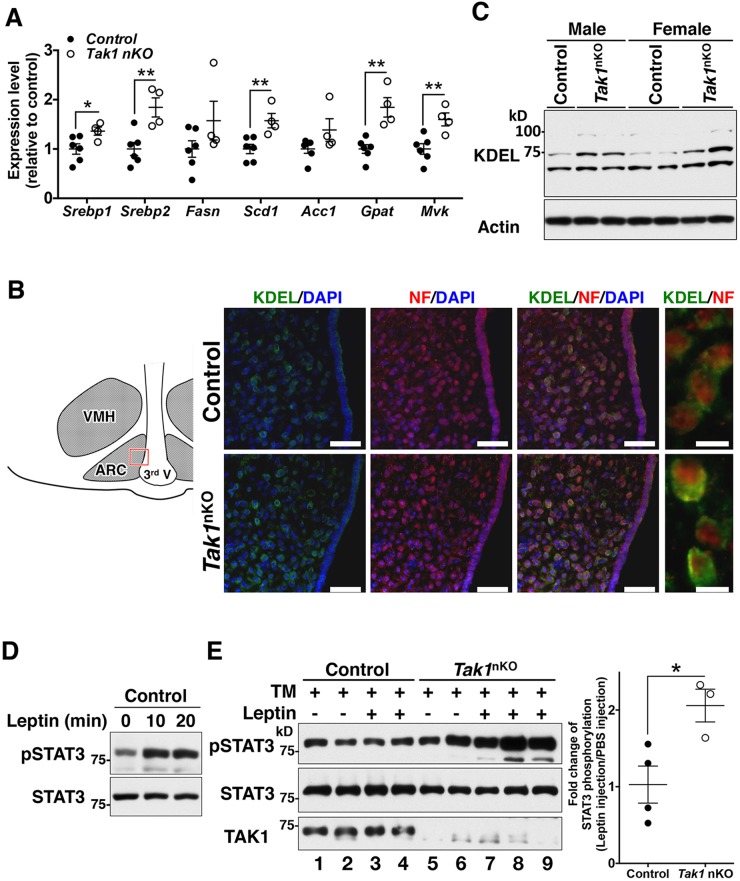
Prolonged ER stress disrupts cellular homeostasis and is known to be associated with the development of various human diseases. The central nervous system (CNS) is highly susceptible to ER stress, which is illustrated by the fact that chronic ER stress is causally associated with many types of neurodegenerative diseases and neuron-derived metabolic disorders (Cai, 2013; Lindholm et al., 2006). Our results described above demonstrate that Tak1 deficiency alleviates ER stress in cultured cells, which led us to examine the possibility that inhibition of TAK1 improves the pathology of ER-stress-associated neuronal disorders. An adipose-tissue-derived hormone, leptin, is the central regulator of appetite by targeting appetite-associated hypothalamic neurons and activating the intracellular JAK2–STAT3 signaling pathway, leading to feeding suppression (Oswal and Yeo, 2010). A chronic HFD is known to induce ER stress in hypothalamic neurons, which inhibits leptin-induced STAT3 activation and impairs proper appetite control (Ozcan et al., 2009). To test whether Tak1 deficiency is protective against ER-stress-induced blockade of leptin signaling, we first utilized Tak1-deficient fibroblasts expressing exogenously introduced leptin receptor b (a splice isoform of Lepr) and monitored leptin-induced phosphorylation of STAT3 in the presence and absence of the ER stressor tunicamycin. An 8-h treatment with tunicamycin significantly diminished leptin-induced STAT3 phosphorylation in wild-type fibroblasts, whereas tunicamycin-treated Tak1-deficient fibroblasts still responded to leptin (Fig. S2E). We then examined whether Tak1 deficiency alleviates ER-stress-induced leptin resistance in vivo. We generated CNS-specific Tak1-deficient mice (hereafter referred to as Tak1nKO mice) by crossing Tak1flox/flox mice to a Nestin-Cre transgenic line that is widely used to achieve gene deletion in the CNS (Zhang et al., 2008; Zimmerman et al., 1994). In Tak1nKO mice, the levels of TAK1 protein in the CNS were reduced at embryonic day 18.5 and effectively diminished by postnatal day 12 (Fig. S3A) – the Nestin-Cre system effectively directs recombination starting from late embryonic period (Liang et al., 2012). As heterozygous CNS-specific Tak1 deletion (Tak1flox/+ Nestin-Cre) did not reduce TAK1 protein levels (Fig. S3A), we used wild-type (Tak1flox/flox no-Cre) and Tak1flox/+ Nestin-Cre littermate or age-matched mice as controls for Tak1nKO mice. Tak1nKO mice were slightly smaller than littermate controls (as discussed later), but they exhibited no overt abnormalities during our experimental period (up to 12 months), consistent with a recent report (Goldmann et al., 2013). There were no detectable differences in the amount of food intake (Fig. S3B), body temperature (Fig. S3C) and percent visceral fat (Fig. S3D) between control and Tak1nKO mice upon feeding with a normal diet. We first examined whether Tak1 deficiency in the CNS increases SREBP activities and ER volume as it does in fibroblasts. Indeed, SREBP-target genes – Srebp1, Srebp2, Scd1, Gpat and Mvk – were upregulated in the hypothalamus of Tak1nKO mice (Fig. 4A). We also detected an increase in Fasn and Acc1 expression levels in the Tak1nKO hypothalamus, although they did not reach a statistical significance level (Fig. 4A). Double-immunofluorescence staining of neuronal marker neurofilament 200 (also known as Nefh) and KDEL-containing proteins in the hypothalamus demonstrated that ER volume was increased in the hypothalamic neurons of Tak1nKO mice (Fig. 4B), which was confirmed by immunoblotting of the hypothalamic extracts (Fig. 4C). We then asked whether Tak1-deficient hypothalamic neurons are resistant to ER stress. We injected tunicamycin into the third ventricle of Tak1nko and control mice, which is known to induce ER stress in the hypothalamus (Zhang et al., 2008). We observed ER-stress-induced activation of the UPR both in control and Tak1nKO hypothalamus. Consistent with the UPR observed in cultured fibroblasts, Xbp1 splicing occurred at comparable levels between control and Tak1nKO hypothalamus (Fig. S4A). We also examined PERK phosphorylation by detecting retarded migration of PERK on SDS-PAGE (Fig. S4B, top panel). Injection of tunicamycin induced a shift in PERK migration in both control and Tak1nKO hypothalamus. These slower migrating smear bands were diminished by phosphatase treatment, indicating these are phosphorylated forms of PERK. We note here that the intensity of the phosphorylated PERK band was much lower in the hypothalamic PERK compared to that in fibroblasts (see Fig. S1H), and we were unable to detect phosphorylated PERK with an antibody against phosphorylated PERK in the hypothalamus. PERK activation might be weaker in the hypothalamus compared to that in fibroblasts under our experimental settings. In this context, caspase-3 cleavage was slightly induced in the control hypothalamus (Fig. S4B). We found that caspase-3 cleavage was completely attenuated by Tak1 deficiency (Fig. S4B), suggesting that Tak1 deficiency alleviates ER stress in the hypothalamus without altering the UPR, as it does in cultured fibroblasts.
Fig. 4.
CNS-specific deletion of Tak1 protects mice from ER-stress-induced leptin resistance. (A) mRNA levels of SREBP-target genes in control (Tak1flox/flox no-Cre and Tak1flox/+ Nestin-Cre) and Tak1nKO hypothalamus from 8-week-old mice were analyzed by performing quantitative real-time PCR. All mRNA levels were quantified relative to Gapdh gene expression and presented as fold change relative to levels in control mice. Control, n=6; Tak1nKO, n=4; mean±s.e.m.; *P<0.05; **P<0.01. (B) The arcuate nucleus (ARC) of the hypothalamic sections from 8-week-old control and Tak1nKO male mice were analyzed by immunofluorescence staining with anti-KDEL (green) and anti-neurofilament-200 (red, NF) antibodies, and DAPI (blue). The schematic diagram indicates the region of ARC shown in the pictures. VMH, ventromedial hypothalamic nucleus; 3rd V, the third ventricle. Scale bars: 50 μm (left three panels); 10 μm (far-right panels). (C) The hypothalamic extract from 16-week-old control and Tak1nKO male (left three lanes) and female (right four lanes) mice fed a HFD for 12 weeks were analyzed by immunoblotting with an anti-KDEL-motif antibody. Each lane represents an individual mouse. (D) Intraperitoneal injection of leptin induces STAT3 phosphorylation (pSTAT3) in the hypothalamus. 16-week-old control mice that had been fed a normal diet were fasted for 18 h. PBS or 1 μg of leptin per gram of body weight was injected intraperitoneally, and the hypothalamus was isolated at the indicated time points after injection. STAT3 phosphorylation was analyzed by immunoblotting. (E) 8-week-old control and Tak1nKO mice (fed a normal diet) were fasted for 12 h. 20 μg of tunicamycin was injected by intracerebroventricular injection. After 6 h, vehicle (PBS) or 1 μg of leptin per gram of body weight was injected intraperitoneally, and hypothalamic STAT3 phosphorylation at 15 min post injection was analyzed by immunoblotting. Total amounts of STAT3 and TAK1 proteins are also shown. Each lane represents an individual mouse. The graph (at right) shows the quantification of the intensities of phosphorylated STAT3 normalized to total the intensity of STAT3, including data from additional samples not shown in the immunoblot. Control, n=4; Tak1nKO, n=3; mean±s.e.m.; *P<0.05; mean±s.e.m.
We then examined whether Tak1 deletion improves ER-stress-induced leptin resistance in vivo. We injected tunicamycin into the third ventricle of the brain of Tak1nko and control mice, and subsequently leptin was administered to the mice by using intraperitoneal injection. As expected, leptin injection effectively induced phosphorylation of STAT3 in the hypothalamus of untreated mice (Fig. 4D), whereas tunicamycin infusion completely blocked leptin-induced STAT3 phosphorylation in the hypothalamus of control mice (Fig. 4E, lanes 1–4). In contrast, tunicamycin failed to block leptin-induced STAT3 phosphorylation in the hypothalamus of Tak1nKO mice (Fig. 4E, lanes 5–9). These data suggest that deletion of Tak1 alleviates ER stress and prevents leptin resistance in both in vitro and in vivo settings.
CNS-specific deletion of Tak1 protects mice against HFD-induced leptin resistance and hyperphagic obesity
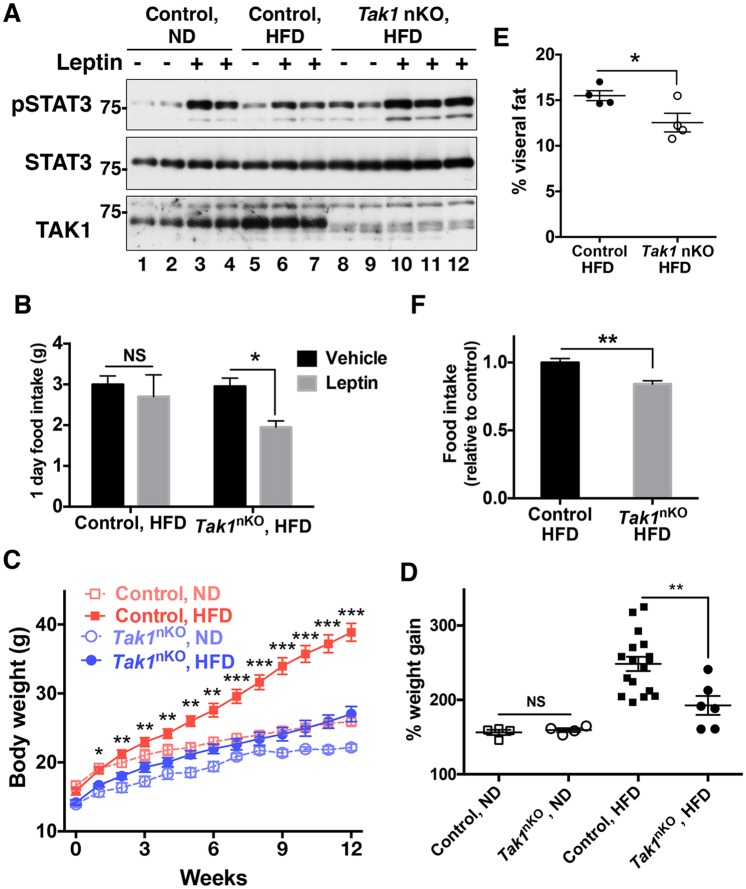
As Tak1 deletion prevented ER-stress-induced leptin resistance, we next asked whether Tak1 deficiency in the CNS protects mice against HFD-induced leptin resistance and subsequent obesity. To this end, we placed 4-week-old mice on a HFD for 12 weeks and examined leptin sensitivity in the hypothalamus. Intraperitoneal injection of leptin effectively induced STAT3 phosphorylation in the hypothalamus of normal-diet-fed control mice (Fig. 5A, lanes 1–4), whereas the effect of leptin was much smaller in HFD-fed control mice (Fig. 5A, lanes 5–7). This indicates that a HFD induced leptin resistance in control mice in our experimental paradigm. In contrast, leptin injection effectively induced STAT3 phosphorylation in the hypothalamus of Tak1nKO mice on a HFD (Fig. 5A, lanes 8–12). Consistent with this finding, intraperitoneal injection of leptin failed to suppress the food intake of HFD-fed wild-type mice, whereas leptin effectively suppressed the food intake of HFD-fed Tak1nKO mice (Fig. 5B). These results suggest that CNS-specific Tak1 deletion prevents development of leptin resistance under a HFD. Furthermore, a HFD resulted in a marked increase of the body weight of control mice compared to that of mice on a normal-feeding diet, whereas Tak1nKO mice showed resistance to HFD-induced excessive weight gain (Fig. 5C). We note here that the initial body weights of Tak1nKO mice at week 0 of the diet (4-week-old mice) (mean±s.e.m., 14.08 g±0.3630 g, n=10) were slightly smaller than those of control mice (mean±s.e.m., 15.99 g±0.4747 g, n=21) (Fig. 5C, week 0). We have not yet explored the pathology underlying this slightly runted phenotype of Tak1nKO mice, and it might contribute to the inability to gain weight. However, the percent change in body weight of Tak1nKO mice was comparable to that of control mice on the normal-feeding diet (Fig. 5D), suggesting that the ability to gain weight was not compromised in Tak1nKO mice. In contrast, HFD-induced weight gain was profoundly attenuated by Tak1 deletion (Fig. 5D). This protective effect against HFD feeding was not observed in mice that were heterozygous for CNS-specific Tak1 deletion (Tak1flox/+ Nestin-Cre) (Fig. S3F), demonstrating that expression of Nestin-Cre alone did not cause observable effects under our experimental settings, although an earlier study reported a marginal reduction in weight gain upon Nestin-Cre expression (Harno et al., 2013). Thus, CNS-specific Tak1 deletion is the cause of resistance to HFD-induced excessive weight gain. The adiposity of HFD-fed Tak1nKO mice was smaller than that of control mice (Fig. 5E). As expected, HFD-fed Tak1nKO mice showed slightly reduced circulating leptin levels compared to HFD-fed control mice, as it reflects body fat levels, although the difference in the leptin levels did not reach statistical significance (Fig. S3E). Consistent with the improved leptin sensitivity, food intake of HFD-fed Tak1nKO mice was lower than that of control mice (Fig. 5F). These results demonstrate that deletion of Tak1 in the CNS prevents HFD-induced leptin resistance and hyperphagic obesity, which is likely to be owing to improved ER function.
Fig. 5.
CNS-specific deletion of Tak1 protects mice from HFD-induced leptin resistance and obesity. (A,B) Control (Tak1flox/flox no-Cre and Tak1flox/+ Nestin-Cre) mice that had been fed a normal diet (ND) or HFD for 12 weeks, and Tak1nKO (Tak1nKO, HFD) mice, were fasted for 18 h. PBS or 1 μg of leptin per gram of body weight was then injected intraperitoneally. (A) The hypothalamus was isolated at 15 min post injection, and STAT3 phosphorylation (pSTAT3) was analyzed by immunoblotting. Each lane represents an individual mouse. (B) Food intake for 24 h after leptin injection was measured. Control with HFD, n=5; Tak1nKO with HFD, n=6; mean±s.e.m.; NS, not significant; *P<0.05. (C–F) 4-week-old control and Tak1nKO male mice were fed either a normal diet or HFD for 12 weeks. (C) Mice were weighed weekly. Control with normal diet, n=4; control with HFD, n=17; Tak1nKO with normal diet, n=4; Tak1nKO with HFD, n=6; mean±s.e.m.; P-values shown are the comparison between control with HFD and Tak1nKO with HFD; *P<0.05; **P<0.01; ***P<0.001. Control mice included wild-type (Tak1flox/flox no-Cre) and Tak1flox/+ Nestin-Cre mice. (D) Percent body weight change at week 12 of the data shown in C was calculated, and all data points were plotted. The initial weights at week 0 are designated as 100%. Mean±s.e.m.; NS, not significant; **P<0.01. (E) Visceral fats were isolated and weighed at the end of the experiment shown in A. n=4 per group; mean±s.e.m.; *P<0.05. (F) At 11 weeks after feeding with a normal diet or a HFD, food intake was measured daily, and cumulative food intake for 1 week was calculated. Control with HFD; n=9; Tak1nKO with HFD; n=5; mean±s.e.m.; **P<0.01.
DISCUSSION
Our unexpected finding that Tak1 deficiency is protective against ER stress led us to investigate the mechanism of TAK1 regulation of ER stress. Here, we show that TAK1 regulation of ER volume is the cause of ER-stress resistance in Tak1-deficient cells and that improved ER function in Tak1-deficient cells can prevent HFD-induced leptin resistance. TAK1 is an intracellular signaling intermediate that is activated by inflammatory stimuli and that functions to transmit a signal from the receptor proximal molecules – such as a complex of TNF receptor, TNF-receptor-activated factors (TRAFs) and receptor-interacting protein kinase 1 (RIPK1) – to downstream signaling cascades – such as IκB-kinase–NF-κB and MAPKK–MAPKs (Mihaly et al., 2014). Interestingly, hypothalamic NF-κB and JNK are known to be activated with HFD feeding, and ablation of NF-κB or JNK alleviates HFD-induced leptin resistance (Sabio et al., 2010; Tsaousidou et al., 2014; Zhang et al., 2008). This raises the possibility that Tak1 deficiency reduces the activity of NF-κB and/or JNK in the hypothalamus, which could participate in protection of leptin resistance. We found that a HFD activated TAK1 and JNK in the hypothalamus (Fig. S3G). However, JNK activity was unaltered by Tak1 deletion (Fig. S3G); hence, JNK is unlikely to be the downstream target of TAK1 in the hypothalamus. In contrast, NF-κB activity, monitored by electrophoresis mobility shift assay (EMSA) (Fig. S3G) and phosphorylation of p65 (also known as RELA) (Fig. S3H), was not detectably upregulated in our experimental settings, even under conditions where TAK1 was activated. Our detection efficiency of NF-κB activation could be lower than that in an earlier study (Zhang et al., 2008). Nonetheless, Tak1 deficiency altered neither the DNA-binding activity of NF-κB (Fig. S3G) nor p65 phosphorylation (Fig. S3H). We note here that endothelial-specific Tak1 deletion also does not alter NF-κB activity in the blood vessels (Morioka et al., 2012). Collectively, TAK1 signaling is not always associated with JNK and NF-κB activity in vivo, which is consistent with the fact that ablation of NF-κB or MAPKs does not result in tissue abnormalities similar to those of Tak1-deficient tissue (del Barco Barrantes et al., 2011; Mihaly et al., 2014; Sabapathy et al., 2001; Voisin et al., 2010). TAK1 regulation of lipogenesis, but not of NF-κB or JNK, is primarily involved in hypothalamic alterations in CNS-specific Tak1-deficient mice. Our results reveal that SREBP-dependent lipogenesis is a previously unrecognized target of TAK1, and it might be a crucial downstream consequence of TAK1 activity, not only in the brain but also in other tissues, which warrants further investigations.
Targeting ER stress in the brain is a promising therapeutic approach against ER-stress-associated neuronal diseases (Zhang and Kaufman, 2008). Nevertheless, a practical method to alleviate ER stress in affected humans is still limited. Intracerebroventricular administration of chemical chaperones, such as 4-phenylbutyrate (4-PBA) and tauroursodeoxycholic acid (TUDCA) are known to improve HFD-induced obesity and several neurodegenerative diseases in the mouse models (Cohen and Kelly, 2003; Ozcan et al., 2009), yet their effects on human diseases are undetermined, and their lack of substrate specificity is likely to be problematic (Aridor, 2007). This has inspired recent chemical screens, which have been used to identify compounds that improve ER functions and can reverse metabolic dysfunctions in the mouse model (Fu et al., 2015; Liu et al., 2015). In the present study, we demonstrate that deletion of Tak1 protects cells from ER stress, both in an in vitro cell culture system and in the CNS. Importantly, Tak1nKO mice did not show overt histopathological abnormalities, and pharmacological inhibition of TAK1 in the brain is reported not to cause adverse effects but rather to be associated with resistance in the mouse model of cerebral ischemia and experimental traumatic brain injury (Neubert et al., 2011; Zhang et al., 2013). Thus, TAK1 is likely to be a low-risk target for therapeutic approaches in the brain. Our findings reveal that TAK1–SREBP regulation of ER membrane biogenesis is crucially involved in susceptibility to ER stress, which raises the possibility that targeting TAK1 could be an alternative and suitable therapeutic approach to treat ER-stress-associated neuronal diseases.
Chronic inflammation is one of the fundamental pathological processes associated with a number of human diseases, including neurodegenerative diseases, metabolic disorders and cardiovascular pathologies, which are often accompanied by ER stress (Hotamisligil, 2006, 2010; Kim et al., 2008; Minamino and Kitakaze, 2010; Zhang and Kaufman, 2008; Zhang et al., 2008). However, the molecular mechanism linking inflammation and ER stress is poorly understood. Here, we demonstrate that TAK1 negatively regulates cellular ER-stress tolerance by inhibiting SREBP-dependent ER membrane biogenesis. Given that TAK1 is activated by a diverse set of inflammatory stimuli, including IL-1 and TNF, one possible mechanism linking inflammation and ER stress is that activation of TAK1 by inflammatory cytokines inhibits ER membrane biogenesis through downregulation of SREBP-dependent lipogenesis, resulting in functional impairment of the ER. Indeed, our data demonstrate that HFD feeding – an inducer of inflammation in the hypothalamus (Thaler et al., 2012) – activated TAK1 in the hypothalamus (see Fig. S3G), which could reduce lipogenesis and ER functional capacity. TAK1 activation could be a previously uncharacterized link between inflammation and ER stress in the context of human diseases.
MATERIALS AND METHODS
Mice and cell culture
Tak1-floxed (Tak1flox/flox) mice and Tak1-deficient fibroblasts have been described previously (Sato et al., 2005). Nestin-Cre transgenic mice were obtained from the Jackson Laboratories (Tronche et al., 1999). All strains used were backcrossed at least seven times to C57BL/6 mice. Tak1-deficient keratinocytes have been described previously (Omori et al., 2006). Littermate and age-matched wild-type (Tak1flox/flox no-Cre) and heterozygous Tak1 deletion (Tak1flox/+ Nestin-Cre) mice, which were phenotypically indistinguishable, were used as control in all mouse experiments. Fibroblasts were cultured in Dulbecco's modified Eagle's medium supplemented with 10% bovine growth serum (Hyclone) and 50 IU/ml penicillin–streptomycin at 37°C under 5% CO2. Keratinocytes were cultured in Ca2+-free Eagle's minimal essential medium (BioWhittaker) supplemented with 4% Chelex-treated bovine growth serum, 10 ng/ml of human epidermal growth factor (Invitrogen), 0.05 mM CaCl2 and 50 IU/ml penicillin–streptomycin at 33°C under 8% CO2. 60% of calories from fat (high fat) diet (D12492, Research Diets) and 13.4 kcal% fat control diet (5001, Lab Diet) were used. To test leptin sensitivity, 1 μg of recombinant murine leptin (Pepro Tech) per gram of body weight was injected intraperitoneally. All animal experiments were conducted with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Plasmids and reagents
Leptin-receptor-b-expressing plasmid has been described previously (Ozcan et al., 2009). Plasmids were transfected into the cells using TransIT-X2 Reagent (Mirus Bio LLC). Tunicamycin, thapsigargin and fatostatin were purchased from Millipore. PstI restriction enzyme was purchased from New England Biolabs.
Crystal Violet assay
Cells were plated on 24-well dishes 24 h before staining at a concentration of 2×104 cells per well. Cells were treated with either tunicamycin or thapsigargin for 18 h. For fatostatin treatment, cells were pretreated with 10 μg/ml of fatostatin 24 h before ER-stress induction. Cells were then washed with PBS and fixed in 10% formalin, and stained with 0.1% Crystal Violet. The dye was eluted in 50% ethanol, 0.1 M sodium citrate and analyzed at 595 nm using a SmartSpec™ 3000 instrument (Bio-Rad).
Immunoblotting
Protein extracts from fibroblasts and the hypothalamus were prepared using an extraction buffer containing 20 mM Hepes, pH 7.4, 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 2 mM EGTA, 10 mM NaF, 2 mM DTT, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 20 μM aprotinin and 0.5% Triton X-100. The extracts were resolved on SDS-PAGE and transferred onto Hypond-P membranes (GE Healthcare). The membranes were immunoblotted with the indicated antibodies, and the bound antibodies were visualized with horseradish peroxidase-conjugated antibodies against rabbit or mouse IgG using the ECL western blotting system (GE Healthcare, 1:10,000). The primary antibodies used were against: TAK1 (described previously, Ninomiya-Tsuji et al., 1999, 1:500), phosphorylated TAK1 (Thr187) (Cell Signaling Technology, 1:1000), β-actin (Sigma, AC15, 1:1000), PERK (Cell Signaling Technology, C33E10, 1:1000), phosphorylated PERK (Thr980) (Cell Signaling Technology, 16F8, 1:1000), IRE1α (Cell Signaling Technology, 14C10, 1:1000), calnexin (Santa Cruz Biotechnology, H-70, 1:200), KDEL (ENZO, 10C3, 1:1000), STAT3 (Cell Signaling Technology, D1A5, 1:1000), phosphorylated STAT3 (Tyr705) (Cell Signaling Technology, D3A7, 1:2000), JNK1/2 (Santa Cruz Biotechnology, sc571, 1:200), phosphorylated JNK (Thr183/Tyr185) (Cell Signaling Technology, 9251, 1:1000), phosphorylated p65 NF-κB (Ser276) (Cell Signaling Technology, 1:1000) and p65 (Santa Cruz Biotechnology, C20, 1:1000). Intensities of bands were quantified using the ImageJ software.
Phosphatase treatment and PERK immunoblotting
To dephosphorylate proteins, protein extracts were incubated with antarctic phosphatase (New England Biolabs) at 30°C for 30 min. To determine PERK phosphorylation, protein extracts that had been treated with or without phosphatase were separated in a 7% polyacrylamide gel and analyzed by immunoblotting.
Immunofluorescence microscopy analysis of fibroblasts
Fibroblasts were seeded on glass coverslips in 6-well plates 24 h prior to fixation. Cells were fixed with 100% methanol for 2 min at −20°C, blocked with PBS containing 3% bovine serum albumin (Santa Cruz Biotechnology) for 30 min at room temperature and then incubated with anti-KDEL antibody (ENZO, 10C3, 1:1000) followed by incubation with anti-mouse IgG conjugated with Alexa-Fluor-594 (1:500). The coverslips were mounted with 50% glycerol and were examined by using a fluorescence microscope (model BX41; Olympus) and camera (model XM10; Olympus) at room temperature. For quantification of the KDEL-positive area, three areas randomly photographed with the same exposure time were used. Each picture contained more than 70 cells, and the KDEL-staining area was quantified using ImageJ software. To calculate the average ER volume in a single cell, the total KDEL-staining area was divided by the cell number.
Immunofluorescence microscopy analysis of hypothalamic sections
Mice were euthanized with CO2 inhalation. The brains were rapidly removed and fixed with 4% paraformaldehyde at room temperature for 3 h. The brains were then transferred to 20% sucrose and kept at 4°C overnight. The brains were embedded in optimum cutting temperature (OCT) compound and frozen immediately. Cryosections (8 μm) were post-fixed with 100% methanol for 1 min at room temperature and blocked with PBS containing 3% bovine serum albumin for 30 min at room temperature, and then incubated with anti-KDEL (ENZO, 10C3, 1:500) and anti-neurofilament-200 (Sigma, 1:200) antibodies followed by incubation with anti-mouse IgG conjugated with Alexa-Fluor-488 (1:500) and anti-rabbit IgG conjugated with Alexa-Fluor-594 (1:500). The sections were stained with DAPI and were mounted onto coverslips with 50% glycerol, and then were examined with a fluorescence microscope (model BX41; Olympus) and camera (model DP80; Olympus) at room temperature.
Electron microscopy analysis
Fibroblasts were seeded on 100-mm dishes 24 h prior to fixation. Cells were fixed in Karnovsky's fixative, 2% paraformaldehyde (electron microscopy grade, Electron Microscopy Sciences) and 2.5% glutaraldehyde (electron microscopy grade, Electron Microscopy Sciences) in PBS overnight at 4°C, and subsequently post-fixed in 2% OsO4 for 1 h at 4°C in the dark. Cells were embedded in 2% agarose, dehydrated and thin-sectioned on a Leica UC6rt (Leica Microsystems) at 80-nm thickness on a DDK diamond knife (Delaware Diamond Knives). Sections were stained with both 4% aqueous uranyl acetate and Reynold's lead citrate before viewing on a JEOL JEM-1200EX transmission electron microscope (JEOL USA) at 80 kV. Images were collected on Kodak 4489 film (Eastman Kodak), which was then scanned at 1200 dpi using an Epson Perfection 4870 Photo flatbed scanner (Epson America). Digital images were then processed and labeled using Photoshop CS5.1 (Adobe Systems).
Quantitative real-time PCR analysis
Total RNA was isolated from fibroblasts and the hypothalamus using Trizol (Invitrogen) or the RNeasy kit (Qiagen), respectively, and transcribed into cDNA using MultiScribe reverse transcriptase (Life Technologies). The expression level of Chop, Atf4, Xbp1S, Grp78, Srebp1, Srebp2 and SREBP-target genes were determined by quantitative real-time PCR and normalized to the level of Gapdh. The following primers were used: Chop-forward, 5′-CAGGAAACGAAGAGGAAGAATCAA-3′; Chop-reverse, 5′-GCTTTGGGATGTGCGTGTGA-3′; Atf4-forward, 5′-ATGGCCGGCTATGGATGAT-3′; Atf4-reverse, 5′-CGAAGTCAAACTCTTTCAGATCCATT-3′; Xbp1S-forward, 5′-GAGTCCGCAGCAGGTG-3′; Xbp1S-reverse, 5′-GTGTCAGAGTCCATGGGA-3′; Grp78-forward, 5′-GACTGCTGAGGCGTATTTGG-3′; Grp78-reverse, 5′-AGCATCTTTGGTTGCTTGTCG-3′; Srebp1-forward, 5′-GATCAAAGAGGAGCCAGTGC-3′; Srebf1-reverse, 5′-TAGATGGTGGCTGCTGAGTG-3′; Srebf2-forward, 5′-GGATCCTCCCAAAGAAGGAG-3′; Srebf2-reverse, 5′-TTCCTCAGAACGCCAGACTT-3′; Fasn-forward, 5′-AAGGCTGGGCTCTATGGATT-3′; Fasn-reverse, 5′-GGAGTGAGGCTGGGTTGATA-3′; Scd1-forward, 5′-CTGACCTGAAAGCCGAGAAG-3′; Scd1-reverse, 5′-GCGTTGAGCACCAGAGTGTA-3′; Acc1-forward, 5′-TGTTGGGAGTTGTGTGTGGG-3′; Acc1-reverse, 5′-AGTGTGTGAGCAGGAAGGAC-3′; Gpat-forward, 5′-CAACACCATCCCCGACATC-3′; Gpat-reverse, 5′-GTGACCTTCGATTATGCGATCA-3′; Mvk1-forward, 5′-GGGACGATGTCTTCCTTGAA-3′; Mvk1-reverse, 5′-GAACTTGGTCAGCCTGCTTC-3′; Gapdh-forward, 5′-GAAGGTCGCTGTGAACGGA-3′; Gapdh-reverse, 5′-GTTAGTGGGGTCTCGCTCCT-3′.
Xbp1 splicing assay
Unspliced and spliced forms of Xbp1 were amplified from cDNA using the following primers. Xbp1-forward, 5′-CCTTGTGGTTGAGAACCAGG-3′; Xbp1-reverse, 5′-CTAGAGGCTTGGTGTATAC-3′. This results in production of unspliced Xbp1 (Xbp1U, 451 bp), spliced Xbp1 (Xbp1S, 425 bp) and a hybrid of unspliced and spliced Xbp1 (Xbp1H, about 500 bp) DNA fragments. To clearly distinguish the DNA fragments of unspliced and spliced Xbp1, PCR products were incubated with PstI restriction enzyme, which specifically digests unspliced Xbp1, at 37°C overnight. PCR products were then separated by electrophoresis in a 2.5% agarose gel and visualized with ethidium bromide staining.
13C lipid fluxomics analysis
Wild-type and Tak1-deficient fibroblasts were plated at 5×106 on 100-mm plates, and incubated with 4 mM [1,2-13C] acetate (Sigma) for 0 or 12 h. Cells were then washed with 150 mM ammonium acetate and quenched in liquid nitrogen. Lipids were extracted and analyzed by performing gas-chromatography-coupled mass spectrometry (Agilent 69890N GC-5975 MS detector). Metabolites were identified by matching the retention time and mass to authentic standards. Isotope peak areas were integrated using MassHunter Quantitative Analysis vB.07.00 software (Agilent Technologies). Peak areas were corrected for natural isotope abundance, and data were normalized to cell protein content before analysis of metabolite fluxes for fatty acid metabolites. The lipogenic flux is shown with the percentages of palmitic and stearic (C16:0 and C18:0) acids containing exogenous 2, 4 and 6 carbon units (M+2, M+4 and M+6) from [1,2-13C] acetate per unlabeled endogenous M+0 palmitic and stearic acids, respectively.
Knockdown of SREBPs
siRNAs targeting Srebp1 and Srebp2, and control siRNA were obtained from Sigma (SREBP1, 5′-CACAGGAGGACAUCUUGCUGCUUCU-3′; SREBP2, 5′-CGAGGAUCAUCCAGCAGCCUUUGAU-3′; control siRNA, 5′-UUCUCCGAACGUGUCACGU-3′). Fibroblasts were transfected with the siRNAs using TransIT-X2 Reagent (Mirus Bio LLC).
Hypothalamus isolation
Isolation of the hypothalamus has been described previously (Zhang et al., 2008). The hypothalami were homogenized using glass Dounce tissue grinders, and the proteins were dissolved in the extraction buffer described above.
Visceral fat measurement
16-week-old mice were euthanized with CO2 inhalation. Epididymal, retroperitoneal, mesenteric and subcutaneous fat pads were carefully removed and weighed.
Leptin sensitivity test
Individually housed mice were fasted for 18 h and injected with PBS by intraperitoneal injection. Food intake was measured for the following 24 h (Control). One week later, the same mice were fasted for 18 h and injected with 1 μg/g of body weight of leptin by intraperitoneal injection to test leptin sensitivity. Food intake was measured for the following 24 h.
Preparation of plasma sample and leptin ELISA
Individually housed mice were fasted for 18 h and peripheral blood was collected from the facial vein into a 1.5 ml tube containing 1 μl of 0.5 M EDTA and centrifuged at 2000 rpm for 5 min. Supernatant was kept as plasma. Plasma leptin levels were measured using a leptin (mouse) ELISA kit (ENZO). The assay was conducted according to the manufacturer's protocols.
Measuring mouse body temperature
Rectal temperature was measured using TCAT-2DF controller (Physitemp Instruments).
Intracerebroventricular injection of tunicamycin
Intracerebroventricular injection of tunicamycin has been described previously (Ozcan et al., 2009). Briefly, 8-week-old mice were anesthetized, and 26-gauge syringes (Hamilton) were inserted into the third ventricle under stereotaxic control using stereotaxic apparatus (−1.8 mm anteroventral, 0.0 mm lateral, 5.0 mm dorsoventral from bregma). Then, 20 μg of tunicamycin was injected over 5 min. After surgery, the head skin was sewed using surgical sutures (Henry Schein).
Electrophoresis mobility shift assay
For the electrophoresis mobility shift assay (EMSA), oligonucleotides for the consensus NF-κB binding DNA site were purchased from Promega. The binding reaction contained 32P radiolabeled oligonucleotide probe, 50 μg of tissue extracts, 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5), 500 ng of poly(deoxyinosinic-deoxycytidylic) acid (poly dI-dC) (GE Healthcare) and 10 μg of bovine serum albumin to a final concentration of 15 μl. The reaction mixture was incubated at 25°C for 30 min, separated on a 5% (w/v) polyacrylamide gel and visualized with autoradiography.
Flow cytometric analysis
Fibroblasts were fixed with 2% paraformaldehyde in PBS for 15 min at room temperature and analyzed with a flow cytometer (BD Biosciences LSR II). Data were analyzed using FlowJo software (Tree Star).
Statistical analyses
Statistical analyses were performed using two-tailed Student's t-test for comparing the means of two groups. *P<0.05; **P<0.01; ***P<0.001; NS, not significant when P>0.05.
Acknowledgements
We thank North Carolina State biological animal facility for technical support, Dr Akira for Tak1-loxed mice, Dr Valerie for electron microscopy analysis and Dr Myers for providing LepRb-plasmid. The lipid flux study utilized Core Services supported by grant DK089503 from the National Institutes of Health to the University of Michigan.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
K.S., S.M., G.T. and J.N.-T. conceived the study; K.S. and N.M. performed experiments; K.S. and J.N.-T. wrote the original draft of the manuscript; K.S., S.M., H.T.G., H.H., K.M. and J.N.-T. reviewed and edited the manuscript; H.T.G. and J.N.-T. acquired funding.
Funding
This work was supported by National Institutes of Health [grant numbers GM068812 and GM112986 to J.N.-T.; and NS062182 to H.T.G.]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.180505/-/DC1
References
- Aridor M. (2007). Visiting the ER: the endoplasmic reticulum as a target for therapeutics in traffic related diseases. Adv. Drug Deliv. Rev. 59, 759-781. 10.1016/j.addr.2007.06.002 [DOI] [PubMed] [Google Scholar]
- Bettermann K., Vucur M., Haybaeck J., Koppe C., Janssen J., Heymann F., Weber A., Weiskirchen R., Liedtke C., Gassler N. et al. (2010). TAK1 suppresses a NEMO-dependent but NF-κB-independent pathway to liver cancer. Cancer Cell 17, 481-496. 10.1016/j.ccr.2010.03.021 [DOI] [PubMed] [Google Scholar]
- Cai D. (2013). Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol. Metab. 24, 40-47. 10.1016/j.tem.2012.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen F. E. and Kelly J. W. (2003). Therapeutic approaches to protein-misfolding diseases. Nature 426, 905-909. 10.1038/nature02265 [DOI] [PubMed] [Google Scholar]
- del Barco Barrantes I., Coya J. M., Maina F., Arthur J. S. C. and Nebreda A. R. (2011). Genetic analysis of specific and redundant roles for p38alpha and p38beta MAPKs during mouse development. Proc. Natl. Acad. Sci. USA 108, 12764-12769. 10.1073/pnas.1015013108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engin F. and Hotamisligil G. S. (2010). Restoring endoplasmic reticulum function by chemical chaperones: an emerging therapeutic approach for metabolic diseases. Diabetes Obes. Metab. 12 Suppl. 2, 108-115. 10.1111/j.1463-1326.2010.01282.x [DOI] [PubMed] [Google Scholar]
- Fu S., Yalcin A., Lee G. Y., Li P., Fan J., Arruda A. P., Pers B. M., Yilmaz M., Eguchi K. and Hotamisligil G. S. (2015). Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Sci. Transl. Med. 7, 292ra98 10.1126/scitranslmed.aaa9134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann T., Wieghofer P., Müller P. F., Wolf Y., Varol D., Yona S., Brendecke S. M., Kierdorf K., Staszewski O., Datta M. et al. (2013). A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618-1626. 10.1038/nn.3531 [DOI] [PubMed] [Google Scholar]
- Harno E., Cottrell E. C. and White A. (2013). Metabolic pitfalls of CNS Cre-based technology. Cell Metab. 18, 21-28. 10.1016/j.cmet.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Hetz C. and Mollereau B. (2014). Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat. Rev. Neurosci. 15, 233-249. 10.1038/nrn3689 [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2006). Inflammation and metabolic disorders. Nature 444, 860-867. 10.1038/nature05485 [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2010). Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900-917. 10.1016/j.cell.2010.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi S., Aoyama T., Miura K., Osterreicher C. H., Kodama Y., Miyai K., Akira S., Brenner D. A. and Seki E. (2010). Disruption of TAK1 in hepatocytes causes hepatic injury, inflammation, fibrosis, and carcinogenesis. Proc. Natl. Acad. Sci. USA 107, 844-849. 10.1073/pnas.0909781107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T. and Peterson P. A. (1990). Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 9, 3153-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajino-Sakamoto R., Omori E., Nighot P. K., Blikslager A. T., Matsumoto K. and Ninomiya-Tsuji J. (2010). TGF-beta-activated kinase 1 signaling maintains intestinal integrity by preventing accumulation of reactive oxygen species in the intestinal epithelium. J. Immunol. 185, 4729-4737. 10.4049/jimmunol.0903587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamisuki S., Mao Q., Abu-Elheiga L., Gu Z., Kugimiya A., Kwon Y., Shinohara T., Kawazoe Y., Sato S.-I., Asakura K. et al. (2009). A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem. Biol. 16, 882-892. 10.1016/j.chembiol.2009.07.007 [DOI] [PubMed] [Google Scholar]
- Kim I., Xu W. and Reed J. C. (2008). Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 7, 1013-1030. 10.1038/nrd2755 [DOI] [PubMed] [Google Scholar]
- Liang H., Hippenmeyer S. and Ghashghaei H. T. (2012). A Nestin-cre transgenic mouse is insufficient for recombination in early embryonic neural progenitors. Biol. Open 1, 1200-1203. 10.1242/bio.20122287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Wootz H. and Korhonen L. (2006). ER stress and neurodegenerative diseases. Cell Death Differ. 13, 385-392. 10.1038/sj.cdd.4401778 [DOI] [PubMed] [Google Scholar]
- Liu J., Lee J., Salazar Hernandez M. A., Mazitschek R. and Ozcan U. (2015). Treatment of obesity with celastrol. Cell 161, 999-1011. 10.1016/j.cell.2015.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Lawrence D. A., Marsters S., Acosta-Alvear D., Kimmig P., Mendez A. S., Paton A. W., Paton J. C., Walter P. and Ashkenazi A. (2014). Opposing unfolded-protein-response signals converge on death receptor 5 to control apoptosis. Science 345, 98-101. 10.1126/science.1254312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaly S. R., Ninomiya-Tsuji J. and Morioka S. (2014). TAK1 control of cell death. Cell Death Differ. 21, 1667-1676. 10.1038/cdd.2014.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino T. and Kitakaze M. (2010). ER stress in cardiovascular disease. J. Mol. Cell. Cardiol. 48, 1105-1110. 10.1016/j.yjmcc.2009.10.026 [DOI] [PubMed] [Google Scholar]
- Morioka S., Inagaki M., Komatsu Y., Mishina Y., Matsumoto K. and Ninomiya-Tsuji J. (2012). TAK1 kinase signaling regulates embryonic angiogenesis by modulating endothelial cell survival and migration. Blood 120, 3846-3857. 10.1182/blood-2012-03-416198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S., Broglie P., Omori E., Ikeda Y., Takaesu G., Matsumoto K. and Ninomiya-Tsuji J. (2014). TAK1 kinase switches cell fate from apoptosis to necrosis following TNF stimulation. J. Cell Biol. 204, 607-623. 10.1083/jcb.201305070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka S., Sai K., Omori E., Ikeda Y., Matsumoto K. and Ninomiya-Tsuji J. (2016). TAK1 regulates hepatic lipid homeostasis through SREBP. Oncogene (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers M. G., Cowley M. A. and Münzberg H. (2008). Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 70, 537-556. 10.1146/annurev.physiol.70.113006.100707 [DOI] [PubMed] [Google Scholar]
- Neubert M., Ridder D. A., Bargiotas P., Akira S. and Schwaninger M. (2011). Acute inhibition of TAK1 protects against neuronal death in cerebral ischemia. Cell Death Differ. 18, 1521-1530. 10.1038/cdd.2011.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Kishimoto K., Hiyama A., Inoue J.-I., Cao Z. and Matsumoto K. (1999). The kinase TAK1 can activate the NIK-I kappaB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252-256. 10.1038/18465 [DOI] [PubMed] [Google Scholar]
- Omori E., Matsumoto K., Sanjo H., Sato S., Akira S., Smart R. C. and Ninomiya-Tsuji J. (2006). TAK1 is a master regulator of epidermal homeostasis involving skin inflammation and apoptosis. J. Biol. Chem. 281, 19610-19617. 10.1074/jbc.M603384200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omori E., Morioka S., Matsumoto K. and Ninomiya-Tsuji J. (2008). TAK1 regulates reactive oxygen species and cell death in keratinocytes, which is essential for skin integrity. J. Biol. Chem. 283, 26161-26168. 10.1074/jbc.M804513200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswal A. and Yeo G. (2010). Leptin and the control of body weight: a review of its diverse central targets, signaling mechanisms, and role in the pathogenesis of obesity. Obesity 18, 221-229. 10.1038/oby.2009.228 [DOI] [PubMed] [Google Scholar]
- Oyadomari S. and Mori M. (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381-389. 10.1038/sj.cdd.4401373 [DOI] [PubMed] [Google Scholar]
- Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Gorgun C. Z. and Hotamisligil G. S. (2006). Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137-1140. 10.1126/science.1128294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan L., Ergin A. S., Lu A., Chung J., Sarkar S., Nie D., Myers M. G. Jr and Ozcan U. (2009). Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35-51. 10.1016/j.cmet.2008.12.004 [DOI] [PubMed] [Google Scholar]
- Ron D. and Walter P. (2007). Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519-529. 10.1038/nrm2199 [DOI] [PubMed] [Google Scholar]
- Sabapathy K., Kallunki T., David J.-P., Graef I., Karin M. and Wagner E. F. (2001). c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J. Exp. Med. 193, 317-328. 10.1084/jem.193.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabio G., Cavanagh-Kyros J., Barrett T., Jung D. Y., Ko H. J., Ong H., Morel C., Mora A., Reilly J., Kim J. K. et al. (2010). Role of the hypothalamic-pituitary-thyroid axis in metabolic regulation by JNK1. Genes Dev. 24, 256-264. 10.1101/gad.1878510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H. (2012). Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol. Sci. 33, 522-530. 10.1016/j.tips.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Sato S., Sanjo H., Takeda K., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Matsumoto K., Takeuchi O. and Akira S. (2005). Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087-1095. 10.1038/ni1255 [DOI] [PubMed] [Google Scholar]
- Sato S., Sanjo H., Tsujimura T., Ninomiya-Tsuji J., Yamamoto M., Kawai T., Takeuchi O. and Akira S. (2006). TAK1 is indispensable for development of T cells and prevention of colitis by the generation of regulatory T cells. Int. Immunol. 18, 1405-1411. 10.1093/intimm/dxl082 [DOI] [PubMed] [Google Scholar]
- Schneeberger M., Dietrich M. O., Sebastian D., Imbernon M., Castano C., Garcia A., Esteban Y., Gonzalez-Franquesa A., Rodriguez I. C., Bortolozzi A. et al. (2013). Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172-187. 10.1016/j.cell.2013.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S., Prinz W. A., Thorn K. S., Voss C. and Walter P. (2009). Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 187, 525-536. 10.1083/jcb.200907074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimano H. (2001). Sterol regulatory element-binding proteins (SREBPs): transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 40, 439-452. 10.1016/S0163-7827(01)00010-8 [DOI] [PubMed] [Google Scholar]
- Sitia R. and Braakman I. (2003). Quality control in the endoplasmic reticulum protein factory. Nature 426, 891-894. 10.1038/nature02262 [DOI] [PubMed] [Google Scholar]
- Sriburi R., Jackowski S., Mori K. and Brewer J. W. (2004). XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35-41. 10.1083/jcb.200406136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I. and Ron D. (2011). Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat. Cell Biol. 13, 184-190. 10.1038/ncb0311-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler J. P., Yi C.-X., Schur E. A., Guyenet S. J., Hwang B. H., Dietrich M. O., Zhao X., Sarruf D. A., Izgur V., Maravilla K. R. et al. (2012). Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153-162. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronche F., Kellendonk C., Kretz O., Gass P., Anlag K., Orban P. C., Bock R., Klein R. and Schutz G. (1999). Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat. Genet. 23, 99-103. 10.1038/12703 [DOI] [PubMed] [Google Scholar]
- Tsaousidou E., Paeger L., Belgardt B. F., Pal M., Wunderlich C. M., Bronneke H., Collienne U., Hampel B., Wunderlich F. T., Schmidt-Supprian M. et al. (2014). Distinct roles for JNK and IKK activation in agouti-related peptide neurons in the development of obesity and insulin resistance. Cell Rep. 9, 1495-1506. 10.1016/j.celrep.2014.10.045 [DOI] [PubMed] [Google Scholar]
- Voeltz G. K., Rolls M. M. and Rapoport T. A. (2002). Structural organization of the endoplasmic reticulum. EMBO Rep. 3, 944-950. 10.1093/embo-reports/kvf202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin L., Saba-El-Leil M. K., Julien C., Fremin C. and Meloche S. (2010). Genetic demonstration of a redundant role of extracellular signal-regulated kinase 1 (ERK1) and ERK2 mitogen-activated protein kinases in promoting fibroblast proliferation. Mol. Cell. Biol. 30, 2918-2932. 10.1128/MCB.00131-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P. and Ron D. (2011). The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081-1086. 10.1126/science.1209038 [DOI] [PubMed] [Google Scholar]
- Wiest D. L., Burkhardt J. K., Hester S., Hortsch M., Meyer D. I. and Argon Y. (1990). Membrane biogenesis during B cell differentiation: most endoplasmic reticulum proteins are expressed coordinately. J. Cell Biol. 110, 1501-1511. 10.1083/jcb.110.5.1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won J. C., Jang P.-G., Namkoong C., Koh E. H., Kim S. K., Park J.-Y., Lee K.-U. and Kim M.-S. (2009). Central administration of an endoplasmic reticulum stress inducer inhibits the anorexigenic effects of leptin and insulin. Obesity 17, 1861-1865. 10.1038/oby.2009.194 [DOI] [PubMed] [Google Scholar]
- Woods S. C., Seeley R. J., Porte D. Jr and Schwartz M. W. (1998). Signals that regulate food intake and energy homeostasis. Science 280, 1378-1383. 10.1126/science.280.5368.1378 [DOI] [PubMed] [Google Scholar]
- Zhang K. and Kaufman R. J. (2008). From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455-462. 10.1038/nature07203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Zhang G., Zhang H., Karin M., Bai H. and Cai D. (2008). Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61-73. 10.1016/j.cell.2008.07.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Hu Y., Sun Q., Zhao J., Cong Z., Liu H., Zhou M., Li K. and Hang C. (2013). Inhibition of transforming growth factor beta-activated kinase 1 confers neuroprotection after traumatic brain injury in rats. Neuroscience 238, 209-217. 10.1016/j.neuroscience.2013.02.022 [DOI] [PubMed] [Google Scholar]
- Zimmerman L., Lendahl U., Cunningham M., McKay R., Parr B., Gavin B., Mann J., Vassileva G. and McMahon A. (1994). Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 12, 11-24. 10.1016/0896-6273(94)90148-1 [DOI] [PubMed] [Google Scholar]