Abstract
Cardiac delayed rectifier potassium channels conduct outward potassium currents during the plateau phase of action potentials and play pivotal roles in cardiac repolarization. These include IKs, IKr and the atrial specific IKur channels. In this chapter, we will review the molecular identities and biophysical properties of these channels. Mutations in the genes encoding delayed rectifiers lead to loss- or gain-of-function phenotypes, disrupt normal cardiac repolarization and result in various cardiac rhythm disorders, including congenital Long QT Syndrome, Short QT Syndrome and familial atrial fibrillation. We will also discuss the possibility and prospect of using delayed rectifier channels as therapeutic targets to manage cardiac arrhythmia.
Keywords: delayed rectifiers, IKs, IKr, IKur, Long QT Syndrome, Short QT Syndrome, atrial fibrillation
I. Delayed rectifiers in the heart: IKs, IKr and IKur
Cardiac action potentials are characterized by an initial depolarization followed by a prolonged depolarization, or plateau phase, before a return to the resting potential. In these cells, sodium channels provide large inward currents that drive rapid depolarization (phase 0) followed by subsequent minor repolarization (phase 1) resulting from transition of sodium channels into a non-conducting inactivated state, as well as activation of transient outward potassium currents (Ito). The plateau phase of the action potential, a period in which membrane potentials become relatively stable for up to several hundred milliseconds, follows. During the plateau phase (phase 2), calcium entry via L-type calcium channels triggers contraction. Counter-balancing the calcium influx, potassium ions pass through the membrane in the outward direction: the plateau phase is thus a balance of inward and outward currents. Unlike the Ito currents that terminate quickly, the slower potassium channel currents persist during the plateau phase, contribute to the repolarization of the cell and eventually terminate the action potential (phase 3) as the balance of currents tips in the outward direction [1]. (Figure 1) Early electrophysiologists noticed such outward potassium conductance that lasts throughout the plateau phase, and referred to these currents as “delayed rectifier” currents. The delayed rectifiers, in concert with other ion channels, essentially determine the waveform as well as action potential duration (APD), and thus play critical roles in cardiac physiology and pathophysiology. Disruption of the normal functions of delayed rectifier channels renders the heart susceptible to abnormal electrical activity, and ultimately predisposes the heart to arrhythmia.
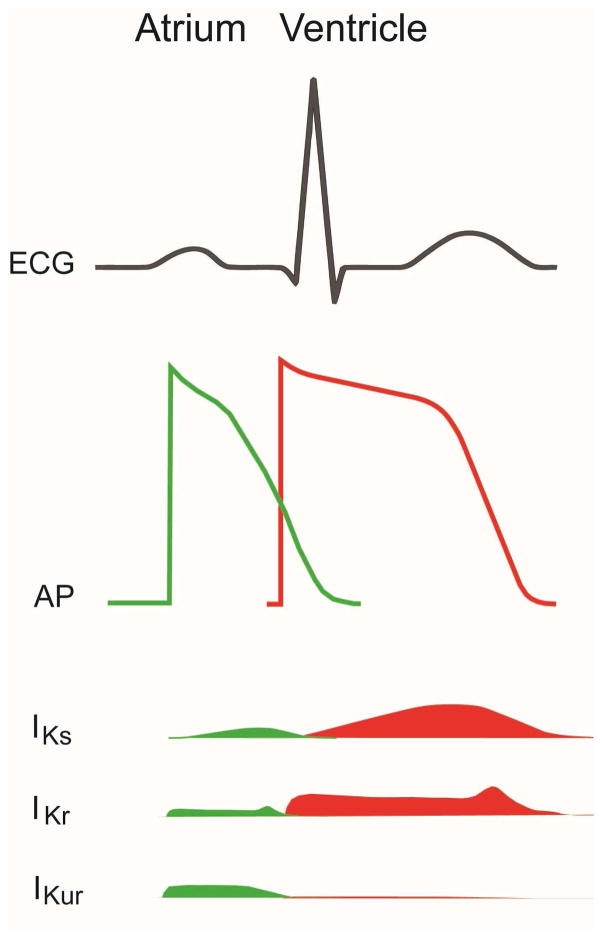
Figure 1.
Schematics of waveforms of ECG, cardiac action potentials and delayed rectifier potassium currents. Top panel: ECG waveforms showing P wave (atrial depolarization), QRS complex (ventricle depolarization) and T wave (ventricle repolarization). Middle panel: cardiac action potential from atrium (green) and ventricle (red). Lower panels: delayed rectifier currents from IKs, IKr and IKur during cardiac repolarization and their tissue (atrial in green and ventricle in red) distributions.
The identities of the delayed rectifiers, however, remained elusive because of a lack of proper pharmacological tools and knowledge of ion channels as functional proteins in cell membrane. It was not until 1969 that Noble and Tsien, using an elegant quantitative approach, demonstrated the existence of two distinct components (which they called Ix1 and Ix2) of the outward currents in the plateau potentials in cardiac Purkenje fibers [2, 3]. The two components differ mainly in activation kinetics, one rapid and one slow. Noble and Tsien’s formalism was disputed by some investigators based on technical issues with the preparations used and the basis of delayed rectification remained controversial. Twenty years later, Sanguinetti and Jurkiewicz confirmed Noble and Tsien’s seminal findings by pharmacologically separating the two components with the use of E4031, a benzenesulfonamide antiarrhythmic agent that selectively blocked the rapid component, which they named IKr accordingly. The remaining slow component was given the name IKs [4]. The terms IKs and IKr have since been widely used to describe the delayed rectifier currents in cardiac myocytes of various species [4–10]. In the atria, an additional ultrarapid component IKur, is prominent during the plateau phase [11–15].
The rapid progress in molecular biology and genetics in the 90’s resulted in the discoveries of the molecular identities and architecture of IKr and IKs channel complexes, defined previously only through pharmacology. Remarkably, these discoveries were all associated with studies of the congenital Long QT Syndromes (LQTS), cardiac rhythm disorders caused by mutations in genes coding for ion channels or channel-associated proteins with a common functional phenotype: prolongation of cellular APD and QT interval of the electrocardiogram. Loss of function mutations of the two prominent delayed rectifiers IKs and IKr with dysfunctional trafficking or channel gating properties have been shown to cause congenital LQTS, underscoring the critical importance of the delayed rectifiers in cardiac physiology.
IKs, IKr, and IKur channels all fall into the superfamily of voltage-gated potassium channels. These channels are formed from tetramers of membrane-spanning proteins that possess a voltage sensing domain (VSD) as well as a pore domain. IKs channels are activated slowly by depolarizing voltages during the plateau phase. The pore-forming subunit is KCNQ1 (also known as KvLQT1 or Kv7.1), first identified by positional cloning and its linkage to LQTS variant 1 (LQT1) [16]. Like other voltage-gated potassium channel, it has six transmembrane domains and is composed of a voltage-sensing domain (S1–4) and a pore domain (S5 and S6) as well as the intracellular N- and C-termini. Four such subunits form a functional channel. However it is KCNE1 (also known as minK), a single transmembrane protein [17], that co-assembles with KCNQ1 and imparts to the KCNQ1 channel its unique slow kinetics similar to that of the native IKs recorded in cardiac myocytes [18, 19]. Thus, the physiologically relevant activity of the IKs channel requires co-assembly of both KCNQ1 and KCNE1. Like KCNQ1, mutations in KCNE1 are also associated with LQTS variant 5 (LQT5) [20, 21]. IKr currents are conducted by hERG channels (also known as Kv11.1 or KCNH2) [22], which was first cloned in the brain as a homolog of the Drosophila “ether-a-go-go” (EAG) potassium channel [23], and was later shown to link to LQTS variant 2 (LQT2) [24]. Similar to IKs channels, the hERG channel is a tetramer of four identical subunits, each with 6 transmembrane domains that form the VSD and the pore. MiRP1 (or KCNE2), a single transmembrane protein homologous to KCNE1, was shown to associate with HERG channels and alter it biophysical properties, and was linked to LQTS variant 6 (LQT6) [25]. However, the exact role of MiRP1 in the molecular composition of IKr channels is disputed [26]. IKur channels have more recently been identified to be composed of Kv1.5 alpha subunits [27, 28] encoded by KCNA5 and are a major contributor to atrial repolarization. (Figure 2)
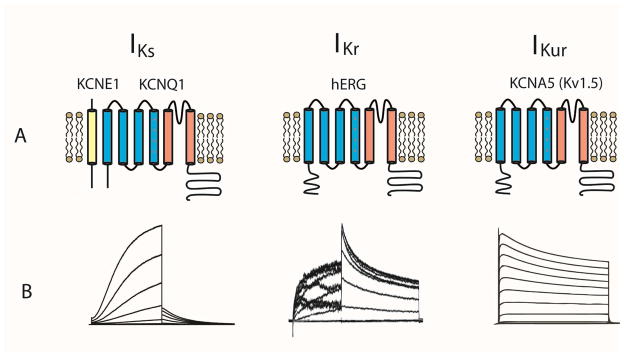
Figure 2.
Cardiac delayed rectifier channels include IKs, IKr and the atrial specific IKur channels and are all voltage-gated potassium channels. Panel A: molecular composition and architecture of cardiac delayed rectifier channels. IKs channels are composed of pore-forming KCNQ1 and auxiliary KCNE1 subunits. IKr channels are composed of hERG subunits. IKur channels are composed of KCNA5 (Kv1.5) subunits. Each α–subunit has six transmembrane helices (S1–6). S1–4 is the voltage sensing domain with positively charged residues on S4 as the voltage sensor. S5–6 is the pore domain that conducts ionic currents. Panel B: recordings of current voltage relationships of IKs, IKr and IKur channels. IKur current traces. Adapted from Decher, N., et al., Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. J Biol Chem, 2004. 279(1): p. 394–400; with permission.
While both are activated by depolarizing voltages during the plateau phase of cardiac action potentials, IKs and IKr channels differ biophysically in many respects [4]. Their different functions can be studied using selective blockers for these currents, E4031 for IKr [4] and Chromanol 293B for IKs [29]. IKr plays the largest role in repolarization in normal conditions and is characterized by prominent inward rectification caused by voltage dependent inactivation. That is, at more positive potentials during the plateau phase, the channels are inactivated and conduct smaller outward current. As repolarization progresses, IKr channels recover from inactivation and produce a large resurgent outward current that repolarizes the membrane potential. IKs channels, however, have little inactivation and activate slowly and gradually impact cellular repolarization (Figure 2). The role of IKs is heightened under certain stressors including adrenergic stimulation wherein IKs currents are larger, more rapidly activate, and slowly deactivate to allow for shortened action potentials that can maintain adequate diastolic filling times even in the face of accelerated heart rate. Under conditions of IKr blockade, the role of IKs also increases and can become the largest contributor to cellular repolarization. Together these channels are critical determinants of the duration and morphology of cardiac action potentials, and consequently impact the QT interval measured in the electrocardiogram (ECG). Inhibition of these channels resulting from either inherited mutations or drug block can lead to Long QT Syndrome associated with increased risk of life threatening arrhythmias.
Following the groundbreaking discoveries of the roles of IKr and IKs in LQTS, it has now become clear that these channels also underlie other cardiac rhythm disorders such as short QT Syndrome (SQTS) and familial atrial fibrillation [30–33]. In recent years, genome wide association studies (GWAS) have revealed that KCNQ1, KCNE1 and hERG were among the common variant loci associated with QT-interval variations [34, 35], thus highlighting again the significance of delayed rectifier channels in cardiac function. The search for precise pharmacological regulation of these channels has now expanded in seeking therapeutic approaches to manage a wide range of clinical disorders.
II. Delayed rectifiers and cardiac rhythm disorders
1. Congenital and Acquired Long QT Syndromes
Congenital LQTS are genetic cardiac rhythm disorders of a common clinical phenotype: delayed repolarization which manifests on body surface ECG as prolonged QT intervals. As a result, abnormal electrical activity such as early afterdepolarizations (EAD) can trigger severe ventricular tachycardia (very often in the form of Torsade de Pointes, or TdP), leading to syncope or sudden death. Clinically, congenital LQTS includes the autosomal recessive Romano Ward Syndrome, which affects approximately 1 in 7000 people, and the very rare Jervell and Lange-Nielsen syndrome, which is accompanied by hearing loss and is autosomal dominant. Most cases of congenital LQTS are caused by loss of function mutations in either KCNQ1 or hERG channels (LQT1 and LQT2, respectively). Less frequent but with marked severity is LQT3, which is due to gain of function (due to impaired inactivation) mutations in SCN5A, the gene coding for the Nav1.5 cardiac sodium channel alpha subunit. Rare genotypes include mutations in KCNE1, inward rectifiers, calcium channels, adaptor protein AKAP9 etc [36–44]. LQTS may also be induced by drugs. In this case, it is the hERG channels that are most often blocked. In the following sections, we will discuss the different types of congenital LQTs caused by dysfunctional delayed rectifier channels and their associating binding partners, including KCNE1 and AKAP9, as well drug induced LQTS caused by hERG block.
KCNQ1 and LQT1
Loss-of-function KCNQ1 mutations contribute to 42–49% of all long QT genotypes [45, 46]. At the cellular level, mutations exert negative impact on IKs channel function through various mechanisms [47, 48]. Some mutations may cause reduction in current densities at physiologically relevant voltages through biophysical effects on the gating of IKs [49]. Deficiency in membrane trafficking of mutant channel subunits is a common mechanism and may lead to fewer functional channels on the cell surface and in turn a reduction of repolarization reserve [50–54]. Mutations may also affect IKs channel tetramerization and assembly [55]. Some mutations occur at sites critical for interaction with key molecules involved in functional modulation. For example, clusters of basic residues (R190, R195, R243, H258, R259, K352, R360 and K362) located in the intracellular linker or proximal C-terminus are critical for PIP2 regulation. Mutations of most of them are associated with LQT1 [48, 56–58]. A leucine zipper motif located in the KCNQ1 C-terminus is responsible for co-assembly with the adaptor molecule Yotiao (also known as AKAP9) that recruits PKA and protein phosphatase 1 (PP1) to the IKs macromolecular complex. Thus this macromolecular complex allows for precise and rapid adrenergic modulation of the channel. Mutation within the leucine zipper at residue G589 was shown to minimize PKA-dependent phosphorylation of the IKs channel and is linked to LQT1 [59]. Unravelling this pathway provided mechanistic insight into the known risk of cardiac events of LQT1 patients during exercise, when sympathetic nerve activity is elevated. When co-expressed with wild type KCNQ1 to mimic the heterogeneity of LQT1, some mutants show a propensity for a dominant negative effect, while others do not affect the function of wild type channels [51, 60]. Not surprisingly, patients who carry dominant negative mutations (functional expression reduced >50%) show longer QTc and significantly higher risk for cardiac events, compared with those who carry mutations with haploinsufficiency phenotypes (functional expression reduced <50%) [61].
The locations of mutations on KCNQ1 correlate with clinical phenotypes and therapeutic responses. To date, more than 200 genetic variations have been identified in KCNQ1 to cause LQT1. LQT1 mutations have been found in the KCNQ1 C-terminus (32%), pore (29%, S5–6), the intracellular linkers (20%), voltage-sensing domain (11%), extracellular link (6%) and N-terminus (2%) (Figure 3A) [62]. In two separate studies, Shimizu et al [63] and Moss et al [61] found that cumulative probability of cardiac events was significantly higher in patients who carry mutations in the KCNQ1 transmembrane domains than those who carry mutations in the C-terminus (Figure 3B). Patients with missense mutation in the cytoplasmic loops of KCNQ1 exhibit the highest risks for cardiac events and the best response to β–blockers [64].
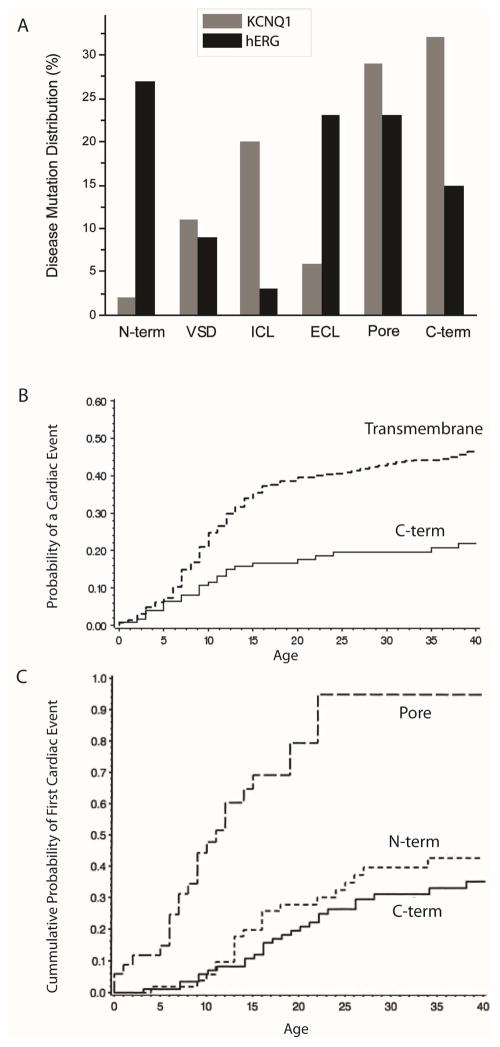
Figure 3.
A. Locations of LQTS mutations in KCNQ1 (grey bars) and hERG (black bars). N-term: N-terminus; VSD: voltage-sensing domain (S1–4); ICL: intracellular loops; ECL: extracellular loops; Pore: channel pore (S5–6); C-term: C-terminus. Data from Jackson et al (BMC Evol Biol 2008) [62]. B. Comparison of probability of a cardiac event in LQT1 patients with mutations in KCNQ1 transmembrane domain and C-terminus. Adapted from Moss et al (Circulation 2007) [61]. C. Comparison of cumulative probability of first cardiac event in LQT2 patients with mutations in hERG pore domain, N- and C-terminus. Adapted from Moss, A.J., et al., Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation, 2002. 105(7): p. 794–9; with permission.
KCNE1 and LQT 5
KCNE1 co-assembles with KCNQ1 and alters the biophysical properties of the channel profoundly. It slows both activation and deactivation kinetic, shifts activation voltage dependence in the positive direction and significantly increases current amplitude [18, 19]. Co-expression of KCNQ1 and KCNE1 results in functional channels with biophysical properties similar to the native IKs channels [18, 19]. Loss of function mutations of KCNE1 lead to congenital LQTS variant 5 (LQT5) in the forms of both Romano-Ward and Jervell and Lange-Nielson Syndromes [21], albeit at much lower prevalence (1.7–3% of all LQTS) [45, 46] compared with LQT1, LQT2 and LQT3. About twenty KCNE1 mutations have been identified to cause LQT5. The majority of them are located in either the transmembrane domain or the cytoplasmic C-terminus [65]. At cellular level, LQT5 disease pathogenesis involves various mechanisms. For example, KCNE1 L51H, a JLN mutation, does not express on the cell membrane, and does not functionally interact with KCNQ1. Other KCNE1 mutations, such as V47F, D76N and W87R, undergo normal trafficking to the cell membrane, but exert distinct impacts on co-expressed KCNQ1: both V47F and W87R functionally interact with KCNQ1 and alter IKs gating, while D76N is a dominant negative mutation that severely suppresses current amplitude [66, 67]. Seebohm et al found that serum- and glucocorticoid-inducible kinase 1 (SGK1) upregulate IKs currents by facilitating exocytosis of KCNQ1 channel in a RAB-11 dependent manner. KCNE1 D76N disrupts this mechanism and results in lower functional expression of IKs current [68].
Much progress has been made in determining the mechanisms underlying KCNE1 effects on the biophysical properties of KCNQ1. KCNE1 makes extensive contact with different parts of the KCNQ1 channel, including the pore domain [69–72], extracellular domain [73–75] and the voltage sensing domain [76]. The physiologically critical stoichiometry of KCNQ1 and KCNE1 remains to be confirmed. KCNQ1/KCNE1 ratios of 4:2 and 4:4 [77–80] have been reported. KCNE1 has been shown to alter the movement of the KCNQ1 voltage sensor using various methods, such as substituted cysteine accessibility methods [73] and voltage clamp fluorometry [81–83].
AKAP9 and LQT11
LQT1 patients are more likely to experience cardiac events (68% of all events) during exercise or periods of elevated sympathetic nervous system activity [84]. The autonomic modulation of delayed rectifier potassium channel and its potential roles in the etiology of LQTs have long been recognized. Kass and Walsh first reported that the IKs channel is regulated by PKA at physiological temperatures [85]. Marx et al later demonstrated that such regulation requires Yotiao (or AKAP9), an A-kinase anchoring protein, which functions as an adaptor that presents PKA and PP1 to the IKs channel so that the channel phosphorylation state can be finely controlled by adrenergic pathways[59]. Subsequent studies have revealed that AKAP9 plays a central role in regulating the cAMP/PKA levels in the compartmentalized microenvironment surrounding the IKs channels [86, 87]. AKAP9 coordinates the actions of two pairs of enzymes: PKA and protein phosphatase 1 (PP1) that phosphorylate or dephosphorylate the channel, respectively, and adenylyl cyclase and phosphodiesterase that synthesize or hydrolyze cAMP, respectively, which in turn stimulate PKA [88]. As a result, the phosphorylation level of IKs channels is tightly regulated by the macromolecular signaling complex anchored by AKAP9. During sympathetic activation, which increases heart rate subsequent to physical or emotional stressors, IKs channel activity is enhanced by PKA to shorten QTc accordingly and to allow for sufficient diastolic filling time as heart rate increases. LQT11 is an LQTS variant associated with mutations in AKAP9. A role of AKAP9 in LQTS was discovered in a study of a case of a suspected LQTS patient who was found to carry a mutation (S1570L) located in a region on AKAP9 critical for interaction with IKs channels. The proband’s family members were also found to carry the same mutation and had a history of LQTS. In vitro analysis showed that this AKAP9 mutation reduced the response of IKs channels to cAMP and associate with LQT [89], and thus, like LQT1 and LQT5, arrhythmia risk is increased during exercise. A recent study suggested that AKAP9 is a genetic modifier of QT intervals in people who carry the LQT1 mutation, confirming the role of AKAP9 in cardiac electrophysiology [90].
hERG and LQT2
39–45% of all LQTS have been attributed to loss of function mutations in HERG [45, 46]. To date, nearly 500 hERG variations have been found to associate with LQT2 [91]. Both haploinsufficiency and dominant negative phenotypes have been found in hERG mutants. Deletion mutants such as ΔI500-F508, or frameshift mutants such as Δbp1261, do not form functional channels by themselves and do not co-assemble with wild type subunits. As a result, hERG channel expression is reduced drastically (haploinsuffienciency) as only tetramers of the remaining wild-type channels can pass current. On the other hand, some missense mutations such as N470D, A561V and G628S suppress the function of wild type channel, causing dominant negative effects [92]. At the molecular and cellular level, many LQT2 mutations in hERG lead to reduction in membrane trafficking associated with protein misfolding, ER retention and subsequent protein degradation [93–95]. A recent comprehensive analysis confirmed that trafficking deficiency contributes to loss of function phenotypes for the majority of LQT2 mutations in hERG [91]. Therefore, pharmacological agents such as thapsigargin [96] and fexofenadine [97] that restore normal trafficking offer a potential therapy for LQT2 [98–101]. While trafficking defects are common, LQT2 mutations may also reduce total IKr by affecting channel gating. For example, G584S was shown to enhance inactivation [102] whereas a series of mutations (F29L, N33T, G53R, R56Q, C66G, H70R, A78P, L86R) [103] in the HERG N-terminal PAS domain [104], a hotspot for channel regulation and LQT2 mutations, was shown to accelerate deactivation. Mutations that affect both gating and trafficking have also been reported [105]. Loss-of-function can also occur as a consequence of altered gene transcription [106, 107]. Whatever the underlying mechanism, loss of IKr contributes to a reduction in net outward current during the repolarization phase of cardiac action potentials, and hence QT prolongation in the ECG.
Compared to LQT1 mutations that are largely clustered in the KCNQ1 pore, intracellular linker and C-terminus, LQT2 mutations are more widely distributed throughout the protein with a significant presence in the extracellular linker and the N-terminus. 21% of all LQT2 mutations were found in the N-terminal PAS domain, 23% in the pore region, 23% in the extracellular linkers and 15% in the C-terminus (Figure 3A) [62]. Locations of the mutations not only correlate with cellular phenotypes, but also clinical risks. Most pore mutations exert dominant negative effects on wild type channels [91]. As such, patients with missense mutations in the channel pore region (S5-pore-S6) carry the highest risk of arrhythmia (Figure 3C) [108, 109].
hERG and drug induced LQT
A significant number of patients (estimates range from 2–8.8%) who receive anti-arrhythmic agents develop drug–induced arrhythmia [110]. This problem is most pronounced with the use of Class III anti-arrhythmic drugs such as dofetilide, E-4031, and MK-499 [111–113]. These drugs are known to block hERG channels with a high affinity thus causing drug-induced loss of hERG channel function. The QT interval prolongation that results [114, 115] predisposes patients to ventricular arrhythmia, including TdP, in a manner that parallels clinical manifestation of congenital LQTS. It is now abundantly clear that drug-induced arrhythmia is not limited to anti-arrhythmic agents [110]. As examples, drugs including antibiotics (such as clarithromycin, erhthromycn), antipsychotics (such as chloropromazine, haloperidol, pimozide, thioridazine), antihistamines (such as astemizole, terfenadine) and gastric prokinetics (such as cisapride) have all been linked to hERG block and TdP [116]. Because of the widespread off target inhibition of hERG channels, virtually all drug development programs must include testing for hERG channel inhibition over drug concentrations that are proposed for clinical regimens.
Reminiscent of delayed rectifier blockade by quaternary ammonium described by Armstrong [117], most of the HERG blockers require an open channel to gain access to the channel pore [111, 114, 118, 119]. The binding of the blockers to their receptor sites inside the channel pore is state dependent: blockers bind to the open channels and dissociate from the closed channel. However, the closing of the channel gates may trap the dissociated blockers inside the closed channels, if the size of the blocker is small enough to fit the limited space of the inner cavities of the channel. Such “drug trapping” causes extremely slow recovery from channel block [114, 118]. Mitcheson et al observed that MK499, a methanesulphonanilide derivative with size too large to fit the cavity of Shaker and KcsA channels, was trapped inside closed hERG, suggesting that the inner cavity of hERG is probably larger than that of Shaker or KcsA [120]. The larger cavity is one potential reason for the unique susceptibility of hERG to blockade by small molecules. Multiple receptors sites for hERG blockers have been determined and are all located on the S6: a pair of polar residues (Thr 623 and Ser 624) sit just below the selectivity filter and a pair of aromatic residues (Tyr 652 and Phe 656) in the lower part of the S6 helix [121–123]. Apart from direct channel block, some drugs may also cause inhibition of membrane trafficking of hERG subunits [124, 125]. Arsenic trioxide [126], geldanamycin [127], pentamidine [128, 129] and probucol [130, 131] have been shown to inhibit trafficking without causing channel block. Other reports have also suggested that some previously described hERG blockers may also cause additional inhibition of trafficking and are thus termed dual inhibitors [124].
2. Short QT Syndrome
Algra et al first noticed that patients with shortened QT interval (<400ms) had risks of sudden deaths as high as those with prolonged QT [132]. Since then, cases of sporadic and familial Short QT Syndromes (SQTS) have been reported [133–135]. Current diagnostic criteria for short QT is 330–370 ms (QTc) [136, 137]. Patients with SQTS may develop atrial fibrillation, syncope, polymorphic VT, VF and sudden death. To date, four gain-of-function mutations of hERG have been reported to cause SQTS variant 1 (SQT1). Both N558K [138, 139] and T618I [140] cause reduction in hERG inactivation, abolishes rectification and subsequently produces greater current during the plateau phase of cardiac action potentials. R1135H causes slower deactivation, which in turn may cause greater “resurgent “current during phase 3 of action potential and results in shortened repolarization. An ECG of patient with R1135H showed not only shortened QT, but also a Brugada pattern [141, 142]. I560T significantly increased peak current density and shifted inactivation curve to a more positive voltage range [143]. Mutations in KCNQ1 are associated with SQTS variant 2 (SQT2) and lead to gain of function of IKs by multiple mechanisms. For example, V307L accelerated activation kinetics and left-shifted voltage dependent activation thus resulting in gain of function [144]. The F279I mutation was shown to decrease the association with KCNE1, and thus accelerate activation and left shift voltage dependent activation [145]. Also, R259H increased current density and caused slower deactivation and faster activation [146]. Drugs that reduce outward potassium currents, such as hydroquinidine [147, 148], dysopyramide [149, 150] and propafenone [151] have been shown effective for SQT1.
3. Familial atrial fibrilation
Familial aggregation is common among patients with idiopathic atrial fibrillation (or lone atrial fibrillation) without underlying cardiovascular diseases. Up to 30% of these patients have a positive family history [30, 152–155]. The relative risk of atrial fibrillation (AF) is significantly higher in individuals with a positive family history [156]. These findings suggest a strong genetic predisposition for familial AF. Mutations in all three delayed rectifier channels (IKs, IKr and IKur) have been associated with familial AF in single gene Mendelian fashion [155, 157–172]. (see Table I for a list of AF mutations identified in KCNQ1, hERG and Kv1.5) Followed by the first report of S140G mutation in KCNQ1 [159] in 2003, multiple mutations of KCNQ1 have been identified to associate with AF. Most of them are gain-of-function mutations that are located in the voltage sensing domain and patients have both familial AF and SQTS. The biophysical properties of KCNQ1 S140G and V141M have been studied extensively. The two affected residues are located in the S1 domain. Both mutations cause extremely slow deactivation and negative shift of voltage–dependent activation [160]. The mutant channels slow deactivation such that insufficient time passes during diastolic intervals for channels to fully deactivate. Over a series of beats this results in a high percentage of channels accumulating in the open state leading to a large, seemingly time-independent outward current. Most of the identified gain-of-function KCNQ1 mutations associated with AF show similar slowed deactivation kinetics to various degrees [163–165, 167, 168]. Intriguingly, while the majority of AF mutations identified in KCNQ1 demonstrate gain-of -function, a few loss-of-function mutations have also been found. For example, A302V is retained in the ER, conducts very small current [158] and was previously shown as a LQT mutation [173]. Two hERG mutations, N588K [138, 174] and K897T [169], have also been shown to associate with AF.
Table I.
List of AF mutations identified in KCNQ1, hERG and Kv1.5 channels.
| channel | mutation | location | function | Clinical phenotype | reference |
|---|---|---|---|---|---|
| KCNQ1 | A46T | N | gain | normal or long QT, AF | [158] |
| S140G | S1 | gain | normal or long QT, AF | [159, 160] | |
| V141M | S1 | gain | short QT, AF | [160, 161] | |
| Q147R | S1 | loss | long QT, AF | [162] | |
| R195W | intracellular | gain | normal or long QT, AF | [158] | |
| S209P | S3 | gain | normal QT, AF | [163] | |
| G229D | S4 | gain | normal QT, AF | [164] | |
| R231C | S4 | gain | normal or long QT, AF | [165, 166] | |
| R231H | S4 | gain | normal or long QT, AF | [167] | |
| V241F | S4 | gain | normal QT, AF | [168] | |
| A302V | pore | loss | long QT, AF | [158] | |
| R670K | C | gain | normal QT, AF | [158] | |
| HERG | N588K | pore | gain | short QT, AF | [138, 174] |
| K897T | C | loss/gain | long or short QT, AF | [169] | |
| Kv1.5 | 71-81del | N | loss | AF | [155] |
| E48G | N | gain | AF | [170] | |
| Y155C | N | loss | AF | [170] | |
| A305T | S1–2 | gain | AF | [170] | |
| D322H | S1–2 | gain | AF | [170] | |
| E375X | S4 | loss | AF | [171] | |
| D469E | pore | loss | AF | [170] | |
| P488S | pore | loss | AF | [170] | |
| T527M | pore | loss | AF | [172] | |
| A576V | C | loss | AF | [172] | |
| E610K | C | loss | AF | [172] |
More recently, more than ten mutations in Kv1.5 (KCNA5), the alpha subunit of the atrial specific IKur channel, have been identified in familial and idiopathic AF patients [155, 170–172]. Olsen et al first reported in 2006 that a mutation (E375X) in the S4 domain led to truncation and loss-of –function of Kv1.5 and was associated with idiopathic AF [171]. Yang et al identified an 11 amino acid residue deletion in Kv1.5 in two probands with strong family history of AF. The mutant channel conducted smaller currents. However the deletion occurred in a functional motif for tyrosine kinase signaling and rendered the channel insensitive to Src tyrosine kinase regulation important for stretch response. This may disrupt the physiological response to stretch and result in shortening of APD [155]. In a recent study, a series of non-synonymous mutations (E48G, Y155C, A305T, D322H, D469E and P488S) were identified in patients with lone AF. These include both gain-of –function (E48G, A305T and D322H) and loss-of-function ((Y155C, D469E and P488S) mutations that are distributed on all major functional domains of Kv1.5 [170]. Conceptually, increased function of potassium channels may lead to shortening of the effective refractory period (ERP) and increased propensity for re-entrant arrhythmia such as AF [175]. This supports the findings that most of the mutations found in AF patients showed gain of function phenotype. On the other hand, recent studies have revealed that multiple loss-of-function mutations in both IKs and IKur channels are linked to AF. The exact mechanism is still unclear. It was shown that Kv1.5 dysfunction in human atrial myocyte caused prolonged APD associated with a propensity of early after depolarizations and rendered the atria prone to arrhythmogenesis [171].
III. Delayed rectifier channels as therapeutic targets for cardiac arrhythmia
Given their pivotal roles in cardiac repolarization as well as various rhythm disorders, delayed rectifier channels have long been considered promising therapeutic targets for cardiac arrhythmia. Class III antiarrhythmic agents such as amiodarone, sotalol, and dronedarone primarily block these channels and prolong APD. These drugs are used for both ventricular and atrial arrhythmias. Activators of both hERG and KCNQ1 channels have been sought after as potential therapies to correct LQT. Several molecules have been identified to up-regulate hERG or KCNQ1 function [176], but their clinical efficacies have yet to be confirmed.
More recently, pharmaceutical companies have attempted to develop atrial specific therapy for AF using Kv1.5 as target. The primary pharmacological approach to treat re-entrant arrhythmia such as AF is to stop the re-entry circuit by lengthening the effective refractory period (ERP), which is essentially determined by APD [175]. Blockers of delayed rectifiers prolong APD and are thus common therapy for AF. Clinically, sotalol (IKr blocker) and amiodarone (multichannel blocker) are effective agents that are able to convert AF to and maintain sinus rhythm [177, 178]. However, blocking delayed rectifier channels such as IKr that are expressed in the ventricles may cause drug-induced LQTS, TdP and mortality [179]. As a result, development of atrial specific therapy for AF has received much attention in recent years. Because of its unique atrial specific expression, Kv1.5 is an ideal pharmacological target for AF treatment that may avoid the undesired proarrhythmic activities [180]. Numerous Kv1.5 blockers have been in development, but to date, most of them have failed to reach clinical trials for various reasons [181]. Vernakalant developed by Cardiome has been shown effective to convert acute onset AF to sinus rhythm and was approved in Europe. Vernakalant inhibits two atrial specific potassium channels, IKur (Kv1.5) and IKAch (Kir3.1/3.4) at low concentration, but is able to block Ito and INa at higher concentration [182]. Recent study suggests its efficacy is likely due to its rapid unbinding sodium channel blockade [183]. XEN-D0101 developed by Xention is another selective Kv1.5 inhibitor [184] and was show effective for AF in animal models [176].
IV. Summary and Future Directions
Delayed rectifier channels conduct outward currents during the plateau phase of cardiac action potentials and play critical roles in the timing of cardiac repolarization. Extensive studies on the biophysical properties of these channels closely combined with molecular genetics and clinical medicine have established a clear “central dogma” that causally connects dysfunctional ion channels, abnormal repolarization and arrhythmia. This is especially true in the field of congenital LQTS where a large body of literature describing hundreds of mutations in the IKs and IKr channels as well as their cellular and clinical phenotypes. In recent years, new evidence has emerged to associate mutations in these channels with other inherited cardiac rhythm disorders, such as familial fibrillation and short QT syndromes. In these disorders, many questions remain unanswered. For example, while most KCNQ1 mutations associated with AF cause gain of function, a few loss-of-function mutations have been identified in familial AF patients. It is curious that both gain- and loss-of-function mutations in the same channel may result in the same type of arrhythmia. Overlap in clinical phenotypes is another intriguing phenomenon. For example, HERG R1135H was first identified in a SQTS patient, whose ECG also showed a Brugada pattern [141, 142, 185]. To understand these questions, it is imperative to understand the functional consequences of gene mutations in multiple systems that include, but are not limited to heterologous expression systems, animal models, and the use of patient-specific induced pluripotent stem cells. Great challenges also remain in the search for safe therapy of cardiac arrhythmia using delayed rectifiers as targets. Despite the effort to develop channel specific or region specific therapies, proarrhythmic activity still poses major hurdles and causes major safety concerns. With growing knowledge of potassium channel structure-function relationships, the application of high-throughput screening using automated patch clamp system, and expanded roles of human induced pluripotent stem cell-derived myocytes as pharmacological profiling platforms, we expect more molecules may emerge to modulate channel function with better specificity and safety profiles.
Key points.
Cardiac delayed rectifier potassium channels conduct outward potassium currents during the plateau phase of action potentials and play pivotal roles in cardiac repolarization.
The rapid progress in molecular biology and genetics in the 90’s resulted in the discoveries of the molecular identities and architecture of IKr and IKs channel complexes.
Inherited mutations or drug block of the delayed rectifier channels cause cardiac arrhythmias.
Delayed rectifier potassium channels may be used as therapeutic targets.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85(4):1205–53. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 2.Noble D, Tsien RW. Reconstruction of the repolarization process in cardiac Purkinje fibres based on voltage clamp measurements of membrane current. J Physiol. 1969;200(1):233–54. doi: 10.1113/jphysiol.1969.sp008690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noble D, Tsien RW. Outward membrane currents activated in the plateau range of potentials in cardiac Purkinje fibres. J Physiol. 1969;200(1):205–31. doi: 10.1113/jphysiol.1969.sp008689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III antiarrhythmic agents. J Gen Physiol. 1990;96(1):195–215. doi: 10.1085/jgp.96.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Z, Fermini B, Nattel S. Rapid and slow components of delayed rectifier current in human atrial myocytes. Cardiovasc Res. 1994;28(10):1540–6. doi: 10.1093/cvr/28.10.1540. [DOI] [PubMed] [Google Scholar]
- 6.Liu DW, Antzelevitch C. Characteristics of the delayed rectifier current (IKr and IKs) in canine ventricular epicardial, midmyocardial, and endocardial myocytes. A weaker IKs contributes to the longer action potential of the M cell. Circ Res. 1995;76(3):351–65. doi: 10.1161/01.res.76.3.351. [DOI] [PubMed] [Google Scholar]
- 7.Balser JR, Bennett PB, Roden DM. Time-dependent outward current in guinea pig ventricular myocytes. Gating kinetics of the delayed rectifier. J Gen Physiol. 1990;96(4):835–63. doi: 10.1085/jgp.96.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh KB, et al. Delayed-rectifier potassium channel activity in isolated membrane patches of guinea pig ventricular myocytes. Am J Physiol. 1991;260(4 Pt 2):H1390–3. doi: 10.1152/ajpheart.1991.260.4.H1390. [DOI] [PubMed] [Google Scholar]
- 9.Li GR, et al. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996;78(4):689–96. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- 10.Apkon M, Nerbonne JM. Characterization of two distinct depolarization-activated K+ currents in isolated adult rat ventricular myocytes. J Gen Physiol. 1991;97(5):973–1011. doi: 10.1085/jgp.97.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bou-Abboud E, Nerbonne JM. Molecular correlates of the calcium-independent, depolarization-activated K+ currents in rat atrial myocytes. J Physiol. 1999;517( Pt 2):407–20. doi: 10.1111/j.1469-7793.1999.0407t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyle WA, Nerbonne JM. A novel type of depolarization-activated K+ current in isolated adult rat atrial myocytes. Am J Physiol. 1991;260(4 Pt 2):H1236–47. doi: 10.1152/ajpheart.1991.260.4.H1236. [DOI] [PubMed] [Google Scholar]
- 13.Boyle WA, Nerbonne JM. Two functionally distinct 4-aminopyridine-sensitive outward K+ currents in rat atrial myocytes. J Gen Physiol. 1992;100(6):1041–67. doi: 10.1085/jgp.100.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Fermini B, Nattel S. Sustained depolarization-induced outward current in human atrial myocytes. Evidence for a novel delayed rectifier K+ current similar to Kv1.5 cloned channel currents. Circ Res. 1993;73(6):1061–76. doi: 10.1161/01.res.73.6.1061. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Fermini B, Nattel S. Delayed rectifier outward current and repolarization in human atrial myocytes. Circ Res. 1993;73(2):276–85. doi: 10.1161/01.res.73.2.276. [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, et al. Positional cloning of a novel potassium channel gene: KVLQT1 mutations cause cardiac arrhythmias. Nat Genet. 1996;12(1):17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- 17.Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242(4881):1042–5. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- 18.Barhanin J, et al. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384(6604):78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 19.Sanguinetti MC, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384(6604):80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 20.Schulze-Bahr E, et al. KCNE1 mutations cause jervell and Lange-Nielsen syndrome. Nat Genet. 1997;17(3):267–8. doi: 10.1038/ng1197-267. [DOI] [PubMed] [Google Scholar]
- 21.Splawski I, et al. Mutations in the hminK gene cause long QT syndrome and suppress IKs function. Nat Genet. 1997;17(3):338–40. doi: 10.1038/ng1197-338. [DOI] [PubMed] [Google Scholar]
- 22.Sanguinetti MC, et al. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81(2):299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 23.Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91(8):3438–42. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curran ME, et al. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80(5):795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 25.Abbott GW, et al. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97(2):175–87. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- 26.Weerapura M, et al. A comparison of currents carried by HERG, with and without coexpression of MiRP1, and the native rapid delayed rectifier current. Is MiRP1 the missing link? J Physiol. 2002;540(Pt 1):15–27. doi: 10.1113/jphysiol.2001.013296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng J, et al. Antisense oligodeoxynucleotides directed against Kv1.5 mRNA specifically inhibit ultrarapid delayed rectifier K+ current in cultured adult human atrial myocytes. Circ Res. 1997;80(4):572–9. doi: 10.1161/01.res.80.4.572. [DOI] [PubMed] [Google Scholar]
- 28.Rampe D, et al. Voltage- and time-dependent block by perhexiline of K+ currents in human atrium and in cells expressing a Kv1.5-type cloned channel. J Pharmacol Exp Ther. 1995;274(1):444–9. [PubMed] [Google Scholar]
- 29.Busch AE, et al. Inhibition of IKs in guinea pig cardiac myocytes and guinea pig IsK channels by the chromanol 293B. Pflugers Arch. 1996;432(6):1094–6. doi: 10.1007/s004240050240. [DOI] [PubMed] [Google Scholar]
- 30.Andalib A, Brugada R, Nattel S. Atrial fibrillation: evidence for genetically determined disease. Curr Opin Cardiol. 2008;23(3):176–83. doi: 10.1097/HCO.0b013e3282fa7142. [DOI] [PubMed] [Google Scholar]
- 31.Tucker NR, Ellinor PT. Emerging directions in the genetics of atrial fibrillation. Circ Res. 2014;114(9):1469–82. doi: 10.1161/CIRCRESAHA.114.302225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giudicessi JR, Ackerman MJ. Potassium-channel mutations and cardiac arrhythmias--diagnosis and therapy. Nat Rev Cardiol. 2012;9(6):319–32. doi: 10.1038/nrcardio.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abriel H, Zaklyazminskaya EV. Cardiac channelopathies: genetic and molecular mechanisms. Gene. 2013;517(1):1–11. doi: 10.1016/j.gene.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 34.Pfeufer A, et al. Common variants at ten loci modulate the QT interval duration in the QTSCD Study. Nat Genet. 2009;41(4):407–14. doi: 10.1038/ng.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arking DE, et al. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat Genet. 2014;46(8):826–36. doi: 10.1038/ng.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bezzina CR, Lahrouchi N, Priori SG. Genetics of sudden cardiac death. Circ Res. 2015;116(12):1919–36. doi: 10.1161/CIRCRESAHA.116.304030. [DOI] [PubMed] [Google Scholar]
- 37.Mizusawa Y, Horie M, Wilde AA. Genetic and clinical advances in congenital long QT syndrome. Circ J. 2014;78(12):2827–33. doi: 10.1253/circj.cj-14-0905. [DOI] [PubMed] [Google Scholar]
- 38.Tester DJ, Ackerman MJ. Genetics of long QT syndrome. Methodist Debakey Cardiovasc J. 2014;10(1):29–33. doi: 10.14797/mdcj-10-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartz PJ, Ackerman MJ. The long QT syndrome: a transatlantic clinical approach to diagnosis and therapy. Eur Heart J. 2013;34(40):3109–16. doi: 10.1093/eurheartj/eht089. [DOI] [PubMed] [Google Scholar]
- 40.Crotti L, et al. Congenital long QT syndrome. Orphanet J Rare Dis. 2008;3:18. doi: 10.1186/1750-1172-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cummings S, Priori S. Genetics of cardiac arrhythmias. Minerva Med. 2011;102(3):209–22. [PubMed] [Google Scholar]
- 42.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104(4):569–80. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 43.Goldenberg I, Moss AJ. Long QT syndrome. J Am Coll Cardiol. 2008;51(24):2291–300. doi: 10.1016/j.jacc.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 44.Towbin JA, Vatta M. Molecular biology and the prolonged QT syndromes. Am J Med. 2001;110(5):385–98. doi: 10.1016/s0002-9343(00)00715-4. [DOI] [PubMed] [Google Scholar]
- 45.Splawski I, et al. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102(10):1178–85. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 46.Napolitano C, et al. Genetic testing in the long QT syndrome: development and validation of an efficient approach to genotyping in clinical practice. JAMA. 2005;294(23):2975–80. doi: 10.1001/jama.294.23.2975. [DOI] [PubMed] [Google Scholar]
- 47.Peroz D, et al. Kv7.1 (KCNQ1) properties and channelopathies. J Physiol. 2008;586(7):1785–9. doi: 10.1113/jphysiol.2007.148254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dvir M, et al. Recent molecular insights from mutated IKS channels in cardiac arrhythmia. Curr Opin Pharmacol. 2014;15:74–82. doi: 10.1016/j.coph.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Eldstrom J, et al. Mechanistic basis for LQT1 caused by S3 mutations in the KCNQ1 subunit of IKs. J Gen Physiol. 2010;135(5):433–48. doi: 10.1085/jgp.200910351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dahimene S, et al. The N-terminal juxtamembranous domain of KCNQ1 is critical for channel surface expression: implications in the Romano-Ward LQT1 syndrome. Circ Res. 2006;99(10):1076–83. doi: 10.1161/01.RES.0000250262.12219.95. [DOI] [PubMed] [Google Scholar]
- 51.Sato A, et al. Novel mechanisms of trafficking defect caused by KCNQ1 mutations found in long QT syndrome. J Biol Chem. 2009;284(50):35122–33. doi: 10.1074/jbc.M109.017293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmitt N, et al. The novel C-terminal KCNQ1 mutation M520R alters protein trafficking. Biochem Biophys Res Commun. 2007;358(1):304–10. doi: 10.1016/j.bbrc.2007.04.127. [DOI] [PubMed] [Google Scholar]
- 53.Gouas L, et al. New KCNQ1 mutations leading to haploinsufficiency in a general population; Defective trafficking of a KvLQT1 mutant. Cardiovasc Res. 2004;63(1):60–8. doi: 10.1016/j.cardiores.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 54.Wilson AJ, et al. Abnormal KCNQ1 trafficking influences disease pathogenesis in hereditary long QT syndromes (LQT1) Cardiovasc Res. 2005;67(3):476–86. doi: 10.1016/j.cardiores.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 55.Schmitt N, et al. A recessive C-terminal Jervell and Lange-Nielsen mutation of the KCNQ1 channel impairs subunit assembly. EMBO J. 2000;19(3):332–40. doi: 10.1093/emboj/19.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matavel A, Medei E, Lopes CM. PKA and PKC partially rescue long QT type 1 phenotype by restoring channel-PIP2 interactions. Channels (Austin) 2010;4(1):3–11. doi: 10.4161/chan.4.1.10227. [DOI] [PubMed] [Google Scholar]
- 57.Thomas AM, et al. Characterization of a binding site for anionic phospholipids on KCNQ1. J Biol Chem. 2011;286(3):2088–100. doi: 10.1074/jbc.M110.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaydman MA, et al. Kv7.1 ion channels require a lipid to couple voltage sensing to pore opening. Proc Natl Acad Sci U S A. 2013;110(32):13180–5. doi: 10.1073/pnas.1305167110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marx SO, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295(5554):496–9. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- 60.Bianchi L, et al. Mechanisms of I(Ks) suppression in LQT1 mutants. Am J Physiol Heart Circ Physiol. 2000;279(6):H3003–11. doi: 10.1152/ajpheart.2000.279.6.H3003. [DOI] [PubMed] [Google Scholar]
- 61.Moss AJ, et al. Clinical aspects of type-1 long-QT syndrome by location, coding type, and biophysical function of mutations involving the KCNQ1 gene. Circulation. 2007;115(19):2481–9. doi: 10.1161/CIRCULATIONAHA.106.665406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson HA, Accili EA. Evolutionary analyses of KCNQ1 and HERG voltage-gated potassium channel sequences reveal location-specific susceptibility and augmented chemical severities of arrhythmogenic mutations. BMC Evol Biol. 2008;8:188. doi: 10.1186/1471-2148-8-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimizu W, et al. Mutation site-specific differences in arrhythmic risk and sensitivity to sympathetic stimulation in the LQT1 form of congenital long QT syndrome: multicenter study in Japan. J Am Coll Cardiol. 2004;44(1):117–25. doi: 10.1016/j.jacc.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 64.Barsheshet A, et al. Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to beta-blocker therapy in type 1 long-QT syndrome. Circulation. 2012;125(16):1988–96. doi: 10.1161/CIRCULATIONAHA.111.048041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hedley PL, et al. The genetic basis of long QT and short QT syndromes: a mutation update. Hum Mutat. 2009;30(11):1486–511. doi: 10.1002/humu.21106. [DOI] [PubMed] [Google Scholar]
- 66.Bianchi L, et al. Cellular dysfunction of LQT5-minK mutants: abnormalities of IKs, IKr and trafficking in long QT syndrome. Hum Mol Genet. 1999;8(8):1499–507. doi: 10.1093/hmg/8.8.1499. [DOI] [PubMed] [Google Scholar]
- 67.Harmer SC, Tinker A. The role of abnormal trafficking of KCNE1 in long QT syndrome 5. Biochem Soc Trans. 2007;35(Pt 5):1074–6. doi: 10.1042/BST0351074. [DOI] [PubMed] [Google Scholar]
- 68.Seebohm G, et al. Long QT syndrome-associated mutations in KCNQ1 and KCNE1 subunits disrupt normal endosomal recycling of IKs channels. Circ Res. 2008;103(12):1451–7. doi: 10.1161/CIRCRESAHA.108.177360. [DOI] [PubMed] [Google Scholar]
- 69.Melman YF, et al. KCNE1 binds to the KCNQ1 pore to regulate potassium channel activity. Neuron. 2004;42(6):927–37. doi: 10.1016/j.neuron.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Melman YF, et al. Structural determinants of KvLQT1 control by the KCNE family of proteins. J Biol Chem. 2001;276(9):6439–44. doi: 10.1074/jbc.M010713200. [DOI] [PubMed] [Google Scholar]
- 71.Melman YF, Krumerman A, McDonald TV. A single transmembrane site in the KCNEencoded proteins controls the specificity of KvLQT1 channel gating. J Biol Chem. 2002;277(28):25187–94. doi: 10.1074/jbc.M200564200. [DOI] [PubMed] [Google Scholar]
- 72.Nakajo K, et al. KCNQ1 subdomains involved in KCNE modulation revealed by an invertebrate KCNQ1 orthologue. J Gen Physiol. 2011;138(5):521–35. doi: 10.1085/jgp.201110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakajo K, Kubo Y. KCNE1 and KCNE3 stabilize and/or slow voltage sensing S4 segment of KCNQ1 channel. J Gen Physiol. 2007;130(3):269–81. doi: 10.1085/jgp.200709805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X, et al. KCNQ1 and KCNE1 in the IKs channel complex make state-dependent contacts in their extracellular domains. J Gen Physiol. 2008;131(6):589–603. doi: 10.1085/jgp.200809976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chung DY, et al. Location of KCNE1 relative to KCNQ1 in the I(KS) potassium channel by disulfide cross-linking of substituted cysteines. Proc Natl Acad Sci U S A. 2009;106(3):743–8. doi: 10.1073/pnas.0811897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nakajo K, Kubo Y. Nano-environmental changes by KCNE proteins modify KCNQ channel function. Channels (Austin) 2011;5(5):397–401. doi: 10.4161/chan.5.5.16468. [DOI] [PubMed] [Google Scholar]
- 77.Chen H, et al. Charybdotoxin binding in the I(Ks) pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40(1):15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 78.Morin TJ, Kobertz WR. Counting membrane-embedded KCNE beta-subunits in functioning K+ channel complexes. Proc Natl Acad Sci U S A. 2008;105(5):1478–82. doi: 10.1073/pnas.0710366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plant LD, et al. Individual IKs channels at the surface of mammalian cells contain two KCNE1 accessory subunits. Proc Natl Acad Sci U S A. 2014;111(14):E1438–46. doi: 10.1073/pnas.1323548111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakajo K, et al. Stoichiometry of the KCNQ1 - KCNE1 ion channel complex. Proc Natl Acad Sci U S A. 2010;107(44):18862–7. doi: 10.1073/pnas.1010354107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osteen JD, et al. KCNE1 alters the voltage sensor movements necessary to open the KCNQ1 channel gate. Proc Natl Acad Sci U S A. 2010;107(52):22710–5. doi: 10.1073/pnas.1016300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ruscic KJ, et al. IKs channels open slowly because KCNE1 accessory subunits slow the movement of S4 voltage sensors in KCNQ1 pore-forming subunits. Proc Natl Acad Sci U S A. 2013;110(7):E559–66. doi: 10.1073/pnas.1222616110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barro-Soria R, et al. KCNE1 divides the voltage sensor movement in KCNQ1/KCNE1 channels into two steps. Nat Commun. 2014;5:3750. doi: 10.1038/ncomms4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz PJ, et al. Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103(1):89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 85.Walsh KB, Kass RS. Regulation of a heart potassium channel by protein kinase A and C. Science. 1988;242(4875):67–9. doi: 10.1126/science.2845575. [DOI] [PubMed] [Google Scholar]
- 86.Terrenoire C, et al. The cardiac IKs potassium channel macromolecular complex includes the phosphodiesterase PDE4D3. J Biol Chem. 2009;284(14):9140–6. doi: 10.1074/jbc.M805366200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Y, et al. The A-kinase anchoring protein Yotiao facilitates complex formation between adenylyl cyclase type 9 and the IKs potassium channel in heart. J Biol Chem. 2012;287(35):29815–24. doi: 10.1074/jbc.M112.380568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen L, Kass RS. A-kinase anchoring protein 9 and IKs channel regulation. J Cardiovasc Pharmacol. 2011;58(5):459–13. doi: 10.1097/FJC.0b013e318232c80c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen L, et al. Mutation of an A-kinase-anchoring protein causes long-QT syndrome. Proc Natl Acad Sci U S A. 2007;104(52):20990–5. doi: 10.1073/pnas.0710527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Villiers CP, et al. AKAP9 is a genetic modifier of congenital long-QT syndrome type 1. Circ Cardiovasc Genet. 2014;7(5):599–606. doi: 10.1161/CIRCGENETICS.113.000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson CL, et al. Large-scale mutational analysis of Kv11.1 reveals molecular insights into type 2 long QT syndrome. Nat Commun. 2014;5:5535. doi: 10.1038/ncomms6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sanguinetti MC, et al. Spectrum of HERG K+-channel dysfunction in an inherited cardiac arrhythmia. Proc Natl Acad Sci U S A. 1996;93(5):2208–12. doi: 10.1073/pnas.93.5.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Z, et al. HERG channel dysfunction in human long QT syndrome. Intracellular transport and functional defects. J Biol Chem. 1998;273(33):21061–6. doi: 10.1074/jbc.273.33.21061. [DOI] [PubMed] [Google Scholar]
- 94.Anderson CL, et al. Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation. 2006;113(3):365–73. doi: 10.1161/CIRCULATIONAHA.105.570200. [DOI] [PubMed] [Google Scholar]
- 95.Gong Q, Jones MA, Zhou Z. Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J Biol Chem. 2006;281(7):4069–74. doi: 10.1074/jbc.M511765200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delisle BP, et al. Thapsigargin selectively rescues the trafficking defective LQT2 channels G601S and F805C. J Biol Chem. 2003;278(37):35749–54. doi: 10.1074/jbc.M305787200. [DOI] [PubMed] [Google Scholar]
- 97.Rajamani S, et al. Pharmacological rescue of human K(+) channel long-QT2 mutations: human ether-a-go-go-related gene rescue without block. Circulation. 2002;105(24):2830–5. doi: 10.1161/01.cir.0000019513.50928.74. [DOI] [PubMed] [Google Scholar]
- 98.Mehta A, et al. Re-trafficking of hERG reverses long QT syndrome 2 phenotype in human iPS-derived cardiomyocytes. Cardiovasc Res. 2014;102(3):497–506. doi: 10.1093/cvr/cvu060. [DOI] [PubMed] [Google Scholar]
- 99.Smith JL, et al. Pharmacological correction of long QT-linked mutations in KCNH2 (hERG) increases the trafficking of Kv11.1 channels stored in the transitional endoplasmic reticulum. Am J Physiol Cell Physiol. 2013;305(9):C919–30. doi: 10.1152/ajpcell.00406.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ayon RJ, Fernandez RA, Yuan JX. Mutant hERG channel traffic jam. Focus on “Pharmacological correction of long QT-linked mutations in KCNH2 (hERG) increases the trafficking of Kv11.1 channels stored in the transitional endoplasmic reticulum”. Am J Physiol Cell Physiol. 2013;305(9):C916–8. doi: 10.1152/ajpcell.00256.2013. [DOI] [PubMed] [Google Scholar]
- 101.Robertson GA, January CT. HERG trafficking and pharmacological rescue of LQTS-2 mutant channels. Handb Exp Pharmacol. 2006;171:349–55. doi: 10.1007/3-540-29715-4_14. [DOI] [PubMed] [Google Scholar]
- 102.Zhao JT, et al. Not all hERG pore domain mutations have a severe phenotype: G584S has an inactivation gating defect with mild phenotype compared to G572S, which has a dominant negative trafficking defect and a severe phenotype. J Cardiovasc Electrophysiol. 2009;20(8):923–30. doi: 10.1111/j.1540-8167.2009.01468.x. [DOI] [PubMed] [Google Scholar]
- 103.Chen J, et al. Long QT syndrome-associated mutations in the Per-Arnt-Sim (PAS) domain of HERG potassium channels accelerate channel deactivation. J Biol Chem. 1999;274(15):10113–8. doi: 10.1074/jbc.274.15.10113. [DOI] [PubMed] [Google Scholar]
- 104.Morais Cabral JH, et al. Crystal structure and functional analysis of the HERG potassium channel N terminus: a eukaryotic PAS domain. Cell. 1998;95(5):649–55. doi: 10.1016/s0092-8674(00)81635-9. [DOI] [PubMed] [Google Scholar]
- 105.Balijepalli SY, et al. Mechanism of loss of Kv11.1 K+ current in mutant T421M-Kv11.1-expressing rat ventricular myocytes: interaction of trafficking and gating. Circulation. 2012;126(24):2809–18. doi: 10.1161/CIRCULATIONAHA.112.118018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gong Q, et al. Nonsense mutations in hERG cause a decrease in mutant mRNA transcripts by nonsense-mediated mRNA decay in human long-QT syndrome. Circulation. 2007;116(1):17–24. doi: 10.1161/CIRCULATIONAHA.107.708818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gong Q, et al. A splice site mutation in hERG leads to cryptic splicing in human long QT syndrome. J Mol Cell Cardiol. 2008;44(3):502–9. doi: 10.1016/j.yjmcc.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moss AJ, et al. Increased risk of arrhythmic events in long-QT syndrome with mutations in the pore region of the human ether-a-go-go-related gene potassium channel. Circulation. 2002;105(7):794–9. doi: 10.1161/hc0702.105124. [DOI] [PubMed] [Google Scholar]
- 109.Shimizu W, et al. Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol. 2009;54(22):2052–62. doi: 10.1016/j.jacc.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Haverkamp W, et al. The potential for QT prolongation and pro-arrhythmia by non-anti-arrhythmic drugs: clinical and regulatory implications. Report on a Policy Conference of the European Society of Cardiology. Cardiovasc Res. 2000;47(2):219–33. doi: 10.1016/s0008-6363(00)00119-x. [DOI] [PubMed] [Google Scholar]
- 111.Spector PS, et al. Class III antiarrhythmic drugs block HERG, a human cardiac delayed rectifier K+ channel. Open-channel block by methanesulfonanilides. Circ Res. 1996;78(3):499–503. doi: 10.1161/01.res.78.3.499. [DOI] [PubMed] [Google Scholar]
- 112.Tseng GN. I(Kr): the hERG channel. J Mol Cell Cardiol. 2001;33(5):835–49. doi: 10.1006/jmcc.2000.1317. [DOI] [PubMed] [Google Scholar]
- 113.Baskin EP, Lynch JJ., Jr Comparative effects of increased extracellular potassium and pacing frequency on the class III activities of methanesulfonanilide IKr blockers dofetilide, D-sotalol, E-4031, and MK-499. J Cardiovasc Pharmacol. 1994;24(2):199–208. [PubMed] [Google Scholar]
- 114.Carmeliet E. Voltage- and time-dependent block of the delayed K+ current in cardiac myocytes by dofetilide. J Pharmacol Exp Ther. 1992;262(2):809–17. [PubMed] [Google Scholar]
- 115.Kiehn J, et al. Molecular physiology and pharmacology of HERG. Single-channel currents and block by dofetilide. Circulation. 1996;94(10):2572–9. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- 116.Hancox JC, et al. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol Ther. 2008;119(2):118–32. doi: 10.1016/j.pharmthera.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Armstrong CM. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971;58(4):413–37. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang T, Snyders DJ, Roden DM. Ibutilide, a methanesulfonanilide antiarrhythmic, is a potent blocker of the rapidly activating delayed rectifier K+ current (IKr) in AT-1 cells. Concentration-, time-, voltage-, and use-dependent effects. Circulation. 1995;91(6):1799–806. doi: 10.1161/01.cir.91.6.1799. [DOI] [PubMed] [Google Scholar]
- 119.Kiehn J, et al. Mapping the block of a cloned human inward rectifier potassium channel by dofetilide. Mol Pharmacol. 1996;50(2):380–7. [PubMed] [Google Scholar]
- 120.Mitcheson JS, Chen J, Sanguinetti MC. Trapping of a methanesulfonanilide by closure of the HERG potassium channel activation gate. J Gen Physiol. 2000;115(3):229–40. doi: 10.1085/jgp.115.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lees-Miller JP, et al. Molecular determinant of high-affinity dofetilide binding to HERG1 expressed in Xenopus oocytes: involvement of S6 sites. Mol Pharmacol. 2000;57(2):367–74. [PubMed] [Google Scholar]
- 122.Mitcheson JS, et al. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci U S A. 2000;97(22):12329–33. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vandenberg JI, et al. hERG K(+) channels: structure, function, and clinical significance. Physiol Rev. 2012;92(3):1393–478. doi: 10.1152/physrev.00036.2011. [DOI] [PubMed] [Google Scholar]
- 124.Nogawa H, Kawai T. hERG trafficking inhibition in drug-induced lethal cardiac arrhythmia. Eur J Pharmacol. 2014;741:336–9. doi: 10.1016/j.ejphar.2014.06.044. [DOI] [PubMed] [Google Scholar]
- 125.Dennis A, et al. hERG channel trafficking: novel targets in drug-induced long QT syndrome. Biochem Soc Trans. 2007;35(Pt 5):1060–3. doi: 10.1042/BST0351060. [DOI] [PubMed] [Google Scholar]
- 126.Ficker E, et al. Mechanisms of arsenic-induced prolongation of cardiac repolarization. Mol Pharmacol. 2004;66(1):33–44. doi: 10.1124/mol.66.1.33. [DOI] [PubMed] [Google Scholar]
- 127.Ficker E, et al. Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ Res. 2003;92(12):e87–100. doi: 10.1161/01.RES.0000079028.31393.15. [DOI] [PubMed] [Google Scholar]
- 128.Cordes JS, et al. Pentamidine reduces hERG expression to prolong the QT interval. Br J Pharmacol. 2005;145(1):15–23. doi: 10.1038/sj.bjp.0706140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kuryshev YA, et al. Pentamidine-induced long QT syndrome and block of hERG trafficking. J Pharmacol Exp Ther. 2005;312(1):316–23. doi: 10.1124/jpet.104.073692. [DOI] [PubMed] [Google Scholar]
- 130.Guo J, et al. Involvement of caveolin in probucol-induced reduction in hERG plasma-membrane expression. Mol Pharmacol. 2011;79(5):806–13. doi: 10.1124/mol.110.069419. [DOI] [PubMed] [Google Scholar]
- 131.Guo J, et al. Identification of IKr and its trafficking disruption induced by probucol in cultured neonatal rat cardiomyocytes. J Pharmacol Exp Ther. 2007;321(3):911–20. doi: 10.1124/jpet.107.120931. [DOI] [PubMed] [Google Scholar]
- 132.Algra A, et al. QT interval variables from 24 hour electrocardiography and the two year risk of sudden death. Br Heart J. 1993;70(1):43–8. doi: 10.1136/hrt.70.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gussak I, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94(2):99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 134.Gussak I, Bjerregaard P. Short QT syndrome--5 years of progress. J Electrocardiol. 2005;38(4):375–7. doi: 10.1016/j.jelectrocard.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 135.Gaita F, et al. Short QT Syndrome: a familial cause of sudden death. Circulation. 2003;108(8):965–70. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 136.Priori SG, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10(12):1932–63. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 137.Gollob MH, Redpath CJ, Roberts JD. The short QT syndrome: proposed diagnostic criteria. J Am Coll Cardiol. 2011;57(7):802–12. doi: 10.1016/j.jacc.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 138.Brugada R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109(1):30–5. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 139.Cordeiro JM, et al. Modulation of I(Kr) inactivation by mutation N588K in KCNH2: a link to arrhythmogenesis in short QT syndrome. Cardiovasc Res. 2005;67(3):498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 140.Sun Y, et al. A novel mutation in the KCNH2 gene associated with short QT syndrome. J Mol Cell Cardiol. 2011;50(3):433–41. doi: 10.1016/j.yjmcc.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 141.Itoh H, et al. A novel KCNH2 mutation as a modifier for short QT interval. Int J Cardiol. 2009;137(1):83–5. doi: 10.1016/j.ijcard.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 142.Wilders R, Verkerk AO. Role of the R1135H KCNH2 mutation in Brugada syndrome. Int J Cardiol. 2010;144(1):149–51. doi: 10.1016/j.ijcard.2008.12.177. [DOI] [PubMed] [Google Scholar]
- 143.Harrell DT, et al. Genotype-dependent differences in age of manifestation and arrhythmia complications in short QT syndrome. Int J Cardiol. 2015;190:393–402. doi: 10.1016/j.ijcard.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 144.Bellocq C, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109(20):2394–7. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 145.Moreno C, et al. A new KCNQ1 mutation at the S5 segment that impairs its association with KCNE1 is responsible for short QT syndrome. Cardiovasc Res. 2015;107(4):613–23. doi: 10.1093/cvr/cvv196. [DOI] [PubMed] [Google Scholar]
- 146.Wu ZJ, et al. Characterization of a Chinese KCNQ1 mutation (R259H) that shortens repolarization and causes short QT syndrome 2. J Geriatr Cardiol. 2015;12(4):394–401. doi: 10.11909/j.issn.1671-5411.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gaita F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol. 2004;43(8):1494–9. doi: 10.1016/j.jacc.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 148.Giustetto C, et al. Long-term follow-up of patients with short QT syndrome. J Am Coll Cardiol. 2011;58(6):587–95. doi: 10.1016/j.jacc.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 149.Dumaine R, Antzelevitch C. Disopyramide: although potentially life-threatening in the setting of long QT, could it be life-saving in short QT syndrome? J Mol Cell Cardiol. 2006;41(3):421–3. doi: 10.1016/j.yjmcc.2006.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.McPate MJ, et al. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J Mol Cell Cardiol. 2006;41(3):563–6. doi: 10.1016/j.yjmcc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 151.Bjerregaard P, Gussak I. Short QT syndrome: mechanisms, diagnosis and treatment. Nat Clin Pract Cardiovasc Med. 2005;2(2):84–7. doi: 10.1038/ncpcardio0097. [DOI] [PubMed] [Google Scholar]
- 152.Darbar D, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41(12):2185–92. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 153.Ellinor PT, et al. Familial aggregation in lone atrial fibrillation. Hum Genet. 2005;118(2):179–84. doi: 10.1007/s00439-005-0034-8. [DOI] [PubMed] [Google Scholar]
- 154.Patton KK, et al. Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol. 2005;28(7):630–8. doi: 10.1111/j.1540-8159.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 155.Yang T, et al. Novel KCNA5 mutation implicates tyrosine kinase signaling in human atrial fibrillation. Heart Rhythm. 2010;7(9):1246–52. doi: 10.1016/j.hrthm.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fox CS, et al. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291(23):2851–5. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 157.Mahida S, et al. Monogenic atrial fibrillation as pathophysiological paradigms. Cardiovasc Res. 2011;89(4):692–700. doi: 10.1093/cvr/cvq381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Steffensen AB, et al. IKs Gain- and Loss-of-Function in Early-Onset Lone Atrial Fibrillation. J Cardiovasc Electrophysiol. 2015;26(7):715–23. doi: 10.1111/jce.12666. [DOI] [PubMed] [Google Scholar]
- 159.Chen YH, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299(5604):251–4. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 160.Restier L, Cheng L, Sanguinetti MC. Mechanisms by which atrial fibrillation-associated mutations in the S1 domain of KCNQ1 slow deactivation of IKs channels. J Physiol. 2008;586(Pt 17):4179–91. doi: 10.1113/jphysiol.2008.157511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hong K, et al. De novo KCNQ1 mutation responsible for atrial fibrillation and short QT syndrome in utero. Cardiovasc Res. 2005;68(3):433–40. doi: 10.1016/j.cardiores.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 162.Lundby A, et al. KCNQ1 mutation Q147R is associated with atrial fibrillation and prolonged QT interval. Heart Rhythm. 2007;4(12):1532–41. doi: 10.1016/j.hrthm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 163.Das S, et al. Mutation in the S3 segment of KCNQ1 results in familial lone atrial fibrillation. Heart Rhythm. 2009;6(8):1146–53. doi: 10.1016/j.hrthm.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hasegawa K, et al. A novel KCNQ1 missense mutation identified in a patient with juvenile-onset atrial fibrillation causes constitutively open IKs channels. Heart Rhythm. 2014;11(1):67–75. doi: 10.1016/j.hrthm.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 165.Bartos DC, et al. R231C mutation in KCNQ1 causes long QT syndrome type 1 and familial atrial fibrillation. Heart Rhythm. 2011;8(1):48–55. doi: 10.1016/j.hrthm.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Henrion U, et al. Overlapping cardiac phenotype associated with a familial mutation in the voltage sensor of the KCNQ1 channel. Cell Physiol Biochem. 2012;29(5–6):809–18. doi: 10.1159/000178470. [DOI] [PubMed] [Google Scholar]
- 167.Bartos DC, et al. A KCNQ1 mutation causes a high penetrance for familial atrial fibrillation. J Cardiovasc Electrophysiol. 2013;24(5):562–9. doi: 10.1111/jce.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ki CS, et al. A KCNQ1 mutation causes age-dependant bradycardia and persistent atrial fibrillation. Pflugers Arch. 2014;466(3):529–40. doi: 10.1007/s00424-013-1337-6. [DOI] [PubMed] [Google Scholar]
- 169.Sinner MF, et al. The non-synonymous coding IKr-channel variant KCNH2-K897T is associated with atrial fibrillation: results from a systematic candidate gene-based analysis of KCNH2 (HERG) Eur Heart J. 2008;29(7):907–14. doi: 10.1093/eurheartj/ehm619. [DOI] [PubMed] [Google Scholar]
- 170.Christophersen IE, et al. Genetic variation in KCNA5: impact on the atrial-specific potassium current IKur in patients with lone atrial fibrillation. Eur Heart J. 2013;34(20):1517–25. doi: 10.1093/eurheartj/ehs442. [DOI] [PubMed] [Google Scholar]
- 171.Olson TM, et al. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15(14):2185–91. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 172.Yang Y, et al. Novel KCNA5 loss-of-function mutations responsible for atrial fibrillation. J Hum Genet. 2009;54(5):277–83. doi: 10.1038/jhg.2009.26. [DOI] [PubMed] [Google Scholar]
- 173.Yang T, et al. Biophysical properties of 9 KCNQ1 mutations associated with long-QT syndrome. Circ Arrhythm Electrophysiol. 2009;2(4):417–26. doi: 10.1161/CIRCEP.109.850149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hong K, et al. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16(4):394–6. doi: 10.1046/j.1540-8167.2005.40621.x. [DOI] [PubMed] [Google Scholar]
- 175.Nattel S. New ideas about atrial fibrillation 50 years on. Nature. 2002;415(6868):219–26. doi: 10.1038/415219a. [DOI] [PubMed] [Google Scholar]
- 176.Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov. 2009;8(12):982–1001. doi: 10.1038/nrd2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roy D, et al. Amiodarone to prevent recurrence of atrial fibrillation. Canadian Trial of Atrial Fibrillation Investigators. N Engl J Med. 2000;342(13):913–20. doi: 10.1056/NEJM200003303421302. [DOI] [PubMed] [Google Scholar]
- 178.Qin D, et al. Comparative effectiveness of antiarrhythmic drugs for rhythm control of atrial fibrillation. J Cardiol. 2015 doi: 10.1016/j.jjcc.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 179.Waldo AL, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996;348(9019):7–12. doi: 10.1016/s0140-6736(96)02149-6. [DOI] [PubMed] [Google Scholar]
- 180.Ravens U, et al. Atrial selectivity of antiarrhythmic drugs. J Physiol. 2013;591(Pt 17):4087–97. doi: 10.1113/jphysiol.2013.256115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ford JW, Milnes JT. New drugs targeting the cardiac ultra-rapid delayed-rectifier current (I Kur): rationale, pharmacology and evidence for potential therapeutic value. J Cardiovasc Pharmacol. 2008;52(2):105–20. doi: 10.1097/FJC.0b013e3181719b0c. [DOI] [PubMed] [Google Scholar]
- 182.Vizzardi E, et al. A new antiarrhythmic drug in the treatment of recent-onset atrial fibrillation: vernakalant. Cardiovasc Ther. 2013;31(5):e55–62. doi: 10.1111/1755-5922.12026. [DOI] [PubMed] [Google Scholar]
- 183.Comtois P, et al. Mechanisms of atrial fibrillation termination by rapidly unbinding Na+ channel blockers: insights from mathematical models and experimental correlates. Am J Physiol Heart Circ Physiol. 2008;295(4):H1489–504. doi: 10.1152/ajpheart.01054.2007. [DOI] [PubMed] [Google Scholar]
- 184.Ford J, et al. Human electrophysiological and pharmacological properties of XEN-D0101: a novel atrial-selective Kv1.5/IKur inhibitor. J Cardiovasc Pharmacol. 2013;61(5):408–15. doi: 10.1097/FJC.0b013e31828780eb. [DOI] [PubMed] [Google Scholar]
- 185.Wang Q, et al. Gain-of-function KCNH2 mutations in patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2014;25(5):522–30. doi: 10.1111/jce.12361. [DOI] [PubMed] [Google Scholar]
- 186.Decher N, et al. Molecular basis for Kv1.5 channel block: conservation of drug binding sites among voltage-gated K+ channels. J Biol Chem. 2004;279(1):394–400. doi: 10.1074/jbc.M307411200. [DOI] [PubMed] [Google Scholar]