Abstract
Chronic stress is known to affect serotonin (5HT) neurotransmission in the brain and to alter body temperature. Body temperature is controlled in part, by the medial preoptic area of the hypothalamus (mPOA). To investigate the effect of chronic stress on 5HT and how it affects body temperature regulation, we examined whether exposure to a chronic unpredictable stress paradigm (CUS) produces long-term alterations in thermoregulatory function of the mPOA through decreased 5HT neurotransmission. Adult male Sprague-Dawley rats underwent 21 days of CUS. Four days after last stress exposure, basal body temperature in the home cage and body temperature in a cold room maintained at 10°C were recorded. CUS rats had significantly higher subcutaneous basal body temperature at 13:00 h compared to unstressed (NoStress) rats. Whereas the NoStress rats were able to significantly elevate body temperature from basal levels at 30 and 60 min of exposure to the cold room, the CUS rats showed a hypothermic response to the cold. Treatment during CUS with metyrapone, a corticosterone synthesis inhibitor, blocked stress-induced decrease in body temperature in response to the cold challenge. CUS also decreased 5HT transporter protein immunoreactivity in the mPOA and 5HT2A/C agonist injection into the mPOA after CUS exposure caused stressed rats to exhibit a sensitized hyperthermic response to cold. These results indicate that CUS induced changes to the 5HTergic system alters mPOA function in thermoregulation. These findings help explain mechanisms underlying chronic stress induced disorders such as chronic fatigue syndrome wherein long lasting thermoregulatory deficits are observed.
Keywords: Chronic unpredictable stress, medial preoptic area, thermoregulation, hyperthermia, serotonin
Introduction
Chronic stress is physiologically and emotionally demanding. Repeated exposures to unpredictable stressors can dysregulate normal stress responses to cause pathological alterations in mood, cognition and physiological function (McEwen, 2003). One such physiological function affected by stress is thermoregulation where chronic stress exposure causes sustained elevation in body temperature up to 60 days after termination of stress (Timmerman et al., 1992; Endo & Shiraki, 2000; Oka et al., 2001; Matuszewich & Yamamoto, 2003; Hayashida et al., 2010). This long-lasting elevation in body temperature is attributed to a shift in brain set-point temperature, but the mechanism underlying this long-term shift in set-point is unknown (Kluger et al., 1987; Briese & Cabanac, 1991).
Body temperature is homeostatically regulated by the preoptic area (POA) of the hypothalamus, a known thermoregulatory center of the brain. The POA integrates thermosensory information from peripheral and central afferents and activates brain regions such as the dorsomedial hypothalamus and the raphe nuclei to permit appropriate responses to the thermal input (Boulant, 1981; Rusyniak et al., 2008b; Nakamura, 2011). The POA receives serotonergic (5HTergic) innervation from the dorsal and medullary raphe nuclei and administration of 5HT or 5HT2A/C agonist into the POA elevates body temperature (Myers & Yaksh, 1969; Myers, 1981; Lin et al., 1998; Hale et al., 2011). Conversely, 5HT1A agonist induced stimulation of presynaptic 5HT1A auto-receptors decreases 5HT release in the POA and decreases body temperature (Lin et al., 1998). These results indicate that 5HT in the POA plays a major role in thermoregulation.
5HT is also known to be a key modulator of stress effects. The midbrain 5HTergic system is involved in mediating both normal and pathological effects of stress (Lucki, 1998; Chaouloff et al., 1999). Stressors can activate the raphe nuclei and acutely increase 5HT released at efferent targets (Kawahara et al., 1993; Adell et al., 1997) such as the cortex, amygdala and hypothalamus (Abrams et al., 2004; Hale & Lowry, 2011). Therefore, 5HT neurotransmission at these postsynaptic targets can be altered by chronic exposure to stress and cause functional deficits in these brain regions to result in abnormal behavioral output.
Taken together, the above findings suggest that the 5HTergic system is modulated by stress and plays an important role in thermoregulation. Since a major target of 5HTergic innervation is the hypothalamus, stress induced dysregulation of 5HT neurotransmission can affect POA function under basal conditions and in response to challenges to the thermoregulatory system. Based on the importance of 5HT in mediating both stress and hyperthermic responses, we hypothesize that exposure to chronic unpredictable stress (CUS) will cause thermoregulatory dysfunction during a cold room challenge due to stress induced changes to 5HTergic function in the medial preoptic area (mPOA).
Methods
Animals
All experiments were conducted in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Toledo Institutional Animal Care and Use Committee. Adult male Sprague-Dawley rats between 175–199g were ordered from (Harlan, Indianapolis IN). These rats are over 60 days old at the start of the experiment and are considered young adults. The rats weighed around 180g at the start and 275g at the end of the experiments. Rats were housed 2 per cage, under a 6:00 h – 18:00 h light cycle and 18:00 h – 6:00 h dark cycle, in housing rooms maintained around 23°C. The rats had free access to food and water and were allowed to acclimate to the housing facility for 4 days before experimentation began.
Drug treatments
Metyrapone (2-Methyl-1,2-di-3-pyridyl-1-propanone; 50mg/kg, Mety) (Sigma Cat. 856525) was dissolved in 10% ethanol vehicle and injected intraperitoneally (ip.),15 min prior to each stressor. The dose of Mety used has been shown to block stress induced elevations in plasma corticosterone (CORT) (Roozendaal et al., 1996; Johnson & Yamamoto, 2010). The control group received 10% ethanol vehicle (ml/kg) injections ip.
Chronic unpredictable stress (CUS) paradigm
Rats were either handled daily or exposed to a chronic unpredictable stress (CUS) paradigm consisting of 21 days of twice daily exposure to mild stressors. The CUS paradigm was used to prevent habituation and adaptation to stress and models unanticipated stressful experiences that occur in humans (Katz et al., 1981; Willner et al., 1992). The stress schedule is provided in Table 1. Briefly, rats underwent isolation; altered light/dark cycle exposure; were deprived of food and water; restrained using commercially available medium flat-bottom plexiglass rodent restrainers (Harvard Instruments) outside the home-cage; placed in an orbital shaker set at 30 RPM; exposed to a cold room maintained at 10°C; placed in a 15″X10″X24″bucket containing 18″ of water maintained between 24–26°C; placed in cages where the bedding was saturated with room temperature water; underwent social stress where rats were exposed to a different novel male rat in a weight range similar to the stressed rats every time this stress was applied. On day 10 of CUS, temperature transponders (IPTT-300 transponder, BMDS) were injected subcutaneously (sc) to measure body temperature remotely during cold room challenge. The transponders were injected under the loose skin 1 inch above the hind limb on the right side. There is no evidence of brown adipose tissue (BAT) in this region (Cannon & Nedergaard, 2004), thereby eliminating confounds due to thermogenic activity of the BAT affecting the readings of the temperature transponders. A separate group of rats received both sc and intraperitoneally (ip) transponder injections to compare differences in temperature readings based on transponder placement.
Table 1.
Chronic unpredictable stress schedule.
| Day1 | 10:00 h | 60 min cold room with cage mate | 14:00h | 60 min restraint |
| Day 2 | 11:00 h | 3 hrs lights off | 18:00 h | Lights on overnight |
| Day 3 | 11:30 h | 3 min group swim | 17:00 h | Food and water deprivation overnight |
| Day 4 | 9:30 h | 60 min wet bedding | 15:00 h | 60 min social stress |
| Day 5 | 11:00 h | 60 min restraint | 14:30 h | 90 min cage rotation |
| Day 6 | 10:00 h | 3 hr lights off | 17:00 h | Isolation overnight |
| Day 7 | 10:00 h | 3 min group swim | 13:00 h | 60 min cold room with cage mate |
| Day 8 | 9:30 h | 60 min wet bedding | 16:00 h | 60 min restraint |
| Day 9 | 11:30 h | 30 min cage rotation | 14:30 h | 60 min social stress |
| Day 10 | 9:30 h | 60 min cold room with cage mate | 15:30 h | 60 min restraint |
| Day 11 | 10:30 h | 3 hr lights off | 18:00 h | Lights on overnight |
| Day 12 | 10:00 h | 3 min group swim | 17:30 h | Food and water deprivation overnight |
| Day 13 | 10:00 h | 90 min wet bedding | 14:00 h | 30 min social stress |
| Day 14 | 11:30 h | 60 min restraint | 15:30 h | 60 min cage rotation |
| Day 15 | 9:30 h | 3 hr lights off | 17:00 h | Isolation overnight |
| Day 16 | 10:30 h | 3 min group swim | 13:30 h | 60 min cold room with cage mate |
| Day 17 | 11:30 h | 30 min wet bedding | 18:00 h | Food and water deprivation overnight |
| Day 18 | 11:00 h | 90 min social stress | 15:00 h | 30 min cage rotation |
| Day 19 | 9:00 h | 3 hr lights off | 14:00 h | 60 min restraint |
| Day 20 | 11:00 h | 3 min group swim | 17:30 h | Lights on overnight |
| Day 21 | 10:00 h | 30 min wet bedding | 13:30 h | 60 min social stress |
Cold room exposure
To study long-term thermoregulatory changes due to stress, rats were exposed to a cold room challenge 4 days after exposure to last stressor and Mety treatment. This time-point was chosen in order to study CUS induced persistent changes in thermoregulation without confounds of drug exposure or acute effects of stress. These experiments were conducted at the same time of day (13:00 h) to account for diurnal fluctuations in body temperature. Basal body temperature in the home cage was recorded remotely from the implanted temperature transponders at 13:00 h. Rats were then removed from the home cage, housed individually, and transported to a cold room maintained at 10°C. Body temperature was remotely recorded at 10 min intervals during the 1 hr exposure to the cold room. At the end of 60 min in the cold room, the rats were returned back to the home cage and basal temperature was once again recorded at 18:00 h, at the start of the animals’ wake cycle. In experiments involving drug treatments, transponders were only implanted sc., since differences in cold room responses was more apparent with the sc. transponder placement.
Bilateral cannulae implantation surgery
One day after CUS, rats were anesthetized with ketamine (75 mg/kg, ip) and xylazine (5 mg/kg, ip) and were secured into a stereotaxic apparatus. Guide cannulae (26 gauge stainless steel) were implanted bilaterally above the mPOA (−0.8 mm Bregma, 0.5 mm Lateral, 8 mm Ventral) (Paxinos and Watson, 1982) and rats were allowed to recover for 3 days. Four days after the end of CUS, rats were temporarily anesthetized using isoflurane. The stylet from the outer guide cannulae were removed, and an injection cannulae made from silica tubing (Polymicro Technologies Cat.1068150018) of outer diameter 153 μm epoxyed to a 5 μl glass micropipette (Drummond scientific company Cat. 5-000-1001), was filled with either artificial cerebrospinal fluid (aCSF) or 5HT2A/C agonist (−)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) (Sigma Cat. D101). The silica injection cannula was inserted 1 mm past the guide cannula so that the tip was positioned in the medial preoptic area (mPOA) (−0.8mm Bregma, 0.5mm Lateral, 9mm Ventral) (Paxinos and Watson, 1982). Either aCSF or 10nM DOI (0.25 μl) was microinjected bilaterally into the mPOA. This dose of DOI has been shown to elicit a cardiovascular and hyperthermic response when injected into the hypothalamus (Lin et al., 1998; Bell et al., 1999). Twenty minutes after injection, rats were placed in the cold room and body temperatures were recorded. After the end of the cold room experiments, brains were extracted and verified for probe placement.
Western blotting
Western blotting for SERT was carried out in order to semi-quantitatively measure the amount of change in SERT in the mPOA between Stress and NoStress groups. Rats were decapitated four days after CUS corresponding to the time-point of exposure to the cold room. These rats received no drug treatments during the CUS procedure. Brains were extracted and frozen on dry ice. Two 500 μm thick sections were taken through the anterior hypothalamus between 0.3 to −1.3 mm Bregma rostro-caudally. The mPOA was dissected 1 mm from either side of the 3rd ventricle below the anterior commissure. The dissected sample was homogenized in 500 μl of ice cold 0.32 M sucrose and the suspension was centrifuged at 800 X g for 24 min at 4°C. The supernatant was collected and centrifuged for 17 min at 22,000 X g at 4°C. The supernatant was discarded and the pellet was re-suspended in 50 μl ice-cold Millipore purified water to obtain the synaptosomal fraction. Bradford protein assay was used to quantify the amount of protein in the synaptosomal fraction. Samples were then vortexed with lithium dodecyl sulfate (LDS) sample buffer without any heating and stored in −80°C. Protein (10μg) was loaded into each lane of 4–12% Bis-Tris gel and ran at 150 V for 90 min (running buffer: 50 mM MOPS (3-morpholinopropanesulfonic acid); 50 mM Tris base; 0.1% SDS; 1 mM EDTA; pH 7.7). Gels were then transferred at 28 V for 2 hrs on to polyvinylidene difluoride (PVDF) membrane (transfer buffer: 25 mM Bicine; 25 mM Bis-Tris; 1 mM EDTA; ph 7.2). The membrane was then blocked for 1 hr in Tris-Buffered Saline (TBST) (10 mM Tris; 150 mM NaCl; pH 8.0 with 0.5% Tween-20) containing 5% non-fat dry milk. The membranes were incubated overnight in Gt X SERT antibody at 1:1000 dilution (Santa Cruz, Cat. sc-1458). The following day, blots were washed 6 times, for 5 minutes each in TBST and incubated in horseradish peroxidase conjugated RbXGt secondary (Santa Cruz, Cat. sc-2004) for 2 hrs. This primary has been well studied and shown to be specific for SERT (McLane et al., 2007). Serotonin transporter (SERT) immunoreactive bands were visualized using enhanced chemiluminescent detection and quantified using LAS-4000 mini luminescent analyzer (FujiFilm, Stamford, CT). Blots were then washed for 10 min in TBST and incubated in Ms X actin antibody (Millipore, Cat. MAB1501) at 1:2500 dilution for 1 hr at room temperature, followed by horseradish peroxidase conjugated goat-anti-mouse secondary (Santa Cruz, Cat. sc-2005) for 30 min and developed. SERT immunoreactive bands were then normalized to corresponding actin on each blot.
Data analysis
Data analyses were conducted using SigmaPlot 11.0 and SAS software. Analyses of SERT protein expression were performed using unpaired t-test. Data analysis was conducted on temperatures obtained from basal transponder readings and those obtained at 30 min and 60 min in the cold room. A two-way repeated measures ANOVA followed by post-hoc Tukey’s multiple-comparison test was conducted to analyze results from body temperatures of NoStress and Stress groups that did not undergo any drug treatments. For all other experimental results, a three-way repeated measures ANOVA (Stress X Treatment X Time) followed by least squares multiple-comparison test was conducted. Rats receiving saline injection during CUS were also included in the cold room data analysis. Deviation from basal temperature was calculated as temperature in the cold room – basal temperature measured before cold room exposure. All data are presented as mean ± SEM and significance was determined at p < 0.05.
Results
CUS effects on basal body temperature
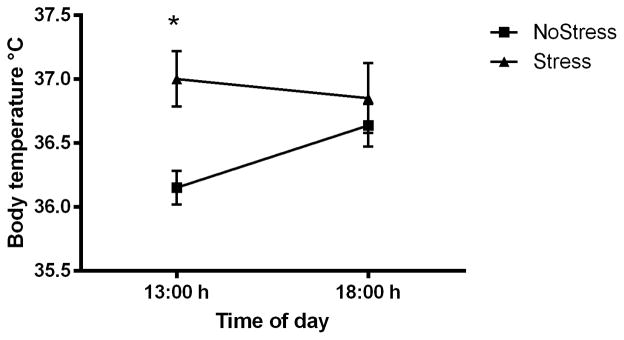
Body temperature was recorded from implanted subcutaneous (sc) transponders in NoStress and Stress rats to determine if 21 days of CUS affected basal resting temperature in the home cage at 13:00 h and 18:00 h, 4 days after exposure to CUS (Figure 1). The 13:00 h basal temperature was obtained prior to cold room exposure and the 18: 00 h temperature was taken after cold room exposure in the home cages. A two-way repeated measures ANOVA indicated a significant main effect of stress (F(1,12) = 6.14, p < 0.05). Stressed rats had significantly elevated body temperature of 37.00 ± 0.22°C at 13:00 h compared to NoStress rats (36.15 ± 0.13°C) (q = 4.34, p < 0.01). There was no significant difference in body temperature between the two groups when measured at 18:00 h in ambient temperature.
Figure 1.
Basal subcutaneous (sc) body temperature measured four days after 21 days of chronic unpredictable stress. Stressed rats significantly increased body temperature of 37.00 ± 0.22°C at 13:00 h compared to NoStress rats (36.15 ± 0.13°C). Values are mean ± SEM, (*, p < 0.01) (n = 6–8 per group).
CUS effects on body temperatures in cold ambient condition
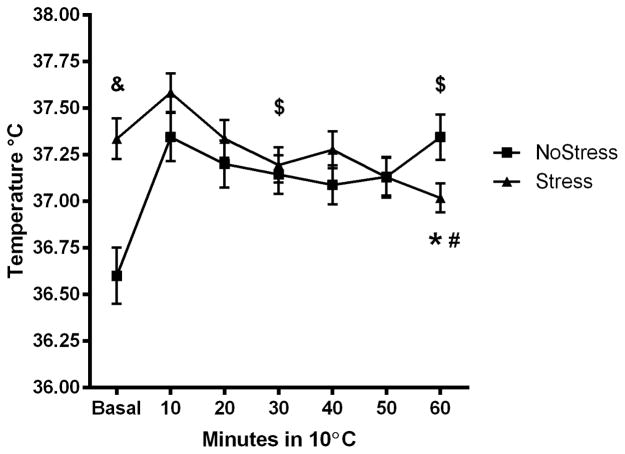
To determine if exposure to CUS caused protracted changes to thermoregulatory responses, thermogenesis during a cold room challenge was tested 4 days after termination of the CUS procedure. Stress and NoStress controls were placed in a cold room maintained at 10°C for 1 hour, 4 days after the end of the CUS procedure and the sc. temperatures was noted (Figure 2A). A significant main effect of Time (F(2,90) = 8.085, p < 0.001) and an interaction between Stress and Time spent in the cold room (F(2,90) = 33.11, p < 0.001) on body temperature was noted. The NoStress group significantly elevated body temperature from basal at 30 min (q = 9.11, p < 0.001) and 60 min (q = 11.63, p < 0.001). The basal body temperature was significantly different between NoStress and Stress rats (q = 8.19, p < 0.001) and the body temperature of the NoStress rats was significantly higher than the Stress rats at 60 min (q = 3.18, p < 0.05). Stressed rats showed a significant decrease in body temperature from basal after 60 min exposure to the cold room (q = 4.22, p < 0.05).
Figure 2.
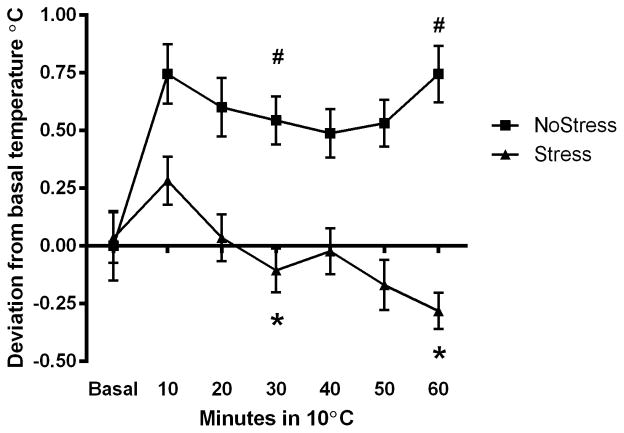
Body temperature (sc) of NoStress and Stress rats in the cold. Rats were placed in a cold room 4 days after last stressor and sc body temperature was recorded. Data were analyzed at basal, 30 and 60 min time points in the cold room. A) Body temperature in the cold room. Body temperature of Stress rats at 60 min in the cold room was significantly lower than basal (*, p < 0.05) and NoStress rats at 60 min in the cold room (#, p < 0.05). The basal temperature between NoStress and Stress were significantly different (&, p < 0.001). NoStress group significantly elevated body temperature from basal in the cold room at 30 and 60 min ($, p < 0.001) B) Change in body temperature in the cold room compared to basal. Body temperature of Stress rats was significantly lower than NoStress rats at 30 and 60 min in the cold room (*, p < 0.001). NoStress rats significantly elevated body temperature at 30 and 60 min in the cold room (#, p < 0.01) (n = 23–24 per group).
In order to illustrate difference in response to the cold room between NoStress and Stress, body temperature data was analyzed and graphed as change from basal temperature (Figure 2B). These results show a significant main effect of Stress (F(1,90) = 74.13, p < 0.001) and an interaction between Stress and time spent in the cold room (F(2,90) = 11.59, p < 0.001). The NoStress group significantly elevated body temperature from basal at 30 min (q = 5.43, p < 0.001) and 60 min (q = 6.91, p < 0.001). The body temperature of the NoStress rats was significantly higher than the Stress rats at 30 min (q = 7.21, p < 0.001) and 60 min (q = 10.01, p < 0.001).
Body temperature in the cold room obtained from transponders placed subcutaneously and intraperitoneally within the same rat
To determine if core body temperature differed from subcutaneous measures, temperature-transponders were implanted into the peritoneal cavity (ip.) and sc. within the same rat. The change from basal temperature in the cold room obtained from sc. transponders in NoStress and Stress groups at 30 min was (NoStress = 0.81 ± 0.22°C, Stress = −0.23 ± 0.18°C) and at 60 min (NoStress = 0.91 ± 0.18°C, Stress = −0.42 ± 0.14°C). A significant main effect of Stress (F(1,24) = 21.79, p < 0.001) and an interaction between Stress and time spent in the cold room (F(2,24) = 10.20, p < 0.001) on body temperature was noted. The NoStress group significantly elevated body temperature from basal at 30 min (q = 5.45, p < 0.01) and 60 min (q = 6.19, p < 0.001). The body temperature of the NoStress rats was significantly higher than the Stress rats at both 30 min (q = 6.09, p < 0.001) and 60 min (q = 7.74, p < 0.001) (Figure not shown).
The change from basal temperature in the cold room obtained from transponders placed ip. was an increase in NoStress and Stress groups at 30 min (NoStress = 1.80 ± 0.24°C, Stress = 0.98 ± 0.19°C) and at 60 min (NoStress = 1.84 ± 0.24°C, Stress = 1.05 ± 0.24°C). The ip. temperature results indicated a main effect of Stress (F(1,28) = 5.03, p < 0.05) and Time spent in the cold room (F(2,28) = 36.31, p < 0.001) and an interaction between Stress and Time (F(2,28) = 4.93, p < 0.05). The NoStress and Stress groups significantly elevated body temperature from basal at 30 min (NoStress q = 9.71, p < 0.001; Stress q = 4.49, p < 0.05) and 60 min (NoStress q = 10.45, p < 0.001; Stress q = 4.82, p < 0.01). However, the elevation in body temperature of the NoStress rats was significantly higher than the Stress rats at both 30 min (q = 3.72 p < 0.05) and 60 min (q = 4.01, p < 0.01) (Figure not shown).
Effect of CORT synthesis inhibition during CUS on basal body temperature and body temperature in response to cold ambient condition
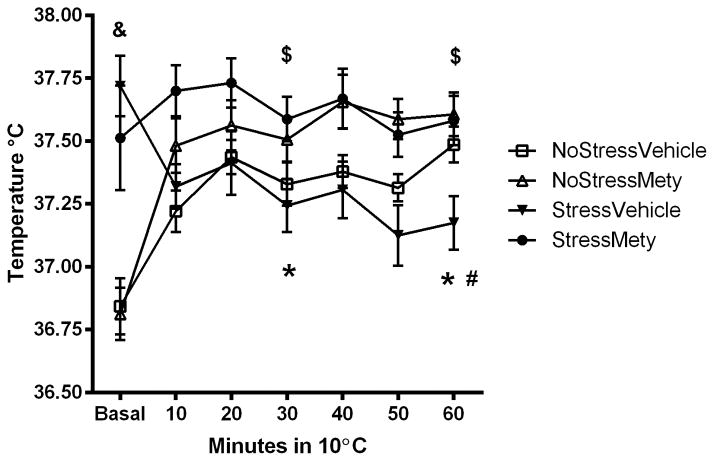
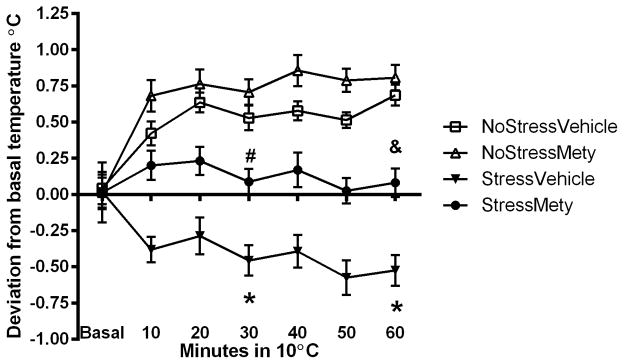
To determine if CORT synthesis inhibition affected thermoregulation during cold exposure, rats received either vehicle or Mety injections during CUS and sc-body temperature was recorded in the cold room 4 days after the end of the stress procedure (Figure 3A). A three-way repeated measures ANOVA showed a significant interaction between Stress, Treatment and Time in the cold room (F(11,113) = 12.84, p < 0.001). The NoStress groups significantly elevated body temperature in the cold room compared to basal: (NoStressVehicle at 30 min (t = −3.67, p < 0.001) and at 60 min (t = −7.19, p < 0.001); NoStressMety at 30 min (t = −4.48, p < 0.001) and 60 min (t = −5.00, p < 0.001)). The StressVehicle group significantly decreased body temperature in the cold room compared to basal at 30 min (t = 3.84, p < 0.001) and 60 min (t = 6.51, p < 0.001) in the cold. The basal temperature was significantly different between the NoStress and Stress groups: (StressVehicle vs. NoStressMety (t = −4.69, p < 0.01); StressVehicle vs. NoStressVehicle (t = −5.46, p < 0.01); StressMety vs. NoStressMety (t = −3.11, p < 0.01); StressMety vs. NoStressVehicle (t = 3.39, p < 0.01)). Furthermore, after 60 min exposure to the cold room, there was a significant difference in temperature between NoStressMety vs. StressVehicle (t = 3.44, p < 0.001) and StressMety vs. StressVehicle (t = 3.24, p < 0.01).
Figure 3.
Body temperature (sc) in cold of Metyrapone (Mety) pretreated rats. Rats were treated with vehicle or 50 mg/kg Mety, 15 min before each stressor. Data were analyzed at basal, 30 and 60 min time points in the cold room. A) 4 days after last stressor, rats were placed in a cold room and body temperature was recorded. StressVehicle group significantly decreased body temperature in the cold at 30 and 60 min compared to basal (*, p < 0.001). StressVehicle rats were significantly lower than NoStressMety and StressMety at 60 min in the cold (#, p < 0.05). The NoStressVehicle and NoStressMety rats significantly increased body temperature at 30 min and 60 min in the cold ($, p < 0.001). The basal body temperatures between NoStress and Stress groups were significantly different (&, p < 0.001). B) Deviation from basal body temperature in the cold room. Body temperature of StressVehicle rats was significantly lower than all other groups and Mety pretreatment prevented the lack of elevation in body temperature in the cold room (* p < 0.05, StressVehicle vs. all other groups; #, p < 0.05 StressMety vs. NoStressMety; &, p < 0.05, StressMety vs. NoStressMety and NoStressVehicle). (n = 14–16 per group).
To assess the differences between each treatment group in response to the cold room, body temperature data was analyzed as change from basal temperature (Figure 3B). There was a significant interaction between Stress, Treatment and Time (F(11,113) = 20.28, p < 0.0001). During cold room exposure, all comparisons except between NoStressMety and NoStressVehicle were significantly different from each other at 30 min: (NoStressMety vs. StressVehicle (t = 5.89, p < 0.001); NoStressMety vs. StressMety (q = 2.82, p < 0.001); NoStressVehicle vs. StressVehicle (t = 5.3, p < 0.001); NoStressVehicle vs. StressMety (t = −2.02, p < 0.001); and StressMety vs. StressVehicle (q = 2.77, p < 0.001)). After 60 min in the cold room, the StressMety group was significantly different from NoStressMety and NoStressVehicle groups (t = 3.23, p < 0.01 and t = −3.15, p < 0.01, respectively), and the StressVehicle group had a significantly lower body temperature compared to all other groups (StressVehicle vs. NoStressVehicle (t = 9.70, p < 0.001); StressVehicle vs NoStressMety (t = 7.46, p < 0.001); StressVehicle vs. StressMety (t = 3.42, p < 0.001)).
SERT in mPOA
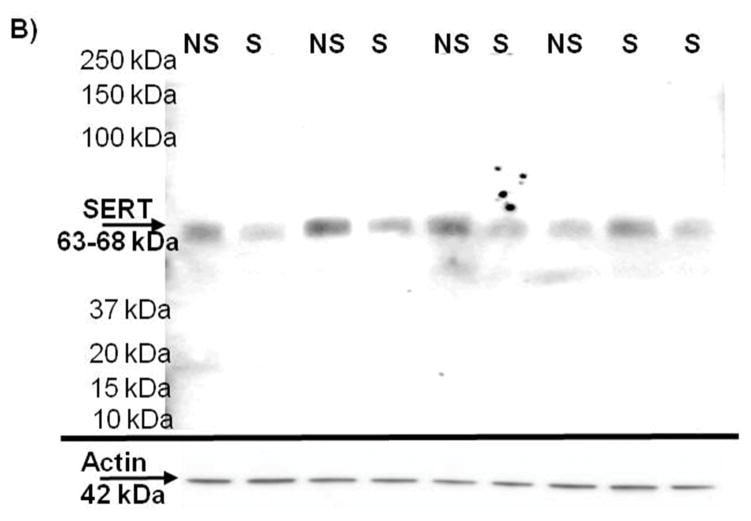
To determine if there was a decreased 5HT innervation of the mPOA, SERT immunoreactivity was measured 4 days after last stressor (Figure 4), in NoStress and Stress rats that did not undergo any drug pretreatment during CUS. SERT immunoreactivity was decreased in the mPOA synaptosomes of the Stress group and the difference of the means between the two groups was 43.66 ± 16.38% (t = 2.67, p < 0.05).
Figure 4.
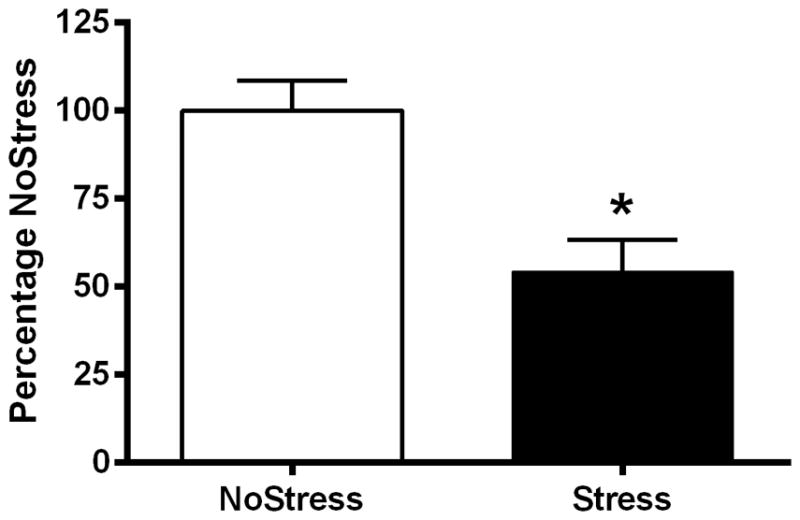
Serotonin transporter (SERT) immunoreactivity in the synaptosomes of the medial preoptic area (mPOA). A) Exposure to CUS significantly decreased SERT immunoreactivity in the mPOA 4 days after last stressor. (*, p < 0.05) (n = 5–7 per group). B) Representative Western blot for SERT immunoreactivity with actin loading control.
5HT2A/C receptor activation in mPOA
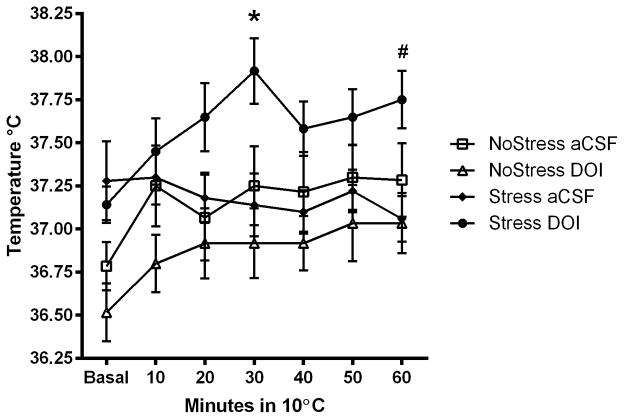
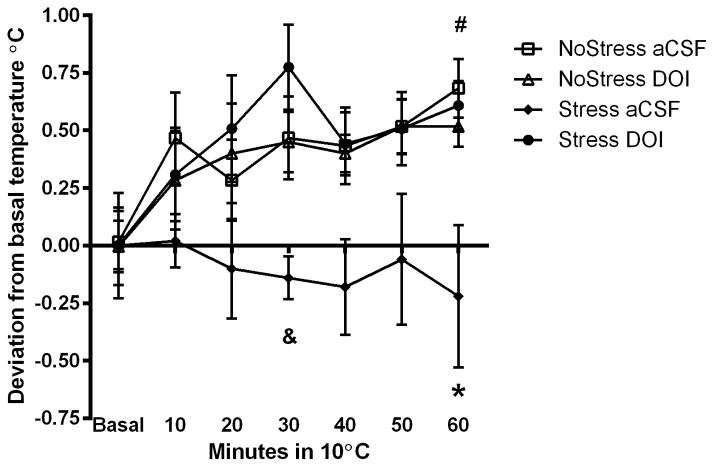
To determine if CUS-induced deficits in the cold room can be prevented by 5HT2A/C receptor activation in the mPOA specifically, aCSF or 5HT2A/C agonist DOI was injected bilaterally into the mPOA and sc. temperature was recorded in the cold (Figure 5A). A significant interaction between Stress, Treatment and Time spent in the cold room (F(11,33) = 12.63, p < 0.001) was noted. The NoStress+aCSF, NoStress+DOI, Stress+DOI rats significantly increased body temperature from basal in the cold room at 30 min (NoStress+aCSF (t = −3.54, p < 0.01); NoStress+DOI (t = −3.16, p < 0.01); Stress+DOI (t = −5.25, p < 0.01)) and 60 min (NoStress+aCSF (t = −2.87, p < 0.01); NoStress+DOI (t = −6.54, p < 0.01); Stress+DOI (q = −7.14, p < 0.01)). The Stress+DOI rats had significantly higher temperatures at 30 min compared to NoStress+DOI (t = −3.65, p < 0.001), NoStress+aCSF (t = 2.85, p < 0.01) and Stress+aCSF (t = 3.13, p < 0.01), and from Stress+aCSF (t = 3.53, p < 0.01) at 60 min in the cold room.
Figure 5.
Body temperature (sc) during cold exposure after serotonin 2A/C receptor agonist DOI injection into the mPOA. Data were analyzed at basal, 30 and 60 min time points in the cold room. A) 4 days after last stressor, DOI was injected 20 min before rats were placed in a cold room and body temperature was recorded. Stress+DOI significantly increased body temperature at 30 min compared to NoStress+aCSF, NoStress+DOI and Stress+aCSF (*, p < 0.05) and at 60 min was significantly different from Stress+aCSF (#, p < 0.05). B) NoStress controls and Stress+DOI treated rats elevated their sc. body temperatures from basal levels in response to cold room exposure 4 days after last stressor. Stress+aCSF treatment significantly decreased body temperature in the cold room compared to all other groups and DOI treatment in Stress rats prior to cold room blocked this effect. (*, p < 0.01, Stress+aCSF vs. all other groups at 60 min; &, p < 0.01; Stress+aCSF vs. Stress+DOI at 30 min; #, p < 0.05, Stress+DOI, NoStress+DOI and NoStress+aCSF at 60 min vs. basal) (n = 5–6 per group).
To examine differences in response to the cold room within each treatment group, body temperature data was analyzed as change from basal temperature (Figure 5B). Significant interactions between Stress, Treatment and Time in the cold room (F(11,33) = 7.88, p < 0.001) were revealed. Post-hoc tests indicate that cold room body temperatures of Stress+aCSF rats was significantly lower than Stress+DOI at the 30 min (t = 4.43, p < 0.001) and 60 min time points (t = 3.82, p < 0.001). Additionally, there was a significant increase in body temperature after 60 min in the cold room compared to basal temperature in the NoStress+aCSF, NoStress+DOI and Stress+DOI groups (t = −2.07, p < 0.05; t = −2.93, p < 0.01 and t = −3.88, p < 0.001, respectively), and NoStress+aCSF and NoStress+DOI groups significantly increased body temperature in the cold room at 60 min compared to Stress+aCSF (t = 3.30, p < 0.01 and t = 2.97, p < 0.01, respectively).
Discussion
Rats exposed to CUS had elevated basal sc-body temperature and decreased sc-body temperature when exposed to cold ambient conditions, unlike non-stressed controls. The decrease in sc-body temperature in response to a cold challenge was blocked by the systemic administration of a CORT synthesis inhibitor Mety prior to each stressor during CUS exposure. Furthermore, the mPOA of CUS rats exhibited a loss of SERT immunoreactivity and injection of the 5HT2A/C receptor agonist DOI into the mPOA prior to cold exposure elevated sc-body temperature of CUS rats in response to a cold challenge.
CUS elevated basal sc-body temperature during the light cycle when measured 4 days after termination of the stress period (Figure 1). Basal body temperature is affected by the circadian rhythm in naïve animals such that body temperature is elevated during the dark phase and lower in the light phase, corresponding to the animals’ wake and sleep cycles, respectively (Ushijima et al., 2006). Rats exposed to CUS maintained an elevated sc-body temperature at 13:00 h and 18:00 h before and after cold room exposure, whereas NoStress rats show significantly lower basal sc-body temperature at 13:00 h and increase it at 18:00 h corresponding to the onset of their wake cycle, suggesting a CUS induced alteration of the circadian effect on body temperature. The current results illustrated that CUS exposure disrupted this normal pattern of body temperature changes, particularly during the sleep cycle, long after termination of stress. These long-lasting increases in body temperature indicate sustained changes to central thermoregulatory function. Interestingly, the CUS paradigm appears to model the interaction between psychological stress and depression as patients diagnosed with depression have elevated nocturnal body temperature (Willner et al., 1992; Duncan, 1996; Willner, 1997). Furthermore, there are reports that psychological stress to humans causes an increase in basal body temperature that remains elevated for years (Willner, 1997; Oka et al., 2001).
The elevation in basal body temperature appears to be mediated by stress induced changes to the thermoregulatory response of the mPOA (Long et al., 1990; Briese & Cabanac, 1991; Hayashida et al., 2010), but the mechanisms underlying these changes are unknown. The mPOA has warm-sensitive neurons to keep heat-production and heat-loss in equilibrium to maintain homeostatic body temperature under normal ambient conditions. CUS shifts this equilibrium to favor increased thermogenesis by disinhibiting mPOA innervations to the dorsomedial hypothalamus, resulting in elevated basal body temperature (Morrison & Nakamura, 2011). Hence, we hypothesized that if stress changes mPOA function, it is likely that stress will also alter responses to a challenge of the thermoregulatory system. Furthermore, challenging the thermoregulatory system could reveal stress-induced changes within the mPOA during active thermoregulation that is not apparent under normal ambient conditions. Therefore, we challenged rats by exposure to cold ambient conditions. NoStress rats responded with typical elevations in body temperatures measured from both sc and ip transponders whereas Stressed rats showed a decrease in sc temperature over the course of 60 min in the cold (Figure 2A). Prior studies have shown that rats exposed to cold ambient conditions typically show increased body temperature and heart rate, and decreased tail temperature indicating that the elevation in body temperature in the cold room is due to a combination of increased thermogenesis and decreased heat dissipation (Ishiwata et al., 2002; Ishiwata et al., 2004). The elevation of body temperature in the cold depends on cutaneous vasoconstriction, brown adipose tissue thermogenesis and shivering, all of which are mediated by the mPOA (Rusyniak et al., 2008a; Morrison & Nakamura, 2011). Therefore, a decrease in sc-body temperature of the CUS rats in the cold compared to NoStress could indicate a dysfunction of mPOA that differentially affects sc-body temperature regulation. These differences could be due to a decrease in heat-generation and an increase in heat-loss produced by chronic stress exposure. Alternatively, the dissociation between ip and sc temperature of CUS rats could indicate an enhanced thermoregulatory response to conserve body heat in the cold.
Chronic exposure to stress alters the circadian rhythm of circulating plasma CORT and stress induced elevations in CORT (Pitman et al., 1988; Ishikawa et al., 1995). Since acute stress-induced hyperthermia and CORT elevations follow a similar time course (Veening et al., 2004), we examined if CUS-induced chronic elevation in CORT dysregulates normal CORT responses and contributes to the lack of hyperthermia in the cold room in stressed rats. Therefore, Mety, a compound that inhibits the 11-β-hydroxylation of deoxycorticosterone into CORT, was administered to rats prior to stress (Sonino, 1982). Roozendaal et al. (1996), have shown that Mety administration to stressed rats attenuated the stress-induced elevation in plasma CORT concentration but had no effect on CORT of non-stressed rats suggesting that Mety blocks increases in newly synthesized CORT in response to stress, without affecting normal basal levels of CORT. Our results show that StressVehicle and StressMety groups had similar elevated basal sc-body temperatures but a differential response to the cold challenge as the StressVehicle rats decreased sc-body temperature and the StressMety group maintained sc-body temperature in the cold (Figure 3A and 3B). The elevated basal sc-body temperature in CUS rats despite Mety treatment suggests that factors independent of CORT are involved in mediating elevations in basal temperature, and that basal temperature maintenance and response to cold challenge are differentially regulated. Since CORT is a downstream activator of stress responses and Mety treatments block only stress induced CORT synthesis without affecting an upstream initiator of the stress response such as corticotrophin releasing factor (CRF), CRF and CORT are likely involved in the thermoregulatory dysfunction due to stress.
CRF containing neurons in the paraventricular nucleus of the hypothalamus initiate stress responses (Leonard, 2006). The activity of the CRF neurons is tightly regulated by inputs from forebrain and brainstem afferents involving neurotransmitter systems such as GABA, norepinephrine and 5HT (Smith & Vale, 2006). CRF neurons of the hypothalamus send projections to the dorsal raphe (DRN), the largest 5HTergic nucleus in the brain (Kirby et al., 2000; Maier & Watkins, 2005). The DRN in turn, innervates the mPOA. Thus, chronic stress exposure can alter not only CORT but also CRF and consequently 5HT content or 5HT release in the mPOA to affect thermoregulatory function (Morimoto et al., 1993; Leonard, 2006).
5HT in the mPOA plays an important role in thermoregulation such that 5HT microinjection into the mPOA increases body temperature (Feldberg & Myers, 1965; Werner & Bienek, 1985). Furthermore, 5,6-dihydroxytryptamine injections into the anterior hypothalamus/POA lesions 5HT innervations and causes hyperthermia under basal ambient conditions and decreases body temperature in a cold environment (Myers, 1975). Therefore, to ascertain if there indeed was a decrease in 5HT in the mPOA produced by CUS, we examined SERT immunoreactivity as a marker of 5HT terminals. Figure 4A shows a decrease in SERT immunoreactivity in the mPOA of CUS rats and is suggestive of a decrease in 5HT innervation within the mPOA. It is also possible that SERT may be down-regulated by diminished release of 5HT from otherwise normal innervations by 5HT terminals. Interestingly, there is evidence of decreased 5HT immunoreactivity in the anterior hypothalamus of rats exhibiting depressive symptoms (Veenema et al., 2006) and depletion of 5HT using a synthesis blocker has been shown to decrease SERT mRNA in the mid-brain (Linnet et al., 1995). Regardless of whether there is a decrease in 5HT innervation or release after CUS exposure, a decrease in SERT could indicate a decrease in 5HTergic function in the mPOA.
Based on the findings of changes in SERT, we hypothesized that the inability of stressed rats to elevate sc-body temperature in response to a cold challenge would be mitigated by 5HT agonist injection into the mPOA. In fact, the microinjection of the 5HT2A/C agonist DOI into the mPOA prior to cold room exposure increased sc-body temperature of CUS rats in a cold environment (Figure 5A and 5B). DOI administered to NoStress rats did not affect their ability to increase sc-body temperature in the cold room similar to NoStress+aCSF rats indicating that DOI by itself did not alter body temperature. However, DOI administered to stressed rats increased sc-body temperature in the cold beyond other groups, suggesting that 5HT2A/C receptor activity may be increased or sensitized in stressed rats to compensate for stress induced changes to 5HTergic function in the mPOA. Interestingly, it has been shown that stress potentiated methamphetamine-induced increases in body temperature and CORT secretion and that these increases are blocked by the 5-HT2 antagonist ketanserin (Doyle & Yamamoto, 2010). Overall, the current results suggest that the lack of a hyperthermic response to the cold is due to a CUS-induced decrease in 5HT2A/C receptor activity caused by diminished 5HT in the mPOA.
Conclusions
The results suggest that chronic stress exposure can decrease 5HT in the mPOA to cause long-lasting thermoregulatory dysfunction. These results may help explain chronic stress disorders such as chronic fatigue syndrome where sustained low-grade psychogenic fever is characteristic (Komaroff et al., 1996; Oka et al., 2001). Therefore, understanding the effects of stress on mPOA function would have far reaching consequences in treating diseases associated with thermoregulatory dysfunction.
Acknowledgments
We would like to thank Kaila Mugford and Amy Ferng for assistance with the chronic unpredictable stress procedure. This work was supported by the National Institute of Health Grant DA007606.
Footnotes
Declaration of Interest
The authors declare no competing interests.
References
- Abrams JK, Johnson PL, Hollis JH, Lowry CA. Anatomic and functional topography of the dorsal raphe nucleus. Ann N Y Acad Sci. 2004;1018:46–57. doi: 10.1196/annals.1296.005. [DOI] [PubMed] [Google Scholar]
- Adell A, Casanovas JM, Artigas F. Comparative study in the rat of the actions of different types of stress on the release of 5-HT in raphe nuclei and forebrain areas. Neuropharmacology. 1997;36:735–741. doi: 10.1016/s0028-3908(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Bell A, Butz B, Alper R. Cardiovascular responses produced by microinjection of serotonin-receptor agonists into the paraventricular nucleus in conscious rats. Journal of cardiovascular pharmacology. 1999;33:175–180. doi: 10.1097/00005344-199902000-00001. [DOI] [PubMed] [Google Scholar]
- Boulant J. Hypothalamic mechanisms in thermoregulation. Federation proceedings. 1981;40:2843–2850. [PubMed] [Google Scholar]
- Briese E, Cabanac M. Stress hyperthermia: physiological arguments that it is a fever. Physiology & behavior. 1991;49:1153–1157. doi: 10.1016/0031-9384(91)90343-m. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Doyle JR, Yamamoto BK. Serotonin 2 receptor modulation of hyperthermia, corticosterone, and hippocampal serotonin depletions following serial exposure to chronic stress and methamphetamine. Psychoneuroendocrinology. 2010;35:629–633. doi: 10.1016/j.psyneuen.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan W. Circadian rhythms and the pharmacology of affective illness. Pharmacology & therapeutics. 1996;71:253–312. doi: 10.1016/s0163-7258(96)00092-7. [DOI] [PubMed] [Google Scholar]
- Endo Y, Shiraki K. Behavior and body temperature in rats following chronic foot shock or psychological stress exposure. Physiology & behavior. 2000;71:263–268. doi: 10.1016/s0031-9384(00)00339-5. [DOI] [PubMed] [Google Scholar]
- Feldberg W, Myers R. CHANGES IN TEMPERATURE PRODUCED BY MICRO-INJECTIONS OF AMINES INTO THE ANTERIOR HYPOTHALAMUS OF CATS. The Journal of physiology. 1965;177:239–245. doi: 10.1113/jphysiol.1965.sp007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale M, Dady K, Evans A, Lowry C. Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Experimental neurology. 2011;227:264–278. doi: 10.1016/j.expneurol.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Hale MW, Lowry CA. Functional topography of midbrain and pontine serotonergic systems: implications for synaptic regulation of serotonergic circuits. Psychopharmacology (Berl) 2011;213:243–264. doi: 10.1007/s00213-010-2089-z. [DOI] [PubMed] [Google Scholar]
- Hayashida S, Oka T, Mera T, Tsuji S. Repeated social defeat stress induces chronic hyperthermia in rats. Physiology & behavior. 2010;101:124–131. doi: 10.1016/j.physbeh.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Ohdo S, Watanabe H, Hara C, Ogawa N. Alteration in circadian rhythm of plasma corticosterone in rats following sociopsychological stress induced by communication box. Physiology & behavior. 1995;57:41–47. doi: 10.1016/0031-9384(94)00192-8. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Hasegawa H, Yazawa T, Otokawa M, Aihara Y. Functional role of the preoptic area and anterior hypothalamus in thermoregulation in freely moving rats. Neuroscience letters. 2002;325:167–170. doi: 10.1016/s0304-3940(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Saito T, Hasegawa H, Yazawa T, Otokawa M, Aihara Y. Changes of body temperature and extracellular serotonin level in the preoptic area and anterior hypothalamus after thermal or serotonergic pharmacological stimulation of freely moving rats. Life sciences. 2004;75:2665–2675. doi: 10.1016/j.lfs.2004.04.040. [DOI] [PubMed] [Google Scholar]
- Johnson BN, Yamamoto BK. Chronic stress enhances the corticosterone response and neurotoxicity to +3,4-methylenedioxymethamphetamine (MDMA): the role of ambient temperature. J Pharmacol Exp Ther. 2010;335:180–189. doi: 10.1124/jpet.110.171322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz R, Roth K, Carroll B. Acute and chronic stress effects on open field activity in the rat: implications for a model of depression. Neuroscience and biobehavioral reviews. 1981;5:247–251. doi: 10.1016/0149-7634(81)90005-1. [DOI] [PubMed] [Google Scholar]
- Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M. Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett. 1993;162:81–84. doi: 10.1016/0304-3940(93)90565-3. [DOI] [PubMed] [Google Scholar]
- Kirby L, Rice K, Valentino R. Effects of corticotropin-releasing factor on neuronal activity in the serotonergic dorsal raphe nucleus. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;22:148–162. doi: 10.1016/S0893-133X(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Kluger M, O’Reilly B, Shope T, Vander A. Further evidence that stress hyperthermia is a fever. Physiology & behavior. 1987;39:763–766. doi: 10.1016/0031-9384(87)90263-0. [DOI] [PubMed] [Google Scholar]
- Komaroff A, Fagioli L, Doolittle T, Gandek B, Gleit M, Guerriero R, Kornish R, Ware N, Ware J, Bates D. Health status in patients with chronic fatigue syndrome and in general population and disease comparison groups. The American journal of medicine. 1996;101:281–290. doi: 10.1016/S0002-9343(96)00174-X. [DOI] [PubMed] [Google Scholar]
- Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13:268–276. doi: 10.1159/000104854. [DOI] [PubMed] [Google Scholar]
- Lin MT, Tsay HJ, Su WH, Chueh FY. Changes in extracellular serotonin in rat hypothalamus affect thermoregulatory function. Am J Physiol. 1998;274:R1260–1267. doi: 10.1152/ajpregu.1998.274.5.R1260. [DOI] [PubMed] [Google Scholar]
- Linnet K, Koed K, Wiborg O, Gregersen N. Serotonin depletion decreases serotonin transporter mRNA levels in rat brain. Brain Research. 1995;697:251–253. doi: 10.1016/0006-8993(95)00906-7. [DOI] [PubMed] [Google Scholar]
- Long N, Vander A, Kluger M. Stress-induced rise of body temperature in rats is the same in warm and cool environments. Physiology & behavior. 1990;47:773–775. doi: 10.1016/0031-9384(90)90093-j. [DOI] [PubMed] [Google Scholar]
- Lucki I. The spectrum of behaviors influenced by serotonin. Biol Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Matuszewich L, Yamamoto BK. Long-lasting effects of chronic stress on DOI-induced hyperthermia in male rats. Psychopharmacology (Berl) 2003;169:169–175. doi: 10.1007/s00213-003-1498-7. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McLane M, Hatzidimitriou G, Yuan J, McCann U, Ricaurte G. Heating induces aggregation and decreases detection of serotonin transporter protein on western blots. Synapse (New York, NY) 2007;61:875–876. doi: 10.1002/syn.20438. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotrophin-releasing factor (CRF-41) in psychological stress in rats. The Journal of physiology. 1993;460:221–229. doi: 10.1113/jphysiol.1993.sp019468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Nakamura K. Central neural pathways for thermoregulation. Frontiers in bioscience (Landmark edition) 2011;16:74–104. doi: 10.2741/3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers RD. Impairment of thermoregulation, food and water intakes in the rat after hypothalamic injections of 5,6-dihydroxytryptamine. Brain Res. 1975;94:491–506. doi: 10.1016/0006-8993(75)90232-2. [DOI] [PubMed] [Google Scholar]
- Myers RD. Serotonin and thermoregulation: old and new views. J Physiol (Paris) 1981;77:505–513. [PubMed] [Google Scholar]
- Myers RD, Yaksh TL. Control of body temperature in the unanaesthetized monkey by cholinergic and aminergic systems in the hypothalamus. J Physiol. 1969;202:483–500. doi: 10.1113/jphysiol.1969.sp008822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. American journal of physiology Regulatory, integrative and comparative physiology. 2011;301:28. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Oka T, Oka K, Hori T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosomatic medicine. 2001;63:476–486. doi: 10.1097/00006842-200105000-00018. [DOI] [PubMed] [Google Scholar]
- Pitman D, Ottenweller J, Natelson B. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiology & behavior. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Bohus B, McGaugh JL. Dose-dependent suppression of adrenocortical activity with metyrapone: effects on emotion and memory. Psychoneuroendocrinology. 1996;21:681–693. doi: 10.1016/s0306-4530(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Rusyniak D, Ootsuka Y, Blessing W. When administered to rats in a cold environment, 3,4-methylenedioxymethamphetamine reduces brown adipose tissue thermogenesis and increases tail blood flow: effects of pretreatment with 5-HT1A and dopamine D2 antagonists. Neuroscience. 2008a;154:1619–1626. doi: 10.1016/j.neuroscience.2008.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyniak D, Zaretskaia M, Zaretsky D, DiMicco J. Microinjection of muscimol into the dorsomedial hypothalamus suppresses MDMA-evoked sympathetic and behavioral responses. Brain Research. 2008b;1226:116–123. doi: 10.1016/j.brainres.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S, Vale W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues in clinical neuroscience. 2006;8:383–395. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmerman R, Thompson J, Noordzij H, van der Meer J. Psychogenic periodic fever. The Netherlands journal of medicine. 1992;41:158–160. [PubMed] [Google Scholar]
- Ushijima K, Morikawa T, To H, Higuchi S, Ohdo S. Chronobiological disturbances with hyperthermia and hypercortisolism induced by chronic mild stress in rats. Behavioural brain research. 2006;173:326–330. doi: 10.1016/j.bbr.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Veenema A, Blume A, Niederle D, Buwalda B, Neumann I. Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. The European journal of neuroscience. 2006;24:1711–1720. doi: 10.1111/j.1460-9568.2006.05045.x. [DOI] [PubMed] [Google Scholar]
- Veening J, Bouwknecht J, Joosten HJ, Dederen P, Zethof TJ, Groenink L, van der Gugten J, Olivier B. Stress-induced hyperthermia in the mouse: c-fos expression, corticosterone and temperature changes. Progress in neuro-psychopharmacology & biological psychiatry. 2004;28:699–707. doi: 10.1016/j.pnpbp.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Werner J, Bienek A. The significance of nucleus raphe dorsalis and centralis for thermoafferent signal transmission to the preoptic area of the rat. Experimental brain research. 1985;59:543–547. doi: 10.1007/BF00261345. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]