Abstract
Objectives
To evaluate the effectiveness of behaviour changing interventions targeting ordering of thyroid function tests.
Design
Systematic review.
Data sources
MEDLINE, EMBASE and the Cochrane Database up to May 2015.
Eligibility criteria for selecting studies
We included studies evaluating the effectiveness of behaviour change interventions aiming to reduce ordering of thyroid function tests. Randomised controlled trials (RCTs), non-randomised controlled studies and before and after studies were included. There were no language restrictions.
Study appraisal and synthesis methods
2 reviewers independently screened all records identified by the electronic searches and reviewed the full text of any deemed potentially relevant. Study details were extracted from the included papers and their methodological quality assessed independently using a validated tool. Disagreements were resolved through discussion and arbitration by a third reviewer. Meta-analysis was not used.
Results
27 studies (28 papers) were included. They evaluated a range of interventions including guidelines/protocols, changes to funding policy, education, decision aids, reminders and audit/feedback; often intervention types were combined. The most common outcome measured was the rate of test ordering, but the effect on appropriateness, test ordering patterns and cost were also measured. 4 studies were RCTs. The majority of the studies were of poor or moderate methodological quality. The interventions were variable and poorly reported. Only 4 studies reported unsuccessful interventions but there was no clear pattern to link effect and intervention type or other characteristics.
Conclusions
The results suggest that behaviour change interventions are effective particularly in reducing the volume of thyroid function tests. However, due to the poor methodological quality and reporting of the studies, the likely presence of publication bias and the questionable relevance of some interventions to current day practice, we are unable to draw strong conclusions or recommend the implementation of specific intervention types. Further research is thus justified.
Trial registration number
CRD42014006192.
Keywords: thyroid function tests, test ordering, behaviour changing interventions, systematic review, medical tests
Strengths and limitations of this study.
The current systematic review was conducted following the methods recommended by the Cochrane Collaboration. We worked to a prespecified protocol and consider our findings to be robust.
This is the first review focusing specifically on the effectiveness of interventions designed to reduce unnecessary ordering of thyroid function tests.
The evidence suggests that, in general, such interventions are effective in reducing the volume, changing the pattern of ordering, improving compliance with guidelines or reducing the cost of thyroid function tests ordered. Whether such changes reflect more appropriate test ordering remains unclear as measures of appropriateness were rarely reported.
However, the poor quality of evidence, the significant heterogeneity in study design and the likely presence of publication bias and selective reporting did not allow strong conclusions and more specific recommendations to be made and precluded pooling the result from the individual studies.
Introduction
Thyroid dysfunctions including hypothyroidism and hyperthyroidism are among the most common medical conditions with prevalence 3.82% (3.77–3.86%) and incidence 259.12 (254.39–263.9) cases per 100 000/year in Europe.1 Both undertreatment and overtreatment of these conditions may have serious consequences for the patient's health and, therefore, correct and timely diagnosis and monitoring are important.2 3
The diagnosis of thyroid dysfunctions, however, is challenging as they present with common and non-specific symptoms: a range of laboratory investigations, such as thyroid-stimulating hormone (TSH), free thyroxine (FT4) and free tri-iodothyronine (FT3) are readily available to rule them in or out. In the UK alone 10 million thyroid function tests (TFTs) are ordered each year at an estimated cost of £30 million.4
Although national guidelines for the use of TFTs exist,4 a recent audit of general practitioners' (GPs) ordering patterns conducted by our group in the South West of England found that there is a sixfold variation in the rates of test requests between different practices. The study also demonstrated that only about 24% of this variation could be accounted for by variation in the prevalence of hypothyroidism and socioeconomic deprivation.5 The National Health Service (NHS) Atlas of Variation in Diagnostic Services published in November 20136 reported even more extreme variation in the annual rate of TFTs ordered by GPs per practice population across different primary care trusts in England. In this report, the estimated annual rate for TSH ordered by GPs ranged from 6.2 to 355.8 per 1000 practice population (57-fold variation). The reported numbers for FT4 and FT3 were 14.6–231.1 (16-fold) and 0.42–17.0 (40-fold) per 1000 practice population, respectively (p. 122).
A qualitative study we conducted identified a wide range of mechanisms that might be responsible for the variation, including the presence of inappropriate test ordering.7 Given the continuous rise of thyroid test requests,8 9 which is disproportionate to the increase in the incidence and prevalence of thyroid conditions,9 and the fact that these investigations make up a significant proportion of all laboratory tests ordered in primary care,10 there is a need to help clinicians avoid inappropriate thyroid testing. Such testing not only increases laboratory workload and wastes scarce resources but may also have a negative impact on patients' health through further unnecessary tests and inappropriate treatment.11
The effectiveness of interventions designed to reduce the number of unnecessary medical tests has already been evaluated in a number of systematic reviews.12–16 Owing to their broad scope, however, the results are too general and of little help when it comes to designing interventions that target specific test ordering behaviour. The effect of the same intervention may vary considerably across different tests, even when they belong to the same diagnostic modality.10 17–19 We conducted a systematic review investigating the effect of behavioural interventions on the ordering of TFTs. We believed a more narrowly focused approach with respect to target behaviour might produce more applicable results and thus better inform the development and implementation of interventions specifically designed to improve TFT ordering.
Methods
In conducting the review, we followed the recommendations of the Cochrane Collaboration.20 MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews were searched using a predefined search strategy (see online supplementary appendix 1). The original search covered the period up until November 2013 and was updated on 1 May 2015. Also, the bibliographies of the included studies and other relevant publications were scrutinised for additional articles. Studies were selected independently by two reviewers (ZZ and RA) with all disagreements resolved through discussion and, if necessary, arbitration by a third reviewer (CH or BV). In the first round, all electronically identified citations were screened at title and abstract level. Full-text copies of potentially relevant articles were retrieved for full-text screening. Studies were included in the review if they met the following prespecified criteria:
Evaluated the effectiveness of interventions designed to reduce the number of inappropriately ordered TFTs (regardless of whether they were the only targeted tests or not).
Were randomised controlled trials (RCTs), non-randomised controlled studies or single-group before and after studies (including both those with trend before and after and those with just one time point before and after).
The outcomes were one or more of the following: change in the total number of TFTs, the number of inappropriately ordered tests, the test-related expenditure or health benefits to individual patients (eg, the number of unnecessary tests or treatments avoided).
Reported the specific effect that the intervention had on the targeted TFTs.
bmjopen-2015-010065supp_appendix1.pdf (255.5KB, pdf)
Studies that targeted TFTs along with other tests and reported only the average effect (across all tests) were excluded. We included all studies that used the rate of inappropriately ordered TFTs as an outcome measure regardless of the definitions they used. Appropriateness of test ordering is usually judged against local protocols or guidelines that may vary from place to place or change over time. We accepted all definitions even when they were outdated or did not fit in with the current UK guidelines. We did not use the setting and the targeted clinicians' characteristics as inclusion criteria but explored, as far as possible, their potential impact on the study outcomes. The methodological quality of the included studies was assessed independently by ZZ and RA using the Effective Public Health Practice Project tool which allows the assessment of all study designs with the same rubric.21 The method of synthesis was narrative; meta-analysis was not used because of the anticipated clinical heterogeneity, particularly in terms of the interventions. The framework for the analysis was based on an existing typology of behaviour change intervention types:12 15
Educational interventions;
Guideline and protocol development and implementation;
Changes to funding policy;
Reminders of existing guidelines and protocols;
Decision-making tools, including test request forms and computer-based decision support;
Audit and feedback.
All work conformed with a protocol defined and published ahead of the review being started (PROSPERO, registration number CRD42014006192).
Results
The initial electronic searches produced 1282 hits of which, after removing duplicates, 869 were screened at title and abstract level and 99 were selected for full-text screening. Twenty five of these papers, with two additional papers identified through backward citation searching, met our prespecified criteria, and were included in the review.10 17–19 22–44 The update search identified another 131 records of which, after screening the titles and abstracts, 7 were selected for full-text screening and 1 met the inclusion criteria.45 It should be noted that two papers46 47 were excluded because in these studies TFTs were allocated to the control arm and, therefore, were not affected by the interventions. Thus, the total number of papers included in the review was 28 of which 2 reported on the same study, the second reporting a long-term follow-up.30 31 The selection process and the reasons for full-text exclusion are detailed in figure 1.
Figure 1.
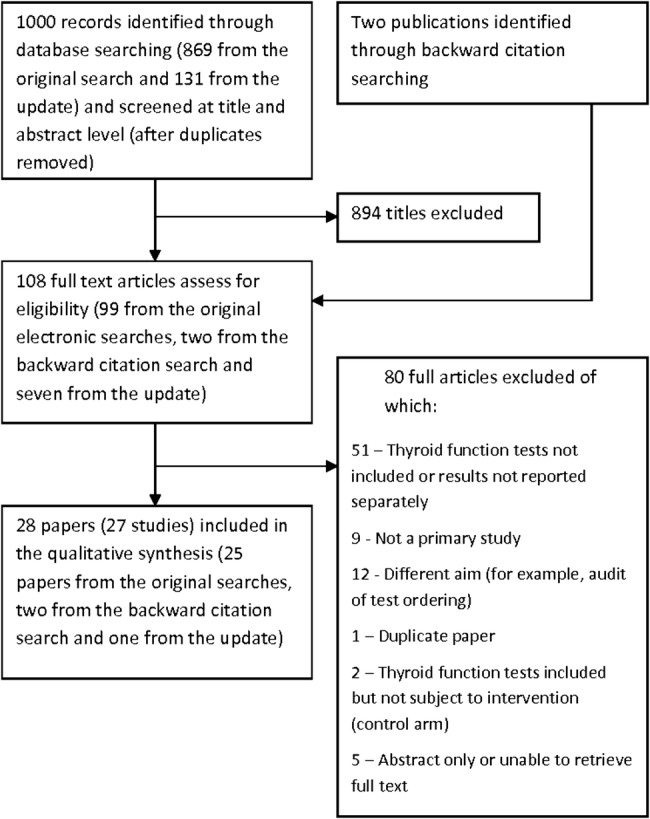
Flow chart of the selection process.
Study characteristics
The characteristics of the included studies are summarised in table 1 and the evaluated interventions are presented in table 2. Ten studies were conducted in the USA, six in the UK and the rest in Australia (n=3), France (n=3), Canada (n=2), the Netherlands (n=1), Sweden (n=1) and New Zealand (n=1). All studies were published in English, except for one in Dutch, which was partly translated by a native Dutch speaker with a background in healthcare research.38 The papers were published between 197943 and 2014:45 seven of them were published before 1991, nine between 1991 and 2000, and 12 after 2000. Fourteen studies were conducted in a hospital setting including general and psychiatric hospitals, medical assessment units, emergency departments and a supraregional liver unit, with the remainder in primary care or community settings (table 1).
Table 1.
Characteristics of the included studies
| Study and country | Study design | Setting | Targeted test users | Target tests | Thyroid tests |
|---|---|---|---|---|---|
| Adlan et al,22 UK | Before and after; single site | Medical assessment unit (acutely ill hospital patients) | Physicians | TFTs only | TSH, FT4, FT3, TPOAb, TRAb |
| Baker et al,23 UK | Cluster RCT | GP practices | GPs, locums, GPs in training and nurses | 5 frequently ordered laboratory tests suspected of being inappropriately ordered | TSH and FT4 |
| Berwick and Coltin,17 USA | Controlled cross-over; 3 sites | Ambulatory centres at health maintenance organisation | Internists and adult nurse practitioners | 13 laboratory and imaging tests suspected of being excessively ordered | TT4 |
| Chu et al,24 Australia | Before and after; single site | Adult tertiary referral teaching hospital ED | Interns and residents | Frequently ordered blood tests suspected of being excessively ordered | TFTs unspecified |
| Cipullo and Mostoufizadeh,19 USA | Before and after; single site | Community hospital | Medical staff (unspecified) | A range of high-volume laboratory procedures | TFTs unspecified but change in TT3 rate used as a measure of impact |
| Daucourt et al,25 France | Cluster RCT | General and psychiatric hospitals | Physicians | TFTs only | TSH, FT4, FT3, TRH test |
| Dowling et al,26 USA | Before and after; single site | Innercity community health centre | Family practice residents | TSHs only (complete blood count with differential used as a comparator) | TSH |
| Emerson and Emerson,27 USA | Before and after; single site | University medical centre | Residents | All laboratory tests | TSH, FT3, FT4, TT3, TT4 (individual and cascade) |
| Feldkamp and Carey,28 USA | Before and after; single site | Metropolitan hospital and 22 satellite clinics (inpatients and outpatients) | Physicians | TFTs only | TSH, TT3, TT4 |
| Gama et al,18 UK | Controlled study; single site | District general hospital (inpatients and outpatients) | General medicine physicians | All laboratory tests | TFTs unspecified |
| Grivell et al,29 Australia | Before and after; single site | Tertiary care community hospital | Consultants | 55 most commonly requested laboratory tests or test groups | TT4 |
| Hardwick et al,44 Canada | Before and after; multiple sites | All non-hospital-based laboratories in British Columbia | All users of non-hospital-based laboratories | TFTs only | TT3, TT4 |
| Horn et al,45 USA | Interrupted time series with a parallel control group; multiple sites | Alliance of 5 multispecialty group practices | Primary care physicians | 27 laboratory tests | TSH |
| Larsson et al,30 Mindemark and Larsson31 (follow-up), Sweden | Before and after; multiple sites | Primary healthcare centres | GPs and laboratory technicians | Various laboratory tests | TSH, FT4, TT4, TT3 |
| Nightingale et al,32 UK | Before and after; single site | Supraregional liver unit at teaching hospital | House officers | Various laboratory tests | TSH |
| Rhyne and Gehlbach,43 USA | Before and after; single site | Family medicine centre | Physicians | TFTs only | Thyroid function panel including TT4 and TT3 |
| Schectman et al,33 USA | Controlled study; single site | Primary care health maintenance organisation | Physicians, physician assistants and nurse practitioners | TFTs only | TSH, TT4, TT3 |
| Stuart et al,34 Australia | Before and after; single site | Urban public hospital ED | Consultants, registrars, junior medical officers and casual medical staff | All laboratory tests | TFTs unspecified |
| Thomas et al,10 UK | Cluster RCT | Primary care practices in 1 NHS covered area | Family practitioners | 9 laboratory tests suspected of being inappropriately ordered | TSH |
| Tierney et al,35 USA | RCT; single site | Academic general medicine practice | Physicians (residents and faculty) | 8 commonly ordered diagnostic tests | TSH |
| Tomlinet al,36 New Zealand | Controlled study; multiple sites | New Zealand primary care | All GPs on the New Zealand Medical Council's register compared with locum GPs and other medical specialists | TFTs only (but related programmes targeted inflammatory response tests and tests for infectious diarrhoea) | TSH, FT3, FT4 |
| Toubert et al,37 France | Before and after; single site | Teaching hospital | Physicians (various specialties, including endocrinologists) | TFTs only | TSH, FT3, FT4, TPOAb, TRAb, TgAb |
| van Gend et al,38 The Netherlands | Before and after; multiple sites | GP practices in 1 geographical area | GPs | 15 laboratory tests | TSH, FT4 |
| van Walraven et al,39 Canada | Retrospective interrupted time series; multiple sites | All private non-hospital-based laboratories in Ontario | Physicians ordering tests from non-hospital laboratories | 7 laboratory tests; 6 unaffected tests were chosen as controls | TSH, TT4, TT3 |
| Vidal-Trécan et al,40 France | Before and after; multiple sites | A network of 50 non-profit university hospitals in the Paris region | Physicians | TFTs only | TSH, TT4, TT3, FT3, FT4 |
| Willis and Datta,41 UK | Before and after; single site | Medical admissions unit at a district general hospital | Nurses | Three potentially inappropriately requested test sets: thyroid profile, lipid profile and coagulation screen | Thyroid profile (unspecified) |
| Wong et al,42 USA | Controlled study; single site | University teaching hospital | Physicians | TFTs, creatinine kinase and lactate dehydrogenase isoenzyme | TSH, TT4, TT3 |
ED, emergency department; FT3, free tri-iodothyronine; FT4, free thyroxine; GP, general practitioner; NHS, National Health Service; RCT, randomised controlled trial; TFTs, thyroid function tests; TgAb, thyroglobin antibody; TPOAb, thyroid peroxidase antibody; TRAb, thyrotropin receptor antibody; TRH, thyrotropin-releasing hormone test; TSH, thyroid-stimulating hormone; TT3, total tri-iodothyronine; TT4, total thyroxin.
Table 2.
Characteristics of the included studies: interventions
| Study and country | Setting | Educational programmes | Guidelines and protocols | Changes to funding | Reminders | Decision tools | Audit and feedback |
|---|---|---|---|---|---|---|---|
| Single-mechanism interventions | |||||||
| Adlan et al,22 UK | Hospital | X | |||||
| Berwick and Coltin,17 USA* | Primary care | X | XX | ||||
| Chu et al,24 Australia | Hospital | X | |||||
| Cipullo and Mostoufizadeh,19 USA | Hospital | X | |||||
| Daucourt et al,25 France* | Hospital | X | X | ||||
| Emerson and Emerson,27 USA | Primary care | X | |||||
| Feldkamp and Carey,28 USA | Hospital | X | |||||
| Gama et al,18 UK | Hospital | X | |||||
| Grivell et al,29 Australia | Hospital | X | |||||
| Horn et al,45 USA | Primary care | X | |||||
| Larsson et al,30 Mindemark and Larsson31 (follow-up), Sweden | Primary care | X | |||||
| Schectman et al,33 USA | Primary care | X | |||||
| Tierney et al,35 USA | Primary care | X | |||||
| Thomas et al,10 UK* | Primary care | X | |||||
| Multifaceted interventions | |||||||
| Baker et al,23 UK | Primary care | X | X | ||||
| Daucourt et al,25 France* | Hospital | X | X | ||||
| Dowling et al,26 USA | Primary care | X | X | ||||
| Hardwick et al,44 Canada | Primary care | X | X | ||||
| Nightingale et al,32 UK | Hospital | X | X | X | |||
| Rhyne and Gehlbach,43 USA | Primary care | X | X | ||||
| Schectman et al,33 USA* | Primary care | X | X | X | |||
| Stuart et al,34 Australia | Hospital | X | X | X | |||
| Thomas et al,10 UK* | Primary care | X | X | ||||
| Tomlin et al,36 New Zealand | Primary care | X | X | X | |||
| Toubert et al,37 France | Hospital | X | X | ||||
| van Gend et al,38 The Netherlands | Primary care | X | X | ||||
| van Walraven et al,39 Canada | Primary care | X | X | X | |||
| Vidal-Trécan et al,40 France | Hospital | X | X | X | |||
| Willis and Datta,41 UK | Hospital | X | X | ||||
| Wong et al,42 USA | Hospital | X | X | ||||
XX—two independent interventions of the same type.
*Study comparing directly two alternative interventions (Schectman et al33 had a non-comparative single-intervention design in its first phase and a multifaceted comparative design in the second phase; Thomas et al10 compared a single-mechanism (reminders) vs multifaceted (feedback plus reminders) interventions; Daucourt et al25 compared two single-mechanism interventions with their combination and usual practice defined as simple diffusion of guidelines).
Education, guidelines/protocols and audit/feedback were the most common types of intervention employed. Reminders and decision tools were less commonly used and changes to funding were assessed in only two studies (table 2). Only three studies reported evaluation of computer-based test ordering, two of which were quite old, published in 198835 and 1994,32 respectively. The recent one45 evaluated only a limited aspect of computerised test ordering—the display of costs of tests being ordered.
The median duration of the interventions was 12 months (IQR 6–12 months, range 2 days to 36 months) and four studies examined test ordering after the intervention had ended.26 30 31 33 35 Description of the interventions was usually limited and often insufficient to allow replication. Where detail was provided, it revealed significant variability in the design and implementation of interventions superficially belonging to the same category. For instance, educational interventions varied in terms of content, intensity and frequency, method of delivery, who delivered and who received the intervention as well as other characteristics which are likely to affect their effectiveness and appropriateness for different contexts and purposes. Most interventions were targeted at both senior and junior doctors. In four studies, only junior doctors were included;24 26 27 32 four studies included other medical staff, such as nurses, physician assistants and laboratory technicians,17 23 30 33 and in one study, the intervention was specifically directed at nurses41 (table 1).
In terms of targeted tests, 10 studies focused exclusively on TFTs22 25 26 28 33 36 37 40 43 44 while the rest targeted either a selection of laboratory tests suspected of being overutilised or had a wider scope including imaging as well as laboratory investigations. Of the targeted TFTs, five studies focused exclusively on TSH,10 26 32 35 45 two on TSH and FT4,23 38 four reported an average result without specifying the individual tests18 24 34 41 and the remaining studies targeted other combinations (table 1). As the studies spanned a long period of time, different generations of tests were used and the guidelines against which the appropriateness of test ordering was judged varied. However, in most studies, the recommended testing strategy was based on TSH as a single first-line test for suspected thyroid dysfunction and for monitoring patients on thyroid replacement hormones.
The effectiveness of the evaluated interventions with respect to TFTs is summarised in table 3 with additional information provided in table 4. The effect of the interventions was measured using a range of outcomes. Thus, 22 studies measured changes in the volume of test ordering expressed either as the absolute number of tests ordered for a period of time or normalised by the number of registered patients, visits or a similar parameter. To capture the effect on the pattern of test ordering, seven studies reported separate results for different TFTs27 28 36 37 40 42 44 and three studies measured the change in the ratios of two different tests (for instance, whether the ratio ‘TSH:all TFTs’ has increased as a result of new guidelines recommending TSH as a single first-line test).30 31 36 38 More direct evaluation of the appropriateness of testing was carried out by measuring adherence to protocols or guidelines25 26 32 33 37 43 with two of these studies reporting underutilisation as well as overutilisation.25 26 Five studies reported effectiveness in terms of expenditure34–36 39 44 and one study reported an estimate of the number of tests avoided as a result of the intervention.39 In one study, researchers made an effort to investigate whether the evaluated intervention (in this case, a test-ordering protocol) had had any adverse effects on patient outcomes by conducting an audit of 4000 case notes and concluded that “No adverse patient outcomes relating to underutilisation of investigations attributable to the protocol were identified.” (ref. 34, p. 133).
Table 3.
Summary of results: effectiveness of the interventions with respect to thyroid function tests
| Outcome | Study | Intervention |
Direction | Large effect | Statistically significant change | RCT design | Notes on outcome measures | |
|---|---|---|---|---|---|---|---|---|
| Single-mechanism interventions | ||||||||
| Test numbers or rates | Adlan et al22 | Guidelines | + | + | + | − | Per cent admissions offered TFTs | |
| Berwick and Coltin17 | Education (test specific) | + | − | NR | − | Per 100 encounters | ||
| Berwick and Coltin17 | Feedback on cost | + | − | NR | − | Per 100 encounters | ||
| Berwick and Coltin17 | Feedback on yield | − | + | NR | − | Per 100 encounters | ||
| Chu et al24 | Guidelines | + | + | + | − | Per 100 ED visits | ||
| Cipullo and Mostoufizadeh19 | Guidelines | + | − | NR | − | Per discharge | ||
| Gama et al18 | Feedback | + | + | + | − | Per outpatient visit | ||
| Emerson and Emerson27 | Request form redesign | + | − | + | − | |||
| Feldkamp and Carey28 | Guidelines | + | − | NR | − | Per 1000 patients | ||
| Grivell et al29 | Feedback | − | + | NR | − | |||
| Horn et al45 | Display of cost of tests being ordered | + | − | − | − | Per 1000 visits | ||
| Schectman et al33 | Educational memorandum | + | − | + | − | Per patient | ||
| Thomas et al10 | Feedback | + | − | + | + | Per 10 000 registered patients | ||
| Thomas et al10 | Reminders | + | − | + | + | Per 10 000 registered patients | ||
| Appropriateness (compliance) | Daucourt et al25 | Pocket memory card | + | − | − | + | Proportion of TFTs ordered in accordance with the guidelines | |
| Daucourt et al25 | Request form redesign | + | + | + | + | Proportion of TFTs ordered in accordance with the guidelines | ||
| Schectman et al33 | Educational memorandum | + | + | + | − | Compliance with TSH-only strategy | ||
| Schectman et al33 | Reminders | + | − | + | − | Compliance with TSH-only strategy | ||
| Expenditure | Tierney et al35 | Display of computer- generated probability estimates |
+ | − | − | + | Per visit | |
| CV | Berwick and Coltin17 | Education (test specific) | − | − | NR | − | ||
| Berwick and Coltin17 | Feedback on cost | + | + | NR | − | |||
| Berwick and Coltin17 | Feedback on yield | + | + | NR | − | |||
| Pattern | Emerson and Emerson27 | Request form redesign | + | + | NR | − | Sought to shift to TSH and thyroid cascade | |
| Feldkamp and Carey | Guidelines | + | + | NR | − | Sought to shift to TSH and TSH-based algorithm | ||
| Larsson et al28 and Mindemark and Larsson31 | Years 1–2 | Education | + | − | + | − | Sought to shift to TSH; and reduce ordering of TT3 and FT4 relative to TSH. Summary based on TSH/all TFTs ratios | |
| Larsson et al28 and Mindemark and Larsson31 | Years 2–6 | Education | − | − | + | − | Sought to shift to TSH; and reduce ordering of TT3 and FT4 relative to TSH. Summary based on TSH/all TFTs ratios | |
| Multiple-mechanism interventions | ||||||||
| Test numbers or rates | Baker et al | Education and guidelines | + | − | − | + | Per 1000 registered patients | |
| Daucourt et al | Pocket memory card and request form redesign | + | − | + | + | Proportion of TFTs ordered in accordance with the guidelines | ||
| Dowling et al26 | Education and feedback | + | − | + | − | Per patient visit | ||
| Hardwick et al44 | Funding policy and guidelines | + | − | NR | − | |||
| Rhyne and Gehlbach43 | Education and guidelines | + | + | + | − | Per 100 encounters | ||
| Schectman et al | Feedback and reminders | + | − | + | − | Per patient | ||
| Thomas et al10 | Feedback and reminders | + | − | + | + | Per 10 000 registered patients | ||
| Tomlin et al36 | Education and feedback and guidelines | + | + | + | − | Per year per GP | ||
| Toubert et al37 | Guidelines and reminders | + | + | NR | − | |||
| van Walraven et al39 | Guidelines and funding policy | + | + | + | − | Summary based on decrease in the proportion of TT4 and T3RU | ||
| van Walraven et al39 | Guidelines and request form redesign | + | − | + | − | Summary based on decrease in TSH utilisation | ||
| Vidal-Trécan et al40 | Education and guidelines and request form redesign | + | − | NR | − | Summary based on the total number of TFTs | ||
| Willis and Datta41 | Education and guidelines | + | + | + | − | Per admission | ||
| Wong et al42 | Guidelines and request form redesign | + | + | NR | − | |||
| Appropriateness (compliance) | Dowling et al26 | Education and feedback | + | + | + | − | Per cent TSH indicated | |
| Nightingale et al32 | Education and feedback and protocol management system | + | + | NR | − | Per cent patients requiring a particular investigation according to protocol who were actually tested | ||
| Rhyne and Gehlbach43 | Education and guidelines | + | − | − | − | Per cent ‘high’ and ‘low’ indications | ||
| Schectman et al33 | Feedback and reminders | + | − | − | − | |||
| Toubert et al37 | Guidelines and reminders | + | + | + | − | Per cent appropriate | ||
| Expenditure | Hardwick et al44 | Funding policy and guidelines | + | + | NR | − | ||
| Stuart et al34 | Education and feedback and guidelines | + | + | + | − | |||
| Tomlin et al36 | Education and feedback and guidelines | + | − | NR | − | |||
| Pattern | Hardwick et al44 | Funding policy and guidelines | + | − | NR | − | Sought to decrease proportion of TT3 as TFTs requested. Summary based on per cent of TFTs TT3 | |
| Tomlin et al36 | Education and feedback and guidelines | + | + | + | − | Sought to shift to TSH. Summary based on per cent TFTs TSH alone | ||
| Toubert et al37 | Guidelines and reminders | + | + | NR | − | Sought to shift to TSH. Summary based on per cent TFTs TSH alone | ||
| van Gend et al38 | Feedback and test form redesign | + | + | NR | − | Sought to shift away from TT4. Summary based on FT4:TSH ratio | ||
| Vidal-Trecan et al40 | Education and guidelines and request form redesign | + | − | NR | − | Sought to shift to TSH. Summary based on the proportion of FT3 and TSH | ||
| Wong et al42 | Guidelines and request form redesign | + | + | NR | − | Sought to decrease ordering of ‘complete’ thyroid panel to more selective use of individual tests | ||
Direction: ‘+’ indicates result favours behaviour change intervention; ‘−’ opposite. Large effect: ‘+’ indicates risk difference is ≥20%; ‘−’ <20%. Statistically significant change: ‘+’ indicates 95% CI do not include no effect or p<0.05; ‘−’ opposite. RCT design: ‘+’ indicates study design is RCT; ‘−’ not RCT.
CV, coefficient of variation; ED, emergency department; FT3, free tri-iodothyronine; FT4, free thyroxine; GP, general practitioner; NR, indicates item not reported; RCT, randomised controlled trial; T3RU, tri-iodothyronine resin uptake; TFTs, thyroid function tests; TSH, thyroid-stimulating hormone; TT3, total tri-iodothyronine; TT4, total thyroxin.
Table 4.
Summary of results: additional information
| Study and country | N at baseline | Intervention and outcome measure | Level at baseline | Level postintervention |
Effect on outcome | |
|---|---|---|---|---|---|---|
| Randomised controlled study designs | ||||||
| Baker et al23 UK | Practices: 17 (I) 16 (C) |
Intervention(s): G+FB Measure(s): median (IQR) number of TFTs per 1000 registered patients |
17.4 (8.0, 39.5) (I) 22.7 (10.4, 30.9) (C) |
19.8 (6.2, 42.3) (I) at 6 months 19.5 (10.3, 31.1) (C) at 6 months 17.7 (6.6, 43.3) (I) at 9 months 17.3 (10.1, 34.0) (C) at 9 months 13.2 (6.3, 35.7) (I) at 12 months 20.9 (13.3, 35.3) (C) at 12 months |
|
|
| Daucourt et al,25 France | Hospital wards:
|
Intervention(s): PMC, TRF, PMC+TRF Measure(s): GCR |
NA | 77.9% (95% CI 68.9% to 87.0%) (MPC+TRF) 82.6% (95% CI 73.1% to 92.1%) (TRF) 73.4% (95% CI 56.7% to 90.1%) (MPC) 62.0% (95% CI 47.7% to 76.4%) (C) |
|
|
| Thomas et al,10 UK | Practices:
|
Intervention(s): FB, R, FB+R Measure(s): median (IQR) TFT requests per 10 000 patients per practice |
750 (515–1329) (C) 829 (476–1412) (FB) 961 (476–1338) (R) 891 (392–1277) (FB+R) |
795 (552–1466) (C) 802 (432–1359) (FB) 891 (490–1250) (R) 800 (287–1077) (FB+R) |
|
|
| Tierney et al,35 USA | Scheduled visits:
|
Intervention(s): display of computer-generated probability estimates Measure(s): charges per scheduled visit (in USA$) |
NA | 1.25 (C) 1.12 (I) |
|
|
| Non-randomised controlled study designs | ||||||
| Berwick and Coltin,17 USA | 35 internists and 30 adult nurses at 3 centres (total number of visits not given) |
Intervention(s): TSE, FBC and FBY Measure(s): per cent change in the number of TT4 ordered per 100 encounters and the CV of test ordering rates |
TT4 tests per 100 encounters:
|
Change: | CV: |
|
| C: +1.7% | +15.7% | |||||
| TSE: −15.9% | +0.5% | |||||
| FBC: −12.1% | −23.6 | |||||
| FBY: +34.0% | −28.2 | |||||
| Gama et al,18 UK | Outpatient visits:
|
Intervention(s): FB Measure(s): TFTs per outpatient visit (mean, range) |
0.17 (0.12–0.22) (I) 0.05 (0.04–0.06) (C) |
0.13 (0.11–0.17) (I) 0.06 (0.05–0.08) (C) |
|
|
| Schectman et al,33 USA | 1425 patients, 30 clinicians (group distribution not given) |
Intervention(s): R and R+FB Measure(s): Compliance Mean (SE) number of TFTs ordered per patient |
68% (R) 65% (R+FB) 1.68 (0.04) |
81% (R) at 6 months 77% (R) at 12 months 64% (R+FB) at 6 months 80% (R+FB) at 12 months 1.37 (0.03) at 2/12 (following EM) 1.32 (0.05) at 6-month follow-up 1.49 (0.04) at 1-year follow-up |
|
|
| Tomlin et al,36 New Zealand | GPs:
|
|
223.6 (I) 33.8 (C) 144.2 (I) 29.1 (C) 41.6 (I) 11.0 (I) NR 2.4:1 7.1:1 NR |
215.2 (I) 32.0 (C) 80.7 (I) 25.3 (C) 26.6 (I) 9.4 (C) 21% decrease (I) NR (C) 3.0:1 8.5:1 −19.8% (I) −9.5% (C) |
TSH showed small decrease (4%, p<0.01) in the I group and no change in the C group (p<0.11). FT4 and FT3 decreased by 44.1% and 36.0%, respectively (p<0.01) in the I group and 13.1% and 14.6%, respectively, in the C group (p<0.01). In the I group, the proportion of TSH as the sole test ordered increased from 43.2% to 65.2% (p<0.001). Ratios of TSH to FT4 increased from 2.4:1 to 3.0:1 and TSH to FT3 from 7.7:1 to 8.5:1. Simultaneous testing of TSH and FT4 and/or FT3 decreased by 41.1% and there was a decrease in the net TFT expenditure (no p values given). |
|
| Wong et al,42 USA | NR |
Intervention(s): G+TRF Measure(s): tests per month |
Intervention tests (months to intervention):* T4 (RIA) and T3RU:
CK:
|
Intervention tests (months after intervention was introduced):* T4 (RIA) and T3RU:
CK:
|
Distributing guidelines through a bulletin alone failed to produce effect but in combination with request form redesign it led to restructuring of test ordering patterns with decrease of ‘complete’ thyroid panel and increase of hyperthyroid and hypothyroid panels and thyroid function screen. No changes in T4 (RIA) and T3RU but the number of T3 (RIA) and TSH tests ordered per month fell on the average to 38% and 61%, respectively, of the mean monthly rates at which these tests had been ordered in the preceding 18 months. No changes were observed in the ordering of the control tests. Statistical significance of the above results is NR. Not all data presented here! |
|
| Interrupted time series | ||||||
| Horn et al,45 | Average monthly orders per 1000 patient visits (TSH):
|
Intervention: display of cost of tests being ordered Measure(s): comparison of change-in-slope of the monthly ordering rates between intervention and control physicians for 12 months preintervention and 6 months postintervention |
Per cent change in monthly order rates (TSH, preintervention):
|
Per cent change in monthly order rates (TSH, postntervention):
|
The difference in the rate of change preintervention to postintervention was 0.7% decrease in the I group and 0.3% increase in the C group; none of these results was significant at p value <0.002 (2-sided Bonferroni-adjusted p value). Across all 27 evaluated tests, a statistically significant modest decrease in ordering rates of intervention physicians compared with control physicians was observed in 5 tests. |
|
| Van Walraven et al,39 Canada | NR |
Intervention(s): G+CFP; G+TRF Measure(s): avoided tests, utilisation rate and cost |
1 July 1991:
|
G+CFP:
|
G+CFP led to 96% decrease in the TT4 utilisation (p=0.02) and decrease in T3RU. Guidelines plus removing TSH ‘tick box’ from the request form resulted in 12% decrease in TSH utilisation (p=0.03). All interventions together resulted in a decrease of 626 098 tests, which saved $2 010 400, including $29 664 in the final year. |
|
| Before and after study designs | ||||||
| Adlan et al,22 UK | Admissions:
|
Intervention(s): G Measure(s): proportion of admitted patients offered TFTs |
53.8% (857 out of 1593) | 21.7% (255 out of 1176) | Significant reduction (32.1%, p<0.001) in the proportion of admitted patients offered TFTs | |
| Chu et al,24 Australia | ED visits:
|
Intervention(s): G Measure(s): mean number of TFTs ordered per 100 ED visits |
2.2 (20-week preintervention period) | 1.6 (20-week postintervention period) | Significant reduction in the mean number of TFTs (0.6 tests per 100 ED presentations, p=0.001) The mean number of all blood tests ordered per 100 ED presentations fell by 19% (p=0.001) and the mean cost fell by 17% (p=0.001). |
|
| Cipullo and Mostoufizadeh,39 USA | NA |
Intervention(s): G Measure(s): mean tests utilisation (T3 per discharge) |
0.006 (1 year before) | 0.005 (1 year after) |
The number of T3 uptake per discharge decreased by 17%. Most of the other targeted tests also showed decline in utilisation. Statistical significance NR. |
|
| Dowling et al,26 USA | Patient visits: 10 961 (pre), 6606 (post),3024 (at 5 months follow-up) |
Intervention(s): E+FB Measure(s): proportion of indicated TSH (out of all TSHs performed) Rate of TSHs ordered per patient visit (total number of TSHs and visits) Rate of indicated TSH per visit Rate of non-indicated TSHs per visit |
28% (25 of 90) 1 per 122 (90 in 10 961) 1 per 438 1 per 169 |
65% (15 of 23) 43% (9 of 21) (at 5 months follow-up) 1 per 287 (23 in 6 606) 1 per 178 (21 in 3 024) (at 5 months follow-up) 1 per 440 1 per 336 (at 5 months follow-up) 1 per 826 1 per 252 (at 5 months follow-up) |
The proportion of indicated TSHs increased significantly (p<0.001) while TSHs per patient visit decreased significantly (p<0.0001) in the intervention period but both showed some decline at 5 months follow-up. The rate of indicated TSHs per visit did not change significantly while the rate of non-indicated TSHs per visit decreased drastically in the intervention period but increased again at follow-up. Data for the control test, CBC with differential, is not shown here but the rate of ordering showed steady decline even in the follow-up and the rates of ordering both indicated and non-indicated CBCs decreased at the end of the intervention, although the statistical significance of these results was NR. |
|
| Emerson and Emerson,27 USA | Unclear |
Intervention(s): TRF Measure(s): number of tests/panels ordered preintervention to postintervention |
TSH: 5300* FT4: 750 TT4: 1700 TT3: 800 FTI/T3RU: 900 TFT cascade: NA Combined TSH and cascade: 5250 |
TSH: 3000* FT4: 1450 TT4: 200 TT3: 500 FTI/T3RU: 100 TFT cascade: 1700 Combined TSH and cascade: 4750 |
TFT testing decreased significantly (p<0.01) with a shift towards FT4 and thyroid cascade. Across all tests, the total number of tests remained the same (due to an increase in the number of patients) but the number of tests per patient visit showed significant decrease (p<0.01). |
|
| Feldkamp and Carey,28 USA | Sequential TFT requests: 1000 (pre) 463 (post, 1 year) 625 (post, 3 years) |
Intervention(s): G Measure(s): percentage of different TFTs and combinations: |
Prealgorithm: | 1 year | 2 years | ‘TSH only’ and DRTSH accounted for 92.4% of all TFTs 3 years after the introduction of the algorithm. The other combinations gradually decreased. However, the statistical significance of these results is NR. |
| TFT only | 33.3% | 44.8% | 32.2% | |||
| DRTSH algorithm | – | 24.4% | 60.2% | |||
| TSH+TT4+T3RU | 16.6% | 3.9% | 1.3% | |||
| TT4 only | 10.0% | 4.8% | 0.8% | |||
| TT4+T3RU | 6.8% | 2.8% | 1.1% | |||
| Others (including TT3) | – | – | 3.0% | |||
| TFTs per 1000 patients: | Prealgorithm: | Postalgorithm: DRTSH: |
Tests/1000 | |||
| TSH | 832 | 982 | 1000 | |||
| TT4 | 667 | 216 | 202 | |||
| T3RU | 234 | 159 | 202 | |||
| Total | 1 733 | 1359 | 1404 | |||
| Difference | – | 374 | 329 | |||
| Grivell et al,29 Australia | NR |
Intervention(s): FB Measure(s): ratio of thyroxin requests postintervention to preintervention |
NA | 1.20 | Thyroxin requests in the postintervention period were 1.2 times the requests in the preintervention period but the statistical significance of this result was NR. | |
| Hardwick et al,44 Canada | NR |
Intervention(s): G+CFP Measure(s): proportion (number) of different TFTs: |
1974/75 | 1976/77 | 1978/79 | Overall decline from baseline to 3 years postintervention (1978/79) with shift towards TT4 which accounted for 80.4% of all TFT investigations in the last period. Expenditure also decreased to 34% of the expected charges by the end of the study period. The statistical significance of these results was NR. |
| TT3 | 21.8% (29 004) | 19.0% (35 101) | 4.7% (7502) | |||
| TT4 | 51.8% (68 912) | 50.8% (93 988) | 80.4% (128 343) | |||
| ETR | 26.4% (35 183) | 30.2% (55 798) | 14.9% (23 703) | |||
| Total | 100% (133 099) | 100% (184 887) | 100% (159 548) | |||
| 1975/76 | 1977/78 | |||||
| TT3 | 20.4% (33 334) | 11.8% (19 255) | ||||
| TT4 | 49.6% (81 004) | 62.3% (101 805) | ||||
| ETR | 29.8% (48 832) | 25.9% (42 346) | ||||
| Total | 100% (163 170) | 100% (163 406) | ||||
| Horn et al,45 | Physicians: 153 (I) 62 (C) |
Intervention(s): Display of cost of tests being ordered (computer-based ordering system) Measure(s): difference in per cent change in monthly orders between I and C group (orders per 1000 patient visits) |
Baseline average monthly order rate (orders per 1000 visits):
|
Per cent change in monthly order rate:
|
Difference:
|
Monthly order rates for TSH decreased slightly in the I group and increased in the C group but the difference was not statistically significant (p=0.04; because 27 tests were analysed, the study used a 2-sided Bonferroni-adjusted p value of <0.002 to determine statistical significance). |
| Larsson et al,30 Sweden | 19 primary care centres |
Intervention(s): E Measure(s): mean ratios of the requests for related tests: |
1996 | 1997 | Significant decrease in TT3/TSH (difference between mean ratios 0.073, SD=0.089, p=0.0012) and non-significant decrease in FT4/TSH (difference 0.032, SD=0.116, p=0.13). As recommended, the ratio of TSH to all TFTs increase significantly (difference −0.017, SD=0.041, p=0.048). |
|
| TT3/TSH | 0.129 | 0.056 | ||||
| FT4/TSH | 0.333 | 0.301 | ||||
| TSH/total number of TFTs | 0.124 | 0.141 | ||||
| Mindemarkand Larsson31 (follow-up of Larsson 1999) | Median ratios: | 1997 | 2004 | 7 years after the intervention TT3/TSH and (TT4+FT4)/TSH were not significantly different from those at the end of the original study period, thus showing no decay in the intervention's effect. However, THS/all TFTs showed slight decrease (difference −0.043) which was statistically significant (p<0.05). Most of the other tests’ ratios also remained stable. |
||
| TT3/TSH | 0.029 | 0.022 | ||||
| (TT4+FT4)/TSH | 0.273 | 0.237 | ||||
| TSH/all TFTs | 0.115 | 0.072 | ||||
| Nightingale et al,32 UK | Number of patients: 654 (before) 833 (after) |
Intervention(s): PMS+E+FB Measure(s): change in compliance |
55%* | 85%* | Compliance of TSH requests increased but results are given only as a graph and the statistical significance is NR. Across all tests, the total number of tests requested per patient day declined by 17% (p<0.001). |
|
| Rhyne and Gehlbach,43 Canada | NR |
Intervention(s): G+E Measure(s):
|
October to December 1976 1.00 January to March 1977 Approximately 1.10 45% 29% |
June to August 1977 Approximately 0.70 September to November 1977 Approximately 1.00 53% 19% |
Significant decrease in the number of TFP ordered per encounter in the 3 months after the intervention (p<0.05) but return to baseline in the following 3 months. Results given only as a graph. The proportion of FTP for ‘high indications’ increased and that for ‘low indications’ decreased but was not statistically significant (p<0.05). Senior residents decreased their TFP ordering rate while that of junior residents increased (p<0.05). | |
| Schectman et al,33 USA | 1425 patients, 30 clinicians (group distribution not given) |
Intervention(s): EM Measure(s): compliance rate Mean (SE) number of TFTs ordered per patient |
35% 1.68 (0.04) |
67% at 2 months (following EM) 1.37 (0.03) at 2 months (following EM) 1.49 (0.04) at 1 year follow-up |
|
|
| Stuart et al,34 Australia | NR; annual census of 42 500 patients |
Intervention(s): E+G+FB Measure(s): mean cost of TFT (in $A) per patient |
0.426 | 0.047 | TFT ordering decreased by 89% and showed a significant decrease in cost per patient (−89% difference (95% CI −55% to −123%; p<0.001). Across all tests, there was 40% decrease in the ordering of tests with test utilisation falling from a mean of $39.32/patient to $23.72/patient (p<0.001). Tests not allowed to be ordered for ED patients, such as TFT, showed the greatest decrease. The effect was sustained at 18 months follow-up. |
|
| Toubert et al,37 France | 800-bed hospital with annual census of 25 266 inpatients and 242 013 outpatients |
Intervention(s): G+R Measure(s): number of TFTs: |
1996 | 1997 | 1998 | A substantial decrease in the total number of TFTs mainly due to a decrease in the number of FT3 and FT4; a decrease in the relative proportion of FT3. Single TSH order forms increased from 23% to 50%, while TSH+FT4+FT3 decreased. The statistical significance of these results was NR. The percentage of appropriate tests increased from 42.5% (95%CI 39.9% to 45.1%) in 1996 to 72.4% (95%CI 69.8% to 75%) in 1997 (p<0.0001) but there was some decrease in 1998 (no p value given). (Data for thyroid antibodies is not presented here.) |
| Total TSH tests | 1329 | 1119 | 1062 | |||
| Total FT4 tests | 1011 | 535 | 539 | |||
| Total FT3 tests | 715 | 247 | 226 | |||
| Total number of TFTs | 3145 | 1901 | 1827 | |||
| Patterns: | ||||||
| Single TSH | 305 | 563 | 512 | |||
| TSH+FT4 | 319 | 313 | 333 | |||
| TSH+FT3 | 23 | 25 | 20 | |||
| TSH+FT4+FT3 | 682 | 218 | 197 | |||
| FT4+FT3 | 10 | 4 | 9 | |||
| All TFT request forms | 1339 | 1123 | 1071 | |||
| Appropriateness: | 42.5% | 72.4% | 70.7% | |||
| van Gend et al,38 The Netherlands | NR |
Intervention(s): TRF+FB Measure(s): ratio of FT4 (removed from) to TSH (left on the request form) |
1992 0.96 (2498:2608) |
1993 0.31 (1180:3747) 1994 0.28 (1436:5040) |
There was a decrease in the FT4:TSH ratio indicating that the intervention had impact on test ordering patterns but the statistical significance of the results was NR. | |
| Vidal-Trécan et al,40 France |
June 1995: all TFTs: 27 945 |
Intervention(s): E+G+TRF Measure(s): number (%) of TFTs |
June 1995 | June 1998 | 11% decrease in the total number of TFTs even though the number of admissions increased by 2% and the number of outpatient visits by 6%. The proportion of FT4 tests remained the same (33%); the proportion of FT3 measurements decreased by 6% and the proportion of TSH tests increased. The statistical significance of the results was NR. |
|
| Total TFTs | 100% (27 945) | 88.7% (24 794) | ||||
| FT3 | 20% (5491) | 14% (3534 of 24 794) | ||||
| TT3 | 1% (339) | 1% (371 of 24 794) | ||||
| FT4 | 33% (9301) | 33% (8125 of 24 794) | ||||
| TT4 | 2% (478) | 1% (238 of 24 794) | ||||
| TSH | 44% (12 336) | 51% (12 526 of 24 794) | ||||
| Willis and Datta,41 UK | An average of 950 patients and 309 thyroid profiles per month |
Intervention(s): E+G Measure(s): mean (SD) number of TFT profiles per admission |
0.32 (0.05) | 0.08 (0.01) | Significant decrease in the number of requested thyroid profiles (p<0.001). Across all tests, a significant change between the total number of sets requested per admission before (7.5 (0.87)) and after the intervention (5.9 (0.33)), p<0.001. |
|
*Approximate reading off a graph.
C, control group; CBC, complete blood count; CFP, changes to funding policy; CK, creatinine kinase; CV, coefficient of variation; DRTSH, directed thyroid testing algorithm; E, education; ED,emergency department; EM, educational memorandum; ETR, effective thyroxin ratio; FB, feedback; FBC, feedback on cost; FBY, feedback on yield; FT3, free tri-iodothyronine; FT4, free thyroxine; FTI, free thyroid index; G, guidelines/protocol; GCR, guideline conformity rate, the proportion of TFTs ordered in accordance with the guidelines; GP, general practitioner; I, Intervention group; LDH, lactic dehydrogenase isoenzyme; NA, not available; NR, not reported; PMC, pocket memory card; PMS, protocol management system; R, reminders; T3 (RIA), tri-iodothyronine radioimmunoassay; T3RU, tri-iodothyronine resin uptake; T4 (RIA), thyroxin radioimmunoassay; TFP, thyroid function panel; TFTs, thyroid function tests; TRF, test request form redesign; TSE, test-specific education; TSH, thyroid-stimulating hormone; TT3, total tri-iodothyronine; TT4, total thyroxin.
Study quality
The results from the methodological quality assessment are presented in table 5. In terms of study design, four were RCTs,10 23 25 35 five studies were non-randomised controlled studies,17 18 33 36 42 two were interrupted time series39 45 and the remaining were single-group studies with just one time point measurement before and after. Most of the studies were of poor or moderate quality; the main issues being selection bias, lack of blinding and failure to control for confounders.
Table 5.
Results from the methodological quality assessment of the included studies using the EPHPP tool
| Study | Selection bias | Study design | Confounders | Blinding | Data collection method | Withdrawals and dropouts | Global rating |
|---|---|---|---|---|---|---|---|
| Randomised controlled trials | |||||||
| Baker et al23 | Strong | Strong | Strong | Moderate | Strong | Strong | Strong |
| Daucourt et al25 | Moderate | Strong | Strong | Strong | Moderate | Strong | Strong |
| Thomas et al10 | Strong | Strong | Strong | Strong | Strong | Strong | Strong |
| Tierney et al35 | Strong | Strong | Strong | Moderate | Strong | Strong | Strong |
| Non-randomised controlled studies | |||||||
| Berwick and Coltin17 | Moderate | Strong | Moderate | Weak | Strong | Weak | Weak |
| Gama et al18 | Weak | Moderate | Weak | Weak | Strong | Strong | Weak |
| Schectman et al33 | Moderate | Strong | Weak | Moderate | Strong | Strong | Moderate |
| Tomlin et al36 | Strong | Strong | Weak | Moderate | Strong | Moderate | Moderate |
| Wong et al42 | Weak | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Interrupted time series | |||||||
| Horn et al45 | Moderate | Moderate | Moderate | Weak | Strong | Moderate | Moderate |
| van Walraven et al39 | Strong | Moderate | Moderate | Strong | Strong | Strong | Strong |
| Before and after studies | |||||||
| Adlan et al22 | Weak | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Chu et al24 | Weak | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Cipullo and Mostoufizadeh19 | Weak | Moderate | Weak | Weak | Weak | Weak | Weak |
| Dowling et al26 | Weak | Moderate | Weak | Weak | Weak | Strong | Weak |
| Emerson and Emerson27 | Weak | Moderate | Weak | Weak | Strong | Weak | Weak |
| Feldkamp and Carey28 | Weak | Moderate | Weak | Weak | Strong | Weak | Weak |
| Grivell et al29 | Weak | Moderate | Weak | Weak | Strong | Weak | Weak |
| Hardwick et al44 | Strong | Moderate | Moderate | Strong | Strong | Strong | Moderate |
| Larsson et al30 | Moderate | Moderate | Weak | Moderate | Strong | Strong | Moderate |
| Nightingale et al32 | Weak | Moderate | Weak | Weak | Strong | Weak | Weak |
| Rhyne and Gehlbach43 | Weak | Moderate | Weak | Moderate | Strong | Weak | Weak |
| Stuart et al34 | Weak | Moderate | Weak | Moderate | Moderate | Moderate | Weak |
| Toubert et al36 | Weak | Moderate | Weak | Moderate | Moderate | Weak | Weak |
| van Gend et al38 | Strong | Moderate | Weak | Moderate | Strong | Strong | Moderate |
| Vidal-Trécan et al40 | Moderate | Moderate | Moderate | Moderate | Strong | Strong | Strong |
| Willis and Datta41 | Weak | Moderate | Weak | Moderate | Strong | Weak | Weak |
Effectiveness of the interventions
Single-mechanism interventions
Fourteen studies10 17–19 22 24 25 27–31 33 35 45 evaluated the effectiveness of the following single-mechanism interventions (tables 2–4): educational programmes (one controlled and two before and after studies),17 30 33 guidelines and protocols (four before and after studies),19 22 24 28 reminders (two RCT and one controlled study),10 25 33 decision-making tools (two RCTs, one interrupted time series and one before and after study),25 27 35 45 and audit and feedback (two controlled and two before and after studies).17 18 29 33 The study by Schectman et al33 was a two-stage study before and after design in the first part and a comparative design in the second. The majority of the evaluated single-mechanism interventions were effective in decreasing test-related expenditure,35 the volume of test ordering,17 18 22 24 27–29 33 changing the pattern of TFT ordering19 27 28 30 31 or increasing compliance25 33 in accordance with the recommended practice.
Two of these studies reported data on test ordering once the intervention was discontinued. Mindemark and Larsson31 investigated the effect of the 2-day educational programme originally evaluated by Larsson et al30 in a before and after study. Eight years after the programme was delivered, they found that the ratios between pairs of different TFTs was similar to that measured at the end of the original study (1 year after the delivery of the programme). Only the ratio ‘TSH:all TFTs’ showed slight but statistically significant decrease which the authors explained with the recommendation given to participants to analyse TSH in elderly patients who had not been tested in the previous 2–3 years (table 4). Although impressive, the observed result is difficult to explain with the educational programme alone as other contextual factors are likely to have contributed to the persistence of the effect.
Tierney et al35 reported that 6 months after the intervention (display of computer-generated probability estimates evaluated in an RCT) the difference between intervention and control group has disappeared and the main outcome—charges per scheduled visit—has returned to baseline (table 4).
Three studies reported unsuccessful interventions: an RCT of good methodological quality demonstrated that a reminder in the form of a memorandum pocket card was unsuccessful in increasing compliance with the recommended thyroid testing strategy;25 a poor quality before and after study showed that monthly feedback given to consultants for a period of 1 year was unable to decrease the ordering rates of a number of laboratory tests;29 and an interrupted time series of moderate methodological quality demonstrated that displaying the cost of tests at the time of ordering was moderately effective in a small number of tests but did not affect the ordering of TFTs.45 The former two studies were conducted in a hospital setting and the latter was a primary care study.
The authors of the before and after study explained the failure of the intervention by the prevailing institutional culture; the fact that the clinical units ordering the largest proportion of tests showed little concern and attributed their requesting pattern to clinical workload and the nature of their patients; and by the fact that the feedback was provided to consultants only, on their request, while many of the tests were ordered by junior doctors unaffected by the intervention.29 The authors of the interrupted time series study surveyed all intervention and non-intervention physicians to investigate their perceptions regarding the intervention and healthcare costs in general. They found that while nearly all participants endorsed the need for cost containment and found the display of costs informative, 50% of them reported that the displays ‘rarely’ or ‘never’ impacted their decision to order the tests.45
Berwick and colleagues who compared two different types of feedback, on cost and on yield, with test-specific education reported mixed results. Across all tests, only feedback on cost showed statistically significant effect, whereas for the TFTs test-specific education had the largest effect. With regard to reducing variability in test ordering, the two forms of feedback but not education led to a positive change. The statistical significance of the results specific to TFTs, however, was not reported.17
Multifaceted interventions
Sixteen studies evaluated interventions that relied on more than one mechanism to change test ordering behaviour (tables 2–4).10 23 25 26 32–34 36–44 Ten of them combined the introduction of guidelines or protocols with audit and feedback,23 education,41 43 redesign of a test request form,39 42 changes to funding policy,39 44 education plus audit and feedback,34 36 reminders,37 or education plus a test request form.40 Of the remaining six studies, three evaluated the combination of audit and feedback with education,26 reminders10 or a problem-oriented test request form;38 one study evaluated the effectiveness of a computer-based protocol management system enhanced with audit and feedback and education;32 one compared an educational memorandum followed by a reminder with the same educational memorandum followed by a reminder and feedback;33 and one compared a combination of pocket memory card (reminder) and redesign of test request form with the same single-mechanism interventions and usual practice defined as simple diffusion of guidelines.25
With the exception of one study,23 all reported that the evaluated multifaceted interventions were effective in decreasing the volume of test ordering,10 23 26 33 36 37 39–41 43 44 changing the pattern of test ordering in accordance with the recommended practice,26 33 37–40 42–44 avoiding unnecessary testing39 and/or decreasing test-related expenditure.34 36 39
Two before and after studies reported data on test ordering once the intervention was discontinued.26 33 Dowling and colleagues evaluated the effectiveness of education plus feedback and measured the rate of TSH ordered per patient visit and the indicated and non-indicated TSH per visit. Despite the initial statistically significant effect, 5 months after the intervention was discontinued, all indicators showed some decline. Schectman et al33 evaluated the effect of educational memorandum followed by reminder or reminder and feedback on compliance and the mean number of TFTs ordered per patients. The interventions increased compliance and led to significant decrease in test ordering which continued 6 months after the interventions but increased again at 1 year follow-up (table 4).
The study that reported an unsuccessful intervention was an RCT of good methodological quality and was conducted in primary care. It targeted the use of five frequently ordered laboratory tests including TSH and FT4 and evaluated the effectiveness of a combination of guidelines and feedback. The authors explained the failure of the intervention by the following: the feedback was provided for 1 year only, the participating practices did not volunteer to take part in the study and the guidelines might not have been sufficient to predispose physicians to change their test ordering behaviour.23
Owing to the significant heterogeneity and poor methodological quality of the studies, we deemed pooling the results inappropriate and were unable to use statistical methods to investigate the impact of various study and intervention characteristics on the reported outcomes. Visual inspection of the data suggests, however, that differences such as intervention type, study design, setting and year of publication have little or no impact on the reported effectiveness. We created a spreadsheet which can be used by the readers to explore this themselves by sorting the results according to different study characteristics (see online supplementary appendix 2).
bmjopen-2015-010065supp_appendix2.pdf (50.4KB, pdf)
Discussion
Main findings
This systematic review of behaviour change interventions designed to modify the ordering of TFTs found 27 studies. Several intervention types were evaluated including education, guidelines and protocols, audit and feedback, decision-making tools, changes to funding policy and reminders, either alone or in combination, in either primary or hospital care, and targeting clinicians of different seniority. Most of the studies were of poor or moderate quality and many of the interventions were poorly described.
In these studies, it appears that behaviour change interventions were, in general, effective in reducing the volume, changing the pattern of test ordering, improving compliance with guidelines or reducing the cost of TFTs ordered. Whether such changes reflect more appropriate test ordering, however, was unclear as in the majority of studies measures of appropriateness were undefined, and thus unreported. No study investigated directly any impact on patient outcomes. Only five studies observed the effect of the intervention on test ordering for more than 12 months28 34 36 37 44 and only four reported the persistence of effect once the intervention had ended,26 30 31 33 35 of which three reported return to baseline or decline within 1 year.26 33 35
Although not the subject of an a priori subgroup analysis, multifaceted interventions did not appear to be more effective than single-mechanism ones. The specific type of intervention(s) appeared less important than the interaction between various intervention-specific variables and the implementation of the intervention in a specific context. For instance, feedback could be very successful if there was a strong institutional support for change26 or completely ineffective if the changes it was trying to introduce clashed with the dominant institutional culture.29 Even within a single study, the same intervention performed differently in different clinical circumstances (eg, inpatients vs outpatients)18 or different interventions seemed to be effective for different type of tests.17 45 As Mindemark and Larsson31 put it “…the most decisive factor for the success of a strategy in optimizing test ordering is not the nature of the intervention itself, but rather its design and implementation in a given setting.” (p. 485)
The effectiveness of the interventions depended to some extent on the outcomes the researchers had chosen to measure. These outcomes reflected specific assumptions about what constitutes inappropriate test ordering and how this could be changed. ‘Appropriateness’ is to a large extent, a value judgement, incorporating elements of importance of the diagnosis, benefits from early diagnosis (and the converse, harms from delays in diagnosis), burden and unpleasantness of the test, plus economic considerations. These aspects were rarely—if ever—explicitly reported as part of the rationale for the intervention and selection of the outcome measure. It was simpler to examine the volume of testing or the shift in the pattern of TFTs ordered which most studies did, but clinically, this is a blunt measure of appropriateness.
For instance, in one study a one-off educational event encouraged GPs to use TSH as a single first-line test30 and the intervention was reported to have a long-term effect.31 The following ratios were used as an outcome measures: ‘TSH:all TFTs’ (which was expected to increase) and ‘T3:TSH’ and ‘FT4:TSH’ (which were expected to decrease as a result). Therefore, the intervention did not address inappropriate ordering of TSH and the chosen outcomes could not capture a possible ‘shift’ where doctors ordered inappropriately more TSHs while ordering fewer T3 and FT4 tests. An interrupted time series analysis which investigated the effect of a series of interventions over a period of several years clearly demonstrates such a possibility.39
Strengths and limitations of study
We conducted the current systematic review using the methods recommended by the Cochrane Collaboration working to a prespecified protocol and consider our findings to be robust. This is the first review focusing specifically on the effectiveness of interventions designed to reduce unnecessary ordering of TFTs. The identified evidence is directly relevant to this particular test ordering behaviour and could be used to guide the design and implementation of future intervention programmes as well as the development of research projects that could address the identified gaps in knowledge. The main limitation is that the quality of evidence did not allow strong conclusions and more specific recommendations to be made. Furthermore, the disparate methods, populations of study, interventions and outcome measures made pooled synthesis of results impossible. Thus, we have chosen to present the results as a narrative synthesis. Similarly, although we strongly suspect that publication bias and selective reporting of outcomes may be operating, particularly for the non-randomised study designs, we could neither investigate nor attempt to quantify the potential impact. We think it is likely that publication bias has exaggerated the results, but is highly unlikely to completely account for the overall beneficial pattern observed. That the more rigorous designs gave less marked and even negative results (tables 3 and 4 and online supplementary appendix 2) adds a note of caution however.
Comparison with other studies
We are not aware of another systematic review with a similar focus. Other systematic reviews have examined the effectiveness of behavioural interventions designed to influence test ordering as a whole.12–16 Our review focused on a specific diagnostic scenario—the use of laboratory tests to diagnose and monitor thyroid dysfunction.
Clinical interpretation of the results
Superficially, based on the predominant pattern of favourable results from the included studies, there would seem to be little doubt that where there is evidence that TFTs ordering needs to be modified, the interventions employed in the included studies could be used to effectively reduce volume, improve compliance, change the pattern of testing or reduce cost. However, we believe some caution is required. The most fundamental issue is that in many included studies, the detail about the nature of the intervention is insufficient for implementation; the situation is particularly acute where the target behaviour is appropriateness, because the value of achieving compliance is completely dependent on the definition of appropriateness being used and how it was derived. The problem concerning insufficient definition of the intervention has been noted before and is, we believe, particularly relevant here.48 Although, additional details may exist outside the published paper and be obtained through personal contact with the investigators, much information about the interventions is likely to be unavailable, particularly for older studies.
Similarly, the current applicability of many of the interventions can be questioned. The circumstances operating in many of the studies may not be similar to the challenges today. A simple example from the study by Thomas et al,10 clearly applicable to UK primary care, is that the rates of test ordering were in the region of 800 per 10 000 practice patients, whereas the equivalent rates in a recent study in the South West were 2500 per 10000.5 The importance of this is accentuated by the fact that interventions do not seem to have been designed in the light of investigations to understand the origin of the difficulties underlying the behaviour they were attempting to address. Thus, in primary care, it is widely accepted that the reason why GPs order tests is frequently not for medical reasons,49 yet most interventions we encountered, such as education and guidelines, assume that lack of medical knowledge is the underlying difficulty. Our own investigations of GPs' reasons why TFT ordering rates might vary, included many factors such as quality of computer systems, communication with hospital systems, general attitude to risk, involvement of other members of the primary care team in test ordering and patient expectations, all issues which are unlikely to have been addressed by any of the interventions we observed.7 Our concerns about openness to bias of the included studies and possibility of publication and outcome reporting bias reinforce our circumspection about whether the evidence reviewed is good enough to implement.
Given the fact that the majority of TFTs are ordered by GPs in the UK5 and that guidelines for the use of these tests in primary care already exist (though appear to be ineffective), interventions that raise clinicians' awareness of these guidelines, ‘translate’ them into easy to follow rules and embed them in decision aids are potentially effective combination. This review suggests that most interventions succeed (albeit in the limited way described above), so it is probable that an intervention can be designed that would work in UK primary care. Other factors will still be relevant, as highlighted by the qualitative study, such as lack of communication, problems with storage and retrieval of previous results, and lack of local protocols that structure the ordering of TFTs in accordance with the existing guidelines.7
Conclusions and policy implications
The systematic review we have conducted indicates that behaviour change interventions can modify TFT ordering. While a starting point for implementation, we do not believe the evidence base is complete and strongly recommend further research. As well as overcoming the limitations highlighted concerning bias, improving details of the interventions to be implemented and improving applicability to current challenges, new research can also address questions barely touched on by the existing evidence base. Such questions include the effectiveness of interventions like computerised test ordering systems (order.com's) in primary care, how to maintain the effect on test ordering over several years and cost-effectiveness. The scale of the problem is important in this regard. While a few of the included studies had large effects, most only had small effects which would not be large enough to impact for instance on the sixfold variation in test ordering in primary care observed in the South West5 or the even more extreme variation observed across the UK.6
The current study also demonstrates that even though reviewing the evidence with the target behaviour clearly in mind is a more productive approach than looking at similar behaviour change interventions applied to a wide variety of targets, such an approach has limitations. For instance, many studies targeting a wide range of test ordering behaviour reported only average effect, even when it was clear that the effect on the ordering of different tests was quite different. This limited the number of studies available for inclusion and probably accounted for the small number of studies evaluating specific interventions such as those based on computerised test ordering systems. Moreover, even when the studies reported test-specific effects, they rarely investigated the reasons for this variation and failed to provide explanation of the observed differences. Given the poor methodological quality of many of the included studies, this made it difficult to draw reliable conclusions and make recommendations. This suggests that although similar reviews to this looking at the effectiveness of behaviour change interventions on modifying the ordering of other routine tests would be helpful, a novel approach may be necessary. Such approach could focus on similar test ordering behaviours rather than similar tests and could incorporate wider range of evidence able to demonstrate not only the effectiveness of different interventions but also to provide insight in the mechanisms behind specific behaviour modifications.
Acknowledgments
The authors would like to thank Rebecca Hardwick, associate research fellow at ESMI, University of Exeter Medical School, who took part in the early discussions of the results and Dr Astrid Janssens, research fellow in child health, University of Exeter Medical School, who helped with the translation of the Dutch paper.
Footnotes
Contributors: ZZ, BV, CH, RA, WTH, SF, AP and JTC wrote the protocol. MR developed the search strategy and did the electronic searches. ZZ and RA screened the titles and abstracts and selected studies for inclusion. ZZ and RA carried out the data extraction and the methodological quality assessment. CH and BV provided advice and arbitration on the selection process, data extraction and methodological quality assessment. ZZ, BV, CH, RA, WTH, SF, AP and JH took part in the analysis of results. ZZ and CH wrote the original draft and prepared the summary of results tables and the other authors revised the draft critically for important intellectual content and approved the final version of the paper. ZZ and CH are the guarantors.
Funding: This research was funded by the National Institute for Health Research (NIHR) Collaboration for Leadership for Applied Health Research and Care for the South West Peninsula.
Disclaimer: The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Garmendia Madariaga A, Santos Palacios S, Guillén-Grima F et al. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab 2014;99:923–31. 10.1210/jc.2013-2409 [DOI] [PubMed] [Google Scholar]
- 2.Bahn Chair RS, Burch HB, Cooper DS et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid 2011;21:593–646. 10.1089/thy.2010.0417 [DOI] [PubMed] [Google Scholar]
- 3.Jonklaas J, Bianco AC, Bauer AJ et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association task force on thyroid hormone replacement. Thyroid 2014;24:1670–751. 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association for Clinical Biochemistry; British Thyroid Association; British Thyroid Foundation. UK guidelines for the use of thyroid function tests. July 2006. http://www.btf-thyroid.org/images/stories/pdf/tft_guideline_final_version_july_2006.pdf (accessed 8 Jun 2015).
- 5.Vaidya B, Ukoumunne OC, Shuttleworth J et al. Variability in thyroid function test requests across general practices in south-west England. Qual Prim Care 2013;21:143–8. [PubMed] [Google Scholar]
- 6. The NHS Atlas of Variation in Diagnostic Services. Reducing unwarranted variation to increase value and improve quality: RighCare; November 2013. http://www.rightcare.nhs.uk/downloads/Right_Care_Diagnostics_Atlas_hi-res.pdf.
- 7.Hardwick R, Heaton J, Griffiths G et al. Exploring reasons for variation in ordering thyroid function tests in primary care: a qualitative study. Qual Prim Care 2014;22:256–61. [PubMed] [Google Scholar]
- 8.Bayram C, Valenti L, Britt H. Orders for thyroid function tests—changes over 10 years. Aust Fam Physician 2012;41:555. [PubMed] [Google Scholar]
- 9.Leese GP, Flynn RV, Jung RT et al. Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: the Thyroid Epidemiology Audit and Research Study (TEARS). Clin Endocrinol (Oxf) 2008;68:311–16. 10.1111/j.1365-2265.2007.03051.x [DOI] [PubMed] [Google Scholar]
- 10.Thomas RE, Croal BL, Ramsay C et al. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: a cluster randomised trial. Lancet 2006;367:1990–6. 10.1016/S0140-6736(06)68888-0 [DOI] [PubMed] [Google Scholar]
- 11.Taylor PN, Iqbal A, Minassian C et al. Falling threshold for treatment of borderline elevated thyrotropin levels—balancing benefits and risks: Evidence from a large community-based study. JAMA Intern Med 2014;174:32–9. 10.1001/jamainternmed.2013.11312 [DOI] [PubMed] [Google Scholar]
- 12.Solomon DH, Hashimoto H, Daltroy L et al. Techniques to improve physicians’ use of diagnostic tests: a new conceptual framework. JAMA 1998;280:2020–7. 10.1001/jama.280.23.2020 [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg JM. Physician utilization: the state of research about physicians’ practice patterns. Med Care 2002;40:1016–35. 10.1097/01.MLR.0000032181.98320.8D [DOI] [PubMed] [Google Scholar]
- 14.Grossman RM. A review of physician cost-containment strategies for laboratory testing. Med Care 1983;21:783–802. 10.1097/00005650-198308000-00003 [DOI] [PubMed] [Google Scholar]
- 15.Oxman AD, Thomson MA, Davis DA et al. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ 1995;153:1423–31. [PMC free article] [PubMed] [Google Scholar]
- 16.Young DW. Improving laboratory usage: a review. Postgrad Med J 1988;64:283–9. 10.1136/pgmj.64.750.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berwick DM, Coltin KL. Feedback reduces test use in a health maintenance organization. JAMA 1986;255:1450–4. 10.1001/jama.1986.03370110072024 [DOI] [PubMed] [Google Scholar]
- 18.Gama R, Nightingale PG, Broughton PM et al. Feedback of laboratory usage and cost data to clinicians: does it alter requesting behaviour? Ann Clin Biochem 1991;28(Pt 2):143–9. 10.1177/000456329102800204 [DOI] [PubMed] [Google Scholar]
- 19.Cipullo JA, Mostoufizadeh M. Bringing order to test orders: one lab's story. CAP Today 1996;10:20–2. [PubMed] [Google Scholar]
- 20.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions Version 5.1.0. The Cochrane Collaboration; 2011. (updated March 2011) http://www.cochrane-handbook.org [Google Scholar]
- 21.Effective Public Health Practice Project. Quality Assessment Tool for Quantitative Studies. http://www.ephpp.ca/tools.html (accessed Apr 2015).
- 22.Adlan MA, Neel V, Lakra SS et al. Targeted thyroid testing in acute illness: achieving success through audit. J Endocrinol Invest 2011;34:e210–13. 10.3275/7480 [DOI] [PubMed] [Google Scholar]
- 23.Baker R, Falconer Smith J, Lambert PC. Randomised controlled trial of the effectiveness of feedback in improving test ordering in general practice. Scand J Prim Health Care 2003;21:219–23. 10.1080/02813430310002995 [DOI] [PubMed] [Google Scholar]
- 24.Chu KH, Wagholikar AS, Greenslade JH et al. Sustained reductions in emergency department laboratory test orders: impact of a simple intervention. Postgrad Med J 2013;89:566–71. 10.1136/postgradmedj-2012-130833 [DOI] [PubMed] [Google Scholar]
- 25.Daucourt V, Saillour-Glénisson F, Michel P et al. A multicenter cluster randomized controlled trial of strategies to improve thyroid function testing. Med Care 2003;41:432–41. 10.1097/01.MLR.0000053216.33277.A4 [DOI] [PubMed] [Google Scholar]
- 26.Dowling PT, Alfonsi G, Brown MI et al. An education program to reduce unnecessary laboratory tests by residents. Acad Med 1989;64:410–12. 10.1097/00001888-198907000-00017 [DOI] [PubMed] [Google Scholar]
- 27.Emerson JF, Emerson SS. The impact of requisition design on laboratory utilization. Am J Clin Pathol 2001;116:879–84. 10.1309/WC83-ERLY-NEDF-471E [DOI] [PubMed] [Google Scholar]
- 28.Feldkamp CS, Carey JL. An algorithmic approach to thyroid function testing in a managed care setting. 3-year experience . Am J Clin Pathol 1996;105:11–16. 10.1093/ajcp/105.1.11 [DOI] [PubMed] [Google Scholar]
- 29.Grivell AR, Forgie HJ, Fraser CG et al. Effect of feedback to clinical staff of information on clinical biochemistry requesting patterns. Clin Chem 1981;27:1717–20. [PubMed] [Google Scholar]
- 30.Larsson A, Biom S, Wernroth ML et al. Effects of an education programme to change clinical laboratory testing habits in primary care. Scand J Prim Health Care 1999;17:238–43. 10.1080/028134399750002476 [DOI] [PubMed] [Google Scholar]
- 31.Mindemark M, Larsson A. Long-term effects of an education programme on the optimal use of clinical chemistry testing in primary health care. Scand J Clin Lab Invest 2009;69:481–6. 10.1080/00365510902749123 [DOI] [PubMed] [Google Scholar]
- 32.Nightingale PG, Peters M, Mutimer D et al. Effects of a computerised protocol management system on ordering of clinical tests. Qual Health Care 1994;3:23–8. 10.1136/qshc.3.1.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schectman JM, Elinsky EG, Pawlson LG. Effect of education and feedback on thyroid function testing strategies of primary care clinicians. Arch Intern Med 1991;151:2163–6. 10.1001/archinte.1991.00400110027006 [DOI] [PubMed] [Google Scholar]
- 34.Stuart PJ, Crooks S, Porton M. An interventional program for diagnostic testing in the emergency department. Med J Aust 2002;177:131–4. [DOI] [PubMed] [Google Scholar]
- 35.Tierney WM, McDonald CJ, Hui SL et al. Computer predictions of abnormal test results. Effects on outpatient testing. JAMA 1988;259:1194–8. [PubMed] [Google Scholar]
- 36.Tomlin A, Dovey S, Gauld R et al. Better use of primary care laboratory services following interventions to ‘market’ clinical guidelines in New Zealand: a controlled before-and-after study. BMJ Qual Saf 2011;20:282–90. 10.1136/bmjqs.2010.048124 [DOI] [PubMed] [Google Scholar]
- 37.Toubert ME, Chevret S, Cassinat B et al. From guidelines to hospital practice: reducing inappropriate ordering of thyroid hormone and antibody tests. Eur J Endocrinol 2000;142: 605–10. 10.1530/eje.0.1420605 [DOI] [PubMed] [Google Scholar]
- 38.van Gend JM, van Pelt J, Cleef TH et al. [Quality improvement project ‘laboratory diagnosis by family physicians’ leads to considerable decrease in number of laboratory tests]. Ned Tijdschr Geneeskd 1996;140:495–500. [PubMed] [Google Scholar]
- 39.van Walraven C, Goel V, Chan B. Effect of population-based interventions on laboratory utilization: a time-series analysis. JAMA 1998;280:2028–33. 10.1001/jama.280.23.2028 [DOI] [PubMed] [Google Scholar]
- 40.Vidal-Trécan G, Toubert ME, Coste J et al. Reducing the number of T3 orders in the Paris hospital network: towards better appropriateness of thyroid function test prescription. Ann Endocrinol (Paris) 2003;64:210–15. [PubMed] [Google Scholar]
- 41.Willis EA, Datta BN. Effect of an educational intervention on requesting behaviour by a medical admission unit. Ann Clin Biochem 2013;50(Pt 2):166–8. 10.1258/acb.2012.012100 [DOI] [PubMed] [Google Scholar]
- 42.Wong ET, McCarron MM, Shaw ST Jr. Ordering of laboratory tests in a teaching hospital. Can it be improved? JAMA 1983;249:3076–80. 10.1001/jama.1983.03330460058036 [DOI] [PubMed] [Google Scholar]
- 43.Rhyne RL, Gehlbach SH. Effects of an educational feedback strategy on physician utilization of thyroid function panels. J Fam Pract 1979;8:1003–7. [PubMed] [Google Scholar]
- 44.Hardwick DF, Morrison JI, Tydeman J et al. Structuring complexity of testing: a process oriented approach to limiting unnecessary laboratory use. Am J Med Technol 1982;48:605–8. [PubMed] [Google Scholar]
- 45.Horn DM, Koplan KE, Senese MD et al. The impact of cost displays on primary care physician laboratory test ordering. J Gen Intern Med 2014;29:708–14. 10.1007/s11606-013-2672-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman LS, Shihab HM, Thiemann D et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med 2013;173:903–8. 10.1001/jamainternmed.2013.232 [DOI] [PubMed] [Google Scholar]
- 47.Shalev V, Chodick G, Heymann AD. Format change of a laboratory test order form affects physician behavior. Int J Med Inf 2009;78:639–44. 10.1016/j.ijmedinf.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 48.Glasziou P, Meats E, Heneghan C et al. What is missing from descriptions of treatment in trials and reviews? BMJ 2008;336:1472–4. 10.1136/bmj.39590.732037.47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Weijden T, van Bokhoven MA, Dinant GJ et al. Understanding laboratory testing in diagnostic uncertainty: a qualitative study in general practice. Br J Gen Pract 2002;52:974–80. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2015-010065supp_appendix1.pdf (255.5KB, pdf)
bmjopen-2015-010065supp_appendix2.pdf (50.4KB, pdf)


