Abstract
Background
Atrial fibrillation (AF) is associated with increased risk for thromboembolism and death; however, the relationships between cardiac structure and function and adverse outcomes among individuals with AF are incompletely understood.
Methods
The ENGAGE AF –TIMI 48 study tested the once-daily oral factor Xa inhibitor edoxaban in comparison to warfarin for the prevention of stroke (ischemic or hemorrhagic) or systemic embolism in 21,105 subjects with nonvalvular AF and increased risk for thromboembolic events (CHADS2 ≥ 2). In a prospective substudy of 971 subjects who underwent transthoracic echocardiography at baseline, we used Cox proportional hazards models to evaluate the associations between cardiac structure and function and the risks for death and thromboembolism (ischemic stroke, TIA, or systemic embolism).
Results
Over a median follow up of 2.5 years, 89 (9.2%) deaths and 48 (4.9%) incident thromboembolic events occurred in 971 subjects. In models adjusted for CHADS2 score, aspirin use, and randomized treatment, larger LV end diastolic volume index (HR: 1.49 [95%CI: 1.16,1.91] per 1 SD [12.9 ml/m2]) and higher LV filling pressures measured by E/′e (HR: 1.32 [95%CI: 1.08,1.61] per 1 SD [4.6]) were independently associated with increased risks for death. E/e′ > 13 significantly improved prediction of death beyond clinical factors alone. No features of cardiac structure and function were independently associated with thromboembolism in this population. Findings were similar when adjusted for CHA2DS2-VASc in place of CHADS2.
Conclusions
In a contemporary population of patients with atrial fibrillation at increased risk for thromboembolic events, larger LV size and higher filling pressures were significantly associated with increased risk for death, but neither left atrial nor left ventricular measures were associated with thromboembolic risk. LV size and filling pressures may help identify AF patients at increased risk of death.
Keywords: Atrial fibrillation, ENGAGE AF-TIMI 48, Echocardiography, Thromboembolism, Stroke, Death
Introduction
Atrial fibrillation (AF) is common, increasing in prevalence, and is associated with increased risks for thromboembolism, stroke, and death.[1, 2] As non-fatal thromboembolism and stroke are associated with high morbidity, a key consideration in managing the AF patient includes thromboembolic risk assessment to inform antithrombotic recommendations.[3] Despite current risk prediction tools, such as CHADS2 and CHA2DS2-VASc, decisions regarding whether to anticoagulate and choosing antithrombotic therapies in AF remain challenging.[1] Anticoagulation is associated with reduction in the risk of fatal and non-fatal strokes; however, thromboembolism accounts for a minority of deaths among AF patients.[2, 4, 5] Recent evidence demonstrates that death occurs more commonly in AF than stroke, and despite therapeutic advances in stroke prevention, mortality rates in AF have not substantially improved over the last decade.[2, 6] Therefore, a particular need exists to understand factors that contribute to the risk of death in AF, which may inform clinical management strategies aimed at decreasing mortality.
Cardiac structure and function in AF may be of prognostic value in refining thromboembolic and mortality risk assessments.[7-9] However, there are conflicting data regarding the relationships between cardiac structure and function in AF and the risk of thromboembolism.[10-13] Inconsistent findings may in part be related to limitations of prior echocardiographic studies which did not include contemporary measures of left ventricular (LV) and left atrial (LA) structure and function. To understand the impact of cardiac structure and function on the risks of thromboembolism and death, we evaluated the prognostic significance of cardiac structure and function in relation to thromboembolic events and all-cause mortality among subjects enrolled in the pre-specified echocardiographic sub-study of Effective aNticoaGulation with factor xA next Generation in AF-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48). We hypothesized that left atrial dysfunction will be associated with increased risk of thromboembolic events and death among individuals with atrial fibrillation.
Material and methods
Study Population
ENGAGE AF–TIMI 48 was a multinational, randomized (1:1:1), double-blind, double-dummy non-inferiority design trial comparing the efficacy and safety of two dosing regimens of once-daily edoxaban (high and low dose with dose reductions for patients with decreased clearance of edoxaban) versus warfarin titrated to an INR of 2.0 to 3.0 in subjects with a history of AF.[14] The study included AF subjects at moderate to high risk for thromboembolic events based upon a CHADS2 ≥ 2. Inclusion and exclusion criteria and the primary results demonstrating non-inferiority of edoxaban compared with warfarin for the prevention of stroke or systemic embolism have been reported.[14, 15] Briefly, eligible subjects were men or women ≥ 21 years old with a history of AF of any duration documented by an electrocardiogram within the prior 12 months and in whom anticoagulation was indicated. Key exclusion criteria were: AF due to reversible causes, severe renal dysfunction (creatinine clearance < 30 ml/min), high bleeding risk, moderate or severe mitral stenosis, or a mechanical valve in any position. Mitral regurgitation was not excluded. Rate or rhythm control strategy was not specified by the study protocol and was at the discretion of the local physician. The study protocol complied with the Declaration of Helsinki and was approved by institutional review boards at each site. Written informed consent was obtained in all patients.
The prospectively designed echocardiographic sub-study of ENGAGE AF-TIMI 48 was performed at 133 sites worldwide between 2009 and 2011. The echocardiographic procedures, protocol, and results of a baseline cross-sectional analysis of the ENGAGE AF-TIMI 48 Echocardiographic sub-study population prior to the availability of longitudinal outcomes have been previously reported.[8] In the current report, the same echocardiographic sub-study population is now examined with regards to longitudinal associations between baseline cardiac structure and function and the outcomes of thromboembolism and death over a median of 2.5 years of follow up. Subjects were invited prior to randomization to voluntarily participate with echocardiographic imaging obtained within the first week after randomization. Standard 2D and Doppler transthoracic echocardiography was performed with images sent to the echocardiography core laboratory at Brigham and Women's Hospital, Boston, MA. Conventional echocardiographic analyses were performed by technicians blinded to clinical information and treatment assignment, with all study measurements confirmed by a board certified cardiologist and echocardiographer.[8] Reproducibility of echocardiographic measurements was good to excellent with an intra-observer intraclass correlation coefficient of 0.95 (0.91–0.99) and inter-observer intraclass correlation coefficient of 0.84 (0.75–0.93).[8]
Echocardiographic analyses
Echocardiography was performed according to American Society of Echocardiography guidelines.[8, 16, 17] LV volumes and ejection fraction (LVEF) were calculated by the modified Simpson's method. LV mass was calculated from LV linear dimensions using the ASE recommend formula (0.8 × (1.04[(LVIDd + PWTd + SWTd)3 - (LVIDd)3]) + 0.6 g) and indexed to body surface area, with LV hypertrophy (LVH) defined as LV mass index (LVMI) >115 g/m2 in men or >95 g/m2 in women. LV geometry was categorized as normal (relative wall thickness ≤0.42 and no LVH) or abnormal (relative wall thickness >0.42 or LVH).
LA diameter was the 2D anterior-posterior length in the parasternal long axis view. LA maximum volume was measured by the modified Simpson's method using apical 4- and 2-chamber views at the end-systolic frame preceding mitral valve opening, and was indexed to body surface area to derive LA volume index (LAVI). Similarly, LA minimum volume was measured at the end diastolic frame preceding mitral valve closure. LA emptying fraction was calculated as: 100 × (maximum volume – minimum volume)/maximum volume (Figure 1).[18, 19]
Figure 1.
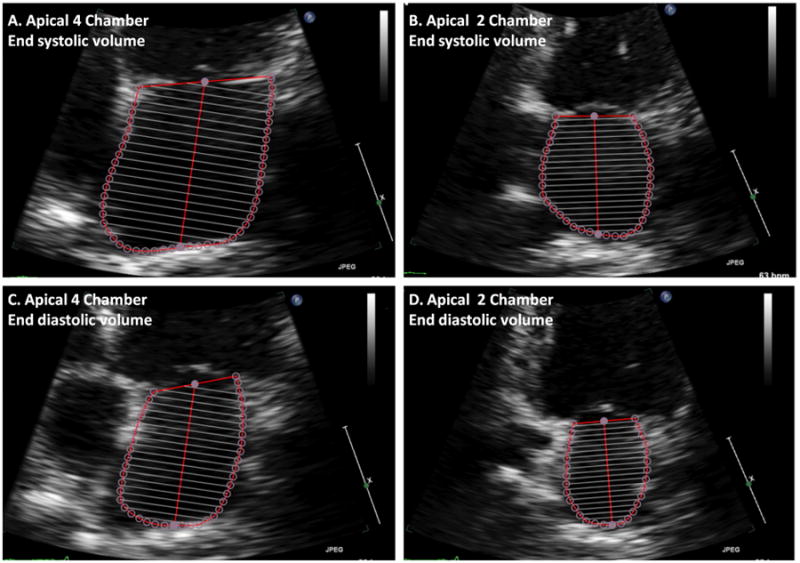
Calculation of left atrial emptying fraction from the apical 4 and 2 chamber views.
Left atrial volume is calculated by the method of disks from focused apical 4 and 2 chamber views at maximum (end ventricular systole) and minimum (end ventricular diastole) volume. The left atrial emptying fraction is calculated from these volumes as 100 × (maximum – minimum)/maximum. In this example, the left atrial emptying fraction is 54%.
Early transmitral velocity (E) was measured by pulsed wave Doppler from the apical 4-chamber view with the sample volume positioned at the tip of the mitral leaflets. Peak lateral and septal mitral annular early relaxation velocities (e′) were assessed using tissue Doppler imaging. LV filling pressures were estimated by E wave divided by average e′ velocities (E/e′). Right ventricular systolic pressure was calculated from the peak tricuspid regurgitant velocity using the simplified Bernoulli equation and assuming a right atrial pressure of 10 mmHg. Final values for all parameters were taken as the mean of measurements from three cardiac cycles.
Outcomes
The primary endpoints for this analysis were time to a) thromboembolic event (defined as ischemic stroke, transient ischemic attack (TIA) or systemic embolic event (SEE)), and b) death from any cause. All events were adjudicated by an independent clinical end-point committee whose members were blinded to study assignment. The median follow up period was 2.5 years (IQR: 2.3, 2.8).
Statistical methods
Subjects were stratified into groups according to those who did and did not have a thromboembolic event in follow up or those who were alive or dead. Summary statistics for clinical characteristics and cardiac structure function were calculated with results presented as medians (interquartile ranges) and counts (percentages), for continuous and categorical data, respectively. Statistical comparisons were made between subjects who did or did not have the outcome of interest in follow up using χ2, Fisher's exact, or Wilcoxon rank sum tests, as appropriate. Incidence rates for thromboembolism and death per 100 person years were calculated. Multivariable Cox proportional hazard models were used to assess the association between covariates and the risk of thromboembolism and death. The echocardiographic substudy was not powered to assess effect modification of treatment (warfarin vs. high dose edoxaban vs. low dose edoxaban) by features of cardiac structure and function on the outcomes of thromboembolism or death. However, to control for treatment assignment in analyzing the association between cardiac structure and function and outcomes, randomization was included as a covariate in multivariable models. To examine the incremental value of cardiac structure and function on the prediction of the outcomes beyond clinical factors, receiving operating curve analyses were performed. All analyses were performed using Stata 11.2 (Stata Corp., College Station, Tx) with p values < 0.05 considered statistically significant.
Results
Clinical Characteristics
Over a median follow up of 2.5 years (IQR 2.3, 2.8), incident thromboembolic events occurred in 48 subjects (Table 1). Thromboembolic events were more frequent among subjects with higher CHADS2 scores. The frequency of thromboembolic events did not differ according to paroxysmal, persistent, or permanent AF. Aspirin use was significantly lower among subjects who had a thromboembolic event as compared to those who did not. In contrast, there was no significant difference with regards to the occurrence of thromboembolism during follow up in relation to randomization to warfarin or edoxaban.
Table 1. Clinical characteristics of atrial fibrillation subjects of the ENGAGE AF-TIMI 48 echocardiography substudy stratified according to thromboembolic event or death in follow up.
| Characteristic | No TE N = 923 | + TE N = 48 | p | Alive N = 882 | Dead N =89 | p |
|---|---|---|---|---|---|---|
| Age, yrs | 73 (65,79) | 74.5 (67,79) | 0.53 | 73 (65,78) | 76 (66,82) | 0.031 |
| Sex, male | 606 (66) | 31 (65) | 0.88 | 571 (65) | 66 (74) | 0.075 |
| Caucasian | 850 (92) | 46 (96) | 0.34 | 811 (92) | 85 (96) | 0.23 |
| CHADS2 | 0.045 | 0.001 | ||||
| 2 | 451 (49) | 24 (50) | 440 (50) | 35 (39) | ||
| 3 | 275 (30) | 8 (17) | 248 (28) | 35 (39) | ||
| 4 | 147 (16) | 9 (19) | 143 (16) | 13 (15) | ||
| 5-6 | 50 (5) | 7 (15) | 51 (6) | 6 (7) | ||
| Heart Failure | 499 (54) | 22 (46) | 0.27 | 465 (53) | 56 (63) | 0.066 |
| Hypertension | 872 (94) | 45 (94) | 0.83 | 833 (94) | 84 (94) | 0.98 |
| Age > 75 yrs | 418 (45) | 24 (50) | 0.52 | 394 (45) | 48 (54) | 0.094 |
| Diabetes mellitus | 326 (35) | 20 (42) | 0.37 | 306 (35) | 40 (45) | 0.054 |
| Stroke/TIA | 240 (26) | 18 (38) | 0.08 | 237 (27) | 21 (24) | 0.51 |
| Vascular Disease | 389 (42) | 25 (52) | 0.18 | 366 (42) | 48 (55) | 0.018 |
| Smoking, current | 96 (10) | 4 (8) | 0.81 | 89 (10) | 11 (12) | 0.50 |
| Obese (BMI > 30 kg/m2) | 388 (42) | 19 (40) | 0.74 | 371 (42) | 36 (40) | 0.77 |
| Baseline heart rate, bpm | 72 (63,81) | 74 (63,81) | 0.66 | 72 (62,80) | 73 (67,82) | 0.17 |
| CKD (CrCl 30- 60 ml/min) | 306 (34) | 19 (40) | 0.40 | 285 (33) | 40 (47) | 0.007 |
| Type of AF | 0.22 | 0.98 | ||||
| Paroxysmal | 298 (32) | 21 (44) | 294 (33) | 25 (28) | ||
| Persistent | 196 (21) | 7 (15) | 183 (21) | 20 (22) | ||
| Permanent | 429 (46) | 20 (42) | 405 (46) | 44 (49) | ||
| BMI, kg/m2 | 29 (26,33) | 29 (25,34) | 0.68 | 29 (26,33) | 28 (25,32) | 0.15 |
| Cr Cl, ml/min | 72 (55,92) | 64 (47,93) | 0.15 | 72 (56,92) | 63 (41,88) | 0.003 |
| Anti-arrhythmic med | 177 (19) | 9 (19) | 0.94 | 173 (20) | 13 (15) | 0.32 |
| ACEI or ARB | 538 (58) | 27 (56) | 0.77 | 512 (58) | 53 (60) | 0.82 |
| Beta-blocker | 624 (68) | 33 (69) | 0.87 | 594 (67) | 63 (71) | 0.55 |
| Diuretic | 923 (100) | 48 (100) | n/a | 882 (100) | 89 (100) | n/a |
| Aspirin use at baseline | 285 (31) | 8 (17) | 0.037 | 271 (31) | 22 (25) | 0.24 |
| Randomized | 0.17 | 0.69 | ||||
| warfarin | 316 (34) | 11 (23) | 297 (34) | 30 (34) | ||
| Edoxaban (low) | 315 (34) | 22 (46) | 303 (34) | 34 (38) | ||
| Edoxaban (high) | 292 (32) | 15 (31) | 282 (32) | 25 (28) |
Data shown as count (%) or median (IQR). TE = thromboembolic; TIA = transient ischemic attack; BMI = body mass index; CKD = chronic kidney disease; Cr Cl = creatinine clearance.
A total of 89 deaths occurred over the follow up period (Table 1). Death was more frequent among subjects with higher CHADS2 score. Vascular disease and chronic kidney disease were also more common among subjects who died. Aspirin use did not significantly differ between those who were alive versus dead at follow up. Similarly, no significant difference was observed with regards to death in relation to randomization to warfarin or edoxaban.
Cardiac Structure and Function
Left ventricular size, mass, ejection fraction, and filling pressures did not significantly differ between subjects who had a thromboembolic event in follow up as compared with those who did not (Table 2). LA size and function were also similar between these two groups.
Table 2. Cardiac structure and function in atrial fibrillation subjects in the ENGAGE AF-TIMI 48 echocardiography substudy stratified according to thromboembolic event or death in follow up.
| Characteristic | No TE N = 923 | + TE N = 48 | p | Alive N = 882 | Dead N = 89 | p |
|---|---|---|---|---|---|---|
| Sinus rhythm at echo | 298 (32) | 23 (48) | 0.028 | 295 (33) | 26 (29) | 0.48 |
| Left ventricular | ||||||
| LVEF, % | 59 (53,61) | 59 (57,61) | 0.061 | 59 (54,61) | 57 (44,60) | 0.003 |
| LVEF < 50% | 210 (23) | 7 (15) | 0.19 | 185 (21) | 32 (36) | 0.001 |
| LVEDVI, ml/m2 | 56 (51,62) | 57 (53,63) | 0.32 | 56 (51,62) | 58 (53,69) | 0.010 |
| LV mass, g | 136 (113,172) | 145 (120,178) | 0.50 | 135 (112,171) | 151 (125,183) | 0.003 |
| LVMI, g/m2 | 68 (58,88) | 72 (60,91) | 0.33 | 68 (58,88) | 76 (63,98) | 0.003 |
| Abnl LV geometry* | 258 (28) | 18 (38) | 0.15 | 252 (29) | 24 (27) | 0.75 |
| Left atrial | ||||||
| LA diameter, cm | 3.6 (3.4,3.8) | 3.5 (3.4,3.8) | 0.17 | 3.6 (3.4,3.8) | 3.7 (3.4,4.0) | 0.055 |
| LAVI, ml/m2 | 33 (26,39) | 33 (27,38) | 0.95 | 33 (26,39) | 34 (28,45) | 0.031 |
| LAVI > 34 ml/m2 | 413 (45) | 19 (40) | 0.55 | 386 (44) | 46 (52) | 0.18 |
| LA emptying fraction, % | 38 (30,45) | 40 (33,49) | 0.12 | 38 (31,46) | 35 (27,43) | 0.015 |
| Doppler | ||||||
| TDI e′ avg, cm/s | 7.7 (6.2,9.1) | 7.2 (6.6,9.0) | 0.65 | 7.7 (6.2,9.1) | 7.1 (5.9,9.1) | 0.18 |
| E/e′ avg | 10.7 (8.6,13.8) | 10.2 (7.9,12.4) | 0.22 | 10.6 (8.5,13.5) | 12.3 (9.3,16.7) | 0.004 |
| E/e′ ≥ 13 | 403 (44) | 20 (42) | 0.88 | 367 (42) | 56 (63) | <0.001 |
| ≥ Moderate MR | 93 (11) | 6 (13) | 0.69 | 85 (10) | 14 (17) | 0.07 |
| **RVSP, mmHg | 32 (29,36) | 34 (28,39) | 0.57 | 32 (28,36) | 36 (30,42) | <0.001 |
Data shown as count (%) or median (IQR). TE = thromboembolic; LVEF = left ventricular ejection fraction; LVEDVI = left ventricular end diastolic volume index; LVMI = left ventricular mass index; Abnl = abnormal; LA = left atrial; LAVI = left atrial volume index; TDI = tissue Doppler imaging; e′ = mitral annular early diastolic velocity; E/e′= transmitral E wave velocity/e; MR = mitral regurgitation'.
Abnormal LV geometry defined as hypertrophy or concentric remodeling.
RVSP measurable in 587/971 (60%) of subjects.
In contrast, subjects who died, as compared with survivors, had significantly larger LV size and mass, with lower ejection fractions as well (Table 2). LA size was significantly larger among subjects who died and LA function, as assessed by emptying fraction, was also significantly lower. LV filling pressures, measured by E/e′, were significantly higher among subjects who died as compared with those who survived.
Risks of thromboembolism and death
Thromboembolism occurred in 4.9% of the study population at a rate of 2.0% per year (95% CI: 1.5,2.7). Death occurred in 9.2% of subjects at a rate of 3.6% per year (95%CI: 2.9,4.5). Cardiovascular death accounted for the majority (72%) of deaths, of which 53%, 16%, and 16% were due to sudden cardiac or unwitnessed death, heart failure, and hemorrhagic or ischemic stroke, respectively.
In multivariable adjusted models, no features of cardiac structure and function were associated with thromboembolic risk independent of CHADS2 score (Figure 2). In contrast, larger LV end diastolic volume index (LVEDVI) (HR: 1.49, 95%CI 1.16, 1.91) and higher E/e′ (HR: 1.32, 95% CI 1.08, 1.61) were both significantly and independently associated with increased risk for death.
Figure 2.
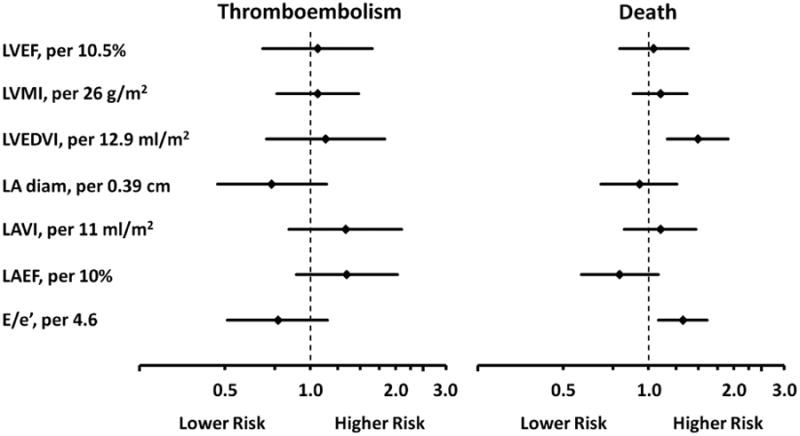
Forest plot of multivariable adjusted hazard ratios for the risk of thromboembolism or death according to features of cardiac structure and function among subjects with atrial fibrillation in the ENGAGE AF-TIMI 48 echocardiography sub-study.
Multivariable adjusted hazard ratios (point) and 95%CI (line) from Cox proportional hazards models. Covariates included CHADS2 score, aspirin use at baseline, randomization to warfarin, edoxaban (low dose), edoxaban (high dose), and all of the features of cardiac structure and function shown. Hazard ratios reflect risk per 1 standard deviation increase. LA diam = left atrial diameter. Other abbreviations as in Table 2.
In an additional analysis, CHA2DS2-VASc was included in the model in place of CHADS2 and the significant associations between LVEDVI (HR: 1.49, 95%CI 1.16, 1.91) and E/e′ (HR: 1.31, 95%CI 1.07, 1.61) with death did not appreciably change.
Sensitivity analyses
We performed several additional analyses to examine the influence of age as continuous variable, sex, creatinine clearance, rhythm at the time of echocardiography, type of atrial fibrillation (paroxysmal, persistent, or permanent), and mitral regurgitation on each of the outcomes. Inclusion of these covariates did not change the significant associations between LVEDVI, E/e′, and death (data not shown). Similarly, inclusion of these covariates did not change the findings for thromboembolism. In addition, age, sex, creatinine clearance, rhythm at the time of echocardiography, type of atrial fibrillation, and severity of mitral regurgitation were not significantly associated with risk of thromboembolism or death (P > 0.05 for all).
Left atrial anterior-posterior diameter
LA enlargement was defined as an anterior-posterior diameter ≥ 4.0 cm and included in univariable and multivariable adjusted Cox models. Using this definition, LA enlargement was not a significant predictor of thromboembolism (HR: 0.39, 95%CI 0.11, 1.38) or death (HR: 1.16, 95%CI 0.60, 2.27).
Incremental value of LVEDVI or E/e′ on the prediction of death
In receiver operating curve analyses, addition of E/e′ > 13 to clinical factors significantly improved prediction of death (AUC 0.71, 95% CI 0.64, 0.77) compared with a model based upon clinical factors alone (AUC 0.67 95% CI 0.60, 0.73), P = 0.031. In contrast, the inclusion of left ventricular enlargement, defined as a LVEDVI > 75 ml/m2, did not substantially improve prediction of death over clinical variables alone, P = 0.70 (Figure 3).
Figure 3.
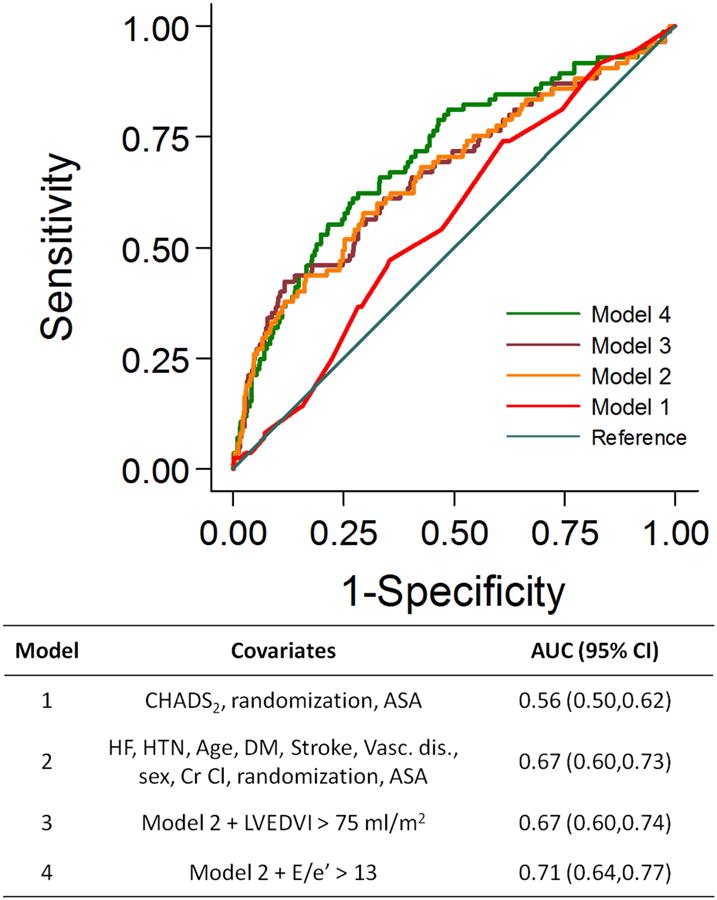
The incremental value of E/e′ beyond clinical factors in the prediction of death among subjects with atrial fibrillation in the ENGAGE AF-TIMI 48 echocardiography substudy.
Receiver operating curve analyses for the incremental value of left ventricular size and filling pressures compared with clinical factors for the prediction of death. The inclusion of left ventricular end diastolic volume index > 75 ml/m2 did not significantly improve prediction of death (P = 0.70 for model 3 vs. model 2). In contrast, addition of E/e′ > 13 to clinical factors did significantly improve prediction of death (P = 0.031 for model 4 vs. model 2).
Discussion
Among 971 subjects with AF and CHADS2 ≥ 2 enrolled in the ENGAGE AF-TIMI 48 echocardiographic sub-study, we found that death was a more frequent event than thromboembolism over a median of 2.5 years of follow up. Features of cardiac structure and function, including larger LV size and higher LV filling pressures were significantly and independently associated with increased risks for death. Additionally, E/e′ > 13 significantly improved prediction of death beyond clinical factors alone. In contrast, no conventional features of cardiac structure and function associated with higher or lower risk of thromboembolic events in follow up.
AF patients are at increased risk for stroke and death. Although death is a more frequent event than stroke, relatively less attention has been given to predicting the risk of death in AF.[2, 4, 20, 21] We found that increased LVEDVI and E/e′ were significantly associated with increased risks of death, independent of CHADS2 or CHA2DS2-VASc. E/e′ has been previously validated as a non-invasive measure of LV filling pressures in AF patients and retrospective analyses indicate that higher E/e′ is associated with an increased risk for death.[22, 23] Our findings in this relatively large (n=971) multicenter prospective echocardiographic sub-study validate the results of the prior smaller retrospective studies (ranging in size from 27 to 230 patients) and extend them by demonstrating the incremental value of E/e′ > 13 beyond clinical factors for the prediction of death. We also demonstrated that increased LV size is associated with all-cause mortality. Larger LV size and higher LV filling pressures may be indicative of volume overload conditions, such as heart failure or chronic kidney disease. The complex associations between AF and heart failure are well recognized and heart failure may be an important mediator of the risk of sudden cardiac death among AF patients.[2, 24-26] Indeed, sudden cardiac death and heart failure were the leading causes of cardiovascular death in this cohort. We also found a non-significant trend towards higher mortality among AF patients with prevalent heart failure. Moreover, the association between larger LV size and higher LV filling pressures and mortality was independent of creatinine clearance in this population without severe renal dysfunction. Therefore, our findings may provide additional insights into markers of and contributors to death in AF. Our findings also emphasize the clinical importance of measuring and reporting LV size and filling pressures among AF patients. Greater attention to LV size and, in particular, filling pressures may not only help clinicians risk stratify AF patients, but also inform therapies directed at reverse LV remodeling (e.g. renin-angiotensin-aldosterone axis inhibitors and/or beta-blockers) and reducing filling pressures (e.g. diuretics) that may be of benefit, although these hypotheses remain to be formally tested.
In contrast to mortality, conventional features of cardiac structure and function were not associated with either higher or lower thromboembolic risk among moderate to high risk AF subjects based upon CHADS2 score. This may in part be explained by the relatively small number of thromboembolic events in follow up that may have limited statistical power. However, these findings are consistent with results from another large study of cardiac structure and function in AF.[13] Additionally, as the ENGAGE AF-TIMI 48 study enrolled subjects with a CHADS2 score ≥ 2, the findings may indicate that among AF patients already at moderate to high risk based upon clinical factors, cardiac structure and function does not independently predict increased risk for thromboembolic outcomes. Conversely, no feature of cardiac structure and function, e.g. smaller LA size or higher LVEF, was associated with lower thromboembolic risk sufficient to alter management recommendations regarding whether to anticoagulate patients with a CHADS2 score ≥ 2. Whether cardiac structure and function is of prognostic significance among lower risk AF patients, i.e. CHADS2 ≤ 1 remains to be prospectively tested.
We previously demonstrated that larger LA size, abnormal LV geometry, and elevated LV filling pressures were significantly associated with higher CHADS2 score as a marker of stroke risk in this population.[8] However, there was greater power to identify differences with CHADS2 than to detect differences in actual thromboembolic outcomes.[8] The CHADS2 score has also been shown to be predictive of death in addition to stroke; therefore, our previous findings may have reflected the relationship between cardiac structure and function and mortality risk.[27] The lack of significant association between features of cardiac structure and function and thromboembolic risk in this population may also be related to the limitations of transthoracic echocardiography to characterize the left atrial appendage, which is believed to be a source of thrombus in AF.[28]
Although we evaluated a relatively large contemporary AF population, limitations should be noted. The irregular rhythm of AF will lead to beat to beat variability in echocardiographic assessment; however, we averaged measures of cardiac structure and function over multiple cardiac cycles. Though we did not directly assess the correlation between E/e′ and invasively measured left ventricular filling pressures, previous literature supports the high correlation between E/e′ and left ventricular end diastolic pressure in atrial fibrillation.[17, 23] In this echocardiographic sub-study of ENGAGE AF-TIMI 48, we did not find that conventional volumetric measures of left atrial structure and function were associated with the clinical outcomes of thromboembolism and death. The lack of statistically significant association may reflect the insensitivity of volume based left atrial measures for predicting these adverse outcomes or type II error. We cannot exclude that unmeasured characteristics of left atrial structure and function may be associated with adverse clinical outcomes among individuals with atrial fibrillation. For example, novel measures of cardiac mechanics, such as strain and strain rate from speckle tracking echocardiography, may provide additional prognostic information and is a future direction.[29] Furthermore, due to ENGAGE AF-TIMI 48 eligiblity criteria, we could not study whether left atrial structure and function is associated with prognosis among lower risk, i.e. CHADS2 < 2, or unanticoagulated patients with atrial fibrillation. The analysis was based upon cardiac structure and function at baseline and does not account for changes over time that may contribute to thromboembolic risk. Right ventricular function was not assessed. Information regarding the duration of atrial fibrillation was not available. By design, ENGAGE AF-TIMI 48 enrolled a moderate to high risk AF population (CHADS2 ≥ 2) and therefore our analysis does not include the entire spectrum of AF patients, particularly younger patients. Similarly, we evaluated AF subjects who volunteered to participate in an echocardiographic substudy of the larger ENGAGE AF-TIMI 48 clinical trial in which all subjects were anticoagulated (median time in therapeutic range for those randomized to warfarin was 72%) and therefore due to inclusion and exclusion criteria the results may not be applicable to the broader AF population.
Conclusions
In a moderate to high risk population of AF subjects enrolled in the echocardiographic sub-study of ENGAGE AF-TIMI 48, we found that measures of cardiac structure and function were associated with an increased risk of death, which was a more frequent event than thromboembolism, for which these measures were not predictive. LV size and filling pressures may help identify AF patients at increased risk of death and could be targets for therapies directed at improving survival.
Highlights.
In 971 moderate to high risk subjects (CHADS2 ≥ 2) with atrial fibrillation enrolled in the echocardiographic sub-study of ENGAGE AF-TIMI 48, death was a more frequent than thromboembolism
Larger left ventricular size and higher filling pressures were associated with increased risks of death
Neither left atrial nor left ventricular measures associated with thromboembolic risk
LV size and filling pressures may help identify AF patients at increased risk of death
Acknowledgments
The ENGAGE AF-TIMI 48 study was funded by Daiichi Sankyo Pharma Development (Edison, NJ). Support was provided by the National Heart, Lung, and Blood Institute training grants (T32 HL094301-02; K12 HL109019). Dr. Giugliano is the principal investigator for the ENGAGE AF-TIMI 48 study, has served as a consultant and had received honoraria from Bristol-Myers Squibb, Janssen, Daiichi Sankyo, Merck, Pfizer and Sanofi, and reports grants (through the Brigham and Women's Hospital) from Daiichi Sankyo, Merck, Johnson & Johnson, Sanofi, and AstraZeneca. EMA, EB, and SDS have received research support from Daiichi Sankyo. CTR and SDS have consulted for Daiichi Sankyo. MFM is an employee of Daiichi Sankyo. CTR has received honoraria for consulting from Alere, Beckman Coulter, and Boerhinger-Ingelheim.
Abbreviations
- AF
Atrial fibrillation
- LV
Left ventricular
- LA
Left atrial
- LVEF
Left ventricular ejection fraction
- LVMI
Left ventricular mass index
- LVH
Left ventricular hypertrophy
- LAVI
Left atrial volume index
- LVEDVI
Left ventricular end diastolic volume index
Footnotes
Clinical Trial Registration: http://www.clinicaltrials.gov; ID = NCT00781391
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lip GY, Tse HF, Lane DA. Atrial fibrillation. Lancet. 2012;379:648–61. doi: 10.1016/S0140-6736(11)61514-6. [DOI] [PubMed] [Google Scholar]
- 2.Piccini JP, Hammill BG, Sinner MF, Hernandez AF, Walkey AJ, Benjamin EJ, et al. Clinical course of atrial fibrillation in older adults: the importance of cardiovascular events beyond stroke. European Heart Journal. 2014;35:250–6. doi: 10.1093/eurheartj/eht483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 4.Marijon E, Le Heuzey JY, Connolly S, Yang S, Pogue J, Brueckmann M, et al. Causes of death and influencing factors in patients with atrial fibrillation: a competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128:2192–201. doi: 10.1161/CIRCULATIONAHA.112.000491. [DOI] [PubMed] [Google Scholar]
- 5.Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955–62. doi: 10.1016/S0140-6736(13)62343-0. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain AM, Gersh BJ, Alonso A, Chen LY, Berardi C, Manemann SM, et al. Decade-long trends in atrial fibrillation incidence and survival: a community study. Am J Med. 2015;128:260–7 e1. doi: 10.1016/j.amjmed.2014.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Providencia R, Trigo J, Paiva L, Barra S. The role of echocardiography in thromboembolic risk assessment of patients with nonvalvular atrial fibrillation. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2013;26:801–12. doi: 10.1016/j.echo.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Gupta DK, Shah AM, Giugliano RP, Ruff CT, Antman EM, Grip LT, et al. Left atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. 2014;35:1457–65. doi: 10.1093/eurheartj/eht500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khumri TM, Idupulapati M, Rader VJ, Nayyar S, Stoner CN, Main ML. Clinical and echocardiographic markers of mortality risk in patients with atrial fibrillation. The American Journal of Cardiology. 2007;99:1733–6. doi: 10.1016/j.amjcard.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 10.Dittrich HC, Pearce LA, Asinger RW, McBride R, Webel R, Zabalgoitia M, et al. Left atrial diameter in nonvalvular atrial fibrillation: An echocardiographic study. Stroke Prevention in Atrial Fibrillation Investigators. American heart journal. 1999;137:494–9. doi: 10.1016/s0002-8703(99)70498-9. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA: the Journal of the American Medical Association. 2001;285:2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.No authors listed. Echocardiographic predictors of stroke in patients with atrial fibrillation: a prospective study of 1066 patients from 3 clinical trials. Archives of Internal Medicine. 1998;158:1316-20.
- 13.Olshansky B, Heller EN, Mitchell LB, Chandler M, Slater W, Green M, et al. Are transthoracic echocardiographic parameters associated with atrial fibrillation recurrence or stroke? Results from the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) study. Journal of the American College of Cardiology. 2005;45:2026–33. doi: 10.1016/j.jacc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. The New England Journal of Medicine. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 15.Ruff CT, Giugliano RP, Antman EM, Crugnale SE, Bocanegra T, Mercuri M, et al. Evaluation of the novel factor Xa inhibitor edoxaban compared with warfarin in patients with atrial fibrillation: design and rationale for the Effective aNticoaGulation with factor xA next GEneration in Atrial Fibrillation-Thrombolysis In Myocardial Infarction study 48 (ENGAGE AF-TIMI 48) American Heart Journal. 2010;160:635–41. doi: 10.1016/j.ahj.2010.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 2009;22:107–33. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 18.Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, et al. Left atrial size: physiologic determinants and clinical applications. Journal of the American College of Cardiology. 2006;47:2357–63. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 19.Gutman J, Wang YS, Wahr D, Schiller NB. Normal left atrial function determined by 2-dimensional echocardiography. The American Journal of Cardiology. 1983;51:336–40. doi: 10.1016/s0002-9149(83)80061-7. [DOI] [PubMed] [Google Scholar]
- 20.Miyasaka Y, Barnes ME, Bailey KR, Cha SS, Gersh BJ, Seward JB, et al. Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. Journal of the American College of Cardiology. 2007;49:986–92. doi: 10.1016/j.jacc.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 21.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946–52. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 22.Okura H, Takada Y, Kubo T, Iwata K, Mizoguchi S, Taguchi H, et al. Tissue Doppler-derived index of left ventricular filling pressure, E/E′, predicts survival of patients with nonvalvular atrial fibrillation. Heart. 2006;92:1248–52. doi: 10.1136/hrt.2005.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn DW, Song JM, Zo JH, Chai IH, Kim HS, Chun HG, et al. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography. 1999;12:927–31. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 24.Reinier K, Marijon E, Uy-Evanado A, Teodorescu C, Narayanan K, Chugh H, et al. The association between atrial fibrillation and sudden cardiac death: the relevance of heart failure. JACC Heart Fail. 2014;2:221–7. doi: 10.1016/j.jchf.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Melgaard L, Gorst-Rasmussen A, Lane DA, Rasmussen LH, Larsen TB, Lip GY. Assessment of the CHA2DS2-VASc Score in Predicting Ischemic Stroke, Thromboembolism, and Death in Patients With Heart Failure With and Without Atrial Fibrillation. JAMA: the Journal of the American Medical Association. 2015;314:1030–8. doi: 10.1001/jama.2015.10725. [DOI] [PubMed] [Google Scholar]
- 26.Bajaj NS, Bhatia V, Sanam K, Ather S, Hashim T, Morgan C, et al. Impact of atrial fibrillation and heart failure, independent of each other and in combination, on mortality in community-dwelling older adults. The American Journal of Cardiology. 2014;114:909–13. doi: 10.1016/j.amjcard.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 27.Oldgren J, Alings M, Darius H, Diener HC, Eikelboom J, Ezekowitz MD, et al. Risks for stroke, bleeding, and death in patients with atrial fibrillation receiving dabigatran or warfarin in relation to the CHADS2 score: a subgroup analysis of the RE-LY trial. Annals of Internal Medicine. 2011;155:660–7, W204. doi: 10.7326/0003-4819-155-10-201111150-00004. [DOI] [PubMed] [Google Scholar]
- 28.Jordan RA, Scheifley CH, Edwards JE. Mural thrombosis and arterial embolism in mitral stenosis; a clinico-pathologic study of fifty-one cases. Circulation. 1951;3:363–7. doi: 10.1161/01.cir.3.3.363. [DOI] [PubMed] [Google Scholar]
- 29.Kojima T, Kawasaki M, Tanaka R, Ono K, Hirose T, Iwama M, et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. European Heart Journal Cardiovascular Imaging. 2012;13:227–34. doi: 10.1093/ejechocard/jer281. [DOI] [PubMed] [Google Scholar]


