Abstract
Elevated intraocular pressure (IOP) is causally implicated in the pathophysiology of primary open-angle glaucoma (POAG). The molecular mechanisms responsible for elevated IOP remain elusive, but may involve aberrant expression and signaling of transforming growth factor (TGF)-β2 within the trabecular meshwork (TM). Consistent with previously published studies, we show here that exogenous addition of TGF-β2 to cultured porcine anterior segments significantly attenuates outflow facility in a time-dependent manner. By comparison, perfusing segments with a TGFβRI/ALK-5 antagonist (SB-431542) unexpectedly elicited a significant and sustained increase in outflow facility, implicating a role for TM-localized constitutive expression and release of TGF-β2. Consistent with this thesis, cultured primary or transformed (GTM3) quiescent human TM cells were found to constitutively express and secrete measurable amounts of biologically-active TGF-β2. Disrupting monomeric GTPase post-translational prenylation and activation with lovastatin or GGTI-298 markedly reduced constitutive TGF-β2 expression and release. Specifically, inhibiting the Rho subfamily of GTPases with C3 exoenzyme similarly reduced constitutive expression and secretion of TGF-β2. These findings suggest that Rho GTPase signaling, in part, regulates constitutive expression and release of biologically-active TGF-β2 from human TM cells. Localized constitutive expression and release of TGF-β2 by TM cells may promote or exacerbate elevation of IOP in POAG.
Keywords: TGF-β2, Trabecular Meshwork, Rho GTPase, Glaucoma, Intraocular Pressure
1. Introduction
Glaucoma remains a leading cause of blindness worldwide. By the year 2020, it is projected that nearly 80 million people will be affected by this disorder (Quigley and Broman, 2006). In the United States, over 2 million individuals aged 40 years or older are currently diagnosed with primary open-angle glaucoma (POAG), the most prevalent form of this disease (Friedman et al., 2004). While the pathophysiology of POAG remains unclear, elevated intraocular pressure (IOP) is considered a key risk factor for the development and progression of POAG. The genesis of elevated IOP in POAG has been largely attributed to an increase in resistance to aqueous humor (AH) outflow through the trabecular meshwork (TM) within the conventional AH outflow pathway.
Increased outflow resistance and IOP elevation in POAG have been strongly correlated with aberrantly elevated levels of a variety of soluble factors within the AH. Of particular interest is transforming growth factor (TGF)-β2, an anti-proliferative/anti-inflammatory cytokine implicated in the pathogenesis of a variety of disorders, including glaucoma. Compared to age-matched healthy control subjects, the content of biologically-active TGF-β2 in the AH of POAG patients is increased approximately 60–70% (Inatani et al., 2001; Picht et al., 2001; Min et al., 2006). A pathogenic role of TGF-β2 in POAG is further supported by early ex vivo studies using cultured human, monkey, or porcine anterior segments (Gottanka et al., 2004; Bachmann et al., 2006; Fleenor et al., 2006; Bhattacharya et al., 2009). TGF-β2 was shown in these studies to markedly elevate IOP in a time-dependent manner, possibly by a mechanism involving increased production and deposition of fibrillar extracellular material within the TM (Gottanka et al., 2004). Moreover, manipulating the content of human TGF-β2 protein in the AH of rodents in vivo similarly elevates IOP (Robertson et al., 2010; Shepard et al., 2010; McDowell et al., 2013; Swaminathan et al., 2014; Hill et al., 2015).
In vitro, TGF-β2 has been shown to markedly enhance the synthesis and secretion of extracellular matrix (ECM) proteins, plasminogen activator inhibitor (PAI)-1, and tissue transglutaminase in cultured human TM cells (Welge-Lussen et al., 2000; Fleenor et al., 2006; Fuchshofer et al., 2007; Wordinger et al., 2007; Tovar-Vidales et al., 2011), while selectively attenuating activity of matrix metalloproteinase (MMP)-2 in a PAI-1 dependent manner (Fuchshofer et al., 2003). TGF-β2 facilitated induction of ECM synthesis and secretion in TM cells is largely regulated by canonical Smad3-mediated signaling mechanisms (Fuchshofer et al., 2009; Tovar-Vidales et al., 2011; McDowell et al., 2013). In contrast, TGF-β2 mediated changes in actin stress fiber organization and contractility utilize non-canonical small monomeric Rho GTPase/Rho kinase (ROCK) signaling pathways (Pattabiraman and Rao, 2010; Han et al., 2011; Von Zee et al., 2012; Pattabiraman et al., 2014). These studies collectively raise awareness that TGF-β2 mediated increases in outflow resistance and IOP in POAG may involve a concerted activation of both canonical (Smad) and non-canonical (Rho/ROCK) signaling pathways.
Early studies utilizing healthy human anterior segments report localization of TGF-β2 to limbal as well as lens epithelial cells, the conjunctival stroma, and the ciliary body (Pasquale et al., 1993; Saika et al., 2000). Additional studies in cultured porcine (Tripathi et al., 1994a) and human (Cao et al., 2003; Luna et al., 2011; Tovar-Vidales et al., 2011) TM cells also demonstrate TM-localized constitutive expression of TGF-β2. By comparison, there remains a paucity of data elucidating the mechanisms which regulate endogenous expression of this cytokine. In this study, constitutive expression and release of biologically-active TGF-β2 in human TM cells is shown to be regulated, in part, by Rho GTPase signaling. We propose that localized Rho GTPase-mediated constitutive expression and release of TGF-β2 by TM cells may promote or exacerbate elevation of IOP in POAG.
2. Methods
2.1. Anterior Segment Perfusion
Anterior segments were prepared from intact porcine globes obtained fresh from a local abattoir (Park Packing, Chicago, IL) and cultured within 6h using previously established methods (Keller et al., 2009). Briefly, globes were bisected aseptically at the equator, and the iris, lens, and vitreous were gently removed to minimize pigment dispersion. The prepared anterior segment was subsequently mounted to a custom-made organ culture chamber and perfused at a constant flow rate of 4.5 μl/min with pre-warmed Dulbecco’s Modified Eagle’s Medium (DMEM) containing 4 mM GlutaMAX-I supplemented with 2.5 μg/ml amphotericin B, 100 U/ml penicillin and 100 μg/ml streptomycin (Life Technologies, Grand Island, NY). Porcine anterior segments were cultured in a humidified tissue culture incubator under an atmosphere of 5% CO2/95% air at 37°C and allowed a 24h pressure stabilization (washout) period. Segments that did not exhibit stable outflow facilities within 24h were discarded. Anterior segments exhibiting stable pressures ranging from 8–15 mmHg were perfused under constant pressure with fresh DMEM containing either vehicle (400 nM HCl) or activated (Von Zee et al., 2012) recombinant human TGF-β2 (10 ng/ml; R&D Systems, Minneapolis, MN). A subgroup of anterior segments which exhibited stable pressures modestly exceeding 15 mmHg following an initial 24h washout period were perfused under constant pressure with fresh DMEM containing either vehicle (0.08% DMSO) or the TGFβRI/ALK-5 antagonist SB-431542 (10 μM; Sigma-Aldrich, St. Louis, MO). IOP was monitored in real time and recorded every 3 minutes using a PowerLab 8/35 data acquisition system equipped with LabChart Pro software for data analysis (AD Instruments, Colorado Springs, CO). Outflow facility (C) was calculated using the formula (F/P), where F represents the flow rate (4.5 μl/min), while P represents pressure (mm Hg). Changes in outflow facility were calculated as (Cexperimental/Cbaseline−1) × 100.
2.2 Histology
At the conclusion of perfusion experiments, porcine anterior segments were immediately fixed in 4% phosphate-buffered paraformaldehyde for 24h at 4°C. Sample wedges from opposite poles of fixed anterior segments were dehydrated in increasing concentrations of alcohol, embedded in paraffin, and sectioned on a sliding microtome (4 μm thickness). Paraffin-embedded sections were rehydrated in decreasing concentrations of ethanol, and stained with hematoxylin and eosin. Stained sections were qualitatively observed on a Leica upright DM 4000B microscope and photographed at 10x magnification using Neurolucida software (MBF Bioscience, Williston, VT).
2.3. Human Trabecular Meshwork Cell Culture
The use of human material in this study was approved by the Edward Hines Jr. VA Hospital institutional review board. Fresh corneoscleral rims were obtained (Illinois Eye Bank, Chicago, IL) at time of corneal transplant and primary human TM cell cultures were prepared using a collagenase-free procedure as we have previously described (Von Zee et al., 2009; Von Zee et al., 2012). Primary human TM cell cultures were maintained in Eagle’s Minimum Essential Medium containing 2 mM L-glutamine supplemented with 5% adult bovine serum, 10% fetal bovine serum, 50 μg/ml gentamicin, 2.5 μg/ml amphotericin B, and a mixture of essential (Life Technologies) and non-essential amino acids (Sigma-Aldrich). Individual TM cell lines were restricted to less than 6 passages. An SV40-transformed human TM cell line derived from a patient with glaucoma (GTM3; Alcon Laboratories) was maintained in DMEM containing 4 mM GlutaMAX-I supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) as previously described (Von Zee and Stubbs, 2011). Primary and transformed human TM cell cultures were maintained at 37°C under a humidified atmosphere of 5% CO2/95% air.
2.4. Experimental Treatment
Prior to use in cell culture, lovastatin (Calbiochem, Billerica, MA) was chemically activated by alkaline hydrolysis as we have previously described (Von Zee et al., 2009). Transformed human TM cells were cultured to confluency and treated x 24h with vehicle (0.01% ethanol) or activated lovastatin (10 μM). To inhibit post-translational isoprenylation and functional activation of small monomeric GTPases, primary or transformed human TM cells were incubated x 24h in the absence (0.6% DMSO) or presence of selective inhibitors of farnesyl transferase (FTI-277, 20 μM) or geranylgeranyl transferase-I (GGTI-298, 20 μM; Calbiochem). To inhibit de novo mRNA synthesis, transformed human TM cells were pre-treated x 1h with actinomycin D (1 μg/ml; Sigma-Aldrich) prior to incubation with GGTI-298 (20 μM). To determine the role of specific Rho subfamily GTPases in facilitating constitutive TGF-β2 expression, GTM3 cells were treated x 24h with specific inhibitors of Rac1 (NSC23766, 20 μM; Calbiochem), Cdc42 (ML141, 20 μM; Calbiochem), RhoA/B/C (C3 exoenzyme, 10 μg/ml; Cytoskeleton, Denver, CO), or p160ROCK (Y-27632, 10 μM; Tocris Biosciences, Minneapolis, MN).
2.5. Real-time RT-PCR
Total RNA was extracted from primary or transformed human TM cells using TRIzol reagent, and reverse-transcribed using Super Script III First Strand Synthesis system (Life Technologies) as described previously (Von Zee et al., 2009; Von Zee et al., 2012). TGF-β2, collagen (COL1A1), or GAPDH cDNA sequences were amplified by real-time PCR on a Mini-Opticon PCR detection system using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). The following human-specific primer pairs were used: TGF-β2 (sense, 5′-GCCCACTTTCTACAGACCCTACTTCAG; antisense, 5′-GGACTTTATAGTTTTCTGATCACCACTGG), COL1A1 (sense, 5′-GATTCCCTGGACCTAAAGGTGC; antisense, 5′-AGCCTCTCCATCTTTGCCAGCA). GAPDH (sense, 5′-TCCCTCAAGATTGTCAGCAA; antisense, 5′-AGATCCACAACGGATACATT) primers were used as a reference control. Optimized amplification steps of 94°C × 5 min followed by 94°C × 45s, annealing at optimized temperatures (59°C for TGF-β2, 60°C for COL1A1, 62°C for GAPDH) for 30s, and elongation at 72°C × 60s were used. For each sample, the specificity of the real-time reaction product was determined by melting curve analysis. Reaction efficiencies were typically >90%. The endogenous expression of GAPDH was unaltered with drug treatment. Data are therefore expressed as relative fold-changes in constitutive TGF-β2 mRNA content normalized to GAPDH.
2.6. TGF-β2 ELISA
The content of biologically-active TGF-β2 present in cell culture media was quantified using a commercially available human-specific ELISA kit (R&D Systems) according to manufacturer’s instructions. The working range for this human-specific TGF-β2 ELISA kit is 31–2000 pg/ml. Media from transformed or primary human TM cells cultured in 6-well cell culture plates were harvested, clarified by centrifugation (700g × 5min), and supernatant stored at −80°C until use. Thawed samples were aliquoted (100 μl) to microtiter wells pre-coated with a monoclonal antibody against human TGF-β2. Samples were read at 450 nm with a 540 nm correction, and results expressed as picograms of biologically-active TGF-β2.
2.7. Statistical Analysis
Results are expressed as mean ± SEM of triplicate cultures, repeated at least one additional time unless otherwise specified. Parametric data were analyzed by Student’s t-test or two-way ANOVA with a Bonferroni’s multiple comparison post-hoc analysis, as indicated. In all cases, p < 0.05 was considered statistically significant.
3. Results
3.1. Disrupting Endogenous TGF-β2 Signaling Enhances Outflow Facility
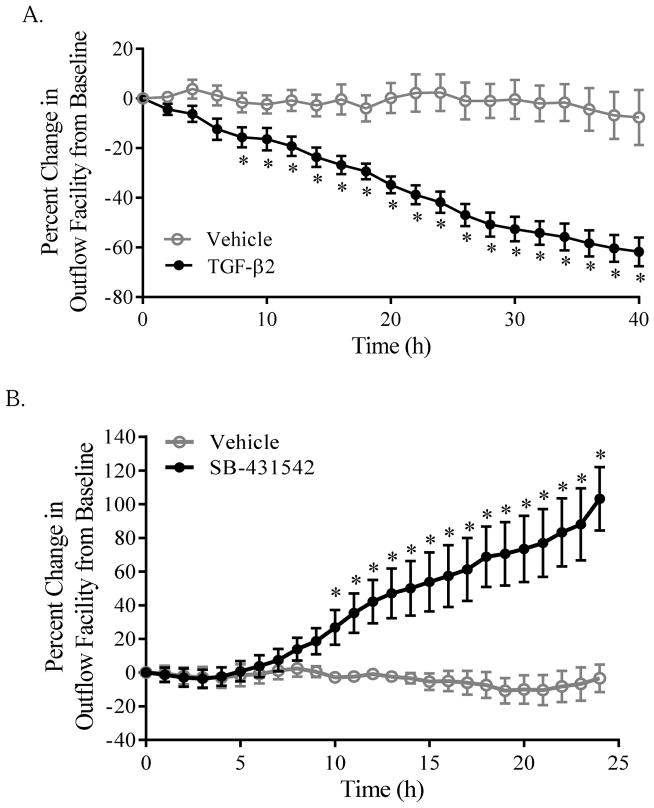
Consistent with previously reported findings (Gottanka et al., 2004; Bachmann et al., 2006; Fleenor et al., 2006; Bhattacharya et al., 2009), perfusing stabilized cultured porcine anterior segments with exogenous recombinant TGF-β2 elicits a marked time-dependent and sustained reduction in outflow facility when compared to vehicle perfused (400 nM HCl) paired segments. Within 8h, segments perfused with added TGF-β2 (10 ng/ml) exhibited a steady, significant decrease in outflow facility, corresponding to a rise in IOP that exceeded 21 mmHg (Fig. 1A, TGF-β2). In contrast, outflow facility within pair-matched vehicle-treated control segments remained stable for the duration of the experiment (Fig. 1A, Vehicle).
Figure 1. Disrupting endogenous TGF-β2 signaling enhances outflow facility in cultured porcine anterior segments.
(A) Stabilized porcine anterior segments were perfused with either vehicle (400 nM HCl) or TGF-β2 (10 ng/ml), as indicated. Data shown are the pooled means ± SEM from two separate experiments (n=5 per group) and expressed as percent change in stabilized outflow facility. (B) Stabilized porcine anterior segments were perfused with either vehicle (0.08% DMSO) or SB-431542 (10 μM), as indicated. Data shown are the means ± SEM of two separate experiments (n = 5–6 per group) and expressed as percent change in stabilized outflow facility. In each case, * p < 0.05; two-way ANOVA with Bonferroni’s post-hoc analysis.
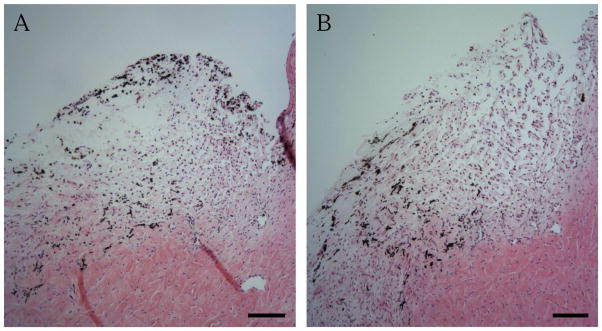
Whereas it is well-established that exogenous recombinant TGF-β2 significantly and reproducibly reduces outflow facility ex vivo, we addressed whether endogenously produced TGF-β2 would similarly elicit changes in outflow facility. To do so, a subgroup of porcine anterior segments which exhibited stable baseline IOPs modestly exceeding 15 mmHg following a 24h washout period was challenged with vehicle (0.08% DMSO) or SB-431542, a selective TGFβRI/ALK-5 antagonist. Segments with stable baseline IOPs over 15 mmHg perfused with vehicle showed no appreciable change in outflow facility over time (Fig. 1B, Vehicle). By comparison, we observed a time-dependent sustained and significant increase in outflow facility within 10h following perfusion with 10 μM SB-431542 (Fig. 1B, SB-431542). Importantly, no appreciable changes in TM cellularity or tissue morphology were observed in hematoxylin and eosin stained sections of these anterior segments (Fig. 2), demonstrating that observed changes in outflow facility were not the result of TM cell death. Application of SB-431542 (10 μM) similarly did not elicit appreciable changes in filamentous actin organization or cell shape, nor in constitutive expression of collagen (COL1A1) mRNA, in cultured human TM cells (data not shown).
Figure 2. Disrupting endogenous TGF-β2 signaling does not affect TM integrity.
Representative light photomicrographs of porcine TM tissue perfused x24h with (A) vehicle (0.08% DMSO) or (B) SB-431542 (10 μM) and stained with hematoxylin and eosin. Results shown are representative of 2 individual eyes per condition. Bar: 100 μm
3.2. Human TM cells Express and Secrete Biologically-Active TGF-β2
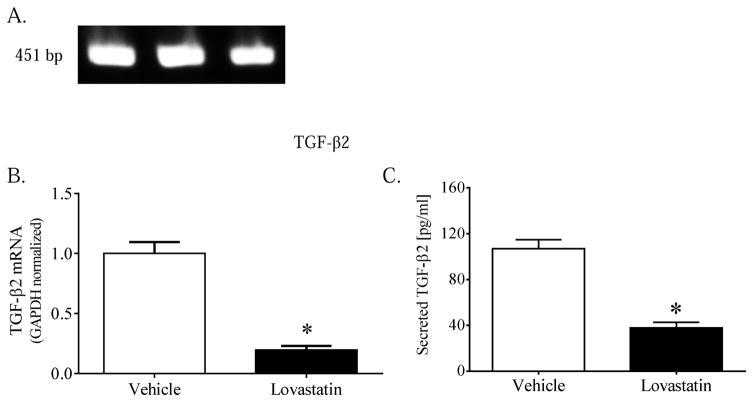
Quiescent primary or transformed human TM cells expressed quantifiable levels of TGF-β2 mRNA. Amplification using human-specific TGF-β2 primers yielded by agarose gel electrophoresis a single robust band migrating at the calculated amplicon size of 451 bp (Fig. 3A). Compared to vehicle-treated controls, TM cells cultured x 24h in the presence of activated lovastatin (10 μM) exhibited a marked 80% reduction in constitutive TGF-β2 mRNA content (Fig. 3B).
Figure 3. Lovastatin attenuates TGF-β2 mRNA expression and protein secretion.
(A) TGF-β2 mRNA expressed in vehicle-treated transformed human TM cell cultures was amplified by RT-PCR using human-specific primers with a calculated amplicon size of 451 bp. Data shown are products from three separate cultures resolved on an ethidium bromide-impregnated agarose mini-gel. (B, C) Confluent transformed human TM cells were incubated x 24h in the absence (vehicle, 0.01% ethanol) or presence (10 μM) of chemically-activated lovastatin. Data shown are the means ± SEM of (B) GAPDH-normalized TGF-β2 mRNA content (n=9) or (C) biologically-active TGF-β2 protein (n=6) present in media collected from cells treated as indicated. *, p < 0.001, Student’s t-test.
To determine the functional relevance of these findings, media collected from quiescent TM cell cultures was assayed by ELISA for the presence of biologically-active TGF-β2 protein. Quiescent vehicle-treated TM cell cultures released into the cell culture media quantifiable amounts (~100 ng/ml) of biologically-active TGF-β2 protein (Fig. 3C). By comparison, media collected from lovastatin-treated cell cultures contained significantly less biologically-active TGF-β2 (Fig. 3C). The level of TGF-β2 in serum-containing media alone was below detectable levels.
3.3. Post-Translational Geranylgeranylation of Rho GTPases Regulates TGF-β2 Expression in Human TM Cells
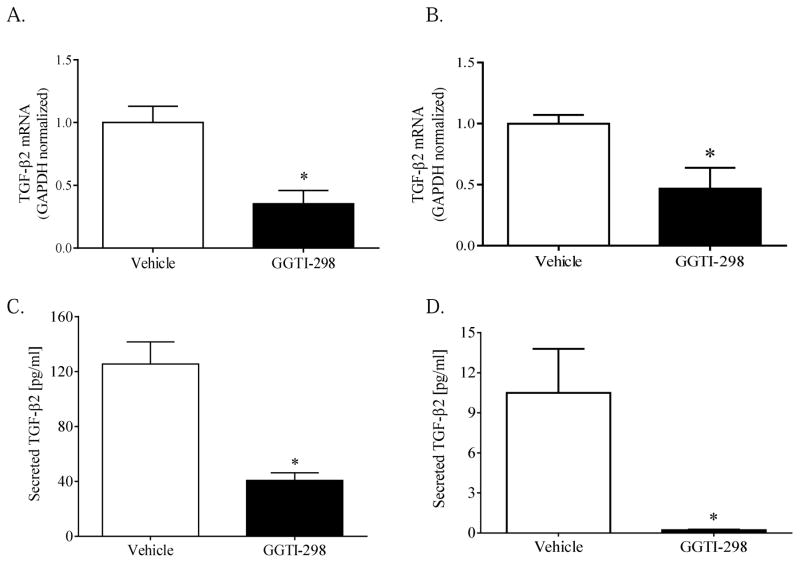
The mechanisms responsible for regulating endogenous TGF-β2 expression within human TM was next assessed. As a direct inhibitor of HMG-CoA reductase, lovastatin disrupts post-translational isoprenylation and functional activation of key monomeric GTPases (Von Zee et al., 2009; Von Zee and Stubbs, 2011; Stubbs and Von Zee, 2012). To determine whether post-translational isoprenylation of GTPases regulates endogenous TGF-β2 mRNA expression, primary or transformed human TM cells were cultured x 24h in media supplemented with vehicle (0.6% DMSO), farnesyltransferase inhibitor-277 (FTI-277, 20 μM), or geranylgeranyltransferase I inhibitor-298 (GGTI-298, 20 μM). Transformed human TM cells cultured in the presence of FTI-277 expressed comparable amounts of TGF-β2 mRNA (1.7 ± 0.4, n= 3), compared to vehicle-treated controls (1.0 ± 0.1, n=9). In contrast, the relative content of TGF-β2 mRNA expressed in GGTI-298 treated transformed cells was approximately 60% less than that expressed in vehicle-treated controls (Fig. 4A). These findings were not unique to the transformed phenotype, as primary human TM cells cultured in the presence of GGTI-298 (20 μM) similarly exhibited a marked reduction in the relative content of TGF-β2 mRNA (Fig. 4B). Disrupting prenyltransferase activity similarly affected the constitutive release of TGF-β2 protein. GGTI-298, but not FTI-277, significantly reduced the amount of biologically-active TGF-β2 protein released into the culture media from transformed (Fig. 4C) or primary (Fig. 4D) human TM cells.
Figure 4. Inhibition of geranylgeranyltransferase I attenuates TGF-β2 expression and secretion.
Confluent transformed (A, C) or primary (B, D) human TM cells were incubated x 24h in the absence (Vehicle, 0.6% DMSO) or presence of GGTI-298 (20 μM) as indicated. Data shown are the means ± SEM of (A, B) GAPDH-normalized TGF-β2 mRNA content (n=7–11) or (C, D) biologically-active TGF-β2 protein (n=3–6) present in media collected from cells treated as indicated. *, p < 0.01, Student’s t-test.
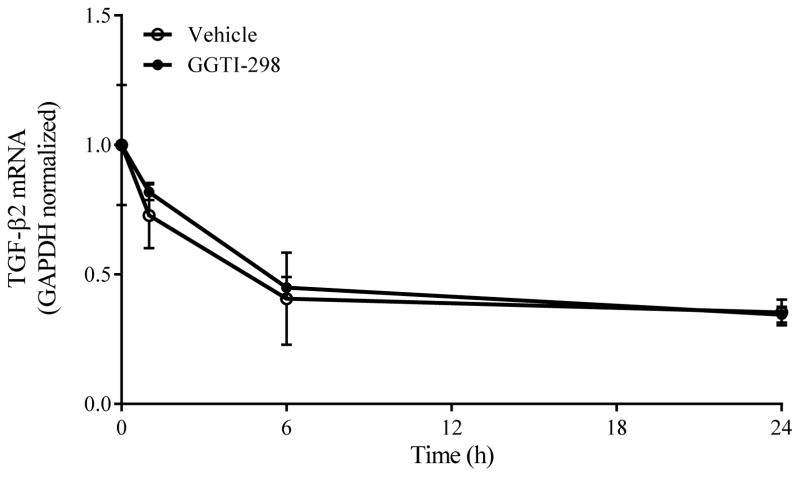
By selectively disrupting post-translational geranylgeranylation of monomeric GTPases, GGTI-298 may have either elicited repression of TGF-β2 gene expression or facilitated destabilization/degradation of TGF-β2 mRNA. To distinguish between these two possibilities, confluent transformed human TM cells were pre-treated x 1h with actinomycin D (1 μg/ml) and subsequently co-cultured for an additional 24h in the absence (0.6% DMSO) or presence of GGTI-298 (20 μM). When assayed in this manner, the content of TGF-β2 mRNA expressed in transformed human TM cells was unaffected by GGTI-298 treatment and gradually declined over the course of 24h at rate that was statistically indistinguishable from vehicle-treated cells (Fig. 5). These findings suggest that disrupting post-translational geranylgeranylation with GGTI-298 attenuates endogenous TGF-β2 mRNA expression in human TM cells by a mechanism other than mRNA destabilization.
Figure 5. Inhibition of geranylgeranyltransferase I does not alter TGF-β2 mRNA stability.
Confluent transformed human TM cells were pre-treated x 1h with actinomycin D (1 μg/ml) and subsequently incubated in the absence (Vehicle; 0.6% DMSO) or presence of GGTI-298 (20 μM). Data shown are the means ± SEM of GAPDH-normalized TGF-β2 mRNA content expressed in cells treated as indicated from a single experiment (n=3 per group), representative of 2–3 separate experiments.
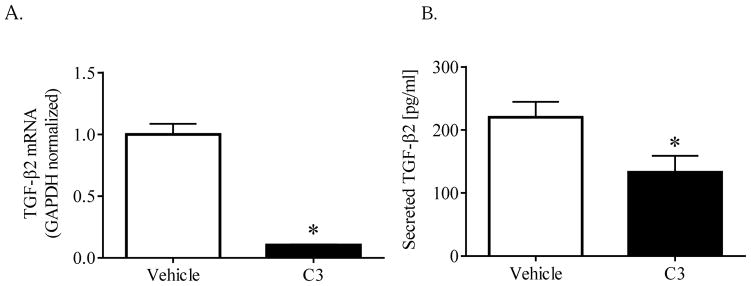
By inhibiting post-translational geranylgeranylation, GGTI-298 may affect the functional activation of multiple monomeric GTPases, including Rho, Rac, Cdc42 and RalA GTPases (Langert et al., 2013; Langert et al., 2014). To identify which subfamily of geranylgeranylated GTPases promotes TGF-β2 mRNA expression, transformed human TM cells were cultured x 24h in the absence or presence of NSC23766 (Rac1 inhibitor; 20 μM), ML141 (Cdc42 inhibitor; 20 μM), C3 exoenzyme (Rho subfamily inhibitor; 10 μg/ml), or Y-27632 (p160 ROCK inhibitor; 10 μM). Cells cultured in the presence of NSC23766, ML141 or Y-27632 expressed and released TGF-β2 in a manner that was indistinguishable from vehicle-treated control cells (data not shown). In contrast, transformed human TM cells cultured in the presence of C3 exoenzyme exhibited a marked 90% reduction in TGF-β2 mRNA content (Fig. 6A). Similarly, the content of biologically-active TGF-β2 released into the culture media by C3-treated cells was significantly reduced by 40% (Fig. 6B). By comparison, expression of collagen (COL1A1) mRNA in C3-treated cells (1.19 ± 0.31) was unchanged compared to cells treated with vehicle (1.00 ± 0.19). These findings strongly implicate a role for the Rho subfamily (RhoA/B/C) of GTPases in selectively promoting TGF-β2 gene expression and release in human TM cells.
Figure 6. Rho GTPases facilitate TGF-β2 expression and secretion.
Confluent transformed human TM cells were incubated x 24h in the absence (PBS) or presence of C3 exoenzyme (10 μg/ml). Data shown are the means ± SEM (n=6) of (A) GAPDH-normalized TGF-β2 mRNA content or (B) biologically-active TGF-β2 protein present in media collected from cells treated as indicated. *, p < 0.05, Student’s t-test.
4. Discussion
Experimental studies strongly support a pathophysiologic role of TGF-β2 at aberrantly elevating IOP. Despite these advancements, however, there remains a paucity of data elucidating the mechanisms regulating constitutive TGF-β2 expression and release. Using an established porcine anterior segment perfusion assay, we replicate in this study previously-reported findings demonstrating marked attenuation of outflow facility in response to exogenously-applied recombinant human TGF-β2. Perfusing anterior segments with a TGFβRI/ALK-5 antagonist (SB-431542), however, unexpectedly elicited a marked increase in outflow facility without markedly altering TM tissue cellularity or morphology. Primary and transformed human trabecular meshwork (TM) cells were found to express, in a Rho GTPase dependent manner, measurable quantities of TGF-β2 mRNA while constitutively releasing into the culture medium quantifiable amounts of biologically-active TGF-β2 protein. These findings show for the first time that endogenous expression and release of biologically-active TGF-β2 in human TM cells is regulated by constitutive Rho GTPase signaling. Localized Rho GTPase-mediated expression and release of TGF-β2 by TM cells may promote or exacerbate elevation of IOP in POAG.
As a therapeutic strategy to minimize scarring following glaucoma filtration surgery, early neutralization studies targeted biologically-active TGF-β2 protein (Cordeiro et al., 2003; Mead et al., 2003). Regrettably, long-term clinical trials proved this strategy to be less than feasible (Khaw et al., 2007). More recently, investigators have turned to targeting TGF-β2 receptors as a means to minimize subconjuctival scarring (Xiao et al., 2009; Sapitro et al., 2010). To our knowledge, disruption of TGF-β2 receptor signaling as a means of enhancing conventional outflow facility, and thus lowering IOP, in ocular hypertensive or POAG patients remains to be experimentally evaluated.
While elevated levels of TGF-β2 protein have been well-described in the AH of POAG patients (Tripathi et al., 1994b; Inatani et al., 2001; Picht et al., 2001; Ochiai and Ochiai, 2002; Ozcan et al., 2004; Yamamoto et al., 2005; Min et al., 2006), possibly due to enhanced synthesis and secretion by human TM cells (Cao et al., 2003; Luna et al., 2011; Tovar-Vidales et al., 2011), the mechanisms regulating constitutive TM-localized expression of this pathogenic cytokine remained largely undefined. Previously, it has been reported that endogenous TGF-β2 mRNA and protein expression in TM cells is modestly regulated by miR-29b (Luna et al., 2011). By comparison, we report here that activation of the Rho GTPase subfamily regulates TGF-β2 mRNA expression and release of biologically-active TGF-β2 protein from human TM cells. To date, the role of miR-29 in regulating RhoA/B/C GTPase expression or signaling is not known, though the miR-29 family is known to negatively regulate Cdc42 mRNA expression in other cell types (Park et al., 2009; Franceschetti et al., 2013).
The human LDS4 gene that encodes for the TGF-β2 protein is regulated by multiple promoter-region specific AP-1, AP-2, SP-1, and ATF-2 transcription factor binding elements, as well as a TATA box and a cAMP response element activated by ATF-1 (Roberts et al., 1991; Kingsley-Kallesen et al., 1999). Whereas induction of TGF-β2 expression by TGF-β1 or all-trans retinoic acid involves direct activation of RhoA/ROCK signaling in other cell types (Shimada et al., 2011; Namachivayam et al., 2015), a growing body of evidence further suggests that Rho GTPases may indirectly affect gene expression by facilitating serum response factor-mediated transcription of c-fos (Hill et al., 1995) or phosphorylation and activation of p38 MAP kinases (Charron et al., 2001; Marinissen et al., 2001). Consistent with these findings, we and others have previously reported a critical role for Rho GTPase signaling in facilitating TGF-β2 mediated induction of endothelin-1 (Von Zee et al., 2012), SPARC (Villarreal et al., 2014), and variety of extracellular matrix-associated genes (Pattabiraman and Rao, 2010; Pattabiraman et al., 2014). Interestingly, pharmacological inhibition of ROCK1 did not alter TGF-β2 mRNA expression and release from TM cells in our study, strongly implicating a role for alternative Rho GTPase signaling mediators, including mDia or LIM Kinase, in facilitating endogenous transcription of TGF-β2. The intermediate pathways activated by Rho GTPases which regulate expression and constitutive release of TGF-β2 protein from TM cells, while evident, remain to be elucidated.
The functional significance of our in vitro findings is underscored by the observed ocular hypotensive effect of SB-431542 in the absence of any observable cellular or morphological effects on the conventional outflow pathway, including changes in cytoskeletal organization or ECM deposition in cultured human TM cells (data not shown). TGF-β2, acting through enhancement of PAI-1 expression, is known to inhibit MMP-2 activity in TM cells (Fuchshofer et al., 2003). As a TGFβRI/ALK-5 antagonist, we speculate that SB-431542 enhances outflow facility in these segments by disrupting endogenous TGF-β2 signaling, including TGF-β2 mediated repression of MMP-2 activity, initiated by localized expression and release by TM cells.
Previously, anterior segment perfusion studies utilizing porcine segments have demonstrated an ocular hypotensive effect of lovastatin or a geranylgeranyl transferase-I inhibitor (Song et al., 2005; Rao et al., 2008). As indirect inhibitors of Rho GTPase subcellular distribution and activation, these studies suggest that lovastatin and geranylgeranyl transferase-I inhibitors enhance outflow through cultured anterior segments by disrupting organization of contractile F-actin stress fibers. Our data build on and expand these findings by suggesting that inhibition of constitutive Rho GTPase signaling may further enhance outflow facility by attenuating endogenous TGF-β2 expression and release.
In conclusion, findings from this study demonstrate Rho GTPase-dependent expression and constitutive release of biologically-active TGF-β2 by human TM cells. We speculate that localized expression and release of TGF-β2 by TM cells may promote or exacerbate elevation of IOP in POAG.
Highlights.
Inhibiting constitutive TGF-β2 expression enhances outflow facility in porcine anterior segments
Cultured human TM cells constitutively express and release active TGF-β2 in a Rho GTPase-dependent manner
Acknowledgments
The authors would like to acknowledge Drs. Donna M. Peters and Jennifer Faralli for assistance with porcine anterior segment perfusion, Dr. Charles Bouchard for assistance with procuring primary human TM cells, and Ms. Angelina Marino for PCR assistance. Supported, in part, by grants from the Department of Veterans Affairs (C7506M and I21RX001593 to CLP; C3638R, B3756-F & I21RX001553 to EBS), 1R21NS08542 to EBS, the Midwest Eye-Banks, the Illinois Society for the Prevention of Blindness, and the Richard A. Peritt Charitable Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Bachmann B, Birke M, Kook D, Eichhorn M, Lutjen-Drecoll E. Ultrastructural and biochemical evaluation of the porcine anterior chamber perfusion model. Invest Ophthalmol Vis Sci. 2006;47:2011–2020. doi: 10.1167/iovs.05-1393. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Gabelt BT, Ruiz J, Picciani R, Kaufman PL. Cochlin expression in anterior segment organ culture models after TGFbeta2 treatment. Invest Ophthalmol Vis Sci. 2009;50:551–559. doi: 10.1167/iovs.08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y, Houren W, Da B, Li Z. Transforming growth factor-β2 gene cloning and protein expression in human trabecular meshwork cells. Journal of Huazhong University of Science and Technology [Medical Sciences] 2003;23:85–87. [Google Scholar]
- Charron F, Tsimiklis G, Arcand M, Robitaille L, Liang Q, Molkentin JD, Meloche S, Nemer M. Tissue-specific GATA factors are transcriptional effectors of the small GTPase RhoA. Genes Dev. 2001;15:2702–2719. doi: 10.1101/gad.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro MF, Mead A, Ali RR, Alexander RA, Murray S, Chen C, York-Defalco C, Dean NM, Schultz GS, Khaw PT. Novel antisense oligonucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003;10:59–71. doi: 10.1038/sj.gt.3301865. [DOI] [PubMed] [Google Scholar]
- Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- Franceschetti T, Kessler CB, Lee SK, Delany AM. miR-29 promotes murine osteoclastogenesis by regulating osteoclast commitment and migration. J Biol Chem. 2013;288:33347–33360. doi: 10.1074/jbc.M113.484568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Wolfs RC, O’Colmain BJ, Klein BE, Taylor HR, West S, Leske MC, Mitchell P, Congdon N, Kempen J. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–538. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Stephan DA, Russell P, Tamm ER. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp Eye Res. 2009;88:1020–1032. doi: 10.1016/j.exer.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003;77:757–765. doi: 10.1016/s0014-4835(03)00220-3. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AH, Welge-Lussen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- Gottanka J, Chan D, Eichhorn M, Lutjen-Drecoll E, Ethier CR. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci. 2004;45:153–158. doi: 10.1167/iovs.03-0796. [DOI] [PubMed] [Google Scholar]
- Han H, Wecker T, Grehn F, Schlunck G. Elasticity-dependent modulation of TGF-beta responses in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:2889–2896. doi: 10.1167/iovs.10-6640. [DOI] [PubMed] [Google Scholar]
- Hill CS, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- Hill LJ, Mead B, Blanch RJ, Ahmed Z, De Cogan F, Morgan-Warren PJ, Mohamed S, Leadbeater W, Scott RA, Berry M, Logan A. Decorin Reduces Intraocular Pressure and Retinal Ganglion Cell Loss in Rodents Through Fibrolysis of the Scarred Trabecular Meshwork. Invest Ophthalmol Vis Sci. 2015;56:3743–3757. doi: 10.1167/iovs.14-15622. [DOI] [PubMed] [Google Scholar]
- Inatani M, Tanihara H, Katsuta H, Honjo M, Kido N, Honda Y. Transforming growth factor-beta 2 levels in aqueous humor of glaucomatous eyes. Graefes Arch Clin Exp Ophthalmol. 2001;239:109–113. doi: 10.1007/s004170000241. [DOI] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Acott TS. Differential effects of ADAMTS-1, -4, and -5 in the trabecular meshwork. Invest Ophthalmol Vis Sci. 2009;50:5769–5777. doi: 10.1167/iovs.09-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaw P, Grehn F, Hollo G, Overton B, Wilson R, Vogel R, Smith Z. A phase III study of subconjunctival human anti-transforming growth factor beta(2) monoclonal antibody (CAT-152) to prevent scarring after first-time trabeculectomy. Ophthalmology. 2007;114:1822–1830. doi: 10.1016/j.ophtha.2007.03.050. [DOI] [PubMed] [Google Scholar]
- Kingsley-Kallesen ML, Kelly D, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 promoter by cAMP-responsive element-binding protein (CREB) and activating transcription factor-1 (ATF-1) is modulated by protein kinases and the coactivators p300 and CREB-binding protein. J Biol Chem. 1999;274:34020–34028. doi: 10.1074/jbc.274.48.34020. [DOI] [PubMed] [Google Scholar]
- Langert KA, Pervan CL, Stubbs EB., Jr Novel role of Cdc42 and RalA GTPases in TNF-alpha mediated secretion of CCL2. Small GTPases. 2014;5 doi: 10.4161/sgtp.29260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langert KA, Von Zee CL, Stubbs EB., Jr Cdc42 GTPases facilitate TNF-alpha-mediated secretion of CCL2 from peripheral nerve microvascular endoneurial endothelial cells. J Peripher Nerv Syst. 2013;18:199–208. doi: 10.1111/jns5.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna C, Li G, Qiu J, Epstein DL, Gonzalez P. Cross-talk between miR-29 and transforming growth factor-betas in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:3567–3572. doi: 10.1167/iovs.10-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinissen MJ, Chiariello M, Gutkind JS. Regulation of gene expression by the small GTPase Rho through the ERK6 (p38 gamma) MAP kinase pathway. Genes Dev. 2001;15:535–553. doi: 10.1101/gad.855801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell CM, Tebow HE, Wordinger RJ, Clark AF. Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice. Exp Eye Res. 2013;116:419–423. doi: 10.1016/j.exer.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead AL, Wong TT, Cordeiro MF, Anderson IK, Khaw PT. Evaluation of anti-TGF-beta2 antibody as a new postoperative anti-scarring agent in glaucoma surgery. Invest Ophthalmol Vis Sci. 2003;44:3394–3401. doi: 10.1167/iovs.02-0978. [DOI] [PubMed] [Google Scholar]
- Min SH, Lee TI, Chung YS, Kim HK. Transforming growth factor-beta levels in human aqueous humor of glaucomatous, diabetic and uveitic eyes. Korean J Ophthalmol. 2006;20:162–165. doi: 10.3341/kjo.2006.20.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namachivayam K, MohanKumar K, Arbach D, Jagadeeswaran R, Jain SK, Natarajan V, Mehta D, Jankov RP, Maheshwari A. All-Trans Retinoic Acid Induces TGF-beta2 in Intestinal Epithelial Cells via RhoA- and p38alpha MAPK-Mediated Activation of the Transcription Factor ATF2. PLoS One. 2015;10:e0134003. doi: 10.1371/journal.pone.0134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai Y, Ochiai H. Higher concentration of transforming growth factor-beta in aqueous humor of glaucomatous eyes and diabetic eyes. Jpn J Ophthalmol. 2002;46:249–253. doi: 10.1016/s0021-5155(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Ozcan AA, Ozdemir N, Canataroglu A. The aqueous levels of TGF-beta2 in patients with glaucoma. Int Ophthalmol. 2004;25:19–22. doi: 10.1023/b:inte.0000018524.48581.79. [DOI] [PubMed] [Google Scholar]
- Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- Pasquale LR, Dorman-Pease ME, Lutty GA, Quigley HA, Jampel HD. Immunolocalization of TGF-beta 1, TGF-beta 2, and TGF-beta 3 in the anterior segment of the human eye. Invest Ophthalmol Vis Sci. 1993;34:23–30. [PubMed] [Google Scholar]
- Pattabiraman PP, Maddala R, Rao PV. Regulation of plasticity and fibrogenic activity of trabecular meshwork cells by Rho GTPase signaling. J Cell Physiol. 2014;229:927–942. doi: 10.1002/jcp.24524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattabiraman PP, Rao PV. Mechanistic basis of Rho GTPase-induced extracellular matrix synthesis in trabecular meshwork cells. Am J Physiol Cell Physiol. 2010;298:C749–763. doi: 10.1152/ajpcell.00317.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picht G, Welge-Luessen U, Grehn F, Lutjen-Drecoll E. Transforming growth factor beta 2 levels in the aqueous humor in different types of glaucoma and the relation to filtering bleb development. Graefes Arch Clin Exp Ophthalmol. 2001;239:199–207. doi: 10.1007/s004170000252. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Peterson YK, Inoue T, Casey PJ. Effects of pharmacologic inhibition of protein geranylgeranyltransferase type I on aqueous humor outflow through the trabecular meshwork. Invest Ophthalmol Vis Sci. 2008;49:2464–2471. doi: 10.1167/iovs.07-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Kim SJ, Noma T, Glick AB, Lafyatis R, Lechleider R, Jakowlew SB, Geiser A, O’Reilly MA, Danielpour D, et al. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp. 1991;157:7–15. doi: 10.1002/9780470514061.ch2. discussion 15–28. [DOI] [PubMed] [Google Scholar]
- Robertson JV, Golesic E, Gauldie J, West-Mays JA. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest Ophthalmol Vis Sci. 2010;51:308–318. doi: 10.1167/iovs.09-3380. [DOI] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Kawashima Y, Okada Y, Yamanaka O, Ohnishi Y, Ooshima A. Immunolocalization of TGF-beta1, -beta2, and -beta3, and TGF-beta receptors in human lens capsules with lens implants. Graefes Arch Clin Exp Ophthalmol. 2000;238:283–293. doi: 10.1007/s004170050354. [DOI] [PubMed] [Google Scholar]
- Sapitro J, Dunmire JJ, Scott SE, Sutariya V, Geldenhuys WJ, Hewit M, Yue BY, Nakamura H. Suppression of transforming growth factor-beta effects in rabbit subconjunctival fibroblasts by activin receptor-like kinase 5 inhibitor. Mol Vis. 2010;16:1880–1892. [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang IH, Jacobson N, Wang WH, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest Ophthalmol Vis Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- Shimada H, Staten NR, Rajagopalan LE. TGF-beta1 mediated activation of Rho kinase induces TGF-beta2 and endothelin-1 expression in human hepatic stellate cells. J Hepatol. 2011;54:521–528. doi: 10.1016/j.jhep.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46:2424–2432. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- Stubbs EB, Jr, Von Zee CL. Prenylation of Rho G-Proteins: a Novel Mechanism Regulating Gene Expression and Protein Stability in Human Trabecular Meshwork Cells. Mol Neurobiol. 2012;46:28–40. doi: 10.1007/s12035-012-8249-x. [DOI] [PubMed] [Google Scholar]
- Swaminathan SS, Oh DJ, Kang MH, Shepard AR, Pang IH, Rhee DJ. TGF-beta2-mediated ocular hypertension is attenuated in SPARC-null mice. Invest Ophthalmol Vis Sci. 2014;55:4084–4097. doi: 10.1167/iovs.13-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar-Vidales T, Clark AF, Wordinger RJ. Transforming growth factor-beta2 utilizes the canonical Smad-signaling pathway to regulate tissue transglutaminase expression in human trabecular meshwork cells. Exp Eye Res. 2011;93:442–451. doi: 10.1016/j.exer.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi RC, Chan WF, Li J, Tripathi BJ. Trabecular cells express the TGF-beta 2 gene and secrete the cytokine. Exp Eye Res. 1994a;58:523–528. doi: 10.1006/exer.1994.1046. [DOI] [PubMed] [Google Scholar]
- Tripathi RC, Li J, Chan WF, Tripathi BJ. Aqueous humor in glaucomatous eyes contains an increased level of TGF-beta 2. Exp Eye Res. 1994b;59:723–727. doi: 10.1006/exer.1994.1158. [DOI] [PubMed] [Google Scholar]
- Villarreal G, Jr, Chatterjee A, Oh SS, Oh DJ, Rhee DJ. Pharmacological regulation of SPARC by lovastatin in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2014;55:1657–1665. doi: 10.1167/iovs.13-12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Zee CL, Langert KA, Stubbs EB., Jr Transforming growth factor-beta2 induces synthesis and secretion of endothelin-1 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2012;53:5279–5286. doi: 10.1167/iovs.11-9289. [DOI] [PubMed] [Google Scholar]
- Von Zee CL, Richards MP, Bu P, Perlman JI, Stubbs EB., Jr Increased RhoA and RhoB protein accumulation in cultured human trabecular meshwork cells by lovastatin. Invest Ophthalmol Vis Sci. 2009;50:2816–2823. doi: 10.1167/iovs.08-2466. [DOI] [PubMed] [Google Scholar]
- Von Zee CL, Stubbs EB., Jr Geranylgeranylation facilitates proteasomal degradation of rho G-proteins in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:1676–1683. doi: 10.1167/iovs.10-6171. [DOI] [PubMed] [Google Scholar]
- Welge-Lussen U, May CA, Lutjen-Drecoll E. Induction of tissue transglutaminase in the trabecular meshwork by TGF-beta1 and TGF-beta2. Invest Ophthalmol Vis Sci. 2000;41:2229–2238. [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Xiao YQ, Liu K, Shen JF, Xu GT, Ye W. SB-431542 inhibition of scar formation after filtration surgery and its potential mechanism. Invest Ophthalmol Vis Sci. 2009;50:1698–1706. doi: 10.1167/iovs.08-1675. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Itonaga K, Marunouchi T, Majima K. Concentration of transforming growth factor beta2 in aqueous humor. Ophthalmic Res. 2005;37:29–33. doi: 10.1159/000083019. [DOI] [PubMed] [Google Scholar]