Abstract
Objective
To investigate the key physical, metabolic, hormonal and cardiovascular characteristics of metabolically healthy obese (MHO) vs. unhealthy obese (MUHO) girls with polycystic ovary syndrome (PCOS).
Design
Cross-sectional study.
Setting
Research center.
Patient(s)
Seventy obese girls with PCOS were divided into 19 MHO and 51 MUHO based on cut points for in vivo insulin sensitivity (within and < 2 SDs of normal-weight girls’ values respectively).
Intervetion(s)
None.
Main Outcome Measure(s)
Body composition, abdominal fat, in vivo insulin sensitivity and secretion (hyperinsulinemic-euglycemic and hyperglycemic clamps respectively), hormonal profile and cardiovascular disease risk markers.
Result(s)
MUHO-PCOS girls had higher waist circumference, visceral adipose tissue, leptin, higher free testosterone, lower SHBG and estradiol, higher Non-HDL cholesterol and atherogenic lipoprotein particle concentrations, smaller HDL particle size, and higher hs-CRP compared with MHO-PCOS. Hepatic and peripheral insulin sensitivity were lower with higher first- and second-phase insulin secretion, but β-cell function relative to insulin sensitivity was lower in MUHO vs. MHO-PCOS. Pair-matching of MHO and MUHO with respect to age and BMI revealed similar findings. MUHO-PCOS girls had larger visceral adiposity, lower insulin sensitivity and β-cell function, worse hormonal profile and severe atherogenic lipoprotein concentrations compared with MHO-PCOS.
Conclusion(s)
MHO-PCOS girls have favorable physical, metabolic, hormonal and CVD characteristics and lower risk biomarkers for type 2 diabetes compared with their MUHO-PCOS peers. A greater understanding of the contrast in this risk phenotype in obese girls with PCOS may have important implications for therapeutic interventions, their outcomes and their durability.
Keywords: PCOS, obese adolescents, metabolic risk
Since 1980s, evidence has accumulated that the presence of obesity-related comorbidities varies widely among obese individuals, with a distinct subgroup who is less prone to the development of metabolic abnormalities (1, 2). These individuals are known as metabolically healthy obese (MHO), and appear to be protected from an increased risk for future type 2 diabetes or cardiovascular disease (CVD) (3). Studies in pediatrics, similar to adults, show that MHO adolescents (defined based on the homeostatic model assessment for insulin resistance and insulin sensitivity) have favorable metabolic, hormonal and cardiovascular characteristics despite having body fat comparable to metabolically unhealthy obese (MUHO) adolescents (4–6).
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting females of the reproductive age and is characterized by menstrual dysfunction, clinical and/or biochemical hyperandrogenism, with or without polycystic ovaries, and insulin resistance (7). More than half of PCOS patients are overweight or obese in the United States (8), with an increased risk for obesity-related comorbidities such as metabolic syndrome, prediabetes or type 2 diabetes and CVD risk (9–11). Obese adolescent girls with PCOS have severe insulin resistance compared with their non PCOS peers (12) with evidence of impairment in β-cell function (13) and high rates of prediabetes and type 2 diabetes (14). Also, approximately 60% of them have the metabolic syndrome (15).
While the existence of the MHO phenotype is well established among obese individuals, men and women, little is known if MHO and MUHO subtypes exist among obese adolescent girls with PCOS. Therefore, the purpose of this study was to investigate the key physical, metabolic, hormonal and cardiovascular characteristics of MHO vs. MUHO adolescent girls with PCOS (MHO-PCOS vs. MUHO-PCOS).
MATERIALS AND METHODS
Patients
Data from 70 overweight/obese girls with a diagnosis of PCOS (age 15.2 ± 0.3 years, BMI 37.0 ± 0.8 kg/m2 [mean ± SE]), recruited from the PCOS Center at Children’s Hospital of Pittsburgh, were used in the present analysis. Eligible patients and their families were informed about the study while being evaluated in the PCOS center and given the opportunity to participate. Additionally, flyers were posted in the medical campus, pediatricians’ offices and city bus routes for interested individuals to contact us to learn about the study and assess eligibility. The diagnosis of PCOS was made based on the presence of clinical signs and symptoms of hyperandrogenism and/or biochemical hyperandrogenemia, oligomenorrhea, and the exclusion of secondary etiologies as per the Endocrine Society Clinical Practice Guidelines and previous publications (7, 12, 13, 16). Inclusion criteria were: 1) PCOS diagnosis as above; 2) age 10–20 years; and postmenarche, and 3) body mass index (BMI) ≥ 85th percentile for age and sex. Girls with pre-existing systemic or psychiatric disease and use of medications that impact carbohydrate or lipid metabolism (oral contraceptive pills [OCP], metformin, anti-epileptics, anti-psychotics, statins and fish oil) were excluded. The study was approved by the institutional review board of the University of Pittsburgh and written informed parental consent and child assent were obtained from all participants before any research participation in accordance with the ethical guidelines of Children’s Hospital of Pittsburgh.
Procedures
All procedures were performed at the Pediatric Clinical and Translational Research Center of Children’s Hospital of Pittsburgh. All participants underwent medical history, physical examination, and hematologic and biochemical tests. Height and weight were assessed to the nearest 0.1cm and 0.1kg, respectively, and used to calculate BMI. Pubertal development was assessed using Tanner criteria (17). Fasting blood samples were collected for determination of traditional lipid profile and lipoprotein particle size and concentration, glucose, insulin, leptin, adiponectin, high-sensitivity C-reactive protein (hs-CRP) and sex hormone profile (total and free testosterone, sex-hormone binding globulin [SHBG], estradiol and dehydroepiandrosterone sulfate [DHEAS]).
Body composition was evaluated with DEXA with measurement of total body fat mass, fat free mass and percent body fat. Abdominal total adipose tissue (TAT), subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) were assessed by computed tomography (CT) at L4–5 intervertebral space (18).
Metabolic Studies
A 2-hr oral glucose tolerance test (OGTT) was performed to assess glucose tolerance status (13, 16). The day following the OGTT or within a 1–4 week period, a hyperinsulinemic-euglycemic clamp, to assess in vivo insulin sensitivity, or a hyperglycemic clamp, to assess insulin secretion, was performed in random order as published before (12, 13, 16).
Fasting hepatic glucose production was measured, after a 10- to 12-hr overnight fast and before the start of the hyperinsulinemic-euglycemic clamp, with a primed (2.2 µmol/Kg) constant infusion of [6,6-2H2] glucose at 0.22 µmol/Kg/min for a total of 2 hours as described before (12, 16). After the 2-hr baseline isotope infusion period, in vivo insulin sensitivity was evaluated during a 3-hr hyperinsulinemic (80 mu/m2/min) -euglycemic clamp (12, 13, 16). Plasma glucose was clamped at approximately 100 mg/dL with a variable rate infusion of 20% dextrose in water. The glucose infusion was adjusted based on arterialized plasma glucose measurements every 5 minutes and blood was sampled every 10 to 15 minutes for determination of insulin concentrations.
First- and second-phase insulin secretion was assessed during a 2-hr hyperglycemic (225 mg/dL) clamp as described before (12, 13, 16). Plasma glucose was increased rapidly to 225 mg/dL by a bolus infusion of 50% dextrose and maintained at that level by a variable rate infusion of 20% dextrose for 2 hours, with frequent measurement of glucose and insulin concentrations.
Biochemical Measurements
Plasma glucose was measured with a glucose analyzer (Yellow Springs Instruments, Yellow Springs, OH) and insulin, leptin, and adiponectin were measured by RIA (16). Hs-CRP was measured by COAG-Nephelometry (Esoterix, formerly Colorado Coagulation). Total testosterone was measured by high-pressure liquid chromatography-tandem mass spectroscopy and DHEAS by RIA in dilute serum after hydrolysis (Esoterix Inc., Calabasas Hills, CA). Free testosterone was measured by equilibrium dialysis and SHBG by immunoradiometric assay. Concentrations of lipoprotein particle size and subclasses were determined using nuclear magnetic resonance spectroscopy at LipoScience using the LipoProfile-2 algorithm (LipoScience, Raleigh, NC) (19). Circulating biomarkers of vascular smooth muscle function, intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1) and E-selectin were quantified using commercially available double-sandwich enzyme-linked immunoassays (R&D Systems, Minneapolis, MN).
Calculations
Fasting hepatic glucose production was calculated during the last 30 min of the 2-hr isotope infusion (-30 to 0 min) according to steady-state tracer dilution equations (13, 16). Hepatic insulin sensitivity was calculated as the inverse of the product of hepatic glucose production and fasting plasma insulin concentration (16, 20). Insulin-stimulated glucose disposal (Rd) was calculated using the average exogenous glucose infusion rate during the final 30 min of the hyperinsulinemic-euglycemic clamp to be equal to the rate of exogenous glucose infusion. Peripheral insulin sensitivity was calculated by dividing the Rd by the steady-state clamp insulin concentration multiplied by 100 (13). During the hyperglycemic clamp, first- and second-phase insulin concentrations were calculated as reported before (13, 16); first phase during the first 10 minutes and second phase from 15–120 minutes. β-cell function relative to insulin sensitivity, i.e., the disposition index (DI), was calculated as the product of insulin sensitivity and first-phase insulin secretion (13, 16).
Statistical Analyses
Independent sample t-tests and chi-square were used to compare characteristics between the two groups (MHO-PCOS vs. MUHO-PCOS). Analysis of covariance was used to compare phenotypes after adjusting for the potential confounding effects of race and Tanner stage. Further, BMI and/or glucose tolerance status were additionally included as covariates. Paired t-test was used to compare MHO to MUHO-PCOS girls pair-matched for age and BMI. Data that did not meet the assumptions for normality were log10 transformed; untransformed data are presented for ease of interpretation. Data were analyzed using PASW 22.0 statistical software package with significance set at P ≤0.05.
RESULTS
Subtyping PCOS Adolescents to Metabolically Healthy (MHO) vs. Metabolically Unhealthy Obese (MUHO)-PCOS
Because insulin resistance is universally accepted to be the linchpin of the dysmetabolic syndrome and its components (21) we chose to define metabolic health based on the cut point for in vivo insulin sensitivity of 49 normal weight healthy adolescent girls (age 13.4 ± 1.9 years and BMI 23.6 ± 2.4 kg/m2 [SD]) who participated in our NIH funded K23 grant investigations of Insulin Resistance in Childhood (6, 22–24). The in vivo insulin sensitivity of the normal weight healthy adolescent girls was 9.1 ± 3.3 mg/kg/min per µU/mL and in the obese girls with PCOS was 1.9 ± 1.0 mg/kg/min per µU/mL [mean ± SD], P<0.0001. The insulin sensitivity of the MHO-PCOS group was chosen to be within 2 SDs of the mean of the normal-weight girls, and the insulin sensitivity of the MUHO-PCOS group was < 2 SDs of the mean of insulin sensitivity of the normal-weight healthy girls (< 2.52 mg/kg/min per µU/mL). Of the 70 obese girls with PCOS, 51 (73%) were categorized as MUHO-PCOS and 19 (27%) as MHO-PCOS (Table 1).
Table 1.
Physical and hormonal characteristics of metabolically healthy obese PCOS (MHO-PCOS) girls and metabolically unhealthy obese (MUHO-PCOS) girls in the total cohort.
| Variables | MHO-PCOS (n=19) |
MUHO-PCOS (n=51) |
P |
Adjusted * P |
|---|---|---|---|---|
| Age (years) | 16.0 ± 0.3 | 14.9 ± 0.3 | 0.065 | |
| Race (AA/AW/Bi) | 2(11%)/13(68%)/4(21%) | 17(33%)/32(63%)/2(4%) | 0.024 | |
| Tanner stage (IV/V) | 0 (0%) / 19 (100%) | 9 (17%) / 42 (83%) | 0.050 | |
| Glycemic status (NGT/IGT) | 14 (74%) / 5 (26%) | 28 (55%) / 23 (45%) | NS | |
| Anthropometrics | ||||
| BMI (kg/m2) | 33.3 ± 0.9 | 38.4 ± 0.2 | 0.007 | <0.0001 |
| BMI percentile | 96.8 ± 0.4 | 98.5 ± 0.2 | <0.0001 | <0.0001 |
| Fat mass (kg) | 40.8 ± 2.2 | 46.6 ± 2.1 | NS | 0.006 |
| Percent body fat (%) | 45.3 ± 0.8 | 46.6 ± 0.7 | NS | 0.123 |
| Fat free mass (kg) | 45.3 ± 1.5 | 49.3 ± 1.1 | 0.053 | <0.0001 |
| Waist circumference (cm) | 97.0 ± 3.2 | 110.2 ± 3.5 | 0.011 | 0.012 |
| Hip circumference (cm) | 113.1 ± 2.4 | 125.9 ± 3.1 | 0.005 | 0.005 |
| VAT (cm2) | 59.1 ± 4.3 | 84.8 ± 5.1 | 0.023 | 0.001 |
| SAT (cm2) | 512.9 ± 28.0 | 612.0 ± 26.9 | 0.051 | 0.003 |
| TAT (cm2) | 572.0 ± 30.1 | 696.7 ± 29.3 | 0.016 | <0.0001 |
| Sex steroid hormonal profile | ||||
| Total testosterone (ng/dL) | 36.2 ± 3.7 | 41.2 ± 2.9 | NS | NS |
| SHBG (nmol/L) | 30.6 ± 3.9 | 18.6 ± 1.4 | <0.001 | 0.002# |
| Free testosterone (pg/mL) | 6.5 ± 1.0 | 9.7 ± 0.9 | 0.013 | 0.003# |
| Estradiol (pg/mL) | 84.2 ± 16.8 | 56.3 ± 6.0 | 0.045 | 0.038# |
| DHEAS (ug/dL) | 197.6 ± 20.7 | 201.0 ± 19.2 | NS | NS |
| Adipokines and inflammatory markers | ||||
| Leptin (ng/mL) | 33.4 ± 2.4 | 43.2 ± 2.8 | 0.027 | 0.042 |
| Adiponectin (ug/mL) | 6.6 ± 0.6 | 5.5 ± 0.3 | NS | 0.095 |
| Leptin/Adiponectin ratio | 5.5 ± 0.5 | 9.3 ± 0.9 | 0.004 | 0.002# |
| hs-CRP (mg/L) | 0.9 ± 0.2 | 2.5 ± 0.5 | 0.013 | 0.007 |
Values are mean ± SEM, or n (%) / n (%) unless otherwise indicated.
P adjusted for race and Tanner stage.
indicates P <0.01 after adjusting for BMI in addition to race and Tanner stage.
NS, not-significant; AA, African-American; AW, American-White; Bi, biracial; NGT, normal glucose tolerance; IGT, impaired glucose tolerance; BMI, body mass index; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; TAT, total adipose tissue; SHBG, sex hormone-binding globulin; DHEAS, dehydroepiandrosterone sulfate.
Physical, Hormonal and Metabolic Characteristics of MHO-PCOS vs. MUHO-PCOS (Tables 1, 2 & 3, Figures 1)
Table 2.
Traditional lipid profile, lipoprotein particle size and concentration, and vascular smooth muscle biomarkers in metabolically healthy obese PCOS (MHO-PCOS) girls and metabolically unhealthy obese (MUHO-PCOS) girls in the total cohort.
| Variables | MHO-PCOS (n=19) |
MUHO-PCOS (n=51) |
P |
Adjusted* P |
|---|---|---|---|---|
| Traditional Lipid Profile | ||||
| Total cholesterol (mg/dL) | 143.4 ± 6.6 | 160.2 ± 4.7 | 0.055 | NS |
| Triglyceride (mg/dL) | 114.5 ± 14.1 | 126.1 ± 8.3 | NS | NS |
| HDL (mg/dL) | 41.1 ± 2.7 | 40.4 ± 1.2 | NS | NS |
| LDL (mg/dL) | 79.4 ± 5.2 | 94.6 ± 4.2 | NS | NS |
| VLDL (mg/dL) | 22.4 ± 2.7 | 24.9 ± 1.6 | NS | NS |
| Non-HDL cholesterol (mg/dL) | 98.4 ± 11.9 | 121.7 ± 4.8 | 0.035 | 0.077 |
| Lipoprotein Particle Size | ||||
| HDL particle size (nm) | 8.9 ± 0.3 | 8.7 ± 0.3 | 0.026 | 0.046† |
| LDL particle size (nm) | 21.2 ± 0.8 | 20.7 ± 0.7 | 0.065 | NS |
| VLDL particle size (nm) | 50.5 ± 2.7 | 54.1 ± 1.8 | NS | NS |
| Lipoprotein Particle Concentration | ||||
| Large VLDL (nmol/L) | 2.5 ± 1.1 | 3.8 ± 0.6 | 0.027 | 0.006#† |
| Medium VLDL (nmol/L) | 17.9 ± 4.1 | 19.5 ± 1.9 | NS | NS |
| Small VLDL (nmol/L) | 21.9 ± 3.3 | 31.1 ± 2.3 | 0.039 | NS |
| IDL (nmol/L) | 21.4 ± 8.8 | 41.5 ± 6.2 | 0.021 | NS |
| Large LDL (nmol/L) | 286.3 ± 48.3 | 260.8 ± 22.8 | NS | NS |
| Medium-small LDL (nmol/L) | 93.6 ± 17.9 | 135.4 ± 11.1 | 0.055 | NS |
| Very small LDL (nmol/L) | 332.0 ± 54.1 | 520.1 ± 48.1 | 0.035 | 0.096 |
| Large HDL (mmol/L) | 4.9 ± 0.7 | 4.5 ± 0.5 | NS | NS |
| Medium HDL (mmol/L) | 4.1 ± 0.6 | 4.2 ± 0.5 | NS | NS |
| Small HDL (mmol/L) | 13.4 ± 1.0 | 16.7 ± 0.7 | 0.021 | 0.065 |
| Vascular Smooth Muscle Biomarkers | ||||
| ICAM-1 (ng/mL) | 172.2 ± 14.6 | 219.1 ± 17.3 | NS | NS |
| VCAM-1 (ng/mL) | 550.0 ± 75.8 | 615.2 ± 47.0 | NS | NS |
| E-selectin (ng/mL) | 37.5 ± 4.6 | 127.6 ± 45.5 | NS | NS |
Values are mean ± SEM.
P adjusted for race and Tanner stage.
indicates P <0.01 after adjusting for BMI in addition to race and Tanner stage.
indicates P <0.01 after adjusting for glucose tolerance status in addition to race and Tanner stage.
NS, not-significant.
Table 3.
Pair-matched metabolically healthy obese (MHO-PCOS) and metabolically unhealthy obese PCOS (MUHO-PCOS) girls for BMI and age; physical, hormonal and metabolic characteristics.
| Variables | MHO-PCOS (n=19) |
MUHO-PCOS (n=19) |
P |
|---|---|---|---|
| Age (years) | 16.0 ± 0.3 | 15.7 ± 0.4 | NS |
| Race (AA/AW/Bi) | 2(11%)/13(68%)/4(21%) | 5(26%)/13(68%)/1(5%) | NS |
| Tanner stage (IV/V) | 0 (0%) / 19 (100%) | 1 (5%) / 18 (95%) | NS |
| Glycemic status (NGT/IGT) | 14 (74%) / 5 (26%) | 11 (58%) / 8 (42%) | NS |
| Anthropometrics | |||
| BMI (kg/m2) | 33.3 ± 0.9 | 34.1 ± 0.9 | NS |
| BMI percentile | 96.8 ± 0.4 | 97.8 ± 0.4 | NS |
| Fat mass (kg) | 40.8 ± 2.2 | 40.4 ± 1.7 | NS |
| Percent body fat (%) | 45.3 ± 0.9 | 44.8 ± 0.9 | NS |
| Waist circumference (cm) | 97.0 ± 3.2 | 101.0 ± 3.6 | NS |
| VAT (cm2) | 59.2 ± 4.3 | 87.7 ± 7.7 | 0.003 |
| SAT (cm2) | 513.0 ± 28 | 537.5 ± 34 | NS |
| TAT (cm2) | 572 ± 30.1 | 625.1 ± 37.8 | NS |
| Sex steroid hormonal profile | |||
| Total testosterone (ng/dL) | 36.2 ± 3.7 | 46.8 ± 6.1 | NS |
| SHBG (nmol/L) | 30.6 ± 3.8 | 19.5 ± 2.8 | 0.009 |
| Free testosterone (pg/mL) | 6.5 ± 1.0 | 11.6 ± 2.1 | 0.018 |
| Estradiol (pg/mL) | 84.2 ± 16.8 | 51.1 ± 6.4 | 0.051 |
| DHEAS (ug/dL) | 197.6 ± 20.7 | 201.0 ± 19.2 | NS |
| Adipokines and inflammatory markers | |||
| Leptin (ng/mL) | 33.4 ± 2.4 | 35.7 ± 2.7 | NS |
| Adiponectin (ug/mL) | 6.6 ± 0.6 | 5.3 ± 0.3 | 0.059 |
| Leptin/Adiponectin ratio | 5.5 ± 0.5 | 7.0 ± 0.5 | 0.047 |
| hs-CRP (mg/L) | 0.9 ± 0.2 | 1.3 ± 0.2 | NS |
| Traditional lipid profile | |||
| Total cholesterol (mg/dL) | 143.4 ± 6.6 | 165.1 ± 8.1 | 0.038 |
| VLDL (mg/dL) | 22.9 ± 2.8 | 24.8 ± 2.5 | 0.016 |
| Lipoprotein Particle Concentration | |||
| Large VLDL (nmol/L) | 2.5 ± 1.1 | 3.4 ± 0.6 | 0.040 |
| Medium-small LDL (nmol/L) | 93.6 ± 17.9 | 131.3 ± 16.2 | 0.047 |
| Very small LDL (nmol/L) | 332.0 ± 54.1 | 504.3 ± 74.3 | 0.032 |
Values are mean ± SEM, or n (%) / n (%) unless otherwise indicated. NS, not-significant.
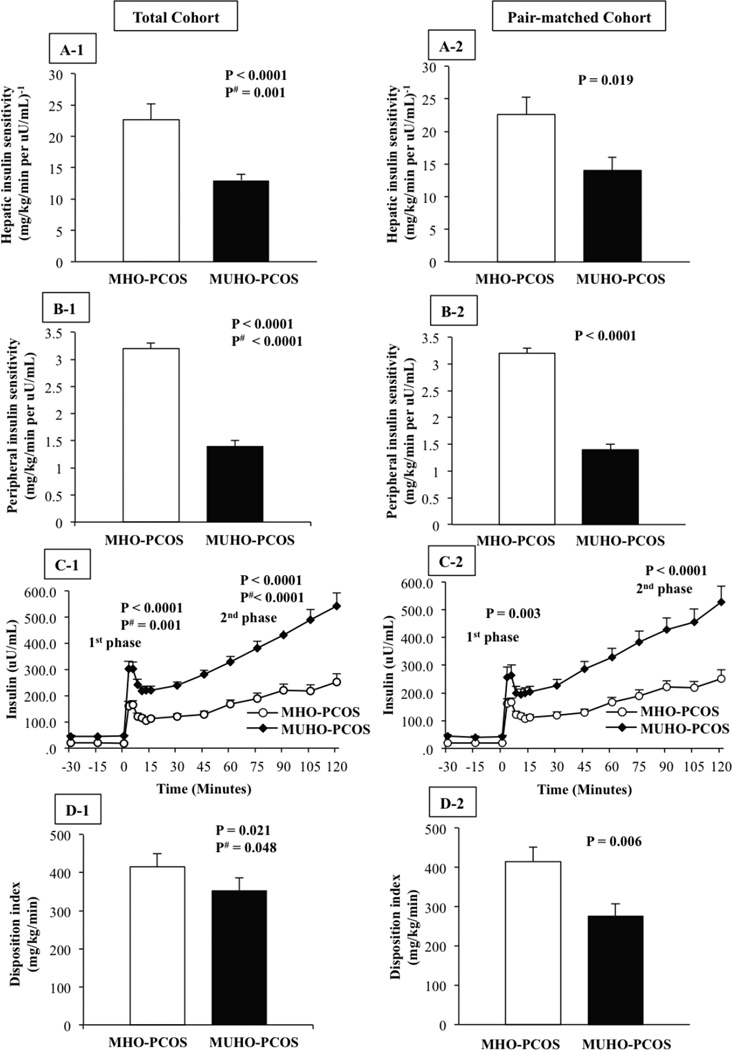
Figure 1. Left Panel: Total cohort (A1-D1), Right Panel: pair-matched cohort (A2-D2).
Hepatic insulin sensitivity (A), peripheral insulin sensitivity (B), insulin concentrations during the hyperglycemic clamp (C), and β-cell function relative to insulin sensitivity (disposition index) (D) in metabolically healthy (MHO-PCOS) versus metabolically unhealthy obese girls with PCOS (MUHO-PCOS). Adjusted P is for race, Tanner stage and BMI.
Table 1 shows the phenotypic characteristics of MHO- and MUHO-PCOS groups. MUHO-PCOS girls were slightly younger, with less advanced Tanner stage and more African-Americans than the MHO-PCOS group. MUHO-PCOS girls had higher BMI, fat mass, waist circumference, abdominal TAT, SAT and VAT compared with the MHO-PCOS (Table 1). For the hormonal profile, MUHO-PCOS girls had lower SHBG and estradiol, higher free testosterone levels and higher leptin to adiponectin ratio compared with the MHO-PCOS before and after adjusting for race, Tanner stage and BMI. Higher leptin and hs-CRP were observed in MUHO vs. MHO-PCOS before and after adjustment for race and Tanner stage (Table 1).
Hepatic and peripheral insulin sensitivity were lower in MUHO-PCOS girls vs. MHO-PCOS (Figure 1A1 and B1). This was compensated by higher first- and second-phase insulin secretion in the MUHO-PCOS group (Figure 1C1). However, β-cell function relative to insulin sensitivity, the DI was significantly lower in the MUHO-PCOS girls (Figure 1D1). These differences remained significant after adjusting for race, Tanner stage and BMI.
Table 2 depicts the differences between the two groups, before and after adjustment for race, Tanner stage, BMI and/or glucose tolerance status, in traditional lipid profile and lipoprotein particle size and concentration. HDL particle size was significantly smaller and large VLDL concentration was significantly higher in MUHO-PCOS compared with MHO-PCOS girls before and after adjustment. Small VLDL, intermediate-density lipoprotein (IDL), medium small LDL, very small LDL and small HDL concentrations were significantly higher in MUHO-PCOS girls; however, after statistical adjustment, these differences were no longer significant. Circulating smooth muscle biomarkers of ICAM-1, VCAM-1 and E-selectin were not different between the two groups.
In multiple regression analyses with age, race, VAT and insulin sensitivity as the independent variables, insulin sensitivity independently explained 19% of variance in second-phase insulin secretion (partial r=−0.434, P=0.001), 15% of variance in β-cell function relative to insulin sensitivity (partial r=0.381, P=0.004) and 9% of variance in leptin/adiponectin ratio (partial r=−0.305, P=0.033). However, both VAT and insulin sensitivity together and independently contributed to the variance in SHBG (partial r=0.277 and 0.548 respectively, P<0.05), to free testosterone (partial r=−0.274 and −0.460 respectively, P<0.05), and to estradiol (partial r=0.427 and 0.398 respectively, P<0.05).
To avoid a potential confounding effect of the difference in age and BMI between the two groups in the total cohort, we pair-matched 19 MHO- and 19 MUHO-PCOS girls with respect to age and BMI (Table 3). Despite similar age, BMI, fat mass and percent body fat, MUHO-PCOS girls had significantly greater VAT, lower SHBG and estradiol, higher free testosterone, higher leptin/adiponectin ratio, higher total and LDL cholesterol, and higher large VLDL, medium-small LDL and very small LDL concentrations compared with their pair-matched MHO-PCOS peers (Table 3). Hepatic and peripheral insulin sensitivity, and β-cell function relative to insulin sensitivity were lower in MUHO-PCOS girls pair-matched for age and BMI to MHO-PCOS girls (Figure 1A2-D2).
DISCUSSION
The present investigation reveals that MUHO-PCOS adolescent girls compared with MHO-PCOS unmatched or pair-matched for age and BMI have worse physical, hormonal and metabolic features characterized by: 1) larger abdominal visceral adiposity, 2) worse sex steroid hormone profile, 3) higher risk for type 2 diabetes manifested in lower insulin sensitivity and lower β–cell function relative to insulin sensitivity, 4) greater CVD risk manifested in an atherogenic lipoprotein lipid profile against the backdrop of severe insulin resistance and hyperinsulinemia.
PCOS is not only a hormonal and reproductive disorder, but also a cluster of metabolic abnormalities driven by insulin resistance and hyperinsulinemia in the presence or absence of obesity (25, 26). Whether or not insulin resistance plays a role in obesity-associated comorbidites has led to the popular concept of the metabolically healthy versus unhealthy obese phenotypes. The former describes a subtype of obese youth and adults who exhibit metabolic health despite having excess body fat (1–6). However, despite the significant obesity in a large proportion of PCOS women and girls and despite the severe insulin resistance which is part of the syndrome, there is a lack of data regarding metabolically healthy vs. unhealthy phenotypes in PCOS. Amato et al. examined MHO/MUHO phenotypes among lean and overweight/obese Caucasian women with PCOS using four different criteria (BMI, waist to hip ratio, at-risk category suggested by Androgen Excess Society and visceral adiposity index) (27). Of these criteria, visceral adiposity index better differentiated the risk in the MUHO group who had lower luteal progesterone levels, higher prevalence of metabolic syndrome and low β-cell function derived from an oral glucose tolerance test, compared with the MHO group. More recently, Tosi et al. divided lean and overweight/obese Caucasian women with PCOS into insulin resistant and insulin sensitive groups defined based on below or above the 25th percentile of the healthy women’s glucose disposal rate during the hyperinsulinemic-euglycemic clamp (28). They showed that insulin resistant women with PCOS had worse metabolic and hormonal profile including hyperinsulinemia, dyslipidemia and hyperandrogenemia compared with insulin sensitive women with PCOS. However, since the aforementioned studies were comprised of women with a wide range of BMI, including lean women, it is possible that different degrees of adiposity may have confounded the comparisons. To date, to our knowledge, no study has examined the “healthy obese” phenotype and its characteristics in adolescent girls with PCOS.
Of obese girls with PCOS recruited from the PCOS Center at Children’s Hospital of Pittsburgh, 73% were classified as MUHO, while 27% were categorized as MHO based on insulin sensitivity cut off derived from healthy normal-weight adolescent girls. Despite no differences in percent body fat between the two groups of the total cohort, the MUHO-PCOS girls had 30% larger abdominal visceral adipose tissue, an important determinant of insulin sensitivity and metabolic syndrome in youth (29). This finding was further confirmed by our BMI- and age- pair matched analysis and in agreement with previous studies on obese insulin resistant vs. insulin sensitive youth (4, 6). Further, consistent with the knowledge that abdominal adiposity is associated with systemic inflammatory response (30), MUHO-PCOS girls who exhibited increased VAT had higher hs-CRP concentrations with higher leptin and higher leptin to adiponectin ratio. Given that inflammation could be the link between insulin resistance, obesity and type 2 diabetes (31), the present findings would suggest that MUHO-PCOS girls are at heightened risk for type 2 diabetes. In fact, this seems to be the case based on our in vivo evaluation of insulin sensitivity and β-cell function demonstrating that the pathophysiological components of type 2 diabetes are worse in MUHO-PCOS than MHO-PCOS girls. Both hepatic and peripheral insulin sensitivity were significantly lower in MUHO vs MHO-PCOS girls. More importantly, despite their hyperinsulinemia manifested in higher fasting, first- and second-phase insulin secretion, MUHO-PCOS girls had significantly lower β-cell function relative to insulin sensitivity, i.e., DI, which is shown to be the strongest metabolic predictor of future type 2 diabetes (32, 33). Thus, our data suggest that MUHO-PCOS girls compared with their MHO-PCOS peers manifest an enhanced risk of type 2 diabetes based on their lower β-cell function and higher inflammation.
The more severe insulin resistance along with the accompanying hyperinsulinemia in MUHO-PCOS girls is associated with lower SHBG and higher free testosterone concentrations despite comparable total testosterone levels. This was also the case when the two groups were pair-matched for BMI and age. It is known that insulin resistance and compensatory hyperinsulinemia inhibit hepatic synthesis of SHBG, followed by increasing the proportion of testosterone that circulates in the unbound form (i.e., free testosterone) (34). Thus, MUHO-PCOS girls may have more untoward androgenic effects due to the higher biologically active free testosterone levels compared with MHO-PCOS girls despite similar total testosterone levels.
With respect to the risk of CVD, MUHO-PCOS girls appear more severely affected than MHO-PCOS girls. Among the traditional lipid profile, only non-HDL cholesterol level was significantly higher in MUHO-PCOS girls. However, a thorough evaluation of lipoprotein particle size and concentration unveiled the higher risk of atherogenesis in MUHO-PCOS girls as evidenced by the significantly small HDL particle size, and higher large VLDL, very small LDL and small HDL concentrations compared to MHO-PCOS. These findings of significant abnormalities in lipoproteins in the absence of pronounced alterations in the traditional lipid profile have been described in other high-risk CVD conditions, such as prediabetes in adults (35) and youth (36) and women with PCOS (37). However, there are limited data regarding non-traditional lipoprotein profile in adolescent girls with PCOS. Given that alterations in lipoprotein particle size and subclass composition may better differentiate CVD risk in obese youth compared with traditional lipid measures (38), our observations in MUHO-PCOS girls would suggest that the atherosclerotic process may be exacerbated in the metabolically unhealthy phenotype with PCOS. These significant differences in atherogenic lipoprotein particle concentrations were present also when the two groups were pair-matched for BMI and age. Nevertheless, we did not observe any differences in ICAM-1, VCAM-1 and E-selectin between MHO vs. MUHO-PCOS girls. Although these circulating biomarkers have been shown to be elevated in the context of obesity in adults (39), limited and inconsistent data have been reported in obese youth (40, 41). Thus, it is possible that alterations in these vascular smooth muscle biomarkers of endothelial function might evolve over time and with aging against the backdrop of persistent obesity, insulin resistance and high risk atherogenic lipoproteins into adulthood.
The strengths of the present investigation include: a) a first-time evaluation of metabolically healthy vs. unhealthy phenotypes in adolescent girls with PCOS, and b) the comprehensive assessment from physical, to hormonal, to state-of-the-art metabolic tests for a thorough probing to compare and contrast MHO vs. MUHO-PCOS girls. Additionally, the results of the analyses from pair-matching of the MHO- to MUHO-PCOS for BMI and age lends further support to our findings from the total cohort showing that the observed phenotypic, hormonal and metabolic differences are not due to BMI differences between the two groups. Potential perceived limitations would be that a cut point for defining MHO- vs. MUHO-PCOS girls is not a universal criteria although we strictly obtained this value from the robust measurement of in vivo insulin sensitivity in normal-weight girls. In addition, our study includes only obese PCOS girls; however the objective was to define the “obese healthy vs. unhealthy” phenotypes. It would be of scientific curiosity to examine if in normal-weight PCOS, categorizing girls according to insulin sensitivity may reveal similar healthy vs. unhealthy phenotypes. Further, it remains to be determined if any of the physical or biochemical contrasting markers between the two groups in the present study could be used in a separate and new set of participants using ROC analyses to identify patients at risk for poor metabolic health. From a clinical perspective such investigations could prove beneficial to the practicing clinician by providing a simple biomarker in identifying those “metabolically unhealthy” PCOS patients for individualized management. Even though our cross-sectional data reveal heightened risk for type 2 diabetes and atherogenesis in MUHO-PCOS girls, longitudinal studies are needed. Lastly, it would be worthwhile to examine whether MHO-PCOS girls preserve their metabolic health over time as they get older, or whether MUHO-PCOS girls respond differently to lifestyle modifications or pharmacological treatment compared with MHO-PCOS peers.
In summary, our data indicate that MHO-PCOS girls have a more favorable risk profile for type 2 diabetes, atherogenesis, inflammation and androgenemia than MUHO-PCOS girls despite similar adiposity. Longitudinal studies will unravel if these differences persist over time and whether or not therapeutic responses differ between the two groups. The translation of our findings to clinical practice remains of importance and should be pursued.
Acknowledgments
The author thank all the research participants and their parents, without whom science would not advance; Nancy Guerra, C.R.N.P., for her assistance; Resa Stauffer for her laboratory expertise; and the nursing staff of the Pediatric Clinical and Translational Research Center for their outstanding care of the participants and meticulous attention to the research. This study was supported by K24 HD01357 to SA, Richard L. Day Endowed Chair to SA, HT and JYK, Children’s Hospital of Pittsburgh PCTRC UL1TR000005 to CTSA and RR024153 to GCRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no conflicts of interest to disclose
REFERENCES
- 1.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1504. doi: 10.1053/meta.2001.27213. [DOI] [PubMed] [Google Scholar]
- 2.Samocha-Bonet D, Dixit VD, Kahn CR, Leibel RL, Lin X, Nieuwdorp M, et al. Metabolically healthy and unhealthy obese--the 2013 Stock Conference report. Obes Rev. 2014;15:697–708. doi: 10.1111/obr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 4.Weiss R, Taksali SE, Dufour S, Yeckel CW, Papademetris X, Cline G, et al. The "obese insulin-sensitive" adolescent: importance of adiponectin and lipid partitioning. J Clin Endocrinol Metab. 2005;90:3731–3737. doi: 10.1210/jc.2004-2305. [DOI] [PubMed] [Google Scholar]
- 5.Vukovic R, Mitrovic K, Milenkovic T, Todorovic S, Soldatovic I, Sipetic-Grujicic S, et al. Insulin-sensitive obese children display a favorable metabolic profile. Eur J Pediatr. 2013;172:201–206. doi: 10.1007/s00431-012-1867-5. [DOI] [PubMed] [Google Scholar]
- 6.Bacha F, Saad R, Gungor N, Arslanian SA. Are obesity-related metabolic risk factors modulated by the degree of insulin resistance in adolescents? Diabetes Care. 2006;29:1599–1604. doi: 10.2337/dc06-0581. [DOI] [PubMed] [Google Scholar]
- 7.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 9.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–1935. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 10.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 11.Legro RS, Kunselman AR, Dunaif A. Prevalence and predictors of dyslipidemia in women with polycystic ovary syndrome. Am J Med. 2001;111:607–613. doi: 10.1016/s0002-9343(01)00948-2. [DOI] [PubMed] [Google Scholar]
- 12.Lewy VD, Danadian K, Witchel SF, Arslanian S. Early metabolic abnormalities in adolescent girls with polycystic ovarian syndrome. J Pediatr. 2001;138:38–44. doi: 10.1067/mpd.2001.109603. [DOI] [PubMed] [Google Scholar]
- 13.Arslanian SA, Lewy VD, Danadian K. Glucose intolerance in obese adolescents with polycystic ovary syndrome: roles of insulin resistance and beta-cell dysfunction and risk of cardiovascular disease. J Clin Endocrinol Metab. 2001;86:66–71. doi: 10.1210/jcem.86.1.7123. [DOI] [PubMed] [Google Scholar]
- 14.Palmert MR, Gordon CM, Kartashov AI, Legro RS, Emans SJ, Dunaif A. Screening for abnormal glucose tolerance in adolescents with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87:1017–1023. doi: 10.1210/jcem.87.3.8305. [DOI] [PubMed] [Google Scholar]
- 15.Coviello AD, Legro RS, Dunaif A. Adolescent girls with polycystic ovary syndrome have an increased risk of the metabolic syndrome associated with increasing androgen levels independent of obesity and insulin resistance. J Clin Endocrinol Metab. 2006;91:492–497. doi: 10.1210/jc.2005-1666. [DOI] [PubMed] [Google Scholar]
- 16.Tfayli H, Ulnach JW, Lee S, Sutton-Tyrrell K, Arslanian S. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311–1319. doi: 10.1210/jc.2010-2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanner JM. Growth and maturation during adolescence. Nutr Rev. 1981;39:43–55. doi: 10.1111/j.1753-4887.1981.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Kuk JL, Hannon TS, Arslanian SA. Race and gender differences in the relationships between anthropometrics and abdominal fat in youth. Obesity (Silver Spring) 2008;16:1066–1071. doi: 10.1038/oby.2008.13. [DOI] [PubMed] [Google Scholar]
- 19.Otvos JD, Jeyarajah EJ, Bennett DW, Krauss RM. Development of a proton nuclear magnetic resonance spectroscopic method for determining plasma lipoprotein concentrations and subspecies distributions from a single, rapid measurement. Clin Chem. 1992;38:1632–1638. [PubMed] [Google Scholar]
- 20.Miyazaki Y, Glass L, Triplitt C, Wajcberg E, Mandarino LJ, DeFronzo RA. Abdominal fat distribution and peripheral and hepatic insulin resistance in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2002;283:E1135–E1143. doi: 10.1152/ajpendo.0327.2001. [DOI] [PubMed] [Google Scholar]
- 21.Reaven GM. Relationships among insulin resistance, type 2 diabetes, essential hypertension, and cardiovascular disease: similarities and differences. Journal of clinical hypertension. 2011;13:238–243. doi: 10.1111/j.1751-7176.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bacha F, Saad R, Gungor N, Arslanian SA. Adiponectin in youth: relationship to visceral adiposity, insulin sensitivity, and beta-cell function. Diabetes Care. 2004;27:547–552. doi: 10.2337/diacare.27.2.547. [DOI] [PubMed] [Google Scholar]
- 23.Lee S, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–56. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 24.Hannon TS, Bacha F, Lin Y, Arslanian SA. Hyperinsulinemia in African-American adolescents compared with their American white peers despite similar insulin sensitivity: a reflection of upregulated beta-cell function? Diabetes Care. 2008;31:1445–1447. doi: 10.2337/dc08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht Baldauff N, Arslanian S. Optimal management of polycystic ovary syndrome in adolescence. Arch Dis Child. 2015;100:1076–1083. doi: 10.1136/archdischild-2014-306471. [DOI] [PubMed] [Google Scholar]
- 26.Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Amato MC, Guarnotta V, Forti D, Donatelli M, Dolcimascolo S, Giordano C. Metabolically healthy polycystic ovary syndrome (MH-PCOS) and metabolically unhealthy polycystic ovary syndrome (MU-PCOS): a comparative analysis of four simple methods useful for metabolic assessment. Hum Reprod. 2013;28:1919–1928. doi: 10.1093/humrep/det105. [DOI] [PubMed] [Google Scholar]
- 28.Tosi F, Di Sarra D, Kaufman JM, Bonin C, Moretta R, Bonora E, et al. Total body fat and central fat mass independently predict insulin resistance but not hyperandrogenemia in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:661–669. doi: 10.1210/jc.2014-2786. [DOI] [PubMed] [Google Scholar]
- 29.Lee S, Gungor N, Bacha F, Arslanian S. Insulin resistance: link to the components of the metabolic syndrome and biomarkers of endothelial dysfunction in youth. Diabetes Care. 2007;30:2091–2097. doi: 10.2337/dc07-0203. [DOI] [PubMed] [Google Scholar]
- 30.Saito T, Murata M, Otani T, Tamemoto H, Kawakami M, Ishikawa SE. Association of subcutaneous and visceral fat mass with serum concentrations of adipokines in subjects with type 2 diabetes mellitus. Endocr J. 2012;59:39–45. doi: 10.1507/endocrj.ej11-0132. [DOI] [PubMed] [Google Scholar]
- 31.Dandona P, Aljada A, Bandyopadhyay A. Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol. 2004;25:4–7. doi: 10.1016/j.it.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 32.Abdul-Ghani MA, Williams K, DeFronzo RA, Stern M. What is the best predictor of future type 2 diabetes? Diabetes Care. 2007;30:1544–1548. doi: 10.2337/dc06-1331. [DOI] [PubMed] [Google Scholar]
- 33.Utzschneider KM, Prigeon RL, Faulenbach MV, Tong J, Carr DB, Boyko EJ, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32:335–341. doi: 10.2337/dc08-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 35.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 36.Burns SF, Lee S, Bacha F, Tfayli H, Hannon TS, Arslanian SA. Pre-diabetes in overweight youth and early atherogenic risk. Metabolism. 2014;63:1528–1535. doi: 10.1016/j.metabol.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phelan N, O'Connor A, Kyaw-Tun T, Correia N, Boran G, Roche HM, et al. Lipoprotein subclass patterns in women with polycystic ovary syndrome (PCOS) compared with equally insulin-resistant women without PCOS. J Clin Endocrinol Metab. 2010;95:3933–3939. doi: 10.1210/jc.2009-2444. [DOI] [PubMed] [Google Scholar]
- 38.Benson M, Hossain J, Caulfield MP, Damaso L, Gidding S, Mauras N. Lipoprotein subfractions by ion mobility in lean and obese children. J Pediatr. 2012;161:997–1003. doi: 10.1016/j.jpeds.2012.05.060. [DOI] [PubMed] [Google Scholar]
- 39.Ferri C, Desideri G, Valenti M, Bellini C, Pasin M, Santucci A, et al. Early upregulation of endothelial adhesion molecules in obese hypertensive men. Hypertension. 1999;34:568–573. doi: 10.1161/01.hyp.34.4.568. [DOI] [PubMed] [Google Scholar]
- 40.Desideri G, De Simone M, Iughetti L, Rosato T, Iezzi ML, Marinucci MC, et al. Early activation of vascular endothelial cells and platelets in obese children. J Clin Endocrinol Metab. 2005;90:3145–3152. doi: 10.1210/jc.2004-1741. [DOI] [PubMed] [Google Scholar]
- 41.Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92:3025–3032. doi: 10.1210/jc.2007-0619. [DOI] [PubMed] [Google Scholar]