Abstract
Validated methods are needed for the analysis of natural product secondary metabolites. These methods are particularly important to translate in vitro observations to in vivo studies. Herein, a method is reported for the analysis of the key secondary metabolites, a series of flavonolignans and a flavonoid, from an extract prepared from the seeds of milk thistle [Silybum marianum (L.) Gaertn. (Asteraceae)]. This report represents the first UHPLC MS-MS method validated for quantitative analysis of these compounds. The method takes advantage of the excellent resolution achievable with UHPLC to provide a complete analysis in less than 7 min. The method is validated using both UV and MS detectors, making it applicable in laboratories with different types of analytical instrumentation available. Lower limits of quantitation achieved with this method range from 0.0400 μM to 0.160 μM with UV and from 0.0800 μM to 0.160 μM with MS. The new method is employed to evaluate variability in constituent composition in various commercial S. marianum extracts, and to show that storage of the milk thistle compounds in DMSO leads to degradation.
Keywords: milk thistle, Silybum marianum, flavonolignans, silymarin, validation
Graphical abstract

1. Introduction
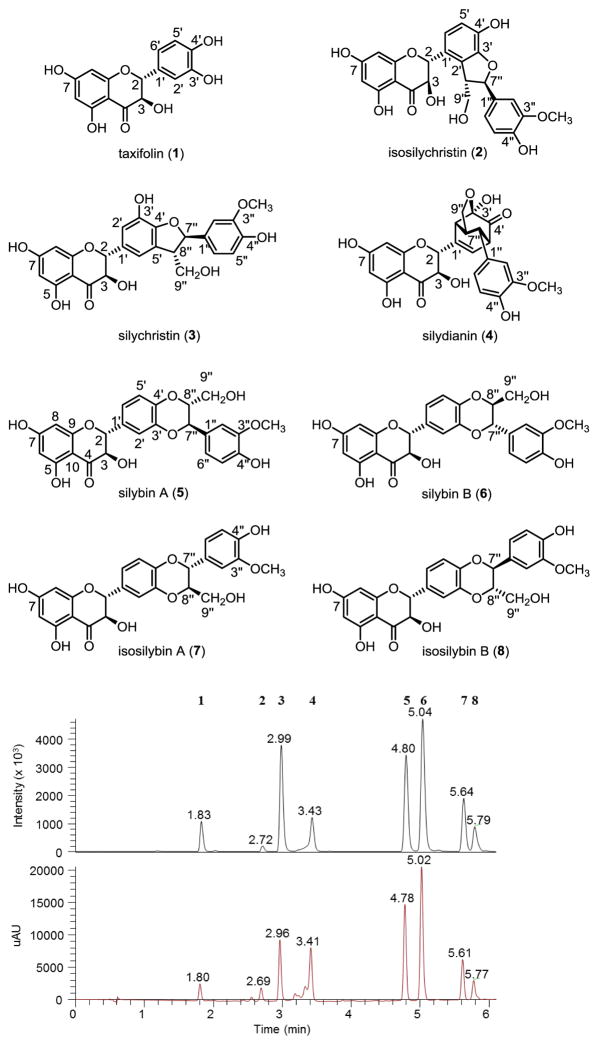
The medicinal herb, milk thistle [Silybum marianum (L.) Gaertn. (Asteraceae)], has been used since antiquity, particularly for hepatoprotective applications [1], and more recently, for prostate cancer chemoprevention [2]. In the modern herbal pharmacopeia, there are two main milk thistle preparations [3]. Silymarin is an extract of the seeds (achenes) and consists of the flavonoid taxifolin (1) and at least seven flavonolignans [isosilychristin (2), silychristin (3), silydianin (4), silybin A (5), silybin B (6), isosilybin A (7), and isosilybin B (8); Fig. 1], along with other minor constituents. This extract can be partially purified to form the other main preparation, termed either silybin or silibinin, which is largely a 1:1 mixture of 5 and 6.
Figure 1.
Structures of compounds 1–8. The numerical order of the compounds corresponds to their elution order in the MS and UV chromatograms for silymarin extract Madaus 37501.
After over a decade of studying the chemistry of flavonolignans from milk thistle, methods have been developed to separate and identify the flavonolignan diastereoisomers on the gram scale [4]. This supply, in turn, has assisted with the examination of the cytoprotective properties of the milk thistle compounds, where they have demonstrated activity in inhibiting virus infection, preventing oxidative stress, and modulating cellular metabolic and inflammatory status [5–7]. For effective studies of the biological activity of milk thistle preparations, both in vitro and in vivo, knowledge of the quantity and identity of bioactive constituents in study material is needed. To address this need, the purpose of this study was to develop a validated method for the quantitative determination of milk thistle compounds in extracts and preparations.
In the past few years, there have been three studies devoted to the chemical characterization of milk thistle products. Li et al [8] developed a 40 min HPLC-MS method for analysis of silibinin (a 1:1 mixture of 5 and 6) in plasma. Hadad et. al. [9] developed an 8 min HPLC method (employing monolithic columns) for analysis of seven compounds from silymarin. The compounds were detected by relying on their absorbance at 288 nm. Finally, Wang et. al. [10] employed UHPLC coupled to electrospray ionization mass spectrometry (ESI-MS) for identification of seven compounds from silymarin. Similar to the approach employed by Wang et. al. [10], the method described herein relies on UHPLC ESI-MS. A major advantage of this approach is the rapid analysis times that it facilitates (in this case, 6 min.). However, unlike the previously published UHPLC ESI-MS method [10], our method is extended to include both quantitation and identification. Indeed, herein is the first validated method for quantitative analysis of all eight major bioactive components of silymarin (1–8).
2. Experimental
2.1. Materials and Chemicals
The seven flavonolignans and taxifolin (Fig. 1) were isolated as described previously [4], and were all of a high purity (≥98%) as determined by UHPLC-UV analysis. Constituent levels were quantified in a number of different silymarin extracts using the methods described herein. Two separate batches of silymarin (product number 345066, lot numbers 37501 and 286061) from Euromed, S.A. (Barcelona, Spain), which is a part of the Madaus Group (Cologne, Germany), a batch of silymarin from Indena S.p.A. (Milan, Italy) (lot number 27691; the source for the isolation of the pure compounds), and two separate batches of silymarin from Sigma-Aldrich (St. Louis, MO, USA) (product numbers S0292 and 254924; lot numbers BCBJ0393V and 05503PG) were analyzed. Caffeine, HPLC grade acetonitrile, and mass spectrometry grade formic acid, methanol (MeOH), and water (H2O) were purchased from Fisher Scientific (Waltham, MA, USA).
2.2. Preparation of Samples and Standards
All compounds were dissolved separately in 1:1 CH3CN:H2O (with 655 μM caffeine as internal control) to produce 5.24 mM stock solutions. An equimolar master mixture of the eight analytes was prepared at concentrations of 655 μM for each individual component (and 655 μM caffeine). This master mixture was serially diluted 2-fold to produce 17 standard mixtures ranging in concentration from 328 μM to 0.005 μM. A separate series of quality control samples (QC) was prepared by separately diluting the 655 μM master mixture to 16.4 μM, 1.64 μM, and 0.164 μM (high, medium, and low QCs). A series of silymarin mixtures was prepared in 1:1 CH3CN:H2O at 2.53 mg/mL, and diluted 100-fold prior to analysis.
2.3. UHPLC-UV-MS Quantitative Analysis
UHPLC analyses were conducted utilizing a Waters Acquity UHPLC system (Milford, MA, USA) equipped with an autosampler, photodiode array detector (PDA), column manager, and binary solvent manager. An HSS-T3 C18 column (100 mm × 2.1 mm i.d., 1.8 μm packing, from Waters) operated at 50 °C was used for all chromatographic analyses. The gradient system consisted of 0.1% formic acid in MeOH (B) and 0.1% formic acid in H2O (A), at a flow rate of 0.5 mL/min. A gradient from 30–55% B over 6.0 min followed by reequilibration at 30% B for 0.6 minutes was used. All samples and standards were analyzed in triplicate using 3 μL injections, with the exception of the QC samples, which were injected in quintuplicate.
The UHPLC system was coupled to a Thermo Scientific TSQ Quantum Access triple quadrapole mass spectrometer (Waltham, MA, USA) with a heated electrospray ionization (HESI) source. Analyses were conducted in the positive ion mode, with a spray voltage of 3800 V, vaporizer and capillary temperatures of 360 °C and 380 °C, respectively, and sheath gas and auxiliary gas of 50 and 45 (arbitrary units), respectively. Tube lens offset and skimmer offset were 122 V and 0 V, respectively.
Quantitative analysis was performed using UV data collected at 288 nm and multiple reaction monitoring (MRM) on the mass spectrometer (MS). The UV and MS data were collected simultaneously for each injection. MRM transitions employed for each compound are given in Table 1. For each analyte, three additional MRM transitions were observed to verify peak identity, and a table of the transitions observed during each of the acquisition segments is provided (Table S1). UV and MS data were collected and analyzed using Xcaliber software (version 2.2, from Thermo Scientific). Peak detection and peak areas were determined using the ICIS algorithm for MS data and the Avalon algorithm for UV data. All calibration curves were generated in Xcaliber using 1/X weighting to create linear curve fits that emphasize the lower concentration calibration points. External calibration was employed for this analysis.
Table 1.
Multiple reaction monitoring transitions observed.a
| Analyte | Retention time (min) | Precursor ion (m/z) | Product ion (m/z) | Collision energy (eV) |
|---|---|---|---|---|
| Caffeine | 1.19 | 195.1 [M+H]+ | 138.2 | 22 |
| 1 | 1.82 | 305.1 [M+H]+ | 259.1 | 13 |
| 2 | 2.70 | 483.1 [M+H]+ | 153.0 | 22 |
| 3 | 2.97 | 453.1 [M-CH2OH +H]+ | 435.1 | 12 |
| 4 | 3.42 | 483.1 [M+H]+ | 153.0 | 30 |
| 5 | 4.79 | 483.1 [M+H]+ | 465.1 | 10 |
| 6 | 5.03 | 483.1 [M+H]+ | 465.1 | 10 |
| 7 | 5.63 | 483.1 [M+H]+ | 329.0 | 10 |
| 8 | 5.79 | 483.1 [M+H]+ | 329.0 | 10 |
The dwell time for all transitions was 0.040 min.
2.4. Method validation
The identities of the standards were confirmed by NMR and mass spectrometry analyses [4, 11, 12], as well as by comparison of elution time with other identified standards. Linearity of the calibration curves was assessed by least-squares analysis. Precision and accuracy were determined by calculating the relative standard deviation (RSD) and relative error (RE), respectively, for replicate injections. For the purpose of this study, RE is defined as the percent difference between the mean measured concentration of three replicate injections of each standard concentration, and the nominal concentration of that standard. All analyses were performed in triplicate on three different days. Repeatability was evaluated based on the RSD and RE for triplicate analyses in a single day, while intermediate precision was determine based on the interday RSD and RE. The lower limit of quantitation (LLOQ) was defined as the lowest concentration of a given analyte that could be measured with an intraday precision below 15%. For the MS data analysis, the upper limit of quantitation (ULOQ) was defined as the highest concentration where the relationship between MS signal and concentration was linear (as evident by R2 > 0.9995). For the UV data analysis, the ULOQ was set at the highest concentration measured in this study, as the upper limit for linearity in UV signals was not reached. All standard curves were plotted using standard concentrations between, and including, the LLOQ and the ULOQ. The linear dynamic range for each analyte was defined as being all concentrations between, and including, the LLOQ and the ULOQ. The linearity of all of the UV standard curves was verified by each having an R2 >0.9999.
Sets of high, medium, and low QC samples were placed at the beginning, middle, and end of the sample sets for each day of analysis, and the intraday and interday results for RE and RSD were measured to verify method and instrument stability across the days of analysis. Caffeine was included as an internal control in all standard and QC samples to monitor consistency in retention times and linearity of both dilutions and instrument response.
2.5. Matrix effects
Matrix effects were examined by comparison of quantitation results for silymarin samples spiked with blank solvent versus the results for the same samples spiked with an equal volume of a solution containing all eight of the analytes. Matrix effects were determined by subtracting the area for the analyte of interest in the unspiked extract (Amatrix) from the area in the spiked extract (Amatrix, spiked), and dividing by the area for the analyte in solvent without matrix (Asolvent) (equation 1).
| Equation 1 |
2.6. Evaluation of compound stability
UHPLC analyses were completed utilizing a Waters Acquity UHPLC system similar to the one described in Section 2.3. All degraded samples used for this experiment were from the same batches of the eight compounds as were used for the validation study. Degraded samples were prepared at 500 μM in DMSO from pure (non-degraded) dry stocks of each analyte and stored at room temperature for 6 months (191 days) before analysis. The fresh samples of each compound were prepared in MeOH from the same dry stocks of the compounds (stored dry at 4 °C over the same 6 months) immediately before analysis.
3. Results and Discussion
3.1. Method validation
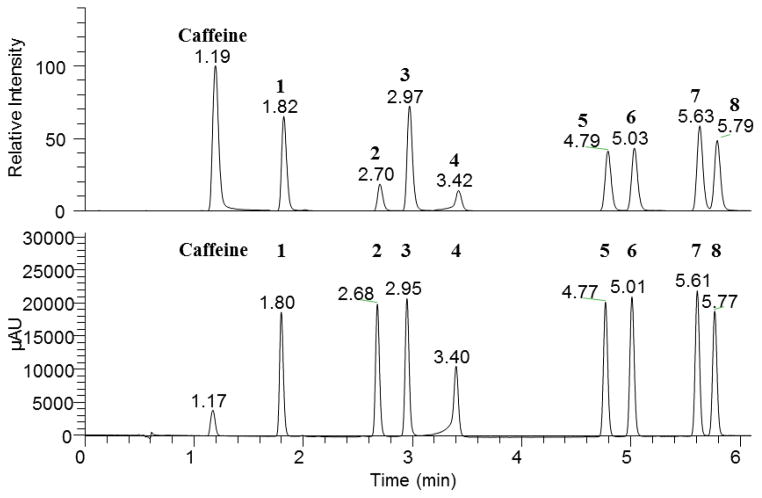
Calibration curves for all standards exhibited coefficients of determination (R2) greater than 0.9995 for MS data (Table 2) and 0.9999 for UV data (Table 3). Baseline resolution of all analytes was achieved (Fig. 2). Precision and accuracy for the MS and UV data are given in Tables 4 and 5, respectively. For all standard concentrations at or above the MS LLOQ, the RSD of all compounds using MS data was below 12% (intraday) and 7.0% (interday); and the RE for MS data remained below 7.8% (intraday) and 6.3% (interday) (Table 4). For all standard concentrations at or above the UV LLOQ, the RSD for all compounds using UV data was below 7.2% (intraday) and 8.4% (interday); and the RE for UV data remained below 12% (intraday) and 9.2% (interday) (Table 5). These findings indicate sufficient accuracy and precision for a validated analytical method. According to the FDA Guidance for Industry: Bioanalytical Method Validation, the target values for precision and accuracy of validated methods are ≤15% [13]. All measurements in the linear dynamic range for each analyte had a precision and accuracy of <15% (Table 4 and Table 5).
Table 2.
Parameters of calibration curves for the MS data.
| Analyte | Slope (± SD) x 103 | Intercept (± SD) x 103 | r2 | LLOQa (μM) | ULOQb (μM) |
|---|---|---|---|---|---|
| 1 | 973.8 (2.9) | 11.7 (12) | 0.9998 | 0.0800 | 10.2 |
| 2 | 315.0 (1.7) | 1.4 (7.2) | 0.9995 | 0.0800 | 10.2 |
| 3 | 1379.5 (4.4) | 29.6 (18) | 0.9997 | 0.0800 | 10.2 |
| 4 | 376.5 (1.4) | −1.1 (6.3) | 0.9995 | 0.160 | 10.2 |
| 5 | 822.9 (1.9) | 22.3 (7.8) | 0.9997 | 0.0800 | 10.2 |
| 6 | 831.4 (3.5) | 25.7 (15) | 0.9995 | 0.0800 | 10.2 |
| 7 | 798.6 (3.5) | 5.9 (15) | 0.9996 | 0.0800 | 10.2 |
| 8 | 748.5 (2.4) | 2.1 (10) | 0.9996 | 0.0800 | 10.2 |
Lower limit of quantitation
Upper limit of quantitation
Table 3.
Parameters of calibration curves for the UV data.
| Analyte | Slope (± SD) | Intercept (± SD) | r2 | LLOQa (μM) | ULOQb (μM) |
|---|---|---|---|---|---|
| 1 | 4495.3 (2.6) | 15 (260) | 1.0000 | 0.0400 | 328 |
| 2 | 5068.8 (2.8) | 9 (280) | 1.0000 | 0.0400 | 328 |
| 3 | 5412.3 (3.4) | 28 (350) | 1.0000 | 0.0400 | 328 |
| 4 | 4460.8 (6.3) | −372 (690) | 0.9999 | 0.160 | 328 |
| 5 | 5474.8 (2.3) | 98 (240) | 1.0000 | 0.0400 | 328 |
| 6 | 5790.5 (2.8) | 101 (280) | 1.0000 | 0.0400 | 328 |
| 7 | 5937.4 (2.9) | 67 (290) | 1.0000 | 0.0400 | 328 |
| 8 | 5156.0 (2.4) | 16 (240) | 1.0000 | 0.0400 | 328 |
Lower limit of quantitation
Upper limit of quantitation
Figure 2.
MS and UV chromatograms of an equimolar mixture of analytes 1–8 at 10.24 μM and the internal standard caffeine.a
aPeak at 1.19/1.17 min is caffeine, which was added to monitor for drift in response and retention time.
Table 4.
Intraday and interday precision and accuracy using MS detection.
| Analyte | Concentration of Standard Solution Injected (μM) | Intraday | Interday | ||
|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| 0.0800 | 2.0 | 1.9 | 2.1 | 0.51 | |
| 0.160 | 1.2 | 2.0 | 1.2 | 0.65 | |
| 0.320 | 3.0 | 1.6 | 2.4 | 0.82 | |
| 0.640 | 2.8 | 0.73 | 0.12 | 0.63 | |
| 1 | 1.28 | 0.092 | 1.3 | 1.6 | 0.080 |
| 2.56 | 0.89 | 0.19 | 0.37 | 0.28 | |
| 5.12 | 1.0 | 0.55 | 0.18 | 0.39 | |
| 10.2 | 1.3 | 0.53 | 0.34 | 0.26 | |
| 0.0800 | 7.2 | 1.6 | 0.49 | 2.0 | |
| 0.160 | 8.2 | 0.76 | 2.7 | 0.28 | |
| 0.320 | 2.4 | 1.5 | 3.0 | 1.6 | |
| 0.640 | 1.6 | 1.4 | 0.61 | 2.1 | |
| 2 | 1.28 | 0.98 | 2.3 | 1.1 | 1.2 |
| 2.56 | 1.1 | 0.054 | 2.3 | 0.93 | |
| 5.12 | 2.5 | 0.38 | 1.0 | 0.074 | |
| 10.2 | 2.5 | 0.021 | 0.24 | 0.25 | |
| 0.0800 | 0.35 | 7.8 | 1.6 | 6.3 | |
| 0.160 | 2.8 | 0.85 | 1.5 | 0.64 | |
| 0.320 | 0.83 | 0.62 | 0.99 | 0.52 | |
| 0.640 | 1.7 | 3.8 | 0.95 | 3.4 | |
| 3 | 1.28 | 0.60 | 2.4 | 0.51 | 2.3 |
| 2.56 | 0.50 | 0.47 | 1.2 | 0.69 | |
| 5.12 | 1.6 | 0.79 | 0.090 | 0.89 | |
| 10.2 | 0.34 | 1.0 | 0.36 | 1.1 | |
| 0.160 | 4.4 | 6.5 | 7.0 | 1.4 | |
| 0.320 | 3.4 | 5.6 | 5.2 | 0.40 | |
| 0.640 | 5.1 | 0.25 | 0.33 | 0.012 | |
| 4 | 1.28 | 2.6 | 0.55 | 1.3 | 0.50 |
| 2.56 | 0.45 | 1.6 | 1.7 | 0.37 | |
| 5.12 | 2.7 | 0.95 | 0.70 | 0.45 | |
| 10.2 | 0.64 | 0.056 | 0.57 | 0.37 | |
| 0.0800 | 8.0 | 1.7 | 2.4 | 0.20 | |
| 0.160 | 6.1 | 7.2 | 5.7 | 1.1 | |
| 0.320 | 1.9 | 4.4 | 3.8 | 0.70 | |
| 0.640 | 3.1 | 3.0 | 1.5 | 1.3 | |
| 5 | 1.28 | 1.7 | 0.019 | 2.3 | 0.74 |
| 2.56 | 0.45 | 2.0 | 1.4 | 0.59 | |
| 5.12 | 1.5 | 0.28 | 1.1 | 0.17 | |
| 10.2 | 0.22 | 0.41 | 0.67 | 0.070 | |
| 0.0800 | 12 | 0.71 | 4.5 | 4.4 | |
| 0.160 | 9.5 | 0.0050 | 1.9 | 1.4 | |
| 0.320 | 4.9 | 1.0 | 2.3 | 3.5 | |
| 0.640 | 4.9 | 0.79 | 4.3 | 1.0 | |
| 6 | 1.28 | 2.9 | 1.5 | 0.66 | 1.0 |
| 2.56 | 2.4 | 1.2 | 0.87 | 0.30 | |
| 5.12 | 1.5 | 0.24 | 0.63 | 0.33 | |
| 10.2 | 1.9 | 0.032 | 0.55 | 0.079 | |
| 0.0800 | 3.7 | 5.9 | 5.9 | 1.5 | |
| 0.160 | 7.9 | 4.3 | 3.0 | 1.3 | |
| 0.320 | 3.1 | 3.0 | 1.7 | 1.1 | |
| 0.640 | 3.2 | 1.3 | 2.0 | 0.98 | |
| 7 | 1.28 | 0.11 | 0.12 | 1.5 | 0.22 |
| 2.56 | 1.3 | 0.92 | 0.92 | 0.75 | |
| 5.12 | 2.1 | 1.7 | 0.50 | 1.2 | |
| 10.2 | 1.5 | 0.59 | 0.56 | 0.38 | |
| 0.0800 | 4.5 | 2.7 | 0.94 | 1.9 | |
| 0.160 | 5.4 | 7.6 | 5.1 | 2.4 | |
| 0.320 | 2.8 | 3.5 | 1.8 | 1.7 | |
| 0.640 | 3.6 | 3.8 | 3.0 | 0.72 | |
| 8 | 1.28 | 2.0 | 0.58 | 1.9 | 1.0 |
| 2.56 | 1.6 | 2.5 | 1.1 | 1.8 | |
| 5.12 | 0.48 | 0.47 | 0.23 | 0.68 | |
| 10.2 | 1.3 | 0.20 | 0.41 | 0.16 | |
Table 5.
Intraday and interday precision and accuracy using UV detection.
| Analyte | Concentration of Standard Solution Injected (μM) | Intraday | Interday | ||
|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| 0.0400 | 1.8 | 2.4 | 6.0 | 1.5 | |
| 0.0800 | 6.6 | 6.4 | 0.12 | 6.5 | |
| 0.160 | 1.5 | 1.4 | 4.7 | 0.95 | |
| 0.320 | 1.4 | 1.2 | 0.97 | 0.11 | |
| 0.640 | 0.36 | 0.33 | 1.5 | 1.2 | |
| 1.28 | 0.088 | 0.40 | 1.4 | 2.0 | |
| 2.56 | 0.25 | 0.72 | 0.85 | 1.7 | |
| 1 | 5.12 | 1.2 | 0.50 | 0.90 | 1.5 |
| 10.2 | 0.22 | 0.70 | 0.41 | 1.2 | |
| 20.5 | 0.52 | 0.78 | 0.32 | 0.94 | |
| 41.0 | 0.037 | 1.1 | 0.42 | 0.70 | |
| 81.9 | 0.033 | 0.19 | 0.23 | 0.26 | |
| 164 | 0.067 | 0.051 | 0.27 | 0.073 | |
| 328 | 0.21 | 0.29 | 0.061 | 0.26 | |
| 0.0400 | 5.8 | 0.89 | 3.7 | 2.8 | |
| 0.0800 | 0.49 | 0.43 | 1.3 | 0.86 | |
| 0.160 | 1.3 | 6.0 | 1.6 | 4.4 | |
| 0.320 | 1.7 | 0.010 | 0.19 | 0.020 | |
| 0.640 | 0.94 | 0.92 | 1.0 | 0.24 | |
| 1.28 | 0.47 | 1.2 | 1.2 | 1.4 | |
| 2.56 | 0.38 | 1.0 | 0.85 | 1.4 | |
| 2 | 5.12 | 1.4 | 1.5 | 0.74 | 1.2 |
| 10.2 | 0.47 | 1.1 | 0.33 | 1.0 | |
| 20.5 | 0.61 | 1.0 | 0.49 | 0.75 | |
| 41.0 | 0.12 | 0.88 | 0.76 | 0.48 | |
| 81.9 | 0.12 | 0.066 | 0.27 | 0.011 | |
| 164 | 0.038 | 0.031 | 0.14 | 0.042 | |
| 328 | 0.21 | 0.27 | 0.16 | 0.15 | |
| 0.0400 | 2.9 | 11 | 2.8 | 8.5 | |
| 0.0800 | 5.3 | 3.8 | 3.5 | 1.1 | |
| 0.160 | 3.7 | 3.9 | 0.80 | 3.4 | |
| 0.320 | 1.7 | 0.11 | 0.75 | 0.23 | |
| 0.640 | 0.94 | 1.4 | 1.7 | 1.2 | |
| 1.28 | 2.8 | 1.6 | 1.2 | 2.4 | |
| 2.56 | 0.087 | 2.2 | 0.55 | 2.3 | |
| 3 | 5.12 | 1.3 | 2.1 | 0.63 | 1.9 |
| 10.2 | 0.52 | 1.4 | 0.26 | 1.4 | |
| 20.5 | 1.0 | 1.5 | 0.65 | 1.1 | |
| 41.0 | 0.10 | 0.90 | 0.75 | 0.52 | |
| 81.9 | 0.16 | 0.019 | 0.30 | 0.050 | |
| 164 | 0.063 | 0.057 | 0.15 | 0.053 | |
| 328 | 0.20 | 0.33 | 0.19 | 0.20 | |
| 0.160 | 0.91 | 12 | 2.2 | 9.2 | |
| 0.320 | 1.7 | 2.5 | 4.2 | 4.9 | |
| 0.640 | 1.2 | 0.58 | 1.2 | 2.0 | |
| 1.28 | 4.3 | 1.4 | 1.7 | 2.9 | |
| 2.56 | 0.49 | 2.5 | 2.0 | 3.0 | |
| 4 | 5.12 | 1.0 | 3.4 | 0.61 | 3.4 |
| 10.2 | 1.3 | 3.6 | 0.078 | 3.6 | |
| 20.5 | 1.1 | 2.5 | 0.50 | 2.2 | |
| 41.0 | 0.49 | 1.8 | 0.50 | 1.4 | |
| 81.9 | 0.0076 | 0.23 | 0.092 | 0.18 | |
| 164 | 0.66 | 0.15 | 0.20 | 0.10 | |
| 328 | 0.20 | 0.69 | 0.23 | 0.59 | |
| 0.0400 | 4.4 | 5.0 | 8.4 | 0.31 | |
| 0.0800 | 2.1 | 0.82 | 3.1 | 1.6 | |
| 0.160 | 1.1 | 1.4 | 2.8 | 1.8 | |
| 0.320 | 2.3 | 1.3 | 0.20 | 1.2 | |
| 0.640 | 1.4 | 1.2 | 1.8 | 0.26 | |
| 1.28 | 0.88 | 0.29 | 0.82 | 0.32 | |
| 2.56 | 0.66 | 0.69 | 0.81 | 0.67 | |
| 5 | 5.12 | 1.1 | 0.48 | 0.29 | 0.60 |
| 10.2 | 0.29 | 0.16 | 0.35 | 0.0013 | |
| 20.5 | 0.65 | 0.62 | 0.34 | 0.33 | |
| 41.0 | 0.21 | 0.78 | 0.85 | 0.40 | |
| 81.9 | 0.15 | 0.10 | 0.35 | 0.0011 | |
| 164 | 0.10 | 0.0085 | 0.17 | 0.027 | |
| 328 | 0.21 | 0.18 | 0.16 | 0.072 | |
| 0.0400 | 4.4 | 7.1 | 6.1 | 0.97 | |
| 0.0800 | 1.0 | 4.7 | 4.0 | 0.47 | |
| 0.160 | 2.6 | 4.2 | 1.2 | 3.5 | |
| 0.320 | 0.92 | 2.8 | 2.3 | 0.70 | |
| 0.640 | 0.96 | 0.52 | 0.99 | 0.31 | |
| 1.28 | 0.84 | 0.86 | 0.82 | 0.37 | |
| 2.56 | 0.65 | 1.1 | 0.81 | 0.95 | |
| 6 | 5.12 | 0.84 | 0.59 | 0.43 | 0.83 |
| 10.2 | 0.15 | 0.13 | 0.43 | 0.17 | |
| 20.5 | 0.64 | 0.89 | 0.28 | 0.59 | |
| 41.0 | 0.21 | 0.93 | 0.95 | 0.55 | |
| 81.9 | 0.14 | 0.12 | 0.38 | 0.032 | |
| 164 | 0.11 | 0.015 | 0.19 | 0.056 | |
| 328 | 0.21 | 0.21 | 0.17 | 0.11 | |
| 0.0400 | 5.8 | 3.5 | 2.3 | 1.4 | |
| 0.0800 | 6.8 | 6.5 | 2.9 | 3.4 | |
| 0.160 | 2.9 | 4.4 | 3.4 | 3.2 | |
| 0.320 | 1.9 | 1.5 | 1.2 | 0.29 | |
| 0.640 | 0.75 | 0.16 | 1.2 | 0.40 | |
| 1.28 | 1.2 | 1.2 | 1.4 | 0.38 | |
| 2.56 | 0.76 | 0.16 | 1.0 | 0.078 | |
| 7 | 5.12 | 1.1 | 0.15 | 0.38 | 0.20 |
| 10.2 | 0.42 | 0.27 | 0.31 | 0.013 | |
| 20.5 | 0.47 | 0.60 | 0.35 | 0.40 | |
| 41.0 | 0.051 | 0.93 | 0.88 | 0.43 | |
| 81.9 | 0.18 | 0.11 | 0.39 | 0.036 | |
| 164 | 0.13 | 0.037 | 0.18 | 0.038 | |
| 328 | 0.26 | 0.18 | 0.18 | 0.070 | |
| 0.0400 | 7.2 | 5.8 | 1.0 | 4.8 | |
| 0.0800 | 4.1 | 1.4 | 6.1 | 0.62 | |
| 0.160 | 1.8 | 2.5 | 1.2 | 2.4 | |
| 0.320 | 0.035 | 1.5 | 1.0 | 0.28 | |
| 0.640 | 1.0 | 1.5 | 1.4 | 1.2 | |
| 1.28 | 0.55 | 1.4 | 1.6 | 0.18 | |
| 2.56 | 0.93 | 0.46 | 0.99 | 0.39 | |
| 8 | 5.12 | 1.3 | 0.65 | 0.48 | 0.16 |
| 10.2 | 0.62 | 0.67 | 0.32 | 0.41 | |
| 20.5 | 0.53 | 0.55 | 0.35 | 0.39 | |
| 41.0 | 0.061 | 0.92 | 0.91 | 0.41 | |
| 81.9 | 0.21 | 0.11 | 0.41 | 0.055 | |
| 164 | 0.14 | 0.057 | 0.18 | 0.016 | |
| 328 | 0.25 | 0.16 | 0.18 | 0.059 | |
3.2. Comparison of linear dynamic range with MS and UV detection
One of the goals of this study was to compare MS and UV detectors for quantitative analysis of silymarin components (Fig. 2). The MS and UV detection techniques provided different linear dynamic ranges (Table 2 and Table 3). The linear dynamic range for the MS analyses was determined to be 0.0800–10.2 μM for all compounds except compound 4, which demonstrated a linear dynamic range of 0.160–10.2 μM (Table 2). Above a concentration of 10.2 μM, signal saturation was observed with MS analysis for all of the silymarin constituents investigated. This saturation was not observed up to the highest concentration investigated when the UV detector was employed. For the UV data, the linear dynamic range was 0.0400–328 μM for all compounds except compound 4, and 0.160–328 μM for compound 4 (Table 3). Saturation at high concentration with MS analysis, particularly of highly polar small molecules, is a common occurrence [14]. However, contrary to the results observed in this study, it is generally the case that MS detection provides lower LLOQ than those observed with UV. Additional ionization methods were evaluated (positive and negative ESI and APCI) and positive mode HESI demonstrated the best sensitivity at the flow rates used in this study. For these studies, the S/N was observed to be higher with the use of the MS detector than the UV detector (Fig. S1). However, peak areas at low concentrations for multiple injections with MS were less repeatable than those measured with UV, leading to a better LLOQ with the UV detector.
3.3. Quantitative analysis of compounds in silymarin mixtures
A series of five commercial silymarin preparations from three different manufacturers was analyzed to determine their total content of compounds 1 through 8 as well as the absolute quantity of each compound individually in the mixtures. The composition of these mixtures varied significantly (at the 95% confidence interval, according to t-test, Table 6), both between different manufacturers and between batches from the same manufacturer. The two batches of silymarin extracts from Madaus showed the highest total content of all eight analytes (62% and 70% by MS), while the two silymarin extracts from Sigma showed the lowest total analyte content (47% and 54%), and the Indena silymarin extract showed a total analyte content of 57%. For compound 4, the percent composition differed more between Madaus batches (1.4% and 14%) than did the average percent composition for compound 4 for each of the manufacturers (2.5% for Sigma, 7.0% for Indena, and 7.8% for Madaus). These data, which were confirmed with both MS and UV detection (see Section 3.4), reaffirm previous studies of the variability amongst different commercial preparations of silymarin [15], which could be related to differences in growth conditions for the S. marianum plants [16] or general inconsistencies in extraction/processing procedures observed with many herbal medicines [17, 18]. For biological studies with S. marianum preparations, it is imperative to consider batch- and vendor-specific variation in individual and total flavonolignan content.
Table 6.
Comparison of UV and MS results for silymarin extracts. Percent composition of compounds 1–8 in various commercial silymarin extracts.
| Extract | Total† | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Madaus | UV | 67 | 2.71 (0.01) | 1.89 (0.02) | 8.63 (0.14) | 11.00 (0.03) | 13.91 (0.02) | 19.49 (0.08) | 5.48 (0.02) | 3.46 (0.02) |
| 37501 | MS | 70 | 2.65 (0.04) | 1.95 (0.05) | 8.79 (0.07) | 14.18 (0.10) | 13.85 (0.15) | 19.38 (0.36) | 5.58 (0.05) | 3.53 (0.07) |
| Madaus | UV | 63 | 2.84 (0.04) | 0.54 (0.01) | 13.40 (0.09) | 1.43 (0.02) | 15.29 (0.05) | 22.62 (0.13) | 4.56 (0.03) | 1.97 (0.02) |
| 286061 | MS | 62 | 2.81 (0.08) | 0.52 (0.04) | 13.33 (0.09) | 1.44 (0.09) | 15.17 (0.20) | ALR | 4.64 (0.07) | 1.94 (0.03) |
| Indena | UV | 56 | 4.13 (0.02) | 1.30 (0.01) | 11.53 (0.06) | 5.48 (0.04) | 10.16 (0.02) | 15.94 (0.11) | 4.917 (0.001) | 2.50 (0.02) |
| 27691 | MS | 57 | 4.13 (0.05) | 1.22 (0.05) | 11.60 (0.05) | 7.02 (0.01) | 10.29 (0.11) | 15.68 (0.04) | 4.92 (0.14) | 2.52 (0.01) |
| Sigma | UV | 46 | 3.17 (0.01) | 0.84 (0.01) | 10.15 (0.05) | 3.27 (0.04) | 9.17 (0.04) | 13.60 (0.04) | 3.84 (0.01) | 1.97 (0.04) |
| BCBJ0393V | MS | 47 | 3.00 (0.08) | 0.89 (0.06) | 10.25 (0.05) | 4.05 (0.07) | 9.20 (0.07) | 13.93 (0.20) | 3.87 (0.07) | 1.99 (0.09) |
| Sigma | UV | 54 | 2.60 (0.03) | 0.437 (0.003) | 13.14 (0.05) | 0.83 (0.02) | 12.50 (0.03) | 18.42 (0.06) | 4.20 (0.01) | 1.62 (0.01) |
| 05503PG | MS | 54 | 2.61 (0.01) | 0.40 (0.02) | 13.08 (0.05) | 0.82 (0.05) | 12.51 (0.06) | 18.49 (0.24) | 4.22 (0.08) | 1.70 (0.04) |
Total percentage of the silymarin extract contributed by the eight compounds being measured in this study
Shaded values for percent composition indicate a statistically significant difference (at the 95% confidence interval) between the percent composition measured by MS versus UV detection according to the t-test. Unshaded values showed no statistically significant difference between MS and UV data.
ALR = above linear range of the MS detection method
BQL = below quantitation limit
ND = not detected
3.4. Consistency of MS and UV results for quantitative analysis
In most cases, the MS and UV data provided very similar results for the total content of compounds 1–8 in silymarin extracts (Table 6). For all compounds, with the exception of compound 4, no statistically significant difference was observed between concentrations determined with MS and UV (at the 95% confidence level, according to t-test) (Table 6). Significant differences were observed between MS and UV data for compound 4, due to matrix interference in the UV data as described in Section 3.5.
3.5. Matrix effects
Matrix effects were not observed in either MS (Fig. S2) or UV (Fig. S3) analysis for all of the compounds (1–8), except for compound 4, which exhibited a clear matrix effect in the UV analysis (Table 7). This matrix effect can be ascribed to contaminants with UV absorbance that co-elute in the peak front for compound 4 (Fig. S3). The UV contaminants are of different mass than compound 4, and are therefore, resolved from this compound with the MS detector and do not interfere with MS analysis (Fig. S2). For this reason, MS detection appears to be preferable for quantitative analysis of compound 4.
Table 7.
Examination of matrix effects by UV and MS for silymarin extracts. Each value represents the percent of the spiked analyte that was observed.a
| Extract | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Madaus | UV | 101 | 101 | 100 | 80 | 101 | 99 | 100 | 100 |
| 37501 | MS | 99 | 98 | 93 | 82 | 89 | 86 | 99 | 92 |
| Madaus | UV | 99 | 101 | 100 | 76 | 96 | 94 | 99 | 102 |
| 286061 | MS | 97 | 99 | 87 | 94 | 87 | 75 | 99 | 95 |
| Indena | UV | 103 | 102 | 105 | 80 | 106 | 106 | 103 | 103 |
| 27691 | MS | 99 | 102 | 91 | 98 | 99 | 97 | 100 | 99 |
| Sigma | UV | 103 | 102 | 108 | 78 | 105 | 108 | 104 | 104 |
| BCBJ0393V | MS | 102 | 100 | 97 | 97 | 99 | 96 | 104 | 100 |
| Sigma | UV | 101 | 100 | 103 | 76 | 102 | 104 | 100 | 101 |
| 05503PG | MS | 100 | 98 | 90 | 95 | 94 | 83 | 102 | 94 |
Values close to 100 indicate no matrix effect observed.
Matrix interference due to ionization suppression is a common problem in analyses via mass spectrometry [19]. In the case of silymarin, however, the lack of matrix interference indicates that ionization suppression is not an issue. This is likely due to the fact that the extract is somewhat purified by the manufacturer. Thus, either MS or UV appear to be acceptable detection techniques for quantitative analysis of silymarin components, with the exception of compound 4.
3.6. Stability in DMSO
A series of samples of the pure compounds from silymarin at 500 μM were examined by UHPLC-UV (after being exposed to DMSO at room temperature for 6 months). These DMSO-exposed sample chromatograms are compared for analytes 1–4 in Fig. S4 and for analytes 5–8 in Fig. S5 with an identical analysis of the freshly prepared samples of the same batches of compounds that had been stored at 4 °C as a dry powder over the same time period. For each compound, a single new peak was observed in the DMSO-exposed sample. For compounds 3, and 5– 8, the new degradation peak was greater than 8% of the total area. For the samples stored dry, no degradation products were detected. Consistent with similar findings on other classes of natural products [20], these data suggest that dry storage of silymarin components is preferable to storage in DMSO for maintaining compound integrity. However, for the standard and QC samples used in the quantitative study, no degradation peaks were observed, indicating stability in 1:1 CH3CN:H2O over the time frame of the quantitative analysis.
4. Conclusion
In summary, herein is described a rigorous, validated method for the quantitative analysis of eight silymarin components in complex extracts. This method should be applicable to other S. marianum preparations, provided that appropriate controls are included to evaluate potential interference that may occur in more complex matrices [19]. For the studies described herein, both mass spectrometry and UV detection were effective for quantitative analysis. The UV detection method had the advantage of an expanded linear dynamic range, particularly at high concentrations, whereas the MS method was less prone to matrix interference for compound 4. Additionally, data are provided that indicate the importance of method of storage for maintaining integrity and biological activity of silymarin constituents. Not surprisingly, dry storage of constituents was preferable to prolonged storage in DMSO. Finally, these studies indicated that significant variation is observed in bioactive component concentrations for silymarin extract preparations among different manufacturers and even from different batches from the same manufacturer. Thus, analytical characterization is critical to establish constituent levels in silymarin preparations prior to conducting biological evaluations. The method published herein should prove useful towards conducting this characterization.
Supplementary Material
Highlights.
A rapid, validated method for the quantitation of seven flavonolignans and the flavonoid, taxifolin, all isolated from milk thistle (Silybum marianum), was developed.
The method utilized UPLC coupled to both a mass spectrometer and a photodiode detector.
The suite of compounds was evaluated in a variety of milk thistle products, quantifying variability.
The extent of breakdown of these compounds was evaluated upon storage in DMSO.
Acknowledgments
This research was supported by the National Institutes of Health/National Center for Complementary and Integrative Health via a supplement to grant R01 AT006842. The authors thank Dr. D.A. Todd of UNCG for valuable suggestions and advice.
Appendix A. Supplementary Data
Supplementary data associated with this article can be found, in the online version, at (insert doi upon publication).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polyak SJ, Oberlies NH, Pecheur EI, Dahari H, Ferenci P, Pawlotsky JM. Silymarin for HCV infection. Antivir Ther. 2013;18:141–147. doi: 10.3851/IMP2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: From bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 3.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 4.Graf TN, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (milk thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med. 2007;73:1495–1501. doi: 10.1055/s-2007-990239. [DOI] [PubMed] [Google Scholar]
- 5.Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, Oberlies NH. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci USA. 2010;107:5995–5999. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morishima C, Shuhart MC, Wang CC, Paschal DM, Apodaca MC, Liu Y, Sloan DD, Graf TN, Oberlies NH, Lee DY, Jerome KR, Polyak SJ. Silymarin inhibits in vitro T cell proliferation and cytokine production in hepatitis C virus infection. Gastroenterology. 2010;138:671–681. doi: 10.1053/j.gastro.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovelace ES, Wagoner J, MacDonald J, Bammler T, Bruckner J, Brownell JL, Beyer R, Zink E, Kim Y-M, Kyle J, Webb-Robertson B-J, Waters K, Metz T, Farin F, Oberlies NH, Polyak SJ. Silymarin Suppresses Cellular Inflammation By Inducing Reparative Stress Signaling. J Nat Prod. 2015 doi: 10.1021/acs.jnatprod.5b00288. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li W, Han JP, Li ZW, Li XX, Hou SP, Liu CX. Preparative chromatographic purification of diastereomers of silybin and their quantification in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B. 2008;862:51–57. doi: 10.1016/j.jchromb.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 9.Hadad GM, Emara S, Abdel-Salam RA. Validated Optimized Method for Simultaneous Analysis of Active Silymarin Components and Dimethyl-4,4'-dimethoxy-5,6,5',6'-dimethylene Dioxybiphenyl-2,2'-dicarboxylate in a Pharmaceutical Preparation by Use of a Monolithic Silica C-18 Column. Chromatographia. 2009;70:217–221. [Google Scholar]
- 10.Wang KW, Zhang H, Shen LQ, Du QZ, Li JR. Rapid separation and characterization of active flavonolignans of Silybum marianum by ultra-performance liquid chromatography coupled with electrospray tandem mass spectrometry. J Pharm Biomed Anal. 2010;53:1053–1057. doi: 10.1016/j.jpba.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano JG, Lankin DC, Graf TN, Friesen JB, Chen SN, McAlpine JB, Oberlies NH, Pauli GF. HiFSA fingerprinting applied to isomers with near-identical NMR spectra: the silybin/isosilybin case. J Org Chem. 2013;78:2827–2839. doi: 10.1021/jo302720h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sy-Cordero AA, Day CS, Oberlies NH. Absolute configuration of isosilybin A by X-ray crystallography of the heavy atom analogue 7-(4-bromobenzoyl)isosilybin A. J Nat Prod. 2012;75:1879–1881. doi: 10.1021/np3005369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA Guidance for Industry: Bioanalytical Method Validation. http://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents/document/ucm070107.pdf.
- 14.Alfaro CM, Uwakweh AO, Todd DA, Ehrmann BM, Cech NB. Investigations of analyte-specific response saturation and dynamic range limitations in atmospheric pressure ionization mass spectrometry. Anal Chem. 2014;86:10639–10645. doi: 10.1021/ac502984a. [DOI] [PubMed] [Google Scholar]
- 15.Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 16.Shokrpour M, Mohammadi SA, Moghaddam M, Ziai SA, Javanshir A. Variation in flavonolignan concentration of milk thistle (Silybum marianum) fruits grown in Iran. J Herbs Spices Med Plants. 2008;13:55–69. [Google Scholar]
- 17.Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM. Variability in commercial ginseng products: an analysis of 25 preparations. Am J Clin Nutr. 2001;73:1101–1106. doi: 10.1093/ajcn/73.6.1101. [DOI] [PubMed] [Google Scholar]
- 18.Booker A, Suter A, Krnjic A, Strassel B, Zloh M, Said M, Heinrich M. A phytochemical comparison of saw palmetto products using gas chromatography and 1H nuclear magnetic resonance spectroscopy metabolomic profiling. J Pharm Pharmacol. 2014;66:811–822. doi: 10.1111/jphp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–3030. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 20.McCloud TG. High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules. 2010;15:4526–4563. doi: 10.3390/molecules15074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.