Abstract
Background
Asthma is the most common chronic disease in childhood and places a significant burden on public and private health systems. This retrospective cohort analysis utilised administrative healthcare claims data (US Clinformatics™ Multiplan database; compliant with the US Department of Health & Human Services Health Insurance Portability and Accountability Act) to characterise asthma exacerbations requiring intervention in a US paediatric patient population.
Methods
Patients aged > 1–17 years with a recorded asthma diagnosis and receiving treatment were identified in the US Clinformatics™ Multiplan database over a 9-year period (2004–2012). Both incident and prevalent cases of asthma were included, with the most recently recorded asthma diagnosis designated as the index date. The 12-month period following the index date was analysed for asthma exacerbations, defined as an event requiring treatment with systemic corticosteroid or resulting in an asthma-related hospitalisation or emergency department visit.
Results
Data from 734,114 children with asthma (41.5 % females, 58.5 % males) were analysed, of this cohort 34.4 % experienced ≥ 1 exacerbation during the follow-up period. The proportion who experienced ≥ 1 exacerbation increased from 28.9 % in 2004 to 36.3 % in 2012, based on the reported index date. Their mean annual exacerbation frequency was 1.4; 85.8 % of exacerbations were defined by systemic corticosteroids use. A consistent trend of increased exacerbation incidence in the fall and early winter was observed, in particular exacerbations defined by systemic corticosteroid use. A greater proportion of asthma-related hospitalisations were associated with younger age.
Conclusions
Approximately one-third of children experienced ≥ 1 exacerbation in real-world clinical practice. A targeted treatment approach with a focus on those with a history of recurrent exacerbations is recommended to improve asthma control. This targeted approach could also minimise the frequent systemic corticosteroid exposure particularly at an early age when side effects of systemic corticosteroids are more pronounced.
Keywords: Asthma, Exacerbations, Paediatric, Systemic corticosteroids
Background
Asthma has been estimated to affect up to 10 % of children in the US [1]. Furthermore, the prevalence of uncontrolled asthma in children remains high despite widespread availabilityy of effective controller medications [2–5]. Lack of adherence to maintenance therapies such as inhaled corticosteroids, may lead to poor asthma control, particularly in older children [6]. Poor asthma control is associated with increased frequency of exacerbations [7, 8] and consequent hospitalisation [2]. In view of the high prevalence of uncontrolled asthma, and considering the impact of exacerbations on patients in terms of reduced quality of life and lost school days [9], and on healthcare costs [10], improving the diagnosis and management of paediatric asthma is a high public health priority.
Asthma exacerbations are acute worsening of symptoms that often require treatment with systemic corticosteroids, visits to the emergency department (ED), or hospital admission [11]. Exacerbations are closely associated with asthma-related morbidity and mortality and are responsible for considerable healthcare costs [10]. Children account for approximately one third of all asthma-related hospitalisations [12]. Despite improvements in asthma management and advances in therapeutics, there has not been a reduction in the reported incidence of exacerbations in paediatric asthma [5]. Previously reported findings from controlled clinical trials indicate that asthma exacerbations in children [9, 13], as well as in adults [14, 15], are predictive of future exacerbations. However, data from randomised controlled trials are often not representative of real-world clinical practice.
To this end, we report the findings of a retrospective cohort study performed using a US administrative healthcare claims data to establish the frequency and type of exacerbations in 734,114 paediatric patients with asthma. Data were obtained from the Clinformatics™ DataMart Multiplan (IMPACT), a product of OptumInsight Life Sciences, Inc. (Eden Praire, MN), formerly the Integrated Healthcare Information Services database. The Clinformatics™ Multiplan database contains anonymised healthcare claims data, including information on inpatient and outpatient medical services and pharmacy dispensing claims, compliant with the Health Insurance Portability and Accountability Act (HIPAA) (US Department of Health & Human Services) on more than 90 million commercially insured patients, primarily from the UnitedHealth Group [16].
In this study, we analysed the annual frequency of paediatric exacerbations in real-world clinical practice and examined any association of age group or gender with exacerbation frequency. Additionally, the longitudinal nature of the dataset was utilised to investigate exacerbation recurrence and seasonal trends in exacerbations.
Methods
Study design
Patients aged > 1–17 years with asthma were identified in the Clinformatics™ Multiplan administrative healthcare claims database in the US by the identification of an outpatient or inpatient claim coded with asthma (ICD9 493.xx) during the period January 1, 2004 and ending on December 31, 2013; the index date was defined as the date of the most recently recorded asthma medical code during the study period that met all inclusion and exclusion criteria. The study period (2004–2013), included a 10-year observation period, and data were assessed in a post-hoc analysis, since the original study period was up to 3 years. Patients identified in the database were required to have at least one recorded asthma medication (e.g. short-acting beta agonist, inhaled corticosteroid [ICS], ICS plus long-acting beta agonist [LABA], leukotriene receptor antagonist [LTRA] or omalizumab) dispensing within +/–3 months of asthma diagnosis, and at least 12 months of data coverage subsequent to the asthma index date.
Patients were excluded from the cohort if their records indicated a history of cystic fibrosis or chronic obstructive pulmonary disease.
Study endpoints
The event of interest in this retrospective cohort study was asthma exacerbations, defined as treatment with systemic corticosteroids administered both orally and via injection, asthma-related ED visits, or asthma-related hospitalisations. Asthma related ED visits and hospital admissions were determined by the presence of an asthma primary diagnosis and/or discharge diagnosis.
Statistical analysis
Data were extracted from the Clinformatics™ Multiplan database and maintained and analysed using SAS v9.2 software (SAS Institute Inc., Cary, NC, USA). All patient-level data were anonymised before being made available for analysis in this study and US data collection was compliant with HIPAA. Therefore, use of these data in clinical research is exempt from Institutional Review Board review.
ICD-9 codes were used to select patients with asthma and healthcare utilisation associated with asthma. The subsequent 12-month period was queried for recorded systemic corticosteroid tablet treatment of 3 or more days (systemic corticosteroids administered via injection were not required to have a minimum duration). ED visits and hospitalisations with an associated asthma-related diagnosis code were also captured during this period. Systemic corticosteroid dispensing occurring less than 7 days apart was considered a single event.
We assessed the overall incidence and type of exacerbation, and the impact of patient demographic factors (age and gender) on exacerbation frequency. Exacerbation recurrence and the relationship between use of controller medications (e.g. LTRA, ICS, ICS/LABA, systemic corticosteroid, omalizumab, theophylline) and exacerbation frequency were also assessed. A sensitivity analysis was performed to evaluate the effect of exclusion of patients in the cohort whose claims history indicated comorbid chronic inflammatory disease (Table 1). The presence of a comorbid chronic inflammatory disease that may have required the use of systemic corticosteroids at any time during the patient’s medical history was defined as having an ICD-9 code as listed in Table 1.
Table 1.
Chronic inflammatory diseases with ICD-9 codes included in the sensitivity analysis
| Chronic inflammatory disease | ICD9 code |
|---|---|
| Systemic lupus erythematosus | (710.0) |
| Juvenile ankylosing spondylitis | (720.0) |
| Scleroderma | (710.1) |
| Inflammatory bowel disease | (555.x) |
| Vasculitis | (447.6) |
| Multiple sclerosis | (340) |
| Psoriasis | (696.x) |
| Juvenile rheumatoid arthritis | (714.3, 714.30, 714.31, 714.32, 714.33) |
| Juvenile dermatomyositis | (710.3) |
| Idiopathic thrombocytopenic purpura | (287.31) |
| Necrotizing enterocolitis | (777.5) |
| Kawasaki disease | (446.) |
| Celiac disease | (579.0) |
| Sickle cell anaemia | (282.6x) |
Data were interpreted descriptively. Based on previous analyses of the Clinformatics™ Multiplan databases, it was determined that the number of paediatric asthma patients likely to be present in the database would be sufficient to achieve the aims of the study.
Results
Overview of patient population and exacerbation data
Data for 734,114 paediatric patients with asthma who met the criteria for data inclusion were analysed (Table 2). The overall study population comprised more males than females (58.5 % vs. 41.5 %); age ranged from > 1 to 17 years.
Table 2.
Demographic and exacerbation data derived from the study cohort
| Characteristics | No exacerbations | ≥1 exacerbation | Total | ||||
|---|---|---|---|---|---|---|---|
| n | Column % | n | Column % | Row % | n | Column % | |
| Total | 481,822 | 100.0 | 252,292 | 100.0 | 34.4 | 734,114 | 100.0 |
| Gender | |||||||
| Male | 276,033 | 57.3 | 153,216 | 60.7 | 35.7 | 429,249 | 58.5 |
| Female | 205,789 | 42.7 | 99,076 | 39.3 | 32.5 | 304,865 | 41.5 |
| Age at date of asthma diagnosis | |||||||
| ≤ 1 year | 25,495 | 5.3 | 20,437 | 8.1 | 44.5 | 45,932 | 6.3 |
| > 1–2 years | 24,329 | 5.0 | 20,226 | 8.0 | 45.4 | 44,555 | 6.1 |
| > 2–3 years | 25,722 | 5.3 | 19,414 | 7.7 | 43.0 | 45,136 | 6.1 |
| 4–12 years | 262,219 | 54.4 | 133,291 | 52.8 | 33.7 | 395,510 | 53.9 |
| 13–17 years | 144,057 | 29.9 | 58,924 | 23.4 | 29.0 | 202,981 | 27.6 |
| Asthma index datea | |||||||
| 2004 | 41,964 | 10.3 | 17,066 | 8.9 | 28.9 | 59,030 | 9.8 |
| 2005 | 45,312 | 11.1 | 18,947 | 9.9 | 29.5 | 64,259 | 10.7 |
| 2006 | 51,128 | 12.6 | 21,546 | 11.2 | 29.6 | 72,674 | 12.1 |
| 2007 | 45587 | 11.2 | 20,957 | 10.9 | 31.5 | 66,544 | 11.1 |
| 2008 | 38,942 | 9.6 | 18,952 | 9.9 | 32.7 | 57,894 | 9.7 |
| 2009 | 40,484 | 10.0 | 20,025 | 10.4 | 33.1 | 60,509 | 10.1 |
| 2010 | 38,010 | 9.4 | 19,044 | 9.9 | 33.4 | 57,054 | 9.5 |
| 2011 | 42,088 | 10.4 | 19,975 | 10.4 | 32.2 | 62,063 | 10.4 |
| 2012 | 62,761 | 15.4 | 35,703 | 18.6 | 36.3 | 98,464 | 16.5 |
aValues are for patients 4–17 years at asthma index date
In the study cohort, 34.4 % experienced ≥ 1 exacerbation during the 12-month follow-up period (males 60.7 %; females 39.3 %). Increasing age was associated with decreased frequency of exacerbations and reduced incidence of exacerbation recurrence (Table 3). The overall mean exacerbation frequency for patients experiencing ≥ 1 exacerbation during the follow-up period was 1.4 events per person (standard deviation = 0.9), with a higher incidence of recurrence observed in the younger age groups compared to the older children and adolescents (Table 2 and Fig. 1a). The proportion of patients with at least one exacerbation during the 12-month follow-up period increased from 2004 to 2012 (Table 2). No difference in frequency of recurrent exacerbations by gender was observed (Fig. 1b).
Table 3.
Asthma exacerbation frequency among the study cohort paediatric asthma patients, by gender and age group
| Number of exacerbations during 12 month follow-up period | Total | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ≥4 | ||
| n (%) | n (%) | n (%) | n (%) | n (%) | N | |
| Overall | 481,822 (65.6) | 189,457 (25.8) | 43,592 (5.9) | 12,278 (1.7) | 6,965 (1.0) | 734,114 |
| Gender | ||||||
| Female | 205,789 (67.5) | 75,394 (24.7) | 16,517 (5.4) | 4,530 (1.5) | 2,635 (0.9) | 304,865 |
| Male | 276,033 (64.3) | 114,063 (26.6) | 27,075 (6.3) | 7,748 (1.8) | 4,330 (1.0) | 429,249 |
| Age groups | ||||||
| 0–1 years | 25,495 (55.5) | 14,078 (30.7) | 4,218 (9.2) | 1,359 (3.0) | 782 (1.7) | 45,932 |
| > 1–2 years | 24,329 (54.6) | 14,176 (31.8) | 4,084 (9.2) | 1,282 (2.9) | 684 (1.5) | 44,555 |
| > 2–3 years | 25,722 (57.0) | 13,904 (30.8) | 3,742 (8.3) | 1,138 (2.5) | 630 (1.4) | 45,136 |
| 4–12 years | 262,219 (66.3) | 101,711 (25.7) | 22,297 (5.6) | 6,077 (1.5) | 3,206 (0.8) | 395,510 |
| 13–17 years | 144,057 (71.0) | 45,588 (22.5) | 9,251 (4.6) | 2,422 (1.2) | 1,663 (0.8) | 202,981 |
Fig. 1.
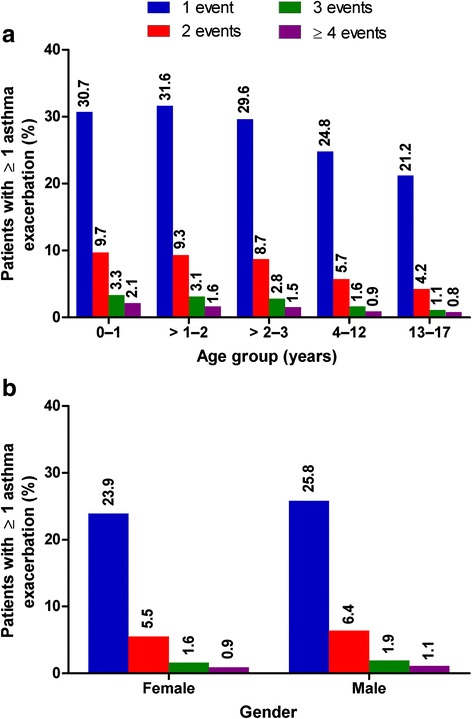
Exacerbation frequency during the 12-month follow-up period by a age group and b gender
A sensitivity analysis of the study population excluding patients with any history of comorbid chronic inflammatory disease showed that exclusion of these patients (n = 13,036) did not affect the overall findings, with the overall exacerbation frequency being near identical (1.4 vs. 1.4).
Exacerbation type
Summary statistics on exacerbation type data were generated (Table 4). Systemic corticosteroid-defined exacerbations (i.e., outpatient treatment) accounted for nearly 85.8 % of recorded events. Among the observed exacerbations, there were a greater proportion of ED visit-defined exacerbations (not precluding the use of systemic corticosteroids) compared with hospitalisation-defined exacerbations (11.8 % versus 2.4 %, respectively). Although a greater proportion of asthma-related hospitalisations were associated with younger age, there was no variation by gender.
Table 4.
Asthma patients with ≥ 1 exacerbation (n = 252,292) by first exacerbation type recorded during the study period
| Specified type | n (%) |
|---|---|
| Hospitalisation | 6,080 (2.4) |
| Emergency department visit | 29,713 (11.8) |
| Systemic corticosteroida | 216,499 (85.8) |
aIncludes corticosteroid injection
Seasonal distribution of exacerbations
Seasonal variation in exacerbation frequency was analysed for all exacerbations and by type of exacerbations (Fig. 2). A trend of seasonality was observed where the exacerbation frequency was the highest in the fall and early winter and lowest during the month of July. The trend was most pronounced for systemic corticosteroid-defined exacerbations, though a similar trend can be seen for ED visit-defined and hospital-defined exacerbations.
Fig. 2.
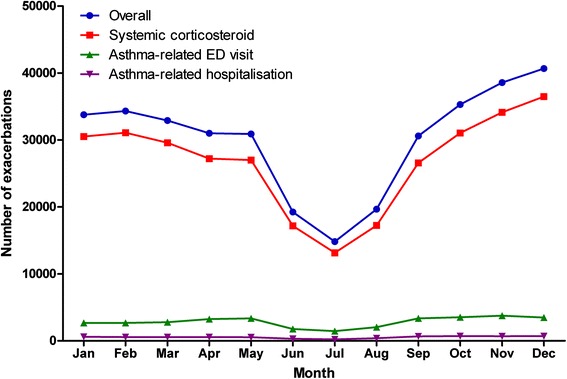
Asthma exacerbations in paediatric patients with asthma by month, overall and by exacerbation type
Association between controller medication use and exacerbation frequency
Exacerbation frequency among patients in the cohort was analysed, overall and by age and gender, according to whether or not there was evidence that the patient had been prescribed asthma controller medication at the asthma index date (Fig. 3). A trend whereby patients prescribed controller medication at baseline were more likely to have had ≥ 1 exacerbation during the 12-month follow-up period was identified; no effect of gender was seen. Although there was no significant variation in exacerbation rates between groups defined by asthma controller use at baseline or gender, a trend towards a higher rate of exacerbations with younger age was observed (Table 5). There were very few reports of exacerbations with omalizumab; 18 males with a mean (SD) exacerbation frequency of 1.50 (0.86) and 18 females with 1.83 (1.47) exacerbations. As there were so few cases, these data have not been included in Table 5.
Fig. 3.
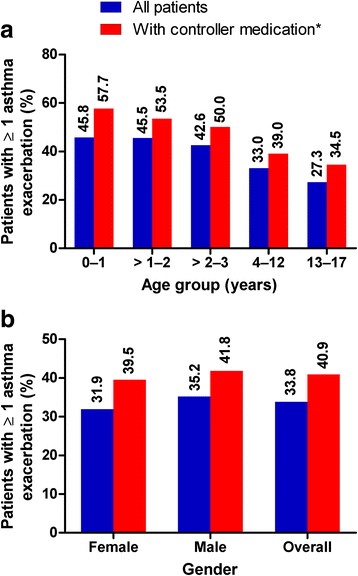
Proportion of patients with ≥ 1 exacerbation during the 12-month follow-up by a age and b gender. *The subset of asthma patients on controller medications was defined as follows: patients who had received an asthma controller medication (LTRA, ICS, ICS/LABA, OCS or injectable steroid, omalizumab, theophylline) within +/-3 months of the index date
Table 5.
Mean annual exacerbation rate by asthma therapy
| Age, years | ICSa | ICS/LABA | LTRA | OCS | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean exacerbation rate (SD) | n | Mean exacerbation rate (SD) | n | Mean exacerbation rate (SD) | n | Mean exacerbation rate (SD) | |
| Females | ||||||||
| 0 -1 | 2017 | 1.50 (0.89) | 5 | 1.60 (0.89) | 490 | 1.67 (1.07) | 3502 | 1.42 (0.83) |
| >1 – 2 | 2036 | 1.49 (0.89) | 10 | 2.0 (2.0) | 1060 | 1.59 (1.08) | 3709 | 1.37 (0.74) |
| >2 – 3 | 2084 | 1.46 (0.86) | 17 | 1.35 (0.79) | 1440 | 1.56 (1.0) | 3289 | 1.34 (0.77) |
| 4 – 12 | 11321 | 1.36 (0.83) | 2034 | 1.42 (0.93) | 12209 | 1.44 (0.89) | 20719 | 1.29 (0.81) |
| 13 – 17 | 3693 | 1.35 (0.93) | 3227 | 1.42 (0.93) | 5915 | 1.45 (1.06) | 11070 | 1.39 (1.01) |
| Overall 0-17 | 21151 | 1.40 (0.86) | 5293 | 1.42 (0.93) | 21114 | 1.46 (0.96) | 42289 | 1.34 (0.86) |
| Males | ||||||||
| 0–1 | 4552 | 1.60 (1.02) | 6 | 2.0 (0.63) | 1232 | 1.68 (1.09) | 6112 | 1.47 (0.89) |
| >1–2 | 3950 | 1.52 (0.94) | 22 | 1.91 (1.06) | 2042 | 1.65 (1.08) | 5803 | 1.42 (0.81) |
| >2–3 | 3630 | 1.48 (0.93) | 40 | 1.93 (1.46) | 2446 | 1.56 (1.02) | 5114 | 1.40 (0.89) |
| 4–12 | 19133 | 1.39 (0.84) | 3743 | 1.42 (0.84) | 21698 | 1.45 (0.92) | 32895 | 1.32 (0.84) |
| 13–17 | 4208 | 1.32 (0.85) | 3777 | 1.37 (0.91) | 21698 | 1.45 (0.92) | 13397 | 1.35 (0.92) |
| Overall 0–17 | 35473 | 1.43 (0.89) | 7588 | 1.40 (0.88) | 34104 | 1.45 (1.06) | 42289 | 1.34 (0.86) |
aincludes nebulized medications
ICS inhaled corticosteroid, LABA long-acting beta agonist, LTRA leukotriene receptor antagonist, OCS oral corticosteroid
Discussion
This study used a US population-based healthcare claims database to investigate paediatric exacerbation frequency. These findings suggest that approximately one-third of paediatric asthma patients (<1–17 years of age) will experience an exacerbation within a 12-month period, with a smaller subset of patients (<9 %) experiencing ≥ 2 exacerbations. Despite being a small proportion of the overall population, this patient group with evidence of more severe asthma could have a disproportionate impact on overall healthcare burden.
Diagnosis of asthma in children aged ≤ 5 years, and especially in infants, is a particular challenge [17]. Symptoms typically characteristic of asthma, such as wheezing and cough, are common in this age group and not necessarily indicative of asthma, and viral infections such as bronchiolitis can confound asthma diagnosis; furthermore, diagnostic tests such as spirometry are often not possible because of the requirement for the patient to perform voluntary breathing manoeuvres in a reproducible manner [11]. As only patients with a recorded asthma diagnosis and at least one asthma medication were included in the cohort, it is possible that asthma prevalence in this age group may be misclassified (e.g. under-reported).
It should be noted that, because of limitations of the available data resource, the definition of asthma exacerbations used in this retrospective cohort study differed from the American Thoracic Society and European Respiratory Society consensus definition [18]. While we anticipate that most severe exacerbations (requiring hospitalisation and/or ED visits) be treated with systemic corticosteroids and be recorded in the claims database, moderate or mild exacerbations may not have been fully captured, specifically those that did not require treatment with a systemic corticosteroid. Nearly 86 % of recorded exacerbations were identified through dispensed systemic corticosteroid and approximately 14 % required hospitalisation or ED visits (not precluding the use of systemic corticosteroids). It is unclear why we observed an increase in exacerbation frequency between 2004 and 2012. The US Centers for Disease Control and Prevention has published data showing that hospitalisation frequency rates remained unchanged from 2001 to 2009, whereas the prevalence of asthma increased [19]. These findings suggest that the increased exacerbation frequency observed in our study may be attributable to an increase in oral corticosteroid-defined exacerbations, rather than an increase in hospitalisations. Another possible explanation, of which further exploration may be warranted, is that the increase in exacerbation frequency may result from incremental improvements in data completeness (i.e. better recording of hospitalisations and ED visits and/or improvements in medication dispensing/prescribing data).
Oral corticosteroids are recommended for the first-line treatment of acute exacerbations in children [11]. However, the adverse impact of the chronic use of systemic corticosteroids was assessed recently in a real-world study of patients with severe asthma. Results from this assessment showed that there was a dose-dependent increase in the risk of developing infections, and/or gastrointestinal and cardiovascular complications in patients aged > 12 years who received daily systemic corticosteroids [20]. Although in the present analysis inclusion of systemic corticosteroid prescription data enabled exacerbations that did not require hospitalisation or ED visits to be captured. Systemic corticosteroid use for reasons other than asthma exacerbation could not be ruled out, and therefore some spurious exacerbations may have been recorded. To account for this possibility, a sensitivity analysis was performed evaluating a subset of patients without comorbid chronic inflammatory conditions (for which patients may have been prescribed systemic corticosteroid without having experienced an asthma exacerbation). This sensitivity analysis did not identify any decrease in the frequency of recorded asthma exacerbations.
A seasonal pattern of paediatric asthma exacerbations is well established [21–24]. In particular, a peak in asthma exacerbations and asthma-related hospitalisations in September has been widely observed in children in the Northern Hemisphere [22–25]. Furthermore, a link between asthma exacerbations and falling temperatures has also been observed [26]. Our data reflect these previously reported findings, showing a marked increase in exacerbations from August to September. This autumnal peak coincides with children returning to school and having a greater exposure to viral infections [24]. There is considerable evidence to support a causal link between viral respiratory tract infections and asthma exacerbations in children, with respiratory viruses being detected in over 80 % of paediatric patients who experience asthma exacerbations [27–30].
When the current analysis was restricted to the subset of patients using controller medications at the asthma index date, the proportion of patients experiencing an exacerbation increased. Consistent with previously reported findings, this suggests that exacerbations occur more frequently in patients who have evidence of more severe asthma and are, therefore, more likely to be prescribed ICS, in accordance with asthma management guidelines and medication labelling [11, 31]. By extension, this suggests that if stricter selection criteria (e.g. excluding patients with mild asthma, who represent a large proportion of the overall asthma population) had been used, the reported exacerbation frequency may have been greater than our results suggest. This observation should be interpreted in light of the fact that patients were not stratified by baseline asthma severity, nor does the methodology used take into account adherence or compliance to medication or comparison with patients who were undertreated.
Previously, managed care data from US healthcare claims databases have been used to investigate exacerbation frequency and associated factors in patients of all ages [5, 32]. Results from a study of adults with asthma show that the risk of exacerbation does not appear to change over time, even with high-intensity treatment, but the occurrence of previous exacerbations does increase the risk of subsequent events [33]. In this study, we made use of these data to specifically examine paediatric exacerbation frequency using a retrospective cohort approach. Our findings indicate that the occurrence of exacerbations among children diagnosed with asthma is as high in real-world clinical practice as has been previously suggested by the smaller-scale studies mentioned above. Recurrence of exacerbations requiring treatment with systemic corticosteroid or more intensive intervention is observed in < 10 % of the paediatric asthma population, and the healthcare burden associated with childhood asthma is heightened during the fall season. Although treatment of asthma has improved since 2004 our results suggest a trend towards an increase in exacerbations as the proportion of patients with at least one event, identified in the index date, increased over the course of the study (28.9 % in 2004 to 36.3 % in 2012).
The current findings are relevant as, although there are some results in the published literature describing exacerbation frequency in adults with asthma [33], there are limited data in paediatric patients with asthma identified in a large healthcare claims database, including a breakdown of exacerbation frequency by type. Moreover, the large sample size utilised to quantify the frequency of asthma exacerbations in this paediatric patient population can be considered a strength of the current study.
Conclusions
These findings highlight the potential unmet need in approximately one-third of paediatric patients with asthma who experienced an exacerbation. This is particularly important because paediatric patients are more susceptible to adverse effects associated with the chronic use of systemic corticosteroids. We propose that a more targeted treatment approach with particular focus on those who have a history of recurrent exacerbations, together with improved treatment engagement of patients and their parents or guardians, in combination with the prescription of effective controller medications, could result in meaningful improvements in asthma control in children.
Acknowledgements
Editorial support in the form of development of the draft outline and manuscript first draft in consultation with the authors, editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, referencing and graphic services was provided by Ian Grieve, PhD and Alison Scott, PhD at Gardiner-Caldwell Communications (Macclesfield, UK) and was funded by GSK.
Funding
This study was sponsored and funded by GSK (WEUSRTP4592).
Availability of data and materials
Data are available from the Clinformatics™ DataMart Multiplan (OptumInsight Life Sciences, Inc., Eden Praire, MN), which contains anonymised healthcare claims data on more than 90 million commercially insured patients primarily from the UnitedHealth Group, and is compliant with the Health Insurance Portability and Accountability Act (US Department of Health & Human Services).
Authors’ contributions
RS participated in the design of the study, collected and analysed data and drafted the manuscript. HO participated in the design of the study and drafted the manuscript. NB collected and analysed data and drafted the manuscript.
Competing interests
RY Suruki and HG Ortega were employed by GSK during the conduct of the study and hold stock in GSK. N Boudiaf is a GSK employee and holds GSK stock.
Consent for publication
Not applicable.
Ethics of approval and consent to participate
Not applicable.
Abbreviations
- ED
emergency department
- HIPAA
Health Insurance Portability and Accountability Act
- ICS
inhaled corticosteroids
- LABA
long-acting beta agonist
- LTRA
leukotriene receptor antagonist
- OCS
oral corticosteroid
Footnotes
Robert Y. Suruki and Hector G. Ortega were employed by GSK during the conduct of the study.
References
- 1.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011;32:1–14. [PubMed] [Google Scholar]
- 2.Fuhrman C, Dubus JC, Marguet C, Delacourt C, Thumerelle C, de Blic J, et al. Hospitalizations for Asthma in Children Are Linked to Undertreatment and Insufficient Asthma Education. J Asthma. 2011;48(6):565–71. doi: 10.3109/02770903.2011.580031. [DOI] [PubMed] [Google Scholar]
- 3.Liu AH, Gilsenan AW, Stanford RH, Lincourt W, Ziemiecki R, Ortega H. Status of asthma control in pediatric primary care: results from the pediatric asthma control characteristics and prevalence survey study (ACCESS) J Pediatr. 2010;157(2):276–81. doi: 10.1016/j.jpeds.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 4.O’Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol. 2011;128(2):257–63. doi: 10.1016/j.jaci.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Winer RA, Qin X, Harrington T, Moorman J, Zahran H. Asthma Incidence among Children and Adults: Findings from the Behavioral Risk Factor Surveillance System Asthma Call-back Survey—United States, 2006–2008. J Asthma. 2012;49(1):16–22. doi: 10.3109/02770903.2011.637594. [DOI] [PubMed] [Google Scholar]
- 6.Stern L, Berman J, Lumry W, Katz L, Wang L, Rosenblatt L, et al. Medication compliance and disease exacerbation in patients with asthma: a retrospective study of managed care data. Ann Allergy Asthma Immunol. 2006;97(3):402–8. doi: 10.1016/S1081-1206(10)60808-3. [DOI] [PubMed] [Google Scholar]
- 7.Johnston NW, Sears MR. Asthma exacerbations. 1: epidemiology. Thorax. 2006;61(8):722–8. doi: 10.1136/thx.2005.045161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suissa S, Ernst P. Inhaled corticosteroids: impact on asthma morbidity and mortality. J Allergy Clin Immunol. 2001;107(6):937–44. doi: 10.1067/mai.2001.115653. [DOI] [PubMed] [Google Scholar]
- 9.Covar RA, Szefler SJ, Zeiger RS, Sorkness CA, Moss M, Mauger DT, et al. Factors associated with asthma exacerbations during a long-term clinical trial of controller medications in children. J Allergy Clin Immunol. 2008;122(4):741–7. doi: 10.1016/j.jaci.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanova JI, Bergman R, Birnbaum HG, Colice GL, Silverman RA, McLaurin K. Effect of asthma exacerbations on health care costs among asthmatic patients with moderate and severe persistent asthma. J Allergy Clin Immunol. 2012;129(5):1229–35. doi: 10.1016/j.jaci.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 11.GINA. Global Strategy for Asthma Management and Prevention. 2015. http://www.ginasthma.org. Accessed 23 October 2015.
- 12.Haselkorn T, Szefler SJ, Simons FE, Zeiger RS, Mink DR, Chipps BE, et al. Allergy, total serum immunoglobulin E, and airflow in children and adolescents in TENOR. Pediatr Allergy Immunol. 2009;21(8):1157–65. doi: 10.1111/j.1399-3038.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 13.Martinez FD. Managing childhood asthma: challenge of preventing exacerbations. Pediatrics. 2009;123(Suppl. 3):S146–50. doi: 10.1542/peds.2008-2233D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller MK, Lee JH, Miller DP, Wenzel SE. Recent asthma exacerbations: a key predictor of future exacerbations. Respir Med. 2007;101(3):481–9. doi: 10.1016/j.rmed.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Pavord ID, Korn S, Howarth P, Bleecker ER, Buhl R, Keene ON, et al. Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet. 2012;380(9842):651–9. doi: 10.1016/S0140-6736(12)60988-X. [DOI] [PubMed] [Google Scholar]
- 16.OptumInsight I. Retrospective Database Analysis. 2014. [Google Scholar]
- 17.Hedlin G, Bush A, Lødrup Carlsen K, Wennergren G, De Benedictis FM, Melén E, et al. Problematic severe asthma in children, not one problem but many: a GA2LEN initiative. Eur Respir J. 2010;36(1):196–201. doi: 10.1183/09031936.00104809. [DOI] [PubMed] [Google Scholar]
- 18.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations. Am J Respir Crit Care Med. 2009;180(1):59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 19.Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3(35):1–58. [PubMed] [Google Scholar]
- 20.Lefebvre P, Duh MS, Lafeuille MH, Gozalo L, Desai U, Robitaille MN, et al. Acute and chronic systemic corticosteroid-related complications in patients with severe asthma. J Allergy Clin Immunol. 2015;136(6):1488–95. doi: 10.1016/j.jaci.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 21.Sears MR. Epidemiology of asthma exacerbations. J Allergy Clin Immunol. 2008;122(4):662–8. doi: 10.1016/j.jaci.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Sposato B, Scalese M, Moschini G, Migliorini MG. Can we modulate asthma maintenance treatment level with disease seasonal variations? Eur Rev Med Pharmacol Sci. 2015;19(6):942–9. [PubMed] [Google Scholar]
- 23.Teach SJ, Gergen PJ, Szefler SJ, Mitchell HE, Calatroni A, Wildfire J, et al. Seasonal risk factors for asthma exacerbations among inner-city children. J Allergy Clin Immunol. 2015;135(6):1465–73. doi: 10.1016/j.jaci.2014.12.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Dole KB, Swern AS, Newcomb K, Nelsen L. Seasonal patterns in health care use and pharmaceutical claims for asthma prescriptions for preschool- and school-aged children. Ann Allergy Asthma Immunol. 2009;102:198–204. doi: 10.1016/S1081-1206(10)60081-6. [DOI] [PubMed] [Google Scholar]
- 25.Johnston NW, Johnston SL, Duncan JM, Greene JM, Kebadze T, Keith PK, et al. The September epidemic of asthma exacerbations in children: a search for etiology. J Allergy Clin Immunol. 2005;115(1):132–8. doi: 10.1016/j.jaci.2004.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brzezińska-Pawłowska OE, Rydzewska AD, Łuczyńska M, Majkowska-Wojciechowska B, Kowalski ML, Makowska JS. Environmental factors affecting seasonality of ambulance emergency service visits for exacerbations of asthma and COPD. J Asthma. 2015:1–7 [Epub ahead of print]. [DOI] [PubMed]
- 27.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet. 2004;376(9743):826–34. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, et al. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995;310(6989):1225–9. doi: 10.1136/bmj.310.6989.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loisel DA, Du G, Ahluwalia TS, Tisler CJ, Evans MD, Myers RA, et al. Genetic associations with viral respiratory illnesses and asthma control in children. Clin Exp Allergy. 2015 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 30.Sears MR, Johnston NW. Understanding the September asthma epidemic. J Allergy Clin Immunol. 2007;120(3):526–9. doi: 10.1016/j.jaci.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fleming L, Murray C, Bansal AT, Hashimoto S, Bisgaard H, Bush A, et al. The burden of severe asthma in childhood and adolescence: results from the paediatric U-BIOPRED cohorts. Eur Respir J. 2015;46(5):1322–33. doi: 10.1183/13993003.00780-2015. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor RD, Bleecker ER, Long A, Tashkin D, Peters S, Klingman D, et al. Subacute lack of asthma control and acute asthma exacerbation history as predictors of subsequent acute asthma exacerbations: evidence from managed care data. J Asthma. 2010;47(4):422–8. doi: 10.3109/02770901003605332. [DOI] [PubMed] [Google Scholar]
- 33.Schatz M, Meckley LM, Kim M, Stockwell BT, Castro M. Asthma exacerbation rates in adults are unchanged over a 5-year period despite high-intensity therapy. J Allergy Clin Immunol Pract. 2014;2(5):570–4. doi: 10.1016/j.jaip.2014.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Clinformatics™ DataMart Multiplan (OptumInsight Life Sciences, Inc., Eden Praire, MN), which contains anonymised healthcare claims data on more than 90 million commercially insured patients primarily from the UnitedHealth Group, and is compliant with the Health Insurance Portability and Accountability Act (US Department of Health & Human Services).


