Abstract
This article summarizes the past, present, and future promise of multiphoton excitation fluorescence microscopy for intravital kidney imaging. During the past 15 years, several high-power visual research approaches have been developed using multiphoton imaging to study the normal functions of the healthy, intact, living kidney, and the various molecular and cellular mechanisms of the development of kidney diseases. In this review, the main focus will be on intravital multiphoton imaging of the glomerulus, the structure and function of the glomerular filtration barrier, especially the podocyte. Examples will be given for the combination of two powerful research tools, in vivo multiphoton imaging and mouse genetics using commercially available whole animal models for the detailed characterization of glomerular cell types, their function and fate, and for the better understanding of the molecular mechanisms of glomerular pathologies. One of the new modalities of multiphoton imaging, serial imaging of the same glomerulus in the same animal over several days will be emphasized for its potential for further advancing the field of nephrology research.
Keywords: Multiphoton microscopy, Glomerular filtration barrier, Albumin leakage, Podocyte, Confetti construct, GCaMP, Purinergic signalling
1. Advantages of multiphoton imaging technology
The inaccessibility, structural and functional complexity of renal cell types, anatomical structures, specialized tubulovascular units such as the glomerular filtration barrier and the juxta-glomerular apparatus at the glomerular vascular pole have been key reasons for the development of visual experimental approaches in kidney research. The intricate three-dimensional micro-anatomy of these structures made them difficult to study in their intact environment with other, more conventional approaches. Multiphoton excitation fluorescence microscopy allows deep optical (noninvasive) sectioning of the living kidney tissue with high temporal and spatial (submicron) resolution. Shortly after the first commercial multiphoton microscopes entered the market (around 1996), the first applications of this new imaging technology focused on studying the living juxtaglomerular apparatus, and glomerular and tubular functions [1,2]. The exact timeline of the various applications, and development of multi-photon imaging modalities have been reviewed recently [3,4]. Also, the biophysical principles of multiphoton fluorescence excitation, and its uses for the in vivo imaging of the kidney have been discussed in detail earlier [5–13].
Briefly, the technology is based on the use of nonlinear-pulsed lasers with infrared light (680 to 1300 nm range in current commercial systems). These lasers and microscopes allow, at the focal plane, the simultaneous absorption of two photons of low, equal energy, which can cause excitation of a fluorophore equivalent to the absorption of a single photon of double the energy [7]. In contrast, conventional confocal (one-photon) fluorescence microscopes use high-energy ultraviolet light (UV) or visible lasers (193–694 nm). With multiphoton imaging, these long-wavelength, low-energy photons allow for deeper penetration into living tissues with much less scattering and phototoxic effects. In turn, low phototoxicity allows for longer (real-time) imaging of living tissues and intact organs without interfering with physiological function. Since multiphoton excitation occurs mainly at the focal plane, 100% of emitted (already confocal) fluorescence can be detected, and therefore there is no need for descanning and filtering the emitted fluorescence through pinholes as with conventional confocal imaging [6,7,12]. Even more than 15 years after its initial use, intravital multiphoton imaging remains a top choice experimental technique for investigators to study renal physiology and pathology. The current trends in further technical development of multiphoton imaging include the use of high sensitivity fluorescence detectors (GaAsP), longer wavelength excitation (1300 nm and beyond) for even deeper penetration and third-harmonic generation microscopy, and light-sheet microscopy [14–16].
Quantitative multiphoton imaging modalities have been developed for studying the living intact kidney in various animal models, including the Munich-Wistar-Fromter rat, various mouse strains, and the zebrafish [2,17–22]. Dynamics processes of several glomerular and tubular cell types have been visualized, including glomerular filtration of different molecular weight tracers, glomerular and peritubular capillary blood flow, proximal and distal tubular flow, the concentrating and diluting mechanism and the effects of diuretics, renin granular content, release, and tissue renin activity, mitochondrial metabolism, cell migration and fate, intracellular processes and parameters such as endocytosis and transcytosis, pH, calcium, and many others [2–4,8–13,17,20]. The following chapters will discuss the most recent intravital multiphoton imaging studies of the glomerulus and the glomerular filtration barrier, and the relevant scientific and technical breakthroughs that were made possible by the use of intravital multiphoton imaging.
2. In vivo imaging of the glomerulus and the glomerular filtration barrier
Since most of the relevant morphological and functional observations were based on cell culture models and fixed tissue sections, an important bottleneck in podocyte research has been the lack of an experimental approach that allowed detailed in vivo study of this important cell type. Likewise, a critical barrier in understanding the mechanistic details of glomerular diseases has been the technical limitation to study the glomerular filtration barrier in its native environment in vivo. Intravital multiphoton imaging has solved these technical barriers and allowed the dynamic portrayal of the structure and function of various glomerular cell types with amazing temporal and spatial resolution. In fact, since the first multiphoton imaging studies of the glomerulus were reported [1,2,17,23], several nephrology research laboratories worldwide have established and applied this technology for a variety of studies on the glomerulus [14,22,24–31]. Altogether, these multiphoton imaging studies provided tremendous help for the better understanding of glomerular and kidney diseases.
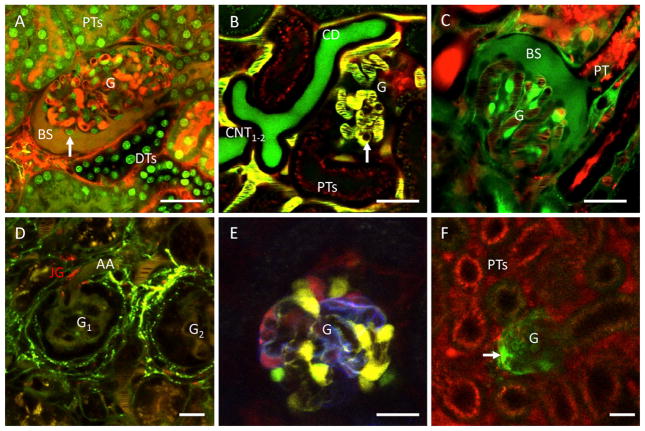
The first in vivo glomerular studies were performed in a specific rat strain, the Munich-Wistar-Fromter rat model that features superficial glomeruli [2,17]. This was due to the highly light scattering and absorbing characteristics of the living kidney tissue (because of the dense peritubular capillary network, massive amount of renal blood flow, fast streaming red blood cells, dense convoluted tubules in the light path, etc.) which limit the depth penetration capability of the multiphoton imaging technique to less than 250 μm under the renal capsule. However, the development of the multiphoton imaging technology and the availability of superficial glomeruli in multiple mouse strains allowed in vivo multiphoton imaging of mouse glomeruli, which has been performed successfully by several laboratories [21,24–28]. The quality and detail of multiphoton imaging for glomerular imaging in vivo is demonstrated in Fig. 1A. Further significant improvements in imaging technology, for example the application of long-wavelength multiphoton excitation (around 1300 nm) can continuously improve depth penetration and the routine multiphoton imaging of deep glomeruli [14].
Fig. 1.
In vivo imaging of glomeruli in the intact mouse kidney. A: optical section of a glomerulus (G), and the adjacent proximal tubules (PTs), and distal tubules (DTs). Cell nuclei are intensely labeled by Hoechst33342 (green). Plasma was labeled by 70 kDa dextran-rhodamine B (red) and lucifer yellow (green), a freely filtered dye given in intravenous bolus. The Bowman’s space (BS) is visible in dark yellow from the mixture of filtered markers. Podocytes are visible around glomerular capillaries in negative (dark), based on their lack of fluorophore uptake (arrow). B: in vivo imaging of blood cells streaming in glomerular capillaries. In addition to the majority of unlabeled (dark) red blood cells, round shaped leukocytes are occasionally visible sticking and rolling in individual capillaries (arrow). Two adjacent connecting tubules (CNT1–2) are seen merging into their common collecting duct (CD). Plasma was labeled yellow by Alexa594-albumin (red) and Lucifer Yellow (green) given in IV bolus. Albumin uptake is visible in proximal tubule segments (PTs, red). C: In vivo labeling and imaging of glomerular endothelial cells and the glomerular and tubular basement membranes using positively charged Lucifer Yellow (green). The cell body of endothelial cells (most intense green), the typical linear network of glomerular and proximal tubular (PT) basement membranes (medium intensity), and the BS are labeled green by Lucifer Yellow. Plasma was labeled by 70 kDa dextran-rhodamine B (red). D: in vivo imaging of renin activity (green) in plasma and renal tissues. Two adjacent glomeruli (G1–2) and their afferent arterioles (AA) are clearly visible. Renin containing juxtaglomerular (JG) cells in the terminal AA are labeled red using LysoTracker-Red. Activity of the fluorogenic renin substrate (green) is visible in both the circulating plasma (intravascular space) and the renal interstitium (most intensely in the JG region around glomeruli). E: multicolor labeling of podocytes in the transgenic Podocin-Confetti mouse model, either by genetically encoded membrane-targeted CFP (cyan), nuclear GFP (green), cytosolic YFP (yellow) or cytosolic RFP (red). F: in vivo image of a single, sclerotic glomerulus (G) in a healthy Podocin–GCaMP3 mouse kidney. The genetically encoded calcium indicator GCaMP3 (green) is expressed and visible only in podocytes. High calcium (intense green) is detected in a subset of podocytes that invade the parietal Bowman’s capsule (arrow). Plasma was labeled by Alexa594-albumin (red) given in IV bolus. High albumin uptake is visible in this nephron’s proximal tubule (PT) segments. Bars = 20 μm.
The initial studies with high-power multiphoton imaging of the glomerular filtration barrier used highly fluorescent molecular tracers of different sizes (Fig. 1A–C) to label the filtrate and measure the permeability of the glomerular filtration barrier to various macromolecules including dextrans, ficoll, albumin, and angiotensinogen. [2,20,28–30,32–35]. Importantly, this approach also helped to visualize the podocytes in negative (Fig. 1A, C) because of their lack of fluorophore uptake. This approach helped to shed light on new microanatomical details of the glomerular filtration barrier, including the subpodocyte space and its important role in puromycin-induced focal segmental glomerulosclerosis, the endothelial surface layer (also called glycocalyx), and angiotensin II-induced albumin vesicle transcytosis in podocytes [3,20,29,31,33]. Fig. 1A–D and several recent studies clearly demonstrate the high spatial and temporal resolution of multi-photon imaging to study complex cell-to-cell interactions between different cell types of the glomerular filtration barrier, and circulating blood cells and other cell and plasma factors in vivo. For example, multiphoton imaging can directly visualize streaming red blood cells and sticking and rolling immune cells in glomerular capillaries (Fig. 1B) [24]. The feature of high lucifer yellow uptake into vascular endothelial cells and into the glomerular and tubular basement membranes (Fig. 1C) can identify these important glomerular filtration barrier layers [20]. Also, the technique of labelling and quantitatively visualizing the glycocalyx in the glomerular endothelium was reported by our laboratory recently [29]. Aging-dependent or enzyme-induced reduction of the glycocalyx depth and coverage was demonstrated by using intravital multiphoton imaging, which was associated with vascular dysfunction and increased albumin leakage in the glomerulus [29]. Importantly, glycocalyx restoration by intravenous injection of wheat germ agglutinin lectin and its adsorption onto the endothelial surface layer significantly improved glomerular albumin permeability [29], which finding may have implications for future therapeutic development.
Multiphoton imaging is capable of detailing the cellular and structural alterations in the glomerular basement membrane, endothelium, and podocyte in response to injury, and the formation of localized microthrombi in glomerular capillaries [20]. In the puromycin model of focal segmental glomerulosclerosis, time-lapse multiphoton imaging visualized podocyte shedding, replacement, and the role of parietal epithelial cells in the pathology of glomerulosclerosis. The rather rapid processes of podocyte death and detachment suggested cell necrosis rather than apoptosis as the mechanism [20]. After shedding, podocytes exited the glomerulus with the glomerular filtrate down the tubular system, or by breaking through the highly permeable parietal epithelial cell layer into the periglomerular interstitium [20]. In addition, migrating cells of unknown origin were seen in both in the parietal and visceral layers of the Bowman’s capsule [20,36], which may play a role in the cellular remodeling of the glomerular filtration barrier.
Intravital multiphoton imaging is also capable of visualizing the cellular and molecular elements of the complex intrarenal renin–angiotensin system, which is an important player in renal pathologies [17,37–40]. Not only renin granular content and release in both the classic vascular site at the juxtaglomerular apparatus and the novel collecting duct site [38], but also the enzyme activity of renin in intact renal tissues can be visualized (Fig. 1D). The principle and use of several fluorescence resonance energy transfer-based fluorogenic renin substrates have been described earlier [12,37]. This fluorescence-based technique permits real-time measurement of renin activity, does not utilize radioactivity, and is conveniently performed within minutes, as opposed to conventional renin assays using radioimmuno-methods. This imaging approach can be very helpful for future studies of the intrarenal renin–angiotensin system in several disease conditions such as diabetes.
3. Combination of intravital imaging and mouse genetics
Although the negative podocyte labeling technique provided much new information, more precise studies of this important cell type required positive cell identification. This has become possible using genetically expressed fluorescent lineage tags (genetic cell fate mapping) in combination with intravital multiphoton imaging [18,19]. This approach was able to accurately identify and visualize parietal epithelial cells and podocytes expressing fluorescent reporter constructs including green fluorescent protein, Confetti, and the calcium-sensitive GCaMP3 [19,22]. Importantly, the multicolor Confetti construct (CFP/GFP/YFP/RFP) as shown in Fig. 1E made it possible to place a unique color identification on a single podocyte and track its fate over time [19]. The use of this in vivo visual approach depicted, for the first time, a highly dynamic glomerular environment, the robust cellular remodelling of the glomerulus by migrating parietal epithelial cells and podocytes in disease conditions, including adriamycin nephropathy (which resembles human focal segmental glomerulosclerosis) and obstructive nephropathy [19]. Visual evidence was shown for the hotly debated issue of the generation of new podocytes, and the presence of long nanotubules interconnecting the parietal and visceral layers of the Bowman’s capsule, which may allow the exchange of material between parietal epithelial cells and podocytes [19].
Other multiphoton imaging studies looked inside living cells to study the role of cytosolic parameters, for example calcium, using the genetically encoded calcium indicator GCaMP3 expressed in podocytes [18]. This approach allowed the detailed investigation of the role of podocyte [Ca2+]i and the related cellular and molecular mechanisms in glomerular disease. In fact, this was the first time that changes in [Ca2+]i were followed in any organ or disease state in vivo. Steady and low baseline, and small angiotensin II-induced changes in podocyte [Ca2+]i were detected, suggesting highly active Ca2+ extrusion and intracellular Ca2+ buffering mechanism in these cells [18]. The application of podocyte injury and glomerular disease models established the role of purinergic calcium signalling via P2Y2 receptors in primary and secondary (propagating) podocyte injury, cell clustering and migration (Fig. 1F) [18]. Future multiphoton imaging studies will use several other commercially available Cre-lox-based mouse genetics tools for intravital imaging of other glomerular, tubular and vascular cells in order to learn new mechanistic insights into kidney disease processes.
4. Serial multiphoton imaging of the same kidney over several days
Perhaps the most exciting new technical development in multiphoton imaging is time-lapse serial images over long time intervals. This will be undoubtedly an important technical advance to better understand the dynamics of slow (on the scale of several hours and days) cellular or molecular processes in the living kidney tissue, such as tissue remodeling by stem or progenitor cells. The technique allows for the visualization of the exact same kidney region (e.g. glomerulus) over days, during the course of disease in the intact living kidney with subcellular resolution [18,19]. No other current technology is capable of accomplishing this. There are several advantages and unique capabilities of this technique, including overcoming glomerular heterogeneity issues, establishing the origin and fate of individual cells, and the dynamics and pattern of their motility and migration over time, and combination with simultaneous functional measurements (e.g. glomerular albumin leakage).
The development and first applications of serial multiphoton imaging made it possible to track the fate of single cells in the intact kidney in vivo using novel fluorescent lineage tagged mouse models, and to study the dynamics of cell motility. This new approach was instrumental in depicting the dynamics and the dramatic changes in the morphology and cellular composition of the injured glomerulus, the robust bridging and migration of visceral podocyte cell clusters to the parietal Bowman’s capsule [18,19]. These studies supported the highly dynamic rather than static nature of the glomerular environment and cellular composition in glomerular disease [19].
Unlike other research techniques, intravital imaging can provide new, important visual clues regarding the pathomechanism of glomerular and tubulointerstitial diseases, but also it can be useful to understand intrinsic mechanisms and dynamics of renal cell/tissue turnover and repair. Future applications of serial multiphoton imaging for the dynamic tracking of individually labeled renal stem and progenitor cells may provide important new knowledge that could be used for the development of novel strategies of nephron and kidney regeneration [36].
Acknowledgments
Financial support and sponsorship
This work was supported in part by US National Institutes of Health grants DK64324 and DK100944, by the American Heart Association grant 15GRNT23040039, and by the American Diabetes Association grant 4-15-CKD-56 to J.P.-P. Thanks to James Burford for his assistance with intravital imaging.
Footnotes
Article presented at the annual seminar “Actualités néphrologiques Jean-Hamburger, hôpital Necker, 2016”.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Peti-Peterdi J, Morishima S, Bell PD, Okada Y. Two-photon excitation fluorescence imaging of the living juxtaglomerular apparatus. Am J Physiol Renal Physiol. 2002;283:F197–201. doi: 10.1152/ajprenal.00356.2001. [DOI] [PubMed] [Google Scholar]
- 2.Dunn KW, Sandoval RM, Kelly KJ, Dagher PC, Tanner GA, Atkinson SJ, et al. Functional studies of the kidney of living animals using multicolor two-photon microscopy. Am J Physiol Cell Physiol. 2002;283:C905–16. doi: 10.1152/ajpcell.00159.2002. [DOI] [PubMed] [Google Scholar]
- 3.Peti-Peterdi J, Burford JL, Hackl MJ. The first decade of using multiphoton microscopy for high-power kidney imaging. Am J Physiol Renal Physiol. 2012;302:F227–33. doi: 10.1152/ajprenal.00561.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peti-Peterdi J, Kidokoro K, Riquier-Brison A. Novel in vivo techniques to visualize kidney anatomy and function. Kidney Int. 2015;88:44–51. doi: 10.1038/ki.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croix CS, Zipfel WR, Watkins SC. Potential solutions for confocal imaging of living animals. Biotechniques. 2007;43:14–9. doi: 10.2144/000112509. [DOI] [PubMed] [Google Scholar]
- 6.Dunn KW, Young PA. Principles of multiphoton microscopy. Nephron Exp Nephrol. 2006;103:e33–40. doi: 10.1159/000090614. [DOI] [PubMed] [Google Scholar]
- 7.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat Methods. 2005;2:932–40. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 8.Ashworth SL, Sandoval RM, Tanner GA, Molitoris BA. Two-photon microscopy: visualization of kidney dynamics. Kidney Int. 2007;72:416–21. doi: 10.1038/sj.ki.5002315. [DOI] [PubMed] [Google Scholar]
- 9.Hall AM, Molitoris BA. Dynamic multiphoton microscopy: focusing light on acute kidney injury. Physiology (Bethesda) 2014;29:334–42. doi: 10.1152/physiol.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molitoris BA, Sandoval RM. Intravital multiphoton microscopy of dynamic renal processes. Am J Physiol Renal Physiol. 2005;288:F1084–9. doi: 10.1152/ajprenal.00473.2004. [DOI] [PubMed] [Google Scholar]
- 11.Peti-Peterdi J. Multiphoton imaging of renal tissues in vitro. Am J Physiol Renal Physiol. 2005;288:F1079–83. doi: 10.1152/ajprenal.00385.2004. [DOI] [PubMed] [Google Scholar]
- 12.Peti-Peterdi J, Toma I, Sipos A, Vargas SL. Multiphoton imaging of renal regulatory mechanisms. Physiology (Bethesda) 2009;24:88–96. doi: 10.1152/physiol.00001.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sipos A, Toma I, Kang JJ, Rosivall L, Peti-Peterdi J. Advances in renal (patho)-physiology using multiphoton microscopy. Kidney Int. 2007;72:1188–91. doi: 10.1038/sj.ki.5002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuh CD, Haenni D, Craigie E, Ziegler U, Weber B, Devuyst O, et al. Long wavelength multiphoton excitation is advantageous for intravital kidney imaging. Kidney Int. 2015 doi: 10.1038/ki.2015.323. http://dx.doi.org/10.1038/ki.2015.323. [DOI] [PubMed]
- 15.Witte S, Negrean A, Lodder JC, de Kock CP, Testa Silva G, Mansvelder HD, et al. Label-free live brain imaging and targeted patching with third-harmonic generation microscopy. Proc Natl Acad Sci U S A. 2011;108:5970–5. doi: 10.1073/pnas.1018743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller PJ, Ahrens MB. Visualizing whole-brain activity and development at the single-cell level using light-sheet microscopy. Neuron. 2015;85:462–83. doi: 10.1016/j.neuron.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Quantitative imaging of basic functions in renal (patho)physiology. Am J Physiol Renal Physiol. 2006;291:F495–502. doi: 10.1152/ajprenal.00521.2005. [DOI] [PubMed] [Google Scholar]
- 18.Burford JL, Villanueva K, Lam L, Riquier-Brison A, Hackl MJ, Pippin J, et al. Intravital imaging of podocyte calcium in glomerular injury and disease. J Clin Invest. 2014;124:2050–8. doi: 10.1172/JCI71702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackl MJ, Burford JL, Villanueva K, Lam L, Susztak K, Schermer B, et al. Tracking the fate of glomerular epithelial cells in vivo using serial multiphoton imaging in new mouse models with fluorescent lineage tags. Nat Med. 2013;19:1661–6. doi: 10.1038/nm.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peti-Peterdi J, Sipos A. A high-powered view of the filtration barrier. J Am Soc Nephrol. 2010;21:1835–41. doi: 10.1681/ASN.2010040378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schiessl IM, Bardehle S, Castrop H. Superficial nephrons in BALB/c and C57BL/6 mice facilitate in vivo multiphoton microscopy of the kidney. PLoS One. 2013;8:e52499. doi: 10.1371/journal.pone.0052499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Endlich N, Simon O, Gopferich A, Wegner H, Moeller MJ, Rumpel E, et al. Two-photon microscopy reveals stationary podocytes in living zebrafish larvae. J Am Soc Nephrol. 2014;25:681–6. doi: 10.1681/ASN.2013020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol. 2006;291:F473–80. doi: 10.1152/ajprenal.00425.2005. [DOI] [PubMed] [Google Scholar]
- 24.Devi S, Li A, Westhorpe CL, Lo CY, Abeynaike LD, Snelgrove SL, et al. Multi-photon imaging reveals a new leukocyte recruitment paradigm in the glomerulus. Nat Med. 2013;19:107–12. doi: 10.1038/nm.3024. [DOI] [PubMed] [Google Scholar]
- 25.Hohne M, Ising C, Hagmann H, Volker LA, Brahler S, Schermer B, et al. Light microscopic visualization of podocyte ultrastructure demonstrates oscillating glomerular contractions. Am J Pathol. 2013;182:332–8. doi: 10.1016/j.ajpath.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Khoury CC, Khayat MF, Yeo TK, Pyagay PE, Wang A, Asuncion AM, et al. Visualizing the mouse podocyte with multiphoton microscopy. Biochem Biophys Res Commun. 2012;427:525–30. doi: 10.1016/j.bbrc.2012.09.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kistler AD, Caicedo A, Abdulreda MH, Faul C, Kerjaschki D, Berggren PO, et al. In vivo imaging of kidney glomeruli transplanted into the anterior chamber of the mouse eye. Sci Rep. 2014;4:3872. doi: 10.1038/srep03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano D, Kobori H, Burford JL, Gevorgyan H, Seidel S, Hitomi H, et al. Multiphoton imaging of the glomerular permeability of angiotensinogen. J Am Soc Nephrol. 2012;23:1847–56. doi: 10.1681/ASN.2012010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salmon AH, Ferguson JK, Burford JL, Gevorgyan H, Nakano D, Harper SJ, et al. Loss of the endothelial glycocalyx links albuminuria and vascular dysfunction. J Am Soc Nephrol. 2012;23:1339–50. doi: 10.1681/ASN.2012010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiessl IM, Castrop H, Angiotensin II. AT2 receptor activation attenuates AT1 receptor-induced increases in the glomerular filtration of albumin: a multi-photon microscopy study. Am J Physiol Renal Physiol. 2013;305:F1189–200. doi: 10.1152/ajprenal.00377.2013. [DOI] [PubMed] [Google Scholar]
- 31.Schiessl IM, Hammer A, Kattler V, Gess B, Theilig F, Witzgall R, et al. Intravital imaging reveals angiotensin II-induced transcytosis of albumin by podocytes. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014111125. http://dx.doi.org/10.1681/ASN.2014111125. [DOI] [PMC free article] [PubMed]
- 32.Russo LM, Sandoval RM, McKee M, Osicka TM, Collins AB, Brown D, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–13. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 33.Salmon AH, Toma I, Sipos A, Muston PR, Harper SJ, Bates DO, et al. Evidence for restriction of fluid and solute movement across the glomerular capillary wall by the subpodocyte space. Am J Physiol Renal Physiol. 2007;293:F1777–86. doi: 10.1152/ajprenal.00187.2007. [DOI] [PubMed] [Google Scholar]
- 34.Yu W, Sandoval RM, Molitoris BA. Rapid determination of renal filtration function using an optical ratiometric imaging approach. Am J Physiol Renal Physiol. 2007;292:F1873–80. doi: 10.1152/ajprenal.00218.2006. [DOI] [PubMed] [Google Scholar]
- 35.Schiessl IM, Kattler V, Castrop H. In vivo visualization of the antialbuminuric effects of the angiotensin-converting enzyme inhibitor enalapril. J Pharmacol Exp Ther. 2015;353:299–306. doi: 10.1124/jpet.114.222125. [DOI] [PubMed] [Google Scholar]
- 36.Peti-Peterdi J, Burford JL, Hackl MJ. Can kidney regeneration be visualized? Nephron Exp Nephrol. 2014;126:86. doi: 10.1159/000360673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang JJ, Toma I, Sipos A, McCulloch F, Peti-Peterdi J. Imaging the renin-angiotensin system: an important target of anti-hypertensive therapy. Adv Drug Deliv Rev. 2006;58:824–33. doi: 10.1016/j.addr.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51:1597–604. doi: 10.1161/HYPERTENSIONAHA.107.107268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toma I, Kang JJ, Peti-Peterdi J. Imaging renin content and release in the living kidney. Nephron Physiol. 2006;103:71–4. doi: 10.1159/000090622. [DOI] [PubMed] [Google Scholar]
- 40.Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest. 2008;118:2526–34. doi: 10.1172/JCI33293. [DOI] [PMC free article] [PubMed] [Google Scholar]