Abstract
Regulatory T cells (Tregs) induced during autoimmunity often become quiescent and unable to resolve disease, suggesting inadequate activation. Resolution of established experimental autoimmune encephalomyelitis (EAE) can be achieved with myelin oligodendrocyte glycoprotein (MOG) fused to reovirus protein σ1 (MOG-pσ1) which activates Tregs, restoring protection, but requiring other regulatory cells to revitalize them. B cells have a dichotomous role in both the pathogenesis and recovery from EAE. While inflammatory B cells contribute to EAE’s pathogenesis, treatment of EAE mice with MOG-pσ1, but not OVA-pσ1, resulted in an influx of IL-10-producing B220+CD5+ B regulatory cells (Bregs) enabling Tregs to recover their inhibitory activity, and in turn, leading to the rapid amelioration of EAE. These findings implicate direct interactions between Bregs and Tregs to facilitate this recovery. Adoptive transfer of B220+CD5− B cells from MOG-pσ1-treated EAE or Bregs from PBS-treated EAE mice did not resolve disease while the adoptive transfer of MOG-pσ1-induced B220+CD5+ Bregs greatly ameliorated EAE. MOG-pσ1-, but not OVA-pσ1-induced IL-10-producing Bregs, expressed elevated levels of BTLA relative to CD5− B cells, as opposed to Tregs or effector T (Teff) cells, whose BTLA expression was not affected. These induced Bregs restored EAE Treg function in a BTLA-dependent manner. BTLA−/− mice showed more pronounced EAE with fewer Tregs but, upon adoptive transfer of MOG-pσ1-induced BTLA+ Bregs, BTLA−/− mice were protected against EAE. Hence, this evidence shows the importance of BTLA in activating Tregs to facilitate recovery from EAE.
Keywords: EAE/MS, Bregs, Tregs, BTLA, mucosal immunity, tolerance
Introduction
B cells have been classically considered a key player in the immune response against microbial infections, mainly through the production of Abs. B cells also have been shown to have a role in the pathogenesis of autoimmune disorders. New clinical interventions have targeted the elimination of B cells for the treatment of rheumatoid arthritis (1), lupus (2), and MS (3). However, an increasing body of evidence supports the existence of a subpopulation of anti-inflammatory IL-10-producing B cells, that critically affect the outcome of autoimmune diseases both in animal models (4-8) and in human clinical disease (9-12).
EAE is a T cell-dependent inflammatory disease, principally mediated by IL-17 (13) and GM-CSF (14). EAE is highly reproducible in mice after immunization of susceptible strains with TCR-reactive peptides, and mimics many aspects of MS (15). Although EAE is predominantly a T cell-mediated disorder, B cells have been shown to play an important pathogenic role in this disease (reviewed by Gray et al in (16). On one hand, autoAb production may increase demyelination and inflammation, worsening the course of the disease (17, 18). Additionally, B cells may function as APCs in early phases of EAE, contributing to the expansion of effector CD4+ T cells (19, 20). On the other hand, B cell-deficient mice develop a more aggressive form of EAE (21-24), and IL-10 production by B cells inhibits the lymphocyte response against auto-Ags, and thus ameliorates EAE (6, 7, 20, 25, 26). As suggested by Matsushita et al, this contradiction may be explained if the existence of regulatory B cells (Bregs) is taken into account (6). While pathogenic B cells increase severity of the disease, Bregs can ameliorate symptoms. Therefore, a balance between pathogenic B cells and Bregs can affect the severity of EAE as well as other autoimmune diseases.
The induction of tolerance in humans is problematic, but some success has been achieved for treating allergies (27, 28) and diabetes (29). Oral tolerance in humans is plagued by the lack of effective means to absorb ingested tolerogens from the gastrointestinal tract. Hence, we postulated that targeting tolerogens or seeking alternative sites of treatment, e.g., nasal, offered a means to implement tolerance. We found that reovirus protein σ1 (pσ1) could facilitate uptake of genetically fused tolerogens, when applied mucosally, and stimulate the induction of tolerogen-specific regulatory T cells (Tregs; 30-33). Pσ1 effectively stimulated Ag-specific tolerance to defined Ags: OVA, proteolipid protein (PLP) peptide 139-151, and myelin oligodendrocyte glycoprotein (MOG29-146) (30-33). PLP139-151 fused to OVA-pσ1 protected mice against PLP139-151-induced EAE while OVA-pσ1 did not (33). MOG-pσ1 protected against MOG35-55-induced EAE, but not OVA- pσ1, MOG, or MOG + pσ1 (32) further demonstrating that the tolerogens must be physically coupled to pσ1 to elicit Ag-specific tolerance. Herein we show that treatment of EAE with MOG-pσ1 increased the percentage of Bregs and their production of IL-10. This treatment provides the regulatory balance needed to resolve disease.
HVEM (Herpes virus entry mediator) belongs to the tumor necrosis factor receptor family. It regulates the immune response in various pathogen and autoimmune settings through interactions with multiple ligands such as Ig superfamily members, BTLA (B and T lymphocyte attenuator) and CD160, as well as, the TNF superfamily ligands, LIGHT and LTα (34, 35). These receptors act bidirectionally having co-stimulatory or co-inhibitory properties. For instance, trans engagement of HVEM with BTLA or LIGHT is co-stimulatory for the HVEM+ cells and promotes cell survival via NF-κB activation (36). Additionally, HVEM engagement of LIGHT potently activates T cells which can lead to T cell-mediated intestinal inflammation (37-39). In contrast, HVEM signaling through BTLA results in the inactivation of the BTLA+ cells mediated by SH2 domain-containing protein tyrosine phosphatase 1 and 2 (40, 41).
Although large numbers of Tregs are found at sites of chronic inflammation, these fail to curb disease (42-45). To address this paradox, we sought to determine the activation status of Bregs since these cells were induced subsequent to MOG-pσ1 treatment. We found elevated numbers of BTLA+ Bregs following MOG-pσ1 treatment. Neutralization of BTLA on Bregs inhibited their capacity to restore suppressive function to EAE Tregs. Thus, we hypothesize that restoration of Treg function is BTLA-dependent. Supporting this hypothesis, adoptive transfer of MOG-pσ1-induced IL-10-producing B220+(CD19+)CD5+, but not B220+(CD19+)CD5neg cells, significantly increased the percentage and activity of CD25+ CD4+ Tregs which reduced EAE severity. Furthermore, these Bregs were protective against EAE in BTLA−/− mice. Such findings underscore the relevance of Bregs and their potential benefit in treating human autoimmune diseases such as MS.
Material and Methods
Preparation of MOG-pσ1 and OVA-pσ1
MOG-pσ1 and OVA-pσ1 fusion proteins were prepared as previously described (31,32) consisting of MOG29-146 or OVA genetically fused to the N-terminus of the whole reovirus pσ1. These recombinant proteins were generated in Pichia pastoris featuring a his-tag to enable their purification (31, 32). Proteins were assessed for purity and quality by Coomassie-stained polyacrylamide gels and by Western blot analysis.
Mice
C57BL/6 females (6-8 wk old; Frederick Cancer Research Facility, National Cancer Institute, Frederick, MD) and breeding colonies of Foxp3-mRFP-transgenic, B cell deficient (μMT), IL-10−/−, and BTLA−/− mice (Jackson Laboratory, Bar Harbor, ME) were maintained at Montana State University Animal Resources Center or the University of Florida Animal Center Services. Mice were kept in individual ventilated cages under high-efficiency particulate absorbing-filtered barrier conditions. All animal care and procedures are in compliance with institutional policies for animal health and well-being.
MOG-pσ1-based therapies and EAE induction
Mice were given a single oral 50 μg dose of MOG-pσ1 or OVA-pσ1 as previously described (30). For EAE induction, mice were challenged s.c. in the flank with 300 - 350 µg of MOG35-55 peptide (Global Peptide Services, LLC, Ft. Collins, CO or Bio-Synthesis, Inc., Lewisville, TX) in 100 µl of IFA (Sigma-Aldrich, St. Louis, MO) containing 400 μg killed Mycobacterium tuberculosis (Difco Laboratories, Detroit, MI) on day 0 followed by i.p. treatments with 200 ng of Bordetella pertussis toxin (List Biological Laboratories, Campbell, CA) on days 0 and 2. Mice were monitored and scored daily for EAE progression (30, 33): 0, normal; 1, a limp tail; 2, hind limb paresis; 3, hind limb paralysis; 4, quadriplegia; 5, moribund state. In most instances, we observed that the PBS-treated EAE C57BL/6 mice made a slow recovery, and generally about days 40-45, exhibited a clinical score of ≤ 1, and these are referred to as “naturally recovered” EAE mice.
Isolation of mononuclear cells from CNS
Prior to dissection, mice were perfused with cold PBS through the left cardiac ventricle. Subsequently, the forebrain and cerebellum were dissected, and the spinal cords (SCs) flushed out with PBS by hydrostatic pressure, minced, and digested with 10 ml HBSS containing 500 U/ml of collagenase (Sigma-Aldrich) for 60-90 min at 37° C with shaking to release mononuclear cells. The tissues were then homogenized in cold HBSS buffer (Sigma-Aldrich) as previously described (30) to obtain a single cell suspension. Centrifuged cells collected from 4-5 mice were pooled, resuspended in 70% Percoll (Sigma-Aldrich), and underlaid with 30% Percoll. Cells were subjected to gradient density centrifugation, and mononuclear cells were isolated from the 30/70 interphase, then washed, and stained for flow cytometry analysis.
Flow cytometry
To block nonspecific Fc receptor binding, lymphocytes were preincubated for 15 min at room temperature with anti-CD16/32 FcR block, and stained 30 minutes in FACS buffer at 4°C with fluorochrome-labeled mAbs against B220, CD1d, CD4, CD5, CD19, CD25, and BTLA (clone 8F4, Biolegend). For intracellular expression of cytokines, lymphocytes were assessed stimulated for 5h with PMA (50 ng/ml) plus ionomycin (500 ng/ml) in the presence of brefeldin A (2 µg/ml), followed by permeabilization with saponin and subsequent staining with mAbs specific IL-10 (clone JES5-16E3) or IL-17 (clone eBio17B7). Nuclear Foxp3 expression by Tregs was measured by FACS analysis as per the manufacturer's instructions (Foxp3 Fix/Perm buffer set, Biolegend). Cells were analyzed using a LSRFortessa flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with Flow Jo software (Tree Star Inc., Ashland, OR).
T cell proliferation and cytokine production
Single-cell splenic and LN suspensions were prepared as previously described (29,30). CD25+CD4+ Tregs and CD25−CD4+ T effector (Teff) cells were purified using a FACSAria (Becton Dickinson) and 50,000 Teff cells were stimulated for 4 days on anti-CD3 mAb-coated wells (5 μg/ml) plus soluble anti-CD28 mAb (5 μg/ml) plus 5 U/ml of human IL-2 (28) Bregs were purified as described below. Splenic Tregs and Bregs were added when indicated at a 1:2 ratio. In some instances, Bregs or Tregs were pretreated (10 μg/ml) with anti-BTLA (clone 6F7; eBioscience) or isotype control mAb when indicated. One μCi per well of [3H]-thymidine was added during the last 12 h of culture. The cells were then harvested, and incorporated [3H]-thymidine was counted using a Beckman LS 6500 scintillation counter. For some experiments, purified CD4+ T cells were restimulated for 4 days with 5 µg/ml MOG35-55 peptide in the presence of irradiated T cell-depleted splenic feeder cells (30-32).
Supernatants from cultured T cells were analyzed for the production of IFN-γ, TGF-β, IL-4, IL-6, IL-10, and IL-17 by ELISA as previously described (32).
Adoptive transfers
Cell-sorting was accomplished using a FACSAria as previously described (30, 32). Briefly, splenic and LN (HNLN and MLN) cells from C57BL/6 and IL-10−/− mice were sorted for Bregs or B cells based on the expression of B220 (or CD19) and CD5. Cell purity exceeded 95%. Sorted cells were kept at 4°C in PBS with 2% FBS until adoptive transfer. Cells were peri-orbitally transferred into anesthetized mice, and the amount transferred is indicated in each experiment.
Statistical analysis
The ANOVA followed by posthoc Tukey test was applied to show differences in clinical scores in treated vs. PBS mice. The Student’s t test was used to evaluate the differences between two groups, and ANOVA was used to test differences between multiple groups. Values p < 0.05 were considered statistically significant.
Results
EAE-derived Tregs are dysfunctional
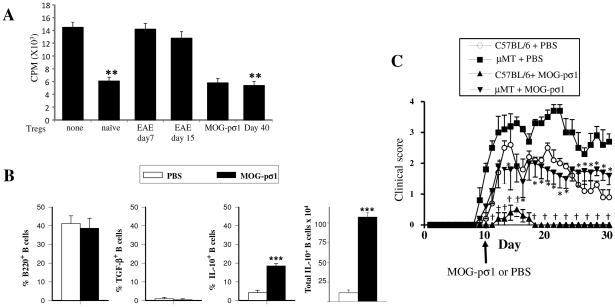
Previous studies have demonstrated the presence of Tregs during EAE and other inflammatory diseases, yet these remained dysfunctional or quiescent and did not reduce inflammation (42-45). Our previous work has shown that the inflammation in EAE mice is rapidly ameliorated with a single low nasal or oral dose of MOG-pσ1 (30, 32). Given this, purified splenic naïve Tregs, EAE-derived Tregs from mice at disease onset (d7 post-challenge [p.ch.], score 1), or Tregs from 15 days after EAE induction (score 3) were added to anti-CD3 plus anti-CD28 stimulated naïve Teff cells to measure inhibition of Teff cell proliferation. Naïve Tregs efficiently inhibited Teff cell proliferation, while EAE Tregs did not (Fig. 1A). Tregs obtained from EAE mice 24 h after treatment with MOG-pσ1 (day 16 p.ch.) were also quite effective in suppressing Teff cell proliferation. Interestingly, Tregs obtained from naturally recovered mice (day 40 p.ch., score 0.5) were as effective as naïve Tregs in suppressing Teff cell proliferation (Fig. 1A) suggesting these Tregs have regained their function.
Figure 1.
EAE derived Tregs are dysfunctional and B cells modulate EAE. (A) Tregs were purified from naïve or EAE mice (day 7 or 15 p.ch., scores 1 and 3 respectively), MOG-pσ1-treated mice (day 15 p.ch.), those naturally recovering from EAE (day 40, scores 0-1), and CD25− CD4+ Teff cells from naïve mice. Tregs were then co-cultured with Teff cells (Tregs/effector ratio1:2) in the presence of anti-CD3 plus anti-CD28 mAbs for 4 days before measuring extent of proliferation using a 3H-thymidine incorporation assay. A representative experiment of 5 is shown; **p ≤ 0.01 versus naïve cell proliferation. (B) C57BL/6 mice (8/group) were induced with MOG35-55 EAE, and 10 days later, treated with PBS or MOG-pσ1. The percentage and total combined LN and splenic B220+ B cells expressing TGF-β+ or IL-10 from individual mice was measured 20 days p.ch.; ***p < 0.001 versus PBS-treated EAE mice. (C) C57BL/6 or B cell-deficient μMT mice were orally dosed with 50 μg MOG-pσ1 or PBS 10 days after EAE challenge and monitored daily for disease course. An average of 5 mice per group is represented; *p < 0.05 versus PBS-dosed μMT, †p < 0.05 versus PBS-dosed C57BL/6 mice.
MOG-pσ1 treatment is only partially effective in μMT mice
MOG-pσ1's therapeutic effect has been shown to be IL-10-dependent (32). To determine the source of IL-10-producing cells, splenic lymphocytes taken from EAE mice previously treated with PBS or MOG-pσ1 were evaluated for their expression of TGF-β and IL-10. In addition to CD4+ T cells producing IL-10 (32), a population of IL-10-producing B cells was also found in MOG-pσ1-treated EAE mice (Fig. 1B). B lymphocytes isolated from EAE mice did not produce TGF-β regardless of the treatment (Fig. 1B). MOG-pσ1 did significantly increase the percentage and total numbers of IL-10-producing B cells (Fig. 1B). Further studies set out to assess the impact of MOG-pσ1 on B cells. C57BL/6 and B cell-deficient (μMT) mice were induced with EAE, and 7 days later, orally treated with 50 μg of MOG-pσ1 or PBS (Fig. 1C). As expected (24, 46), μMT mice developed a more severe and lingering disease, yet treatment of μMT mice with MOG-pσ1 reduced the disease severity (clinical score) by nearly 50%, showing that MOG-pσ1's therapeutic effect is partially dependent on B cells.
MOG-pσ1 treatment augments the IL-10-producing B220+ CD5+ regulatory B cells
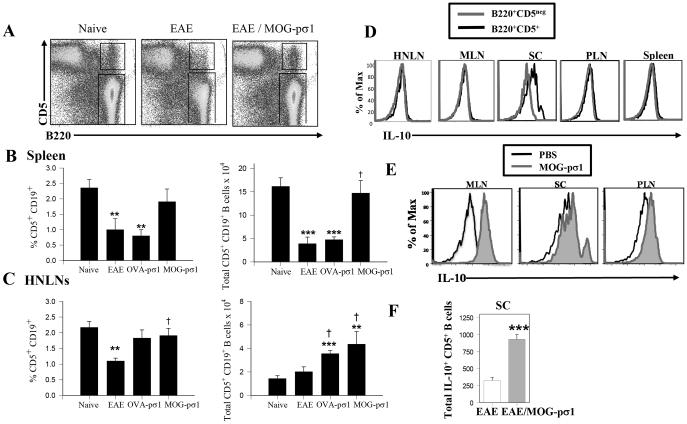
Both CD5+CD1dHi (7, 47) and CD5+ (7) B cell subsets can serve as Bregs. An analysis was performed to determine which of these Bregs was induced following MOG-pσ1 stimulation. When compared to naïve mice, a reduced presence of CD5+ B cells was observed in the spleen (Fig. 2A, B) and in the head and neck lymph nodes (HNLNs; Fig. 2C). Interestingly, treatment of EAE mice with MOG-pσ1, but not OVA-pσ1, restored the CD5+ B cells to levels similar to those of naïve mice (Fig. 2B). In contrast to CD5+ B cells that first decreased in diseased EAE mice and then increased either subsequent to MOG-pσ1 treatment or following natural recovery (Fig. 2A, B), the B220+CD5+CD1dHi cells could be detected (data not shown), but did not increase as notably as did the CD5+ B cells following MOG-pσ1 treatment (Fig. 2A-C). Thus, from this point forward, the described Bregs will be referred to as CD5+ (B220+ or CD19+) B cells. During EAE, the levels of CD5+ B cells diminished, and MOG-pσ1 treatment essentially restored to basal levels in the spleens (Fig. 2B), and the total number of CD5+ B cells increased in the HNLNs (Fig. 2C). OVA-pσ1 treatment failed to restore CD5+ B cells in the spleen (Fig. 2B), but did increase their numbers in the HNLNs (Fig. 2C). These findings support the importance of Bregs in protection conferred by MOG-pσ1 in EAE.
Figure 2.
MOG-pσ1 intervention promotes the development of regulatory B cells (Bregs). EAE was induced on C57BL/6 mice, and 14 days later, mice were treated with PBS or MOG-pσ1. (A) A representative dot-plot showing the percentage of B220+CD5+ Bregs is depicted. Bar graphs indicate mean (± SEM) percentages of and total (B) splenic and (C) HNLN CD19+CD5+ B cells (9 mice/group) in naïve mice, EAE mice, and EAE mice treated with OVA-pσ1 or MOG-pσ1; ***p < 0.001, **p ≤ 0.01, *p < 0.05 versus naïve B cells. (D) EAE mice were evaluated d14 p.ch. for IL-10 production by B220+CD5+ and B220+CD5neg cells in HNLNs, MLNs, SCs, PLNs, and spleens. (E) EAE (day 14 p.ch.) mice (5/group) were analyzed 24h after PBS or MOG-pσ1 treatment for intracellular IL-10 production for pooled SCs, MLNs, and PLNs. (F) The total number of IL-10-producing CD5+ B cells from the SCs of PBS- and MOG-pσ1-treated EAE mice is depicted (right panel); ***p ≤0.001 vs. EAE mice. A representative experiment of three is shown.
The induced Bregs were further analyzed for their capacity to generate IL-10. EAE mice were evaluated 24h following MOG-pσ1 or PBS treatment, and spleens, spinal cords (SCs), HNLNs, mesenteric LNs (MLNs), and peripheral LNs (PLNs) were analyzed for intracellular IL-10 production (Fig. 2D). Only SC-infiltrating Bregs were able to produce IL-10 by the PBS-treated group while MOG-pσ1 treatment increased Breg IL-10 production in the SCs, PLNs, and MLNs (Fig. 2E). Of interest, the total number of IL-10-producing Bregs in the SCs was significantly greater in MOG-pσ1-treated EAE than EAE mice (Fig. 2F). Thus, MOG-pσ1 treatment augments these IL-10-producing CD19+ (B220+) CD5+ Bregs during EAE.
MOG-pσ1-induced CD5+ Bregs are protective against EAE and require IL-10
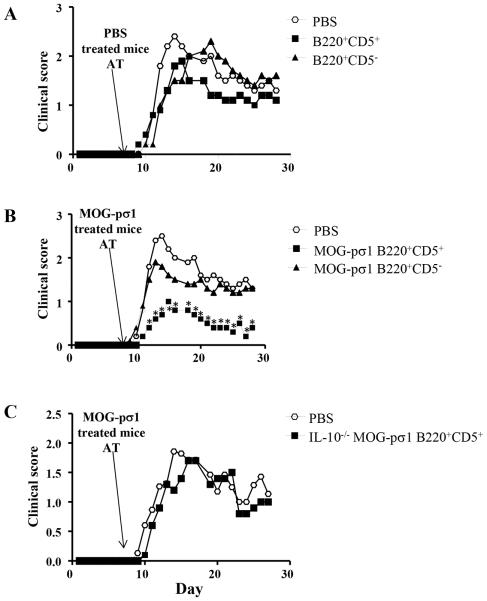
To determine whether MOG-pσ1-induced Bregs are protective against EAE, B cells were isolated from C57BL/6 mice induced with EAE, and treated 2 weeks later with PBS or MOG-pσ1. Twenty-four hours after treatment, combined LN and splenic B cells were cell-sorted into B220+CD5− (inflammatory) B cells and B220+CD5+ Bregs, and each was then adoptively transferred into different C57BL/6 mice that were induced with EAE 7 days earlier (Fig. 3). Neither CD5− nor CD5+ B cells obtained from PBS-treated donors affected clinical manifestation of EAE (Fig. 3A). Donor B220+CD5− B cells from MOG-pσ1-treated mice also failed to ameliorate EAE; however, MOG-pσ1-induced B220+CD5+ Bregs significantly reduced disease severity and accelerated recovery (Fig. 3B; p < 0.01). These findings underscore the importance of this IL-10- producing Bregs. The Breg subset, isolated from IL-10−/− mice, failed to confer protection against EAE when adoptively transferred into C57BL/6 mice with EAE (Fig. 3C). This evidence further confirms the critical role of IL-10+ Bregs induced by MOG-pσ1 in mediating protection against EAE. Notably, Bregs from diseased EAE mice failed to confer such protection.
Figure 3.
MOG-pσ1-, not PBS-induced Bregs, confers protection against EAE. C57BL/6 or IL-10−/− mice (10/group) were induced with EAE and 2 weeks later treated with PBS or MOG-pσ1. Twenty four hours after MOG-pσ1 treatment, combined LNs and splenic B cells were sorted into B220+CD5− B cells and B220+CD5+ B cell subsets, and 2.0x106 B cells were adoptively transferred (AT) into C57BL/6 recipients (5/group) that were already induced with EAE 7 days earlier. (A) PBS-induced B220+CD5− B cells or Bregs failed to ameliorate EAE. (B) MOG-pσ1-induced Bregs, not B220+CD5− B cells were able to significantly reduce EAE severity. (C) In the same experiment, IL-10−/− B220+CD5+ Bregs induced with MOG-pσ1 failed to significantly reduce EAE, indicating that their effect is IL-10-mediated. Panels A and B share the same control PBS group. *p < 0.01 versus PBS-treated mice or recipients adoptively transferred with MOG-pσ1-induced B220+ CD5− B cells.
Adoptive transfer of MOG-pσ1-treated CD5+ Bregs increases the number and activity of CD25+CD4+ Tregs in recipient mice
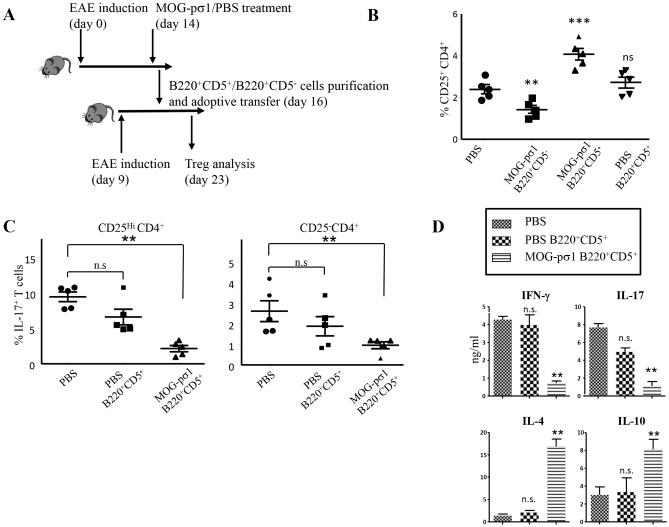
In an effort to understand how Bregs mediate their protection against EAE, both Bregs (B220+CD5+) and inflammatory B (B220+CD5−) cells were purified 48h after MOG-pσ1 treatment of EAE C57BL/6 mice. Subsequently, either of these B cell subsets was adoptively transferred into C57BL/6 mice induced with EAE 7 days earlier (Fig. 4A). At the peak of disease (day 14), the activity and quantity of Tregs were determined in recipients (Fig. 4B). Recipients given MOG-pσ1-induced Bregs showed an increase in the percentage of Tregs unlike those recipients given PBS-induced Bregs or MOG-pσ1-induced CD5− B cells. Such evidence suggests that the MOG-pσ1-induced Bregs become activated and stimulate an influx of Tregs or in turn activate quiescent, disease-induced Tregs. The percentage of IL-17-producing T cells amongst the CD25HiCD4+ and CD25−CD4+ subsets (48) was measured. Mice adoptively transferred with MOG-pσ1-induced Bregs showed significantly fewer IL-17-producing cells than those found in EAE mice and recipients adoptively transferred with Bregs from PBS-treated EAE mice (Fig. 4C, p ≤ 0.01).
Figure 4.
Adoptive transfer of MOG-pσ1-treated B220+CD5+ cells increases both the frequency and activity of CD25+CD4+Tregs in recipient mice. C57BL/6 mice (10/group) were treated with MOG-pσ1 or PBS 2 wks after EAE induction, and B220+CD5+ and B220+CD5− cells were purified 48 h after treatment from combined LNs and spleens. B cells (105 cells/mouse) were adoptively transferred into C57BL/6 mice (5/group) previously induced with EAE 7 days earlier. (A) A schematic depicting the experiment is provided. (B) The percentage of CD25+CD4+ T cells in each group was measured 1 wk later. Only recipients given MOG-pσ1-induced B220+CD5+ Bregs showed an increase in the percentage of CD25+CD4+ T cells; ***p = 0.002, **p < 0.01 versus PBS-treated mice; ns, not significant. (C) Analysis of the percentage of IL-17-expressing cells between CD25HiCD4+ and CD25− CD4+ T cells. Recipients given MOG-pσ1-induced Bregs showed significantly fewer IL-17-producing cells; **p < 0.01; ns, not significant. (D) Bregs stimulate Tregs to produce IL-4 and IL-10 and suppress IFN-γ and IL-17 production. CD25+CD4+ T cells were purified from PBS-treated mice and recipients adoptively transferred with PBS- or MOG-pσ1-induced B220+CD5+ Bregs, and stimulated in vitro with anti-CD3 + anti-CD28 mAbs. Cytokine levels (mean ± SEM from triplicate cultures) in culture supernatants were measured by ELISA 4 days after culture, and values corrected for spontaneous production from unstimulated cells; **p < 0.01 versus PBS-treated mice; ns, not significant. Data are representative from two experiments.
To determine which cytokines correlated with protection induced upon Breg adoptive transfer, cytokine analyses were performed on purified splenic CD25+CD4+ T cells isolated 7 days following adoptive transfer. Tregs obtained from mice adoptively transferred with MOG-pσ1-induced Bregs showed significant elevations in IL-4 and IL-10 when compared to control groups or recipients given PBS-induced Bregs (p < 0.01) (Fig. 4D) with concomitant reductions in IFN-γ and IL-17 (p < 0.01). These results demonstrate that MOG-pσ1-induced Bregs are protective by increasing and activating Tregs to produce regulatory cytokines and to simultaneously inhibit proinflammatory cytokine production.
The influx of Bregs is BTLA+ and important for reactivating Tregs
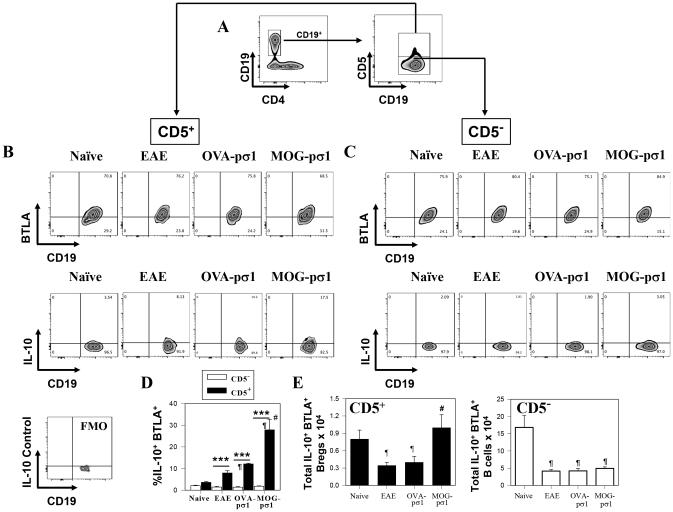
Investigations of how Bregs become activated led to an examination of the role of the BTLA-HVEM-LIGHT pathway, since depending on the ligands’ interactions to their HVEM receptor, this pathway can either be activating or suppressing (34, 35). CD19+CD5+ B cells, from EAE mice treated with MOG-pσ1 (Fig. 5A), expressed BTLA (Fig. 5B, D, E), and these BTLA+ Bregs from MOG-pσ1-treated mice showed enhanced capacity for producing IL-10 unlike those BTLA+ CD5− B cells (Fig. 5C, E). Although IL-10 production was mostly associated with Bregs in all treatment groups, MOG-pσ1 treatment of EAE mice resulted in a much higher percentage of IL-10-producing BTLA+ Bregs than was seen in either naïve mice or EAE mice treated with PBS or OVA-pσ1 (Fig. 5B, D). Thus, MOG-pσ1 enhances the frequency and the total number of IL-10-producing BTLA+ Bregs (Fig. 5B, D, E).
Figure 5.
MOG-pσ1 intervention upon EAE promotes increased BTLA expression/activation of Bregs for enhanced IL-10 production. EAE was induced in C57BL/6 mice, and mice were treated with PBS, OVA-pσ1, or MOG-pσ1 at the peak of disease (day 14), and splenic B cells were subsequently evaluated for BTLA expression. (A) Gating strategy used to identify Bregs staining for CD19 vs. CD4, and CD19+ B cells further analyzed for CD5 expression. (B) CD5+ and (C) CD5− CD19+ B cells from naïve, PBS-treated EAE, and MOG-pσ1-treated EAE mice were analyzed for BTLA and IL-10 expression; a fluorescence minus one (FMO) control histogram was performed for intracellular IL-10 staining. (D) The percent and (E) total IL-10+ BTLA+ CD5+ or CD5− B cells ± SEM from individual mice from naïve (n=9), PBS- (n=11), OVA-pσ1- (n=9), and MOG-pσ1-treated (n=11) mice are shown; ***p < 0.001, *p < 0.05 versus the corresponding CD5− B cell subset; #p < 0.005 versus EAE mice; and ¶p < 0.05 versus naïve mice.
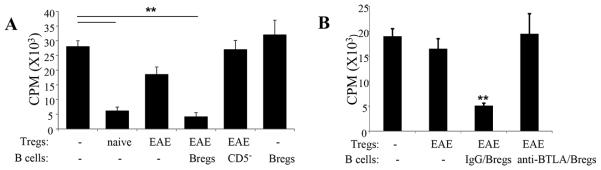
To determine if BTLA expression on Tregs was modulated as a consequence of EAE or MOG-pσ1 treatment, these Tregs were also examined (Table I). The percentage of BTLA+ Tregs did not change significantly as a consequence of treating EAE mice with MOG-pσ1 or OVA-pσ1 (Table I). Likewise, BTLA expression remained unchanged for CD25− CD4+ T cells (Table I). To determine whether Bregs enhanced the functionality of EAE-derived Tregs and contributed to EAE recovery, a T cell suppression assay was performed. To assess their function, splenic Tregs were either purified from naïve or EAE mice, and co-cultured with naïve CD25−CD4+ Teff cells in the presence or absence of MOG-pσ1-induced Bregs. Interestingly, CD5+ Bregs, but not CD5− B cells, were able to completely restore the inhibitory activity of EAE-derived Tregs (Fig. 6A). Of note, Bregs required the presence of Tregs since Bregs alone had no inhibitory effect upon Teff cells, suggesting that Bregs act through Tregs to facilitate their inhibitory activity (Fig. 6A).
Table I.
. Percent BTLA+ Tregs following treatment of EAE mice with PBS, OVA-pσ1, or MOG-pσ1
| %BTLA+ Foxp3+ CD25+ CD4+ T cellsb | %BTLA+ CD25− CD4+ T cellsb | |||
|---|---|---|---|---|
|
| ||||
| Treatment Groupa | Mean ±SEM | p-valuec | Mean ± SEM | p-valuec |
| Naive (no EAE) | 17.52 ± 0.79 | NS | 9.07 ± 2.08 | NS |
| PBS-treated EAE | 26.40 ± 5.64 | - | 13.22 ± 3.29 | - |
| OVA-pσ1- treated EAE |
27.86 ±8.61 |
NS |
14.15 ±5.90 |
NS |
| MOG-pσ1- treated EAE |
30.72 ± 4.95 |
NS |
18.40 ± 5.99 |
NS |
C57BL/6 mice were induced with EAE, and treated with PBS or 50 μg of OVA-pσ1 or MOG-pσ1 orally at the peak of EAE.
FACS analysis was performed on BTLA+ Tregs and CD25 CD4+ T cells isolated from the spleen. Mean ± SEM of 5-6 mice per group is presented.
Statistical significance was calculated by the one-way ANOVA to test differences among the treatment groups relative to PBS-treated EAE mice; NS = not significant.
Figure 6.
Bregs activate EAE Tregs to restore Treg function. (A, B) To test if Bregs can reactivate Tregs in vitro, (A) splenic Tregs obtained from naïve or EAE mice were stimulated as described in Fig. 1A, co-cultured with naïve CD4+ T cells in the absence or presence of CD19+ CD5+ Bregs or CD19+ CD5− B cells obtained from MOG-pσ1-treated EAE mice, and measured their 3H-thymidine incorporation 4 days later (Tregs/B cells/Teff cells ratio1:1:2). As a control, naïve CD4+ T cell proliferation in the presence of B220+ CD5+ cells (without Tregs) was also included. An average of 6 replicates/treatment group is depicted, and these data are representative of three experiments; **p ≤ 0.01 versus naïve T cell proliferation. (B) Tregs obtained from naïve or EAE mice were co-cultured with naïve CD4+ T cells in the presence or absence of B220+CD5+ Bregs (Tregs/Bregs/Teff cells ratio1:1:2), and the extent of CD4 T cell proliferation was measured 4 days later. Where indicated, B220+CD5+ Bregs were pretreated with an anti-BTLA mAb. An average of 6 replicates/treatment group is depicted, and these data are representative of two experiments; **p ≤ 0.01 versus naïve T cell proliferation.
To confirm the relevance of BTLA expression by Bregs, studies were performed to assess whether blocking BTLA on MOG-pσ1-induced Bregs impacts the function of EAE-derived Tregs. MOG-pσ1-induced Bregs were pretreated with an anti-BTLA mAb or its isotype control. Isotype control-treated MOG-pσ1-induced Bregs were able to restore Treg function as evidenced by suppression of CD4+ T cell proliferation (Fig. 6B). In contrast, MOG-pσ1-induced Bregs treated with an anti-BTLA mAb blocked Bregs from interacting with EAE Tregs and the Tregs remained dysfunctional. BTLA−/− Bregs were unable to activate EAE-derived Tregs, confirming the importance of BTLA expression on Bregs.
EAE exacerbation in BTLA−/− mice is the result of the loss of IL-10 induction by Tregs and Bregs
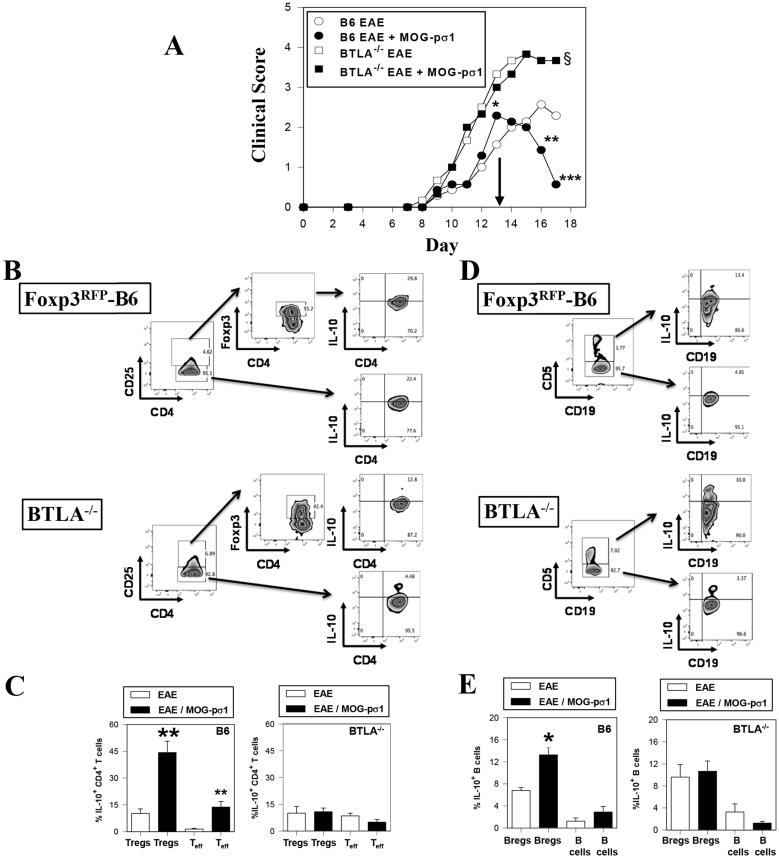
BTLA−/− mice induced with EAE exhibited significantly more severe clinical disease than did C57BL/6 mice (p = 0.027; Fig. 7A), similar to what others have shown (49). Moreover, these BTLA−/− mice were refractive to MOG-pσ1 treatment (Fig. 7A). The observed EAE exacerbation was not due to an intrinsic defect by naïve BTLA−/− Tregs since naïve BTLA−/− Tregs retained the ability to inhibit CD4+ T cell proliferation and were able to proliferate after MOG35-55 stimulation (Supplementary Fig. S1A-B). Further analysis of these BTLA−/− (Foxp3+ CD25+) Tregs from EAE mice showed a failure to produce IL-10 relative to MOG-pσ1-treated BTLA-sufficient mice (Fig. 7B, C). Likewise, there were fewer IL-10-producing Teff cells from BTLA−/− mice when compared to C57BL/6 mice. Analysis of BTLA−/− Bregs also showed their inability to upregulate IL-10 following MOG-pσ1 treatment (Fig. 7D, E). Thus, the absence of BTLA prevents the means to co-stimulate via BTLA, resulting in reduced IL-10 production by both Tregs and Bregs.
Figure 7.
A lack of IL-10 activation in BTLA−/− accounts for increased susceptibility to MOG-induced EAE. (A) Groups of C57BL/6 and BTLA−/− mice were induced with EAE on day 0, and were subsequently treated with PBS (B6 mice, n=7; BTLA−/− mice, n = 6) or MOG-pσ1 (B6 mice, n=7; BTLA−/− mice, n = 6) on day 13 (arrow). Clinical scores were measured throughout the disease; ***p < 0.001, **p < 0.005, *p = 0.036 versus PBS-treated B6 mice; §p < 0.030 for PBS- or MOG-pσ1-treated BTLA−/− mice versus PBS-treated B6 mice. (B) MOG-pσ1-treated BTLA−/− mice failed to show an induction of IL-10. (B-E) Additional groups of PBS- and MOG-pσ1-treated Foxp3RFP-B6 and BTLA−/− mice were treated as described in (A), and four days after treatment, isolated HNLNs were evaluated for IL-10 expression by (B and C) Tregs and Teff cells and (D and E) CD5+ Bregs and CD5− B cells. In BTLA-sufficient mice, MOG-pσ1 was able to significantly induce IL-10 by both (C) Tregs and (E) Bregs while no changes in IL-10 production by these same cells from BTLA−/− mice remained unchanged; **p = 0.003, *p ≤ 0.019 versus cells from EAE mice. Depicted data are representative of two experiments.
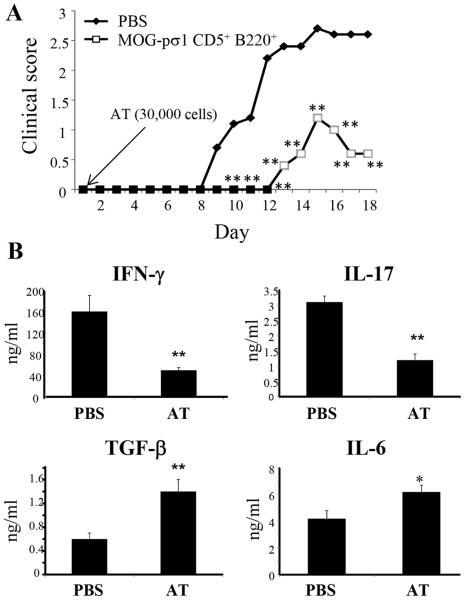
We next asked if MOG-pσ1-induced Bregs could restore Treg function in BTLA−/− mice. To further address Tregs’ dependency on BTLA+ Bregs, C57BL/6-derived BTLA+ Bregs were adoptively transferred into BTLA−/− mice at the time of EAE induction, and recipients were monitored for development of disease. Consistent with the concept of requiring BTLA+ Bregs to complete Treg activation, BTLA−/− recipients exhibited significantly less clinical disease (apparent by both delayed onset and reduced clinical score) than PBS-treated litter mates (**p ≤ 0.01, Fig. 8A). In fact, very few Bregs were required since as few as 3x104 Bregs could effectively dampen EAE.
Figure 8.
Adoptive transfer of Bregs restores protection to EAE in BTLA−/− mice. (A) BTLA+ CD19+CD5+ Bregs were purified from MOG-pσ1-treated C57BL/6 mice and 3x104 Bregs were adoptively transferred (AT) into BTLA−/− mice at the time of EAE induction; clinical scores were measured throughout the disease (5/group, **p ≤ 0.01). (B) Cytokine analyses were performed on purified splenic CD4+ T cells at the peak of the disease (day 18 p.ch.). Purified CD4+ T cells were cultured for 4 days in the presence of MOG35-55 (5 μg/ml) and irradiated APCs. Cytokine production showed significant elevations in TGF-β and IL-6 when compared to PBS mice with concomitant reductions in IFN-γ and IL-17 production (* p < 0.05, **p ≤ 0.01).
To determine which cytokines correlated with protection induced after Bregs’ adoptive transfer, cytokine analyses were performed on purified splenic CD4+ T cells isolated at the peak of the disease (day 18 p.ch.). Cytokine production was significantly elevated in TGF-β and IL-6 as compared to PBS-treated mice with reduced IFN-γ and IL-17 production (*p ≤ 0.05, **p ≤ 0.01; Fig. 8B). No differences in IL-10 production were observed. These results show that MOG-pσ1-induced BTLA+ Bregs have a critical role in maintaining Treg function, and the adoptive transfer of BTLA+ Bregs was sufficient to suppress inflammation and disease as evidenced by reduced clinical scores, and less IFN-γ and IL-17 being produced.
Discussion
MS, the primary cause of paralysis in young adults, is a neurodegenerative disease of the CNS that involves infiltration by activated inflammatory cells, damaging both myelin and axons (13). EAE, a rodent model for MS, recapitulates and provides experimental support for the basic disease mechanism in MS (50). The role of B cells in MS and EAE has generated increasing interest (12, 46, 51-55). Distinct B cell subtypes have recently been associated with EAE disease progression and regulation (6, 7, 16, 53, 56-59), and are at least partially dependent on the choice of antigen (protein or peptide) used to induce the disease (18). A role for B cells has also been suggested in different models of recombinant MOG (rMOG)-induced EAE (18, 60-62). In general, when rMOG was used to induce EAE, pathogenic B cells were induced, and their depletion resulted in less EAE severity (18, 60-62). However, even these studies varied in the type of rMOG used, e.g., human (18) versus mouse (60-62). Whether a greater mobilization of Bregs can be achieved using MOG-pσ1 in rMOG-induced EAE, remains to be determined.
Previous studies have shown that MOG35-55-induced EAE does stimulate anti-MOG35-55 Ab responses (23, 32, 63), but EAE can still be elicited in the absence of B cells (24, 50). Abs induced to different MOG peptides as a result of epitope spreading (64) or immunization with MOG protein do contribute to EAE pathogenicity (60-63, 65). Such evidence is consistent with the notion that Abs to the MOG protein are pathogenic to MS patients (66, 67). In contrast, a suppressive role for B cells has been suspected for many years (24, 68). The existence of a regulatory population of B cells has not yet been widely accepted, mainly because of the lack of a unique set of cell surface markers or a defined transcription factor. In mice, at least three different phenotypes have been associated with Bregs: O’Garra et al (68) first identified the CD5+ subpopulation among B cells responsible for IL-10 production; more recently, Bouaziz and colleagues have described CD1dHiCD5+ B cells, (generally referred as B10 cells) (69) while Evans et al attributed IL-10 production to CD21HiCD23+ cells (2B cells; (4). Recently, a role for GITR ligand-expressing (59), IL-35-producing (70), or PD-L1-expressing B cells (71) controlling autoimmunity independent of IL-10 has also been described, highlighting the complexity of the Breg-Treg interactions, where multiple mechanisms may be involved. Independent of the idea that Bregs constitute a true cell lineage or merely a state of activation by B cells, the production of IL-10 by a subset of B cells has proven critical for the resolution of EAE and other autoimmune disorders both in mice and humans (6, 10, 11, 21, 69). Bregs also have a role in the mechanism of action by Copaxone®, a glatiramer acetate copolymer, approved for the treatment of relapsing-remitting MS (5, 12, 25, 52, 56). In a recent clinical trial, Ireland et al showed that treatment of relapsing-remitting MS patients with glatiramer acetate restored IL-10 production while reducing lymphotoxin-α production by peripheral B cells, thus contributing to the therapeutic effects of glatiramer acetate (53). This finding further supports our contention that IL-10-producing Bregs contribute to suppression of autoimmunity.
Upon encountering Ag, lymphocyte signaling via T or B cell receptor is modulated by a cognate signal from a co-stimulatory or co-inhibitory molecule (72). One such B cell activation signal, CD40, has recently been found to be important for the generation of Bregs (73). Moreover, the role of HVEM and its ligands BTLA, LIGHT, and CD160 has generated interest (30, 35, 44, 74-77). HVEM is often referred to as a “molecular switch” of the immune system since it facilitates either a co-stimulatory or co-inhibitory signal. In addition, HVEM has bidirectional signaling capacity, transducing a co-inhibitory signal by the ligand (BTLA)-positive cells and simultaneously transducing a co-stimulatory signal for the HVEM (receptor)-positive cells (reviewed by Murphy et al., (35)).
BTLA is highly expressed by anergic cells (78), but for Tregs and Teff cells remain unchanged despite EAE disease or MOG-pσ1 treatment (Table I). In EAE mice, we demonstrate that both Breg and Treg function is absent. Regarding the absence of Breg function in EAE mice, purified Bregs isolated from PBS-treated EAE mice adoptively transferred into EAE mice failed to reduce clinical disease (Fig. 3A). In contrast, purified Bregs from MOG-pσ1-treated EAE mice adoptively transferred into EAE mice reduced disease (Fig. 3B). Such reduction in clinical disease was the result of Breg-induced augmentation Tregs noted by the increased number of Tregs and their activation status to protect against EAE. This combination reduced the presence of IL-17-producing encephalitogenic Teff cells (Fig. 4). Although BTLA levels on Bregs were not augmented as a consequence of MOG-pσ1treatment, the percentage of and total BTLA+ Bregs producing IL-10 were enhanced, further showing that MOG-pσ1 activates these Bregs in an Ag-specific fashion. OVA-pσ1’s inability to replicate MOG-pσ1’s action was evidenced by the failure to elevate the percentage of or total number of activated IL-10-producing Bregs. Increases in IL-10 production by MOG-pσ1-treated mice were not significantly increased for CD5− B cells. Aspects of Breg intervention were also recapitulated using a T suppressor assay in which MOG-pσ1-induced Bregs, but not CD5− B cells, reversed the loss of T cell suppressor activity. Furthermore, neutralization of BTLA interfered with the ability of the MOG-pσ1-induced Bregs to restore EAE Treg suppressor activity (Fig. 6). In addition, IL-10 production by BTLA−/− mice with EAE was substantially reduced by both Bregs and Tregs. These mice are refractive to MOG-pσ1 treatment of EAE, and clinical disease remained enhanced relative to BTLA-sufficient mice. Only upon adoptive transfer of BTLA-sufficient Bregs from MOG-pσ1-treated C57BL/6 mice did we observe reduction in EAE. Even though these donor cells were IL-10+, IL-10 production by BTLA−/− mice remained undetectable; however, TGF-β was induced by this treatment suggesting alternative mechanisms may have been activated. The universality of BTLA+ Bregs’ role to suppress EAE remains to be determined. It is important to bear in mind that EAE is a very heterogeneous disease in terms of its induction, clinical and pathological presentations, and mouse susceptibilities, which have raised some concerns about its relevance as a MS model (15, 79).
The results presented here begin to address the paradox of having large numbers of Tregs at sites of chronic inflammation, but still having no apparent effect in subduing this inflammation (42-45). We know that EAE Tregs become activated subsequent MOG-pσ1 treatment since tetramer-specific Foxp3+ Tregs were previously found to express CD69 (30). This stimulation reverses the Treg quiescent status, initiating a cascade of events that leads to the restoration of suppressor cell activity via the production of regulatory cytokines which abate clinical disease. Furthermore, the results from this present study show that Tregs purified from EAE mice remain inactive as evidenced by their inability to suppress polyclonally activated CD4+ T cell proliferation (Fig 1A). In contrast, Tregs from EAE mice treated with MOG-pσ1 showed restored suppressor activity prompting us to study their reactivation. Tregs purified from mice naturally recovering from the disease (5 or 6 weeks after EAE induction) also regained their capacity to inhibit CD4 T cell proliferation to a level similar to those Tregs derived from naïve mice. Future work will be needed to determine if the naturally recovered Tregs share similar suppressor mechanisms as those with the MOG-pσ1-induced Tregs.
Treg engagement of BTLA+ Bregs represents one mechanism for stimulating EAE Tregs. We are currently investigating whether this interaction is HVEM-dependent or whether other co-stimulatory molecules are involved. It has been shown that BTLA interacts in cis with HVEM on cells in which both BTLA and HVEM are expressed (35). The fact that BTLA acts cis with HVEM on Tregs offers one potential pathway by which BTLA+ Tregs are rendered quiescent resulting in the inability to control EAE. Tregs possibly become quiescent during EAE as a consequence of persistent co-inhibitory molecule signaling via BTLA on Tregs with concomitant down-regulation of activation or co-stimulatory molecules. The introduction of BTLA+ cells, much like the Bregs described here, could compete with Tregs’ own BTLA, and be rendered active resulting in suppressor function restoration. Defining which specific ligands are induced on Tregs during EAE, and how these are regulated by MOG-pσ1 is currently being investigated.
The role of B cells in MS and EAE has generated significant interest, especially since the discovery of IL-10-producing B cells (4, 21, 68, 69). Here we show that CD5+ Bregs dramatically restore Tregs’ suppressive properties while B220+CD5−, BTLA− B cells, or Bregs from diseased mice, do not. In fact, adoptive transfer of disease-induced Bregs into EAE mice failed to stimulate Treg production of regulatory cytokines. In contrast, the EAE recipients given MOG-pσ1-induced Bregs stimulated Tregs to generate regulatory cytokines. Furthermore, BTLA−/− mice developed a severe form of EAE (49), but adoptive transfer of wild-type (BTLA+) Bregs restored protection against EAE (Fig. 8). These BTLA-expressing Bregs interact with Tregs, providing an alternative (or synergistic) pathway for Treg rescue. Of note, the derived Bregs, especially those induced with MOG-pσ1, cannot substitute for Tregs since they are unable to directly suppress Teff cells. Rather, they are crucial modulators of Tregs’ activity. In contrast, a recent publication (80) reported that innate B regulatory progenitors (c-kitlowSca-1low CD127+B220+CD19+IgM−CD1dintCD43+) directly suppress CD4+ T cell proliferation. These data suggest a number of regulatory cell subsets, each dampening autoimmune disease.
In summary, these studies show a critical role for BTLA-dependent signaling in the activation of both Bregs and Tregs. The lack or failure of Treg activation has critical consequences for EAE induction, which in turn is important for amelioration of disease by MOG-pσ1. Although previous studies (81, 82) have shown that Bregs are able to selectively induce Foxp3+ Treg proliferation, our work provides critical insights to explain how Treg function can be restored following their quiescent status during autoimmune disease. Moreover, our hypothesis proposes a method to restore Treg function, namely with the addition of therapeutically induced Bregs. Since both regulatory subsets are necessary to reduce EAE, utilizing the HVEM-BTLA pathway has promise in the development of effective therapies for inflammatory diseases such as MS.
Supplementary Material
Acknowledgments
We are grateful to Ms. Jill Bobel for her technical assistance.
This work is supported by U.S. Public Health Service Grant R01 AI-078938.
Abbreviations used in this article
- Breg
regulatory B cell
- BTLA
B and T lymphocyte attenuator
- HNLNs
head and neck lymph nodes
- HVEM
Herpes virus entry mediator
- IKDCs
interferon-producing killer dendritic cells
- LIGHT
lymphotoxin-related inducible ligand that competes for glycoprotein D binding to herpesvirus entry mediator on T cells
- MLNs
mesenteric lymph nodes
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- PLNs
peripheral LNs
- p.ch.
post-challenge
- pσ1
protein sigma one
- SCs
spinal cords
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Edwards JC, G. Cambridge Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology (Oxford) 2001;40:205–211. doi: 10.1093/rheumatology/40.2.205. [DOI] [PubMed] [Google Scholar]
- 2.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, H. T. Group B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 4.Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868–7878. doi: 10.4049/jimmunol.178.12.7868. [DOI] [PubMed] [Google Scholar]
- 5.Kala M, Rhodes SN, Piao WH, Shi FD, Campagnolo DI, Vollmer TL. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp Neurol. 2010;221:136–145. doi: 10.1016/j.expneurol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ochoa-Repáraz J, Mielcarz DW, Haque-Begum S, Kasper LH. Induction of a regulatory B cell population in experimental allergic encephalomyelitis by alteration of the gut commensal microflora. Gut Microbes. 2010;1:103–108. doi: 10.4161/gmic.1.2.11515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun JB, Czerkinsky C, Holmgren J. B lymphocytes treated in vitro with antigen coupled to cholera toxin B subunit induce antigen-specific Foxp3+ regulatory T cells and protect against experimental autoimmune encephalomyelitis. J Immunol. 2012;188:1686–1697. doi: 10.4049/jimmunol.1101771. [DOI] [PubMed] [Google Scholar]
- 9.Bouaziz JD, Calbo S, Maho-Vaillant M, Saussine A, Bagot M, Bensussan A, Musette P. IL-10 produced by activated human B cells regulates CD4+ T-cell activation in vitro. Eur J Immunol. 2010;40:2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- 10.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 11.Flores-Borja F, Bosma A, Ng D, Reddy V, Ehrenstein MR, Isenberg DA, Mauri C. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Sci Transl Med. 2013;5:173ra123. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- 12.Ireland SJ, Blazek M, Harp CT, Greenberg B, Frohman EM, Davis LS, Monson NL. Antibody-independent B cell effector functions in relapsing remitting multiple sclerosis: clues to increased inflammatory and reduced regulatory B cell capacity. Autoimmunity. 2012;45:400–414. doi: 10.3109/08916934.2012.665529. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 14.Codarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. RORgammat drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 15.Batoulis H, Addicks K, Kuerten S. Emerging concepts in autoimmune encephalomyelitis beyond the CD4/TH 1 paradigm. Ann Anat. 2010;192:179–193. doi: 10.1016/j.aanat.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Gray D, Gray M. What are regulatory B cells? Eur J Immunol. 2010;40:2677–2679. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- 17.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 18.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 22.Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ T regulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- 23.Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Begum-Haque S, Sharma A, Christy M, Lentini T, Ochoa-Repáraz J, Fayed IF, Mielcarz D, Haque A, Kasper LH. Increased expression of B cell-associated regulatory cytokines by glatiramer acetate in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;219:47–53. doi: 10.1016/j.jneuroim.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 27.Canonica GW, Passalacqua G. Sublingual immunotherapy in the treatment of adult allergic rhinitis patients. Allergy. 2006;61(Suppl 81):20–23. doi: 10.1111/j.1398-9995.2006.01161.x. [DOI] [PubMed] [Google Scholar]
- 28.Didier A, Malling HJ, Worm M, Horak F, Jäger S, Montagut A, André C, de Beaumont O, Melac M. Optimal dose, efficacy, and safety of once-daily sublingual immunotherapy with a 5-grass pollen tablet for seasonal allergic rhinitis. J Allergy Clin Immunol. 2007;120:1338–1345. doi: 10.1016/j.jaci.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 29.Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, Colman PG, Harrison LC. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60:1237–1245. doi: 10.2337/db10-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huarte E, Rynda-Apple A, Riccardi C, Skyberg JA, Golden S, Rollins MF, Ramstead AG, Jackiw LO, Maddaloni M, Pascual DW. Tolerogen-induced interferon-producing killer dendritic cells (IKDCs) protect against EAE. J Autoimmun. 2011;37:328–341. doi: 10.1016/j.jaut.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rynda A, Maddaloni M, Mierzejewska D, Ochoa-Repáraz J, Maslanka T, Crist K, Riccardi C, Barszczewska B, Fujihashi K, McGhee JR, Pascual DW. Low-dose tolerance is mediated by the microfold cell ligand, reovirus protein sigma1. J Immunol. 2008;180:5187–5200. doi: 10.4049/jimmunol.180.8.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rynda-Apple A, Huarte E, Maddaloni M, Callis G, Skyberg JA, Pascual DW. Active immunization using a single dose immunotherapeutic abates established EAE via IL-10 and regulatory T cells. Eur J Immunol. 2011;41:313–323. doi: 10.1002/eji.201041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rynda A, Maddaloni M, Ochoa-Reparaz J, Callis G, Pascual DW. IL-28 supplants requirement for Treg cells in protein sigma1-mediated protection against murine experimental autoimmune encephalomyelitis (EAE) PLoS One. 2010;5:e8720. doi: 10.1371/journal.pone.0008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87:223–235. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 35.Murphy TL, Murphy KM. Slow down and survive: Enigmatic immunoregulation by BTLA and HVEM. Annu Rev Immunol. 2010;28:389–411. doi: 10.1146/annurev-immunol-030409-101202. [DOI] [PubMed] [Google Scholar]
- 36.Cheung TC, Steinberg MW, Oborne LM, Macauley MG, Fukuyama S, Sanjo H, D'Souza C, Norris PS, Pfeffer K, Murphy KM, Kronenberg M, Spear PG, Ware CF. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc Natl Acad Sci U S A. 2009;106:6244–6249. doi: 10.1073/pnas.0902115106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauri DN, Ebner R, Montgomery RI, Kochel KD, Cheung TC, Yu GL, Ruben S, Murphy M, Eisenberg RJ, Cohen GH, Spear PG, Ware CF. LIGHT, a new member of the TNF superfamily, and lymphotoxin α are ligands for herpesvirus entry mediator. Immunity. 1998;8:21–30. doi: 10.1016/s1074-7613(00)80455-0. [DOI] [PubMed] [Google Scholar]
- 38.Shaikh RB, Santee S, Granger SW, Butrovich K, Cheung T, Kronenberg M, Cheroutre H, Ware CF. Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol. 2001;167:6330–6337. doi: 10.4049/jimmunol.167.11.6330. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Lo JC, Foster A, Yu P, Chen HM, Wang Y, Tamada K, Chen L, Fu YX. The regulation of T cell homeostasis and autoimmunity by T cell-derived LIGHT. J Clin Invest. 2001;108:1771–1780. doi: 10.1172/JCI13827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truong W, Hancock WW, Plester JC, Merani S, Rayner DC, Thangavelu G, Murphy KM, Anderson CC, Shapiro AM. BTLA targeting modulates lymphocyte phenotype, function, and numbers and attenuates disease in nonobese diabetic mice. J Leukoc Biol. 2009;86:41–51. doi: 10.1189/jlb.1107753. [DOI] [PubMed] [Google Scholar]
- 42.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B, Chin YE, Zhang JZ. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19:322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Zhu M, Yu P, Fu YX. Promoting immune responses by LIGHT in the face of abundant regulatory T cell inhibition. J Immunol. 2010;184:1589–1595. doi: 10.4049/jimmunol.0901582. [DOI] [PubMed] [Google Scholar]
- 45.Zhao J, Zhao J, Fett C, Trandem K, Fleming E, Perlman S. IFN-γ and IL-10-expressing virus epitope-specific Foxp3+ T reg cells in the central nervous system during encephalomyelitis. J Exp Med. 2011;208:1571–1577. doi: 10.1084/jem.20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ray A, Mann MK, Basu S, Dittel BN. A case for regulatory B cells in controlling the severity of autoimmune-mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Neuroimmunol. 2011;230:1–9. doi: 10.1016/j.jneuroim.2010.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Boussiotis VA. The role of IL-17-producing Foxp3+ CD4+ T cells in inflammatory bowel disease and colon cancer. Clin Immunol. 2013;148:246–253. doi: 10.1016/j.clim.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watanabe N, Gavrieli M, Sedy JR, Yang J, Fallarino F, Loftin SK, Hurchla MA, Zimmerman N, Sim J, Zang X, Murphy TL, Russell JH, Allison JP, Murphy KM. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 50.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 51.Ray A, Wang L, Dittel BN. IL-10-independent regulatory B-cell subsets and mechanisms of action. Int Immunol. 2015 doi: 10.1093/intimm/dxv033. [DOI] [PubMed] [Google Scholar]
- 52.Van Kaer L. Glatiramer acetate for treatment of MS: regulatory B cells join the cast of players. Exp Neurol. 2011;227:19–23. doi: 10.1016/j.expneurol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ireland SJ, Guzman AA, O'Brien DE, Hughes S, Greenberg B, Flores A, Graves D, Remington G, Frohman EM, Davis LS, Monson NL. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA Neurol. 2014;71:1421–1428. doi: 10.1001/jamaneurol.2014.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 55.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O'Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Begum-Haque S, Christy M, Ochoa-Repáraz J, Nowak EC, Mielcarz D, Haque A, Kasper LH. Augmentation of regulatory B cell activity in experimental allergic encephalomyelitis by glatiramer acetate. J Neuroimmunol. 2011;232:136–144. doi: 10.1016/j.jneuroim.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calderón-Gómez E, Lampropoulou V, Shen P, Neves P, Roch T, Stervbo U, Rutz S, Kuhl AA, Heppner FL, Loddenkemper C, Anderton SM, Kanellopoulos JM, Charneau P, Fillatreau S. Reprogrammed quiescent B cells provide an effective cellular therapy against chronic experimental autoimmune encephalomyelitis. Eur J Immunol. 2011;41:1696–1708. doi: 10.1002/eji.201041041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rafei M, Hsieh J, Zehntner S, Li M, Forner K, Birman E, Boivin MN, Young YK, Perreault C, Galipeau J. A granulocyte-macrophage colony-stimulating factor and interleukin-15 fusokine induces a regulatory B cell population with immune suppressive properties. Nat Med. 2009;15:1038–1045. doi: 10.1038/nm.2003. [DOI] [PubMed] [Google Scholar]
- 59.Ray A, Basu S, Williams CB, Salzman NH, Dittel BN. A novel IL-10-independent regulatory role for B cells in suppressing autoimmunity by maintenance of regulatory T cells via GITR ligand. J Immunol. 2012;188:3188–3198. doi: 10.4049/jimmunol.1103354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dang AK, Jain RW, Craig HC, Kerfoot SM. B cell recognition of myelin oligodendrocyte glycoprotein autoantigen depends on immunization with protein rather than short peptide, while B cell invasion of the CNS in autoimmunity does not. J Neuroimmunol. 2015;278:73–84. doi: 10.1016/j.jneuroim.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Litwak SA, Payne NL, Campanale N, Ozturk E, Lee JY, Petratos S, Siatskas C, Bakhuraysah M, Bernard CC. Nogo-receptor 1 deficiency has no influence on immune cell repertoire or function during experimental autoimmune encephalomyelitis. PLoS One. 2013;8:e82101. doi: 10.1371/journal.pone.0082101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weber MS, Prod'homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, Bernard CC, Martin F, Zamvil SS. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lalive PH, Molnarfi N, Benkhoucha M, Weber MS, Santiago-Raber ML. Antibody response in MOG35-55 induced EAE. J Neuroimmunol. 2011;240-241:28–33. doi: 10.1016/j.jneuroim.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 64.Zhang GX, Yu S, Gran B, Li J, Calida D, Ventura E, Chen X, Rostami A. T cell and antibody responses in remitting-relapsing experimental autoimmune encephalomyelitis in (C57BL/6 x SJL) F1 mice. J Neuroimmunol. 2004;148:1–10. doi: 10.1016/j.jneuroim.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 65.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 66.Lalive PH, Hausler MG, Maurey H, Mikaeloff Y, Tardieu M, Wiendl H, Schroeter M, Hartung HP, Kieseier BC, Menge T. Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult Scler. 2011;17:297–302. doi: 10.1177/1352458510389220. [DOI] [PubMed] [Google Scholar]
- 67.Lalive PH, Menge T, Delarasse C, Della Gaspera B, Pham-Dinh D, Villoslada P, von Budingen HC, Genain CP. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:2280–2285. doi: 10.1073/pnas.0510672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O'Garra A, Howard M. Cytokines and Ly-1 (B1) B cells. Int Rev Immunol. 1992;8:219–234. doi: 10.3109/08830189209055575. [DOI] [PubMed] [Google Scholar]
- 69.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 70.Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E, Ries S, Dang VD, Jaimes Y, Daridon C, Li R, Jouneau L, Boudinot P, Wilantri S, Sakwa I, Miyazaki Y, Leech MD, McPherson RC, Wirtz S, Neurath M, Hoehlig K, Meinl E, Grutzkau A, Grun JR, Horn K, Kuhl AA, Dorner T, Bar-Or A, Kaufmann SH, Anderton SM, Fillatreau S. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan AR, Hams E, Floudas A, Sparwasser T, Weaver CT, Fallon PG. PD-L1hi B cells are critical regulators of humoral immunity. Nat Commun. 2015;6:5997. doi: 10.1038/ncomms6997. [DOI] [PubMed] [Google Scholar]
- 72.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 73.Kleffel S, Vergani A, Tezza S, Ben Nasr M, Niewczas MA, Wong S, Bassi R, D'Addio F, Schatton T, Abdi R, Atkinson M, Sayegh MH, Wen L, Wasserfall CH, O'Connor KC, Fiorina P. Interleukin-10+ regulatory B cells arise within antigen-experienced CD40+ B cells to maintain tolerance to islet autoantigens. Diabetes. 2015;64:158–171. doi: 10.2337/db13-1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Albring JC, Sandau MM, Rapaport AS, Edelson BT, Satpathy A, Mashayekhi M, Lathrop SK, Hsieh CS, Stelljes M, Colonna M, Murphy TL, Murphy KM. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. J Exp Med. 2010;207:2551–2559. doi: 10.1084/jem.20102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 76.Cai G, Freeman GJ. The CD160, BTLA, LIGHT/HVEM pathway: a bidirectional switch regulating T-cell activation. Immunol Rev. 2009;229:244–258. doi: 10.1111/j.1600-065X.2009.00783.x. [DOI] [PubMed] [Google Scholar]
- 77.Sakoda Y, Park JJ, Zhao Y, Kuramasu A, Geng D, Liu Y, Davila E, Tamada K. Dichotomous regulation of GVHD through bidirectional functions of the BTLA-HVEM pathway. Blood. 2011;117:2506–2514. doi: 10.1182/blood-2010-08-301325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hurchla MA, Sedy JR, Gavrieli M, Drake CG, Murphy TL, Murphy KM. B and T lymphocyte attenuator exhibits structural and expression polymorphisms and is highly Induced in anergic CD4+ T cells. J Immunol. 2005;174:3377–3385. doi: 10.4049/jimmunol.174.6.3377. [DOI] [PubMed] [Google Scholar]
- 79.Constantinescu CS, Farooqi N, O'Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br J Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Montandon R, Korniotis S, Layseca-Espinosa E, Gras C, Megret J, Ezine S, Dy M, Zavala F. Innate pro-B-cell progenitors protect against type 1 diabetes by regulating autoimmune effector T cells. Proc Natl Acad Sci U S A. 2013;110:E2199–2208. doi: 10.1073/pnas.1222446110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen X, Jensen PE. Cutting edge: primary B lymphocytes preferentially expand allogeneic FoxP3+ CD4 T cells. J Immunol. 2007;179:2046–2050. doi: 10.4049/jimmunol.179.4.2046. [DOI] [PubMed] [Google Scholar]
- 82.Reichardt P, Dornbach B, Rong S, Beissert S, Gueler F, Loser K, Gunzer M. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.