Abstract
T cells from patients with systemic lupus erythematosus (SLE) display a number of functions including increased early signaling events following engagement of the T cell receptor (TCR). Signaling lymphocytic activation molecule family (SLAMF) cell surface receptors and the X-chromosome-defined signaling lymphocytic activation molecule-associated protein (SAP) adaptor are important in the development of several immunocyte lineages and modulating immune response. Here we present evidence that SAP protein levels are decreased in T cells and in their main subsets isolated from 32 women and 3 men with SLE independently of disease activity. In SLE T cells the SAP protein is also subject to increased degradation by a caspase-3. Forced expression of SAP in SLE T cells simultaneously heightened IL-2 production, calcium (Ca2+) responses and tyrosine phosphorylation of a number of proteins. Exposure of normal T cells to SLE serum IgG, known to contain anti-CD3/TCR antibodies, resulted in SAP downregulation. We conclude that SLE T cells display reduced levels of the adaptor protein SAP probably as a result of continuous T cell activation and degradation by caspase-3. Restoration of SAP levels in SLE T cells corrects the overexcitable lupus T cell phenotype.
Introduction
Systemic lupus erythematosus (SLE) is a chronic multisystem autoimmune disease of unknown etiology that mainly affects women of reproductive age. Clinical symptoms may vary from relatively mild to severe life-threatening manifestations involving vital organs including the kidneys, lungs and the central nervous system (CNS) (1). Multiple cellular and molecular aberrations have been claimed to be associated to the immunopathogenesis of the disease (2). A number of loci across the whole genome and especially the long arm of chromosome 1, where also the genes for the signaling lymphocytic activation molecule family (SLAMF) of cell surface receptors reside, have been identified [reviewed in (3) and (4)]. Associations between SLAMF variants and autoimmune diseases, including SLE (5) (6) and rheumatoid arthritis (7), have been described. Furthermore, SLAMF variants have been linked to specific disease manifestations such as neuropsychiatric lupus (8) or response to TNF blockade in patients with rheumatoid arthritis (9).
The expression of SLAMF7 on B cells and of SLAMF4 on NK and CD8+ T cells is altered in patients with SLE (10) (11) and SLAMF3 and SLAMF6 are also reportedly increased on the cell surface of SLE CD4+ T cells (12) (13). The importance of the SLAMF cell surface receptors in autoimmunity is further underscored by the finding that the lupus prone congenic mouse strains B6.Sle1b [Sle1b] and B6.129chr1b [129chr1b] develop autoantibodies linked to polymorphisms of SLAMF1-7 molecules (14) (15). SLAMF3-deficient mice (129xBALB/c) spontaneously develop autoimmune characteristics including autoantibodies against nuclear antigens, aberrant cytokine production and splenomegaly (16) and SLAMF1, 5 and 6 serve as negative regulators of humoral immune response (17).
One of the characteristic features of the SLAMF members (with the exception of SLAMF2 and SLAMF8–9) is the presence of one or more immunoreceptor tyrosine switch motifs (ITSM), which upon SLAMF engagement, interact with high affinity with the signaling lymphocytic activation molecule-associated protein (SAP, SH2D1A). SAP is a highly conserved, non-polymorphic cytoplasmic protein, predominantly expressed in T cells, NK, NKT cells, eosinophils and platelets. SAP has been shown to be requisite for germinal center formation and hence for both normal humoral responses and autoantibody production (18) (19) (20) (21). Although SAP is considered to act as a natural competitor of SH2-containing phosphatases such as SHP-1 and SHP-2 for binding to the same ITSM motifs (22), subsequent work revealed that it interacts with Fyn (23) (24), possibly with Lck (25), b-PIX (26) and NCK (27) and recruits PKCθ to the immune synapse (28).
Non-transformed T cell lines from SAP-deficient male subjects display an interesting dichotomous CD3/TCR response with elevated [Ca2+]i response and decreased production of IL-2 both of which were corrected following replenishment of SAP (29). This pattern of response was reminiscent of that observed in SLE T cells (30) (31) and prompted us to ask whether SAP expression was altered in SLE T cells.
We show that the expression levels of SAP in T cells from patients with active or inactive SLE are decreased. Following forced expression of SAP both [Ca2+]i response and IL-2 production return to normal. Caspase-3 appears to degrade SAP in SLE T cells. We also show that SLE-derived IgG reduces the levels of SAP in normal T cells. Although the reduction of SAP in the mostly (~90%) female SLE patients appears to be a secondary defect due to continuous T cell activation, restoration of SAP levels by limiting its degradation may warrant clinical attention.
Patients and methods
Patients and controls
Patients (n=35) [32 females and 3 males] fulfilling the American College of Rheumatology criteria for lupus were recruited at the Rheumatology Department at Beth Israel Deaconess Medical Center for the study. 29 age- and sex-matched healthy volunteers were evaluated in parallel. Disease activity score for the patients with SLE was measured using the SLEDAI scoring system. SLEDAI scores ranged between 0 and 16. Demographic and clinical information on the patients with SLE that participated in the study is provided on Supplementary Table S1. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Reagents and antibodies
Murine anti-CD3 clone OKT3 was used for T cell stimulation and was purchased from BioXcell. Affinity purified goat anti-mouse IgG was from Jackson Immunoresearch Laboratories Inc. Antibodies against SAP (clone 1D12), β-actin, as well as goat anti-rabbit, goat anti-mouse and donkey anti-goat horseradish-peroxidase (HPR)-conjugated secondary antibodies were all purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). The anti-phosphotyrosine HRP-conjugated monoclonal antibody (mAb) (clone 4G10) was from Millipore (Temecula, CA). Cycloheximide (CHX) and actinomycin D were purchased from Sigma Aldrich. Pan-caspase inhibitor Z-Val-Ala-Asp-FMK (VAD), caspase-3 inhibitor Z-Asp-Glu-Val-Asp (VEVD) and MG132 proteasome inhibitor were from Enzo LifeSciences (Farmingdale, NY).
T cell purification
Heparinized venous blood was obtained from the study subjects and primary T cells were isolated by negative selection (RosetteSep, Stem Cell Technologies,Vancouver, Canada) according to the manufacturer’s instructions. The percentage of purified T cells was assessed by flow cytometry and was always over 95%. For some experiments total and naïve CD4+ T cells from normal donors and patients with SLE were further purified from total T cells by negative selection using the CD4+ T cell Isolation Kit and the naïve CD4+ T cell Isolation Kit II (both purchased from Miltenyi) according to the manufacturer’s instructions. The positive fractions representing CD8+ T cells and memory/effector CD4+ T cells respectively were also collected. Cells were either directly lysed in order to extract protein and total RNA or were kept overnight at 37°C in RPMI-1640 medium supplemented with 10% FBS, 100mg/ml streptomycin and 100U/ml penicillin in order to be used for experiments.
Serum samples were also collected and stored at −80 °C from all participants. Sera were heat-inactivated at 56 °C for 30 min and centrifuged before added to cell cultures.
Cell cultures
T cell cultures were performed at 2×106 cells/ml density in 24-well plates in complete RPMI-1640 medium. For protein degradation experiments total T cells were cultured in complete medium supplemented with cycloheximide (CHX) at a final concentration 50µg/ml. Cells were collected at 0, 3, 4 and 5 hours after addition of CHX in T cell culture. Cell lysates were extracted and analyzed with Western immunoblot. For certain experiments T cells were pretreated with proteasome inhibitor MG132 (final concentration 20µM) for 1h before the addition of CHX in the culture medium. For pan-caspase and caspase-3 inhibition experiments, T cells were cultured in complete medium with 50µM VAD, 50µM VEVD or equal volume of vehicle DMSO in the presence of 50µg/ml CHX. In some experiments sera from patients with SLE or from normal donors were added to cell cultures at 1% concentration for 6h. In other experiments sera were fractionated using the ProteoExtract Albumin/IgG removal kit (Calbiochem) according to the manufacturer’s instructions and normal T cells were cultured with 1µg/ml of each fraction in complete medium. Purity of each fraction was always assessed by SDS electrophoresis followed by Coomassie staining.
Transfections
Transient transfections of purified total T cells were performed using the Amaxa Human T cell nucleofector system (Lonza, Cologne, Germany). Briefly, 5–10×106 total T cells were rested overnight in complete RMPI 1640 medium and treated with 1µg/ml PHA at a density of 1×106/ml at 37 °C, in 5%CO2. Small interfering silencer RNAs against SAP (siSAP) and non-specific control siRNAs (sicontrol) were purchased from Life Technologies. Plasmid DNA encoding SAP (plSH2D1A) and the control pCMV-XL5 vector (pcDNA) were both from Origene. T cells were resuspended in 100µl of nucleofector solution plus 5µg of plasmid (plSH2D1A or pcDNA) or with various concentrations of siRNA (siSAP or sicontrol) and were transfected. 24h after transfection cells were stimulated with 10µg/ml of soluble anti-CD3 antibody in the presence of 20µg/ml goat anti-mouse mAb for various time points. In every case, transfection efficiency was evaluated by western immunoblots and/or real-time PCR analysis.
Reverse transcription and real-time PCR
Total RNA was extracted from 3–5×106 cells using an RNeasy Mini Kit according to the manufacturer’s instructions (Qiagen, Santa Clarita, CA). Single stranded complementary DNA (cDNA) was synthetized from 200ng of total RNA by using the RNA into cDNA premix (Clontech). Real time amplification was performed using SYBR Green (LightCycler 480 SYBR Green I Master, Roche) with 40 cycles at 95°C for 15sec and 60°C for 1min. All PCR reactions were performed in triplicates. Primer sequences were as follows: IL-2, forward,5´-CAAGAAGGCCACAGAACTGA-3´ and reverse 5’- TGGTTGCTGTCTCATCAGCAT-3´; IFNγ, forward 5´- CTGTTACTGCCAGGACCCAT-3´ and reverse 5’- TCTGTCACTCTCCTCTTTCCA-3´; SAP, forward 5´- GTTCTTGGAGTGCTGAGACAG-3´and reverse 5´- GGCAGACATCAGGATCTTCTCTT-3´; β-actin, forward 5´- AGAGCTACGAGCTGCCTGAC-3´ and reverse 5´- AGCACTGTGTTGGCGTACAG-3´, cyclophilin A forward 5´-TTCATCTGCACTGCCAAGAC-3´ and reverse 5´-TCGAGTTGTCCACAGTCAGC-3´.
Protein extraction and immunoblotting
2×106 T cells were incubated for 45min in ice-cold lysis buffer (10mM Tris, 50mM NaCl, 5mM EDTA and 1% Triton X-100) containing various protease and phosphatase inhibitors (50mM NaF, 1mM Na3VO4, 30mM sodium pyrophosphate, 1mM PMSF, 2µg/ml leupeptin, 2µg/ml aprotinin). Lysates were then centrifuged at 12.000 g for 15min at 4°C to remove insoluble material. Protein concentration was determined using the Coomassie protein assay reagent (Sigma Aldrich). Proteins (20µg per lane) were separated in 4 to12% gradient (wt/vol) Bis-Tris gels (Life Technologies) and transferred to nitrocellulose (Life Technologies) or PVDF (Millipore) membrane. Membranes were blocked for 1h with Tris-buffered saline solution containing 0.05% Tween (TBS-T) and 5% non-fat dry milk at room temperature. Following blocking, membranes were incubated overnight at 4°C with the indicated antibody in blocking solution on a shaking surface and were then washed and incubated with the appropriate secondary HRP-conjugated antibody for 1.5h at room temperature. Detection was performed with the Clarity ECL Western Blotting detection reagents (Biorad) and membranes were visualized by the ChemiDoc XRS+ Molecular Imager (Biorad). Densitometric analysis was performed using the ImageJ software and results are expressed as densitometric ratios of tyrosine phosphorylated proteins or SAP over β-actin ± SEM.
Calcium responses
Five million transfected T cells were stained for CD4 (FITC) and CD8 (PercP), resuspended in RPMI 1640 supplemented with 1% FBS and were loaded with Indo-1 acetoxymethylester (Life Technologies) for 30min at 37°C. Cells were then washed three times with RPMI 1640 at 1500 rpm for 5min and were finally resuspended in RPMI 1640 supplemented with 1% FBS and 1mM CaCl. Data were acquired on an LSR II flow cytometer (BD Biosciences) and were analyzed using FlowJo version 7.6.5 (Tree Star). Samples were allowed to run unstimulated for 60 sec in order to record a baseline fluorescence ratio representing the concentration of intracellular calcium at the resting state. Cells were then stimulated with 10µg/ml anti-CD3 followed by 20µg/ml goat anti-mouse IgG that were added to the tube and the mean fluorescence ratio of violet to blue Indo-1 fluorescence, that is proportionally representative of the free intracytoplasmic calcium concentration, was recorded for a period of 10min. Stimulation with ionomycin (25µg/ml) was used as a positive control at 7 or 9min.
Quantitative determination of IL-2 and IFNγ
Cell culture supernatants were collected and kept at −80°C until ready to be analyzed. IL-2 and ΙFNγ productions were measured by ELISA kits (Elisa Deluxe Max Set, Biolegend). The ODs of the wells were determined using a microplate reader set at 450nm, according to the manufacturer’s instructions.
Statistics
The two-tailed unpaired or paired Student’s t-test, one-way ANOVA and two-way ANOVA were used for data analysis, as appropriate (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.005). Data are presented as mean ± SEM.
Results
Silencing of SAP in healthy human T cells results in increased early signaling events and aberrant cytokine production
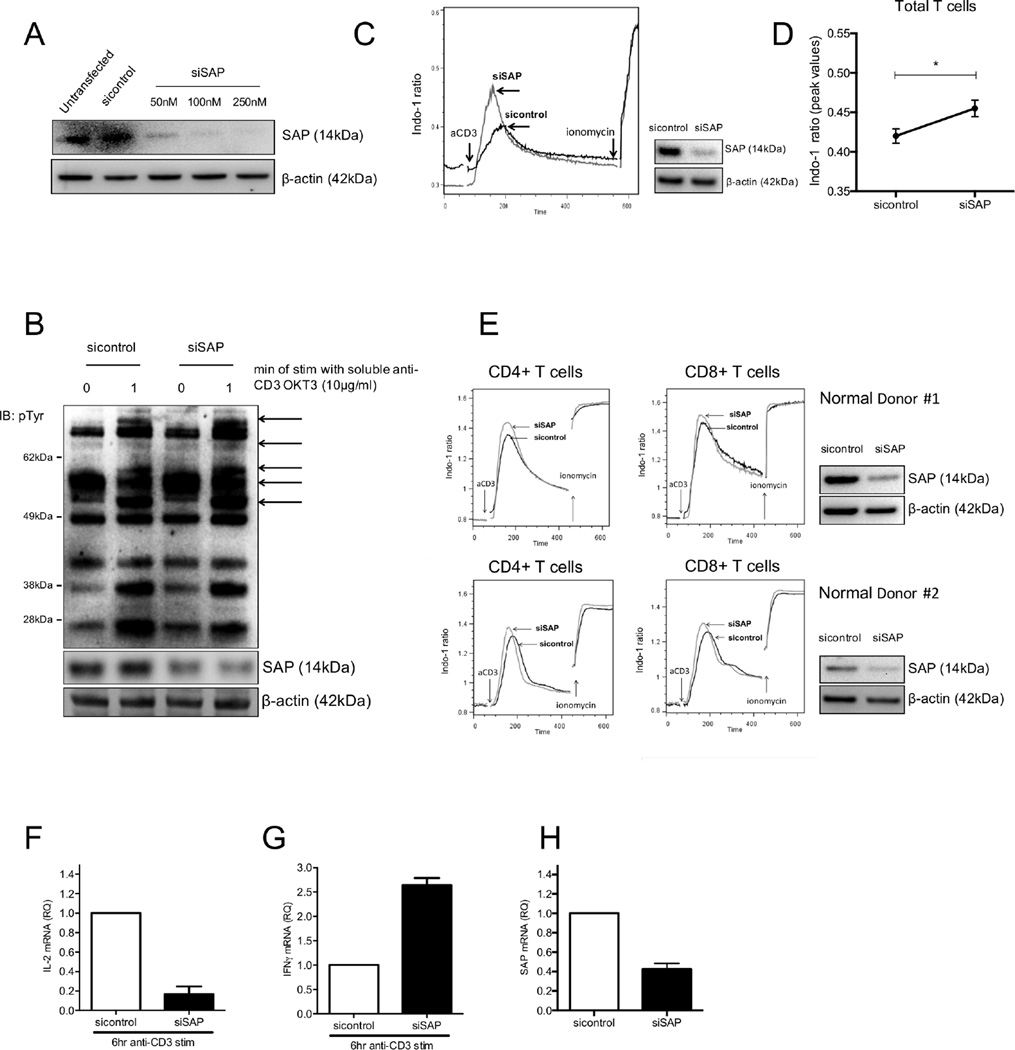
Non-transformed CD4 T cell lines established from SAP-deficient patients display increased free intracytoplasmic calcium responses following anti-CD3 stimulation but decreased IL-2 production (29). We were able to reproduce this functional phenotype in healthy human T cells (n=4) in which SAP was successfully silenced (Fig 1A). Levels of tyrosine phosphorylated proteins after 1 min of TCR-mediated stimulation were higher in SAP-depleted cells compared to T cells transfected with control siRNA (Fig 1B). Furthermore, TCR-triggered free intracytoplasmic calcium responses were higher in total T cells transfected with SAP siRNA (Fig 1C and 1D). This difference was detected in both CD4+ and CD8+ T cell subsets (Fig 1E). Stimulation of normal T cells, in which SAP had been successfully silenced, with an anti-CD3 mAb SAP resulted in significant decrease of IL-2 mRNA expression production and a 2.5 fold increase in IFNγ mRNA levels (Fig. 1F, G and H).
Figure 1. Enhanced TCR-mediated early signal transduction events and aberrant cytokine production in SAP-depleted T cells from healthy individuals.
(A) Total T cells from healthy individuals were transfected with various concentrations of small interfering RNA against SAP (siSAP) or control siRNA (sicontrol). Efficacy of transfection was determined by SAP protein levels by Western blotting. (B) Total T cells (n=4) transfected with siSAP or sicontrol were stimulated 24hr post-transfection with anti-CD3 mAb followed by cross-linking for 60sec and levels of pTyr were evaluated by Western blotting. (C) Increased TCR-triggered free intracytoplasmic calcium flux in normal total T cells (n=3). Cumulative results for total T cells shown in (D) and are expressed as the mean peak value of the Indo-1(violet) to Indo-1 (blue) ratio ± SEM. (E) Representative experiments of increased TCR-initiated free cytoplasmic calcium flux in CD4+ and CD8+ T cell subsets following silencing of SAP (n=3). Impaired IL-2 production (F) and elevated IFNγ production (G) after 6 hrs of anti-CD3 stimulation in normal T cells following knockdown of SAP. Efficiency of transfection, measured by SAP mRNA levels, is shown in (H).
Expression of SAP is reduced in peripheral blood T cells from patients with SLE
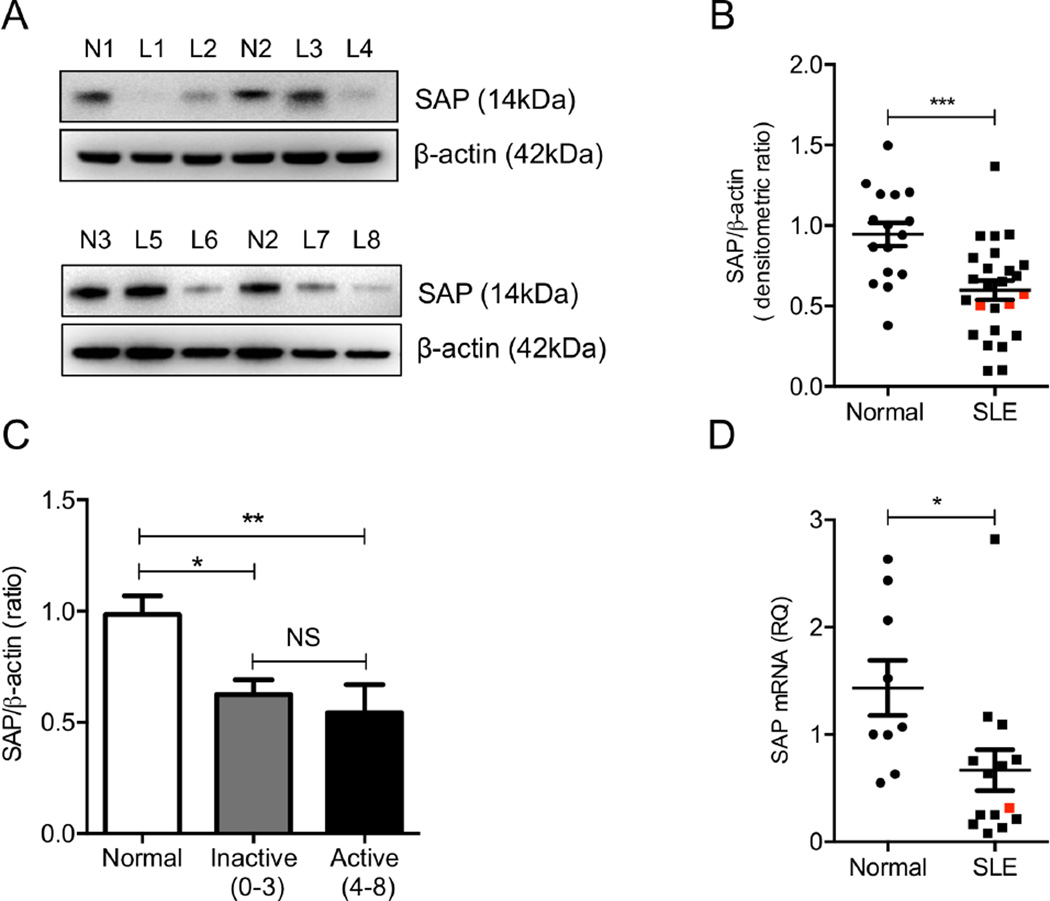
Because SAP-deficient T cells and SAP-depleted primary human T cells (Fig 1) display increased early signaling events followed by decreased IL-2 production, we asked whether SAP levels are decreased in patients with SLE. First we determined the protein levels of SAP in SLE (n=25) and normal (n=16) T cell lysates by Western blotting. SAP protein levels were significantly decreased in patients with SLE compared to normal donors (Fig 2A and B). SAP levels were decreased in both patients with active (SLEDAI, 4–8, n= 9) and inactive disease (SLEDAI, 0–3, n= 16) (Fig 2C). In the relatively small study sample we were not able to identify an association between reduced T cell SAP content and specific SLE clinical manifestations and/or laboratory findings. SAP mRNA levels were also measured in patients with SLE and normal donors with real-time PCR. Consistent with the decrease in protein levels, we observed a 2.1-fold reduction in SAP mRNA levels in SLE T cells, suggesting a potential transcriptional and/or post-transcriptional defect that may lead to suppressed expression of SAP in SLE (Fig 2D). Three men with SLE were included in the protein (Fig. 2B) and one in mRNA (Fig. 2D) expression analysis and although all measurements were below the recorded mean values they were not the lowest among the SLE samples.
Figure 2. Levels of SAP in T cells from patients with SLE and from healthy individuals.
Protein levels of SAP were assessed in whole-cell lysates of T cells obtained from normal donors (n=16) and patients with SLE (n=25) by Western blotting. Two individual representative experiments are shown in (A) and cumulative results, expressed as the mean densitometric ratio of SAP over β-actin ± SEM, are depicted in (B). (C) SAP protein levels are found to be decreased in SLE patients with both inactive and active disease. SAP gene expression was evaluated with qPCR in 9 healthy donors and 14 patients with SLE and results are shown in (D). Red points signify men with SLE. N=Normal Donors and L= SLE patients.
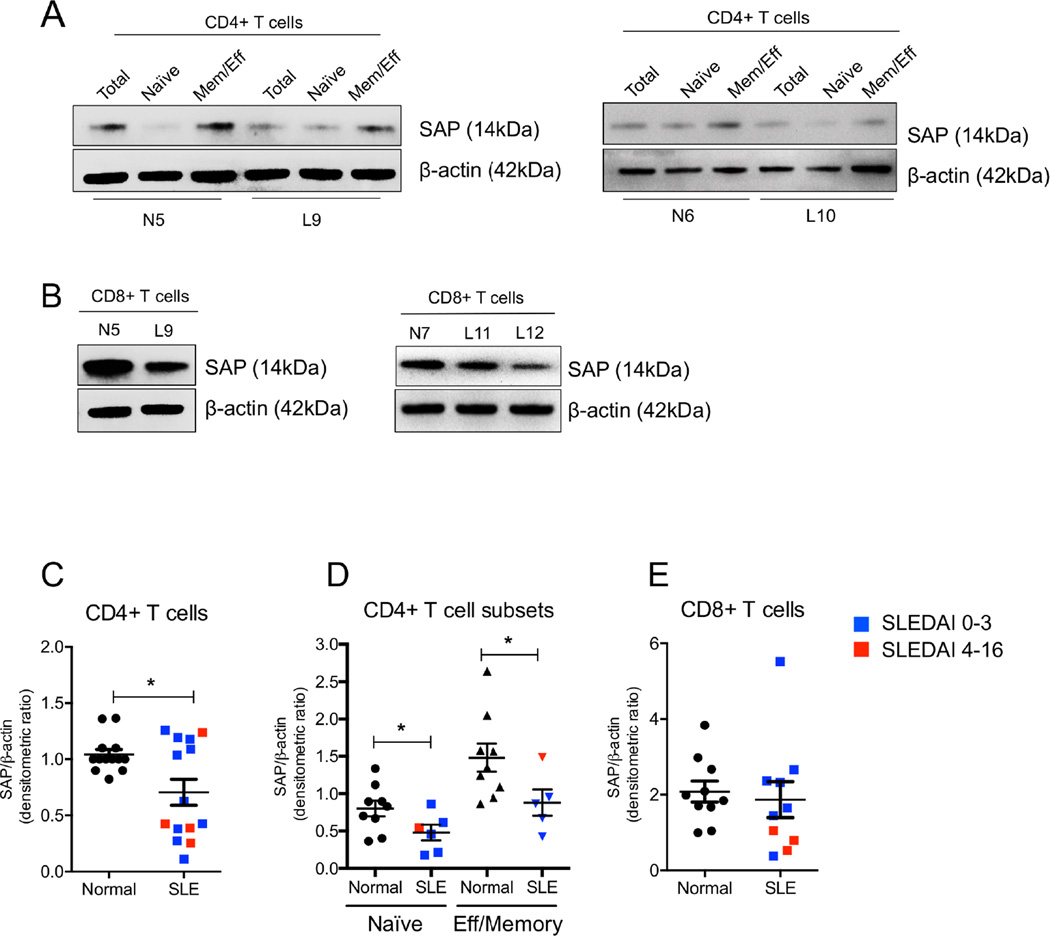
To determine whether all T cell subsets displayed decreased SAP expression we purified CD4+ and CD8+ T cells from the peripheral blood of patients with SLE. We observed that the levels of SAP were significantly lower in SLE CD4+ T cells compared to T cells from normal subjects. We further purified naïve and effector/memory CD4+ T cells from 5 patients with SLE and found that both naïve and effector/memory SLE CD4+ T cells express lower levels of SAP compared to those isolated from healthy sample donors (Fig 3A, C and D). In CD8+ T cells from patients with SLE the expression of SAP displayed a lower but not statistically significant trend (Fig 3B and E). As a minority of SLE patients presented with normal SAP levels, all subsequent mechanistic studies were performed using T cells from those patients who were established to have low SAP levels.
Figure 3. Expression of SAP in CD4+ T cell subsets and CD8+ T cells in SLE patients and healthy controls.
CD4+ T cells and CD8+ T cells were purified from the peripheral blood of healthy individuals and patients with SLE. Naïve CD4+ T cells and effector-memory CD4+ T cells (Mem/Eff) were further isolated from the CD4+ T cell fraction. SAP protein levels were evaluated by Western blotting. (A) SAP expression in total, naïve and effector/memory CD4+ T cells in normal individuals and patients with SLE. (B) Representative Western blots of SAP expression in healthy and SLE CD8+ T cells. The mean densitometric ratio of SAP over β-actin ± SEM is shown for CD4+ T cells (C), CD4+ T cell subsets (D) and CD8+ T cells (E). SLE samples are either depicted as blue squares (SLEDAI: 0– 3) or red squares (SLEDAI: 4–16).
Reconstitution of SAP protein levels normalizes early signal transduction events in SLE T cells
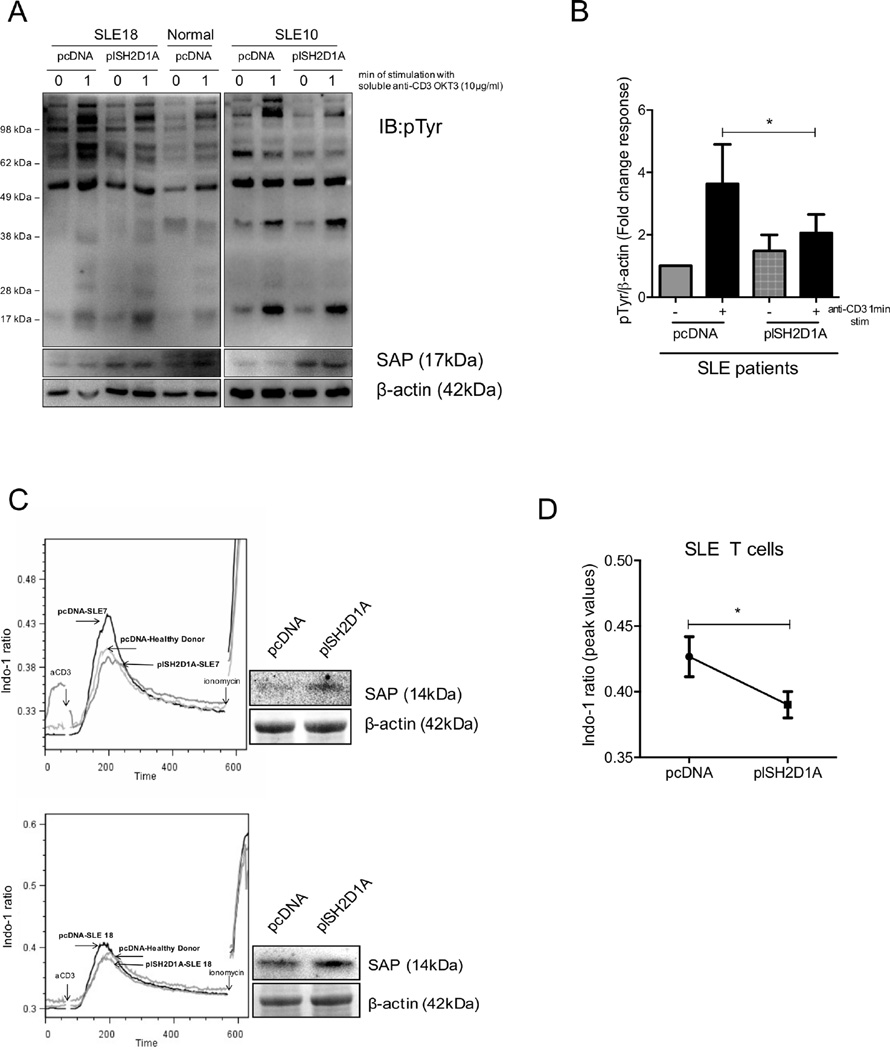
Because SLE T cells display both increased levels of tyrosine phosphorylated proteins following antigenic stimulation as well as heightened calcium responses, we performed experiments to examine whether reconstitution of SAP protein levels in SLE T cells can reverse the aberrant phenotype. To this end, we transfected purified T cells from normal donors and patients with SLE either with a control vector (pcDNA) or a plasmid expressing SAP (plSH2D1A). Transfected T cells were treated with soluble anti-CD3 (10µg/ml) or control IgG followed by cross-linking at 37 °C for 60 sec. Levels of tyrosine phosphorylated proteins in pcDNA-transfected SLE T cells were significantly higher compared to control T cells following 1min of anti-CD3 stimulation in a manner similar to that described before (31) (32). However, when SAP was re-introduced in SLE T cells, the overall amount of tyrosine phosphorylated proteins following TCR engagement was comparable to the tyrosine phosphorylation levels that were induced by anti-CD3 stimulation in control pcDNA-transfected normal T cells (Fig 4A and 4B). There were no differences at the baseline levels of tyrosine phosphorylated proteins in unstimulated normal T cells or in resting SLE T cells that were transfected with pcDNA or plSH2D1A (Fig 4B). We then asked whether restoration of SAP protein expression in T cells from patients with SLE could ameliorate the aberrant calcium responses that are known to characterize lupus T cells (30). Indeed, when we re-introduced SAP in SLE T cells, the peak ratio of free intracellular Ca2+ flux following anti-CD3 cross-linking was lower in SAP-transduced T cells and was comparable to the responses that were elicited in normal T cells (Fig 4C and D). Taken together, our data indicate that reduced SAP protein levels contribute to the hyper-responsiveness exhibited by T cells from patients with SLE and that reconstitution of SAP expression in lupus T cells may reverse, at least in vitro, the aberrant overexcitable phenotype.
Figure 4. Forced expression of SAP in SLE T cells restores TCR-mediated early signal transduction events.
Purified T cells from normal donors (n=4) and patients with SLE (n=4) were transfected either with a control vector (pcDNA) or a SAP-expressing plasmid (plSH2D1A). (A) Levels of tyrosine phosphorylated proteins (pTyr) were evaluated by Western blotting in transfected cells from one healthy individual and two patients with SLE following stimulation with cross-linked soluble anti-CD3 or control IgG at 37 °C for 60 sec (representative experiment). Cumulative results are shown in (B) and are expressed as the fold change of the mean densitometric ratio of pTyr proteins (36–110kDa area) over β-actin ± SEM. (C) Representative histograms demonstrating the peak intracellular calcium [Ca2+]i response in transfected total T cells from normal donors (transfected with control pcDNA) and patients with SLE (transfected with pcDNA or plSH2D1A). Successful transfection for each SLE donor is shown by Western blotting next to each calcium response graph. (D) Results are expressed as the mean peak value of the Indo-1(violet) to Indo-1(blue) ratio ± SEM for a total of 4 independent experiments. aCD3= stimulation with anti-CD3 OKT3 mAb.
Forced SAP expression in SLE T cells restores anti-CD3-mediated IL-2 production
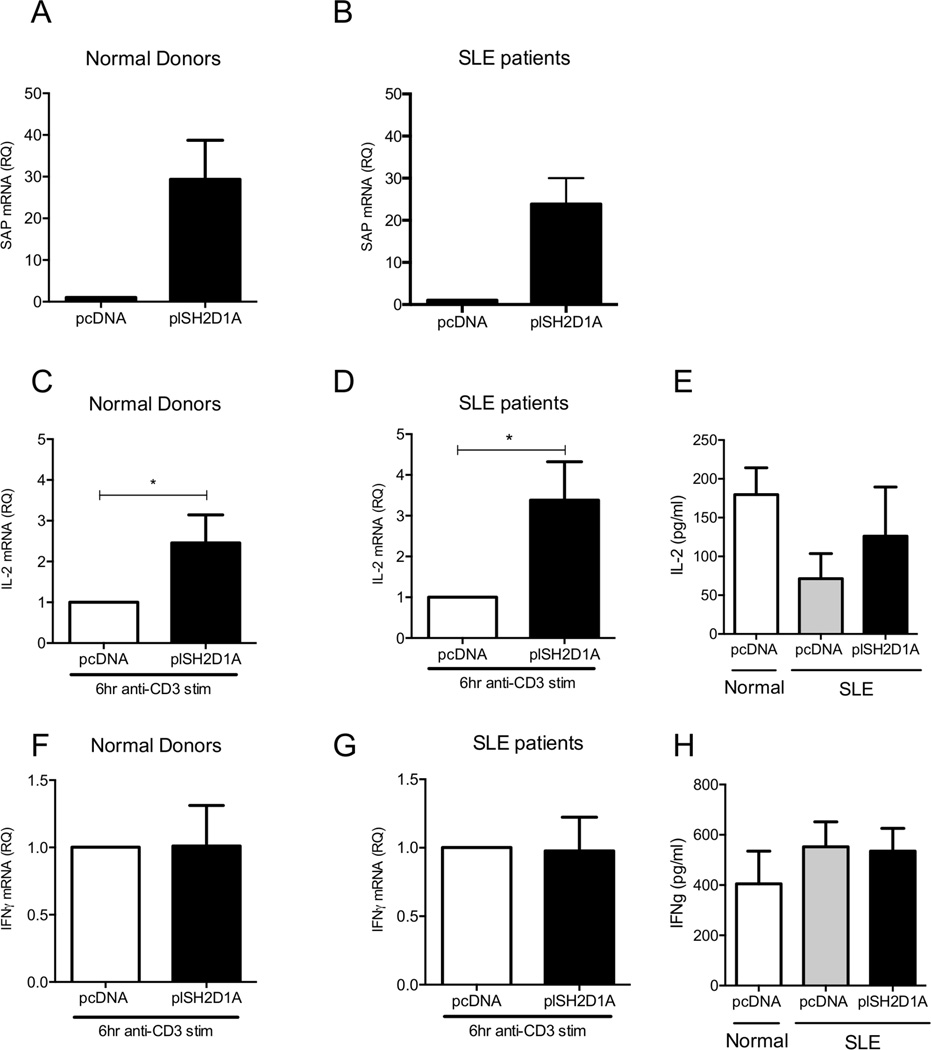
It is well established that despite the hyper-responsiveness that is being manifested by lupus T cells, production of IL-2 following antigenic stimulation is decreased in SLE. IL-2 production in patients with X-Linked Proliferative syndrome (XLP), where SAP is either completely absent or non-functional, is also impaired (33) (29). Therefore, we sought to determine whether reconstitution of SAP protein levels in SLE T cells could restore IL-2 production. Accordingly, we isolated total T cells from patients with SLE or from healthy subjects and transfected them either with a pcDNA or plSH2D1A vector (Fig 5A and B). 24h after transfection T cells were stimulated for 6h with soluble anti-CD3 antibody in the presence of a cross-linker. Following TCR-mediated stimulation SAP-transfected T cells from patients with SLE produced higher amounts of IL-2 compared to pcDNA-transfected SLE T cells (Fig 5D and E). When SAP was overexpressed in normal primary T cells, IL-2 production following anti-CD3 stimulation was also significantly enhanced (Fig 5C). Because it has been previously reported that T cells from SAP-deficient patients produce higher amounts of IFNγ compared to T cells from healthy donors, we wondered whether decreased SAP levels in SLE T cells is responsible for increased ΙFNγ production. As we previously showed, when SAP was silenced in normal T cells the levels of IFNγ mRNA were significantly increased following 6h of TCR-mediated stimulation. However, when SAP was re-introduced in SLE T cells, IFNγ production following stimulation with anti-CD3 remained unaltered, suggesting that at least in patients with SLE increased production of IFNγ following TCR-mediated stimulation is SAP-independent (Fig 5G and H). No change in IFNγ mRNA levels was observed when we overexpressed SAP in normal T cells either (Fig 5F)
Figure 5. Replenishment of SAP levels in SLE T cells rescues IL-2 production following TCR-mediated stimulation.
Purified total T cells from (A) healthy individuals (n=13) and (B) SLE patients (n=9) and were successfully transfected with control pcDNA or a plasmid expressing SAP (plSH2D1A) and levels of SAP were measured by qPCR to validate transfection efficiency. 24 h post-transfection T cells were stimulated with soluble anti-CD3 mAb in the presence of a cross-linker for 6 h. IL-2 and ΙFNγ mRNA levels were assessed in normal donors (C and F) and patients with SLE (D and G) with qPCR. Production of IL-2 and IFNγ was also evaluated by ELISA from the supernatants of transfected T cells following 6 h of anti-CD3 stimulation (E and H).
Exposure to SLE IgG results in decreased SAP expression in normal T cells
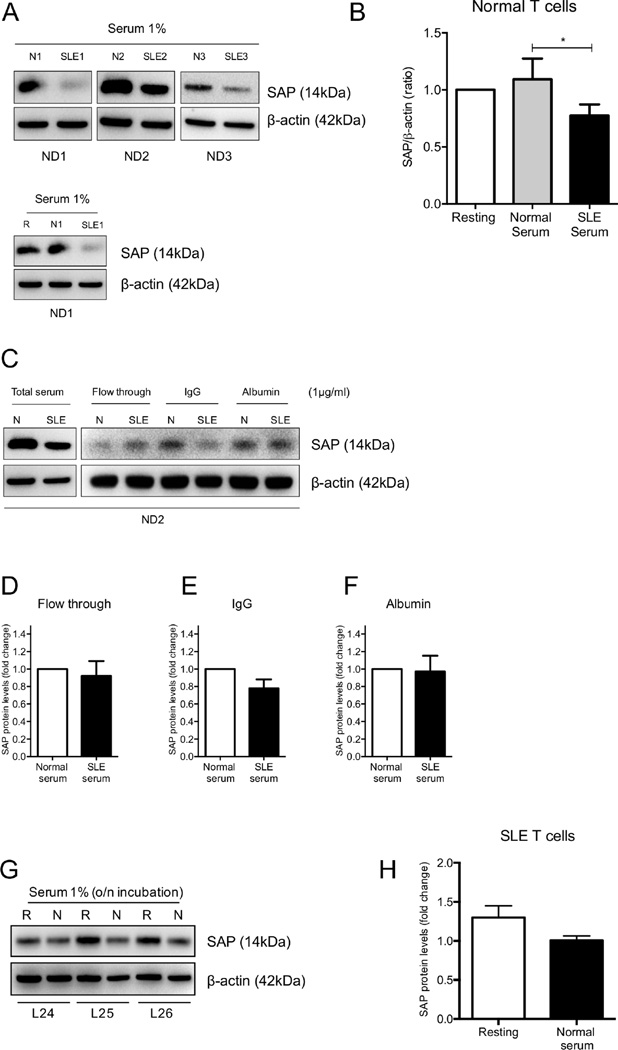
It has been demonstrated that exposure of normal T cells to serum from patients with SLE results in suppression of IL-2 production and this was attributed to the existence of anti-TCR/CD3 IgG autoantibodies in SLE sera (34). Because restoration of SAP protein expression in SLE T cells was able to rescue IL-2 production, we wondered whether downregulation of SAP in lupus T cells represents an effect exerted on T cells by SLE serum. Purified normal T cells were either kept resting in medium supplemented with 1% FBS or were exposed to 1% serum from healthy donors or patients with SLE for 6h. Whole-cell extracts were obtained and SAP content was evaluated with Western immunoblotting. Downregulation of protein levels of SAP was seen in 4 out of 6 normal donors exposed to SLE serum (Fig 6A and B). We then fractionated lupus sera into IgG and non-IgG fractions and normal total T cells were exposed to the two fractions for 6h. Normal T cells exposed to the IgG, but not the non-IgG faction, of lupus sera displayed reduced SAP protein levels (Fig 6C, D, E and F). Exposure of SLE T cells to serum from healthy donors overnight failed to restore SAP expression to normal levels (Fig 6G and H)
Figure 6. Treatment of normal T cells with SLE serum results in SAP downregulation in an IgG-dependent mechanism.
Normal T cells (n=6) were cultured in the presence of 1% serum from patients with SLE, healthy individuals or medium supplemented with 1% FBS for 6 h and SAP protein expression was examined with Western blot. Representative experiments are shown in (A) and cumulative results in (B). Normal T cells were exposed to normal or SLE serum (whole or fractionated) for 6 h and SAP levels were evaluated with Western blot in (C). (D, E and F) Cumulative results of SAP levels following incubation of healthy T cells with fractionated serum from normal donors and patients with SLE are expressed as the fold change of the densitometric ratio of SAP over β-actin ± SEM. Total T cells from patients with SLE (n=3) were exposed to 1% normal serum overnight. SAP levels were examined with Western blotting (G) and results are depicted in (H), expressed as the mean densitometric ratio of SAP over β-actin ± SEM. N=normal serum, SLE=serum from SLE patient, ND=T cells from Normal Donor, L=T cells from SLE patients and R=resting
Caspase 3 degrades SAP protein in T cells from patients with SLE
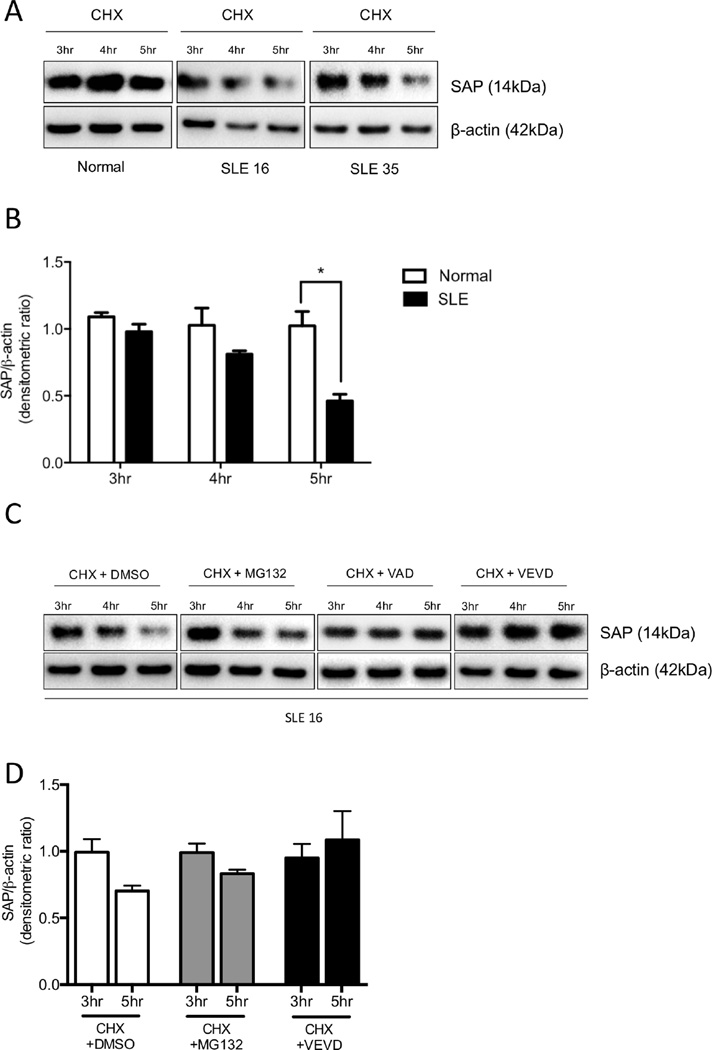
We investigated the potential mechanisms that may be responsible for the reduced SAP protein and mRNA levels in T cells from patients with SLE. First, we explored whether SAP is subject to a higher rate of protein degradation in SLE T cells. We treated normal and lupus T cells with 50µg/ml cycloheximide (CHX) to block protein synthesis and whole-cell lysates were obtained at 0, 3, 4 and 5h after treatment. Western immunoblot analyses showed that SAP protein levels were diminished by 4h of culture in T cells from patients with SLE. On the contrary, cytoplasmic SAP levels remained constant in normal T cells (Fig 7A and B). Because caspase activity, especially caspase-3, is reportedly increased in patients with SLE (35) we asked whether faster SAP protein degradation in SLE T cells is attributed to caspase-mediated cleavage. SLE T cells were cultured with CHX in the presence or absence of a pan-caspase or a caspase-3 inhibitor for 3, 4 and 5h. By inhibiting caspase activity, SAP protein levels were stabilized in SLE T cells (Fig 7C). Conversely, when lupus T cells were incubated with CHX in the presence of the proteasome inhibitor MG132, SAP protein was degraded suggesting that SAP proteolysis is primarily caspase-3 dependent (Fig 7C and D).
Figure 7. SAP undergoes faster protein degradation in SLE T cells compared to normal T cells.
(A) Total T cells from healthy individuals (n=3) and SLE patients (n=5) were cultured in the presence of CHX for up to 5 h to inhibit protein synthesis. Cells were lysed and protein extracts were analyzed for SAP protein levels by Western blotting. Cumulative results are shown in (B) and are expressed as the fold change in densitometric ratio of SAP over β-actin ± SEM. (C) Protein synthesis was arrested with CHX and T cells were incubated in the presence of proteasome inhibitor MG132, pan-caspase inhibitor VAD or caspase-3-specific inhibitor VEVD. SAP levels were evaluated by Western blotting at 3, 4 and 5 h after treatment and cumulative results are shown in (D)
Because SAP mRNA levels were significantly reduced in patients with SLE we examined whether SAP mRNA degradation may also be altered in patients with SLE. We purified T cells from patients with SLE and healthy donors and transcription was arrested by adding actinomycin D (50µg/ml). Levels of SAP mRNA were assessed every 30min for a total of 4h. Levels of the housekeeping gene cyclophilin A were additionally analyzed as an internal stable transcript. We did not observe any differences in the rate of the SAP mRNA degradation in lupus and control donors, suggesting that reduced SAP mRNA may be attributed to transcriptional rather than post-transcriptional defects (data not shown).
Discussion
In the present communication we demonstrate that SAP protein and gene expression is significantly reduced in T cells, and more pronounced, in CD4+ T cell subsets from patients with SLE. Forced expression of SAP in SLE resulted in correction of the hyper-responsive phenotype that characterizes the lupus T cell and increased production of IL-2 to normal levels. Exposure of normal T cells to lupus IgG caused reduction of SAP levels while in SLE T cells, increased caspase-3 activity appears to account for its degradation.
T cells from SAP-deficient individuals display a similarly dichotomous series of events following stimulation which involves increased [Ca2+]i response but limited IL-2 production (29). Similarly, if SAP is silenced in normal T cells, a similar pattern of response was observed (Fig. 1) and this parallels the response of SLE T cells stimulated with an anti-CD3 antibody (30). The increased early signaling events which prevail in SLE T cells have been linked to a rewiring of the CD3 complex with CD3ζ been replaced with FcεRγ and involvement of Syk (36). The experiments which we have presented here imply that decreased SAP levels may also contribute to the “overexcitable” T cell phenotype in SLE T cells. Interestingly, SAP was reported increased in T cells from Chinese patients with SLE and this was linked to decreased expression of miRNA-142-3p/5p (37). Regardless, in our study sample we have included 2 individuals of reported Chinese ancestry in whom the levels of SAP expression were also decreased. Obviously, independent confirmation of the claimed altered SAP expression in SLE T cells is warranted.
Previously (34) we had demonstrated that SLE sera containing anti-CD3/TCR antibodies which cause decreased IL-2 production by limiting the transcriptional activity of the IL-2 promoter due to increased binding of the repressor CREMα. Here we show that lupus sera result in decreased expression of SAP and thereafter to decreased IL-2 production signifying the multiplicity of the molecular abnormalities displayed by SLE T cells and the complexity of its biology. The fact that anti-CD3/TCR antibodies present in lupus sera lead to decreased expression of SAP along with the fact that 90% of the study subjects were women point away from the involvement of genetic causes.
Of clinical relevance is the finding that in SLE T cells SAP is degraded by caspase-3 in a manner similar to that we previously reported for CD3ζ (35). Targeted inhibition of caspase-3 in SLE T cells should lead to the restoration of levels of SAP, CD3ζ and probably other molecules.
The role of SAP in the expression of autoimmunity and in T cell activation and function is unclear (38) (39) (40) (25). Studies have claimed that SAP activates CD3ζ chain and other canonical T cell signaling molecules (40). Because CD3ζ is decreased in SLE T cells (31), we have not been able to address directly whether this occurs also in SLE T cells. Yet, when we downregulated SAP in normal T cells the total expression of CD3ζ was not different compared to cells that have been transfected with control siRNA (data not shown).
SAP-deficient mice do not spontaneously develop autoimmunity and are resistant to pristane-induced lupus (41). Moreover, a spontaneous mutation in SAP in the MRL/lpr strain led to attenuation of the disease (42) and SAP deficiency also ameliorated the lupus phenotype of the Roquinsan/san mice (43). Sustained expression of SAP has also been shown to be a requisite for the progression of collagen-induced arthritis in the early stages of the disease in mice (39). On the other hand, patients with XLP display defective B cell tolerance and transitional B cells in the peripheral blood of XLP patients are enriched in autoreactive clones (44). It was also shown that SAP-deficient T cells from patients with XLP appear to be resistant to regulatory T cell-mediated suppression (44). Decreased SAP mRNA transcripts have been reported in PBMCs from patients with rheumatoid arthritis (45).
In summary, this study presents data demonstrating that expression of SAP is decreased in T cells from patients with SLE and contributes to aberrant early signaling events and decreased IL-2 production. Because caspase-3 accounts for the degradation of SAP, in manner similar to that described for CD3 ζ in SLE T cells, it is proper to consider its pharmaceutical inhibition to restore its expression and correct aberrant cell function (35).
Supplementary Material
Acknowledgments
Work was supported by the National Institutes of Health (grant numbers P01AI065687 and R01AI42269). This work was also supported by a SICPA Foundation grant to D.C.
References
- 1.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 2.Moulton VR, Tsokos GC. T cell signaling abnormalities contribute to aberrant immune cell function and autoimmunity. J Clin Invest. 2015;125:2220–2227. doi: 10.1172/JCI78087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispín JC, Hedrich CM, Tsokos GC. Gene-function studies in systemic lupus erythematosus. Nat Rev Rheumatol. 2013;9:476–484. doi: 10.1038/nrrheum.2013.78. [DOI] [PubMed] [Google Scholar]
- 4.Detre C, Keszei M, Romero X, Tsokos GC, Terhorst C. SLAM family receptors and the SLAM-associated protein (SAP) modulate T cell functions. Semin Immunopathol. 2010;32:157–171. doi: 10.1007/s00281-009-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunninghame Graham DS, Vyse TJ, Fortin PR, Montpetit A, Cai YC, Lim S, McKenzie T, Farwell L, Rhodes B, Chad L, Hudson TJ, Sharpe A, Terhorst C, Greenwood CM, Wither J, Rioux JD, et al. Investigators., CaNIOS GenES. Association of LY9 in UK and Canadian SLE families. Genes Immun. 2008;9:93–102. doi: 10.1038/sj.gene.6364453. [DOI] [PubMed] [Google Scholar]
- 6.Margraf S, Garner LI, Wilson TJ, Brown MH. A polymorphism in a phosphotyrosine signalling motif of CD229 (Ly9, SLAMF3) alters SH2 domain binding and T-cell activation. Immunology. 2015;146:392–400. doi: 10.1111/imm.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki A, Yamada R, Kochi Y, Sawada T, Okada Y, Matsuda K, Kamatani Y, Mori M, Shimane K, Hirabayashi Y, Takahashi A, Tsunoda T, Miyatake A, Kubo M, Kamatani N, Nakamura Y, Yamamoto K. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat Genet. 2008;40:224–229. doi: 10.1038/ng.205. [DOI] [PubMed] [Google Scholar]
- 8.Ota Y, Kawaguchi Y, Takagi K, Tochimoto A, Kawamoto M, Katsumata Y, Gono T, Masuda I, Ikari K, Momohara S, Yamanaka H. Single nucleotide polymorphisms of CD244 gene predispose to renal and neuropsychiatric manifestations with systemic lupus erythematosus. Mod Rheumatol. 2010;20:427–431. doi: 10.1007/s10165-010-0302-x. [DOI] [PubMed] [Google Scholar]
- 9.Cui J, Stahl EA, Saevarsdottir S, Miceli C, Diogo D, Trynka G, Raj T, Mirkov MU, Canhao H, Ikari K, Terao C, Okada Y, Wedrén S, Askling J, Yamanaka H, Momohara S, Taniguchi A, Ohmura K, Matsuda F, Mimori T, Gupta N, Kuchroo M, Morgan AW, Isaacs JD, Wilson AG, Hyrich KL, Herenius M, Doorenspleet ME, Tak PP, Crusius JB, van der Horst-Bruinsma IE, Wolbink GJ, van Riel PL, van de Laar M, Guchelaar HJ, Shadick NA, Allaart CF, Huizinga TW, Toes RE, Kimberly RP, Bridges SL, Jr, Criswell LA, Moreland LW, Fonseca JE, de Vries N, Stranger BE, De Jager PL, Raychaudhuri S, Weinblatt ME, Gregersen PK, Mariette X, Barton A, Padyukov L, Coenen MJ, Karlson EW, Plenge RM. Genome-wide association study and gene expression analysis identifies CD84 as a predictor of response to etanercept therapy in rheumatoid arthritis. PLoS Genet. 2013;9:e1003394. doi: 10.1371/journal.pgen.1003394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JR, Mathew SO, Patel RK, Pertusi RM, Mathew PA. Altered expression of signalling lymphocyte activation molecule (SLAM) family receptors CS1 (CD319) and 2B4 (CD244) in patients with systemic lupus erythematosus. Clin Exp Immunol. 2010;160:348–358. doi: 10.1111/j.1365-2249.2010.04116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kis-Toth K, Comte D, Karampetsou MP, Kyttaris VC, Kannan L, Terhorst C, Tsokos GC. The selective loss of SLAMF4+ CD8+ T cells contributes to the decreased cytotoxic cell activity in systemic lupus erythematosus. Arthritis Rheumatol. 2016;68:164–173. doi: 10.1002/art.39410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee M, Kis-Toth K, Thai TH, Terhorst C, Tsokos GC, et al. SLAMF6-driven co-stimulation of human peripheral T cells is defective in SLE T cells. Autoimmunity. 2011;44:211–218. doi: 10.3109/08916934.2010.530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee M, Rauen T, Kis-Toth K, Kyttaris VC, Hedrich CM, Terhorst C, Tsokos GC. Increased expression of SLAM receptors SLAMF3 and SLAMF6 in systemic lupus erythematosus T lymphocytes promotes Th17 differentiation. J Immunol. 2012;188:1206–1212. doi: 10.4049/jimmunol.1102773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Jr, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Carlucci F, Cortes-Hernandez J, Fossati-Jimack L, Bygrave AE, Walport MJ, Vyse TJ, Cook HT, Botto M. Genetic dissection of spontaneous autoimmunity driven by 129-derived chromosome 1 Loci when expressed on C57BL/6 mice. J Immunol. 2007;178:2352–2360. doi: 10.4049/jimmunol.178.4.2352. [DOI] [PubMed] [Google Scholar]
- 16.de Salort J, Cuenca M, Terhorst C, Engel P, Romero X. Ly9 (CD229) Cell-Surface Receptor is Crucial for the Development of Spontaneous Autoantibody Production to Nuclear Antigens. Front Immunol. 2013;4:225. doi: 10.3389/fimmu.2013.00225. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang N, Halibozek PJ, Yigit B, Zhao H, O'Keeffe MS, Sage P, Sharpe A, Terhorst C. Negative Regulation of Humoral Immunity Due to Interplay between the SLAMF1, SLAMF5, and SLAMF6 Receptors. Front Immunol. 2015;6:158. doi: 10.3389/fimmu.2015.00158. eCollection 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Havenar-Daughton C, Crotty S. Modulation of SAP dependent T:B cell interactions as a strategy to improve vaccination. Curr Opin Virol. 2013;3:363–370. doi: 10.1016/j.coviro.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morra M, Barrington RA, Abadia-Molina AC, Okamoto S, Julien A, Gullo C, Kalsy A, Edwards MJ, Chen G, Spolski R, Leonard WJ, Huber BT, Borrow P, Biron CA, Satoskar AR, Carroll MC, Terhorst C. Defective B cell responses in the absence of SH2D1A. Proc Natl Acad Sci U S A. 2005;102:4819–4823. doi: 10.1073/pnas.0408681102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keszei M, Detre C, Castro W, Magelky E, O'Keeffe M, Kis-Toth K, Tsokos GC, Wang N, Terhorst C. Expansion of an osteopontin-expressing T follicular helper cell subset correlates with autoimmunity in B6.Sle1b mice and is suppressed by the H1-isoform of the Slamf6 receptor. FASEB J. 2013;27:3123–3131. doi: 10.1096/fj.12-226951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, Oettgen H, De Vries JE, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 23.Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- 24.Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- 25.Katz G, Krummey SM, Larsen SE, Stinson JR, Snow AL. SAP facilitates recruitment and activation of LCK at NTB-A receptors during restimulation-induced cell death. J Immunol. 2014;192:4202–4209. doi: 10.4049/jimmunol.1303070. [DOI] [PubMed] [Google Scholar]
- 26.Gu C, Tangye SG, Sun X, Luo Y, Lin Z, Wu J. The X-linked lymphoproliferative disease gene product SAP associates with PAK-interacting exchange factor and participates in T cell activation. Proc Natl Acad Sci U S A. 2006;103:14447–14452. doi: 10.1073/pnas.0606624103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C, Schibli D, Li SS. The XLP syndrome protein SAP interacts with SH3 proteins to regulate T cell signaling and proliferation. Cell Signal. 2009;21:111–119. doi: 10.1016/j.cellsig.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Sanzone S, Zeyda M, Saemann MD, Soncini M, Holter W, Fritsch G, Knapp W, Candotti F, Stulnig TM, Parolini O. SLAM-associated protein deficiency causes imbalanced early signal transduction and blocks downstream activation in T cells from X-linked lymphoproliferative disease patients. J Biol Chem. 2003;278:29593–29599. doi: 10.1074/jbc.M300565200. [DOI] [PubMed] [Google Scholar]
- 30.Vassilopoulos D, Kovacs B, Tsokos GC. TCR/CD3 complex-mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. J Immunol. 1995;155:2269–2281. [PubMed] [Google Scholar]
- 31.Liossis SN, Ding XZ, Dennis GJ, Tsokos GC. Altered pattern of TCR/CD3-mediated protein-tyrosyl phosphorylation in T cells from patients with systemic lupus erythematosus. Deficient expression of the T cell receptor zeta chain. J Clin Invest. 1998;101:1448–1457. doi: 10.1172/JCI1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yi Y, McNerney M, Datta SK. Regulatory defects in Cbl and mitogen-activated protein kinase (extracellular signal-related kinase) pathways cause persistent hyperexpression of CD40 ligand in human lupus T cells. J Immunol. 2000;165:6627–6634. doi: 10.4049/jimmunol.165.11.6627. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H, Zarycki J, Sullivan JL, Jung JU. Abnormal T cell receptor signal transduction of CD4 Th cells in X-linked lymphoproliferative syndrome. J Immunol. 2001;167:2657–2665. doi: 10.4049/jimmunol.167.5.2657. [DOI] [PubMed] [Google Scholar]
- 34.Juang YT, Wang Y, Solomou EE, Li Y, Mawrin C, Tenbrock K, Kyttaris VC, Tsokos GC. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan S, Kiang JG, Fisher CU, Nambiar MP, Nguyen HT, Kyttaris VC, Chowdhury B, Rus V, Tsokos GC. Increased caspase-3 expression and activity contribute to reduced CD3zeta expression in systemic lupus erythematosus T cells. J Immunol. 2005;175:3417–3423. doi: 10.4049/jimmunol.175.5.3417. [DOI] [PubMed] [Google Scholar]
- 36.Enyedy EJ, Nambiar MP, Liossis SN, Dennis G, Kammer GM, Tsokos GC. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 37.Ding S, Liang Y, Zhao M, Liang G, Long H, Zhao S, Wang Y, Yin H, Zhang P, Zhang Q, Lu Q. Decreased microRNA-142-3p/5p expression causes CD4+ T cell activation and B cell hyperstimulation in systemic lupus erythematosus. Arthritis Rheum. 2012;64:2953–2963. doi: 10.1002/art.34505. [DOI] [PubMed] [Google Scholar]
- 38.Chu C, Wang Y, Zhang X, Ni X, Cao J, Xu W, Dong Z, Yuan P, Wei W, Ma Y, Zhang L, Wu L, Qi H. SAP-regulated T Cell-APC adhesion and ligation-dependent and -independent Ly108-CD3ζ interactions. J Immunol. 2014;193:3860–3871. doi: 10.4049/jimmunol.1401660. [DOI] [PubMed] [Google Scholar]
- 39.Zhong MC, Veillette A. The adaptor molecule signaling lymphocytic activation molecule (SLAM)-associated protein (SAP) is essential in mechanisms involving the Fyn tyrosine kinase for induction and progression of collagen-induced arthritis. J Biol Chem. 2013;288:31423–31436. doi: 10.1074/jbc.M113.473736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Proust R, Bertoglio J, Gesbert F. The adaptor protein SAP directly associates with CD3ζ chain and regulates T cell receptor signaling. PLoS One. 2012;7:e43200. doi: 10.1371/journal.pone.0043200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hron JD, Caplan L, Gerth AJ, Schwartzberg PL, Peng SL. SH2D1A regulates T-dependent humoral autoimmunity. J Exp Med. 2004;200:61–66. doi: 10.1084/jem.20040526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Komori H, Furukawa H, Mori S, Ito MR, Terada M, Zhang MC, Ishii N, Sakuma N, Nose M, Ono M. A signal adaptor SLAM-associated protein regulates spontaneous autoimmunity and Fas-dependent lymphoproliferation in MRL-Faslpr lupus mice. J Immunol. 2006;176:395–400. doi: 10.4049/jimmunol.176.1.395. [DOI] [PubMed] [Google Scholar]
- 43.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:61–76. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menard L, Cantaert T, Chamberlain N, Tangye SG, Riminton S, Church JA, Klion A, Cunningham-Rundles C, Nichols KE, Meffre E. Signaling lymphocytic activation molecule (SLAM)/SLAM-associated protein pathway regulates human B-cell tolerance. J Allergy Clin Immunol. 2014;133:1149–1161. doi: 10.1016/j.jaci.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takei Masami, Ishiwata Tetsuyoshi, Mitamura Ko, Fujiwara Shigeyoshi, Sasaki Katsutoshi, Nishi Tatsunari, Kuga Tetsuro, Ookubo Takahiro, Horie Takashi, Ryu Junnosuke, Ohi Hiroyuki, Sawada Shigemasa. Decreased expression of signaling lymphocytic-activation molecule-associated protein (SAP) transcripts in T cells from patients with rheumatoid arthritis. Int. Immunol. 2001;13:559–565. doi: 10.1093/intimm/13.4.559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.