Abstract
Here, we report that novel epidermal growth factor receptor (EGFR) gene fusions comprising the N-terminal of EGFR linked to various fusion partners, most commonly RAD51, are recurrent in lung cancer. We describe five patients with metastatic lung cancer whose tumors harbored EGFR fusions, four of whom were treated with EGFR tyrosine kinase inhibitors (TKIs) with documented anti-tumor responses. In vitro, EGFR-RAD51 fusions are oncogenic and can be therapeutically targeted with available EGFR TKIs and therapeutic antibodies. These results support the dependence of EGFR-rearranged tumors on EGFR-mediated signaling and suggest several therapeutic strategies for patients whose tumors harbor this novel alteration.
Keywords: Epidermal growth factor receptor (EGFR), non-small cell lung cancer (NSCLC), next-generation sequencing (NGS), targeted therapy, gene rearrangement, kinase fusion, kinase domain, tyrosine kinase inhibitor (TKI), erlotinib, afatinib, osimertinib, cetuximab
Introduction
Oncogenic mutations in the epidermal growth factor receptor (EGFR) are found in a subset of patients with non-small cell lung cancer (NSCLC) and serve as important predictive biomarkers in this disease (1–3). These mutations, which most commonly occur as either small in-frame deletions in exon 19 or point mutations in exon 21 (L858R) within the EGFR tyrosine kinase domain, confer constitutive activity and sensitivity to EGFR tyrosine kinase inhibitors (TKIs). Several large phase III clinical trials have shown that patients with EGFR-mutant lung cancer derive superior clinical responses when treated with EGFR TKIs as compared with standard chemotherapy (4–6), and several EGFR inhibitors are already FDA-approved. These trials used PCR-based ‘hotspot’ testing, which typically interrogate for EGFR point mutations and small indels in exons 18–21. More recently, next-generation sequencing (NGS) of tumor samples has allowed for the identification of additional mechanisms whereby the EGF receptor may become aberrantly activated (7,8), further documenting the importance of EGFR signaling in the pathogenesis of lung cancer. Here, we report, for the first time in lung cancer, the presence of oncogenic EGFR fusions, most commonly EGFR-RAD51, which contain the entire EGFR tyrosine kinase domain fused to RAD51, a protein involved in DNA damage responses. We demonstrate that these fusions are oncogenic in pre-clinical studies and show that patients whose tumors harbor EGFR fusions derive significant clinical benefit from treatment with EGFR TKI therapy.
Results
Frequency of EGFR alterations in lung cancer
To determine the frequency of EGFR fusions in lung cancer, we analyzed data from ~10,000 clinical cases (Supplementary Table S1). Fusion events, defined by a genomic breakpoint in EGFR exons 23 through intron 25, were detected in 5 patients, each of which is described below.
Case Reports
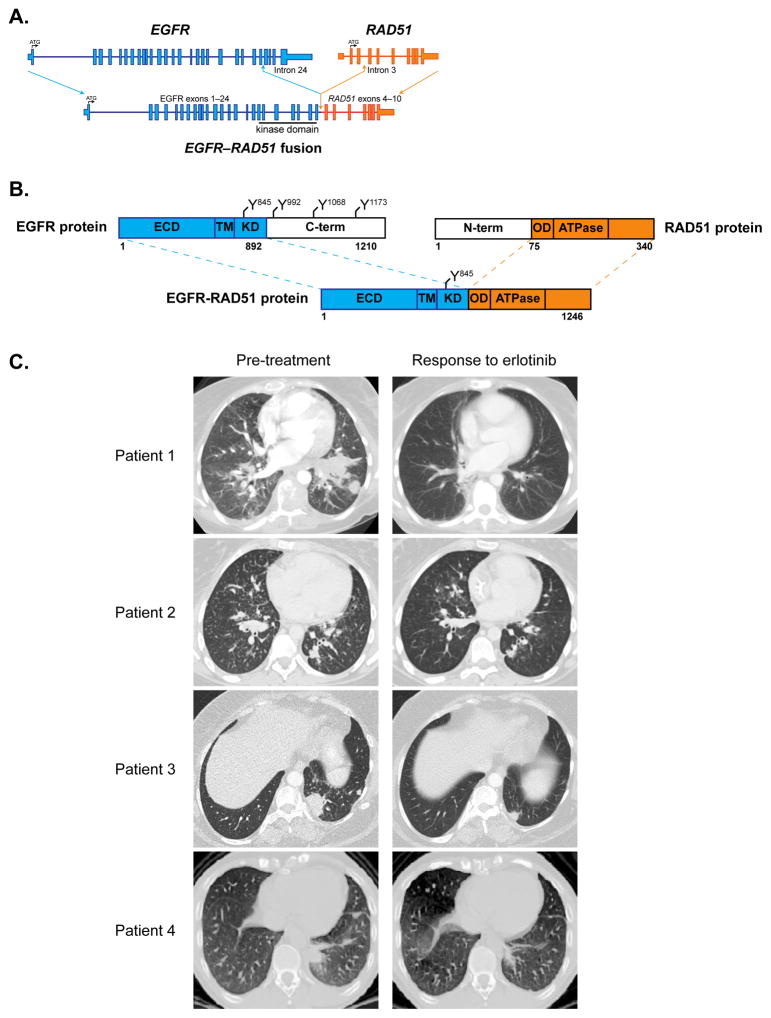
Patient 1, a 35-year-old female, was diagnosed with metastatic lung adenocarcinoma after presenting with generalized weakness and worsening vision. Imaging studies revealed widespread disease in the bone, liver, lymph nodes, adrenal glands, and hard palate (Table 1). MRI showed innumerable metastases in the brain, dura, and left globe, resulting in retinal detachment. She was initially treated with radiotherapy to the brain and spine. Due to significant debility in the setting of tumor-induced disseminated intravascular coagulation (DIC), she was a poor candidate for cytotoxic chemotherapy. A lymph node biopsy was sent for genomic profiling using an extensively validated hybrid capture-based NGS diagnostic platform (FoundationOne®) (9) and found to harbor a novel EGFR rearrangement at exon 25, resulting in the formation of a fusion gene between EGFR and RAD51 (Figs. 1A–B, Supplementary Table S2). The patient was treated with the EGFR TKI, erlotinib. Within two weeks of erlotinib initiation, DIC had resolved (Supplementary Fig. S1A) and the patient experienced clinical improvement with a noticeable decrease in supraclavicular lymphadenopathy and a hard palate metastatic lesion. After six months of treatment, the primary left lung mass and largest two liver lesions had decreased by 69% per RECIST (10) (Fig. 1C, Supplementary Fig. S1B), and the patient experienced an improvement in her functional status. She remained on erlotinib for 8 months, after which she experienced disease progression.
Table 1. Clinical characteristics of patients with non–small cell lung cancer harboring EGFR kinase fusions.
TKI= Tyrosine Kinase Inhibitor. RT= Radiation Therapy. WBI= Whole Brain Irradiation. PR= Partial Response. N/A= Not Applicable. Mets = Metastases.
| Patient No. | Age | Gender | Ethnicity | Diagnosis | Smoking Status | Sites of disease | Prior Treatment | EGFR fusion | EGFR TKI | Best response | Duration of EGFR TKI therapy |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | Female | South Asian | Stage IV lung adenocarcinoma | Never | Lung Lymph nodes Bone Brain Adrenal gland Breast Peritoneum Eye |
|
EGFR-RAD51 | Erlotinib | PR | 8 months |
| 2 | 21 | Female | Caucasian | Stage IV lung adenocarcinoma | 3 pack years | Lung Lymph nodes Bone Brain |
|
EGFR-RAD51 | Erlotinib | PR | 5 months |
| 3 | 43 | Female | Caucasian | Stage IV lung adenocarcinoma | 10 pack years, quit > 10 years ago | Lung Bone Brain |
|
EGFR-PURB | Erlotinib | PR | 20 months, on-going |
| 4 | 38 | Male | Caucasian | Stage IV lung adenocarcinoma | Former light smoker (3 pack years) | Lung Lymph nodes Pleura Bone |
|
EGFR- RAD51 | Erlotinib | PR | 6 months, on-going |
| 5 | 60 | Female | Caucasian | Stage IV lung adenocarcinoma | Never | Lung Lymph nodes Brain |
|
EGFR-RAD51 | N/A | N/A | N/A |
Figure 1. EGFR fusions are clinically actionable.
(A) Scaled representation of EGFR-RAD51 depicting the genomic structure of the fusion. ATG = translational start site. Blue = EGFR. Orange = RAD51.
(B) Schematic representation of EGFR-RAD51 fusion protein domain structure. Numbers correspond to amino acid residues. Y = tyrosine residue. ECD = extracellular domain. TM = transmembrane domain. KD = kinase domain. C-term = carboxyl terminus. N-term = amino terminus. OD = oligomerization domain/section. ATPase = adenylpyrophosphatase. Blue = EGFR. Orange = RAD51.
(C) Serial CT scans from index patients with lung adenocarcinoma harboring EGFR fusions, documenting response to the EGFR TKI, erlotinib. Left images = scans obtained prior to initiation of erlotinib. Right images = scans obtained during erlotinib therapy.
Patient 2, a 21-year-old female, was diagnosed with metastatic lung adenocarcinoma after presenting with right shoulder pain and unintentional weight loss. MRI revealed extensive metastatic disease in the spine and a right paraspinal mass extending into neuroforamina. Additional imaging studies showed metastatic disease in the brain, innumerable lung nodules, lymph nodes, and right acetabulum. Biopsy of an axillary lymph node showed metastatic adenocarcinoma consistent with lung primary. NGS testing revealed an EGFR-RAD51 fusion. The patient received palliative radiotherapy to the spine and brain metastases. Subsequently, the patient reported hemoptysis and dyspnea with exertion. Complete blood count showed a marked drop in platelet number and elevated lactate dehydrogenase, consistent with DIC. She was not a candidate for systemic chemotherapy. She was started on erlotinib approximately 6 weeks after initial presentation. Thrombocytopenia resolved within ten days (Supplementary Fig. S2A), and the patient experienced symptomatic improvement. CT scans obtained 3 months after the initiation of erlotinib showed a significant regression of bilateral miliary nodules as well as a 43% decrease in the index lesions of the left lower lobe (LLL), subcarinal lymph node, and right apical soft tissue mass compared to baseline (Fig. 1C, Supplementary Fig. S2B). The patient remained on erlotinib for 5 months with response, but she is no longer taking this medication due to nonmedical issues.
Patient 3, a 42-year-old female, was diagnosed with metastatic lung adenocarcinoma after presenting with right hip pain. Imaging studies revealed widespread disease including the primary left lower lobe (LLL) lesion, lytic lesions in the right pelvis and acetabulum, and brain metastases. Biopsy of a lung mass was positive for adenocarcinoma. She was initially treated with whole brain radiotherapy and platinum based chemotherapy with a partial response. While receiving chemotherapy, her tumor biopsy sample was sent for NGS testing and found to harbor an EGFR rearrangement at exon 25, resulting in the formation of a fusion gene between EGFR and PURB (Supplementary Table S2, Supplementary Fig. S3A–B). At the time of disease progression on chemotherapy, the patient was treated with erlotinib, resulting in a 48% decrease in the LLL index lesion on-going for 20 months (Fig. 1C, Supplementary Fig. S3C).
Patient 4, a 38-year-old male, was diagnosed with metastatic lung adenocarcinoma after presenting with dyspnea and progressive weakness. Imaging studies showed metastatic disease to the lungs, lymph nodes, pleura, and bone. A pleural biopsy was performed, and NGS testing identified an EGFR-RAD51 fusion. He was initially treated with cisplatin/pemetrexed followed by maintenance pemetrexed. At the time of disease progression, the patient was started on erlotinib, with partial response after 2 cycles of therapy (Fig. 1C, Supplementary Fig. S4). The patient has now received erlotinib for 6 months with continued response.
Patient 5, a 60-year-old female, initially presented with headache, slurred speech, and left foot drag. MRI revealed three enhancing cerebral masses with midline shift. Further imaging studies showed a 4cm mass in the lingula and lymphadenopathy. Biopsy of the lung mass revealed adenocarcinoma. The patient underwent resection of a right frontal tumor followed by radiotherapy. She was treated with four cycles of carboplatin/pemetrexed with partial response followed by pemetrexed maintenance therapy. During this treatment, NGS testing was completed and revealed an EGFR-RAD51 fusion. The patient continues to receive benefit from pemetrexed therapy; she has not yet been treated with an EGFR TKI.
EGFR-RAD51 is oncogenic
We stably expressed EGFR variants in Ba/F3 cells and detected expression of EGFR-RAD51 at the expected molecular weight as compared to EGFR wild-type (WT) and the known oncogenic EGFR-L858R mutation (Fig. 2A). We observed that, analogous to EGFR-L858R, EGFR-RAD51 was able to activate downstream signaling through the MAPK and PI3K/AKT pathways. EGFR-RAD51 was also able to sustain IL-3-independent proliferation of Ba/F3 cells, an activity phenotype associated with the transforming function of other oncogenic tyrosine kinases (Fig. 2B) (11). In parallel, we expressed the same EGFR variants in NR6 cells (which lack endogenous EGFR (12)) (Supplementary Figs. S5A–B). EGFR-RAD51 significantly increased colony formation of NR6 cells in soft agar— a hallmark of tumor cells—as compared to control cells and those expressing EGFR-WT (Supplementary Figs. S5C–D).
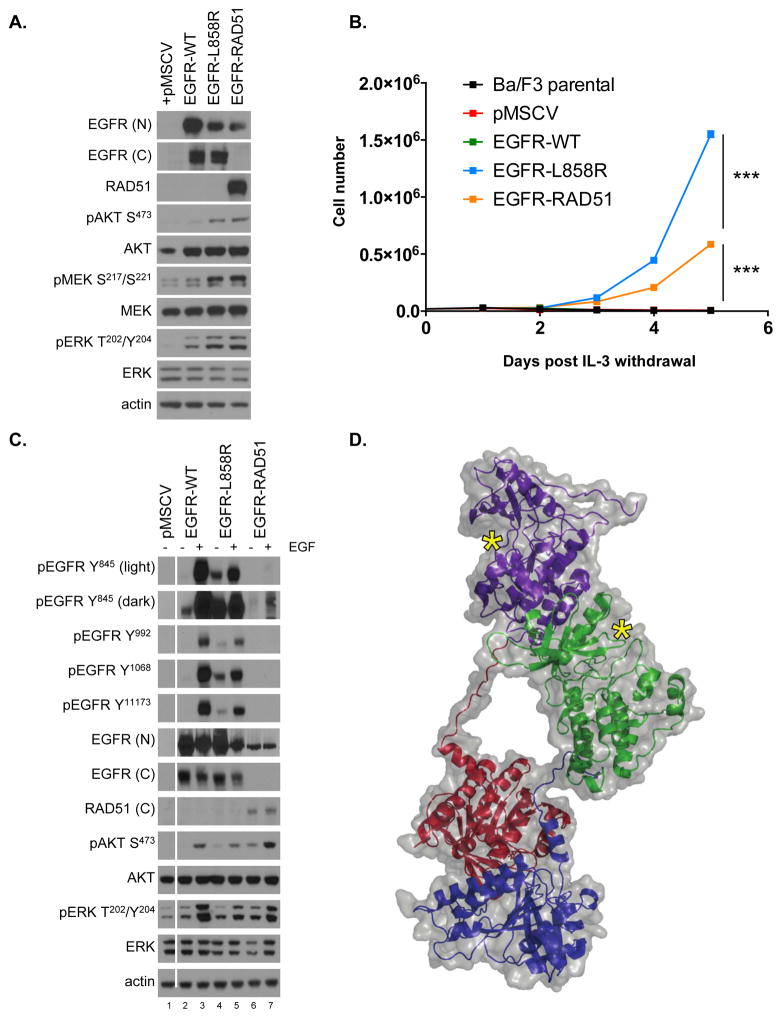
Figure 2. EGFR-RAD51 is an oncogenic EGFR alteration.
(A) Ba/F3 lines stably expressing pMSCV (vector only), EGFR-WT, EGFR-L858R or EGFR-RAD51 were subjected to western blot analysis with indicated antibodies. The three distinct EGFR variants were detected at the anticipated molecular weight (MW) of ~150kD. EGFR-RAD51 fusion is detected with both the N-terminal EGFR antibody [EGFR(N)] and with the RAD51 antibody. There is no cross reactivity between wild-type RAD51 protein, which has MW ~35kD, and the EGFR-RAD51 fusion.
(B) Ba/F3 cells transfected with indicated constructs (pMSCV = vector only) were grown in the absence of IL-3 and counted every 24 hours. *** = p < 0.0001.
(C) Ba/F3 expressing EGFR variants were serum starved for 16 hours, treated with 50 ng/mL EGF for 5 minutes, and subjected to western blot analysis with indicated antibodies.
(D) Ribbon diagram and space-filling model of the EGFR-RAD51 kinase domains illustrating the proposed mechanism of activation. Purple = first EGFR kinase domain; green = second EGFR kinase domain; red = first RAD51 partner; blue = second RAD51 partner, yellow asterisks = active sites.
EGFR contains several autophosphorylation sites in the C-terminal tail of the receptor (including tyrosines 992, 1068, and 1173) that positively regulate the transforming activity of EGFR by mediating downstream proliferative signaling (13). These autophosphorylation sites, which serve as docking sites for signaling adaptor proteins, are lacking in the EGFR-RAD51 fusion (Figs. 1B, 2C). Notably, however, EGFR-RAD51 contains tyrosine 845, a phosphorylation site within the kinase domain which is critical for complete EGFR function and transformation in NSCLC (14). The presence of tyrosine 845 may explain why EGFR-RAD51 is still able to activate downstream oncogenic signaling via the PI3K/AKT and MAPK pathways (Figs. 2A,C). Notably, these EGFR fusions also lack tyrosine 1045, the Cbl binding site which targets EGFR for degradation. Indeed, EGFR-RAD51 is more stable (has a slower turnover rate) compared with the WT EGF receptor (Supplementary Figs. S6A–B), suggesting that EGFR-RAD51 receptor stability might also play a role in its oncogenic properties. Taken together, these data support that EGFR-RAD51 is able to activate tumorigenic signaling and confer an oncogenic phenotype.
Computational modeling of EGFR-RAD51
The EGFR tyrosine kinase is activated through ligand-mediated formation of asymmetric (N-lobe to C-lobe) dimers (15). Kinase fusions, on the other hand, commonly share a mechanism of activation whereby the fusion partner drives dimerization of the kinase and leads to ligand-independent activation (16). Given the presence of RAD51, a self-assembling filamentous protein (17), we hypothesized that the EGFR-RAD51 fusion protein can form such partner-driven dimers. To validate this hypothesis, we modeled EGFR-RAD51 based on available experimental structures of RAD51 and the active asymmetric EGFR dimer. Conformational loop sampling with Rosetta demonstrates that it is geometrically feasible for EGFR kinase subunits to adopt the asymmetric (active) dimeric conformation when fused to RAD51 (Fig. 2D). Further, the concatenation of RAD51 subunits could bring tethered EGFR kinase domains close together, increasing their local concentration, and leading to further EGFR activation (Supplementary Fig. S7). Although this structural modeling demonstrates that the EGFR-RAD51 is geometrically capable of forming active dimers, further experimental data are needed to confirm this mechanism.
EGFR-RAD51 can be therapeutically targeted with existing EGFR inhibitors
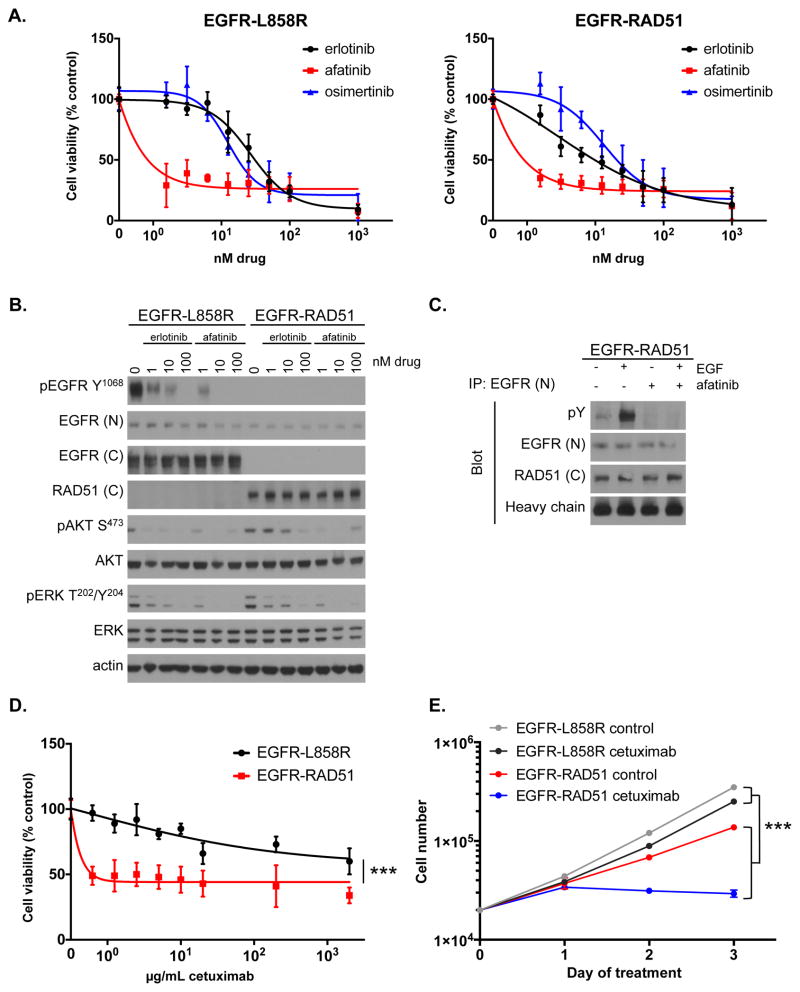
The finding of recurrent EGFR fusions in lung cancer is of particular interest because EGFR TKIs have proven an effective therapeutic strategy for tumors harboring certain EGFR mutations. Therefore, we sought to determine the effectiveness of EGFR TKIs against EGFR-RAD51. We treated Ba/F3 cells expressing EGFR-RAD51 with erlotinib (1st-generation reversible EGFR TKI), afatinib (2nd-generation irreversible EGFR/HER2 TKI), and osimertinib (3rd-generation irreversible EGFR TKI) to assess the effects of these inhibitors on cellular proliferation. EGFR-L858R served as a positive control in this experiment. Each TKI effectively inhibited the growth of Ba/F3 cells expressing EGFR-RAD51 to varying degrees (Fig. 3A, Supplementary Table S3). Downstream MAPK and PI3K/AKT signaling was also inhibited with TKI treatment (Fig. 3B). The on-target effect of EGFR TKIs could be observed when blotting for phospho-tyrosine from immunoprecipitated EGFR-RAD51 protein (Fig. 3C) and when observing the phosphorylation status of tyrosine 845, which is included in the fusion protein (Supplementary Fig. S8). Finally, we tested the effects of the FDA-approved EGFR antibody, cetuximab, in our cell culture models. Cetuximab binds to the EGFR extracellular domain and blocks the binding of growth factors, such as EGF (18). In contrast to EGFR-L858R, proliferation of Ba/F3 cells expressing EGFR-RAD51 was potently inhibited by cetuximab (Figs. 3D–E, Supplementary Fig. S9). Together, these results show that the EGFR-RAD51 can be potently inhibited by a variety of EGFR-targeted agents, suggesting several intriguing clinical avenues.
Figure 3. EGFR-RAD51 is therapeutically targetable with EGFR inhibitors.
(A) Ba/F3 lines stably expressing EGFR-L858R or EGFR-RAD51 were treated with increasing doses of erlotinib, afatinib, or osimertinib for 72 hours. Cell titer blue assays were performed to assess cell viability. Each point represents sextuplicate replicates. Data are presented as the mean percentage of viable cells compared to vehicle control ± standard deviation (s.d.).
(B) Ba/F3 lines stably expressing EGFR-L858R or EGFR-RAD51 were treated with increasing doses of erlotinib or afatinib for two hours and subjected to western blot analysis with indicated antibodies.
(C) Ba/F3 cells stably expressing EGFR-RAD51 were serum starved for 16 hours and then treated with 100 nM afatinib for 1 hour followed by 50 ng/mL EGF for 5 minutes. EGFR was immunoprecipitated from cellular lysates with an antibody targeting the EGFR N-terminus and then subjected to western blot analysis with indicated antibodies.
(D) Ba/F3 cells stably expressing EGFR-L858R or EGFR-RAD51 were treated with increasing doses of cetuximab for 72 hours. Cell titer blue assays were performed to assess cell viability. Each point represents sextuplicate replicates. Data are presented as the mean percentage of viable cells compared to vehicle control ± standard deviation (s.d.).
(E) Ba/F3 cells stably expressing EGFR-L858R or EGFR-RAD51 were treated with 5 μg/mL cetuximab and counted every 24 hours. Each point represents the average of triplicate replicates ± standard deviation (s.d.).
Discussion
Just over ten years ago, ‘canonical’ EGFR point mutations and short indels in the kinase domain were detected retrospectively by PCR-based ‘hotspot’ testing in patients with lung cancer who responded to EGFR TKI therapy (1–3). Assessing for these ‘canonical’ EGFR mutations is now the accepted standard of care worldwide for patients with lung cancer. Here, by utilizing a comprehensive NGS assay that interrogates the entire coding region of EGFR (as well as introns 7, 15, 24, 25, and 26), we identified novel EGFR fusions in lung cancer patients—EGFR-RAD51 and EGFR-PURB—that would otherwise have gone undetected by the standard of care.
While distinct EGFR fusions have previously been observed in glioma (19), this is the first documentation of patients with EGFR fusion positive tumors that derived significant and sustained anti-tumor responses from treatment with the EGFR TKI, erlotinib. Our in vitro work also hints at afatinib being potent against EGFR-RAD51—highlighting that the structural effects of the EGFR mutation, the resultant conformation of the EGFR kinase domain, and the type of EGFR inhibitor are all important factors in determining the efficacy of a specific EGFR TKI against a particular EGFR mutation (20).
Interestingly, EGFR-RAD51 fusions were markedly sensitive to both EGFR TKIs and the EGFR antibody, cetuximab. While some unselected NSCLC patients respond to cetuximab, the monoclonal anti-EGFR antibody adds minimal benefit for most patients—even when combined with chemotherapy (21,22). Previous work has shown that cetuximab sensitivity is correlated with asymmetric dimerization (23). As our modeling suggests that the EGFR-RAD51 fusion is activated by virtue of constitutive dimerization, therefore, these findings suggest a unique molecular cohort that may benefit from cetuximab. Further, these findings provide a rationale for therapeutically targeting this subset of lung cancers with a wide array of anti-EGFR therapies, many of which are already FDA-approved. On-going work will elucidate whether the deregulated RAD51 component of the EGFR-RAD51 fusion protein may also be a therapeutic vulnerability for treatment with platinum-based and PARP-inhibitor therapies.
Our experimental work also demonstrates that EGFR-RAD51 fusions are oncogenic and able to mediate downstream signaling through the MAPK and PI3K/AKT pathways. While this may seem surprising, given that EGFR-RAD51 fusions lack the C-terminal tail known to be important for EGFR signal transduction, analyses of several cancer types have identified EGFR C-terminal deletions as recurrent and transforming events (24–26). The exact mechanism whereby these EGFR C-terminal deletions activate the EGFR kinase domain and confer transforming properties remains unclear. In the case of EGFR-RAD51 fusions, we believe there is a unique role for RAD51 given the specific fusion event’s recurrence. A common characteristic of tyrosine kinase fusions is that the fusion partner (here, RAD51) contributes an oligomerization domain, which promotes activation of the kinase (27). As demonstrated by our structural modeling, EGFR-RAD51 fusion proteins could be activated by virtue of RAD51 oligomerization. Similarly, structural modeling has shown that PURB proteins can self-associate in the absence of nucleic acids (28). Additional experimental work will be required to determine whether activation of EGFR fusion proteins is driven by asymmetric dimerization of the EGFR tyrosine kinase domain, dimerization through the known partner oligomerization interface, or both.
The cases presented here highlight that adjusting our strategy and using newly available tools, such as comprehensive NGS tests, could prove useful in detecting alternative ways in which the EGFR pathway is altered (and can be targeted) in tumors. Based on the observation that the EGFR-RAD51 fusions detected in the first two patients occurred with breakpoints in EGFR intron 24, we defined further fusion events by the presence of a genomic breakpoint in EGFR exons 23 through intron 25. While we limited our investigation to these parameters, we cannot exclude that other EGFR rearrangements may exist in lung cancer outside of these parameters. Refinements in the assay may help discover more clinically relevant EGFR fusions or alterations in the future.
Methods
Cell culture
Ba/F3 cells were purchased from DSMZ. Plat-GP cells were purchased from CellBioLabs. NR6 cells have been previously described (12). Ba/F3 cells were maintained in RPMI 1640 medium (Mediatech, Inc.). NR6 and Plat-GP cells were maintained in DMEM (Gibco). Media was supplemented with 10% heat inactivated fetal bovine serum (Atlanta Biologicals) and penicillin-streptomycin (Mediatech, Inc.) to final concentrations of 100 U/ml and 100 μg/ml, respectively. The Ba/F3 cell line was supplemented with 1 ng/mL murine IL-3 (Gibco). The Plat-GP cell line was cultured in the presence of 10 μg/mL blasticidin (Gibco). All cell lines were maintained in a humidified incubator with 5% CO2 at 37°C and routinely evaluated for mycoplasma contamination. Besides verifying the status of EGFR mutations in cell lines, no additional cell line identification was performed.
In vitro analysis of the EGFR-RAD51 fusion protein
Plasmids containing EGFR-RAD51 were constructed based on the reported genomic sequence (Supplementary Fig. S10). NR6 and Ba/F3 cells were transduced with retrovirus encoding EGFR variants. Functional analyses, including colony formation, proliferation, and response to EGFR inhibitors, were performed as previously described (7).
Immunoprecipitation and immunoblotting
For immunoprecipitation, cells were harvested, washed in PBS, and lysed in NDLB buffer (1% Triton-X-100, 137 mM NaCl, 10% Glycerol, 20 mM Tris·HCl, pH 8.0) with freshly added 40 mM NaF, 1 mM Na-orthovanadate, and protease inhibitor mini tablets (Thermo Scientific). Protein was quantified using protein assay reagent and a SmartSpec plus spectrophotometer (Bio-Rad) per the manufacturer’s protocol. 300–500 μg of lysates were subjected to overnight immunoprecipitation with 2 μg N-term EGFR clone 528 (Santa Cruz#120; 10 μL). Antibody was precipitated with Protein A Dynabeads (Invitrogen). Immunoblotting was then performed as described below. Please see the Supplementary Methods for details of all of the antibodies used in this study.
For immunoblotting, cells were harvested, washed in PBS, and lysed in RIPA buffer (150 mM NaCl, 1% Triton-X-100, 0.5% Na-deoxycholate, 0.1% SDS, 50 mM Tris·HCl, pH 8.0) with freshly added 40 mM NaF, 1 mM Na-orthovanadate, and protease inhibitor mini tablets (Thermo Scientific). Protein was quantified (as detailed above) and 20μg of lysates were subjected to SDS-PAGE. Protein was transferred to PVDF membranes (Millipore) at 1000 mA·hr, blocked in 5% BSA, and incubated with antibodies as detailed above. Detection was performed using Western Lightning ECL reagent (Perkin Elmer) and autoradiography film paper (Denville). Samples analyzed with N-term EGFR clone 528 were prepared under non-reducing and non-boiled conditions.
Cell viability, counting, and clonogenic assays
For viability experiments, cells were seeded at 5,000 cells/well in 96-well plates and exposed to treatment the following day. At 72 hours post drug addition, Cell Titer Blue reagent (Promega) was added, and fluorescence at 570 nm was measured on a Synergy MX microplate reader (Biotek) according to the manufacturer’s instructions. For cell counting experiments, cells were seeded at 10,000 cells/well in 24-well plates in the absence of 1 ng/mL IL-3. Every 24 hours, cells were diluted 20 fold and counted using a Z1 Coulter Counter (Danaher). For cell counting experiments with drug, cells were seeded at 20,000 cells/well in 12-well plates in the absence of 1 ng/mL IL-3. Drugs were added at the following concentrations: erlotinib 500 nM, afatinib 50 nM, osimertinib 500 nM, cetuximab 5 μg/mL. Every 24 hours, cells were diluted 20 fold and counted using a Z1 Coulter Counter (Danaher). Viability assays were set up in sextuplicate, clonogenic assays were set up in triplicate, and cell-counting assays in triplicate. All experiments were performed at least three independent times. Data are presented as the percentage of viable cells compared to control (vehicle only treated) cells. To determine the IC50s, regressions were generated as asymmetric sigmoidal dose-response curves using Prism 6 (GraphPad).
Structural modeling of the EGFR-RAD51 fusion
The sequence of the EGFR-RAD51 fusion protein was used to generate a structural model based on the crystal structure templates 1SZP.PDB (S. cerevisiae Rad51) (17) and 2GS6.PDB (human EGFR) (15). PyMOL version 1.5.0.3 (Schrödinger) was used to combine two monomers of yeast RAD51 and two kinase domains of EGFR into a single template structure for input to modeling. The N-termini of the RAD51 domains were positioned close to the C-termini of the EGFR domains to represent the fusion result. Modeller version 9.14 (29) was then used to generate the dimeric model of the fusion protein structure. The conformational space for the dimer was then sampled using Rosetta version 2015.05 (30). A total of 20,000 independent modeling runs were performed using kinematic loop closure. To illustrate the arrangement of the filament, the crystallographic symmetry records from 1SZP. PDB were then used to construct eight additional copies of the complex in PyMOL.
Clinical data and tumor genotyping
All patient data was acquired under Institutional Review Board (IRB)–approved protocols. Informed consent was obtained from all patients. Samples were de-identified, protected health information reviewed according to the Health Insurance Portability and Accountability Act (HIPAA) guidelines, and studies conducted in accordance with the Declaration of Helsinki. Genomic profiling of tumor samples was performed using a hybrid capture-based NGS diagnostic platform (FoundationOne) (9).
Identification of EGFR genomic alterations in lung cancer diagnostic specimens
The database of >56,000 Foundation Medicine clinical cases (Primary_150929_114735, November 2nd, 2015) was interrogated for rearrangement class events to identify those cases likely to harbor a EGFR fusion event using the FoundationOne Molecular Information Browser v0.8. Cases involving an event with a genomic breakpoint in EGFR exons 23 through intron 25 were manually investigated to evaluate the potential of each individual rearrangement. Exon boundaries were chosen based on the index cases (EGFR-RAD51 harboring a genomic breakpoint in EGFR intron 24).
Statistics and data presentation
All experiments were performed using at least three technical replicates and at least two independent times (biological replicates). For statistical analyses, all biological and technical replicates were pooled to perform an integrated assessment on the differences among groups. To determine the differences in cell counts, time, and dose trends, and in order to account for the dependence of technical replications and repeated measurements, the linear mixed model was used to perform the analysis. The assumption of normality for mixed model was investigated. If necessary, data was transformed using log transformation for the linear mixed model. R3.2.2 (www.R-project.org) was used to perform all statistical analyses.
Each figure or panel shows a single representative experiment with the statistical significance derived from integrated experimental analyses—as described above. Unless indicated otherwise, data is presented as mean ± standard deviation. Western blot autoradiography films were scanned in full color at 600 dpi, desaturated in Adobe Photoshop CC, and cropped in Powerpoint. Genomic and proteomic diagrams were created in Adobe Illustrator CC. Patient images were cropped to highlight the region of interest. No other image alterations were made.
Supplementary Material
Statement of Significance.
We report for the first time the identification and therapeutic targeting of EGFR C-terminal fusions in patients with lung cancer and document responses to the EGFR inhibitor, erlotinib, in 4 patients whose tumors harbored EGFR fusions. Findings from these studies will be immediately translatable to the clinic as there are already several approved EGFR inhibitors.
Acknowledgments
We would like to thank the patients and their families. We are grateful to William Pao and Catherine Meador for critical review of the manuscript.
Financial support: This study was supported in part by the National Institutes of Health (NIH) and National Cancer Institute (NCI) R01CA121210 (CML) and P01CA129243. Research was supported by the 2015 AACR-Genentech BioOncology Career Development Award for Cancer Research on the HER Family Pathway, Grant Number 15-20-18-LOVL (to CML). CML, JNG, and JHS were supported by a V Foundation Scholar-in-Training Award. CML was additionally supported by a Damon Runyon Clinical Investigator Award and a LUNGevity Career Development Award. JNG was additionally supported by F30CA206339 and MSTP grant T32GM007347. Work in the Meiler laboratory is supported through the NIH (R01GM080403, R01GM099842, R01DK097376, R01HL122010, R01GM073151) and the NSF (CHE1305874). TV and BJG were part of the Genomic of Young Lung Cancer study (GoYLC, NCT02273336), which was funded by the Bonnie J. Addario Lung Cancer Foundation, Peter Barker Foundation, Genentech, Beth Longwell Foundation, Schmidt Legacy Foundation, and Upstage Lung Cancer.
Abbreviations
- EGFR
epidermal growth factor receptor
- TKI
tyrosine kinase inhibitor
- NSCLC
non-small cell lung cancer
- PR
partial response
- FDA
The United States Food and Drug Administration
- MAPK
mitogen activated protein kinase
- PI3K
phosphatidylinositol-3-kinase
- Indels
insertions/deletions
Footnotes
Author disclosures: KK has been a consultant for Celgene and has served on advisory boards for Boehringer-Ingelheim and Arog Pharmaceuticals. VP is an employee of Janssen Research & Development, LLC. BES has been a consultant for AstraZeneca and Clovis Oncology in addition to being a speaker for Merck, Celgene, Amgen, and Eisei. KG, DM, JSR, PJS, VAM, and SMA are employees of and have equity interest in Foundation Medicine, Inc. CML has served as a consultant for Pfizer, Novartis, Genoptix, Sequenom, Clovis, and Ariad and has been an invited speaker for Abbott and Qiagen. JNG, YKC, FJG, BJG, EI, TKO, SSR, SKR, BES, TV, AW, HC, YY, JHS, and JM report no conflicts of interest.
Author contributions: Provided direct patient care and generated clinical data: KK, YKC, FJG, BJG, TKO, VP, SSR, SKR, BES, TV, AW. Designed experiments: JNG, EI, CML, JHS, JM. Performed experiments: JNG, EI, YY, JHS. Generated and analyzed preclinical data: JNG, EI, CML, JHS, JM Created figures: JNG, JHS, CML. Wrote the manuscript: JNG, CML. Statistical analysis: HC. Oversaw research: PJS, JSR, DM, VAM, SMA, JM. Reviewed the data and final manuscript: All authors.
References
- 1.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA National Acad Sciences. 2004;101:13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science American Association for the Advancement of Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Mok TS, Wu Y-L, Thongprasert S, Yang C-H, Chu D-T, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 5.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 6.Sequist LV, Yang JC-H, Yamamoto N, O’Byrne K, Hirsh V, Mok T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. Journal of Clinical Oncology. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 7.Gallant J-N, Sheehan JH, Shaver TM, Bailey M, Lipson D, Chandramohan R, et al. EGFR Kinase Domain Duplication (EGFR-KDD) Is a Novel Oncogenic Driver in Lung Cancer That Is Clinically Responsive to Afatinib. Cancer Discovery. 2015;5:1155–63. doi: 10.1158/2159-8290.CD-15-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baik CS, Wu D, Smith C, Martins RG, Pritchard CC. Durable Response to Tyrosine Kinase Inhibitor Therapy in a Lung Cancer Patient Harboring Epidermal Growth Factor Receptor Tandem Kinase Domain Duplication. Journal of Thoracic Oncology. 2015;10:e97–9. doi: 10.1097/JTO.0000000000000586. [DOI] [PubMed] [Google Scholar]
- 9.Frampton GM, Fichtenholtz A, Otto GA, Wang K, Downing SR, He J, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nature Biotechnology Nature Publishing Group. 2013;31:1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 11.Daley GQ, Baltimore D. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210bcr/abl protein. Proc Natl Acad Sci USA. 1988;85:9312–6. doi: 10.1073/pnas.85.23.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pruss RM, Herschman HR. Variants of 3T3 cells lacking mitogenic response to epidermal growth factor. Proc Natl Acad Sci USA. 1977;74:3918–21. doi: 10.1073/pnas.74.9.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 14.Chung BM, Dimri M, George M, Reddi AL, Chen G, Band V, et al. The role of cooperativity with Src in oncogenic transformation mediated by non-small cell lung cancer-associated EGF receptor mutants. Oncogene Nature Publishing Group. 2009;28:1821–32. doi: 10.1038/onc.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J. An allosteric mechanism for activation of the kinase domain of epidermal growth factor receptor. Cell. 2006;125:1137–49. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Shaw AT, Hsu PP, Awad MM, Engelman JA. Tyrosine kinase gene rearrangements in epithelial malignancies. Nat Rev Cancer Nature Publishing Group. 2013;13:772–87. doi: 10.1038/nrc3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conway AB, Lynch TW, Zhang Y, Fortin GS, Fung CW, Symington LS, et al. Crystal structure of a Rad51 filament. Nat Struct Mol Biol. 2004;11:791–6. doi: 10.1038/nsmb795. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Schmitz KR, Jeffrey PD, Wiltzius JJW, Kussie P, Ferguson KM. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–11. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nature Communications Nature Publishing Group. 2014;5:1–10. doi: 10.1038/ncomms5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vivanco I, Robins HI, Rohle D, Campos C, Grommes C, Nghiemphu PL, et al. Differential Sensitivity of Glioma- versus Lung Cancer-Specific EGFR Mutations to EGFR Kinase Inhibitors. Cancer Discovery. 2012;2:458–71. doi: 10.1158/2159-8290.CD-11-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirker R, Pereira JR, Szczesna A, Pawel von J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373:1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 22.Kim ES, Neubauer M, Cohn A, Schwartzberg L, Garbo L, Caton J, et al. Docetaxel or pemetrexed with or without cetuximab in recurrent or progressive non-small-cell lung cancer after platinum-based therapy: a phase 3, open-label, randomised trial. Lancet Oncol. 2013;14:1326–36. doi: 10.1016/S1470-2045(13)70473-X. [DOI] [PubMed] [Google Scholar]
- 23.Cho J, Chen L, Sangji N, Okabe T, Yonesaka K, Francis JM, et al. Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization. Cancer Research American Association for Cancer Research. 2013;73:6770–9. doi: 10.1158/0008-5472.CAN-13-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–77. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho J, Pastorino S, Zeng Q, Xu X, Johnson W, Vandenberg S, et al. Glioblastoma-Derived Epidermal Growth Factor Receptor Carboxyl-Terminal Deletion Mutants Are Transforming and Are Sensitive to EGFR-Directed Therapies. Cancer Research. 2011;71:7587–96. doi: 10.1158/0008-5472.CAN-11-0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing. Cell Elsevier Inc. 2012;150:1107–20. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 28.Rumora AE, Steere AN, Ramsey JE, Knapp AM, Ballif BA, Kelm RJ. Isolation and characterization of the core single-stranded DNA-binding domain of purine-rich element binding protein B (Purβ) Biochemical and Biophysical Research Communications. 2010;400:340–5. doi: 10.1016/j.bbrc.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eswar N, Eramian D, Webb B, Shen M-Y, Sali A. Methods Mol Biol. Vol. 426. Totowa, NJ: Humana Press; 2008. Protein structure modeling with MODELLER; pp. 145–59. [DOI] [PubMed] [Google Scholar]
- 30.Simons KT, Kooperberg C, Huang E, Baker D. Assembly of protein tertiary structures from fragments with similar local sequences using simulated annealing and Bayesian scoring functions. Journal of Molecular Biology. 1997;268:209–25. doi: 10.1006/jmbi.1997.0959. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.