Abstract
To identify nuclear DNA (nDNA) oxidative phosphorylation (OXPHOS) gene mutations using cultured cells, we have developed a complementation system based on retroviral transduction with a full length cDNA expression library and selection for OXHOS function by growth in galactose. We have used this system to transduce the Chinese hamster V79-G7 OXPHOS mutant cell line with a defect in mitochondrial protein synthesis. The complemented cells were found to have acquired the cDNA for the bS6m polypeptide of the small subunit of the mitochondrial ribosome. bS6m is a 14 kDa polypeptide located on the outside of the mitochondrial 28S ribosomal subunit and interacts with the rRNA. The V79-G7 mutant protein was found to harbor a methionine to threonine missense mutation at codon 13. The hamster bS6m null mutant could also be complemented by its orthologs from either mouse or human. bS6m protein tagged at its C-terminus by HA, His or GFP localized to mitochondrion and was fully functional. Through site-directed mutagenesis we identified the probable RNA interacting residues of the bS6m peptide and tested the functional significance of mammalian specific C-terminal region. The N-terminus of the bS6m polypeptide functionally corresponds to that of the prokaryotic small ribosomal subunit, but deletion of C-terminal residues along with the zinc ion coordinating cysteine had no functional effect. Since mitochondrial diseases can result from hundreds to thousands of different nDNA gene mutations, this one step viral complementation cloning may facilitate the molecular diagnosis of a range of nDNA mitochondrial disease mutations.
Keywords: mitochondrial diseases, complementation cloning, OXPHOS, mitochondrial ribosomes, bS6m
INTRODUCTION
The mitochondrial genome consists of one to two thousand nDNA genes and hundreds to thousands of copies per cell of the mitochondrial DNA (mtDNA). The mtDNA codes for 13 essential polypeptides for the mitochondrial energy generating system, oxidative phosphorylation (OXPHOS), plus the 22 tRNAs and the 12S and 16S ribosomal RNAs for their translation on the mitochondria-specific ribosomes. All of the ribosomal proteins and translation factors are nDNA coded.
Mitochondrial dysfunction is being implicated in an increasing array of rare and common diseases. While pathogenic mtDNA mutations can generally be identified due to their maternal inheritance, identification of causal nDNA mitochondrial gene mutations is problematic since anyone of the thousand plus nDNA genes could be responsible. Currently, nDNA mitochondrial gene mutations are identified by whole genome or exome sequencing followed by differentiating among the multiple candidate genes by identifying additional independent cases and/or by functional studies. This can particularly problematic if the clinician is confronted by an isolated case.
An alternative approach for identifying mutant nDNA mitochondrial genes using the cells from a single patient could be to complement the genetic defect by transfection with a cDNA expression library, selection for the clones in which the OXPHOS defect is complemented, and isolation of the complementing plasmid. Here, we use this approach to directly clone a complementing cDNA for a respiration-deficient Chinese hamster cell line V79-G7 with a complete deficiency in mitochondrial protein synthesis [1]. Respiration-deficient cells can grow normally in medium with an abundant supply of glucose for glycolysis, but are unable to grow in medium in which glucose has been substituted by the obligate oxidizable substrate galactose, DME-Gal [2]. The mutated gene cloned for the V79-G7 cell line proved to be a polypeptide of the small subunit of the mitochondrial ribosome.
The defective genes of other respiration-deficient Chinese hamster cell lines have been isolated by more conventional biochemical and somatic cell genetic approaches. These are also able to complement the mutant cells permitting growth in DME-Gal medium. Among these are mutations in two complex I genes, the X-linked MWFE (NDUFA1) and ESSS (NDUFB11) protein genes [3, 4].
The successful restoration of mitochondrial protein synthesis and OXPHOS function in the V79-G7 cell line by complementation with a cDNA expression library validates the use of the random cDNA library complementation for cloning defective OXPHOS genes. The identification of the mutant protein as a subunit of the small mitochondrial ribosome provides a model system to study the importance of mitochondrial protein synthesis in mammalian cells.
EXPERIMENTAL PROCEDURES
Cell lines and cell culture
The V79-G7 cells were derived from the Chinese hamster V79 cell line (CCL93, American Type Culture Collection). Respiration-deficient (res−) cells were grown in DME medium with 4.5 mg/ml glucose (DME-Glu) supplemented with 10% fetal bovine serum (FBS) and nonessential amino acids. With 1 mg/ml galactose substituted for glucose (DME-Gal) and with 10% dialyzed FBS conditions the medium becomes non-permissive for res− cells [2].
Construction of a Chinese hamster retroviral cDNA library
The cDNA library was prepared using the Creator SMART Library Construction Kit (Clontech), starting with 2 μg of total RNA from CCL16-B1 Chinese hamster lung fibroblasts. The library was cloned into the retroviral vector pLib, titered by transforming electro-competent E. coli cells, and the transformed cells were amplified by plating on 30-40 large LB-Agar plates with ampicillin and grown overnight at 37°C, resulting in at least 3 × 106 clones. The colonies were then pooled by scraping the plates, and DNA was prepared using a Plasmid Maxiprep Kit (Qiagen).
Retrovirus production and infection
HEK-293T cells were plated at 3×105 cells/well in 0.5% gelatin coated 6 well plates and incubated overnight. Cells in each well were transfected, using the BioT reagent (Bioland), with 3 μg of DNA consisting of 2 μg of plasmid cDNA library, 0.1 μg of VSVG envelope plasmid, and 0.9 μg MMLV Gag-pol plasmid pHIT 60. At 48-72 hours post transfection the viral supernatants were harvested, centrifuged at 600×g for 5 minutes, and filtered through a 45 micron filter. The viral particles were precipitated from the filtered suspension by adding 8 μg/ml polybrene plus 3 mM CaCl2 and centrifuging at 700×g for 30 min at room temperature on to the adherent hamster mutant cells previously grown overnight in a six-well plate, followed by incubating at 37°C. Seventy-two hours after viral infection the cells were transferred to DME-Gal medium for at least two weeks to select for clones that had been successfully complemented by a cDNA.
Recovery of viral inserts from complemented clones
Genomic DNA was isolated from complemented cell populations using a standard protocol [5], and the complementary cDNA inserts were recovered by PCR using the KlenTaq DNA polymerase mix (Sigma) and primers flanking the multiple cloning site of the pLib vector: the 5' primer 5'AGCCCTCACTCCTTCTCTAG3' and the 3' primer 5'ACCTACAGGTGGGGTCTTTCATTCCC-3'. PCR reactions used 100–300 ng genomic DNA in a 50 μL.
Transfections/ Expression Plasmids
The cloned bS6m cDNA was inserted into the first exon of the polycistronic pTRIDENT-14 neo vector (pIS2104) [3]. Primers used for cloning were the 5’ Primer 5’ACGAATTCGTCGGGGGCCATGCCCCGC-3’, and the 3’Primer 5’ATGCTAGCGATGCCTCGGTGCTAGAGC-3’. The primers have EcoR1 (in the 5’ primer) or Nhe1 (in the 3’ primer) sites for cloning into the unique pTRIDENT-14 neo vector EcoR1 and Nhe1 sites. Consequently, the expressed protein can be tagged with either the HA, His or GFP sequences at the carboxy terminal. The complete complementing retroviral insert was sequenced and the cDNA open reading frame found to code for the hamster bS6m protein from a comparison with human and mouse cDNA sequences from the available databases.
To confirm that the bS6m cDNA complements the protein synthesis mutant, 2 × 105 V79-G7 cells were seeded in a 6-well tissue culture plate overnight, and then transfected with 2.0 μg of plasmid vector carrying bS6m cDNA using 5 μl of BioT transfection reagent (Bioland). Stable transfectants were selected using 800 μg/ml geneticin (G418) added 48 hrs post transfection. After two weeks antibiotic resistant colonies were tested for growth in DME-Gal medium [2]. Pooled bS6m expressing cells were used for further analysis.
Site directed mutagenesis
Site directed mutagenesis of the hamster bS6m cDNA was performed in the pIS2104 plasmid backbone; it was carried out using the Quick Change mutagenesis kit (Promega) [6]. The primers used for the studies are listed in Table 1.
Table 1.
Primers used for mutagenesis:
| R3A-F:TCGGGGGCCATGCCCgcCTACGAGTTGGCTTTG | R3A-R:CAAAGCCAACTCGTAGgcGGGCATGGCCCCCGA |
| Y4F-F:GGGGCCATGCCCCGCTtCGAGTTGGCTTTGGCT | Y4F-R:AGCCAAAGCCAACTCGaAGCGGGGCATGGCCCC |
| E5A-F:GCCATGCCCCGCTACGcGTTGGCTTTGGCTTTG | E5A-R:CAAAGCCAAAGCCAACgCGTAGCGGGGCATGGC |
| R90A-F:GACGTTGATGTGGTTgcACCAAACGTCGTGAAA | R90A-R:TTTCACGACGTTTGGTgcAACCACATCAACGTC |
| V93T-F:GTGGTTAGACCAAACacCGTGAAACACCCTCTG | V93T-R:CAGAGGGTGTTTCACGgtGTTTGGTCTAACCAC |
| K95A-F:AGACCAAACGTCGTGgcACACCCTCTGACCCAG | K95A-R:CTGGGTCAGAGGGTGTgcCACGACGTTTGGTCT |
| S53A-F:CTGCCTTACAGGATGgCCAGCCACGGCCAGCAG | S53A-R:CTGCTGGCCGTGGCTGGcCATCCTGTAAGGCAG |
| S73A-F:TTTTATGCCCCCACAgcTTCTGTGGACAGCATA | S73A-R:TATGCTGTCCACAGAAgcTGTGGGGGCATAAAA |
| S77A-F:ACAAGTTCTGTGGACgcCATATTGGATCACTTG | S77A-R:CAAGTGATCCAATATGgcGTCCACAGAACTTGT |
| S119A-F:GAAGAAAAGCTGTATgCAACAAAGAGGAGAAAG | S119A-R:CTTTCTCCTCTTTGTTGcATACAGCTTTTCTTC |
| K124R-F:TCAACAAAGAGGAGAAgGAAGGCTAGCTACCCT | K124R-R:AGGGTAGCTAGCCTTCcTTCTCCTCTTTGTTGA |
| K125R-F:ACAAAGAGGAGAAAGAgGGCTAGCTACCCTTAC | K125R-R:GTAAGGGTAGCTAGCCcTCTTTCTCCTCTTTGT |
Isolation of mitochondria and mitochondrial fractions
Mitochondria were isolated from 1 × 109 cells, washed twice with PBS buffer and harvested by trypsinization [7]. The pellets were suspended in 5 ml SM buffer (50 mM Tris.HCl, pH 7.4, 0.25 M sucrose, 2 mM EDTA) and homogenized using a tightly fitting Dounce homogenizer (30-35 up/down strokes). The homogenate was centrifuged twice at 625×G for 10 min at 4°C in order to remove unbroken cells and nuclei. The mitochondria were pelleted from the supernatant by centrifugation at 10,000 × g for 20 minutes at 4° C and suspended in 0.1 ml of the SM buffer.
Immunochemical assays and antibodies
For Western blot analysis, cells were lysed in PBS buffer with 2% Dodecyl-β-D-Maltoside (Sigma) on ice for 30 minutes. Solubilized protein supernatants (between 50 and 100 μg) were fractionated by SDS-PAGE and transferred to Immobilon-P (0.2 μ) membranes. Anti-HA and anti-porin antisera were used at 1:5000 dilution, whereas the other antibodies were used at 1:1000 dilution. Horseradish peroxidase-conjugated secondary antibodies (anti-rabbit or anti-mouse) were used at 1:5000 dilution. Signals on the immunoblots were detected using an Enhanced Chemiluminescence system (ECL+Plus from Amersham).
The anti-MWFE antiserum was reported previously [8]. Sources of other antibodies were: anti-COXII, anti-30kDa, and anti-ATP5a (all human) from Molecular Probes, anti-porin from Calbiochem, anti-HA from Covance BabCo, anti-mouse and anti-rabbit secondary antibodies from BioRad Laboratories and Amersham Pharmacia Biotech, respectively.
Blue native polyacrylamide gel electrophoresis (BN-PAGE)
Mitochondrial respiratory complexes were separated by BN-PAGE [8]. Mitochondrial pellets equivalent to 400 μg of protein were solubilized with 800 μg of Dodecyl-β-D-Maltoside (Sigma) in 5 mM 6-aminohexanoic acid, 50 mM imidazole-HCl (pH 7.0) and 10% glycerol. To the solubilized samples Coomassie Brilliant Blue G-250 (Serva) was added at a dye/detergent ratio of 1:5 (w/w). Proteins were separated on a 4-13% acrylamide gradient gel, blotted for 3 h, and destained by washing with 100% methanol.
RESULTS
Complementation by the hamster cDNA library
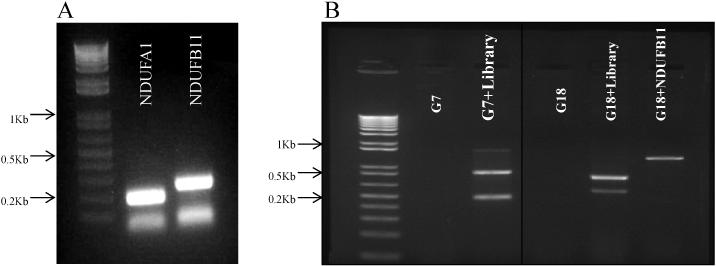
To optimize complementation potential of the hamster cDNA expression library, the cDNAs had to be complete such that the 5’-end would include the methionine start site and any potential N-terminal mitochondrial import sequences, and the library had to be of high complexity to include low abundance mRNAs (Figure 1). Therefore, at each step in preparation of the cDNA and viral libraries we checked for the presence of transcripts for NDUFA1 and NDUFB11 encoding subunits of complex I (Figure 1 and Figure 2).
Figure 1. Characterization of Retroviral Vector Cloned Chinese Hamster cDNA Library.
A: Size distribution of inserts of the CCL16-B1 hamster mRNA-derived cDNA library from cDNA bearing bacterial clones generated by PCR amplification of inserts using primers homologous to the pLib vector for sequences flanking the cDNA inserts and separated on agarose gels. Each lane shows the size of the cDNA insert in the individual clone. B: PCR amplification of NDUFA1 and NDUFB11 cDNAs from the cloned bacterial library using gene-specific primers.
Figure 2. Characterization of the Retroviral cDNA Library and Viral Transduction.
A: PCR amplification of NDUFA1 and NDUFB11 cDNAs from the retroviral RNA library using gene-specific primers. B: PCR amplification of the integrated retroviral cDNA inserts that complemented the protein synthesis defect of V79-G7 (G7 = V79-G7, complementation group VII; G7 + library = retroviral complemented G7 capable of growth in galactose) and the X-linked complex I NDUFB11 ESSS defect of V79-G18 (G18 = V79-G18, Complementation Group II; G18 + Library = retroviral complemented G18 capable of growth in galactose). Retroviral cDNA inserts amplified using flanking pLib vector primers. G18 + NDUFB11 = V79-G18 cells transformed with an NDUFB11 bearing retrovirus as a positive control. Note: The pLib-NDUFB11 PCR fragment (Panel B, last lane) is larger (than the complementing cDNA from library) due to the fact that the EcorI-Not1 fragment (approximately 400bp cDNA and about 0.5kb fragment from the vector backbone) from previously published pTrident-ESSS-HA fragment is cloned into the EcoRI-NotI digested pLib vector.
To optimize the efficiency of transduction with the cDNA expression library, we utilized the pLib retroviral vector packaged in HEK-293T cells expressing MMLV Gag-Pol and the vesicular stomatitis virus (VSV) G-protein associated with the retrovirus coat envelope protein (VSVG). The resulting virus can be produced at high viral titers with a high efficiency of infection (Figure 2, Panel A). The robust ability of the library to complement genetic defects was confirmed by demonstrating that it could complement the NDUFB11 ESSS gene defect in mutant V79-G18 cells [3] (Figure 2, Panel B)
We then used the library to transduce the V79-G7 cells and selected transductants in DME-Gal. Several clones were isolated and analyzed by PCR-based methods to confirm the presence of a retrovirus associated complementing hamster cDNA. One clone was isolated in which the cDNA was able to consistently restore the V79-G7 mitochondrial protein synthesis defect (Figure 2, Panel B). The complementing hamster cDNA was found to be the autosomal gene for the bS6m polypeptide of the small mitochondrial ribosomal subunit.
The bS6m cDNA was subcloned into a modified pTrident expression vector and this construct was able to efficiently complement all defects in the V79-G7 cells (Figure 3). As expected, the restoration of protein synthesis allowed the assembly of other imported proteins into the OXPHOS complexes required for growth in DME-Gal. Specifically, transduction of V79-G7 cells with the bS6m cDNA restored the synthesis of the mitochondrially encoded COXII peptide, generated complex V activity, and permitted recovery of complex I assembly and activity demonstrated by Western blots (Figure 3, panel A) and blue native gels (Figure 3, panel B). The nuclear-encoded MWFE subunit is very unstable and not detectable unless it becomes incorporated into a complex I [8].
Figure 3. Analysis of OXPHOS Complexes in Mitochondria Isolated from bS6m-HA Complemented V79-G7 Mutant Cells.
A: SDS-PAGE western shows restoration of the synthesis of the mtDNA coded COXII and nDNA coded MWFE polypeptides following transduction of the wild type bS6m cDNA into V79-G7 cells. Porin was used as a loading control. B: BN-PAGE western analysis showing that Complex I (probed with MWFE anti-sera) and Complex V (probed with ATPase a anti-sera) are restored by bS6m complementation of the V79-G7 cell line. The 30 kDa complex II subunit was used as a loading control.
When the bS6m cDNAs from mouse and humans were cloned into the pTrident expression vector, all were found to be able to complement the V79-G7 defect. Addition to the C-terminal end of the hamster bS6m peptide of epitope tags (HA, His, Tetracysteine) or polypeptides (EGFP and mini-SOG [9, 10] permitted confirmation that the bS6m protein is efficiently imported into the mitochondrion, with all of the C-terminal tagged constructs being able to complement the V79-G7 defect (Figure 4).
Figure 4. Demonstration that bS6m is Completely Imported into the Mitochondria Monitored by the Import of C-terminus Fluorescently Tagged Protein.
Hamster mutant cell line V79-G7 was stably transfected with a di-cistronic vector expressing the tagged bS6m peptide and transfectants selected in galactose. A: Cryo-EM of the small subunit of the bovine mitochondrial ribosome showing the Location of the bS6m polypeptide. B: bS6m protein C-terminus tagged with EGFP. C: bS6m protein C-terminus tagged with miniSOG tag.
The genotype of the V79-G7 mutant cells
In humans the bS6m gene maps on chromosome 21 at 21q21.3-q22 near or within the “Down's Syndrome Critical Region” [11]. In mammals the gene encompasses two large introns (~51.6 kb and 16.9 kb) and three small exons encoding the 125 amino acids. Alternate genes may also be expressed from this region [12].
To identify the inactivating mutation in the V79-G7 bS6m allele we recovered the bS6m cDNA from V79-G7 cells by RT-PCR and sequenced both strands. A T>C missense mutation was found within the open reading frame leading to substitution of the codon 13 methionine to threonine (M13T) (Figure 5, Panel A and B). To determine if the bS6m M13T substitution is deleterious, V79-G7 cells were transformed with an expression plasmid in which the mutation M13T had been introduced into the wild type bS6m cDNA by site-directed mutagenesis. Stable transfectants were obtained by selecting for the bicistronic plasmid with G418. Surprisingly, cells over-expressing M13T bS6m polypeptide survived selection in DME-Gal media, indicating that this mutated protein is not totally inactive. The bS6m peptide was found to have no mitochondrial targeting sequence at the N-terminus, so an internal peptide sequence must be responsible for mitochondrial import. The M13T mutation does not affect the mitochondrial translocation of the peptide, as indicated by the mitochondrial localization of the GFP-tagged mutated protein (Figure 6, Panel A & B). However, the growth rate of the bS6m M13T over-expressing cells was significantly reduced relative to that of V79-G7 cells transformed with wild type bS6m (Figure 5, Panel C). It is not clear why over-expression of the mutated peptide leads to partial restoration of mitochondrial protein synthesis.
Figure 5. Analysis of bS6m Mutation Identified in the V79-G7 Mutant Cells.
A: Sequence of the mutant bS6m cDNA recovered from the V79-G7 cells showing the T>C transition mutation (upper panel = wild type, lower panel = V79-G7 with arrow indicating the mutant base). B: Interspecies alignment of the bS6m polypeptide with the position V79-G7 M13T missense mutation indicated by the red box. .
Figure 6. Functional Analysis of the bS6m cDNA.
A: Mitochondrial localization of the M13T mutant of the bS6m-GFP cDNA in transformed cells. B: Restoration of the expression of bS6m polypeptide determined by Western blot of mutant (G7) cells, G7 cells complemented with the wild type bS6m cDNA, and G7 complemented with the M13T mutant bS6m. C: Differential growth rate of V79-G7 cells transformed with the wild type hamster bS6m-HA cDNA (blue line) or with a bS6m-HA cDNA into which the M13T missense mutation was introduced by site-directed mutagenesis (red line). The results are average of three independent experiments.
Since the b6Sm gene is autosomal and genomic sequencing revealed only one mutant allele, the V79-G7 cell line may have become homozygous for the mutant allele, or the second bS6m allele may be inactivated by an epigenetic mechanism as previously observed in the “pseudodiploid” cultured Chinese hamster cells [13]. To determine if there was a normal but inactive bS6m allele, V79-G7 cells were grown in 5-aza-cytidine, which reverses the hypermethylation of CpG islands in the promoters of inactivated genes reactivating their expression (http://mpromdb.wistar.upenn.edu). Exposure to 5-aza-cytidine did restore V79-G7 cell growth on galactose. Therefore, the most likely genotype for the V79-G7 cell line is that one allele is mutated harboring the M13T missense substitution in the expressed b6Sm protein, while the second (normal) allele are silenced by methylation.
The mitochondrial ribosomal protein bS6m
The bS6m protein is 125 amino acids in length and has a molecular mass of 14.2 kDa with no canonical cleavable N-terminal transit peptide [14, 15].The polypeptide is conserved from bacterial ribosomes to mammalian mitochondrial ribosomes [14-17]. While the V79-G7 mitochondrial ribosomal function could be restored by transformation with the human or mouse bS6m cDNAs, transformation of V79-G7 cells with a mitochondrially targeted prokaryotic RPS6 gene could not restore mitochondrial protein synthesis.
Combining structural information from cryo-electron microscopy has suggested a model of the small mitochondrial ribosomal subunit at a high resolution [14, 15, 18, 19]. The b6Sm/RPS6 subunit is localized on the surface of the small ribosomal subunit at some distance removed from the interface between the small and large ribosomal subunits and from the active sites for polypeptide synthesis and the mechanism of translation [14, 15, 19] (Figure 4, Panel A). There have been no studies (even in prokaryotic systems) to demonstrate its essential role in translation. However the mammalian peptide has recently been proposed to play a role in contributing a coordination site for a Zinc ion in combination with side chains of b18S and uL10m peptides [15].
Mutagenesis Studies
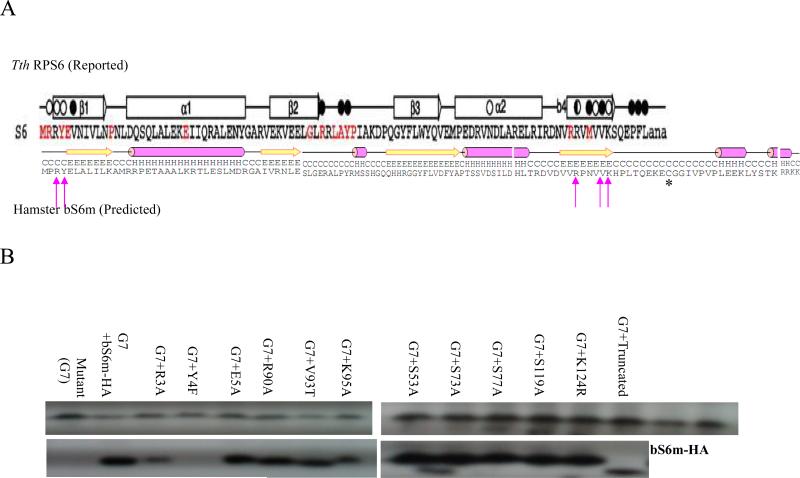
High resolution structures of the S6 subunit in combination with other ribosomal components have revealed a 70 kDa sub-complex in the central domain of the bacterial Thermus thermophilus 30S ribosomal subunit [20]. This complex contains a 104 nucleotide RNA fragment associated with polypeptides S6, S15, and S18. S6 makes contact with the RNA through amino acids arginine (R) 2, tyrosine (Y) 4, asparagine (N) 73, arginine (R) 87, valine (V) 90, and lysine (K) 92 (Thermus thermophilus amino acid numbering). Most of these residues are conserved in the hamster/mammalian protein, where they are renumbered as R3, Y4, R90, V93, and K95 (Figure 7, Panel A). Using site-directed mutagenesis, we changed each of the relevant hamster bS6m amino acid residues and tested the corresponding mutant protein for its ability to complement the V79-G7 null mutant. Replacement of tyrosine 4 with phenylalanine abolished bS6m function. This supports the importance of a hydrogen bond between Y4 and a phosphate in the 12S rRNA [20]. Removing the positively charged side chains of R3 or K95 also reduced bS6m function, but not as severely as alteration of Y4, since the R3 and K95 mutants allowed slow growth in galactose. Alteration of the other amino acid side chains predicted to interact with the 12S rRNA had no effect on the stability the polypeptide (Figure 7, Panel B) or on restoration of mitochondrial protein synthesis assayed by restoration of CoxII peptide (data not shown).
Figure 7. Structure-Function Analysis of bS6m Polypeptide.
A: bS6m amino acid sequence and regional amino acid motifs as determined by analysis of the Thermus thermophilus bacterial RPS6. Arrows indicate the conserved rRNA interacting residues. B: SDS-PAGE western analysis of mRSP6 expression in whole cell lysates of V79-G7 cells stably transfected with a tri-cistronic vector carrying bS6m-HA in which amino acid residues predicted to bind the rRNA from Thermus thermophilus RPS6 have been altered by site directed mutagenesis. G7 = V79-G7.
The mammalian RPS6 protein has a highly charged and conserved C-terminus, KRRKK-COOH. The Thermus thermophilus protein is shorter by 22 residues and does not contain a similar C-terminus motif. This additional motif was shown to have acquired a coordinating Zinc ion function in the mammalian mitochondrial ribosomes [15] probably through a cysteine residue (Figure 7, Panel A). However, deletion of 29 amino acids downstream of histidine 96 including the cysteine residue had no effect on the ribosome stability and activity (Figure 7, Panel B) as the truncated protein could fully restore the mitochondrial function. Hence, the function of the C-terminal amino acids of the bS6m polypeptide remains to be determined.
Proteomic analysis of bovine mitochondrial ribosomal proteins suggested that a substantial fraction of these subunits may be phosphorylated. Predicted phosphorylation scores for several serines in bS6m were very high. However, substitution of the serines 53, 73, 77 and 119 with alanines had no detrimental effect on the polypeptide's stability and function (Figure 7, Panel B).
DISCUSSION
Using a Chinese hamster cell model system we have developed the capacity to directly identify mutated nuclear genes encoding mitochondrial proteins by complementation using a specifically designed retroviral cDNA expression library. This approach was first validated successfully by isolating the X-linked NDUFB11 (ESSS) gene known to be mutated in a complex I deficient cell line characterized previously. The method was then employed to identify the gene defective in the hamster V79-G7 mitochondrial protein synthesis defective cell line. V79-G7 mitochondria retain hydrodynamically “normal” ribosomal subunits and all of the mtDNA transcripts, but are totally deficient in mitochondrial protein synthesis [21]. Our approach identified the missing function as that of the mRPS6/bS6m protein, a highly conserved subunit of prokaryotic and mitochondrial ribosomes. Considerable progress has been made in defining the polypepide composition of the mammalian mitochondrial ribosomes [14, 15, 22-27]. However, the location of the bS6m polypeptide [14, 15] on the surface of the 28S ribosome is known [19], although the actual function of the bS6m polypeptide beyond binding the small rRNA [20] remains to be elucidated (26). Our studies have demonstrated that this protein is absolutely essential for mitochondrial translation, but could not yet distinguish between a defect in initation or elongation. These steps in mitochondrial protein synthesis remain of great interest, since many details remain to be elucidated [28-37]. The absence of a significant 5'UTR on mitochondrial mRNAs poses a challenge in understanding initiation, and it is highly likely that initiation, and elongation are tightly coupled to the insertion of the resulting integral membrane proteins into the inner membrane and into complexes of the electron transport chain.
The availability of the V79-G7 mutant cell line in combination with the complementation by a variety of modified bS6m proteins presents opportunities for a more detailed study of the role of the b6Sm protein and its functional domains. Why, for example, is the C-terminal of the mammalian protein so highly conserved and highly positively charged (KRRKK-COOH) yet expendable? The location of the bS6m gene on chromosome 21 near or within the Down Syndrome Critical Region raises the possibility that duplication of bS6m might contribute to the pathophysiology of Down's syndrome. Down's syndrome cells have recently been found to manifest mitochondrial dysfunction, increased oxidative stress, and elevated somatic mtDNA mutations [38-42]. The cytoplasmic analogue of this protein (RPS6) has been shown to be a key component in cellular signal transduction pathways [43]. Might this be true for the mitochondrial bS6m?
The availability of a cDNA library complementation system should permit the cloning of other mutant genes in respiration-deficient Chinese hamster cell lines. One such complementation group of interest involves an X-linked complex I gene which is not one of the already known structural genes [44].
Valuable insights have been obtained from model systems in which essential mitochondrial proteins can be expressed from inducible promoters, allowing their synthesis/function to be turned on and subsequent biosynthetic mechanisms to be synchronized [45-47]. A cell line with an inducible bS6m construct would allow a further exploration of the assembly of the OXPHOS complexes, the coordination of protein import from the cytosol and protein synthesis in the mitochondrial matrix, and analysis of the possible interaction between the mitochondrial ribosomes and the nucleoid.
Finally, the successful demonstration of the use of a specifically designed cDNA expression library in characterizing mutant genes in respiration-deficient Chinese hamster cells provides us with an approach and methodology to identify the defective genes in human patients with mitochondrial diseases. To take advantage of this system for human studies, we have already prepared a full length and complete retrovirus expression library with human cDNAs. This library has the same properties for transfecting and screening human mutant cell lines as does the hamster library.
Research Highlights.
High throughput functional complementation screening is a viable experimental strategy in determining nuclear mutations affecting mitochondria.
cDNA expression library screening identifies the defective bS6m gene in a previously un-characterized Chinese hamster mutant cell line.
Our approach is a robust alternative for cloning unknown genes with mitochondrial function.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants NS21325, CA182384, NS070298, and DK73691 awarded to D.C.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Ditta G, Soderberg K, Scheffler IE. Chinese hamster cell mutant with defective mitochondrial protein synthesis. Nature. 1977;268:64–67. doi: 10.1038/268064a0. [DOI] [PubMed] [Google Scholar]
- 2.Ditta G, Soderberg K, Landy F, Scheffler IE. The selection of Chinese hamster cells deficient in oxidative energy metabolism. Somatic Cell Genet. 1976;2:331–344. doi: 10.1007/BF01538838. [DOI] [PubMed] [Google Scholar]
- 3.Potluri P, Yadava N, Scheffler IE. The role of the ESSS protein in the assembly of a functional and stable mammalian mitochondrial complex I (NADH-ubiquinone oxidoreductase) Eur. J. Biochem. 2004;271:3265–3273. doi: 10.1111/j.1432-1033.2004.04260.x. [DOI] [PubMed] [Google Scholar]
- 4.Scheffler IE, Yadava N, Potluri P. Molecular genetics of complex I-deficient Chinese hamster cell lines. Biochim. Biophys. Acta. 2004;1659:160–171. doi: 10.1016/j.bbabio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A. Simplified mammalian DNA isolation procedure. Nucleic Acids Res. 1991;19:4293. doi: 10.1093/nar/19.15.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Naismith JH. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008;8:91. doi: 10.1186/1472-6750-8-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trounce IA, Kim YL, Jun AS, Wallace DC. Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol. 1996;264:484–509. doi: 10.1016/s0076-6879(96)64044-0. [DOI] [PubMed] [Google Scholar]
- 8.Yadava N, Potluri P, Smith EN, Bisevac A, Scheffler IE. Species-specific and mutant MWFE proteins. Their effect on the assembly of a functional mammalian mitochondrial complex I. J. Biol. Chem. 2002;277:21221–21230. doi: 10.1074/jbc.M202016200. [DOI] [PubMed] [Google Scholar]
- 9.Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc. 2002;124:6063–6076. doi: 10.1021/ja017687n. [DOI] [PubMed] [Google Scholar]
- 10.Shu X, Lev-Ram V, Deerinck TJ, Qi Y, Ramko EB, Davidson MW, Jin Y, Ellisman MH, Tsien RY. A genetically encoded tag for correlated light and electron microscopy of intact cells, tissues, and organisms. PLoS Biol. 2011;9:e1001041. doi: 10.1371/journal.pbio.1001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sultan M, Piccini I, Balzereit D, Herwig R, Saran NG, Lehrach H, Reeves RH, Yaspo ML. Gene expression variation in Down's syndrome mice allows prioritization of candidate genes. Genome Biol. 2007;8:R91. doi: 10.1186/gb-2007-8-5-r91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buccafusca R, Venditti CP, Kenyon LC, Johanson RA, Van Bockstaele E, Ren J, Pagliardini S, Minarcik J, Golden JA, Coady MJ, Greer JJ, Berry GT. Characterization of the null murine sodium/myo-inositol cotransporter 1 (Smit1 or Slc5a3) phenotype: myo-inositol rescue is independent of expression of its cognate mitochondrial ribosomal protein subunit 6 (Mrps6) gene and of phosphatidylinositol levels in neonatal brain. Mol. Genet. Metab. 2008;95:81–95. doi: 10.1016/j.ymgme.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Au HC, Scheffler IE. A respiration-deficient Chinese hamster cell line with a defect in mitochondrial protein synthesis: rapid turnover of some mitochondrial transcripts. Somat. Cell Mol. Genet. 1997;23:27–35. doi: 10.1007/BF02679953. [DOI] [PubMed] [Google Scholar]
- 14.Amunts A, Brown A, Toots J, Scheres SH, Ramakrishnan V. Ribosome. The structure of the human mitochondrial ribosome. Science. 2015;348:95–98. doi: 10.1126/science.aaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greber BJ, Bieri P, Leibundgut M, Leitner A, Aebersold R, Boehringer D, Ban N. Ribosome. The complete structure of the 55S mammalian mitochondrial ribosome. Science. 2015;348:303–308. doi: 10.1126/science.aaa3872. [DOI] [PubMed] [Google Scholar]
- 16.Koc EC, Burkhart W, Blackburn K, Moyer MB, Schlatzer DM, Moseley A, Spremulli LL. The large subunit of the mammalian mitochondrial ribosome. Analysis of the complement of ribosomal proteins present. J. Biol. Chem. 2001;276:43958–43969. doi: 10.1074/jbc.M106510200. [DOI] [PubMed] [Google Scholar]
- 17.Smits P, Smeitink JA, van den Heuvel LP, Huynen MA, Ettema TJ. Reconstructing the evolution of the mitochondrial ribosomal proteome. Nucleic Acids Res. 2007;35:4686–4703. doi: 10.1093/nar/gkm441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma MR, Booth TM, Simpson L, Maslov DA, Agrawal RK. Structure of a mitochondrial ribosome with minimal RNA. Proc. Natl. Acad. Sci. USA. 2009;106:9637–9642. doi: 10.1073/pnas.0901631106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma MR, Koc EC, Datta PP, Booth TM, Spremulli LL, Agrawal RK. Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell. 2003;115:97–108. doi: 10.1016/s0092-8674(03)00762-1. [DOI] [PubMed] [Google Scholar]
- 20.Agalarov SC, Sridhar Prasad G, Funke PM, Stout CD, Williamson JR. Structure of the S15,S6,S18-rRNA complex: assembly of the 30S ribosome central domain. Science. 2000;288:107–113. doi: 10.1126/science.288.5463.107. [DOI] [PubMed] [Google Scholar]
- 21.Burnett KG, Scheffler IE. Integrity of mitochondria in a mammalian cell mutant defective in mitochondrial protein synthesis. J. Cell Biol. 1981;90:108–115. doi: 10.1083/jcb.90.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amunts A, Brown A, Bai XC, Llacer JL, Hussain T, Emsley P, Long F, Murshudov G, Scheres SH, Ramakrishnan V. Structure of the yeast mitochondrial large ribosomal subunit. Science. 2014;343:1485–1489. doi: 10.1126/science.1249410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spremulli LL. Large-scale isolation of mitochondrial ribosomes from mammalian tissues. Methods Mol. Biol. 2007;372:265–275. doi: 10.1007/978-1-59745-365-3_19. [DOI] [PubMed] [Google Scholar]
- 24.O'Brien TW. Properties of human mitochondrial ribosomes. IUBMB Life. 2003;55:505–513. doi: 10.1080/15216540310001626610. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien TW, O'Brien BJ, Norman RA. Nuclear MRP genes and mitochondrial disease. Gene. 2005;354:147–151. doi: 10.1016/j.gene.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Sylvester JE, Fischel-Ghodsian N, Mougey EB, O'Brien TW. Mitochondrial ribosomal proteins: candidate genes for mitochondrial disease. Genet. Med. 2004;6:73–80. doi: 10.1097/01.gim.0000117333.21213.17. [DOI] [PubMed] [Google Scholar]
- 27.Greber BJ, Boehringer D, Leitner A, Bieri P, Voigts-Hoffmann F, Erzberger JP, Leibundgut M, Aebersold R, Ban N. Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature. 2014;505:515–519. doi: 10.1038/nature12890. [DOI] [PubMed] [Google Scholar]
- 28.Christian B, Haque E, Spremulli L. Ribosome shifting or splitting: it is all up to the EF-G. Mol. Cell. 2009;35:400–402. doi: 10.1016/j.molcel.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Christian BE, Haque ME, Spremulli LL. The effect of spermine on the initiation of mitochondrial protein synthesis. Biochem. Biophys. Res. Commun. 2010;391:942–946. doi: 10.1016/j.bbrc.2009.11.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Christian BE, Spremulli LL. Mechanism of protein biosynthesis in mammalian mitochondria. Biochim. Biophys. Acta. 2012;1819:1035–1054. doi: 10.1016/j.bbagrm.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christian BE, Spremulli LL. Evidence for an active role of IF3mt in the initiation of translation in mammalian mitochondria. Biochemistry. 2009;48:3269–3278. doi: 10.1021/bi8023493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christian BE, Spremulli LL. Preferential selection of the 5'-terminal start codon on leaderless mRNAs by mammalian mitochondrial ribosomes. J. Biol. Chem. 2010;285:28379–28386. doi: 10.1074/jbc.M110.149054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grasso DG, Christian BE, Spencer A, Spremulli LL. Overexpression and purification of mammalian mitochondrial translational initiation factor 2 and initiation factor 3. Methods Enzymol. 2007;430:59–78. doi: 10.1016/S0076-6879(07)30004-9. [DOI] [PubMed] [Google Scholar]
- 34.Haque ME, Elmore KB, Tripathy A, Koc H, Koc EC, Spremulli LL. Properties of the C-terminal tail of human mitochondrial inner membrane protein Oxa1L and its interactions with mammalian mitochondrial ribosomes. J. Biol. Chem. 2010;285:28353–28362. doi: 10.1074/jbc.M110.148262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada S, Okubo T, Tsutsumi M, Takase S, Muramatsu T. Investigation of genetic risk factors associated with alcoholism. Alcohol. Clin. Exp. Res. 1996;20:293A–296A. [PubMed] [Google Scholar]
- 36.Jones CN, Wilkinson KA, Hung KT, Weeks KM, Spremulli LL. Lack of secondary structure characterizes the 5' ends of mammalian mitochondrial mRNAs. RNA. 2008;14:862–871. doi: 10.1261/rna.909208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spencer AC, Spremulli LL. Interaction of mitochondrial initiation factor 2 with mitochondrial fMet-tRNA. Nucleic Acids Res. 2004;32:5464–5470. doi: 10.1093/nar/gkh886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coskun P, Wyrembak J, Schriner SE, Chen HW, Marciniack C, LaFerla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochim. Biophys. Acta. 2012;1820:553–564. doi: 10.1016/j.bbagen.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coskun PE, Wyrembak J, Derbereva O, Melkonian G, Doran E, Lott IT, Head E, Cotman CW, Wallace DC. Systemic mitochondrial dysfunction and the etiology of Alzheimer's disease and down syndrome dementia. J. Alzheimers Dis. 2010;20(Suppl 2):S293–S310. doi: 10.3233/JAD-2010-100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helguera P, Seiglie J, Rodriguez J, Hanna M, Helguera G, Busciglio J. Adaptive downregulation of mitochondrial function in Down syndrome. Cell Metab. 2013;17:132–140. doi: 10.1016/j.cmet.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Helguera P, Pelsman A, Pigino G, Wolvetang E, Head E, Busciglio J. Ets-2 promotes the activation of a mitochondrial death pathway in Down's syndrome neurons. Journal of Neuroscience. 2005;25:2295–2303. doi: 10.1523/JNEUROSCI.5107-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiano L, Busciglio J. Mitochondrial dysfunction and Down's syndrome: is there a role for coenzyme Q(10) ? Biofactors. 2011;37:386–392. doi: 10.1002/biof.184. [DOI] [PubMed] [Google Scholar]
- 43.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 44.Day CE, Scheffler IE. Mapping of the genes of some components of the electron transport chain (complex I) on the X chromosome of mammals. Somatic Cell Genet. 1982;8:691–707. doi: 10.1007/BF01543012. [DOI] [PubMed] [Google Scholar]
- 45.Scheffler IE. Mitochondrial disease associated with complex I (NADH-CoQ oxidoreductase) deficiency. J. Inherit. Metab. Dis. 2015;38:405–415. doi: 10.1007/s10545-014-9768-6. [DOI] [PubMed] [Google Scholar]
- 46.Vogel RO, van den Brand MA, Rodenburg RJ, van den Heuvel LP, Tsuneoka M, Smeitink JA, Nijtmans LG. Investigation of the complex I assembly chaperones B17.2L and NDUFAF1 in a cohort of CI deficient patients. Mol. Genet. Metab. 2007;91:176–182. doi: 10.1016/j.ymgme.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 47.Yadava N, Houchens T, Potluri P, Scheffler IE. Development and characterization of a conditional mitochondrial complex I assembly system. J. Biol. Chem. 2004;279:12406–12413. doi: 10.1074/jbc.M313588200. [DOI] [PubMed] [Google Scholar]