Abstract
Objective
To describe family burden among caregivers of children who survived out-of-hospital cardiac arrest (OH-CA) and who were at high risk for neurologic disability; and examine relationships between family burden, child functioning and other factors during the first year post-arrest.
Design
Secondary analysis of data from the Therapeutic Hypothermia after Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial.
Setting
Thirty-six pediatric intensive care units in the U.S. and Canada.
Patients
Seventy-seven children recruited to the THAPCA-OH trial who had normal pre-arrest neurological functioning and were alive one year post-arrest.
Interventions
Family burden was assessed using the Infant Toddler Quality of Life (ITQOL) Questionnaire for children <5 years of age and the Child Health Questionnaire (CHQ) for children ≥5 years of age at baseline (reflecting pre-arrest status), 3 months and 12 months post-arrest. Child functioning was assessed using the Vineland Adaptive Behavior Scale II, the Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scales, and caregiver perception of global functioning.
Measurements and Main Results
Fifty-six (72.7%) children were male, 48 (62.3%) were white, and 50 (64.9%) were <5 years of age prior to OH-CA. Family burden at baseline was not significantly different from reference values. Family burden was increased at 3 and 12 months post-arrest compared to reference values (p<.001). Worse POPC and PCPC, lower adaptive behavior, lower global functioning and higher family burden all measured 3 months post-arrest were associated with higher family burden 12 months post-arrest (p<0.05). Sociodemographics and pre-arrest child functioning were not associated with family burden 12 months post-arrest.
Conclusions
Families of children who survive OH-CA and have high risk for neurologic disability often experience substantial burden during the first year post-arrest. The extent of child dysfunction 3 months post-arrest is associated with family burden at 12 months. .
Keywords: Caregiver burden, parent, family, pediatric, resuscitation, adaptive behavior
INTRODUCTION
Out-of-hospital cardiac arrest (OH-CA) in children often results in death or poor quality neurologic survival (1–3). Only about 10% of children experiencing OH-CA survive to hospital discharge. Among survivors, global ischemia during cardiac arrest often results in serious cognitive and physical impairments (4–5). Most children surviving OH-CA are discharged home to be cared for by their families. Caregiver burden is important to consider for these families because it can reduce caregiver health, which can further impair the health of the disabled child.
Theoretical models have been proposed to describe relationships between caregiving and caregiver health. Raina et al (6) developed a model of caregiving for parents of disabled children. Constructs proposed in the model to affect caregiver health include the severity of the child’s disability; social and economic characteristics of the family; and parental caregiving demands, intra-psychic factors, coping strategies and social supports. Empirical data exist supporting various components of the model. For example, health problems have been shown to be common among caregivers of disabled children including sleep disorders, depression, substance abuse and self-reported decrements in physical health (7). Additionally, the severity of the child’s disability has been shown to be a predictor of family burden for conditions such as traumatic brain injury (8). Other factors such as reduced social support, higher financial burden, higher help-needs in the household, and poor family coping have been shown to contribute to health risks for caregivers (7, 9).
The Therapeutic Hypothermia after Pediatric Cardiac Arrest Out-of-Hospital (THAPCA-OH) trial was a randomized controlled trial comparing the efficacy of therapeutic hypothermia with that of therapeutic normothermia on survival with good neurobehavioral outcome in children one year after OH-CA (ClinicalTrials.gov, NCT00878644) (10). All children recruited to the THAPCA-OH trial required mechanical ventilation after return of circulation and were at high risk for neurologic disability. Results of the trial showed that neither treatment arm conferred a significant benefit in survival with good functional outcome. In the context of the THAPCA-OH trial, we evaluated the extent of family burden experienced by caregivers (i.e., parents or guardians) of children who survived OH-CA at baseline, and 3 and 12 months post-arrest. We hypothesized that family burden would be associated with child functioning. The objective of the study is to describe family burden among caregivers of children who survived OH-CA and who were at high risk for neurologic disability, and examine relationships between family burden, child functioning and other factors during the first year post-arrest.
MATERIAL AND METHODS
Study Design and Setting
The study is a secondary analysis of data collected in the THAPCA-OH trial (10). The THAPCA-OH trial was conducted at 36 pediatric intensive care units (PICUs) in the United States (U.S.) and Canada between September 1, 2009, and December 31, 2012. Details of the THAPCA-OH trial have been previously published (10–13). The study was approved by the Institutional Review Boards at the Data Coordinating Center at the University of Utah and all sites. Parental permission was obtained for all participants.
Study Participants
Children were eligible for inclusion in the THAPCA-OH trial if they were >48 hours of age and <18 years of age, experienced an OH-CA with chest compressions for at least 2 minutes, and required mechanical ventilation after return of circulation. Major exclusion criteria included the inability to be randomized within 6 hours of return of circulation, a Glasgow Coma Scale motor score of 5 or 6 (14), a decision by the clinicians to withhold aggressive treatment, and OH-CA due to trauma. Of 295 children randomized, 270 had normal pre-arrest neurological functioning and were eligible for inclusion in the THAPCA-OH primary efficacy analysis. Normal pre-arrest neurological functioning was defined as a baseline Vineland Adaptive Behavioral Scale II, second edition (VABS-II) score ≥70 (15), or in the absence of a baseline VABS-II assessment, Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) scores of 1 (normal) or 2 (mild disability) (16). Of these 270 subjects, 87 were alive 12 months post-arrest, 77 of whom had undergone assessment of family burden at baseline and 12 months post-arrest. Of these, 76 also had assessment of family burden at 3 months post-arrest. These 77 children are the subjects of this report.
Data Collection
All baseline measures were completed by caregivers (i.e., parents and/or guardians) at the local sites within 24 hours of randomization. Trained research coordinators assisted caregivers with completing baseline measures and informed caregivers that baseline was intended to reflect pre-arrest status. Three and 12 month measures were performed by the Kennedy Krieger Institute by telephone interviews conducted by trained interviewers unaware of THAPCA-OH treatment group assignment.
Outcome Measures
The primary outcome was family burden 12 months after OH-CA. Family burden was assessed using two scales from the Infant and Toddler Quality of Life Questionnaire (ITQOL) (17, 18) for children <5 years of age, and three scales from the Child Health Questionnaire (CHQ) (19) for children ≥5 years of age. Family burden was assessed at baseline, and 3 and 12 months post-arrest.
The Family Burden scales of the ITQOL cover 2 domains including (1) parent impact-emotion, and (2) parent impact-time (17). The parent impact-emotion domain consists of 7 items in which the parent is asked to rate how much anxiety or worry each of the child characteristics described in the items has caused during the past 4 weeks (i.e., feeding/sleeping/eating habits; physical health; emotional well-being; learning abilities; ability to interact with others; behavior; temperament). The parent impact-time domain consists of 7 items in which the parent is asked to rate how much of his/her time was limited for personal needs due to problems with the child’s personal needs during the past 4 weeks. For each scale, the mean of the responses is transformed to 0–100 with higher scores indicating lesser burden. Normative reference data from a U.S. population are not available, but Dutch reference data exist (18).
The Family Burden scales of the CHQ cover 3 domains including (1) parent impact-emotion, (2) parent impact-time, and (3) family activities (19). The parent impact-emotion and the parent impact-time domains are similar to the corresponding domains of the ITQOL except that the CHQ domains each have 3 items which pertain to the child’s physical health, emotional well-being, and learning abilities. The family activities domain consists of 6 items which assess how often the child’s health or behavior has interfered with family activities during the past 4 weeks. For each scale, the mean of the responses is transformed to 0–100 with higher scores indicating lesser burden. Normative reference data from a U.S. population are available (19).
Independent Variables
Other data included child and family sociodemographics; family functioning as assessed by the General Functioning Scale of the Family Assessment Device (FAD-GF) (20); and child functioning as assessed by the VABS-II (15), the POPC and PCPC scales (16), and caregivers’ perception of global functioning. Sociodemographics and the FAD-GF were assessed at baseline; the VABS-II and global functioning were assessed at baseline and 3 and 12 months; and the POPC and PCPC were assessed at baseline, PICU discharge, and 3 and 12 months.
The FAD-GF is a 12-item measure to distinguish between healthy and unhealthy family functioning (20). Respondents rate how well each item describes their own family using a 4-point scale. Total scale scores are the mean of the item responses and range from 1–4. Higher scores indicate worse family functioning. A score of ≥2 indicates problematic functioning.
The VABS-II is a measure of adaptive behavior from birth through adulthood (15). Adaptive behavior is defined as the individual’s performance on daily life activities necessary for personal and social independence. The VABS-II covers domains of communication, daily living, socialization, and motor skills. The number of items (i.e., tasks) that can be performed in each domain is standardized for the child’s age. In normative U.S. populations, the mean VABS-II score is 100 and the standard deviation is 15. Higher scores indicate better functioning.
The POPC and PCPC are scales to assess overall functional morbidity and cognitive impairment, respectively (16). Both are 6-point graded scales of increasing disability. Scores are 1 for good/normal, 2 for mild disability, 3 for moderate disability, 4 for severe disability, 5 for coma or vegetative state, and 6 for death.
Caregiver perception of global child functioning was assessed by investigator-developed items. At baseline, caregivers were asked “Compared to children of the same age, were your child’s home, school or social activities limited before his/her cardiac arrest?” Response categories were “not limited, limited a little, and limited a lot.” At 3 and 12 months, caregivers were asked (1) “Compared to children of the same age, are your child’s home, school or social activities limited now?” Response categories were “not limited, limited a little, and limited a lot,” and (2) “Thinking about your child since his/her cardiac arrest, has he/she: gained a lot of new skills, gained a few new skills, stayed the same, lost a few skills, or lost a lot of skills.”
Statistical Analyses
Baseline characteristics were summarized using frequencies and percentages for categorical variables and medians and quartiles for continuous variables. The Family Burden scales and VABS-II were summarized at baseline, 3 months, and 12 months using the mean and standard deviation. The Family Burden scales at each time point were compared to reference values using the t-test and the difference between these scales from 3 to 12 months was examined using the paired t-test. Spearman correlations were used to assess associations between the Family Burden scales and the independent variables. The reference values for each Family Burden scale were used to calculate z-scores at month 12. These z-scores were used to categorize Family Burden as mildly elevated/normal (z-score -1.5 to 1.5), moderately elevated (z-score −3 to −1.5) or highly elevated (z-score < −3). All analyses were completed using SAS software v9.4 (Cary, NC).
RESULTS
Baseline characteristics of children and their caregivers are shown in Table 1. Overall, 56 (72.7%) children were male, 48 (62.3%) were white, and 50 (64.9%) were <5 years of age prior to the OH-CA. Child adaptive behavior pre-arrest was similar to reference norms with median VABS-II scores of 99.0 for children <5 years of age and 107.0 for children ≥5 years of age. Seventy-one (92.2%) children had pre-arrest POPC in the good or mild disability range, and 74 (96.1%) had pre-arrest PCPC in the normal or mild disability range. Seventy-four (96.1%) children were globally assessed by their caregivers as not limited or limited a little. Etiology of arrest was a respiratory disorder in 54 (70.1%) children. Thirty-three (42.9%) caregivers had at most a high school diploma or passed a General Education Development (GED) test. Family functioning pre-arrest was reported as normal by 66 (86.8%) caregivers.
Table 1.
Descriptive Characteristics
| Assessment completed at Baseline
|
||
|---|---|---|
| Characteristic | ITQOL (N = 50) | CHQ (N = 27) |
| Age at Randomization (years): Median [Q1, Q3] | 1.9 [0.6, 3.1] | 14.5 [9.8, 16.2] |
| Male | 34 (68.0%) | 22 (81.5%) |
| Race | ||
| American Indian or Alaska Native | 0 (0.0%) | 1 (3.7%) |
| Asian | 0 (0.0%) | 2 (7.4%) |
| Black or African American | 9 (18.0%) | 9 (33.3%) |
| White | 34 (68.0%) | 14 (51.9%) |
| Other/Unknown | 7 (14.0%) | 1 (3.7%) |
| Ethnicity | ||
| Hispanic or Latino | 8 (16.0%) | 6 (22.2%) |
| Not Hispanic or Latino | 40 (80.0%) | 20 (74.1%) |
| Unknown | 2 (4.0%) | 1 (3.7%) |
| Caregivers highest education received | ||
| Some high school or less | 5 (10.0%) | 8 (29.6%) |
| High school graduate or GED | 17 (34.0%) | 3 (11.1%) |
| Vocational school or some college | 13 (26.0%) | 5 (18.5%) |
| College degree | 8 (16.0%) | 6 (22.2%) |
| Graduate or doctoral degree | 7 (14.0%) | 5 (18.5%) |
| Primary etiology of cardiac arrest | ||
| Cardiac | 7 (14.0%) | 8 (29.6%) |
| Respiratory | 39 (78.0%) | 15 (55.6%) |
| Other/Unknown | 4 (8.0%) | 4 (14.8%) |
| Pre-cardiac arrest VABS Adaptive Behavior Composite Score: Median [Q1, Q3] | 99.0 [89.0, 111.0] | 107.0 [92.0, 121.0] |
| Pre-cardiac arrest POPC | ||
| Good = 1 | 41 (82.0%) | 20 (74.1%) |
| Mild disability = 2 | 4 (8.0%) | 6 (22.2%) |
| Moderate disability = 3 | 5 (10.0%) | 1 (3.7%) |
| Pre-cardiac arrest PCPC | ||
| Normal = 1 | 45 (90.0%) | 24 (88.9%) |
| Mild disability = 2 | 2 (4.0%) | 3 (11.1%) |
| Moderate disability = 3 | 3 (6.0%) | 0 (0.0%) |
| Pre-cardiac arrest Global Child Functioning: Caregiver perception of limitations | ||
| Not limited | 43 (87.8%) | 18 (66.7%) |
| Limited a little | 6 (12.2%) | 7 (25.9%) |
| Characteristic | (N = 50) | (N = 27) |
| Limited a lot | 0 (0.0%) | 2 (7.4%) |
| Pre-cardiac arrest FAD Family Functioning | ||
| Healthy Family Functioning | 46 (93.9%) | 20 (74.1%) |
| Unhealthy Family Functioning | 3 (6.1%) | 7 (25.9%) |
Family burden at baseline, and 3 and 12 months post-arrest for children <5 years of age (ITQOL), and for children ≥5 years of age (CHQ) is shown in Tables 2a and b, respectively. Two children whose caregivers completed the ITQOL at baseline were administered the CHQ at 3 and 12 months because the children turned 5 years of age between the baseline and 3 month assessment. Five children whose caregivers completed the ITQOL at baseline and 3 months were administered the CHQ at 12 months because the children turned 5 years of age between the 3 and 12 month assessment. Family burden (i.e., parent impact-emotion, parent impact-time, and family activities) at baseline was not significantly different from reference values for either age group. However, family burden was significantly greater (i.e., lower ITQOL and CHQ domain scores) at 3 and 12 months post-arrest compared to reference values. Family burden tended to improve between 3 and 12 months, but only the parent impact-time scale of the CHQ was significantly different. Adaptive behavior was reduced (i.e., lower VABS-II scores) 3 and 12 months post-arrest compared to reference norms.
Table 2a.
ITQOL Family Burden Measures over Time
| Time point
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Baseline N=50 | P-value* | Month 3 N=48 | P-value* | Month 12 N=43 | P-value* | P-value† | |
| ITQOL: standardized score for Parent Impact - Emotion | ||||||||
| Mean (SD) | 92.1 (10.84) | 90.5 (19.46) | 0.575 | 71.1 (26.91) | <.001 | 76.8 (24.96) | <.001 | 0.119 |
| ITQOL: standardized score for Parent Impact - Time | ||||||||
| Mean (SD) | 93 (10.92) | 91.7 (16.60) | 0.591 | 77.8 (26.64) | <.001 | 77.3 (27.07) | <.001 | 0.784 |
| VABS Adaptive Behavior Composite Score | ||||||||
| Mean (SD) | 100 (15) | 99.0 (15.32) | 73.1 (27.19) | 75.9 (25.67) | ||||
P-value from t-test comparing Family Burden measure to Reference.
P-value from t-test comparing the change in Family Burden measure from month 3 to month 12.
Table 2b.
CHQ Family Burden Measures over Time
| Time point
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Baseline N=27 | P-value* | Month 3 N=28 | P-value* | Month 12 N=34 | P-value* | P-value† | |
| CHQ: standardized score for Parent Impact - Emotion | ||||||||
| Mean (SD) | 80.3 (19.1) | 74.4 (30.48) | 0.322 | 44.6 (35.08) | <.001 | 52.2 (34.41) | <.001 | 0.440 |
| CHQ: standardized score for Parent Impact - Time | ||||||||
| Mean (SD) | 87.8 (19.9) | 87.2 (22.37) | 0.898 | 45.6 (37.45) | <.001 | 55.9 (33.67) | <.001 | 0.007 |
| CHQ: standardized score for Family Activities | ||||||||
| Mean (SD) | 89.7 (18.6) | 87.0 (12.89) | 0.293 | 61.2 (32.71) | <.001 | 63.1 (28.91) | <.001 | 0.697 |
| VABS Adaptive Behavior Composite Score | ||||||||
| Mean (SD) | 100 (15) | 107.0 (16.45) | 64.1 (29.04) | 66.1 (29.51) | ||||
P-value from t-test comparing Family Burden measure to Reference.
P-value from t-test comparing the change in Family Burden measure from month 3 to month 12.
Note: one patient did not complete the month 3 CHQ assessment; N=28 for CHQ domains and N=29 for VABS.
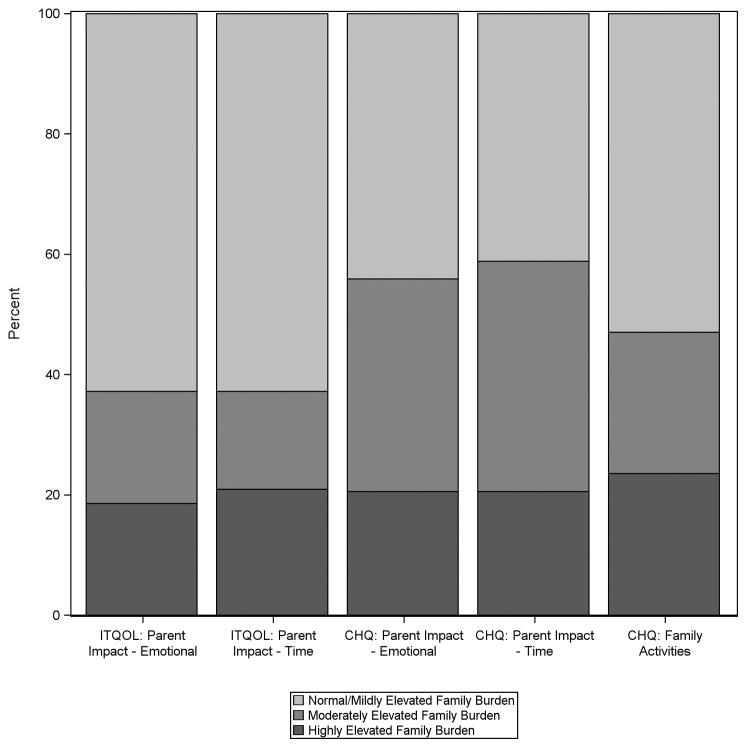
Figure 1 shows the percent of caregivers reporting normal to mildly elevated, moderately elevated, and highly elevated burden for each of the family burden scales 12 months post-arrest. For caregivers of children <5 years of age, 16 (37.2%) reported moderate to highly elevated burden for the parent impact-emotion domain, and 16 (37.2%) reported moderate to highly elevated burden for the parent impact-time domain. For caregivers of children ≥5 years of age, 19 (55.9%) reported moderate to highly elevated burden for the parent impact-emotion domain, 20 (58.8%) for the parent impact-time domain, and 16 (47.0%) for the family activities domain.
Figure 1.
Percent of caregivers reporting normal/mild, moderate and high levels of burden for each assessed family burden domain 12 months after their child’s cardiac arrest.
Correlations of variables with 3 and 12 month family burden domain scores are shown in Tables 3a and b, respectively. Several variables reflecting reduced child functioning prior to 3 months post-arrest were moderately correlated with increased family burden at 3 months post-arrest (Table 3a). Specifically, reduced global functioning pre-arrest was associated with greater caregiver limitations in personal time (i.e., parent impact-time) 3 months post-arrest for children <5 years of age. Worse POPC and PCPC at PICU discharge were associated with increased family burden in all domains 3 months post-arrest for children ≥5 years of age. Worse POPC and PCPC 3 months post-arrest were associated with increased caregiver worry (i.e., parent impact-emotion) in children <5 years of age and with all family burden domains in children ≥5 years of age. Lower adaptive behavior and global functioning 3 months post-arrest were associated with increased family burden in all domains 3 months post-arrest in both age groups. Baseline characteristics including child age, caregiver education, family functioning, child adaptive behavior, and POPC and PCPC were not associated with family burden 3 months post-arrest. Child sex, race and ethnicity were also not associated with family burden 3 months post-arrest (data not shown).
Table 3a.
Month 3 Family Burden Measure Correlations
| Covariate | ITQOL Parent Impact - Emotion | ITQOL Parent Impact - Time | CHQ Parent Impact - Emotion | CHQ Parent Impact - Time | CHQ Family Activities |
|---|---|---|---|---|---|
| Pre-cardiac arrest | |||||
| Age at Randomization (years) | −0.084 | −0.158 | 0.105 | 0.058 | 0.151 |
| Caregiver's highest education received | 0.021 | −0.051 | −0.010 | −0.188 | −0.101 |
| Global Child Functioning: Caregiver perception of limitations | −0.253 | −0.368* | 0.052 | −0.073 | 0.145 |
| Average FAD score | 0.246 | 0.043 | −0.128 | 0.021 | −0.142 |
| VABS Adaptive Behavior Composite Score | 0.138 | 0.023 | 0.071 | 0.045 | 0.231 |
| POPC | −0.126 | −0.152 | 0.311 | 0.138 | 0.231 |
| PCPC | −0.034 | −0.033 | 0.274 | 0.124 | 0.179 |
| PICU discharge | |||||
| POPC | −0.239 | −0.128 | −0.376* | −0.439* | −0.449* |
| PCPC | −0.241 | −0.119 | −0.386* | −0.421* | −0.461* |
| Month 3 | |||||
| VABS Adaptive Behavior Composite Score | 0.511* | 0.325* | 0.557* | 0.423* | 0.585* |
| POPC | −0.415* | −0.217 | −0.563* | −0.477* | −0.620* |
| PCPC | −0.422* | −0.205 | −0.616* | −0.479* | −0.647* |
| Global Child Functioning: Caregiver perception of limitations | −0.460* | −0.487* | −0.483* | −0.382* | −0.466* |
| Global Child Functioning: Caregiver perception of skills | −0.540* | −0.421* | −0.481* | −0.343 | −0.502* |
Indicates p-value < 0.05
Table 3b.
Month 12 Family Burden Measure Correlations
| Covariate | ITQOL Parent Impact - Emotion | ITQOL Parent Impact - Time | CHQ Parent Impact - Emotion | CHQ Parent Impact - Time | CHQ Family Activities |
|---|---|---|---|---|---|
| Pre-cardiac arrest | |||||
| Age at Randomization (years) | 0.027 | 0.033 | -0.306 | -0.021 | -0.112 |
| Caregiver's highest education received | 0.172 | 0.093 | -0.200 | -0.133 | -0.281 |
| Global Child Functioning: Caregiver perception of limitations | -0.079 | -0.124 | -0.151 | -0.067 | -0.048 |
| Average FAD score | -0.065 | 0.084 | 0.063 | 0.263 | 0.130 |
| VABS Adaptive Behavior Composite Score | 0.037 | 0.182 | -0.024 | 0.011 | 0.039 |
| POPC | 0.032 | -0.068 | 0.048 | -0.020 | 0.007 |
| PCPC | 0.167 | 0.030 | -0.093 | -0.109 | -0.178 |
| PICU discharge | |||||
| POPC | -0.331* | -0.316* | -0.397* | -0.323 | -0.190 |
| PCPC | -0.315* | -0.301* | -0.394* | -0.315 | -0.220 |
| Month 3 | |||||
| VABS Adaptive Behavior Composite Score | 0.507* | 0.474* | 0.457* | 0.445* | 0.458* |
| POPC | -0.495* | -0.422* | -0.417* | -0.522* | -0.429* |
| PCPC | -0.429* | -0.381* | -0.424* | -0.514* | -0.457* |
| G−lobal Child Functioning: Caregiver perception of limitations | −0.370* | −0.527* | −0.363* | −0.481* | −0.405* |
| Global Child Functioning: Caregiver perception of skills | −0.450* | −0.408* | −0.330 | −0.459* | −0.435* |
| Corresponding Family Burden measure | 0.687* | 0.543* | 0.528* | 0.843* | 0.687* |
| Month 12 | |||||
| VABS Adaptive Behavior Composite Score | 0.566* | 0.502* | 0.432* | 0.423* | 0.461* |
| POPC | −0.537* | −0.476* | −0.374* | −0.438* | −0.301 |
| PCPC | −0.502* | −0.457* | −0.400* | −0.418* | −0.337 |
| Global Child Functioning: Caregiver perception of limitations | −0.650* | −0.558* | −0.517* | −0.541* | −0.444* |
| Global Child Functioning: Caregiver perception of skills | −0.476* | −0.494* | −0.559* | −0.564* | −0.504* |
Indicates p-value < 0.05
Several variables reflecting reduced child functioning prior to 12 months post-arrest were moderately correlated with increased family burden at 12 months post-arrest (Table 3b). Worse POPC and PCPC at PICU discharge correlated with increased family burden in all domains for children <5 years of age and with increased caregiver worry (i.e., parent impact-emotion) in children ≥5 years of age. Worse POPC and PCPC, lower adaptive behavior, and lower global functioning 3 and 12 months post-arrest were associated with increased family burden in all domains 12 months post-arrest. Family burden at 3 months post-arrest was also moderately to highly correlated with the corresponding family burden scale at 12 months post-arrest. Baseline child and family characteristics were not associated with family burden 12 months post-arrest.
Caregivers of two children ≥5 years of age with VABS-II ≥70 at 12 months post-arrest reported a high degree of worry (i.e., parent impact-emotion scores <10). VABS-II scores declined from 99 at baseline to 92 at 12 months for one of these children, and from 98 to 77 for the other. Caregivers of seven children (three <5 years and four ≥5 years) with VABS-II <70 at 12 months post-arrest reported no burden for at least one family burden domain (i.e., ITQOL or CHQ domain score = 100).
DISCUSSION
Our findings suggest that caregivers of children who survive OH-CA and are at high risk for neurologic disability often experience substantial family burden during the first year post-arrest. Most children in our study had no or mild disability pre-arrest based on VABS-II scores, and were a part of families with healthy family functioning. Family burden pre-arrest was similar to that experienced by families of healthy children. However, following the arrest, family burden increased substantially. At 3 months post-arrest, caregivers reported increased levels of anxiety and worry, and interference with meeting their own personal needs and conducting family activities. Although some gain in ability to meet personal needs was observed by 12 months post-arrest for caregivers of older children, family burden at 12 months remained high for many families.
A recent cross-sectional study from the Netherlands evaluated long-term health status and health-related quality of life among survivors of cardiac arrest in childhood and their parents (4). In this study, parents completed the Family Burden scales of the ITQOL (n=12) or the CHQ (n=33) a median of 5.6 years (range 1.8–11.9 years) after their child’s PICU discharge. Anxiety and worry were increased among parents of children ≥5 years of age compared to reference values. No differences were observed in the parent impact-time or family activity domains for parents of children ≥5 years of age, and no differences were observed in family burden domains for children <5 years of age compared to reference values. In addition to the cross-sectional design and wide post-arrest recruitment period, the Dutch study differed from our study in that it attempted to recruit all children admitted to PICU who survived cardiac arrest, either in-hospital or out-of-hospital arrest, regardless of neurologic status in the immediate post-arrest period. Children in the Dutch study may have been at lower risk for poor functional outcomes and increased family burden compared to those in the THAPCA-OH trial. Despite these differences, findings from the Dutch study and our study, taken together, suggest that family burden after a child’s cardiac arrest is high during the first year post-arrest and gradually decreases over time. This may be related to improvement in coping or child health. Research suggests that many families gradually adapt to their children’s disabilities and their own caregiving situations (7, 21).
Our findings also suggest that family burden 12 months after a child’s OH-CA is associated with child functioning and family burden earlier in the course of illness. Reduced functional and cognitive status assessed by POPC and PCPC at PICU discharge were associated with increased worry 12 months post-arrest for caregivers of children in the younger and older age groups, and with increased interference with self-care for caregivers of younger children only. Caregiver perceptions of their child’s global functioning, POPC and PCPC scores, and child adaptive behavior 3 months post-arrest were all associated with family burden at 12 months. Family burden at 3 months post-arrest was one of the variables most strongly correlated with family burden at 12 months. These findings are important because identifying factors that are associated with increased burden early after a child’s OH-CA may provide increased opportunity for intervention. PICU follow-up clinics have been suggested as one type of intervention to support parents most vulnerable to psychological distress after their child’s critical illness. However, a recent study reported that only a minority of parents were able or willing to participate in a PICU follow-up clinic appointment (22). Follow-up meetings or other interventions to support caregivers of children after OH-CA would need to be developed or adapted for this population, and tested for feasibility, acceptability and impact on family burden.
Our findings are consistent with other research suggesting that the extent of child disability is a predictor of family burden. In a study of pediatric traumatic brain injury, Aitken et al (8) found that the extent of child dysfunction predicted family burden 3 and 12 months post-injury. In a study of acquired brain injury due to trauma and non-traumatic causes, de Kloet et al (23) found that non-traumatic brain injury, severity of injury, and presence of health problems prior to the injury independently predicted impact on the family. Qualitative research has also shown that the extent of child disability impacts parental well-being, especially for mothers (24, 25). Thus, the high degree of family burden reported by caregivers in our study is not surprising given the severity of dysfunction observed among children in the THAPCA-OH trial.
Despite these findings, healthcare professionals should realize that some caregivers whose children function relatively well after OH-CA may still experience substantial burden, and vice versa. For example, caregivers of two children in our older age group who were functioning in the normal range 12 months post-arrest reported a high degree of worry. Although still in the normal range, child functioning had declined for both of these children from baseline to 12 months post-arrest. The family burden observed may have been due to this decline. This pattern of discrepancy between child functioning and family burden (e.g., good function, high burden) was not observed for children in our younger age group. Caregivers of older children may experience more burden because it is easier to recognize deficits in older children as more complex tasks are expected such as those related to self-care and school performance. In contrast, caregivers of 7 children (three <5 years and four ≥5 years) who were functioning below normal at 12 months post-arrest reported no burden for at least one family burden domain. Caregivers of younger children may expect their children to be dependent for daily activities and are therefore less burdened by the disabilities. Additionally, some caregivers may feel social or cultural pressure to answer the questionnaires about family burden in a positive manner.
Sociodemographics, and baseline family and child functioning were not associated with family burden 12 months post-arrest in our study. This is in contrast to other studies reporting an impact of sociodemographics on family burden and child health-related quality of life. Hinojosa et al (26) examined family impact for white, African American and Hispanic parents of children with life-threatening illnesses and public health insurance in Florida. Hispanic parents reported their child’s illness resulted in greater negative impact on the family compared to whites, whereas African Americans were not significantly different from whites. Alonso et al (27) explored health-related quality of life using the ITQOL and CHQ among families two years after pediatric liver transplant. Hispanic race was associated with more parent worry and lower child health-related quality of life. Racial and ethnic variation in family burden may be related to cultural differences in caregiving, and greater caregiving responsibilities for minority families. It is possible that our study did not show a relationship between sociodemographics and family burden because of the severe neurologic dysfunction among children in the THAPCA-OH trial, and the important contribution of neurologic dysfunction to family burden.
Strengths of our study include the multicenter, longitudinal design, and follow-up by centralized trained interviewers. Strengths also include the use of reliable and valid measures to assess family burden, and child and family functioning. Limitations include the use of different family burden measures for children in different age groups. Splitting the primary outcome measure by age group could have reduced statistical power to detect some clinically important relationships. Because the study was part of the larger THAPCA-OH trial in which family burden was a secondary outcome, an a priori power calculation was not performed. Normal reference data from a general U.S. population are not available for the ITQOL. Other limitations include the lack of data from families of children who were eligible for the THAPCA-OH trial but did not consent to participate, and the lack of data on the use of family support services. Additionally, the same caregiver may not have provided data at each time point.
CONCLUSIONS
Caregivers of children who survived OH-CA and were at high risk for neurologic disability often experienced a high degree of family burden during the first year post-arrest. The extent of the child’s disabilities and family burden 3 months post-arrest were associated with family burden at 12 months. Future research should continue to explore factors contributing to family burden after pediatric OH-CA. Greater understanding of risk factors for family burden will allow identification of families most in need of ongoing support.
Acknowledgments
Financial Support: Primary support for the conduct of the THAPCA-OH Trial was funding from NIH U01HL094345 (FWM) and U01HL094339 (JMD). Additional support from the following federal grants contributed to the planning of the THAPCA-OH Trial: NIH, Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), Bethesda, MD, HD044955 (FWM) and HD050531 (FWM). In part support was from the participation of the following research networks: Pediatric Emergency Care Applied Research Network (PECARN) from cooperative agreements U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008; and the Collaborative Pediatric Critical Care Research Network (CPCCRN) from cooperative agreements (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934. At several centers, clinical research support was supplemented by the following grants or Cooperative Agreements: UL1TR000003, P30HD040677, P30HD062171, U07MC09174, UL1 RR 024986, and UL1 TR 000433.
Footnotes
Reprints will not be ordered.
References
- 1.Moler FW, Donaldson AE, Meert K, et al. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topjian AA, Nadkarni VM, Berg RA. Cardiopulmonary resuscitation in children. Curr Opin Crit Care. 2009;15:203–208. doi: 10.1097/mcc.0b013e32832931e1. [DOI] [PubMed] [Google Scholar]
- 3.Donoghue AJ, Nadkarni V, Berg RA, et al. CanAM Pediatric Cardiac Arrest Investigators: Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. An Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 4.Van Zellem L, Buysse C, Madderom M, et al. Long-term neuropsychological outcomes in children and adolescents after cardiac arrest. Intensive Care Med. 2015;41:1057–1066. doi: 10.1007/s00134-015-3789-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Zellem L, Utens EM, Legerstee JS, et al. Cardiac arrest in children: Long-term health status and health-related quality of life. Pediatr Crit Care Med. 2015;16:693–702. doi: 10.1097/PCC.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 6.Raina P, O’Donnell M, Schwellnus H, et al. Caregiving process and caregiver burden: Conceptual models to guide research and practice. BMC Pediatrics. 2004;4:1. doi: 10.1186/1471-2431-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers HG, Chambers JA. Effects of caregiving on the families of children and adults with disabilities. Phys Med Rehabil Clin N Am. 2015;26:1–19. doi: 10.1016/j.pmr.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Aitken ME, McCarthy ML, Slomine BS, et al. Family burden after traumatic brain injury in children. Pediatrics. 2009;123:199–206. doi: 10.1542/peds.2008-0607. [DOI] [PubMed] [Google Scholar]
- 9.Vonneilich N, Ludecke D, Kofahl C. The impact of care on family and health-related quality of life of parents with chronically ill and disabled children. Disabil Rehabil. 2015;23:1–7. doi: 10.3109/09638288.2015.1060267. [DOI] [PubMed] [Google Scholar]
- 10.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med. 2015;372:1898–1908. doi: 10.1056/NEJMoa1411480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moler FW, Silverstein FS, Meert KL, et al. Rationale, timeline, study design, and protocol overview of the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2013;14:e304–e315. doi: 10.1097/PCC.0b013e31828a863a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holubkov R, Clark AE, Moler FW, et al. Efficacy outcome selection in the Therapeutic Hypothermia after Pediatric Cardiac Arrest trials. Pediatr Crit Care Med. 2015;16:1–10. doi: 10.1097/PCC.0000000000000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pemberton VL, Browning B, Webster A, et al. Therapeutic Hypothermia after Pediatric Cardiac Arrest trials: The vanguard phase experience and implications for other trials. Pediatr Crit Care Med. 2013;14:19–26. doi: 10.1097/PCC.0b013e31825b860b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 15.Sparrow S, Cicchetti D, Balla D. Vineland Adaptive Behavior Scales. 2. Minneapolis: Pearson Assessment; 2005. [Google Scholar]
- 16.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 17.HealthActCHQ. Infant and Toddler Quality of Life Questionnaire-97 (ITQOL-97) Boston, MA: HealthActCHQ; 2008. Confidential Scoring Rules. [Google Scholar]
- 18.Raat H, Landgraf JM, Oostenbrink R, et al. Reliability and validity of the Infant and Toddler Quality of Life Questionnaire (ITQOL) in a general population and respiratory disease sample. Qual Life Res. 2007;16:445–460. doi: 10.1007/s11136-006-9134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.HealthActCHQ. The CHQ Scoring and Interpretation Manual. Boston, MA: HealthActCHQ; 2008. [Google Scholar]
- 20.Epstein N, Baldwin L, Bishop D. The McMaster Family Assessment Device. J Marital Fam Ther. 1983;9:171–180. [Google Scholar]
- 21.Hsieh RL, Huang HY, Lin MI, et al. Quality of life, health satisfaction and family impact on caregivers of children with developmental delays. Child Health Care Dev. 2009;35:243–249. doi: 10.1111/j.1365-2214.2008.00927.x. [DOI] [PubMed] [Google Scholar]
- 22.Samuel VM, Colville GA, Goodwin S, et al. The value of screening parents for their risk of developing psychological symptoms after PICU: A feasibility study evaluating a Pediatric Intensive Care Follow-Up Clinic. Pediatr Crit Care Med. 2015;16:808–813. doi: 10.1097/PCC.0000000000000488. [DOI] [PubMed] [Google Scholar]
- 23.De Kloet AJ, Lambregts SAM, Berger MAM, et al. Family impact of acquired brain injury in children and youth. J Dev Behav Pediatr. 2015;36:342–351. doi: 10.1097/DBP.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 24.Mackey S, Goddard LD. The experience of health and wellness in mothers of young children with intellectual disabilities. J Intellect Disabil. 2006;10:305–315. doi: 10.1177/1744629506070055. [DOI] [PubMed] [Google Scholar]
- 25.Smith J, Cheater F, Bekker H. Parents’ experiences of living with a child with a long-term condition: A rapid structured review of the literature. Health Expect. 2015;18:452–474. doi: 10.1111/hex.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinojosa MS, Knapp CA, Madden VL, et al. Caring for children with life-threatening illnesses: Impact on white, African American, and Latino Families. J Pediatr Nurs. 2012;27:500–507. doi: 10.1016/j.pedn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso EM, Neighbors K, Baron FB, et al. Health-related quality of life and family function following pediatric liver transplantation. Liver Transpl. 2008;14:460–468. doi: 10.1002/lt.21352. [DOI] [PubMed] [Google Scholar]