Abstract
Resident T cells in barrier tissues are important in protecting against foreign agents but could also contribute to inflammatory diseases if dysregulated. How T cell homeostasis is maintained in barrier tissues is still poorly understood. Herein we report that resident CD8+ T cells directly support maintenance of regulatory T (Treg) cells in the skin to promote immune homeostasis. Impaired establishment of resident CD8+ T cells due to knockout of the skin-homing chemokine receptor CCR10 resulted in altered balance of resident Treg and CD4+ effector T (Teff) cells in the skin and over-reactive inflammatory responses to cutaneous stimulations. Furthermore, B7.2 expressed on skin CD8+ T cells is involved in supporting survival of Treg cells, likely through interaction with its receptor CTLA-4, which is highly expressed on skin Treg cells. Our findings provide novel insight into T cell homeostatic regulation in the skin and may help understand pathobiology of tissue inflammatory diseases.
INTRODUCTION
Barrier tissues such as the skin are under constant assaults of various environmental agents. Resident T cells in barrier tissues play important roles in protecting against the assaults such as infections (1–3), but could be responsible for development of tissue inflammatory diseases when their homeostasis is dysregulated (4–6). Understanding mechanisms regulating homeostatic presence of T cells in barrier tissues is fundamental to develop cures against tissue inflammatory diseases and infections.
The chemokine receptor CCR10 is expressed on skin-homing and resident T cells (7, 8). It was suggested that through interaction with its skin-specific ligand CCL27, CCR10 regulates migration of T cells during skin inflammatory responses (9, 10). However, a later study found that CCR10-knockout (KO) in T cells did not affect their migration into immunization sites of the skin (11). Using a strain of CCR10-KO/EGFP-knockin (CCR10EGFP/EGFP) mice in which the CCR10-coding sequence is replaced with a DNA fragment coding for enhanced green fluorescent protein (EGFP) to report CCR10 expression (12), we recently demonstrated that CCR10 is critical for T cell migration into the non-inflamed skin (13), suggesting that CCR10 might be important in establishment of skin-resident T cells. Here we report that CCR10-regulated proper establishment of CD8+ T cells in the skin is critical for CD4+ T cell homeostasis to prevent over-reactive inflammatory responses. Furthermore, we found that the B7.2/ligand interaction mediates CD8+ T cell regulation of Treg cells in the skin.
MATERIALS AND METHODS
Mice
CCR10EGFP/EGFP mice were described (12). Wild-type (WT) C57BL/6, Rag1−/−, transgenic OT-I, B7.1−/−B7.2−/−, and Foxp3-RFP (red fluorescent protein) reporter mice were purchased from The Jackson Laboratory (Bar Harbor, ME). OT-I mice on CCR10EGFP/EGFP or B7.2−/− background were generated by proper crossing. All animal experiments were approved by The Pennsylvania State University Institutional Animal Care and Use Committee.
Reagents
Antibodies were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA) or Biolegend (San Diego, CA). 1-Fluoro-2,4-dinitrobenzene (DNFB) and chicken ovalbumin protein (Ova) were purchased from Sigma-Aldrich (St. Louis, MO). Cholera toxin was purchased from List Biological Laboratories (Campbell, CA).
Adoptive OT-I T cell transfer, Ova immunization and challenge
The experiments were performed as reported (13). Briefly, purified naïve splenic OT-I CD8+ T cells (~ 0.5 million) of CCR10EGFP/EGFP, CCR10+/EGFP, B7.2−/− or WT background were i.p. injected into WT mice, which were then immunized with topical application of 50μl PBS solution of Ova (5mg/ml) and cholera toxin (0.5 mg/ml) on the ear or back twice with 7 days in between. For challenge, immunized mice were topically applied with Ova (5mg/ml in PBS) or DNFB (0.5% in 4:1 acetone/olive oil) on un-immunized ear or torso skin 3 months after the Ova immunization.
Transfer of polyclonal T cells into Rag1−/− mice
0.5 million sorter-purified splenic CD8+ T cells of CCR10EGFP/EGFP, CCR10+/EGFP, B7.1−/−B7.2−/− or WT mice were injected into Rag1−/− mice together with 1 million purified WT splenic CD4+ T cells. Recipients were analyzed 1–2 months later.
Skin lymphocyte isolation, flow cytometric (FC) analysis and cell sorting were performed as previously described (13).
Quantitative real-time RT-PCR
RNA extracted from the skin was reverse-transcribed to cDNA, and analyzed by Sybr green real-time PCR with primer pairs for specific cytokines. TNF-α: TTCTATGGCCCAGACCC and GGCACCACTAGTTGGTTGTC; IL-1β: TCTCGCAGCAGCACATCA and CACACCAGCAGGTTATCATCAT; IL-10: ACCAAAGCCACAAAGCAGCC and CCGACTGGGAAGTGGGTGC; β-actin: CCCATCTACGAGGGCTAT and TGTCACGCACGATTTCC.
In vitro T cell co-culture
Skin Treg or CD4+ Teff cells were sorter-purified from Foxp3-REP mice based on RFP signals, co-cultured with purified skin CD8+ cells of WT or B7.1−/−B7.2−/− mice at the 1:2 ratio in presence of IL-2 (2 ng/ml) for one day, and then analyzed for survival by Annexin V staining and flow cytometry. To block the B7.2/ligand interaction, 5 μg/ml anti-B7.2 antibodies (GL1) were added in some cultures.
Statistical analysis
Data are expressed as means ± standard errors (SEM). Two-tailed student T test or ANOVA test with Tukey adjustment was used to determine statistical significance for two or multiple group comparison. P < 0.05 is considered significant.
RESULTS AND DISCUSSION
CCR10 is important for establishment of CD8+ resident T cells in the un-inflamed skin
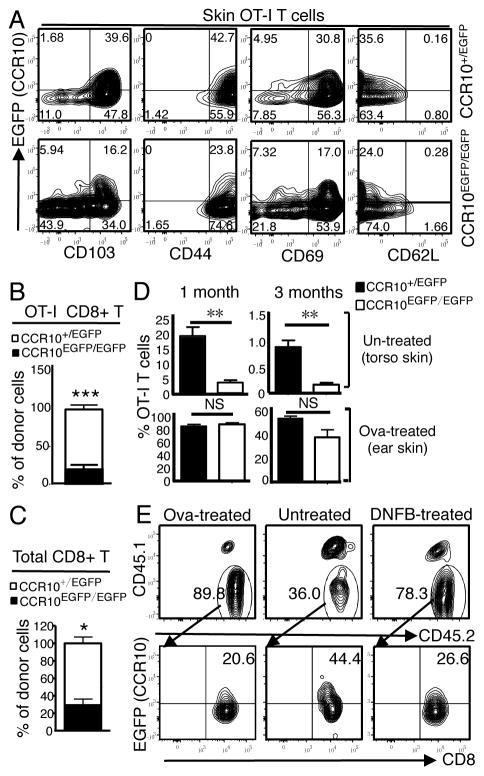
To address whether CCR10 was involved in establishment of resident CD8+ T cells in the skin, we used a T cell transfer model in which naïve Ova-specific transgenic OT-I CD8+ T cells of CCR10+/EGFP or CCR10EGFP/EGFP background were transferred into WT mice (referred as CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice), followed by epicutaneous immunization on ears with Ova, which activated OT-I cells to differentiate into EGFP(CCR10)+ T cells (13). One week after immunization, most EGFP+ OT-I cells of the skin displayed a surface marker expression pattern typical of resident memory T cells (CD103+CD69+CD44+CD62L−) (Fig. 1A). CCR10-KO did not affect the marker expression on CCR10EGFP/EGFP EGFP+ OT-I cells (Fig. 1A). However, when co-transferred into WT mice, CCR10EGFP/EGFP EGFP+ OT-I or polyclonal T cells migrated much less efficiently into the skin than their corresponding CCR10+/EGFP counterparts (Fig. 1B–C), supporting the notion that CCR10 is important for T cell migration into the un-treated skin (13).
Figure 1.
CCR10 is important for establishment of CD8+ resident memory T cells in the un-inflamed skin. A) FC analysis of EGFP(CCR10)+ OT-I cells of the skin of CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice for indicated molecules. Analyzed one week after Ova immunization. Representative of at least 3 experiments. B) Relative contribution of CCR10+/EGFP versus CCR10EGFP/EGFP donor EGFP+ OT-I cells in the skin of WT recipients 1 week after their co-transfer. N=9 pooled of 2 experiments. C) Relative contribution of CCR10+/EGFP and CCR10EGFP/EGFP polyclonal donor EGFP+ T cells in the skin of WT recipients 2 days after their co-transfer. N≥3. D) Average percentages of CCR10+/EGFP and CCR10EGFP/EGFP OT-I cells of total CD8+ T cells in Ova-immunized ear and un-treated torso skin 1 and 3 months after immunization. N≥6 pooled of 3 experiments. E) FC analysis of CCR10+/EGFP OT-I cells (CD45.1−CD45.2+, top row) of Ova-treated, untreated and DNFB-treated skin and their CCR10(EGFP) expression (bottom) 1 week after immunization. Representative of 3 experiments. *P<0.05; **P<0.005; ***P<0.001 and NS: no significant difference (applied to all figures).
We then assessed how CCR10-KO affected establishment of resident OT-1 cells in the skin at the memory phase. One month after immunization, there were many fewer CCR10EGFP/EGFP than CCR10+/EGFP OT-I cells in untreated parts of the skin (torso) and the percentage of EGFP+ CCR10EGFP/EGFP OT-I cells was also lower than CCR10+/EGFP controls (Fig. 1D, Supplemental Fig. 1A). In contrast, percentages of CCR10EGFP/EGFP and CCR10+/EGFP OT-I cells of immunization sites of the skin (ear) were similar (Fig. 1D, Supplemental Fig. 1A). Three months after immunization, percentages of CCR10+/EGFP and CCR10EGFP/EGFP OT-I cells in the un-treated skin were both reduced from their levels at 1 month after immunization (Fig. 1D). However, extents of the reduction were roughly similar for CCR10+/EGFP and CCR10EGFP/EGFP OT-I cells (~20% to ~1% and ~4% to ~0.2%, respectively) (Fig. 1D). There were still no significant difference in the percentage of CCR10EGFP/EGFP and CCR10+/EGFP OT-I cells at original immunization sites (Fig. 1D). These results demonstrate that CCR10-dependent migration of T cells into the un-treated skin is critical for establishment of resident memory T cells but CCR10 is not critical for their retention in the skin. On the other hand, CCR10 is dispensable for migration and establishment of resident memory T cells at immunization sites of the skin.
The different requirements of CCR10 for migration of T cells into un-treated and immunized skin could be due to increased inflammation and/or preferential stimulation of T cells by local antigens at immunization sites. To dissect these, CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice were immunized with Ova on ears and painted with DNFB on one side of the back skin at the same time to induce Ova-specific and -irrelevant inflammation locally. One week after immunization, Ova-immunized ears and DNFB-treated skin had more infiltration of total OT-I cells than the untreated skin, mainly due to increased EGFP− OT-I cells (Fig. 1E), indicating that inflammation overrides the requirement of CCR10 for migration of T cells into the skin.
Defective establishment of CCR10EGFP/EGFP CD8+ resident memory T cells leads to over-reactive inflammatory responses in the skin
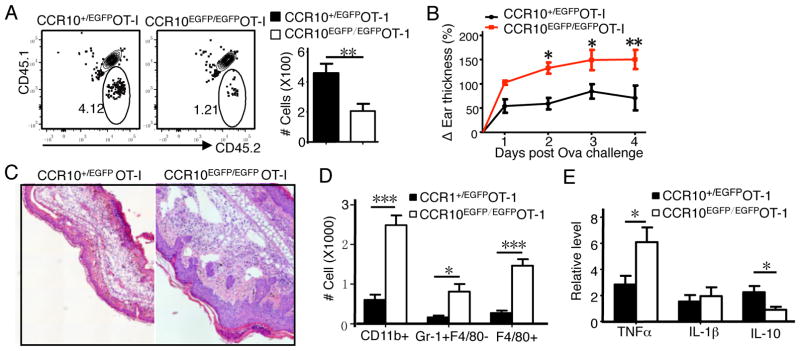
Resident memory T cells play important roles in memory responses. We then challenged Ova-immunized CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice with Ova on the un-immunized skin to induce memory responses 3 months after the immunization. As expected, CCR10EGFP/EGFPOT-ITR mice still had fewer OT-I cells in the skin than CCR10+/EGFPOT-ITR mice 3 days after challenge (Fig. 2A). Surprisingly, however, compared to CCR10+/EGFPOT-ITR mice, CCR10EGFP/EGFPOT-ITR mice had larger thickness increases (Fig. 2B), increased microscopic pathology (Fig. 2C), and increased infiltration of neutrophils (Gr-1+F4/80−) and macrophages (Gr-1−F4/80+) in the challenged skin (Fig. 2D), indicating an overall enhanced inflammation. Compared to CCR10+/EGFPOT-ITR mice, CCR10EGFP/EGFPOT-ITR mice also had enhanced TNF-α and reduced IL-10 expression in the challenged skin (Fig. 2E), suggesting defective immune regulation.
Figure 2.
Defective establishment of CCR10-knockout resident CD8+ memory T cells leads to over-reactive inflammatory responses in the skin. A) FC analysis of OT-I cells in Ova-challenged skin of CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice that were immunized with Ova 3 months earlier. Analyzed on day 3 post challenge. Bar graphs show calculated numbers of the OT-I cells. N=5 pooled of 2 experiments. B) Thickness changes of ears different days after Ova challenge. N=9 for WT and 8 for KO, pooled of 2 experiments. C) H&E staining of ear sections on day 4 post Ova challenge. One representative of 6 mice of 2 experiments. D) Numbers of CD11b+ immune cells, neutrophils (Gr1+CD11b+) and macrophages (F4/80+CD11b+) in Ova-challenged skin day 3 post challenge. N=5 pooled of 2 experiments. E) Levels of TNF-α, IL-1β and IL-10 transcripts in Ova-challenged skin on day 3. Normalized on β-actin levels. N≥6 pooled of 2 experiments.
Regulation of skin CD4+ T cell homeostasis by resident CD8+ T cells
Treg cells are important in immune regulation. We therefore compared their presence in the skin of immunized and/or challenged CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice. There were significantly lower percentages of Foxp3+ Treg cells in the Ova-challenged skin of immunized CCR10EGFP/EGFPOT-ITR than of CCR10+/EGFPOT-ITR mice (Fig. 3A). There were also fewer Treg cells in un-treated skin of immunized CCR10EGFP/EGFPOT-ITR mice than of CCR10+/EGFPOT-ITR mice even before Ova challenge (Fig. 3B). CD4+ T cells of untreated skin of immunized CCR10EGFP/EGFPOT-ITR mice also had lower percentages of IFNγ+ and higher percentages of IL-17+ cells than CCR10+/EGFPOT-ITR controls (Fig. 3C). These results suggest that reduced CCR10EGFP/EGFP OT-I cells in the skin result in defective CD4+ Treg and Teff cell homeostasis. Consistent with this, Ova-immunized CCR10EGFP/EGFPOT-ITR mice also had enhanced skin inflammation in response to DNFB challenge compared to CCR10+/EGFPOT-ITR controls (Fig. 3D–E). The dysregulation of Treg and Teff cells was observed in CCR10EGFP/EGFPOT-ITR mice as early as one week after Ova immunization (Supplemental Fig. 1B and C), suggesting that the modulation of skin CD4+ T cells by infiltrating CCR10+ CD8+ OT-I cells began at the effect phase.
Figure 3.
Skin CD8+ T cells regulate local CD4+ T cell homeostasis. A) FC analysis of gated CD4+ T cells of Ova-challenged skin to detect Foxp3+ Treg cells. Day 7 after challenge. N=6 pooled of 2 experiments. B–C) FC detection of Treg (B), IL-17+ and IFNγ+ subsets (C) in gated CD4+ cells of the torso skin of CCR10EGFP/EGFPOT-ITR and CCR10+/EGFPOT-ITR mice, 3 months after Ova immunization on ears. Bar graphs show average percentages of the different T cell subsets. N=9 pooled of 3 experiments. D) Ear thickness changes different days after DNFB challenge. N=8 pooled of 2 experiments. E) H&E staining of skin sections on day 3 after DNFB treatment. Representative of 5 mice of 2 experiments. F–G) Percentages of CCR10+/EGFP or CCR10EGFP/EGFP CD8+ T cells (F), and Foxp3+, IFNγ+ and IL-17+ CD4+ cells (G) of the skin of Rag1−/− recipient mice one month after co-transfer of CCR10+/EGFP or CCR10EGFP/EGFP CD8+ T cells and WT CD4+ T cells. N=6 pooled of 2 experiments.
To confirm that regulation of CD4+ T cell homoeostasis by CD8+ T cells in the skin was a general process, we used another model in which polyclonal CD8+ T cells of spleens of CCR10EGFP/EGFP or CCR10+/EGFP mice were co-transferred with WT splenic CD4+ T cells into Rag1−/− mice. There were fewer CCR10EGFP/EGFP than CCR10+/EGFP CD8+ donor T cells in the skin of recipients 1 month after transfer (Fig. 3F, Supplemental Fig. 1D), consistent with the requirement of CCR10 for T cell migration into the skin. Associated with this, donor CD4+ cells of the skin of recipients co-transferred with CCR10EGFP/EGFP CD8+ T cells contained lower percentages of Foxp3+ and IFNγ and higher percentages of IL-17+ cells than those of recipients co-transferred with CCR10+/EGFP CD8+ cells (Fig. 3G, Supplemental Fig. 1E–F). Together, these results demonstrate that CD8+ T cells promote CD4+ T cell homeostasis, including supporting Treg cells, in the skin to help immune homeostasis.
B7.2 expressed on resident CD8+ T cells supports Treg cells in the skin
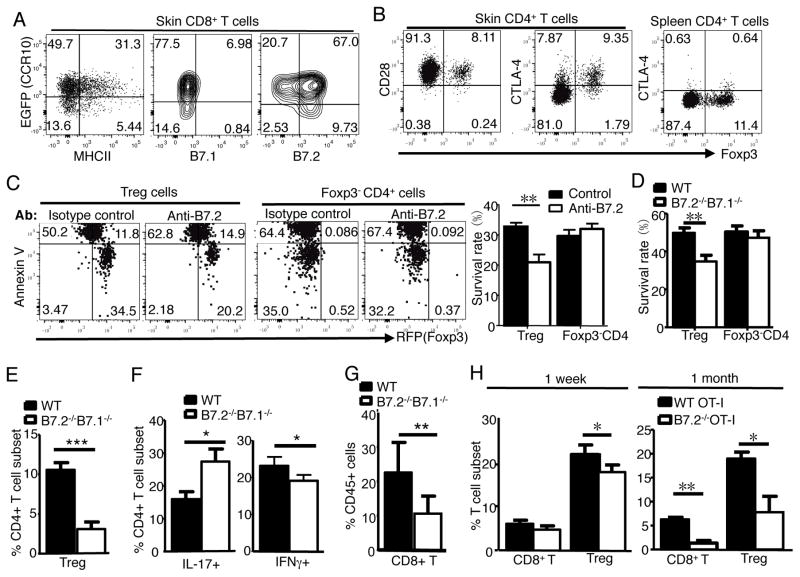
We searched for molecules expressed on skin CD8+ T cells that were involved in supporting Treg cells. Notably, skin CCR10+ CD8+ T cells expressed high levels of the co-stimulatory molecule B7.2 but no or low levels of B7.1, PD-L1, PD-L2, B7H, B7H3 and B7x or MHCII (Fig. 4A, Supplemental Fig. 2A). Of the two receptors for B7.2, CD28 was equally expressed on CD4+ skin Treg and Teff cells while CTLA-4 was uniquely highly expressed on the surface of skin Treg cells (Fig. 4B). While both receptors are involved in maintenance and function of Treg cells (14, 15), CTLA-4/B7.2 interaction has a much higher affinity than CD28/B7.2 interaction (16), and is likely specifically involved in supporting skin Treg cells.
Figure 4.
Resident CD8+ T cells support maintenance of Treg cells in the skin through B7.2/receptor axis. A) Expression of MHCII, B7.1 and B7.2 on skin CD8+ T cells. One representative of 4 analyses of 2 experiments. B) Expression of CD28 and CTLA-4 on skin and splenic Treg and CD4+ Teff cells. Representative of 4 analyses. C) FC analysis of survival of WT Treg and Foxp3− CD4+ Teff cells 1 day after co-culture with WT CD8+ T cells in presence of anti-B7.2 or isotype control antibodies. Bar graphs show percentages of live Annexin V− RFP+ Treg and Annexin V− Teff cells. N=6 pooled of 2 experiments. D) Survival of WT Treg and Foxp3− CD4+ Teff cells 1 day after co-culture with WT or B7.1−/−B7.2−/− CD8+ T cells, performed same as in (C). N=4 pooled of 2 experiments. E–G) Percentages of Treg cells (E), IL-17+ and IFNγ+ CD4+ cells (F) and CD8+ cells (G) in the skin of Rag1−/− mice 1 month after they are transferred with WT or B7.2−/−B7.1−/− CD8+ and WT CD4+ T cells. N=5 pooled of 2 experiments. H) Analysis of WT and B7.2−/− donor OT-I cells and host Treg cells in the torso skin of WT recipients 1 week and 1 month after Ova immunization on ears. N=3 each.
We then tested whether B7.2 was required for CD8+ T cell modulation of skin CD4+ cells by co-culturing skin CD4+ Treg or Teff cells with skin CD8+ cells in vitro in presence of anti-B7.2 antibodies. CD4+ Treg and Teff cells were purified from the skin of Foxp3-RFP mice based on the RFP reporter of Foxp3 expression (17). Based on Annexin V+ staining and loss of RFP signal (due to leakage of cytosolic RFP), significantly higher percentages of Treg cells died and fewer of them remained alive after co-culture with skin CD8+ T cells in presence of anti-B7.2 antibodies than in presence of isotype control antibodies (Fig. 4C). On the other hand, RFP− CD4+ Teff cells co-cultured with skin CD8+ cells in presence of anti-B7.2 or control antibodies had similar survival rates (Fig. 4C). Therefore, B7.2 expressed on CD8+ T cells is important to support survival of Treg but not Teff cells. Supporting this notion, fewer skin Treg cells were alive one day after co-culture with skin B7.2−/−B7.1−/− CD8+ cells than with WT CD8+ cells (Fig. 4D, Supplemental Fig. 2B).
To test whether B7.2 mediated CD8+ T cell regulation of CD4+ T cell homeostasis in vivo, we co-transferred B7.2−/−B7.1−/− or WT CD8+ cells and WT CD4+ cells into Rag1−/− mice. One month after transfer, mice receiving WT CD4+ and B7.2−/−B7.1−/− CD8+ T cells had fewer skin CD4+ Treg cells than those receiving WT CD4+ and WT CD8+ T cells (Fig. 4E, Supplemental Fig. 2C). In addition, skin CD4+ T cells co-transferred with B7.2−/−B7.1−/− CD8+ T cells expressed higher IL-17 and lower IFNγ than those co-transferred with WT CD8+ T cells (Fig. 4F, Supplemental Fig. 2D). There were also fewer B7.2−/−B7.1−/− donor CD8+ T cells in the skin of recipients than WT donor CD8+ T cells (Fig. 4G, Supplemental Fig. 2E), suggesting bidirectional effects of B7.2/ligand interaction on promoting homeostatic maintenance of Treg and CD8+ T cells in the skin.
We further tested whether B7.2-KO in CD8+ OT-I cells would affect skin CD4+ T cell homeostasis in the OT-I cell transfer model, in which B7.2−/− or WT OT-I cells were transferred into WT mice (referred as B7.2−/−OT-ITR and WT OT-ITR mice). One week after Ova immunization on ears, there were slightly lower percentages of Treg cells in the untreated torso skin of B7.2−/−OT-ITR mice than of WT OT-ITR mice while there was no significant difference in infiltration of B7.2−/− and WT OT-I cells into the skin (Fig. 4H, Supplemental Fig. 2F). One month post immunization, there were significantly lower percentages of donor OT-I cells and host Treg cells in the untreated skin of B7.2−/−OT-ITR mice than of WT OT-ITR mice (Fig. 4H, Supplemental Fig. 2F). These results reveal that B7.2-mediated signals are important in homeostatic maintenance of CD8+ T and Treg cells in the skin.
Conclusion remarks
Various subsets of T cells reside in barrier tissues such as skin. Their balanced presence is critical for local immune protection as well as prevents development of inflammatory diseases. Our study reveals a novel process in which CD8+ T cells regulate CD4+ T cell homeostasis by supporting maintenance of Treg cells in the skin and B7.2 expressed on CD8+ T cells is involved in the CD8+ T cell-regulated survival of Treg cells. Considering that CTLA-4 is highly expressed on skin Treg cells and that engagement of CTLA-4 could block activation-induced cell death of T cell hybridoma in vitro (18), it will be interesting to determine whether the B7.2/CTLA-4 interaction plays a role in the CD8+ T cell regulated survival and maintenance of Treg cells in vivo in the future. The general importance of the CD8+ T cell-regulated maintenance of Treg cells in skin homeostasis and inflammation also requires further investigation.
Supplementary Material
Acknowledgments
Research reported in this publication was partly supported by the National Institute of Arthritis, Musculoskeletal and Skin Diseases of NIH under Award Number R01AR064831 (to N.X.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 2.Mueller SN, Zaid A, Carbone FR. Tissue-resident T cells: dynamic players in skin immunity. Front Immunol. 2014;5:332. doi: 10.3389/fimmu.2014.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schenkel JM, Masopust D. Tissue-resident memory T cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hijnen D, Knol EF, Gent YY, Giovannone B, Beijn SJ, Kupper TS, Bruijnzeel-Koomen CA, Clark RA. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J Invest Dermatol. 2013;133:973–979. doi: 10.1038/jid.2012.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheuk S, Wiken M, Blomqvist L, Nylen S, Talme T, Stahle M, Eidsmo L. Epidermal Th22 and Tc17 cells form a localized disease memory in clinically healed psoriasis. J Immunol. 2014;192:3111–3120. doi: 10.4049/jimmunol.1302313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaide O, Emerson RO, Jiang X, Gulati N, Nizza S, Desmarais C, Robins H, Krueger JG, Clark RA, Kupper TS. Common clonal origin of central and resident memory T cells following skin immunization. Nat Med. 2015;21:647–653. doi: 10.1038/nm.3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, Muller A, McClanahan TK, Dieu-Nosjean MC, Orozco R, et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- 8.Jarmin DI, Rits M, Bota D, Gerard NP, Graham GJ, Clark-Lewis I, Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J Immunol. 2000;164:3460–3464. doi: 10.4049/jimmunol.164.7.3460. [DOI] [PubMed] [Google Scholar]
- 9.Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 10.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tubo NJ, McLachlan JB, Campbell JJ. Chemokine receptor requirements for epidermal T-cell trafficking. Am J Pathol. 2011;178:2496–2503. doi: 10.1016/j.ajpath.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jin Y, Xia M, Sun A, Saylor CM, Xiong N. CCR10 is important for the development of skin-specific gammadeltaT cells by regulating their migration and location. J Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia M, Hu S, Fu Y, Jin W, Yi Q, Matsui Y, Yang J, McDowell MA, Sarkar S, Kalia V, et al. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J Allergy Clin Immunol. 2014;134:634–644e610. doi: 10.1016/j.jaci.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 15.Kolar P, Knieke K, Hegel JK, Quandt D, Burmester GR, Hoff H, Brunner-Weinzierl MC. CTLA-4 (CD152) controls homeostasis and suppressive capacity of regulatory T cells in mice. Arthritis Rheum. 2009;60:123–132. doi: 10.1002/art.24181. [DOI] [PubMed] [Google Scholar]
- 16.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 17.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Rocha Dias S, Rudd CE. CTLA-4 blockade of antigen-induced cell death. Blood. 2001;97:1134–1137. doi: 10.1182/blood.v97.4.1134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.