Abstract
The etiology of cardiovascular disease (CVD) is impacted by multiple modifiable and non-modifiable risk factors including dietary choices, genetic predisposition, and environmental exposures. However, mechanisms linking diet, exposure to pollutants, and CVD risk are largely unclear. Recent studies identified a strong link between plasma levels of nutrient-derived Trimethylamine N-oxide (TMAO) and coronary artery disease. Dietary precursors of TMAO include carnitine and phosphatidylcholine, which are abundant in animal-derived foods. Dioxin-like pollutants can upregulate a critical enzyme responsible for TMAO formation, hepatic flavin containing monooxygenase 3 (FMO3), but a link between dioxin-like PCBs, upregulation of FMO3, and increased TMAO has not been reported. Here, we show that mice exposed acutely to dioxin-like PCBs exhibit increased hepatic FMO3 mRNA, protein, as well as an increase in circulating levels of TMAO following oral administration of its metabolic precursors. C57BL/6 mice were exposed to 5 μmol PCB 126/kg mouse weight (1.63 mg/kg). At 48 h post-PCB exposure, mice were subsequently given a single gavage of phosphatidylcholine dissolved in corn oil. Exposure to 5 μmole/kg PCB 126 resulted in greater than 100-fold increase in FMO3 mRNA expression, robust induction of FMO3 protein, and a 5-fold increase in TMAO levels compared with vehicle treated mice. We made similar observations in mice exposed to PCB 77 (49.6 mg/kg twice); stable isotope tracer studies revealed increased formation of plasma TMAO from an orally administered precursor trimethylamine (TMA). Taken together, these observations suggest a novel diet-toxicant interaction that results in increased production of a circulating biomarker of cardiovascular disease risk.
Keywords: Nutrition, TMAO, FMO3, PCB toxicity, cardiovascular disease
1. Introduction
The etiology of cardiovascular disease (CVD) is impacted by multiple modifiable and non-modifiable risk factors including dietary choices, genetic predisposition, and environmental exposures. Exposure to persistent organic pollutants such as dioxins and dioxin-like polychlorinated biphenyls (PCBs) is associated with increased risk of multiple pro-inflammatory human diseases including diabetes, cancer, and CVD [1–9]. Preclinical studies have shown that dioxin-like PCBs may increase oxidative stress and subsequent chronic inflammatory states which can lead to glucose intolerance, alterations of lipid and cholesterol homeostasis, and other risk factors for multiple metabolic diseases [1, 10]. Certain human populations may be variably susceptible to the toxicity of environmental pollutants, and emerging data now implicate the importance of examining interactions between pollutant exposures and nutrition [11].
Nutritional choices have been shown to be strongly related to metabolic diseases, but until recently, mechanisms linking diet, exposure to environmental pollutants, and CVD risk have remained elusive. Interestingly, it appears that diet can either exacerbate the toxicity of environmental pollutants, or in the case of healthful diets, certain nutritional choices may actually reduce risks associated with pollutant exposures [12, 13]. For example, diets high in certain pro-inflammatory fats may exacerbate PCB-induced endothelial dysfunction and atherosclerosis, but diets high in anti-inflammatory bioactive nutrients, such as polyphenols, may block the acute or chronic pro-atherogenic effects of pollutants [12–18].
Preclinical studies using cell culture and animal models of disease have identified multiple mechanisms that might link exposure to dioxin-like PCBs with disease processes. The toxicity of coplanar PCBs, such as PCBs 77 and 126, is primarily induced through constant basal activation of the aryl hydrocarbon receptor (AhR), a transcription factor involved in xenobiotic metabolism. Once bound to a ligand, AhR translocates into the nucleus, and then binds to a xenobiotic responsive element (XRE) in the promoter region of the target genes [19, 20]. A large number of drug-metabolizing enzymes are induced as a result of AhR activation, including the phase I (oxidation), phase II (conjugation), and transporters of the phase III (excretion) metabolic pathways. The activation of these enzymes results in AhR ligands inducing their own metabolism and clearance from the body[21]. Previous work has demonstrated that coplanar PCBs are capable of acting as AhR agonists [20, 22, 23]. The toxicity that is then caused by coplanar PCBs while stimulating AhR and coupled with induction of P450 enzymes is often attributed to the release of reactive oxygen species (ROS) and a subsequent increase in oxidative stress [24]. Increased amounts of ROS and dysregulated redox status have been shown to be heavily involved in many of the risk factors for cardiovascular disease [25], including the development of atherosclerosis [26]. In addition to increasing oxidative stress, coplanar PCBs acting as AhR agonists also induce cellular inflammation that is largely mediated by nuclear factor κB (NF-κB)[22, 27].
Activation of AhR by xenobiotics or endogenous metabolites may lead to deleterious phenotypes such as CVD and diabetes by mechanisms unrelated to Cyp1a1. Recent studies identified a strong link between plasma levels of the nutrient biomarker Trimethylamine N-oxide (TMAO) and coronary artery disease risk in humans [28]. Circulating TMAO is generated from intestinal quaternary nitrogen (+ charged) containing metabolites (predominantly choline and carnitine) [28, 29]. These diet-derived quaternary amines are metabolized by anaerobic gut microbiota to form trimethylamine (TMA). Circulating TMA is then oxidized to form TMAO by hepatic flavin containing monooxygenases (FMO), primarily the FMO3 isoform [30]. Studies in animal models strongly support the concept that FMO and its product TMAO are pro-atherogenic. For example dietary administration of TMAO and its precursors choline and carnitine accelerate atherosclerosis in mice through mechanisms that involve changes in cholesterol metabolism, increased vascular inflammation, and formation of atherosclerotic plaques [28–32]. Downregulation or knock-out of FMO3 leads to decreased levels of TMAO and is athero-protective in these models, whereas increased expression of FMO3 leads to pro-atherogenic lipid profiles (e.g., higher VLDL and LDL) [31, 33]. Additionally, upregulation of FMO3 may be pro-atherogenic or pro-diabetic via mechanisms unrelated to increased production of TMAO. Increases in FMO3 have been linked to alterations of reverse cholesterol transport[31], and downregulation of FMO3 can prevent hyperglycemia, hyperlipidemia, and atherosclerosis in a mouse model of insulin resistance [34]. Importantly, past work has shown that the environmental pollutant TCDD, the strongest known agonist of AhR, can induce the upregulation of FMO3, and this induction was determined to be AhR-dependent [35]. However, the possibility that dioxin-like PCBs may also be able to upregulate FMO3, and result in increased circulating levels of TMAO has not been investigated.
Thus, the current study was designed to investigate whether exposures to dioxin-like PCBs can lead to increased levels of the known pro-atherogenic nutrient biomarker TMAO. Our data provide strong evidence that multiple dioxin-like PCBs cause upregulation of FMO3, and that dietary precursors, when fed to mice pre-exposed to PCBs, increased plasma levels of TMAO. The increase of FMO3 and/or TMAO by certain environmental pollutants represents a novel example of a toxicant/diet interaction, and may prove to be a causative mechanism linking environmental exposures to cardiovascular disease and related pathologies.
2. Materials and Methods
2.1. Animals, diets, and dosing treatments
Three independent in vivo studies were completed:
Experiment 1
For the initial PCB 126 mouse study, C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME) at 2 months of age and were randomly assigned to either vehicle corn oil or PCB 126 groups. Mice were fed chow diet (Teklad Global 18% Protein Rodent Diet; Envigo, United Kingdom) and were gavaged one time with PCB 126 (5 μmol/kg mouse) or vehicle (stripped corn oil; Acros Chemical Company, Pittsburgh, PA). The concentration of PCB 126 used was chosen based on observations in preliminary studies where gavage of 5 μmol PCB 126/kg mouse weight (1.63 mg/kg) showed pro-inflammatory responses in C57/BL6 mice but not wasting syndrome [36]. 48 h after exposure to PCB or vehicle (corn oil), all mice were administered phosphatidylcholine by oral gavage. Phosphatidylcholine (in corn oil) was chosen because it has been shown to be a major dietary precursor for TMAO production [28]. Microvolumes of blood were collected from the tail vein at 3 and 5 h post-choline gavage and at 7 h post via cardiac puncture.
Experiment 2
For the second PCB 126 study, C57BL/6 mice were randomly assigned to either vehicle corn oil or PCB 126 groups. As before, mice were exposed to either vehicle or PCB 126 and 48 h after, all mice were administered phosphatidylcholine by oral gavage. Microvolumes of blood were then collected from the tail vein at 8 and 16 h post-choline gavage and at 24 post via cardiac puncture. Unless otherwise noted, experimental conditions between Experiment 1 and 2 were the same.
Experiment 3
To examine if alterations in TMAO levels were unique to exposure to PCB 126, a third in vivo experiment was conducted that focused on PCB 77; a less potent agonist of AhR. For this PCB 77 study, C57BL/6 mice were again purchased from Jackson Laboratory (Bar Harbor, ME) at 2 months of age and were randomly assigned to either vehicle safflower oil or PCB 77 groups. Mice were fed a10% fat diet (Research Diets- D12450K, New Brunswick, NJ) and were gavaged with PCB 77 (169.9 μmol/kg mouse, 49.6 mg/kg) or vehicle (stripped safflower oil, Dyets, Bethlehem, PA) once at week 1 and again at week 2. This concentration and dosing schedule was chosen based on previous studies from other groups[37]. 48 h after the second PCB77 or vehicle exposure, 5 mice from each group were administered via oral gavage 0.12mg/g body weight d9-TMA (in water), and 30 minutes later blood was collected by cardiac puncture. Trimethl-d9-amine was purchased in the hydrochloride form from Sigma-aldrich (St. Louis, MO)
2.2 Blood and tissue harvesting
In all cases, mice were euthanized with CO2 and quickly exsanguinated. Ethylenediaminetetraacetic acid (EDTA) was added to collected blood samples, briefly mixed, and centrifuged at 2000 g for 15 min at 4 °C to separate blood plasma. Plasma samples were frozen in liquid nitrogen and stored at −80 °C until processing. Livers were harvested for mRNA or protein and stored at −80 °C prior to analysis.
2.3. RNA isolation and polymerase chain reaction (PCR) amplification
Liver samples used to analyze oxidative stress, inflammatory, and metabolic mRNA markers were homogenized and mRNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. mRNA concentrations were then determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA). Reverse transcription was performed using the AMV reverse transcription system (Promega, Madison, MI). mRNA levels were determined by quantitative real-time PCR using a CFX96 Real-Time PCR system (BioRad, Hercules, CA) and SYBR Green master mix (Applied Biosystems) as compared to constitutively expressed 18S using the relative quantification method (ΔΔCt). Primer sequences (see Suppl. Table 1) for SYBR Green reactions were designed using the Primer Express Software 3.0 for real-time PCR (Applied Biosystems) or as in [33] and synthesized by Integrated DNA Technologies, Inc. (Coralville, IA).
2.4. Immunoblotting
Liver samples used for protein analysis were homogenized in extraction RIPA buffer containing protease inhibitors (Pierce, Rockford, IL). Lysed tissue was centrifuged at 10,000 g for 30 min at 4 °C followed by Bradford protein assay (Pierce). Protein samples were separated using 10% SDS-PAGE and subsequently were transferred onto nitrocellulose membranes. Membranes were blocked with 5% non-fat milk buffer and incubated overnight at 4 °C with the following primary antibodies: β-actin (product #A2066, ~42 kD, Sigma, St. Louis, MO), FMO3 (product #126790, ~55 kD, Abcam), NFκB p65 acetylated (product #52175, ~60 kD, Abcam), BAX (product #CS2772, ~21 kD, Cell Signaling), and NQO1 (product #ab34173, ~31 kD, Abcam). After washing, membranes were incubated with secondary antibodies conjugated with horseradish peroxidase and visualized using ECL detection reagents (Thermo, Waltham, MA).
2.5. Quantitation of markers of inflammation in the plasma
Plasma cytokine levels were measured using the Milliplex Map Mouse Adipokine Magnetic Bead Panel (Millipore Corp, Billerica, MA, USA) on the Luminex Xmap MAGPIX system (Luminex Corp, Austin, TX, USA), as per the manufacturer’s instructions. Only PAI-1 levels were statistically altered in PCB-exposed mice.
2.6. Measurement of TMAO and related metabolites by HPLC Electrospray Ionization Tandem Mass Spectrometry
Analysis of TMAO, TMA and choline was carried out using a Shimadzu HPLC coupled with an AB Sciex 4000-Qtrap hybrid linear ion trap triple quadrupole mass spectrometer in multiple reaction monitoring (MRM) mode. d9-TMAO, d9-TMA and d9-choline were used as internal standards. TMAO, TMA and choline were analyzed using a Primesep 100, 3 um, 2.1 X 100 mm column (from SIELC) with a flow rate of 0.5 ml/min and column temperature of 30 C. The mobile phase consisted of water with 0.1% TFA as solvent A and acetonitrile as solvent B. Analysis of TMAO, TMA and choline was achieved using an isocratic flow of 80 % solvent A and 20 % solvent B for 12 min. Sample injection volume was 10 uL. The mass spectrometer was operated in the positive electrospray ionization mode with optimal ion source settings determined by synthetic standards of TMAO, d9-TMAO, TMA, d9-TMA, choline and d9-choline with a declustering potential of 66 V, entrance potential of 10 V, collision energy of 31 V, collision cell exit potential of 6 V, curtain gas of 20 psi, ion spray voltage of 5500 V, ion source gas1/gas2 of 40 psi and temperature of 550 C. MRM transitions monitored were as follows: 60.1/44.4 for TMA, 69.1/49.2 for d9-TMA, 76/59.1 for TMAO, 85.1/66.0 for d9-TMAO, 104.2/60.1 for choline and 113.1/69.1 for d9-choline.
2.6. Data analyses
Data were analyzed using SigmaStat software (Systat Software, Point Richmond, CA). Comparisons between treatments were made by Student’s t-test or two-way ANOVA with post-hoc comparisons of the means when appropriate. Bivariate relationships between plasma TMAO levels and biomarker mRNA or protein levels were assessed using scatterplots. Univariate linear regression models were then carried out to determine unadjusted association between the variables and TMAO levels. Sample sizes for each experiment are indicated in corresponding figure legends (e.g., qRT-PCR mRNA analysis n=5, Western blot protein analysis n=5). A probability value of p < 0.05 was considered statistically significant.
3. Results
3.1 Exposure to coplanar or dioxin-like PCBs increases expression of FMO3
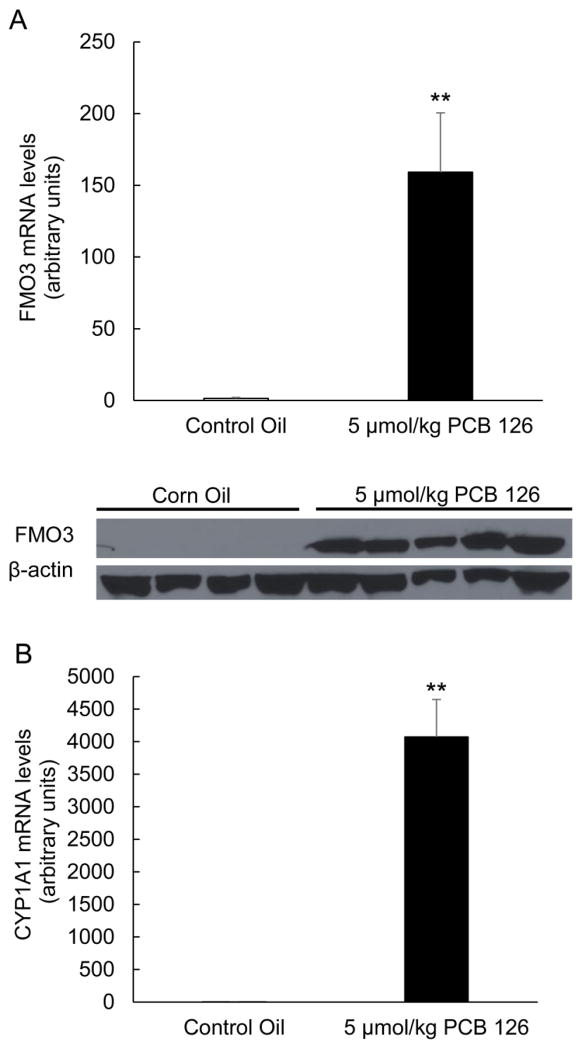
Induction of FMO3 has been shown to be mediated by AhR, and we tested the hypothesis that AhR ligands like coplanar PCBs can induce gene expression changes of FMO3. As seen in Fig. 1A, basal levels of FMO3 mRNA and protein in male C57BL/6 mice are extremely low at baseline, but are highly induced upon exposure to a single dose of PCB 126, a potent ligand of AhR. Upregulation of FMO3 was mirrored by upregulation of Cyp1a1, a consequence of ligand binding and induction of AhR (Fig. 1B). In previous studies, we have shown that this upregulation of FMO3 by PCB 126 appears to be dose dependent in nature (data not shown). Hepatic FMO3 upregulation as a result of PCB exposure was also observed in mice exposed to PCB 77, another coplanar PCB and AhR ligand. (Fig. 4C).
Figure 1.
Exposure to PCB 126 leads to induction of AhR mediated detoxification genes. Relative mRNA and protein levels of FMO3 (A), and relative mRNA levels of Cyp1a1 (B) were determined in mouse liver samples. All mRNA values were determined using the relative quantification method (ΔΔCt) and normalized to control oil. 18S was used as the housekeeping gene for all mRNA quantifications. Protein expression of FMO3 in mouse liver samples was assessed by western blot analysis. Samples were separated through gel electrophoresis and probed with FMO3 primary antibody and β-actin was used as a loading control. Data are presented as mean±S.E.M (*p<0.05, n=5, Student’s t-test).
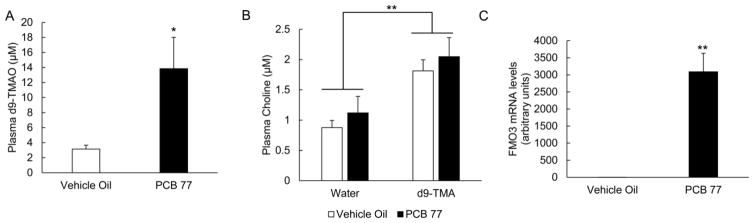
Figure 4.
Exposure to PCB 77 increases the conversion of mass labeled TMA to d9-TMAO. Mice were exposed to either vehicle oil or PCB 77 and 48 h later, half of each group were administered the FMO3 substrate TMA (mass labeled) or vehicle water. Plasma was collected 30 minutes post- d9-TMA administration and measured for d9-TMAO (A) and choline (B) by HPLC/MS MS. MRM transitions monitored included: d9-TMAO-85.1/66.0, choline-104.2/60.1, and d9-choline-113.1/69.1. AUC integration values were determined and all results were normalized to d9-choline internal standard values. No d9-TMAO was observed in mice administered vehicle water. Data are presented as mean±S.E.M (*p<0.05, **p<0.01, n=5 for each group, Student’s t-test for (A) and 2-way ANOVA for (B)).
(C) Relative mRNA levels of FMO3 were determined in mouse liver samples. All mRNA values were determined using the relative quantification method (ΔΔCt) and normalized to control oil. 18S was used as the housekeeping gene for all mRNA quantifications. No statistical differences were determined for FMO3 in mice administered water vs. d9-TMA. Data are presented as mean±S.E.M (*p<0.05, n=10, Student’s t-test).
3.2 Exposure to dioxin-like pollutants increases the formation of TMAO from dietary precursors
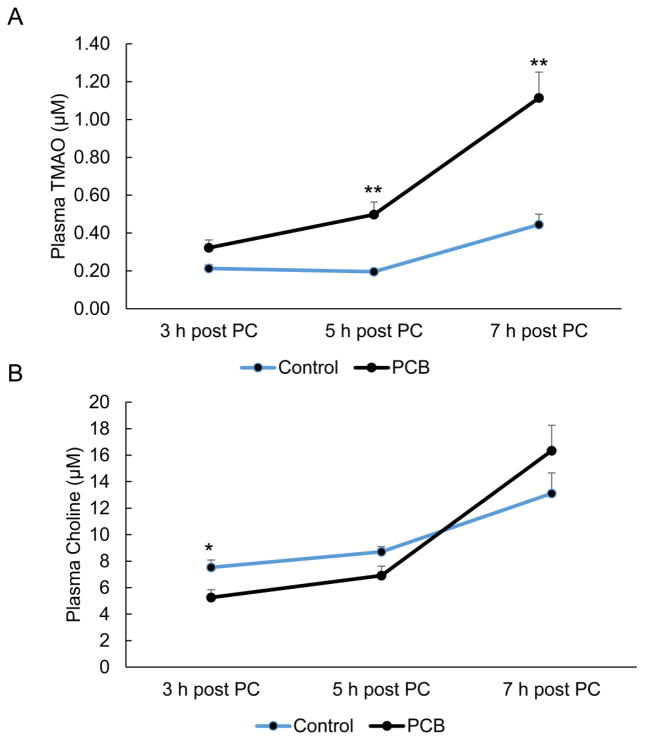
FMO3 catalyzes the oxygenation of TMA to TMAO. Circulating levels of TMAO have been highly correlated with coronary artery disease in humans, but mechanisms linking nutrition and toxicant exposure with higher susceptibility to TMAO formation are still lacking. We adapted and validated a method for quantitation of TMAO using stable isotope dilution HPLC coupled tandem mass spectrometry (Suppl. Fig. 1) and used this to monitor effects of PCB treatment on plasma TMAO levels in mice. Plasma samples from mice exposed to a single bolus of PCB 126 and subsequently administered a dietary choline source by oral gavage of phosphatidylcholine were analyzed for TMAO and related nutrient biomarkers by HPLC/MS MS. As seen in Fig. 2A, circulating levels of TMAO were higher in plasma from mice exposed to PCB 126 in comparison to vehicle oil controls, and these differences were most apparent 5 and 7 h post-choline gavage. Interestingly, in these PCB 126 mice, levels of endogenous TMA were significantly lower at 48 h post-PCB 126 exposure and this difference remained until 5 h post-choline administration (Suppl. Fig. 2). As seen in Fig. 2B, circulating levels of choline were initially diminished in PCB-exposed mice (e.g., 3 h post-phosphatidylcholine gavage), but these differences became insignificant as plasma levels of choline increased throughout time. Since it was observed that levels of TMAO were still increasing after 7 h post-choline gavage, a second study was undertaken which focused on longer-sustained effects of PCB exposure on TMAO levels.
Figure 2.
Exposure to PCB 126 leads to increased plasma levels of TMAO after administration of dietary precursors. Mice were exposed to either vehicle oil or PCB 126 and 48 h later administered a dietary choline source. Plasma was collected at the indicated times, and TMAO (A) and choline (B) levels were measured by HPLC/MS MS. MRM transitions monitored included: TMAO-76/59.1, choline-104.2/60.1, and d9-choline-113.1/69.1. AUC integration values were determined and all results were normalized to D9-choline internal standard values. Data are presented as mean±S.E.M. (*p<0.05, n=3–7, Student’s t-test).
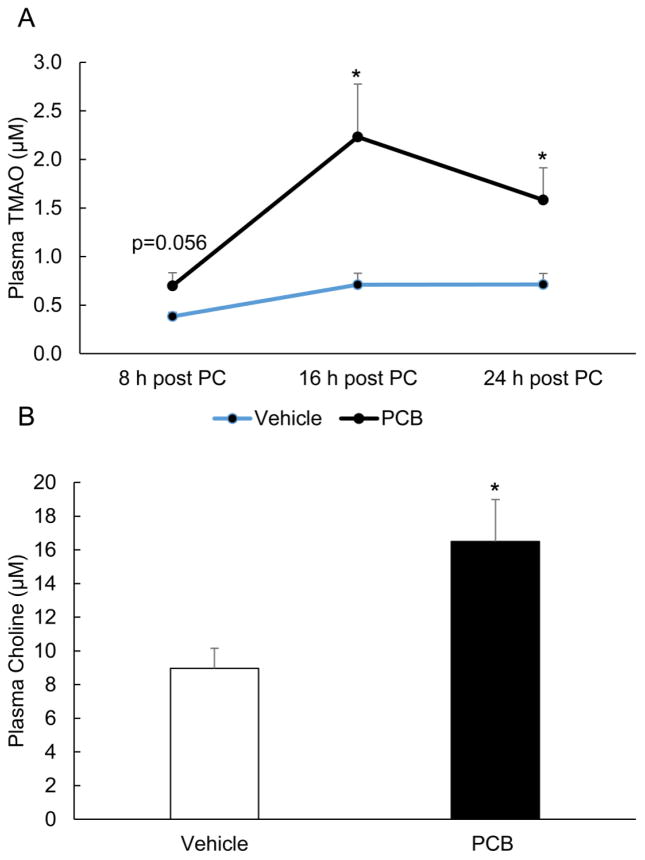
Consistent with the previous experiment, mice were exposed to a single bolus of PCB 126 (5 μmol/kg) and subsequently administered phosphatidylcholine; a substrate that can be metabolized to TMAO. Plasma was collected and analyzed for TMAO, TMA, and choline by HPLC/MS MS. As seen in Fig. 3, circulating levels of TMAO were higher in plasma from mice exposed to PCB 126 as compared to vehicle controls, and these differences peaked at 16 h and remained significantly different even 24 h post-choline administration. In this study, Experiment 2, circulating TMA levels were not significantly different prior to choline administration, but were significantly decreased in PCB 126 exposed mice at 16 h post-choline gavage compared to vehicle oil treated mice (data not shown). Finally, endogenous choline levels were significantly increased in PCB 126 mice at the conclusion of this follow-up study (Fig. 3B). Taken together, these results implicate multiple impacts of PCB 126 on the physiological system of TMAO formation. Generation of TMAO from dietary phosphatidylcholine requires metabolism of this lipid to form TMA by the gut microbiota and subsequent conversion of TMA to TMAO by FMO3 in the liver. Since it was observed in our first two experiments that PCB 126 may have altered TMA and choline levels (and there has been some previous work on the effects of toxicants on the gut microbiota [38, 39]) we attempted to bypass these possibly confounding events by directly measuring effects of PCB exposure on conversion of orally administered mass labeled TMA to circulating TMAO in Experiment 3.
Figure 3.
Exposure to PCB 126 leads to sustained increases in plasma levels of TMAO after administration of dietary precursors. Mice were exposed to either vehicle oil or PCB 126 and 48 h later administered a dietary choline source. Plasma was collected at the indicated times for TMAO quantification (A) and levels were measured by HPLC/MS MS. For choline measurements (B), plasma was collected at the conclusion of the study and levels were measured by HPLC/MS MS. MRM transitions monitored included: TMAO-76/59.1, Choline-104.2/60.1, and d9-choline-113.1/69.1. AUC integration values were determined and all results were normalized to d9-choline internal standard values. Data are presented as mean±S.E.M. (*p<0.05, n=5 for each group, Student’s t-test).
As seen in Fig. 4, the observed conversion of d9-TMA to d9-TMAO was more readily detectable in plasma from mice exposed to PCB 77 (d9-TMAO was entirely absent in mice administered vehicle water instead of d9-TMA). Interestingly, mice in this study were administered d9-TMA for only 30 minutes prior to sacrifice; which implicates the rapid nature of TMA-TMAO conversion. In fact, we have shown previously in mice that administration of d9-TMA via oral gavage results in conversion to d9-TMAO for approximately 4 h, and after this time point, levels of d9-TMAO quickly decrease to negligible amounts. This preliminary observation suggests possible unknown mechanisms of storage or metabolism for TMAO (data not shown). Finally, in Experiment 3 we did not observe strong PCB 77-induced effects on choline or endogenous TMA (Fig. 4B).
3.3 Exposure to PCB 126 modulates multiple pro-inflammatory, oxidative stress, and metabolic mediators
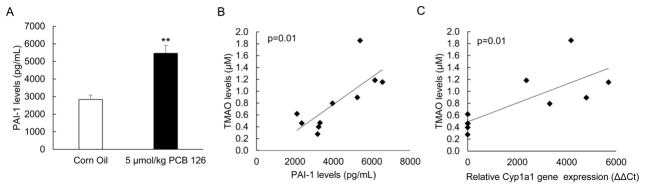
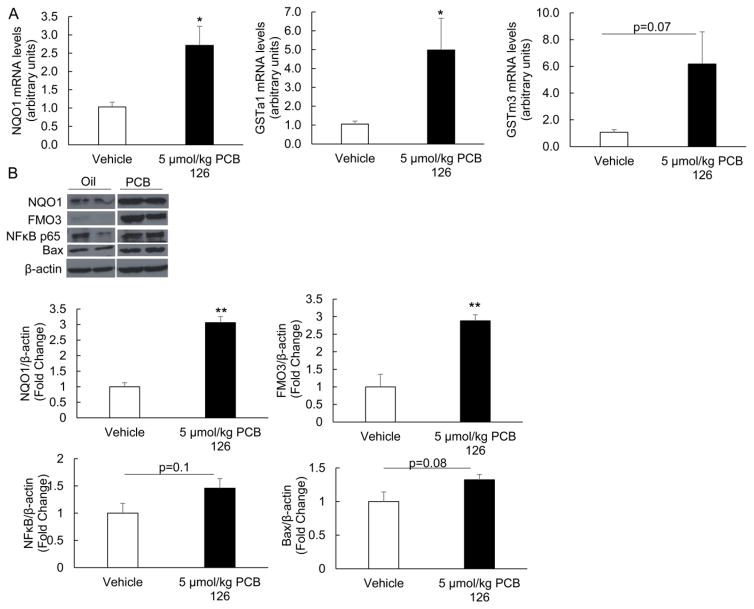
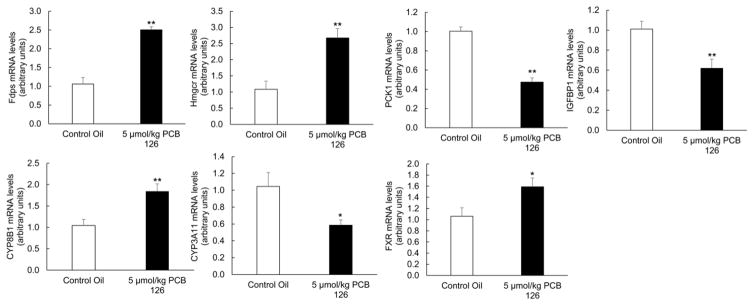
Induction of FMO3 and increased levels of TMAO have been correlated with changes in multiple inflammatory signaling pathways. Also, changes in FMO3 expression have been associated with alterations of metabolic pathways independent of TMAO. Thus, we examined PCB 126 sensitive effects on pro-inflammatory, metabolic, and oxidative stress mediators. In the current study, and as observed previously [14], PCB 126 induced an inflammatory response in livers of exposed mice (Suppl. Fig. 3). Indicators of inflammation including monocyte chemoattractant protein-1 (MCP-1, also referred to as chemokine (C-C motif) ligand 2, CCL2), CCL3 (also referred to as macrophage inflammatory protein-1α, MIP-1α), and Interleukin 13 (IL-13) all trended toward being increased in PCB 126-exposed mice; whereas anti-inflammatory Interleukin 10 (also referred to as human cytokine synthesis inhibitory factor, CSIF) trended toward decreased mRNA expression (Suppl. Fig. 3). To gain a more systemic perspective of the inflammatory state of these mice, plasma levels of Plasminogen activator inhibitor-1 (PAI-1) was examined via Magpix instrumentation. Mice exposed to a single dose of 5 μmol/kg PCB 126 displayed a statistically significant doubling of PAI-1 levels (Fig. 5A). Also, it was determined via linear regression modeling that plasma TMAO levels in these mice significantly correlated with PAI-1 plasma levels and liver Cyp1a1 relative gene expression (Fig. 5B and 5C). Inflammation and oxidative stress are strongly linked, so we examined mRNA and protein levels of multiple redox sensitive mediators in mice from Experiment 2. As seen previously [14], mice exposed to dioxin-like PCB showed increased liver mRNA levels of glutathione S-transferases (GSTa1 and GSTm3) and NAD(P)H dehydrogenase [quinone] 1 (NQO1) (Fig. 6A). Whole cell levels of oxidative stress-sensitive proteins such as NQO1, NF-κB p65 (acetylated form), and BAX (bcl-2-like protein 4) were also more abundant in livers from mice exposed to PCB 126 (Fig. 6B). Mice exposed to PCB 126 also displayed multiple changes in relation to glucose, cholesterol, and lipid synthesis/homeostasis. As seen in Figure 7, cholesterol/bile biosynthesis mediators farnesyl diphosphate synthase (Fdps), HMG-CoA reductase (Hmgcr), Cyp8b1, and farnesoid X receptor (Fxr) were significantly upregulated in PCB 126 exposed mice whereas mRNA levels of Cyp3a11 was decreased. In addition, the expression levels of glucose/insulin related genes phosphoenolpyruvate carboxykinase 1 (Pck1) and insulin like growth factor binding protein 1 (Igfbp1) were significantly downregulated by PCB 126.
Figure 5.
AhR-mediated inflammatory genes are increased in PCB exposed mice and are correlated with plasma TMAO levels (A) Plasma levels of Plasminogen activator inhibitor-1 (PAI-1), a known risk factor for atherosclerosis, was significantly increased in mice exposed to PCB 126. Plasma was collected at the end of the study and PAI-1 total levels were identified using the Magpix system. All data are presented as mean±S.E.M (*p<0.05, **p<0.01, n=5 for each group, Student’s t-test). (B) Plasma PAI-1 levels were plotted against plasma TMAO levels for each mouse (n=5 for each group) and a linear goodness of fit was determined (linear regression). A statistically significant positive correlation between PAI-1 protein levels and plasma TMAO levels was determined (R=0.76, R2=0.57, p=0.01). (C) Relative gene expression values as determined by ΔΔCt method for AhR-target gene Cyp1a1 were plotted against plasma TMAO levels for each mouse (n=5 for each group) and a linear goodness of fit was determined (linear regression). A statistically significant positive correlation between liver Cyp1a1 relative gene expression levels and plasma TMAO levels was determined (R=0.76, R2=0.57, p=0.012).
Figure 6.
Exposure to PCB 126 leads to induction of oxidative stress-sensitive proteins. Relative mRNA (A) and protein expression (B) of multiple redox sensitive markers from mouse liver samples was assessed by real time PCR or western blot analyses. All mRNA values were determined using the relative quantification method (ΔΔCt) and normalized to control oil. 18S was used as the housekeeping gene for all mRNA quantifications. Protein samples were separated through gel electrophoresis and probed with NQO1, FMO3, Bax, and NF κB p65 primary antibodies. In addition to a visualized representative blot, samples were quantified via densitometry and compared to β-actin housekeeping gene. (*p<0.05, **p<0.01, n=5, Student’s t-test).
Figure 7.
Exposure to PCB 126 leads to liver alterations of multiple genes related to glucose, lipid, and cholesterol metabolism/homeostasis. All mRNA values were determined using the relative quantification method (ΔΔCt) and normalized to control oil. 18S was used as the housekeeping gene for all mRNA quantifications. (*p<0.05, **p<0.01, n=5 for each group, Student’s t-test).
In summary, mice treated with PCB 126 and subsequently administered a precursor for TMAO exhibited increased oxidative stress, inflammation, and altered metabolic signaling pathways. These data support our hypothesis that plasma levels of TMAO are correlated with increases in pathological inflammatory markers associated with atherosclerosis and other inflammatory diseases.
Discussion
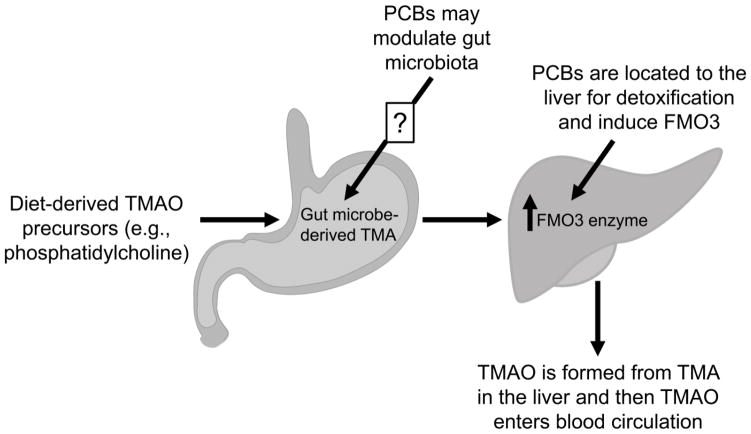
The strong association of plasma TMAO levels with human coronary artery disease risk implies that this molecule is a valid disease risk biomarker. These clinical observations suggest there is a critical need to examine mechanisms involved in the regulation and formation of circulating TMAO levels. This is a complicated process involving dietary intake of methylamine containing metabolic precursors, their conversion to TMA by the gut microbiota and oxidation of TMA to TMAO by hepatic FMO3 (Fig. 8). Our studies identify environmental pollutants as a previously unappreciated modulator of plasma TMAO levels through effects on expression and activity of FMO3 in the liver. These observations raise the possibility that exposure to environmental pollutants like PCBs contributes to interindividual variability in plasma TMAO levels. Our findings also suggest that TMAO levels and/or acute formation of TMAO from dietary precursors might represent a valid biomarker or relatively noninvasive clinical test to monitor alterations in FMO3 expression and activity in environmental pollutant exposed individuals. This latter possibility would be of particular interest because these pollutants bioaccumulate in fatty lipid rich tissues like the liver and adipose tissue and measurement of their levels in the plasma may not always be a reliable measure of body burden or disease risk associated with exposure to these pollutants [40].
Figure 8.
Exposure to dioxin-like PCBs impacts the formation of TMAO. TMAO is formed by the complex breakdown of dietary methylamine containing metabolic precursors. Precursors such as phosphatidylcholine are metabolized by the gut microbiota to form TMA which then undergoes oxidation to TMAO by hepatic FMO3. Exposure to dioxin-like pollutants may impact this pathway by increasing the protein expression of hepatic FMO3 (detailed in this paper), or by altering the gut microbial environment (investigated elsewhere [38, 39]).
The liver is the primary organ for TMAO formation as well as xenobiotic detoxification. Here, we have observed increased expression of the critical TMAO generating enzyme FMO3 in mice exposed to dioxin-like PCBs. This increased FMO3 protein abundance resulted in functional effects on TMA oxidation leading to higher levels of pro-atherogenic TMAO after addition of necessary dietary precursors. In addition, we have observed PCB-induced alterations of other dietary biomarkers (e.g., choline) related to TMAO formation that may implicate the gut microbiota as a possible target of toxicity worthy of future investigation.
TMAO and FMO3 may both play important roles in the pathology of metabolic diseases such as diabetes and CVD. Mice administered TMAO in combination with high fat diet exhibited increased fasting insulin and decreased glucose tolerance [41]. Also, although exposure to TMAO via osmotic pump did not alter blood pressures in normotensive rats, TMAO did prolong angiotensin II-induced hypertensive effects [42]. Although TMAO may indeed possess pro-atherogenic characteristics, emerging research now implicates important pathological roles for FMO3 that may be independent of increased TMAO production [31, 32]. Observations that dietary intake of TMAO precursors are not always linked to increased heart disease risk support this idea [43]. Thus, and as supported by our data, the increased risk of CVD correlated with high plasma levels of TMAO may not only be an independent biomarker for CVD risk, but could also be linked to high expression or activity of FMO3. Increased FMO3 expression has been linked to increases in glucose secretion and lipogenesis, and alterations in cholesterol metabolism and reverse cholesterol transport [31, 32]. Others have shown that knockdown of FMO3 in mice results in decreased levels of Cyp8b1 and FXR [32]; both of these important cholesterol/bile related mediators were increased in our PCB 126 mice (increased expression of FMO3). Also, downregulation of FMO3 has been shown to prevent the development of hyperlipidemia, atherosclerosis, and hyperglycaemia in a mouse model of insulin resistance [33]. It is important to note that in the current study some FMO3-mediated effects may be masked or altered by the strong activation of AhR by dioxin-like PCBs. For example, others have shown overexpression of FMO3 leads to increased Pck1 [31, 32], but here we show exposure PCB 126 downregulates this gluconeogenesis gene. PCB 126-induced downregulation of Pck1 was observed previously and was determined to be to be AhR dependent [44]. As more information related to FMO3’s role in metabolic processes and disease pathologies emerges, it will be important to identify environmental factors, such as dioxin-like PCBs, that can modulate FMO3 expression.
FMO3 is transcriptionally regulated by multiple nuclear receptors and is induced by nutrient and toxicant-derived mediators. Both AhR and FXR seem to play important roles in the regulation of FMO3 transcription and mRNA levels of AhR target genes and FXR itself were significantly upregulated in our PCB 126 exposed mice [30, 45]. Moreover, these receptors may have the ability to cross-talk with each other as well as other nuclear receptors culminating in unique influences on gene expression [46]. However, with the observed strong induction of Cyp1a1, a known AhR target gene, in our PCB treated mice, AhR may be a predominant FMO3 control mechanism for our model. In this current study we determined that plasma levels of TMAO significantly correlated with liver relative Cyp1a1 gene expression levels. It is unknown if cytochrome P450s can oxidize TMA, but this observed correlation may just implicate the importance of AhR in FMO3 regulation. Other groups have also observed robust upregulation of FMO3 by dioxin-like pollutants, and this induction appears to be strongly linked to AhR [35, 47, 48]. More work needs to be done to determine if plasma levels of TMAO or other FMO3 substrate/products pairs may be utilized as relevant biomarkers of PCB or dioxin-like environmental pollutant exposure and associated disease risks.
TMAO levels are strongly linked to human diseases and plasma concentrations are correlated to dietary choices [28, 49]. For example, diets high in red meat, and specifically L-carnitine, produce TMAO and accelerate atherosclerosis in mouse models [29, 42]. Also, human studies have determined that vegans and vegetarians produce less TMAO than their omnivorous counterparts after dietary challenge [29]. This would suggest that altering dietary habits to more closely resemble a vegetarian lifestyle might decrease circulating TMAO and perhaps decrease risks associated with CVD (perhaps through alterations of gut microbiota). Also, studies now implicate a protective role of certain dietary components to counteract the deleterious effects of environmental pollutants. For example, diets high in anti-inflammatory bioactive polyphenols have been shown to decrease the toxicity of PCBs and related toxicants [13, 14, 50]. In this study, we have generated evidence indicating that the complex interplay between toxicant exposure and nutritional profile is important and worthy of future research.
In summary, our current study supports previous work detailing the metabolic-altering and pro-inflammatory nature of dioxin-like PCBs [51–53]. Here, we show that PCB-induced upregulation of FMO3 can lead to increased circulating plasma levels of pro-atherogenic TMAO following administration of diet-derived precursors. More studies are needed to support our hypothesis that PCB-induced increases in TMAO can be a novel mechanism linking nutrition, exposure to environmental pollutants, and CVD and other metabolic disorders.
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Institute of Environmental Health Sciences at the National Institutes of Health [P42ES007380] and the University of Kentucky Agricultural Experiment Station.
Research reported was supported by NIEHS/NIH grant P42ES007380 and the University of Kentucky Agricultural Experiment Station. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The authors would like to thank Dr. Sudha Biddinger for her advice related to the manuscript and for her continued intellectual support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perkins JT, et al. Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res Int. 2016;23(3):2160–72. doi: 10.1007/s11356-015-4479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SA, et al. Associations of organochlorine pesticides and polychlorinated biphenyls with total, cardiovascular, and cancer mortality in elders with differing fat mass. Environ Res. 2015;138:1–7. doi: 10.1016/j.envres.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Pavuk M, et al. Predictors of serum polychlorinated biphenyl concentrations in Anniston residents. Sci Total Environ. 2014;496:624–34. doi: 10.1016/j.scitotenv.2014.06.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavuk M, et al. Serum concentrations of polychlorinated biphenyls (PCBs) in participants of the Anniston Community Health Survey. Sci Total Environ. 2014:473–474. 286–97. doi: 10.1016/j.scitotenv.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aminov Z, et al. Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama. Environ Health. 2013;12:108. doi: 10.1186/1476-069X-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silverstone AE, et al. Polychlorinated biphenyl (PCB) exposure and diabetes: results from the Anniston Community Health Survey. Environ Health Perspect. 2012;120(5):727–32. doi: 10.1289/ehp.1104247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goncharov A, et al. Blood pressure and hypertension in relation to levels of serum polychlorinated biphenyls in residents of Anniston, Alabama. J Hypertens. 2010;28(10):2053–60. doi: 10.1097/HJH.0b013e32833c5f3e. [DOI] [PubMed] [Google Scholar]
- 8.Langer P, et al. Multiple adverse thyroid and metabolic health signs in the population from the area heavily polluted by organochlorine cocktail (PCB, DDE, HCB, dioxin) Thyroid Res. 2009;2(1):3. doi: 10.1186/1756-6614-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lind PM, et al. The dioxin-like pollutant PCB 126 (3,3′,4,4′,5-pentachlorobiphenyl) affects risk factors for cardiovascular disease in female rats. Toxicol Lett. 2004;150(3):293–9. doi: 10.1016/j.toxlet.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Murphy MO, et al. Exercise protects against PCB-induced inflammation and associated cardiovascular risk factors. Environ Sci Pollut Res Int. 2016;23(3):2201–11. doi: 10.1007/s11356-014-4062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hennig B, et al. Nutrition can modulate the toxicity of environmental pollutants: implications in risk assessment and human health. Environ Health Perspect. 2012;120(6):771–4. doi: 10.1289/ehp.1104712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environ Sci Pollut Res Int. 2014;21(10):6410–8. doi: 10.1007/s11356-013-1549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petriello MC, et al. Modulation of persistent organic pollutant toxicity through nutritional intervention: emerging opportunities in biomedicine and environmental remediation. Sci Total Environ. 2014;491–492:11–6. doi: 10.1016/j.scitotenv.2014.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Newsome BJ, et al. Green tea diet decreases PCB 126-induced oxidative stress in mice by up-regulating antioxidant enzymes. J Nutr Biochem. 2014;25(2):126–35. doi: 10.1016/j.jnutbio.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramadass P, et al. Dietary flavonoids modulate PCB-induced oxidative stress, CYP1A1 induction, and AhR-DNA binding activity in vascular endothelial cells. Toxicol Sci. 2003;76(1):212–9. doi: 10.1093/toxsci/kfg227. [DOI] [PubMed] [Google Scholar]
- 16.Hennig B, et al. PCB-induced oxidative stress in endothelial cells: modulation by nutrients. Int J Hyg Environ Health. 2002;205(1–2):95–102. doi: 10.1078/1438-4639-00134. [DOI] [PubMed] [Google Scholar]
- 17.Slim R, et al. The role of methyl-linoleic acid epoxide and diol metabolites in the amplified toxicity of linoleic acid and polychlorinated biphenyls to vascular endothelial cells. Toxicol Appl Pharmacol. 2001;171(3):184–93. doi: 10.1006/taap.2001.9131. [DOI] [PubMed] [Google Scholar]
- 18.Hennig B, et al. Effects of lipids and antioxidants on PCB-mediated dysfunction of vascular endothelial cells (EC) Cent Eur J Public Health. 2000;8(Suppl):18–9. [PubMed] [Google Scholar]
- 19.Korashy HM, El-Kadi AO. The role of aryl hydrocarbon receptor in the pathogenesis of cardiovascular diseases. Drug Metab Rev. 2006;38(3):411–50. doi: 10.1080/03602530600632063. [DOI] [PubMed] [Google Scholar]
- 20.Lim EJ, et al. Coplanar polychlorinated biphenyl-induced CYP1A1 is regulated through caveolae signaling in vascular endothelial cells. Chem Biol Interact. 2008;176(2–3):71–8. doi: 10.1016/j.cbi.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beischlag TV, et al. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Eukaryot Gene Expr. 2008;18(3):207–50. doi: 10.1615/critreveukargeneexpr.v18.i3.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hennig B, et al. Proinflammatory properties of coplanar PCBs: in vitro and in vivo evidence. Toxicol Appl Pharmacol. 2002;181(3):174–83. doi: 10.1006/taap.2002.9408. [DOI] [PubMed] [Google Scholar]
- 23.Xiao L, Zhang Z, Luo X. Roles of xenobiotic receptors in vascular pathophysiology. Circ J. 2014;78(7):1520–30. doi: 10.1253/circj.cj-14-0343. [DOI] [PubMed] [Google Scholar]
- 24.Schlezinger JJ, et al. Uncoupling of cytochrome P450 1A and stimulation of reactive oxygen species production by co-planar polychlorinated biphenyl congeners. Aquat Toxicol. 2006;77(4):422–32. doi: 10.1016/j.aquatox.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 25.Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–23. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- 26.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. Journal of Hypertension. 2000;18(6):655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 27.Wu F, et al. Induction of oxidative stress and the transcription of genes related to apoptosis in rare minnow (Gobiocypris rarus) larvae with Aroclor 1254 exposure. Ecotoxicol Environ Saf. 2014;110:254–60. doi: 10.1016/j.ecoenv.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koeth RA, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–85. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bennett BJ, et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013;17(1):49–60. doi: 10.1016/j.cmet.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Warrier M, et al. The TMAO-Generating Enzyme Flavin Monooxygenase 3 Is a Central Regulator of Cholesterol Balance. Cell Rep. 2015 doi: 10.1016/j.celrep.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih DM, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37. doi: 10.1194/jlr.M051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miao J, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miao J, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tijet N, et al. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol Pharmacol. 2006;69(1):140–53. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- 36.Eske K. Nutritional Sciences. University of Kentucky; 2013. Coplanar PCB-induced inflammation and dietary interventions; p. 197. http://uknowledge.uky.edu/nutrisci_etds/8. [Google Scholar]
- 37.Baker NA, et al. Coplanar polychlorinated biphenyls impair glucose homeostasis in lean C57BL/6 mice and mitigate beneficial effects of weight loss on glucose homeostasis in obese mice. Environ Health Perspect. 2013;121(1):105–10. doi: 10.1289/ehp.1205421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, et al. Persistent Organic Pollutants Modify Gut Microbiota-Host Metabolic Homeostasis in Mice Through Aryl Hydrocarbon Receptor Activation. Environ Health Perspect. 2015;123(7):679–88. doi: 10.1289/ehp.1409055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi JJ, et al. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect. 2013;121(6):725–30. doi: 10.1289/ehp.1306534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MJ, et al. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ Health Perspect. 2011;119(3):377–83. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao X, et al. Dietary trimethylamine N-oxide exacerbates impaired glucose tolerance in mice fed a high fat diet. J Biosci Bioeng. 2014;118(4):476–81. doi: 10.1016/j.jbiosc.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Ufnal M, et al. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol. 2014;30(12):1700–5. doi: 10.1016/j.cjca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 43.Ussher JR, Lopaschuk GD, Arduini A. Gut microbiota metabolism of L-carnitine and cardiovascular risk. Atherosclerosis. 2013;231(2):456–61. doi: 10.1016/j.atherosclerosis.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Gadupudi GS, et al. PCB126-Induced Disruption in Gluconeogenesis and Fatty Acid Oxidation Precedes Fatty Liver in Male Rats. Toxicol Sci. 2016;149(1):98–110. doi: 10.1093/toxsci/kfv215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang Y, et al. Farnesoid X receptor directly regulates xenobiotic detoxification genes in the long-lived Little mice. Mech Ageing Dev. 2013;134(9):407–15. doi: 10.1016/j.mad.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pascussi JM, et al. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- 47.Novick RM, Vezina CM, Elfarra AA. Isoform distinct time-, dose-, and castration-dependent alterations in flavin-containing monooxygenase expression in mouse liver after 2,3,7,8-tetrachlorodibenzo-p-dioxin treatment. Biochem Pharmacol. 2010;79(9):1345–51. doi: 10.1016/j.bcp.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Celius T, et al. Flavin-containing monooxygenase-3: induction by 3-methylcholanthrene and complex regulation by xenobiotic chemicals in hepatoma cells and mouse liver. Toxicol Appl Pharmacol. 2010;247(1):60–9. doi: 10.1016/j.taap.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fogelman AM. TMAO is both a biomarker and a renal toxin. Circ Res. 2015;116(3):396–7. doi: 10.1161/CIRCRESAHA.114.305680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Petriello MC, Newsome B, Hennig B. Influence of nutrition in PCB-induced vascular inflammation. Environ Sci Pollut Res Int. 2013 doi: 10.1007/s11356-013-1549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy MO, et al. Exercise protects against PCB-induced inflammation and associated cardiovascular risk factors. Environ Sci Pollut Res Int. 2015 doi: 10.1007/s11356-014-4062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petriello MC, et al. PCB 126 toxicity is modulated by cross-talk between caveolae and Nrf2 signaling. Toxicol Appl Pharmacol. 2014;277(2):192–9. doi: 10.1016/j.taap.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang W, et al. PCB 126 and other dioxin-like PCBs specifically suppress hepatic PEPCK expression via the aryl hydrocarbon receptor. PLoS One. 2012;7(5):e37103. doi: 10.1371/journal.pone.0037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.