Abstract
Dll4, one of the Notch ligands, is overexpressed in ovarian cancer, especially in tumors resistant to anti–VEGF therapy. Here, we examined the biological effects of dual anti-Dll4 and anti-VEGF therapy in ovarian cancer models. Using Dll4-Fc blockade and anti-Dll4 antibodies (murine REGN1035 and human REGN421), we evaluated the biological effects of Dll4 inhibition combined with aflibercept or chemotherapy in orthotopic mouse models of ovarian cancer. We also examined potential mechanisms by which dual Dll4 and VEGF targeting inhibits tumor growth using immunohistochemical staining for apoptosis and proliferation markers. Reverse phase protein arrays were used to identify potential downstream targets of Dll4 blockade. Dual targeting of VEGF and Dll4 with murine REGN1035 showed superior anti-tumor effects in ovarian cancer models compared to either monotherapy. In the A2780 model, REGN1035 (targets murine Dll4) or REGN421 (targets human Dll4) reduced tumor weights by 62% and 82%, respectively; aflibercept alone reduced tumor weights by 90%. Greater therapeutic effects were observed for Dll4 blockade (REGN1035) combined with either aflibercept or docetaxel P < 0.05 for the combination vs. aflibercept). The superior anti-tumor effects of REGN1035 and aflibercept were related to increased apoptosis in tumor cells compared to the monotherapy. We also found that GATA3 expression was significantly increased in tumor stroma from the mice treated with REGN1035 combined with docetaxel or aflibercept, suggesting an indirect effect of these combination treatments on the tumor stroma. These findings identify that dual targeting of Dll4 and VEGF is an attractive therapeutic approach.
Keywords: Dll4, ovarian cancer, angiogenesis, aflibercept, anti-Dll4 antibodies
Introduction
Ovarian cancer remains the leading cause of death from a gynecologic malignancy in the United States. Although tumor-reductive surgery and taxane- and platinum-based chemotherapy regimens are effective for most patients with primary ovarian cancer, recurrence is common and often deadly. New therapeutic agents are thus needed to improve survival rates and, eventually, to cure this deadly disease.
Vascular endothelial growth factor (VEGF) plays an important role in tumor angiogenesis (1). We have previously demonstrated that the combination of a soluble VEGF decoy receptor (VEGF Trap, also called aflibercept) plus a taxane is effective in decreasing tumor burden in a mouse model of human ovarian cancer(2). The recent success of anti-angiogenic therapies such as bevacizumab and aflibercept in solid tumors, including ovarian cancer, has confirmed the clinical viability of this approach (3). However, not all ovarian cancers are responsive to VEGF inhibitors (4). Moreover, ovarian cancers develop resistance to VEGF inhibitors over time (5, 6), leading to progressive tumor growth. Thus, additional angiogenic signaling pathways will likely need to be targeted to enhance or extend the benefit of anti-angiogenic therapy (7).
Dll4, one of the Notch ligands, plays an important role in tumor angiogenesis (8). VEGF up-regulates Dll4 expression in endothelial cells (9), and in turn, Dll4-Notch signaling modulates the response of endothelial cells to VEGF (10, 11). Blockade of Dll4-Notch signaling results in excessive sprouting of markedly abnormal, tortuous tumor vessels, which, seemingly paradoxically, reduces tumor growth because these abnormal vessels are not perfused (12, 13). Our previous work demonstrated the biological roles of Dll4 in tumor and endothelial cells in ovarian cancer, showing that targeting Dll4 can restore VEGF receptoR2 expression via epigenetic mechanisms (14). Moreover, ovarian cancer patients with increased Dll4 expression in the tumor endothelial cells had poor response to anti-VEGF therapy (14). These findings prompted us to evaluate the biological effects of Dll4 blockade combined with aflibercept in ovarian cancer mouse models.
In this study, we examined the anti-tumor activity of both human- and mouse-derived anti-Dll4 antibodies (REGN421 and REGN1035) with and without aflibercept in orthotopic mouse models of ovarian cancer. We also examined potential mechanisms by which dual Dll4 and VEGF targeting inhibits tumor growth using tumor vessel imaging, immunohistochemical staining for apoptosis and proliferation markers, and reverse phase protein arrays (RPPAs).
Materials and Methods
Reagents and cell cultures
Docetaxel was obtained from Sanofi-Aventis (Bridgewater, NJ). Soluble Dll4 extracellular domain fused to Fc (mouse Dll4-Fc), aflibercept (inhibits the activity of VEGF-A, VEGF-B and PIGF) (15,16), REGN1035 (a surrogate that cross-reacts with mouse Dll4), and REGN421 (a fully human monoclonal antibody directly against Dll4), vehicle, and human Fc control were obtained from Regeneron Pharmaceuticals (Tarrytown, NY) (12, 15,17). REGN421 binds to Dll4 and blocks the interaction of Dll4 with Notch receptors, thereby inhibiting Notch signaling. The human OVCAR3 cell line (2) was kindly provided by T. Hamilton (Fox Chase Cancer Center, Philadelphia, PA). HeyA8 and A2780 cancer cell lines were obtained from the MD Anderson Characterized Cell Line Core Facility (Houston, Texas), which supplies authenticated cell lines. The cell lines were routinely tested to confirm the absence of mycoplasma, and all experiments were performed with cell lines at 60%–80% confluence.
Experimental animals
Female athymic nude mice (NCr-nu) were purchased from the Animal Production Program of the National Cancer Institute, Frederick Cancer Research and Development Center (Frederick, MD). The animals were kept under specific pathogen–free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care. Mice used in these experiments were 5–7 weeks of age. All protocols involving mice were approved by the Committee on Animal Research at the University of California at San Francisco and by the MD Anderson Cancer Center Institutional Animal Care and Use Committee.
Aflibercept, Dll4-Fc, REGN1035, and REGN421 treatments
To test the effects of Dll4-Fc both alone and combined with aflibercept in an OVCAR3 model, we prepared OVCAR-3 cells from ascites fluid of athymic mice inoculated with OVCAR-3 cells, as described in our previous study(2). Briefly, ascites fluid was collected and placed in a 4°C refrigerator for 1 to 2 hours. The supernatant was discarded and the cells were diluted with RPMI 1640 supplemented with 2.0 g/L glucose and 0.3 g/L l-glutamine that had been pre-warmed in a 37°C incubator. Athymic mice (5–7 weeks) were inoculated i.p. with OVCAR-3 cells (2 × 106 cells per mouse in 500 μL RPMI 1640). Two weeks after inoculation, the mice were randomized into 4 groups (10 mice per group). One group was treated with intraperitoneal aflibercept (10 mg/kg body weight) twice weekly plus intravenous Dll4-Fc (25 mg/kg) three times weekly for 3 weeks. The second group was treated with aflibercept alone (10 mg/kg) three times per week. The third group was treated with 25 mg/kg Dll4-Fc alone. The fourth group received the vehicle alone.
Abdominal circumference and body weight were measured twice weekly during treatment. After 5 weeks of treatment the mice were euthanized by CO2 inhalation. The number of tumor nodules and distribution of tumors were recorded. The volumes of the ascites were measured, and tumor tissue was excised, weighed, fixed in 4% paraformaldehyde (pH 7.4) at 4°C for 24 hours, and embedded in paraffin. Paraffin sections (5 μm) were used for immunohistochemical analysis.
Additional experiments were carried out to test the effects of aflibercept, REGN1035, REGN421, and docetaxel in mice inoculated with A2780 and HeyA8 ovarian cancer cells, which we had previously found to be Dll4 positive (14). Following trypsin treatment, these cell cultures were mixed with medium containing 10% fetal bovine serum, were subjected to centrifugation at 1000 rpm for 5 minutes, and were washed in phosphate-buffered saline (PBS). Female athymic nude mice were then injected intraperitoneally with Dll4-positive A2780 cells (2 × 106 cells/0.2 mL) or Dll4-weak positive HeyA8 cells (2.5 × 105 cells/0.2 mL).
Seven days after inoculation, the mice were randomized into seven groups (10 mice per group): (1) a control, (2) docetaxel, (3) aflibercept, (4) REGN1035 or REGN421, (5) docetaxel plus aflibercept, (6) docetaxel plus REGN1035 or REGN421, or (7) aflibercept plus REGN1035 or REGN421. Docetaxel was given intraperitoneally at 35 μg/mouse three times per week, aflibercept was given intraperitoneally at 12.5 mg/kg three times per week, and REGN1035 and REGN421 were given intravenously at 5 mg/kg twice per week (http://www.google.com/patents/WO2010151770A1?cl=en). Fifteen minutes before sacrifice, the mice were infused with 100 μl of Hypoxyprobe TM-1 (pimonidazole HCl, 100mg/kg, NPI, Inc, Burlington, MA) through the tail vein. Mice were killed after 4–6 weeks of therapy, when animals in the control group became moribund. At the time of death, the mouse and tumor weights, and number of nodules were recorded.
Immunohistochemical staining
Paraffin-embedded tumor tissues were used to detect cell proliferation (Ki67), apoptosis (cleaved caspase 3), GATA3, CX3CL1, hypoxia and F4/80 positive cells. Sections were deparaffinized, and rehydrated. After antigen retrieval with citrate buffer (pH 6.0), the sections were blocked with 3% hydrogen peroxide in methanol and protein blocker at room temperature. The sections were then incubated with a monoclonal anti-Ki67 antibody (1:200 dilution; Biocare Medical, Concord, CA), anti–cleaved caspase 3 antibody (1:800; Biocare Medical), or anti–mouse GATA3 antibody (HG3-31) (1:100; Santa Cruz, CA), Rat anti-mouse F4/80 antibody (1:50, Serotec), anti-rabbit polyclonal CX3CL1 (1:100; Abcam), and Hypoxyprobe-1-Mab 1 (1:50; Natural Pharmacia International, Inc., Burlington, MA) overnight at 4°C. After being washed with PBS, all sections were incubated with 4 plus biotinylated goat anti-rabbit (for Ki67 and cleaved caspase 3) or anti-mouse antibody (for GATA3) (Biocare Medical) for 20 minutes. Then, the slides were washed with PBS and incubated with 4 plus streptavidin horseradish peroxidase (Biocare Medical) for 20 minutes.
To detect microvessels as represented by CD31, frozen ovarian tumor sections were fixed in cold acetone for 15 minutes, washed with PBS, blocked with protein blocker (4% fish gelatin), and then incubated with primary antibody against CD31 (1:800; Pharmingen BD Biosciences, San Diego, CA) overnight at 4°C. They were then washed with PBS and incubated with horseradish peroxidase–conjugated goat anti-rat IgG (1:200; Jackson ImmunoResearch Laboratories) for 1 hour. Reactive tissues were visualized using staining with 3,3′-diaminobenzidine (Research Genetics, Huntsville, AL) followed by counterstaining with Gill's hematoxylin (BioGenex Laboratories, San Ramon, CA).
Quantification of microvessel density, cleaved caspase 3, Ki67, GATA3, CX3CL1, hypoxia and F4/80 cells
For quantification of microvessel density, cleaved caspase 3, Ki67, and GATA3, five ovarian tumor samples from each treatment group were examined. To quantify microvessel density for each sample, CD31-positive blood vessels were counted within five randomly selected 0.159-mm2 fields at ×200 magnification. To quantify the expression of cleaved caspase 3, Ki67, GATA3, CX3CL1 and F4/80 cells, we determined the percentage of positive cells in five randomly selected 0.159-mm2 fields at ×200 magnification. The percentage of hypoxic area was determined by subtracting areas of necrosis from total pimonidazole-positive areas and then normalizing by whole section areas.
Reverse transcriptase polymerase chain reaction (RT-PCR)
The relative expression of E-cadherin in ovarian tumors obtained from in vivo studies was determined by RT-PCR. Each RT-PCR used 5 µg total RNA isolated from homogenized tissues treated with a single agent or the combination from each treatment group. GAPDH was used as a control (15). The primer sequences of human E-cadherin primers (991 bp) were as follows: H-E-cadherin-F (forward): 5′-GTGACTGATGCTGATGCCCCCAATACC-3′; H-E-cadherin-R (reverse): 5′-GACGCAGAATCAGAATTAGGAAAGCAAG-3′. The human CX3CL1 primers were obtained from Sigma (St. Louis, MO).
Reverse phase protein array analysis
Tumor tissues collected from in vivo studies were homogenized using a digital homogenizer in the following lysis buffer: 1% Triton X-100, 50 nM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.4), 150 mM MgCl2, 1 mM ethylene glycol tetraacetic acid , 100 mM NaF, 10 mM sodium pyrophosphate, 1 mM Na3VO4, 10% glycerol, and freshly added protease and phosphatase inhibitors. Cellular proteins were denatured using 1% sodium dodecyl sulfate, and five two-fold serial dilutions were performed in lysis buffer containing 1% sodium dodecyl sulfate (dilution buffer). These diluted lysates were arrayed on nitrocellulose-coated FAST slides (Whatman, Vernon Hills, IL) using an Aushon 2470 arrayer (Aushon Biosystems, Billerica, MA). Slides were probed with 176 validated human primary antibodies. The Supercurve Fitting logistical model, developed by the Department of Bioinformatics and Computational Biology at MD Anderson (http://bioinformatics.mdanderson.org/OOMPA), was used to generate a fitted curve for each dilution. For both observed and fitted data, the fitted curve was then plotted with the signal intensities on the y-axis and the log2 values of protein levels on the x-axis. The positive fold changes were calculated by dividing each linear value greater than 1.0 by the average control linear value for each antibody tested. The negative fold changes (for linear values less than 1.0) were calculated using the formula −1/linear fold change were plotted as a bar graph.
Quantification of plasma markers by ELISA assay
Plasma mouse IFN-γ (Catalog number: KMC4021; Life Technologies) level was measured by ELISA assay based on the manufacturer’s instructions. All plasma samples were run in duplicate. A monoclonal antibody specific for IFN-γ was coated onto the wells of the microtiter strips provided. Samples, including standards of known IFN-γ content, control plasma and treated plasma were pipetted into these wells.
Statistical analysis
For the in vivo therapy experiments, 10 mice were used in each group, which provided the power to detect a 50% reduction in tumor size (β error = 0.2). For non-parametric distributions, the Mann-Whitney U test was used. A value of P less than 0.05 with two-tailed testing was deemed statistically significant.
Results
Effects of Dll4-Fc and aflibercept on tumor burden and ascites formation
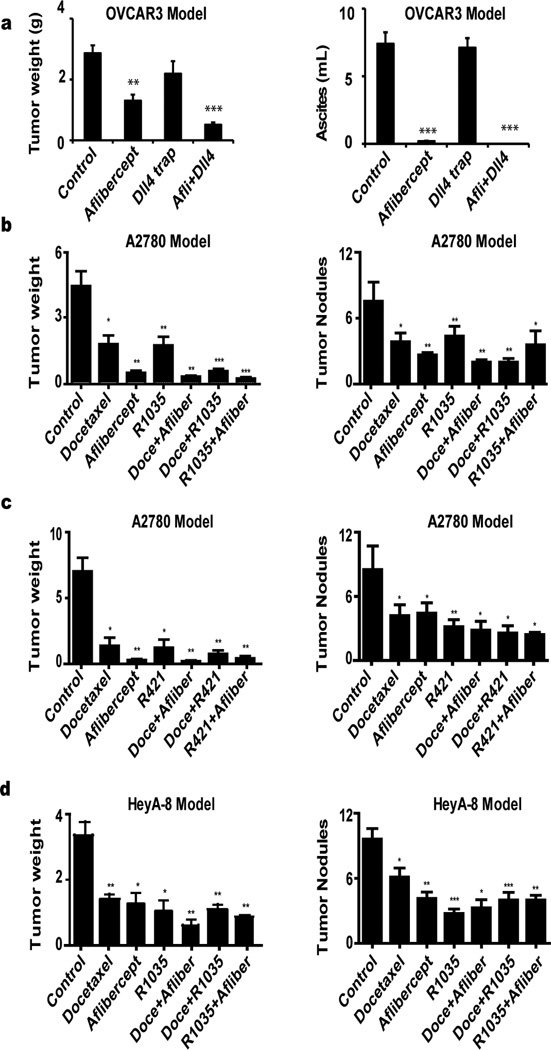
In mice inoculated with OVCAR3 ovarian cancer cells, the mean tumor burden in the group treated with aflibercept plus Dll4-Fc (0.51 ± 0.08 g) was 82% less than that in the control group (2.86 ± 0.26 g; Fig. 1A, left, and Supplementary Fig. 1A). Mean tumor burden in the aflibercept-alone (1.30 ± 0.20 g) and Dll4-Fc alone (2.19 ± 0.41 g) groups were 55% less and 24% less, respectively, than that in the control group (p<0.01). Ascites were almost completely prevented in the aflibercept plus Dll4-Fc–treated and aflibercept-treated groups, compared with the controls (Fig. 1A, right, and Supplementary Fig. 1B).
Figure 1.
In vivo study of Dll4 blockade combined with aflibercept in OVCAR3, A2780, and HeyA8 models. A, mean tumor burden and ascites in OVCAR3-inoculated nude mice treated with aflibercept, Dll4-Fc, or both for 3 weeks. B-C, in vivo Dll4 inhibition by mouse or human anti-Dll4 antibody in the A2780 (B, C) and HeyA8 (D) models. *P < 0.05, compared with control group; **P < 0.01 ; ***P < 0.001.
At the end of the study period (after 4 weeks of treatment), 100% of the control and Dll4-Fc–alone groups and 60% of the aflibercept-alone group had tumors in the hilum of the liver, whereas only 20% of the Dll4-Fc plus aflibercept group had any peri-hepatic tumors. In addition, 90% of the control group, 70% of the Dll4-Fc–alone group, and 50% of the aflibercept-alone group had tumors on the diaphragm. In contrast, no mice in the Dll4-Fc plus aflibercept group displayed diaphragmatic tumors (p<0.05), suggesting that the combined treatment suppressed direct metastasis. Observable behaviors in the mice (e.g., degrees of activity and eating) did not significantly differ between the treatment groups.
In vivo Dll4 inhibition with human or mouse anti-Dll4 antibody
To investigate the translational relevance of Dll4-targeted therapy in ovarian cancer, we next studied the effects of anti-Dll4 antibodies in combination with anti-VEGF treatment in vivo on tumor growth and angiogenesis. To address the biological significance of Dll4 expression in tumor and endothelial cells, we used human (tumor) and mouse (endothelial) anti-Dll4 antibodies and A2780 and HeyA8 cells, which we had previously found to be Dll4 positive. In the mice inoculated with A2780 ovarian cancer cells, treatment with mouse (REGN1035) or human (REGN421) anti-Dll4 antibodies alone resulted in significant growth inhibition compared with the control (62% and 82%, P < 0.05 for both; Fig. 1B, left, and 1C, left, respectively). Aflibercept alone led to reduce tumor weights by 90% less than those of the controls P < 0.05). However, the combination of REGN1035 plus aflibercept resulted in the greatest inhibition of tumor growth compared with the controls (96%, P < 0.0001 for the combination vs. control). Notably, superior therapeutic effects were observed for Dll4 blockade (REGN1035) combined with either aflibercept or docetaxel P < 0.05 for the combination vs. aflibercept; P < 0.01 for the combination vs. REGN1035 or docetaxel) compared to monotherapy.
However, tumor reduction did not differ significantly between the REGN421 combined treated groups and the single-drug treated groups. We also examined the effects of treatment on the number of tumor nodules and body weight in the A2780 model. The number of nodules was significantly lower in all treated groups than in the controls (P < 0.05; Fig. 1B-1C, right), but nodules did not differ between the combination and single-drug treatment groups (Fig.1B-1C, right). Mean body weights did not differ significantly between treatment groups (Supplementary Fig.2A-2B).
In the mice inoculated with HeyA8 ovarian cancer cells, the effects of Dll4 inhibition on ovarian tumor weight were, in general, similar to those in the A2780 model (Fig. 1D, left). In addition, tumor weights in mice treated with single agents were significantly lower than those in the controls: 61% less with aflibercept (P < 0.05), 68% less with REGN1035 (P < 0.05), and 57% less with docetaxel (P < 0.01). However, the extent of reduction in tumor weight and number of nodules did not differ significantly between the combined- and single-drug treatment groups (Fig. 1D, right). Mean mouse body weights did not differ significantly between any treatment groups in the HeyA8 model (Supplementary Fig. 2C).
Effects of Dll4 and VEGF blockade on microvessel density, apoptosis, and proliferation
To identify potential mechanisms underlying the efficacy of Dll4 and VEGF blockade, we examined the effects of Dll4 and VEGF blockade on angiogenesis using CD31 staining in the A2780 ovarian cancer model. As shown in Figure 2, microvessels were less dense in the groups treated with docetaxel alone or aflibercept alone than in the controls (P < 0.01). Furthermore, aflibercept combined with REGN421 or with REGN1035 led to lower microvessel density than the single-drug treatments did (P < 0.05; Fig. 2A, and 2B, respectively). In contrast, vessel density was not significantly changed in the groups treated with REGN421 or REGN1035 with or without docetaxel (Fig. 2A and 2B, respectively), which is consistent with prior reports (12, 14) that Dll4 blockade increased non-productive tumor vascularity.
Figure 2.
Dll4 blockade inhibits angiogenesis and proliferation and induces apoptosis in the A2780 model. A, Proliferation, cleaved caspase 3, and microvessel density (CD31) were detected by IHC staining in A2780-inoculated mice treated with human anti-Dll4 antibody (REGN421), docetaxel, or aflibercept, alone or in combination. B, Proliferation, cleaved caspase 3, and microvessel density (CD31) were detected by IHC staining in A2780-inoculated mice treated with mouse anti-Dll4 antibody (REGN1035) alone or in combination. All pictures were taken at original magnification (×200). Error bars represent the standard error of the mean. *P < 0.05, compared with controls; **P < 0.01 ; ***P < 0.001. MVD: microvessel density.
To determine the effect of Dll4 and VEGF blockade on the induction of apoptosis, we performed immunohistochemical staining for cleaved caspase 3 in the A2780 model. As shown in Figures 2A and 2B, levels of apoptosis were significantly higher in groups treated with REGN421, REGN1035, aflibercept, or docetaxel alone than in controls (P < 0.05 for each). Furthermore, aflibercept combined with REGN1035 led to significantly greater levels of apoptosis in tumor cells than aflibercept alone (P < 0.01 for aflibercept plus REGN1035 vs. aflibercept alone).
In addition, when we examined the effect of Dll4 and VEGF blockade on proliferation in the A2780 model, proliferation was significantly lower with REGN421 than in controls, suggesting a direct effect of REGN421 on ovarian cancer cells. Furthermore, the combinations of aflibercept plus REGN421 and docetaxel plus REGN421 led to less proliferation than the single-drug treatments did (Fig.2A) (P < 0.05). However, proliferation did not differ significantly between the groups treated with REGN1035 plus aflibercept or docetaxel and the single-drug treatment groups (Fig.2B).
RPPA analysis of novel Dll4 downstream targets
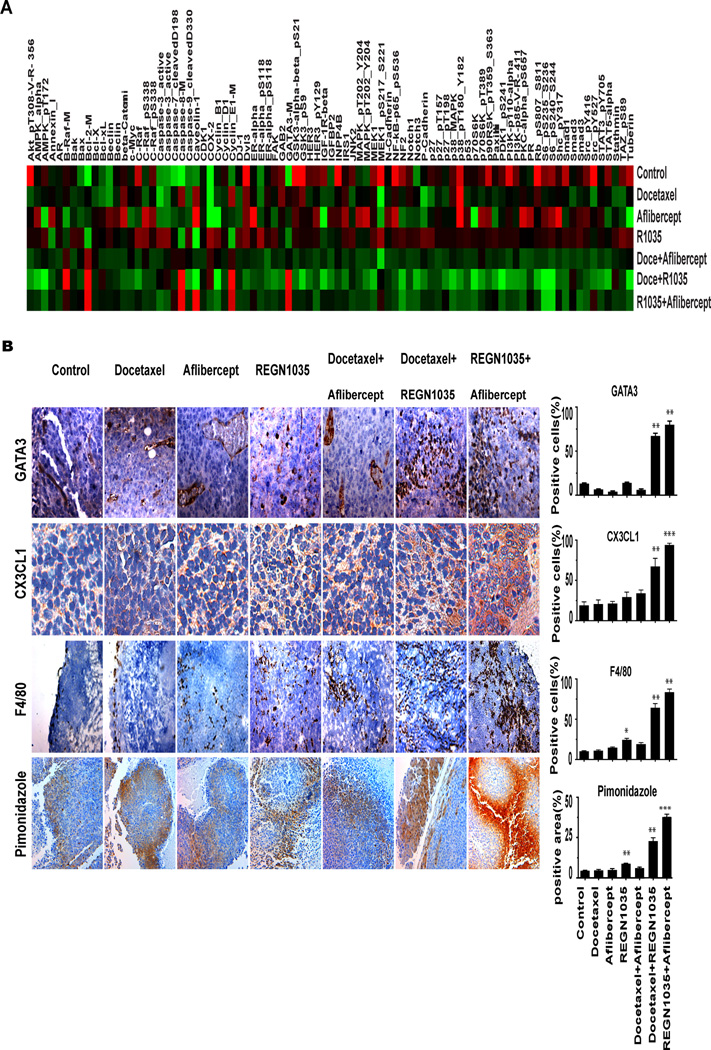
To identify potential downstream targets of Dll4 blockade, we used RPPA to quantify proteins involved in cell cycle, apoptosis, angiogenesis, and adhesion in response to single drug or combination treatments in the A2780-model (Fig. 3A). Analysis of RPPA data revealed that 37 proteins were significantly differentially modulated by Dll4 blockade and the combination. Surprisingly, the expression level of total GATA3, a master transcription factor, was significantly increased in mice treated with REGN1035 combined with docetaxel or aflibercept (Fig. 3A). Immunohistochemical analysis further validated that GATA3 level was significantly higher in mice treated with REGN1035 combined with docetaxel or aflibercept than in the controls (Fig. 3B). To further investigate the potential role of GATA3 in enhancing the efficacy of dual targeting of Dll4 and VEGF signaling in ovarian cancer, we tested GATA 3 interacting genes (CX3CL1 and E-cadherin) in tumors and found that CX3CL1 expression was significantly increased in tumors (Fig.3B) from mice treated with REGN1035 combined with docetaxel or aflibercept, but CX3CL1 was slightly increased in the tumor stroma of mice treated with REGN1035 combined with docetaxel or aflibercept, which could result from an interaction between murine GATA3 and CX3CL1 (Supplementary Figure 3). However, the exact mechanism by which CX3CL1 expression was increased in cancer cells (Fig.3) is not clear. It is possible that murine GATA3 could transactivate human genes in a paracrine manner (Supplementary Figure 4). But the exact mechanisms of direct interaction between GATA3 and CX3CL1 need to be further investigated. F4/80 cells and plasma IFN-γ level were examined in the A2780 model. F4/80 cells and plasma IFN-γ were significantly higher in mice treated with combination of REGN1035 and docetaxel, or aflibercept (Fig.3B and Supplementary Figure 5).
Figure 3.
RPPA analysis of Dll4-Notch targeted genes in A2780-inoculated mice treated with REGN1035, aflibercept, or docetaxel, alone or in combinations. A, heat map of selective target modulation (e.g., GATA 3) in tumor tissues from mice treated with single agent or combination therapy. B, Immunohistochemical staining of GATA3, CX3CL1, hypoxia and F4/80 cells in the A2780 model. All pictures were taken at original magnification (×200). Error bars represent the standard error of the mean. *P < 0.05 compared with controls; **P < 0.01 and ***p<0.001 compared with controls.
Given the role of GATA3 in metastasis inhibition via inhibition of epithelial-mesenchymal transition (EMT) (18–20), we next performed RT-PCR analysis for E-cadherin and found that E-cadherin levels were significantly higher in tumors from the mice treated with REGN1035 combined with docetaxel or aflibercept compared to controls (Supplementary Figure 6) (20). E-cadherin levels were also significantly higher in tumor treated with REGN1035 alone and in those treated with docetaxel plus aflibercept than in the controls.
Discussion
The key findings from this study are that dual targeting of VEGF and Dll4 with murine REGN1035 results in superior anti-tumor effects in ovarian cancer compared to either monotherapy. These effects were related to increased apoptosis, and deregulated angiogenesis, accompanied by induction of hypoxia. The mechanisms by which dual targeting of VEGF and Dll4 signaling significantly reduces tumor growth are not fully understood. We demonstrated that the combination of aflibercept and anti-Dll4 antibody (REGN1035) induced extensive apoptosis in ovarian cancer; this apoptosis may have been due to a profound inhibition of functional tumor vessels. Moreover, down-regulation of Dll4-Notch results in aberrant network formation in tumor angiogenesis (13), and Dll4-Fc reduces tumor growth in part by increasing vascular tortuosity while also decreasing functional flow (8). Other mechanisms have also been reported by our group (14) and others in that the interaction of VEGF inhibition and Dll4 inhibition increases tumor hypoxia and necrosis (11, 21, 22). VEGF not only is a major hypoxia-responsive gene but also up-regulates Dll4 expression (14, 23). Blocking VEGF inhibits neo-vessel sprouting and reduces Dll4 expression in tumors (23). Conversely, Notch signaling can down-regulate VEGF receptoR2 expression via epigenetic mechanisms (14, 23).
In addition, another possible explanation for how dual targeting of VEGF and Dll4 signaling with murine REGN1035 significantly reduces tumor growth involves GATA3 and its interacting targets (e.g., E-cadherin and CX3CL1). These potent effects of combined aflibercept and Dll4 inhibition may be due to increased GATA3 expression under hypoxic conditions, which could result from an indirect effect of these combination treatments on the tumor stroma.
Increased GATA3 expression in triple-negative breast cancer cells has been related to reduced tumorgenicity and metastases by reversing EMT (20). GATA3 can bind GATA-like motif of E-cadherin promoter and could transactivate gene expression, since human and murine GATA3 shows a high degree of amino acid sequence identity and similar patterns of tissue specificity of expression in chicken, murine and human (18,24). Moreover, GATA-3 has also been reported to promote NK cell maturation and IFN-production(25). Tumoral CX3CL expression has been shown to enhance recruitment of NK cells and DCs (26). But, the exact mechanisms by which GATA3 expression in the tumor microenvironment occurs and regulates the human gene expression will require further investigation.
In summary, superior therapeutic effects were observed for Dll4 blockade combined with either aflibercept or docetaxel in ovarian cancer models. Data from the phase I study of REGN421 (R)/SAR153192, a fully-human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors reported clinical response and prolonged SD in patients with ovarian or other solid tumors (Clinical trial information: NCT00871559) (27). Moreover, a phase 1/Ib clinical trial of demcizumab (OMP-21M18, anti-Dll4 antibody, NCT 01952249) in combination with weekly paclitaxel in patients with ovarian cancer is ongoing at our institution. Based on the present work, additional opportunities for clinical development could include combination of anti-Dll4 antibody with anti-VEGF drugs and cytotoxic agents (e.g., taxanes).
Supplementary Material
Acknowledgments
Financial Information
Portions of this work were supported by the National Institutes of Health (P50 CA083639, P50 CA098258, CA109298, UH2 TR000943, and CA177909; to A.K. Sood), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the Department of Defense (OC073399, OC093146; to A.K. Sood), the Judi A. Rees Ovarian Cancer Research Fund, the Marcus Foundation, the Chapman Foundation, the Betty Anne Asche Murray Distinguished Professorship; to A.K. Sood and the institutional Core Grant CA16672 to MD Anderson Cancer Center from the National Institutes of Health. HJD and RAP were supported by a T32 Training Grant (T32CA101642; to H.J.Dalton and R.A. Previs) from the National Cancer Institute, the U.S. Department of Health and Human Services, and the National Institutes of Health.
We thank Sarah Bronson and Dawn Chalaire in the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editing our manuscript. We also thank Regeneron for providing Dll4 antibodies and aflibercept.
Footnotes
The authors report no conflicts of interest.
References
- 1.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11:6966–6971. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 3.Coleman RL, Duska LR, Ramirez PT, Heymach JV, Kamat AA, Modesitt SC, et al. Phase 1–2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol. 2011;12:1109–1117. doi: 10.1016/S1470-2045(11)70244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 5.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel RS, Yu J, Tran J, Man S, Viloria-Petit A, Klement G, et al. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev. 2001;20:79–86. doi: 10.1023/a:1013172910858. [DOI] [PubMed] [Google Scholar]
- 7.Thanapprapasr D, Hu W, Sood AK, Coleman RL. Moving Beyond VEGF for Anti-angiogenesis Strategies in Gynecologic Cancer. Curr Pharm Des. 2012 doi: 10.2174/138161212800626201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston G, Noguera-Troise I, Yancopoulos GD. The Delta paradox: DLL4 blockade leads to more tumour vessels but less tumour growth. Nat Rev Cancer. 2007;7:327–331. doi: 10.1038/nrc2130. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi H, Kume T. Foxc transcription factors directly regulate Dll4 and Hey2 expression by interacting with the VEGF-Notch signaling pathways in endothelial cells. PLoS One. 2008;3:e2401. doi: 10.1371/journal.pone.0002401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans. 2009;37:1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- 11.Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, et al. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 13.Li JL, Harris AL. Crosstalk of VEGF and Notch pathways in tumour angiogenesis: therapeutic implications. Front Biosci. 2009;14:3094–3110. doi: 10.2741/3438. [DOI] [PubMed] [Google Scholar]
- 14.Hu W, Lu C, Dong HH, Huang J, Shen DY, Stone RL, et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer. Cancer Res. 2011;71:6030–6039. doi: 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuhnert F, Chen G, Coetzee S, Thambi N, Hickey C, Shan J, Kovalenko P, Noguera-Troise I, Smith E, Fairhurst J, Andreev J, Kirshner JR, Papadopoulos N, Thurston G. Dll4 Blockade in Stromal Cells Mediates Antitumor Effects in Preclinical Models of Ovarian Cancer. Cancer Res. 2015 Oct 1;75(19):4086–4096. doi: 10.1158/0008-5472.CAN-14-3773. [DOI] [PubMed] [Google Scholar]
- 16.Byrne AT, Ross L, Holash J, Nakanishi M, Hu L, Hofmann JI, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- 17.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 18.Yan W, Cao QJ, Arenas RB, Bentley B, Shao R. GATA3 inhibits breast cancer metastasis through the reversal of epithelial-mesenchymal transition. The Journal of biological chemistry. 2010;285:14042–14051. doi: 10.1074/jbc.M110.105262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu IM, Lai WC, Aprelikova O, El Touny LH, Kouros-Mehr H, Green JE. Expression of GATA3 in MDA-MB-231 triple-negative breast cancer cells induces a growth inhibitory response to TGFss. PLoS One. 2013;8:e61125. doi: 10.1371/journal.pone.0061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou J, Lin JH, Brenot A, Kim JW, Provot S, Werb Z. GATA3 suppresses metastasis and modulates the tumour microenvironment by regulating microRNA-29b expression. Nat Cell Biol. 2013;15:201–213. doi: 10.1038/ncb2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jubb AM, Turley H, Moeller HC, Steers G, Han C, Li JL, et al. Expression of delta-like ligand 4 (Dll4) and markers of hypoxia in colon cancer. Br J Cancer. 2009;101:1749–1757. doi: 10.1038/sj.bjc.6605368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel NS, Li JL, Generali D, Poulsom R, Cranston DW, Harris AL. Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Res. 2005;65:8690–8697. doi: 10.1158/0008-5472.CAN-05-1208. [DOI] [PubMed] [Google Scholar]
- 23.Thurston G, Kitajewski J. VEGF and Delta-Notch: interacting signalling pathways in tumour angiogenesis. Br J Cancer. 2008;99:1204–1209. doi: 10.1038/sj.bjc.6604484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linda JK, Yamamoto M, Leonard MW, Geogre KM, TING P, Engel JD. Murine and Human T-Lymphocyte GATA-3 Factors Mediate Transcription through a cis-Regulatory Element within the Human T-Cell Receptor 8 Gene Enhancer. Mol Cell Biol. 1991;11:2778–2784. doi: 10.1128/mcb.11.5.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samson SI, Richard O, Tavian M, Ranson T, Vosshenrich CA, Colucci F, et al. GATA-3 promotes maturation, IFN-gamma production, and liver-specific homing of NK cells. Immunity. 2003;19:701–711. doi: 10.1016/s1074-7613(03)00294-2. [DOI] [PubMed] [Google Scholar]
- 26.Park MH, Lee JS, Yoon JH. High expression of CX3CL1 by tumor cells correlates with a good prognosis and increased tumor-infiltrating CD8+ T cells, natural killer cells, and dendritic cells in breast carcinoma. J Surg Oncol. 2012;106:386–392. doi: 10.1002/jso.23095. [DOI] [PubMed] [Google Scholar]
- 27.Jimeno AP, Strother RM, Diamond JR, Plato L, Younger A, Messersmith WA, et al. Phase I study of REGN421 (R)/SAR153192, a fully-human delta-like ligand 4 (Dll4) monoclonal antibody (mAb), in patients with advanced solid tumors. J Clin Oncol. 2013;31 doi: 10.1158/1078-0432.CCR-14-2797. (suppl; abstr 2502) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.