Abstract
Attention plays a critical role in action selection. However, the role of attention in eye movements is complicated as these movements can be either voluntary or involuntary, with, in some circumstances (antisaccades), these two actions competing with each other for execution. But attending to the location of an impending eye movement is only one facet of attention that may play a role in eye movement selection. In two experiments, we investigated the effect of arousal on voluntary eye movements (antisaccades) and involuntary eye movements (prosaccadic errors) in an antisaccade task. Arousal, as caused by brief loud sounds and indexed by changes in pupil diameter, had a facilitation effect on involuntary eye movements. Involuntary eye movements were both significantly more likely to be executed and significantly faster under arousal conditions (Experiments 1 & 2), and the influence of arousal had a specific time course (Experiment 2). Arousal, one form of attention, can produce significant costs for human movement selection as potent but unplanned actions are benefited more than planned ones.
The antisaccade task (Hallett 1978; Evdokimidis et al. 2002) is a particularly useful visual task that reveals subjects’ ability to override pre-potent involuntary responses and initiate voluntarily controlled actions. In this task, subjects are presented with a suddenly appearing visual stimulus in the periphery (left or right) and have to suppress an eye movement towards it, in order to look to the opposite empty hemi-field (an antisaccade). Subjects fail frequently to suppress this involuntary response; therefore, a good number of both voluntary movements (antisaccades) and involuntary movements (prosaccadic errors) are made. Within this empirical paradigm, we can test the competitive nature of involuntary versus voluntary control over eye movements and the factors that influence either (or both) type of eye actions.
One critical component that influences the competitive process between involuntary and voluntary eye movements is attention (Posner and DiGirolamo 1998; Duncan 2006). Attention and eye-movements are linked (Rizzolatti et al. 1987) and share many neural mechanisms (Corbetta et al. 1998). Both have an involuntary rapid component where a visual stimulus will likely attract the eye or attention; and in both cases, this component is difficult to suppress. Both have a voluntary, slower component where the eye or attention can be effortfully shifted, even in the absence of a visual stimulus. Several lines of research demonstrate that different components of attention influence eye movements (Klein 1980; Kristjánsson et al. 2001; Kissler and Keil 2008). Orienting attention away from the location where a saccade is to be made impedes eye movements, while orienting attention to the location of an impending saccade improves the accuracy and speed of the subsequent saccade (e.g., Klein 1980). Moreover, under conditions where attention is capacity limited by having an additional task prior to the start of an antisaccade, subjects are faster paradoxically at making the antisaccade (e.g., Kristjánsson et al. 2001). These authors suggested that the involuntary component in the antisaccade task (programming a prosaccadic error) requires attention; therefore, distracting capacity from this process facilitates the voluntary antisaccade. Likewise, monaural sounds (which are spatially congruent) before the start of an antisaccade produce a facilitation of that voluntary action (Kirchner and Colonius 2005). Kirchner and Colonius, like Kristjánsson et al., concluded that the facilitation resulted from easier suppression of the prosaccadic error due to the distraction (capacity limitation) from the sound interfering with the involuntary programming.
However, orienting attention and capacity limits are but two components of attention. As Posner & Boise (1971) originally suggested, attention can be parsed into at least 3 different components: 1) orienting to positions or objects in space; 2) capacity limits; and 3) arousal. Arousal plays an important role in our cognitive capabilities (Robbins and Everitt 1995) from the extreme of lesions to the reticular activating system leaving people in coma (Foltz and Schmidt 1956) to the daily intracortical modulations of cognitive systems by arousal (Munk et al. 1996). While capacity limits of attention can influence beneficially the voluntary component of eye movements (antisaccades), and orienting can impede or facilitate the voluntary component of eye movements, it remains unclear whether arousal has a differential effect on involuntary and/or voluntary eye movements.
There is evidence that arousal plays at least some role in eye movements. Subjects’ eye movements in general are biased towards arousing unpleasant (e.g., LaBar et al. 2000) and pleasant pictures (Calvo and Lang 2005). Moreover, arousing stimuli, such as rewards and punishments (Blaukopf and DiGirolamo 2005; Blaukopf and DiGirolamo 2006; Blaukopf and DiGirolamo 2007; Milstein and Dorris 2007) and emotional pictures (Kissler and Keil 2008), influence not just where people look, but also the speed of saccadic reaction times (sRTs). And, antisaccades in an antisaccade task and prosaccades in a prosaccade task are speeded when a central warning sound is played prior to the eye movement (Kristjánsson et al. 2004). While prosaccades during a prosaccade task, and prosaccadic errors during an antisaccade task are similar in that both eye movements are executed toward the go signal, they are also fundamental different. Prosaccades during a prosaccade task are voluntary and the goal of the participant. Eye movements toward the suddenly appearing light during an antisaccade task are not voluntary as they are against the goals of the subject. Hence, prosaccades during a prosaccadic task contain a significant voluntary component. Whether arousal plays a critical role in purely involuntary actions (prosaccadic errors during an antisaccade task) has yet to be determined.
At least some very basic involuntary processes (e.g., the eye blink reflex and stretch reflex) are influenced by arousal (Bohlin and Graham 1977; Low et al. 1996) or arousing stimuli (i.e., reward, Chen and Wolpaw 1995). Indeed, Low and colleagues (1996) demonstrated that an irrelevant sound sped both a manual choice reaction time to a light, and the eye blink response to the same light. Speeding of an involuntary response via an irrelevant (but arousing stimulus) has a long history; for example, more than 100 years ago, Bowditch (1890) demonstrated that an air puff to the neck facilitates an involuntary knee-jerk reflex as much as 200 ms prior to the stimulus that elicits the reflex.
The current studies were designed to investigate the influence of one component of attention (arousal) on both voluntary and involuntary movements. While the benefits of arousal on some voluntary processes have been demonstrated (e.g., Kristjánsson et al., 2004) and the benefits of arousal on some involuntary processes have also been seen (e.g., Bowditch, 1890), it is unknown what effect arousal will have on these processes when they are paired against each other in the same action selection. Specifically, these studies investigated the influence of arousal on involuntary movements to determine if arousal, a critical component of attention, has a stronger effect on involuntary action as indexed by pro-saccade errors during an antisaccade task. If arousal has a greater impact on involuntary processes than voluntary processes, then, during the antisaccade task in which there is high competition between involuntary actions and voluntary actions, more errors will be made in this task. In today’s highly arousing and stimulating environment, knowing if arousal increases the likelihood of automatic actions even when they are inappropriate for the goals of the subject is critical.
Hence, the aim of Experiment 1 was to investigate the influence of arousal on both voluntary and involuntary eye movements (antisaccades and prosaccadic errors, respectively).
Experiment 1
Methods
Subjects
Thirty-two participants (14 male, 18 female; all aged between 18–35 years) gave informed consent and took part in the experiment. All reported normal or corrected-to-normal vision. Participants were paid for their time. In this and the next experiment, participants’ data were excluded from an analysis if they did not contribute 3 or more trials in a condition, in which all saccadic reaction times were consistent, to provide a stable saccadic reaction time (Blaukopf and DiGirolamo 2005; Blaukopf and DiGirolamo 2006). Of the 32 subjects, two were discarded because they made insufficient voluntary movements to reliably average and seven had insufficient involuntary movements to fill every condition, leaving 23 subjects. All participants gave informed consent and were treated in accordance with the ethical standards of the Declaration of Helsinki and the American Psychological Association.
Materials and Procedure
Stimuli were presented on a 21-inch color monitor placed at a viewing distance of 70 cm from the subject. The resolution of the screen was 1024×768 pixels. Eye movements and pupil size were recorded with an EyeLink 1000™ eye tracker with 1000Hz sampling and ~0.1° of resolution. Prior to the start of the experiment and after each rest, eye position was calibrated, as well as recalibrated after every 8 trials, to ensure accurate tracking of the eye.
Participants completed one block of 96 trials1. Sound stimuli were presented randomly such that 48 trials contained a sound and 48 trials were silent. The twelve sound stimuli were 175ms long samples of grating noises (e.g., dentist drill), presented binaurally at ~72 dB via headphones. We used sounds that were unpleasant to increase the level of arousal with such a short sound.
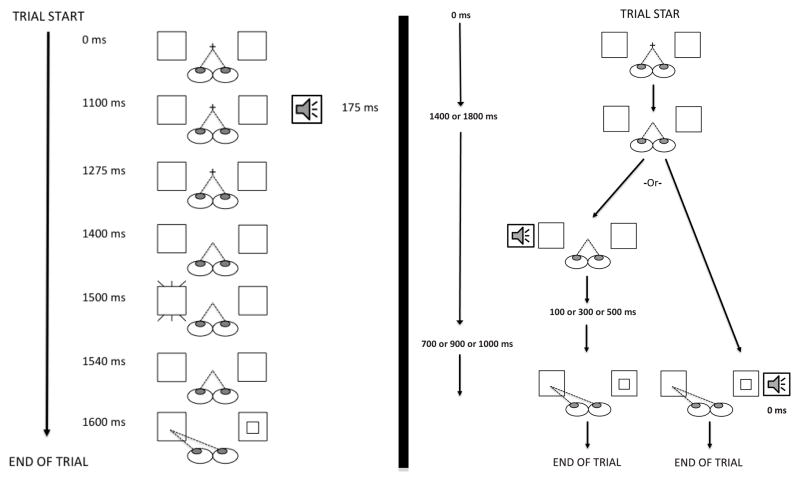
Each trial began with a black screen showing a central white fixation cross (0.4° × 0.4°) flanked by two red outline boxes (2.5° × 2.5°), 7.2° to the left and right of the center of the cross. The fixation was presented for between 1400–1800ms. On each trial, fixation was ensured before the trial proceeded. During half the trials, one of the twelve sounds started 300ms before removal of fixation on half of the trials. In remaining 48, no sound was played and these were the silent trials. 100ms after the fixation cross’s removal, one of the two red boxes flashed white for 40ms. This flash, the cue, served two purposes. First, the cue served as an effective visual warning signal to give participants accurate information, on both sound and silent trials, about when the go signal would occur. Second, the cue increases the number of prosaccadic errors that participants make, yielding sufficient data for meaningful analysis of prosaccadic error reaction times (Fischer and Weber 1996; Fischer et al. 1999; Blaukopf and DiGirolamo 2005; Blaukopf and DiGirolamo 2006; Klein et al. 2007). Sixty ms after the offset of the cue, the go signal, a small white square (0.4°×0.4°), appeared in the center of the red box opposite the one in which the cue appeared. Participants had to make their response, an antisaccade to the opposite box from where the go signal appeared, within 650ms after the onset of the go signal, after which the display cleared (see Figure 1). Participants were instructed to look at the central fixation and to move their eyes to the center of the red box opposite the go signal after the go signal appeared.
Figure 1.
A graphic representation of the time line of a typical sound trial in Experiment 1 (on left) or Experiment 2 (on right).
An eye movement was classified as a saccade when its velocity reached 30 degs/sec, or its acceleration had reached 8000 degs/sec2. Trials in which the eye movements were executed under 80 ms after the onset of the go signal were considered anticipations and discarded (Wenban-Smith and Findlay 1991; Fischer 1996), as were trials where an eye blink occurred. For every trial, the first saccade following the go signal was labeled either a prosaccadic error (involuntary action) if it landed in the box that contained the go signal, or an antisaccade (voluntary action) if it landed in the opposite box from the go signal.
In addition to the location and speed of the eye movement, we also measured pupil dilation as an index of arousal on each trial (Bradley et al. 2008). Previous research has demonstrated that pupil diameter correlates exceptionally well with other physiological measures (increased skin conductance response or increased heart rate) of arousal, and is a good indicator of arousal (Bradley et al. 2008; van Steenbergen et al. 2011). While pupil diameter is a good index of arousal, it also peaks slowly, often 1 to 1.5 secs after the sound (Bradley et al. 2008). For the current study, it is critical that a change in pupil diameter, indexing arousal, was found when the eye movement had to be executed. Hence, we measured the pupil diameter using a 10 ms time window around the onset of the go signal, when the eye movement must be chosen and executed. The error rates (%), sRTs (the time from the appearance of the go signal to the start of the saccade) and pupil diameter were analyzed using analysis of variance (ANOVA) or t tests.
Results
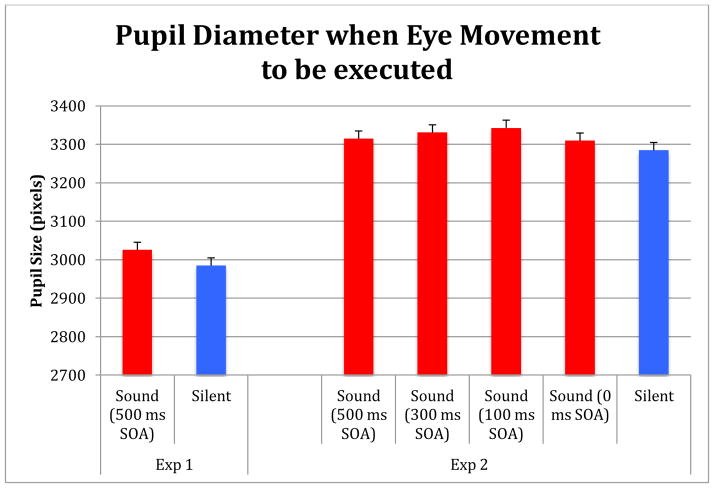
First, we needed to ensure that our sound stimulus actually produced a physiological reaction consistent with arousal and this change in arousal was present when the saccade was to be made. We compared the size of the pupil diameter when the go signal occurred and contrasted the size of the pupil response during sound and silence. As Figure 2 shows, pupil diameter at the time of the go signal was significantly larger on sound trials than silent trials (t(31)= 5.19, p< .0001). Hence, these data demonstrates that the sounds produced a pupillary response consistent with a change in arousal (Bradley et al. 2008). In short, the sound produced a pupillary response consistent with a change in arousal, and most importantly, that change was present when the saccade was to be initiated.
Figure 2.
Pupil diameter when the go signal occurred, indexing a physiological change consistent with arousal during sound trials which was present when the action was to be made.
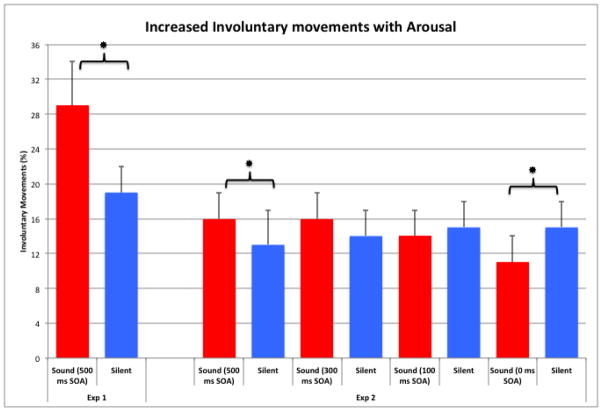
Next, we analyzed the percentage of involuntary actions (errors) and sRTs based on saccade type (voluntary vs. involuntary) and arousal conditions (sound vs. silence). First and foremost, as the left side of Figure 3 shows, involuntary eye movements significantly increased during the sound trials compared to the silent trials, with t(22)= 4.03, p< .002. The presentation of a sound significantly increased the number of involuntary eye movements that participants made. To preview the coming analyses of the sRT data, the sound also facilitated the speed of the involuntary eye movements.
Figure 3.
Percentage of involuntary eye movements in the sound and silent conditions in Experiments 1 & 2. Involuntary saccades were significantly increased under conditions of arousal with a specific time course for the increase in involuntary movements. Asterisks indicate significant differences (p< 0.05) between silent and sound conditions.
As expected, voluntary sRTs were slower than involuntary sRTs (M= 267 ms, SEM= 11 ms vs. M= 183 ms, SEM= 12 ms respectively), F(1,22)=58.9, p< .001. Sound trials had significantly faster sRTs (M= 214 ms, SEM= 12 ms) than silent trials (M= 237 ms, SEM= 10 ms), F(1,22)=17.8, p< .001.
Finally, there was a significant interaction (F(1,22)=6.3, p< .03), involuntary sRTs were sped (relative to silent) more by the sound than the voluntary ones (see Table 1). The benefit effect (difference between silent sRTs minus sound sRTs) for the voluntary eye movements was 7ms and was not significant, with F(1, 22)= 1.84, p= .19. In contrast, the sound’s effect on involuntary eye movements was 40ms and significant, with F(1, 22)= 13.00, p < .003.
Table 1.
Saccadic Reactions Times for Involuntary and Voluntary Eye Movements for Experiments 1 and 2.
| Experiment | SOA (between sound and go signal) | Movement Type | Sound | Silence | *Significant p < 0.05 |
|---|---|---|---|---|---|
| 1 | 500 | Involuntary | 164 (10) | 203(17) | * |
| Voluntary | 264 (12) | 271(11) | |||
| 2 | 500 | Involuntary | 178 (12) | 199(10) | * |
| Voluntary | 241 (8) | 262(9) | * | ||
| 300 | Involuntary | 203 (18) | 198 (10) | ||
| Voluntary | 240 (28) | 263 (10) | |||
| 100 | Involuntary | 214 (21) | 192 (11) | ||
| Voluntary | 281 (12) | 265 (12) | |||
| 0 | Involuntary | 200 (14) | 202 (12) | ||
| Voluntary | 315 (10) | 263 (8) | * |
An alternative reason for more facilitation (increased numbers and faster execution) for involuntary movements can be attributed to the relative speed of the different movements. As involuntary sRTs are significantly faster than voluntary sRTs, the arousing sound occurred closer in time to the involuntary movement onset. It is possible that arousal is equally beneficially for both types of movements, but is quickly dissipating, and the voluntary movement happens too late to retain the full benefit of the arousing stimulus. To try to address this interpretation, we performed an additional analysis where we equated the speed of the voluntary and involuntary sRTs. We ranked separately each subject’s voluntary sRTs and involuntary sRTs. We then iteratively included the fastest voluntary sRT and the slowest involuntary sRT in each condition until parity was achieved. After adding 12 data points in each condition, involuntary sRTs (224 ms) were now numerically slower than, though statistically equivalent to, voluntary sRTs (208 ms). Even though the voluntary sRTs occurred closer in time to the arousing stimulus than involuntary sRTs, the effect of arousal on involuntary movements (M= 66ms, SEM= 12.92) was still significantly greater than the effect on voluntary movements (M= 15ms, SEM = 12.92), with F(1, 22)= 7.8, p< .02. Therefore, the greater effect of arousal on involuntary movements in this experiment is unlikely due to their shorter sRTs that allow them to be executed closer in time to the arousing sound than the voluntary movements. While this analysis is suggestive, a more exact testing of the time course of this arousal effect is required. In Experiment 2, we varied the timing of the sound relative to the go signal to test this hypothesis more explicitly.
Experiment 2
In Experiment 1, following a sound, the number of involuntary eye movements significantly increased and involuntary sRTs were significantly faster compared to silence. While an increase in arousal is one likely mechanism for the changes in the involuntary actions, there are other possibilities that must be ruled out.
Experiment 1 used a well-established version of the antisaccade task that incorporated a pre-cue prior to the flash of the go signal to increase errors (Fischer and Weber 1996; Fischer et al. 1999; Blaukopf and DiGirolamo 2005; Blaukopf and DiGirolamo 2006; Klein et al. 2007). The addition of another factor (the sound on arousal trials) increased the amount of information during the sound trials to be processed relative to the silent trials; hence, increasing the difficulty of the task which might explain more involuntary movements (errors). To test this alternative hypothesis, Experiment 2 used a simplified anti-saccade task without a precue. This minor alteration allows a direct replication of Experiment 1 and decreases the amount of information the participants had to process on each trial.
Second, involuntary sRTs could have been influenced more by arousal as the onsets of these movements are faster than the onsets of voluntary eye movements. Since the arousing sound in Experiment 1 always occurred 500 ms prior to the go signal, the influence of the sound could have dissipated by the onsets of the voluntary movements that are, on average, 80–170 ms slower than involuntary eye movements. In Experiment 1, an additional analysis demonstrated that involuntary actions were significantly more speeded than voluntary actions even when sRTs for the two movement types were equated. However, this analysis, while useful, is not a rigorous test. In Experiment 2, we manipulated the onset of the arousing sound relative to the go signal to test the time course of the influence of the sound on eye movements.
Methods
Subjects
20 participants (8 male, 12 female; aged 18–22 years) gave informed consent and voluntarily took part in the experiment. All reported normal or corrected-to-normal vision. As before, if participants did not contribute a stable sRT in the condition in the analysis, their data were excluded for that comparison.
Procedure
The methods and procedures were identical to Experiment 1 with the following exceptions. The go signal appeared with no intervening pre-cue. To ensure that participants could not predict the onset of the go signal, the interval between the offset of the fixation and the onset of the go signal was varied (1100 ms, 900 ms, 700 ms). As Figure 1 (on right) shows, on sound trials, the interval between the onset of the sound stimulus and the onset of the go signal was also varied (500 ms, 300 ms, 100 ms, 0 ms). Finally, there were 3 blocks of trials with 180 trials in each block. Each block consisted of 36 silence trials, and 36 trials of sound at each SOA (144 sound trials). As in Experiment 1, each factor was counterbalanced and trials randomized within each block. There was a short break between each block, and the calibration procedure was conducted at the beginning of the experiment and after the breaks. A drift correction occurred after every 9 trials.
The proportion of involuntary actions at each SOA was analyzed between the two conditions (silent vs. sound). The sRT data were analyzed based on saccade type (voluntary antisaccade or involuntary prosaccade), and arousal condition (sound or silent) at each SOA (500 ms, 300 ms, 100ms, 0 ms) between the sound and the go signal.
Results
To begin, we again wanted to ensure that our sound stimulus produced a physiological response consistent with an arousal effect. As before, we compared the size of the pupil diameter during sound trials to the silent trials at the go signal. We found a significant difference in the size of the pupil based on our conditions, with F(4, 76), p < .005. Pupil diameter was significantly larger for all sound conditions (p < .01) with the exception of the 0 SOA condition were the effect was marginal (p= .07). Hence, these data demonstrate, as in Exp. 1, that the sounds produced a pupillary response consistent with change in arousal (Bradley et al. 2008) when the eye movement must be selected.
As the right panel of Figure 3 demonstrates, we found a main effect of condition across the SOAs in the number of involuntary movements, with F(4,40)= 3.69, p< 0.02. As before, when the sound occurred 500 ms before the go signal, the number of involuntary eye movements increased significantly during the sound trials compared to silence, t(19)= 2.34, p< .03. These data replicate our previous results in a simpler paradigm demonstrating an increase in the number of involuntary movements following a sound. The number of involuntary movements did not significantly change if the sound preceded the go signal at either the 300 ms or 100 ms SOA (t< 1.5 in both cases). When the sound and the go signal occurred simultaneously (0 ms SOA), the number of involuntary errors did not increase. In fact, participants were significantly less likely, t(15)= 2.32, p< .04, to make an involuntary eye movement in this condition than in the silent condition. These data suggest that the increase in involuntary eye movements in Experiment 1 were not caused by a difficulty in processing based simply on the number of events in the arousal condition (sound and then go signal) compared to the silent condition (just go signal). During the condition in this experiment where participants are processing two events simultaneously (sound and go signal), participants responded by decreasing the number of involuntary errors they made. To preview our RT data, they accomplished this reduction in involuntary movements by significantly slowing down their voluntary sRT. In short, when faced with simultaneous events, participants are more likely to be careful to execute a voluntary response even if sacrificing the speed of that voluntary action.
In all cases of the sRT data, involuntary movements were significantly faster than voluntary movements (p< .001). As in the error rate data, there was a main effect of condition with F(4, 40) = 11.49, p< 0.001. The main analysis of interest was the different between movement type (involuntary vs. voluntary) and condition (silence vs. sound) at each SOA.
As Table 1 indicates, when the sound preceded the go signal by 500 ms (a replication of our condition in Experiment 1), sRTs were significantly faster during the sound conditions than the silent condition, with F(1, 19)= 8.43, p< .01. As in Experiment 1, involuntary sRTs were significantly faster during sound trials than silent trials. In this experiment, however, the sound condition did not significantly interact with the voluntary and involuntary sRT (F<1). In short, the sound produced a speeding in both the involuntary and voluntary movements compared to the same conditions in silence.
When the sound preceded the go signal by 300 ms or 100 ms, there was no effect of the sound on sRT and no interaction, with p> .15 in all cases.
When the sound and the go signal occurred simultaneously (0 SOA), there was a trend toward a significant main effect of condition (sound vs. silent), with F(1, 15)= 4.06, p= .06. This main effect was qualified by a significant interaction (F(1, 15) = 5.50, p< .04). Voluntary sRTs during sound trials were significantly slower than during silence, t(15)= 4.80, p< .001). These data, combined with the error rate data demonstrate that participants are trading off the speed of a voluntary action, under confusing conditions, to ensure that they successfully carry out the voluntary response. There was no effect on involuntary sRTs when the sound occurred with the go signal compared to the silent condition, t < 1.
As in Experiment 1, a sound presented 500 ms prior to a go signal significantly increased the likelihood of executing an involuntary movement and reduced the speed of that involuntary eye movement. These data demonstrate that an arousing sound has a unique influence at 500 ms prior to the start of an eye movement, with increases in number of involuntary eye movements and faster execution.
General Discussion
A sound prior to the execution of an eye movement had a differential effect on voluntary and involuntary eye movements. The sound, which produced a physiological reaction consistent with arousal, significantly increased the number of involuntary actions likely to be executed (Experiments 1 & 2) and significantly increased the speed of involuntary (Experiments 1 & 2) and voluntary eye movements (Experiment 2). Moreover, our data suggest that this influence on involuntary action has a specific time course with an arousing sound 500 ms before the action being critical. Other intervals closer to the go signal were ineffective in producing either an increase in the number of involuntary actions or a speeding in sRT. In Exp. 2, a simultaneous presentation of the sound and the go signal led to significantly less involuntary actions. At this simultaneous interval, participants were able to control the involuntary movement by slowing their action response (voluntary sRTs were significantly slowed), and hence prevent the involuntary response. However, in both experiments, at a 500 ms SOA between the arousing stimulus and the time of action choice, involuntary actions were significantly facilitated with an increase in the number of these involuntary actions and a speeding of the involuntary action. This interval may seem to far-removed from the actual action to be an arousal effect; however, there is a significant history of research (Bowditch and Warren, 1890; Stahl & Rammsayer, 2005) that shows that involuntary actions are influenced at longer fore-period and the fore-period length differs with different involuntary actions and voluntary actions (for a review, see Hackley, 2009). For example, a puff of air to the neck 200 ms prior facilitates the knee-jerk reaction (Bowditch and Warren, 1890); whereas to influence manual reaction times, the warning signal can occur as much as 100 ms after the stimulus to-be-responded-to has occurred (Stahl and Rammsayer 2005). The well-know Yerkes-Dodson curve (Yerkes and Dodson 1908) for arousal has suggested that different levels of arousal change optimal performance with too little or too much arousal impeding behavior. Likewise, differing temporal intervals from the arousal stimulus to the to-be-executed action may have differential effects, even when the level of arousal is constant (i.e., the same arousing stimulus). Moreover, the time course of arousal to influence actions may be different between involuntary and voluntary actions for optimal effects. Within our own data, we demonstrated that the sound stimulus was effective only at 500 ms prior to the onset of the go signal. These data suggest that 500 ms prior to an eye movement is a good point on a temporal curve for arousal effects on involuntary eye movement. It may take the full 500 ms for the physiological reactions to have its influence on the involuntary action. It is important to note that at least 500 ms SOA for a significant effect of arousal on involuntary attention is the lower bound, we do not have data defining the upper bounds. A recent study by van Steenbergen et al. (2011), however, may help to define the upper bound. In this study, positive, and neutral pictures were shown prior to either an antisaccade or prosaccade task. Pupil diameter increased for the negative and positive pictures but not the neutral, demonstrating an arousal effect consistent with this stimulus set (Lang and Greenwald 1988) and with the current data. Relevant for the present discussion, there was no change in error rate for the antisaccade task across the picture types. In short, showing an arousing stimulus did not increase the number of prosaccade errors (involuntary movements). However, the arousing stimulus preceded the task by a minimum of 1700 ms. While it is difficult to reason from a null result, this paper (van Steenbergen et al. 2011) and the current experiments suggests that the arousal effect for involuntary movements starts at 500 ms and has dissipated by 1700 ms maximum.
What does arousal do? Some researchers have argued that increased arousal facilitates sensory analysis of the visual stimuli (Bernstein et al. 1970). Recent work (Deuter et al. 2013) has shown that a startling stimulus 500 ms before a voluntary saccade (either toward or away from the image) benefits the voluntary response with decreased sRTs. These authors argued for sensory and attentional processes reduced by the increased arousal following a startling stimulus. However, other theories argue that since more errors are made in the presence of an arousing accessory stimulus, the effect of arousal is best described as reducing the time for response selection in the absence of any increase in quality of information (Posner 1978; Stahl and Rammsayer 2005; Hackley 2009). Event-related potential data suggest no change in the lateralized readiness potential, suggesting that if arousal influences responses, it must be very early in response selection process (Hackley and Valle-Inclan 1999).
Several papers (e.g., Munoz and Everling 2004) have modeled antisaccade programming as a race between the involuntary prosaccadic programming and the voluntary antisaccadic programming. Given the independent processes competing for fastest execution, Massen (2004) has logically argued that selectively slowing the involuntary processes should reduce the error rate (as less involuntary movements will win; i.e., be executed). Likewise, slowing the voluntary component should increase the error rate (as more involuntary movements will win). Obviously, if both components are slowed or speeded equally, then there should be no change in error rate. This theory has been a useful framework in which to understand the effect of various factors on voluntary and involuntary movements.
Given our present findings, we suggest that the sound influences both movement types as both involuntary and voluntary movements were sped, though the former were more influenced (but see also, Kirchner and Colonius 2005). This effect on voluntary movements would also explain Kirchner & Colonius’s data for speeded antisaccades with spatial congruent sound stimuli. However, the present study also demonstrated increased numbers of involuntary movements (as well as more speeded actions) suggesting a stronger effect on involuntary actions. Involuntary movements occurred more frequently under conditions of sound suggesting that the arousing stimulus enhanced the involuntary components more than the voluntary components. This increase in involuntary movements could be occurring because, during conditions of arousal, the involuntary component is strengthened and won the race (i.e., Munoz & Everling, 2004) more often relative to a non-aroused system. Alternatively, the inhibition of the involuntary component necessary to execute the voluntary movement (i.e., Kristjánsson et al. 2004) isn’t occurring as the involuntary component is too powerful under arousal conditions to inhibit. In either case, the present data suggests more influence of arousal on involuntary than voluntary processes. The effect of increased involuntary actions in our data, likely driven by arousal, are similar in kind to data gathered by Kissler & Keil (2008). Kissler & Keil had participants make an antisaccade away from either a positive or negative valence picture that was also highly arousing. Compared to antisaccade rates during a low arousal neutral stimulus, participants made more prosaccadic errors to both the positive and negative valence stimuli. This data suggests that the valence of the stimulus might not be important in producing an increase in involuntary actions, but rather the arousal is critical to increasing involuntary, automatic actions. In short, what we have shown in the present data is an enhanced involuntary system by arousing stimuli.
While arousal seems to be beneficial to involuntary eye movements (c.f., Kristjánsson et al. 2004, Exp. 5), other aspects of attention may be useful for the voluntary system. In Posner & Boise (1978) originally classification, they distinguished between capacity limits and arousal (see also, Posner and DiGirolamo 1999). Capacity limits are frequently thought of as a limited and finite resource for cognitive processes that can’t easily be split between competing processes (Posner and DiGirolamo 1998). In contrast to arousal, when capacity limits are taxed in an antisaccade task, antisaccades are speeded (e.g., Kristjánsson et al. 2001). Kristjánsson, Chen and Nakayama (2001) suggested that the involuntary component in the antisaccade task (programming a prosaccadic error) requires capacity; therefore, distracting capacity from this process facilitates the voluntary antisaccade. Hence, this aspect of attention plays a critical role in voluntary eye movements. In the current data, we show similar effects when the sound and go signal are simultaneous; participants strategically make more voluntary movements. In contrast, at 500 ms SOA, arousal facilitated the involuntary eye movements.
Our results support the effects of arousal on early response selection in the absence of improvement in quality of sensory information. If arousal led to better processing of the visual stimulus, then involuntary sRTs would indeed be speeded, but voluntary sRTs would have correspondingly slowed due to the increased difficulty in overcoming the stronger prosaccade tendencies (Kristjánsson et al. 2004). Since voluntary sRTs and involuntary sRTs are reduced by the sound, early response selection must be working faster and independently for both the voluntary and involuntary movement. However, arousal facilitates the response selection for the involuntary component. One obvious reason for this may be the difference in neuroanatomical locations and connection of the voluntary and involuntary eye movement network. Voluntary saccades rely more on parietal and frontal cortical areas, while involuntary saccades rely more on the subcortical superior colliculi structures (e.g., Mort et al. 2003). Given the anatomical proximity and reciprocal connections between the superior colliculus and the reticular formation (Chen and May 2000), involuntary saccades may benefit more because of these direct neuroanatomical pathways. As such, involuntary eye actions may be more susceptible to arousal than other involuntary actions because of these neuroanatomical connections; though, both eye blink reflexes and stretch reflexes in the limbs are also increased by arousal.
Involuntary eye movements being influence more strongly by the sound than voluntary ones may have implications for every day action. In today’s high-stimulation environment, most people experience daily periods of high arousal. In these situations, one may be more likely to respond to the powerful stimulus in the environment with a well-learned response rather that a strategic voluntary one. Of course, the current data is limited to involuntary eye movements, but work from our lab (DiGirolamo et al. 2015; DiGirolamo et al. 2016) suggests that this task may be a good marker of more relevant and destructive responding to powerful stimuli in the environment (i.e., drug stimuli in addiction). Other factors (e.g., from genetics to impulsivity) may influence the intensity and the extent that arousal (one component of attention) will drive involuntary actions. Our data suggests that arousal increases the chances of an involuntary, but well-learned, response to a powerful stimulus, but the extent of that influence based on individual variation and generalization to other responses remains unknown.
Footnotes
After completion of this experiment, participants also completed an additional experiment of 96 trials of the same task without warning signals and were asked to rate if they made an error and how confident they were in that judgment. This data was obtained for a different purpose from the present paper.
References
- Bernstein IH, Rose R, Ashe V. Prepatory state effects in intersensory facilitation. Psychonomic Science. 1970;19:113–114. [Google Scholar]
- Blaukopf CL, DiGirolamo GJ. The automatic extraction and use of information from cues and go signals in an antisaccade task. Experimental Brain Research. 2005;167:654–659. doi: 10.1007/s00221-005-0125-8. [DOI] [PubMed] [Google Scholar]
- Blaukopf CL, DiGirolamo GJ. Differential effects of reward and punishment on conscious and unconscious eye movements. Exp Brain Res. 2006;174:786–792. doi: 10.1007/s00221-006-0685-2. [DOI] [PubMed] [Google Scholar]
- Blaukopf CL, DiGirolamo GJ. Reward, context & human behaviour. The Scientific World Journal. 2007;7:626–640. doi: 10.1100/tsw.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlin G, Graham FK. Cardiac deceleration and reflex blink facilitation. Psychophysiology. 1977;14:423–431. doi: 10.1111/j.1469-8986.1977.tb01306.x. [DOI] [PubMed] [Google Scholar]
- Bowditch HP, Warren JW. The knee-jerk reaction and its modifications. Journal of Physiology. 1890;11:25–64. doi: 10.1113/jphysiol.1890.sp000318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45:602–607. doi: 10.1111/j.1469-8986.2008.00654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo MG, Lang PJ. Parafoveal semantic processing of emotional visual scenes. Journal of Experiment Psychology: Human Perception and Performance. 2005;31:502–519. doi: 10.1037/0096-1523.31.3.502. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. The feedback circuit connecting the superior colliculus and the central mesencephalic reticular formation: A direct morphological demonstration. Experimental Brain Research. 2000;131:10–21. doi: 10.1007/s002219900280. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Operant conditioning of the H-reflex in freely moving rats. Journal of Neurophysiology. 1995;73:411–415. doi: 10.1152/jn.1995.73.1.411. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Akbudak E, Conturo TE, et al. A common network of functional areas for attention and eye movements. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Deuter CE, Schilling TM, Kuehl LK, Blumenthal TD, Schachniger H. Startle effecs on saccadic responses to emotional stimuli. Psychophysiology. 2013;50:1056–1063. doi: 10.1111/psyp.12083. [DOI] [PubMed] [Google Scholar]
- DiGirolamo GJ, Smelson D, Guevremont N. Cue-Induced craving in patients with cocaine use disorder predicts cognitive control deficits toward cocaine cues. Addictive Behaviors. 2015;47:86–90. doi: 10.1016/j.addbeh.2015.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGirolamo GJ, Sophis EJ, Daffron JL, Gonzalez G, Romero-Gonzalez M, Gillespie SA. Breakdowns of eye movement control toward smoking cues in young adult light smokers. Addictive Behaviors. 2016;52:98–102. doi: 10.1016/j.addbeh.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. Brain mechanisms of attention. Quarterly Journal of Experimental Psychology. 2006;59:2–27. doi: 10.1080/17470210500260674. [DOI] [PubMed] [Google Scholar]
- Evdokimidis I, Smyrnis N, Constantinidis TS, et al. The antisaccade task in a sample of 2,006 young men. I. Normal population characteristics. Experimental Brain Research. 2002;147:45–52. doi: 10.1007/s00221-002-1208-4. [DOI] [PubMed] [Google Scholar]
- Fischer B. Effects of procues on error rate and reaction times of antisaccades in human subjects. Experimental Brain Research. 1996 doi: 10.1007/BF00229636. [DOI] [PubMed] [Google Scholar]
- Fischer B, Gezeck S, Mokler A. Erroneous prosaccades in a gap-antisaccade-task. In: Becker W, Deubel H, Mergner T, editors. Current oculomotor research: Physiological and psychological aspects. Plenum Publishers; New York: 1999. pp. 53–63. [Google Scholar]
- Fischer B, Weber H. Effects of procues on error rate and reaction time of antisaccades in human subjects. Experimental Brain Research. 1996;109:507–512. doi: 10.1007/BF00229636. [DOI] [PubMed] [Google Scholar]
- Foltz EL, Schmidt RP. The role of the reticular formation in the coma of head injury. Journal of Neurosurgery. 1956;13:145–154. doi: 10.3171/jns.1956.13.2.0145. [DOI] [PubMed] [Google Scholar]
- Hackley S. The speeding of voluntary reaction by a warning signal. Psychophysiology. 2009;46:225–233. doi: 10.1111/j.1469-8986.2008.00716.x. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclan F. Accessory stimulus effects on response selection: does arousal speed decision making? Journal of Cognitive Neuroscience. 1999;11:321–329. doi: 10.1162/089892999563427. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Kirchner H, Colonius H. Cognitive control can modulate intersensory facilitation: Speeding up visual antisaccades with an auditory distracter. Experimental Brain Research. 2005;166:440–444. doi: 10.1007/s00221-005-2383-x. [DOI] [PubMed] [Google Scholar]
- Kissler J, Keil A. Look–don’t look! How emotional pictures affect pro-and anti-saccades. Experiemental Brain Research. 2008;188:215–222. doi: 10.1007/s00221-008-1358-0. [DOI] [PubMed] [Google Scholar]
- Klein RM. Does oculormotor readiness mediate cognitive control of visual attention? In: Nickerson R, editor. Attention and Performance. Lawrence Erlbaum Associates; Hillsdale, NJ: 1980. pp. 259–276. [Google Scholar]
- Klein TA, Endrass T, Kathmann K, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Kristjánsson Á, Chen Y, Nakayama K. Less attention is more in the preparation of antisaccades, but not prosaccades. Nature Neuroscience. 2001;4:1037–1042. doi: 10.1038/nn723. [DOI] [PubMed] [Google Scholar]
- Kristjánsson A, Vandenbroucke MWG, Driver J. When pros become cons for anti- versus prosaccades: Factors with opposite or common effects on different saccade types. Experimental Brain Research. 2004;155:231–244. doi: 10.1007/s00221-003-1717-9. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Mesulam M, Gitelman DR, Weintraub S. Emotional curiosity: modulation of visuospatial attention by arousal is preserved in aging and early-stage Alzheimer’s disease. Neuropsychologia. 2000;38:1734–1740. doi: 10.1016/s0028-3932(00)00077-4. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK. The international affective picture system standardization procedure and initial group results for affective judgments. University of Florida; Gainsville, Florida: 1988. [Google Scholar]
- Low KA, Larson SL, Burke J, Hackley SA. Alerting effects on choice reaction time and the photic eyeblink reflex. Electroencephalography and Clinical Neurophysiology. 1996;98:385–393. doi: 10.1016/0013-4694(96)95085-3. [DOI] [PubMed] [Google Scholar]
- Massen C. Parallel programming of exogenous and endogenous components in the antisaccade task. Quarterly Journal of Experimental Psychology: Section A. 2004;57:475–498. doi: 10.1080/02724980343000341. [DOI] [PubMed] [Google Scholar]
- Milstein DM, Dorris MC. The influence of expected value on saccadic preparation. Journal of Neuroscience. 2007;27:4810–4818. doi: 10.1523/JNEUROSCI.0577-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mort DJ, Perry RJ, Mannan SK, et al. Differential cortical activation during voluntary and reflexive saccades in man. Neuroimage. 2003;18:231–246. doi: 10.1016/s1053-8119(02)00028-9. [DOI] [PubMed] [Google Scholar]
- Munk MHJ, Roelfsema PR, König P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272:271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: The anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Posner MI. Chronometric explorations of mind. Erlbaum; Hillsdale, NJ: 1978. [Google Scholar]
- Posner MI, Boies SJ. Components of attention. Psychological Review. 1971;78:391–408. [Google Scholar]
- Posner MI, DiGirolamo GJ. Executive attention: Conflict, target detection and cognitive control. In: Parasuraman R, editor. The attentive brain. MIT Press; Cambridge, MA: 1998. pp. 401–423. [Google Scholar]
- Posner MI, DiGirolamo GJ. Attention in cognitive neuroscience. In: Gazzaniga M, editor. The Cognitive Neurosciences. 2. MIT Press; Cambridge, MA: 1999. pp. 623–631. [Google Scholar]
- Rizzolatti G, Riggio L, Dascola I, Umilta C. Reorienting attention across the horizontal and vertical meridians: Evidence in favor of a premotor theory of attention. Neuropsychologia. 1987;25:31–40. doi: 10.1016/0028-3932(87)90041-8. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Arousal systems and attention. In: Gazzaniga M, editor. The Cognitive Neurosciences. MIT Press; Cambridge, MA: 1995. pp. 703–720. [Google Scholar]
- Stahl J, Rammsayer TH. Accessory stimulation in the time course of visuomotor information processing: stimulus intensity effects on reaction time and response force. Acta Psychologica. 2005;120:1–18. doi: 10.1016/j.actpsy.2005.02.003. [DOI] [PubMed] [Google Scholar]
- van Steenbergen H, Band GPH, Hommel B. Threat But Not Arousal Narrows Attention: Evidence from Pupil Dilation and Saccade Control. Frontiers in psychology. 2011:2. doi: 10.3389/fpsyg.2011.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenban-Smith MG, Findlay JM. Express saccades: is there a separate population in humans? Exp Brain Res. 1991;87:218–222. doi: 10.1007/BF00228523. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology. 1908;18:459–482. [Google Scholar]