Abstract
IL-10 is a key pleiotropic cytokine that can both promote and curb Th2-dependent allergic responses. Herein we demonstrate a novel role for IL-10 in promoting mast cell expansion and the development of IgE-mediated food allergy. Oral ovalbumin challenge in sensitized BALB/c mice resulted in a robust intestinal mast cell response accompanied by allergic diarrhea, mast cell activation, and a predominance of Th2 cytokines, including enhanced IL-10 expression. In contrast, the development of intestinal anaphylaxis including diarrhea, mast cell activation, and Th2 cytokine production was significantly attenuated in IL-10−/− mice compared to WT controls. IL-10 also directly promoted the expansion, survival, and activation of mast cells, increased FcɛRI expression on mast cells, and enhanced the production of mast cell cytokines. IL-10−/− mast cells had reduced functional capacity, which could be restored by exogenous IL-10. Similarly, attenuated passive anaphylaxis in IL-10−/− mice could be restored by IL-10 administration. The adoptive transfer of WT mast cells restored allergic symptoms in IL-10−/− mice, suggesting that the attenuated phenotype observed in these animals is due to a deficiency in IL-10-responding mast cells. Lastly, transfer of WT CD4 T cells also restored allergic diarrhea and intestinal mast cell numbers in IL-10−/− mice, suggesting that the regulation of IL-10-mediated intestinal mast cell expansion is T cell-dependent. Our observations demonstrate a critical role for IL-10 in driving mucosal mast cell expansion and activation, suggesting that in its absence, mast cell function is impaired, leading to attenuated food allergy symptoms.
Introduction
Activation of intestinal mast cells is a crucial and defining step in the development of anaphylactic responses to ingested food allergens (1, 2). Mast cells drive acute episodes of food allergy resulting in muscular contractions, allergic diarrhea, and anaphylaxis, and their activation is dependent on the production of an effective allergen-specific Th2 response that elicits elevated levels of IgE antibodies. Furthermore, both, Th2 cytokines such as IL-4 and IL-9, as well as IgE, play a central role in promoting intestinal mast cell expansion and driving the allergic response in experimental food allergy models and patients with food allergy (3–6).
IL-10 is an important immunoregulatory cytokine that plays a critical role in the propagation and suppression of immune responses (7–11). Although, first isolated as a mediator of Th2 cell responses (12), it is a pleiotropic cytokine that is produced by many cell types including macrophages, epithelial cells, mast cells and regulatory T cells (Tregs), and has both pro- and anti-inflammatory effects during immune responses (7–9). This is evidenced from its distinctive effects in both promoting the generation of plasma cells from centrocytes (13–15), as well as inducing suppression mediated by Tregs and other T cells (11, 16).
The effects of IL-10 on the development of allergic inflammation have been previously examined using animal models of allergic asthma and atopic dermatitis. While some studies showed that IL-10 is involved in regulating the extent of allergic inflammation and suppressing Th2-mediated effects (consistent with its known immunoregulatory functions), others suggest that IL-10 can promote the development of eosinophilia, airway hyperreactivity (AHR), mucus metaplasia, and IL-5 production during the allergic response (17–25). Furthermore, Geha and colleagues demonstrated that IL-10 can skew dendritic cell function in favor of a Th2-specific response (26), suggesting that IL-10 may play a critical role in maintaining the balance between the progression and resolution of allergic responses. However, the role of IL-10 in the regulation of food allergy remains to be elucidated.
Herein, we demonstrate that IL-10 has proinflammatory effects and modulates the development of food allergy to the experimental food allergen ovalbumin (OVA). Intragastric (i.g.) administration of OVA to sensitized BALB/c mice resulted in a robust intestinal mast cell response accompanied by allergic diarrhea, mast cell activation, and a predominance of Th2 cytokines in the gut. In contrast, IL-10−/− animals were resistant to the development of food allergy. Furthermore, we demonstrate that IL-10 can directly promote mast cell expansion, and has proinflammatory effects resulting in enhanced production of mast cell-derived cytokines. Lastly, adoptive transfer experiments demonstrated that the allergic phenotype as well as intestinal mast cell numbers in IL-10−/− mice can be restored by the transfer of either CD4 T cells or bone marrow-derived mast cells (BMMCs) from WT mice, suggesting that the suppression of intestinal anaphylaxis in IL-10−/− animals may be due to an impairment of mast cell function in the absence of IL-10. Taken together, our observations demonstrate for the first time a critical role for IL-10 in the expansion and function of mast cells as well as in the development of food allergy.
Materials and Methods
Animals
BALB/c mice were purchased from Taconic Farms and Harlan. IL-10−/− mice were a kind gift of Dr. Hans Oettgen (Harvard Medical School) and also purchased from Jackson. All mice were bred in our facility and all animal research was approved by the IACUC of Western New England University.
Food allergy regimen
To induce food allergy, BALB/c and IL-10−/− mice were i.p. immunized with 50 µg chicken egg OVA in 1 mg alum twice as previously described (27). Mice were challenged i.g. with 50 mg OVA on 6 alternating days. One hour after the 6th challenge, mice were sacrificed and assessed as previously described (1, 4).
Measurement of intestinal anaphylaxis
Intestinal anaphylaxis was assessed in challenged mice by scoring the percentage of animals exhibiting allergic diarrhea (27). Briefly, mice were observed for the presence of diarrhea for one hour after the 6th OVA-challenge and were scored as diarrhea-positive or negative.
Histological analysis and enumeration of mast cells
Intestinal mast cells were enumerated as previously described (6). Tissue sections were stained with chloroacetate esterase (CAE) and mast cells were counted in complete cross-sections of jejunum.
Quantitative PCR analysis and ELISAs
Quantitative RT-PCR was performed as previously described (27). Expression of cytokine genes was calculated relative to GAPDH transcripts. ELISAs for mMCP-1 (Affymetrix), IL-4, IL-5 (BD Biosciences), IL-13 (R&D Systems), TNF-α, IL-6, IFN-γ (Biolegend) and OVA-IgE were performed according to manufacturers’ protocols as previously described (6, 27).
Spleen and MLN stimulation
Spleen and mesenteric lymph node (MLN) cells were cultured with medium, 200 µg/ml OVA or anti-CD3 and anti-CD28 and cytokines were enumerated in supernatants as previously described (6).
Flow cytometry
Spleen and MLN cells and BMMCs were incubated with monoclonal antibodies (Biolegend and Affymetrix) against CD3, CD4, CD8, B220, c-Kit and FcɛRI. Flow cytometric analysis was performed using an Accuri C6 cytometer and Flow jo software.
Intracellular cytokine staining
Spleen and MLN cells from experimental mice were isolated and stimulated with 200 µg/ml OVA in the presence of brefeldin A for 6 hours. Surface staining for CD3+CD4+ cells was performed and cells were fixed and permeabilized using a kit from Affymetrix. Intracellular staining for IL-10 (Biolegend) was performed and compared with isotype control.
BMMC culture
BMMCs were generated from naïve WT and IL-10−/− mice and cultured with 10 ng/ml of IL-3 and SCF for 4–8 weeks as previously described (28). Harvested BMMC were > 99% positive for c-Kit and FcεRI.
Carboxyfluorescien succinimidyl ester (CFSE) labeling and transfer of mast cells
WT and IL-10−/− BMMCs were treated with 5 µM CFSE and 1.0×106 labeled cells were injected i.p. into BALB/c and IL-10−/− mice as previously described (27). On the 6th day, mice were sacrificed, and peritoneal cells were examined for the presence of CFSE+c-Kit+FcεRI+ cells by flow cytometry.
In vitro studies
1.0×106 BMMCs were cultured in triplicate with IL-3 and SCF for 7 days. 20 ng/ml recombinant IL-10 (rIL-10) (Peprotech and Biolegend) was added to cell cultures for either 24 hours or 7 days. The number of live cells/well was enumerated. To induce activation, cells were primed with 2 µg/ml DNP-IgE overnight and stimulated with 200 ng/ml DNP-BSA for 1h to assess cytokine expression or for 6 hours to assess cytokine secretion.
β-Hexosaminidase (β-Hex) Assay
BMMCs were cultured in the presence or absence of 20 ng/ml rIL-10 for 24 hours or 6 days as described above. Cells were activated and β-hex activity was assessed as previously described (29).
Passive Anaphylaxis
WT and IL-10−/− were sensitized i.v. with 10 µg DNP-IgE (clone SPE7, Sigma). 24h later, they were challenged i.v. with 100 µg of DNP-BSA, and changes in core body temperature were recorded using subcutaneously placed transponders (Biomedic Data Systems). To assess the effects of IL-10 on the development of passive anaphylaxis, some mice were injected i.p. with 3 µg rIL-10 starting 5 hours before injection with DNP-IgE, followed by 4 µg rIL-10 i.v. concurrently with the DNP-IgE injection. A final dose of 3 µg rIL-10 was administered i.p. one hour before challenge with DNP-BSA.
Adoptive Transfer Experiments
Spleen cells were isolated from naïve BALB/c mice and CD4 T cells were negatively selected using microbeads from Miltenyi Biotec. Approximately, 5×106 CD4+ T cells were injected i.v. into IL-10−/− mice. Two days later, reconstituted mice were subject to the food allergy experimental regimen.
To determine whether the transfer of WT BMMCs can restore the development of intestinal anaphylaxis in IL-10−/− mice, 10×106 WT BMMCs were injected i.v. into IL-10−/− animals prior to food allergy induction. Twenty-four hours later, mice were subject to the food allergy experimental regimen including sensitization and challenge. As controls, IL-10−/− BMMCs were also injected into IL-10−/− mice.
Statistical Analysis
Data are expressed as mean±SEM, unless stated otherwise. Statistical significance comparing different sets of mice (between 2 groups) was determined by the Student’s t-test. In experiments comparing multiple experimental groups or time points, two-way analysis of variance was performed and Bonferroni post-tests were used to compare replicate means.
Results
IL-10 is essential for the development of allergic diarrhea and mast cell activation in OVA-challenged mice
In light of observations suggesting that IL-10 can modulate Th2 responses and have proinflammatory effects during allergic inflammation, we hypothesized that IL-10 may similarly modulate mast cell function during allergic responses in vivo, and chose to study the development of food allergy in IL-10−/− mice.
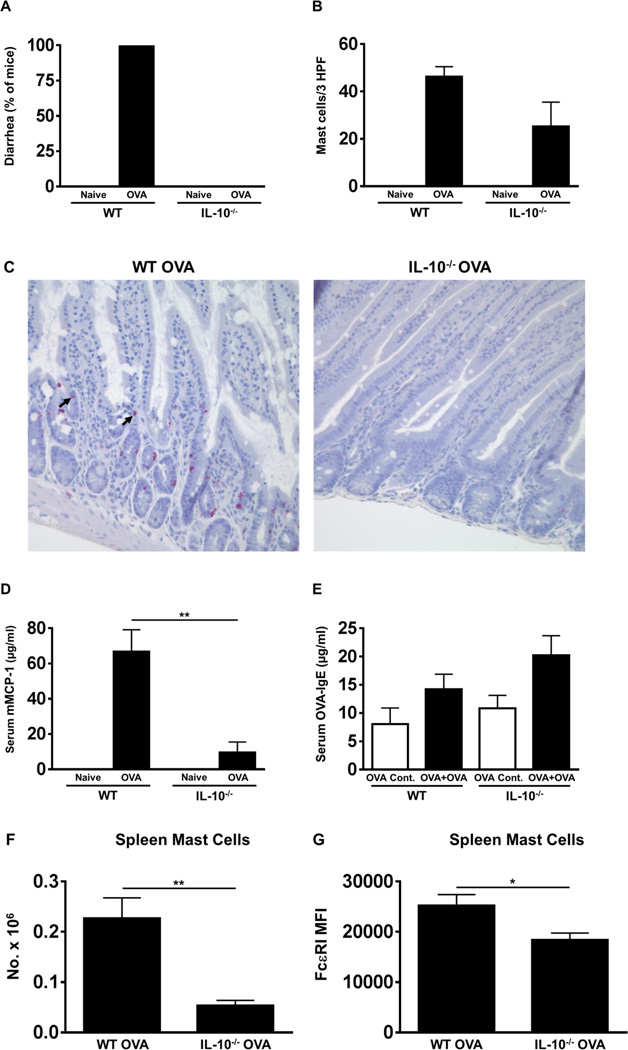
BALB/c wild-type (WT) mice as well as IL-10−/− animals were sensitized and challenged with OVA as described in Materials and Methods and observed for the presence of diarrhea for one hour after the sixth challenge. OVA-challenged WT mice exhibited profuse diarrhea in comparison with unchallenged controls (Fig. 1A). In contrast, no diarrhea could be observed in any of the similarly challenged IL-10−/− mice. Examination of jejunal tissue from sacrificed animals revealed the presence of many degranulating mast cells including intraepithelial mast cells in WT mice as assessed by chloroacetate esterase (CAE) staining (Fig. 1B & C). In contrast, fewer to no undegranulated mast cells were observed in the jejunae of similarly challenged IL-10−/− mice, suggesting that IL-10 may affect the homeostasis of intestinal mast cells during food allergy development (Fig. 1B & C). Similarly, examination of sera revealed the presence of elevated levels of mMCP-1 in OVA-challenged WT mice compared to unchallenged controls (Fig. 1D). In contrast, the levels of this enzyme were significantly diminished in IL-10−/− mice, suggesting that mast cell activation is impaired in the absence of IL-10 (Fig. 1D). However, equivalent levels of OVA-IgE were observed in both groups, suggesting that mast cell activation and allergic diarrhea are attenuated despite the presence of OVA-IgE (Fig. 1E). Further examination revealed that significantly fewer mast cell numbers were also observed in the spleen of IL-10−/− mice as compared with WT controls (Fig. 1F). Moreover, the expression of the high affinity receptor for IgE, FcɛRI, was also decreased in splenic mast cells from IL-10−/− mice (Fig. 1G). Taken together, these data suggest that in the absence of IL-10, mast cell homeostasis and function is impaired leading to the attenuated development of allergic diarrhea in IL-10−/− mice.
Figure 1. OVA-sensitized and challenged IL-10−/− mice fail to develop allergic diarrhea and do not exhibit mast cell activation.
WT BALB/c and IL-10−/− mice were sensitized and challenged with OVA. (A) Percent of diarrhea-positive animals. (B) Numbers of CAE+ jejunal mast cells. (C) Histology depicting CAE-positive jejunal mast cells. Mast cells, including intraepithelial cells are shown by an arrow (D) Serum mMCP-1 levels. (E) Serum OVA-IgE levels. (F) Spleen mast cells. (G) MFI of FcɛRI expression on spleen mast cells. n=10–15 mice/group. *=p<0.05; **=p<0.01 (t-test). Data are representative of 2 independent experiments.
Local Th2 responses are attenuated in OVA-challenged IL-10−/− mice
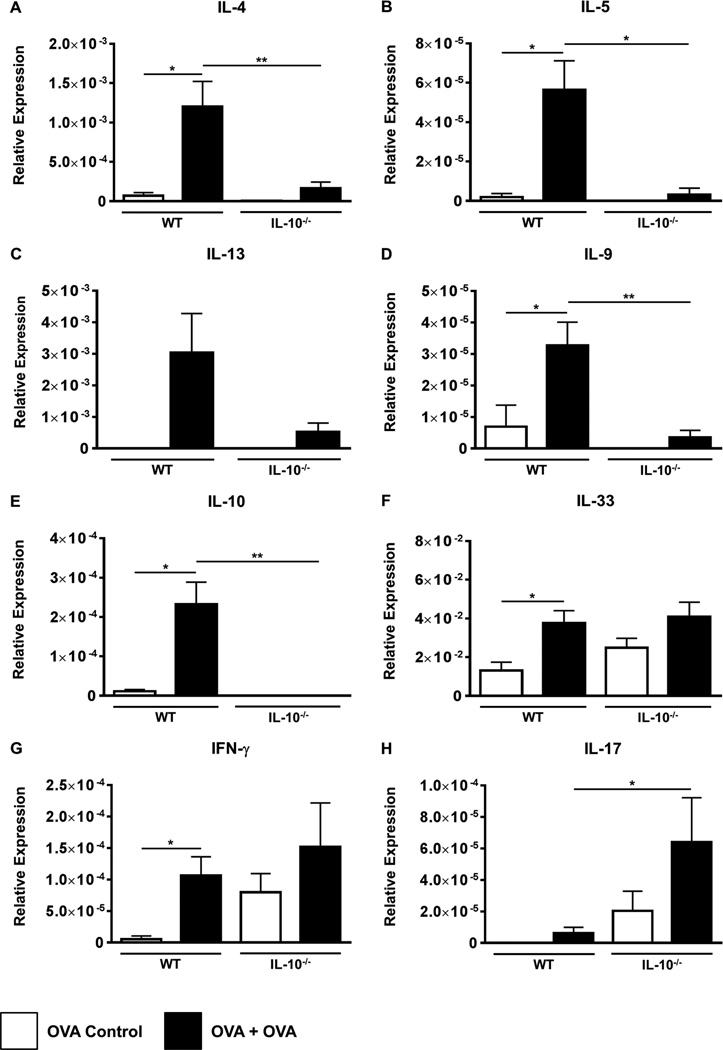
Th2 cells and their cytokines drive the allergic response and play critical regulatory roles in the expansion of discrete mast cell populations (3–5). Assessment of jejunal tissue by RT-PCR revealed enhanced expression of the classic Th2 cytokines IL-4, IL-5, IL-13 and IL-9 in the jejunum of OVA-challenged WT mice compared to unchallenged controls (Figs.2A–D). This was accompanied by significantly increased expression of IL-10 (Fig. 2E). In contrast, the expression of these cytokines was decreased in the jejunae of OVA-challenged IL-10−/− mice (Figs.2A–E). Examination of IL-33, IFN-γ and IL-17 revealed that, while both groups exhibited equivalent expression of IL-33 and IFN-γ (Figs.2F–G), the expression of IL-17 was enhanced in both OVA-challenged IL-10−/− mice and unchallenged controls (Fig. 2H). These data therefore suggest that intestinal Th2 responses are attenuated in IL-10−/− mice during the development of food allergy, and may instead be accompanied by an alternate T cell expression profile such as Th17.
Figure 2. OVA-challenged IL-10−/− animals exhibit attenuated expression of intestinal Th2 cytokines.
BALB/c and IL-10−/− mice were sensitized and challenged with OVA. The expression of mRNA for various cytokines (A–H) was assessed using established Taqman probes and RT-PCR. n=10–15 mice/group. *=p<0.05; **=p<0.01. (student’s t-test). Data are representative of 2 independent experiments.
OVA-challenged IL-10−/− mice retain the capacity to induce systemic Th2 cytokines
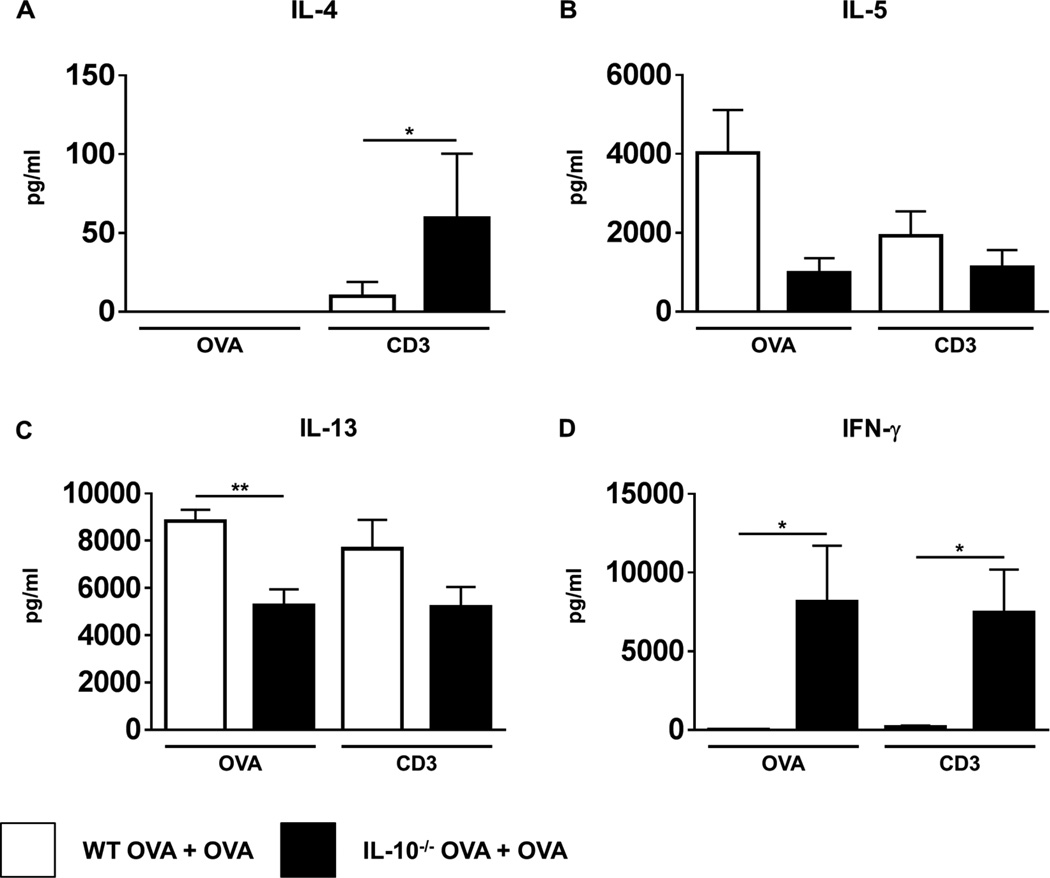
Th2 responses were further assessed by examining MLN and spleen cells for cytokine production in response to OVA stimulation. As expected, enhanced levels of IL-5 and IL-13 were observed in supernatants from both MLN and spleens of OVA-challenged WT mice compared to unchallenged controls (Figs.3B & C and data not shown). IL-4 was not detected in MLN supernatants (Fig. 3A), but enhanced levels were observed in the spleen (data not shown). In contrast, both IL-5 and IL-13 were decreased in IL-10−/− OVA-challenged mice (Figs. 3B & C), and was accompanied by increased secretion of IFN-γ (Fig. 3D). These data therefore suggest that allergen-specific Th2 responses in both the intestines and secondary lymphoid tissue are impaired during food allergy in IL-10−/− mice. This led us to question whether in the absence of IL-10, there may be an intrinsic defect in the development of Th2 cytokines, especially since IL-10−/− mice have been associated with the spontaneous development of Th1-mediated colitis. To determine that this was not the case, cells were further examined after stimulation with T cell agonists. Anti-CD3/CD28 stimulation induced equivalent levels of IL-5 and IL-13 in both OVA-challenged WT and IL-10−/− mice (Figs.3B & C). Furthermore, OVA-challenged IL-10−/− mice exhibited elevated IL-4 and IFN-γ production compared with their WT counterparts (Figs.3A & D). These data therefore confirm that while the induction of Th2 cytokines in the context of food allergy is defective in IL-10−/− mice, they retain the capacity to make enhanced levels of these cytokines, especially IL-4, when polyclonally stimulated.
Figure 3. Impaired Th2 cytokine production by MLN cells from OVA-challenged IL-10−/− mice in response to antigen but not polyclonal stimulation.
MLN cells were stimulated with either OVA or T cell agonists for 72 hours. Levels of the cytokines (A) IL-4 (B) IL-5 (C) IL-13 and (D) IFN-γ were enumerated in the supernatants by ELISA. n=4 mice/group. *=p<0.05; **=p<0.01. (student’s t-test). Data are representative of 2 independent experiments.
IL-10 promotes the expansion of BMMCs and is required for their proliferation in vivo
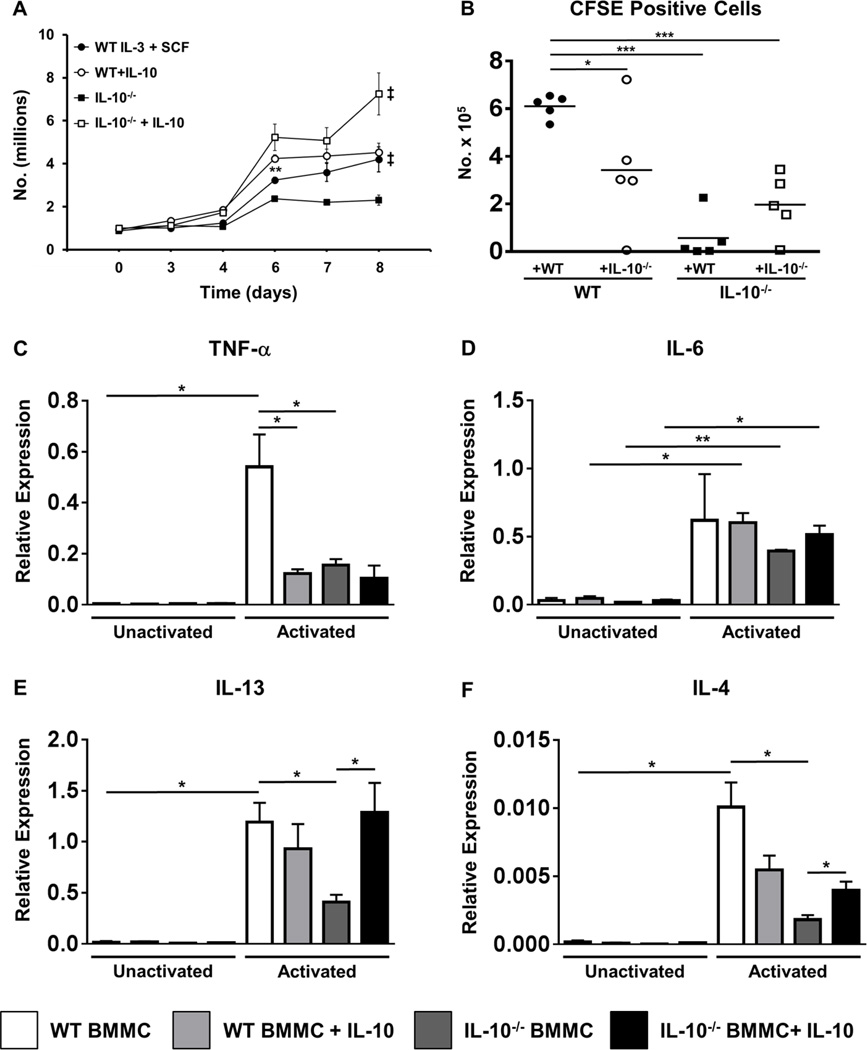
The data described above suggest that IL-10 may regulate mast cell responses during food allergy, resulting in suppression of symptoms such as allergic diarrhea. To further determine whether IL-10 can have stimulatory effects on mast cells, we examined the effects of co-culture with rIL-10 on the proliferation of WT and IL-10−/− BMMCs. WT BMMCs cultured with IL-3 and SCF exhibited a normal course of expansion with increased numbers of cells observed by the last day of culture (Fig. 4A). Addition of rIL-10 at the start of cell culture further amplified their proliferation with notable effects observed between days 4–6. In contrast, IL-10−/− BMMCs exhibited a lower rate of proliferation compared to that observed in WT cells. However, the addition of rIL-10 not only restored the ability of IL-10−/− BMMCs to undergo expansion to WT levels, but also significantly increased their proliferation compared to both untreated and IL-10-treated WT BMMCs (Fig. 4A).
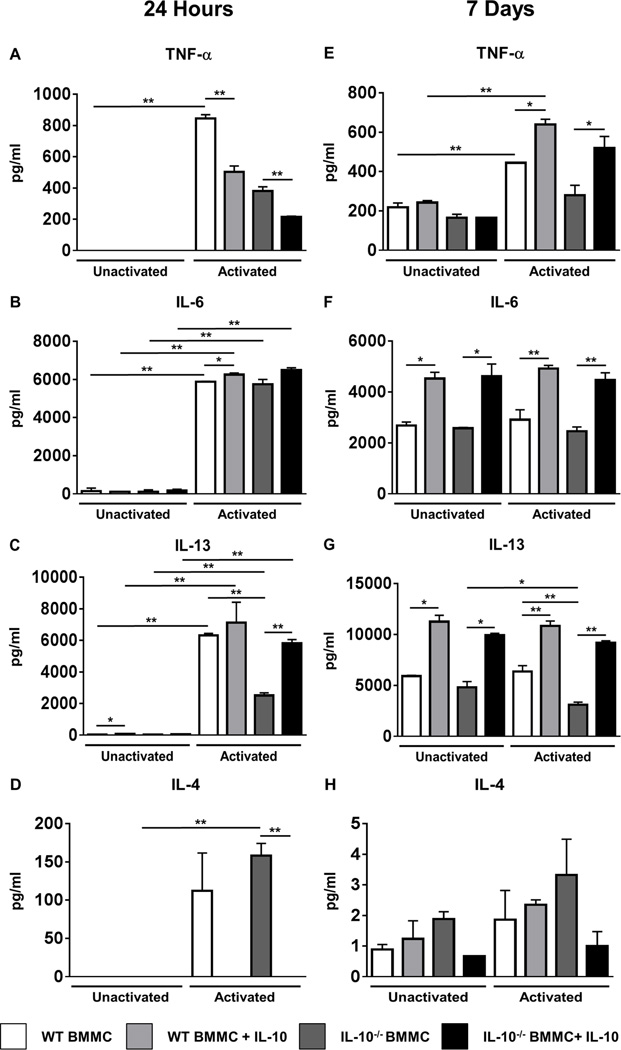
Figure 4. IL-10 promotes mast cell expansion and is required for BMMC cytokine production.
(A) WT and IL-10−/− BMMCs were cultured with IL-3 and SCF, in the presence or absence of rIL-10. Numbers of live cells are shown. (B) CFSE+ WT or IL-10−/− BMMCs were transferred into the peritoneal cavity of WT and IL-10−/− mice. Six days later mice were sacrificed and the number of recovered peritoneal CFSE+c-Kit+ cells is shown. (C–F) BMMCs were cultured in the presence or absence of rIL-10 overnight and were activated with IgE and antigen for one hour. Expression of the cytokines TNF-α, IL-6, IL-13 and IL-4 was assessed by RT-PCR. Data are representative of 3 or more independent experiments. **=p<0.01; *=p<0.05 (student’s t-test). ‡=p<0.0001 by ANOVA.
Since IL-10 could thus regulate the expansion of mast cells in culture, we asked whether it would have similar effects on mast cell expansion and survival in vivo in the context of physiological exposure to other potential mast cell growth factors. Naïve WT and IL-10−/− mice were injected intraperitoneally with CFSE-labeled WT or IL-10−/− BMMCs, and their survival was examined. Six days after transfer, a number of labeled WT cells were recovered from the peritoneum of naïve WT mice as previously observed (27, 28) (Fig. 4B). In contrast, none to fewer numbers of transferred WT BMMCs were recovered from the peritoneum of IL-10−/− mice, suggesting that in the absence of IL-10, mast cell survival may be impaired (Fig. 4B). Similarly, while transfer of IL-10−/− BMMCs into the peritoneum of naïve WT mice led to the recovery of a fair number of labeled mast cells 6 days later, fewer labeled IL-10−/− BMMCs could be recovered from the peritoneum of IL-10−/− mice. These data therefore suggest that IL-10 is needed for the expansion of mast cells during physiological responses in vivo and consequently may regulate mast cell homeostasis during the development of food allergy. Furthermore, the greater survival of IL-10−/− BMMCs in WT mice but not IL-10−/− mice suggests that there is no intrinsic defect in IL-10−/− mast cells per se, and that their reduced function in vivo in IL-10−/− mice is likely due to the deficiency in IL-10.
Co-culture with rIL-10 amplifies cytokine production by mast cells and restores cytokine production capacity in IL-10−/− BMMCs
To determine whether IL-10 also affects cytokine production from activated BMMCs, BMMCs were cultured with rIL-10 and the expression of cytokine genes was assessed after activation. As shown in Figs.4C–F, IgE-mediated activation significantly enhanced the expression of TNF-α, IL-13 and IL-4 in WT BMMCs compared to both unactivated controls and similarly activated IL-10−/− BMMCs. In contrast, similar types of differences in IL-6 were not observed. Furthermore, the addition of rIL-10 to IL-10−/− BMMCs restored the capacity of these cells to express IL-4 and IL-13 post-activation. Interestingly however, the treatment of WT BMMCs with rIL-10 did not have a similar effect and actually suppressed the expression of both TNF-α and IL-4 by WT cells (Figs.4C & F). Cytokine secretion as assessed 24 hours later was also affected in a similar manner, with the exception of IL-4, which was produced at comparable levels by both activated WT and IL-10−/− BMMCs (Figs.5A–D).
Figure 5. Co-culture with rIL-10 amplifies cytokine production and restores cytokine production capacity in IL-10−/− BMMCs.
WT and IL-10−/− BMMCs were cultured in the presence or absence of rIL-10 for 24 hours (A–D) or 7 days (E–F). BMMCs were activated with IgE and antigen for 12 hours and cytokine levels were enumerated in cell culture supernatants by ELISA. Data are representative of 3 or more independent experiments. *=p<0.05; **=p<0.01. (student’s t-test).
Interestingly, the BMMC proliferation experiments described above demonstrated that the effects of IL-10 were more pronounced during the expansion phase of these cells (days 4–7), suggesting that prolonged exposure to rIL-10 may further modulate BMMC cytokine production. As such, we next examined whether co-culture of BMMCs with rIL-10 for 7 days would yield different results compared to those observed above. As shown in Fig. 5E, while the constitutive secretion of TNF-α was observed in all groups tested, activation induced enhanced secretion of TNF-α by WT BMMCs compared to unactivated controls. In contrast, no increase was observed in activated IL-10−/− BMMCs compared to unactivated controls (Fig. 5E). Furthermore, and to our surprise, co-culture with rIL-10 for 7 days not only restored the production of TNF-α by IL-10−/− BMMCs to WT levels, but also significantly enhanced its production by WT BMMCs, suggesting that extended exposure to rIL-10 is able to overcome the initial suppression of this cytokine (Fig. 5E). In contrast, while IL-6 and IL-13 were observed in unactivated controls in all the groups tested, no significant enhancement of these cytokines was observed in both WT and IL-10−/− BMMCs after activation (Figs.5F & G). However, co-culture with rIL-10 significantly increased the levels of IL-6 and IL-13 in both WT and IL-10−/− BMMCs irrespective of IgE-mediated activation. This suggests that IL-10 is able to amplify the constitutive production of these cytokines by BMMCs when exposed to it for prolonged periods of time (Figs.5F & G). No such effect was observed in the case of IL-4 production (Fig. 5H). These data therefore suggest that IL-10 can have different effects on the production of cytokines by BMMCs both constitutively and in the context of IgE-mediated activation, and that extended exposure to IL-10 in the context of inflammation may similarly enhance mast cell cytokine production and regulate mast cell function in vivo.
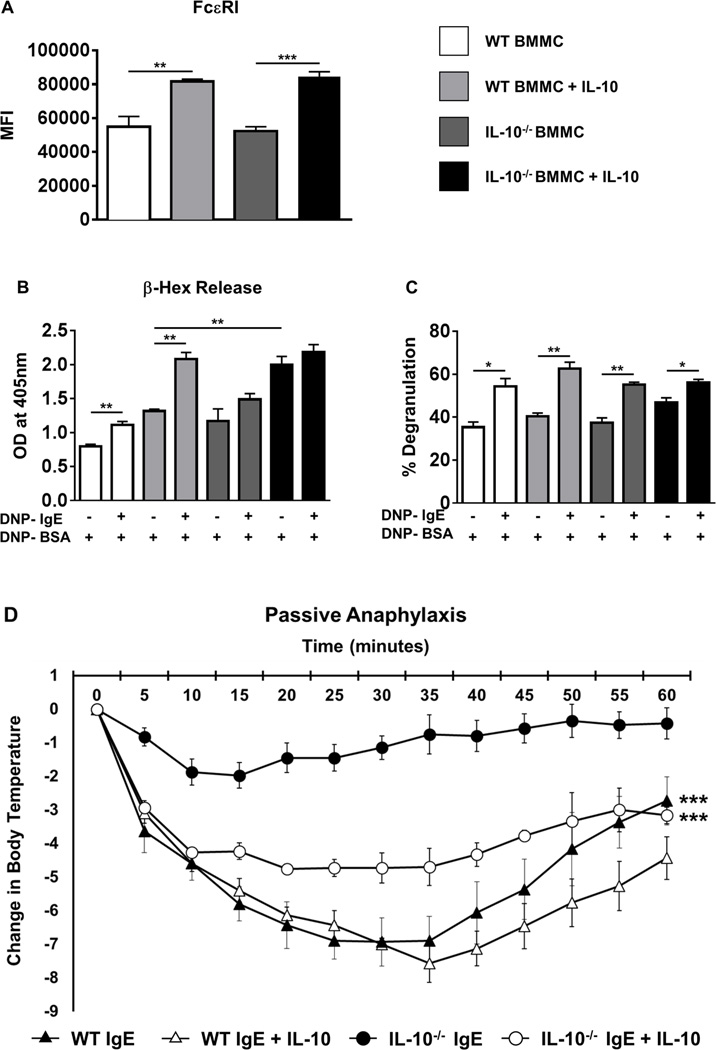
IL-10 promotes expression of FcɛRI, enhances mast cell activation, and is essential for mast cell-mediated passive anaphylaxis in antigen-exposed mice
To determine whether the effects observed above may be due to changes in FcɛRI expression in IL-10−/− BMMCs, we assessed the expression of FcɛRI on BMMCs 4 days after culture. No changes in the MFI of FcɛRI were observed between WT and IL-10−/− BMMCs at this point, suggesting that the activation-induced effects observed above were not due to decreased FcɛRI expression in IL-10−/− BMMCs (Fig. 6A). Surprisingly, however, co-culture with rIL-10 further enhanced the expression of FcɛRI on both WT and IL-10−/− BMMCs in contrast to previously published observations (Fig. 6A). This suggests that IL-10 can enhance the effects of FcɛRI-mediated signaling events by amplifying the expression of FcɛRI on BMMCs in contrast to previous reports (30).
Figure 6. IL-10 enhances BMMC degranulation and restores IgE-mediated passive anaphylaxis in IL-10−/− mice.
(A) BMMCs were cultured in the presence or absence of rIL-10 for 4 days and the levels of FcɛRI expression were assessed by flow cytometry. MFI is shown. (B–C) WT and IL-10−/− BMMCs were cultured in the presence or absence of rIL-10 and activated with IgE and antigen. (B) O.D. values correlating to β-hex release in supernatants and (C) percent degranulation of BMMCs is shown. (D) WT and IL-10−/− mice were passively sensitized with DNP-IgE and challenged with DNP-BSA. A group of WT and IL-10−/− mice received IL-10 prior to sensitization and challenge. Changes in body temperature after challenge are shown. Data are representative of 2 independent experiments. *=p<0.05; **=p<0.01. (student’s t-test). ***=p<0.0001 by ANOVA.
To further investigate the effects of IL-10 on mast cell activation, the extent of mast cell degranulation was examined by measuring release of β-hex into cell culture supernatants. Assessment of optical density revealed that activated WT BMMCs exhibited significantly elevated levels of β-hex compared with unactivated controls (Fig. 6B). In contrast, a similar increase was not observed in activated IL-10−/− BMMCs compared to unactivated controls, suggesting that while IL-10−/− BMMCs constitutively release comparable levels of β-hex as WT BMMCs, IgE-mediated activation does not induce further release of this enzyme. However, IL-10−/− BMMCs had a greater total cellular β-hex content in comparison to WT BMMCs, resulting in increased degranulation as a percent of total by activated IL-10−/− BMMCs (Fig. 6C). Furthermore, while co-culture with rIL-10 resulted in increased constitutive β-hex production by both WT and IL-10−/− BMMCs, activation induced significantly higher release by WT BMMCs as opposed to that observed in IL-10−/− cells (Fig. 6B & C). This suggests that while rIL-10 can enhance the constitutive secretion of β-hex by both WT and IL-10−/− BMMCs, it has no further effect post-activation of IL-10−/− BMMCs.
To assess whether IL-10 has a similar effect on mast cell activation in vivo, WT mice were passively transferred with IgE antibodies and some mice were primed with rIL-10. Antigen exposure induced severe hypothermia in both untreated and IL-10-exposed WT mice, but no further decrease in core body temperature was observed in IL-10-exposed WT animals (Fig. 6D). In contrast, while IL-10−/− mice were resistant to the development of hypothermia after antigen exposure, administration of rIL-10 restored the ability of these animals to undergo passive anaphylaxis (Fig. 6D). These data therefore confirm that IL-10 can influence the extent of mast cell activation and consequently mast cell function both in vitro as well as in vivo.
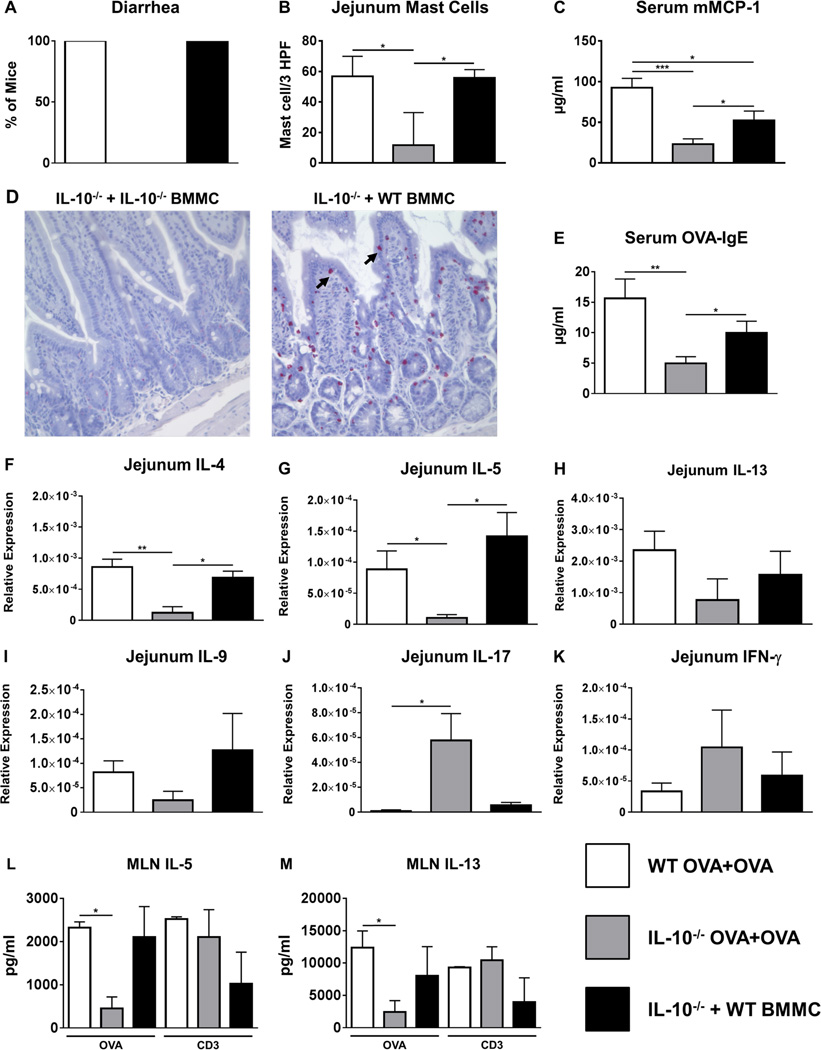
Adoptive transfer of WT BMMCs restores the allergic phenotype in IL-10−/− mice
Since our data demonstrated that IL-10 can affect both mast cell expansion and activation both in cell culture and during food allergy in vivo, we examined whether administration of IL-10−/− mice with WT BMMCs would restore the development of food allergy in IL-10−/− mice. Challenge with OVA induced the development of allergic diarrhea and resulted in increased mast cell numbers in the jejunum, enhanced mMCP-1 levels, and elevated OVA-IgE in WT mice as previously observed (Figs.7A–D). As expected, both IL-10−/− mice as well as IL-10−/− mice reconstituted with IL-10−/− BMMCs mice did not exhibit diarrhea, had fewer intestinal mast cells, and displayed significantly decreased mMCP-1 levels compared to OVA-challenged WT mice (Figs.7A–D and data not shown). Furthermore, decreased levels of OVA-IgE were also observed in these animals contrary to that previously observed (Fig. 1E), suggesting that the production of IgE antibodies may be variable in IL-10−/− mice (Fig. 7E). In contrast, IL-10−/− mice that had been injected with WT BMMCs prior to sensitization not only exhibited diarrhea, but also exhibited significantly higher levels of mMCP-1 and greater intestinal mast cell numbers compared with both IL-10−/− mice and IL-10−/− mice reconstituted with IL-10−/− BMMCs (Figs.7A–D). Three out of five animals had mMCP-1 levels that were comparable to OVA-challenged WT mice. This suggests that the transfer of WT BMMCs can restore the development of allergic symptoms in IL-10−/− mice and enhance mast cell activation and function in challenged animals. Similarly, while OVA-challenged WT and IL-10−/− animals exhibited patterns of intestinal and systemic Th2 cytokine expression and production consistent with our previous observations, the expression of Th2 cytokines in the intestines and their production by OVA-stimulated spleen and MLN cells was also increased in IL-10−/− mice that had been injected with WT BMMCs (Figs.7F–M and Fig. S1). These data therefore suggest that the transfer of WT BMMCs is sufficient to alter the intestinal milieu and induce mast cell-mediated effects such as allergic diarrhea.
Figure 7. Adoptive transfer of WT BMMCs restores allergic diarrhea, mast cell activation and Th2 cytokine production in IL-10−/− mice.
WT and IL-10−/− mice were sensitized and challenged with OVA. A group of IL-10−/− mice received WT BMMCs prior to sensitization. One hour after the 6th challenge, mice were sacrificed. (A) Percent of diarrhea-positive animals (B) intestinal mast cell numbers (C) serum mMCP-1 levels (D) histology depicting CAE-positive cells (mast cells are shown by arrows) (E) serum OVA-IgE levels (F–K) jejunal cytokine expression and (L–M) cytokines secreted by MLN cells in response to OVA stimulation is shown. Data are representative of 2 independent experiments. *=p<0.05; **=p<0.01. (student’s t-test).
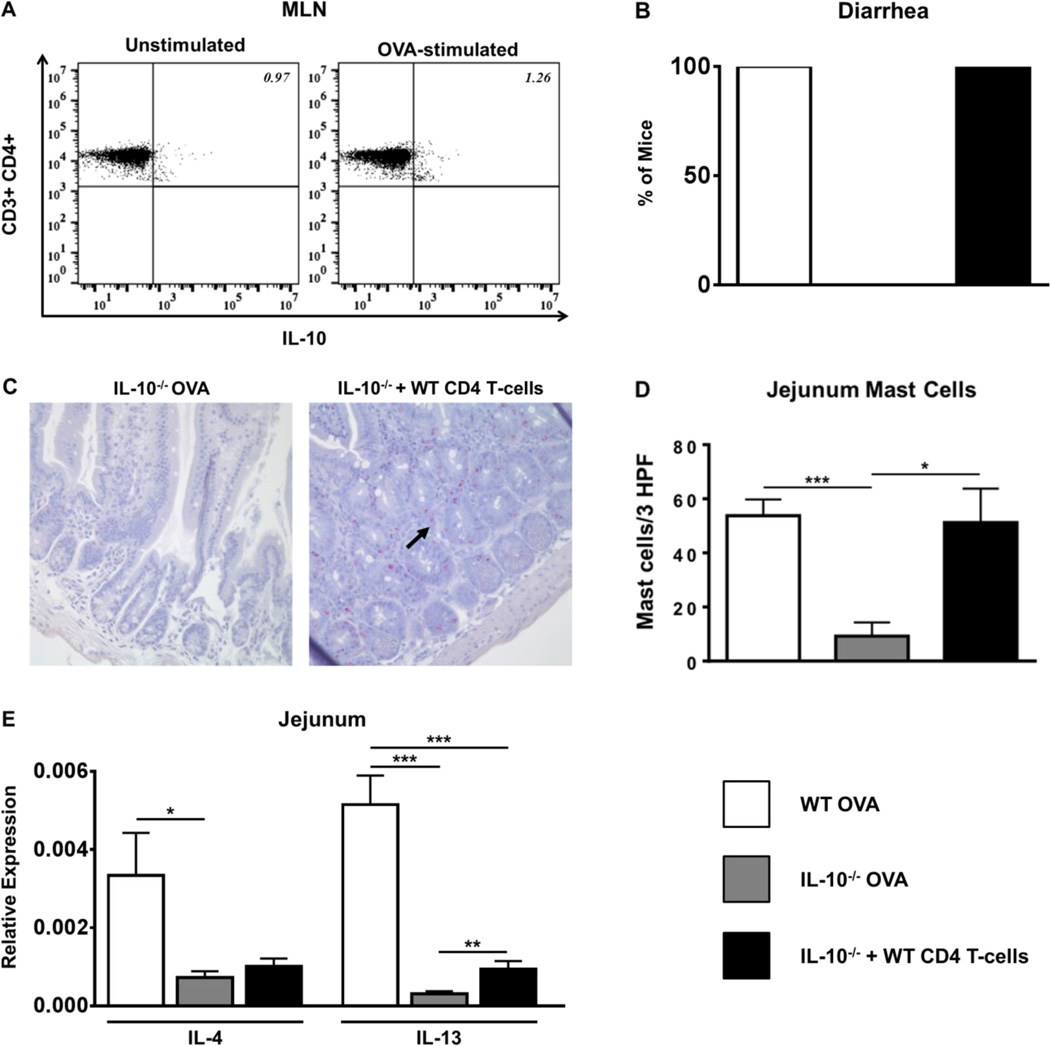
Adoptive transfer of WT CD4 T cells restores allergic diarrhea and intestinal mast cell numbers in IL-10−/− mice
The data described above further confirm the effects of IL-10 during food allergy and suggest that mast cell function is impaired in the absence of IL-10 in IL-10−/− mice. Since Th2 cells are known producers of proinflammatory levels of IL-10 in vivo, we wondered whether the defect in mast cell expansion in IL-10−/− mice may be due to a lack of IL-10-producing Th2 cells during food allergy. To determine whether CD4 T cells can produce IL-10 during food allergy, intracellular staining was performed and both splenic and MLN CD4 T cells from experimental mice were examined for the production of IL-10. Stimulation with OVA resulted in the production of IL-10 by CD4 T cells from both sensitized and OVA-challenged as well as sensitized and OVA-unchallenged BALB/c mice, with slightly higher levels being produced by CD4 T cells from allergic animals (Fig. 8A and data not shown). This suggests that CD4 T cells can produce IL-10 during the development of food allergy in vivo.
Figure 8. Adoptive transfer of WT CD4 T cells restores allergic diarrhea and intestinal mast cell numbers in IL-10−/− mice.
WT and IL-10−/− mice were sensitized and challenged with OVA. A group of IL-10−/− mice received WT CD4 T cells prior to sensitization. One hour after the 6th challenge, mice were sacrificed. (A) WT MLN cells from OVA-sensitized and challenged mice were cultured in the presence or absence of OVA. Intracellular cytokine staining for IL-10 is shown. (B) Percent of diarrhea-positive animals (C) histology depicting CAE-positive cells (mast cells are shown by arrows) (D) intestinal mast cell numbers and (E) jejunal cytokine expression is shown. *=p<0.05; **=p<0.01. (student’s t-test).
To further assess the effects of T cells on the development of food allergy and the expansion of mast cells, we examined whether adoptive transfer of WT CD4 T cells prior to OVA sensitization can restore allergic symptoms in IL-10−/− mice. As expected, OVA challenge resulted in the production of diarrhea in WT BALB/c mice (Fig. 8B), accompanied by increased intestinal mast cell numbers (Fig. 8C & D), and increased expression of Th2 cytokine levels in the intestine (Fig. 8E). In contrast, the absence of diarrhea, and decreased numbers of intestinal mast cells as well as Th2 cytokine expression, were observed in IL-10−/− mice (Figs.8B–E). Reconstitution with WT CD4 T cells not only restored the development of diarrhea (Fig. 8B), but significantly increased the numbers of intestinal mast cells comparable to those observed in WT mice (Figs.8C & D). However, no increase in mMCP-1 levels was observed compared to unreconstituted IL-10−/− animals (data not shown), suggesting that while WT CD4 T cells can promote intestinal mast cell expansion, they are not able to overcome the need for the continuous presence of IL-10, which is required to induce mMCP-1 transcription as previously shown (31). Similarly, while reconstitution increased the intestinal expression of IL-13 compared to IL-10−/− mice, similar increases in IL-4 or IL-5 were not observed in these animals (Fig. 8E and data not shown). Taken together, these data suggest that CD4 T cells can produce IL-10 during the development of food allergy, and the expansion of mast cells in vivo as well as mast cell-mediated effects such as allergic diarrhea may be regulated by IL-10-producing CD4 T cells.
Discussion
IL-10 is an important immunoregulatory cytokine exerting pleiotropic effects on immune responses, depending on the type of response (Th1 versus Th2) and the site of antigen exposure (8, 10, 11, 32, 33). In this study, we demonstrate an important role for IL-10 in regulating the expansion and activation of mast cells and consequently the development of mast cell-mediated food allergy. This is a novel finding, which suggests that IL-10 can drive mucosal mast cell function, and modulate the development of intestinal anaphylaxis. Furthermore, we demonstrate that the regulation of intestinal mast cell expansion by IL-10 in vivo may be CD4 T cell-dependent.
The role of IL-10 in regulating Th2-mediated allergic responses has been controversial. Allergic patients produce elevated levels of IL-10 (34–36), and we have previously observed increased intestinal expression of IL-10 during food allergy (6). Animal models suggest that IL-10 can have discordant effects on the development of allergy (17–20, 24, 37). While some studies suggest that IL-10 can promote eosinophilia, IL-5 production and AHR (19, 20, 22), others showed that it only enhances AHR and not inflammation (18). Similarly, the transgenic expression of IL-10 can induce mucus metaplasia, tissue inflammation and airway remodeling (23). However, Th2 cells engineered to produce IL-10 can prevent the development of AHR and inflammation during asthma (24). Using a different model system, Laouini et al. demonstrated that IL-10 is not only critical for the development of eosinophilia during atopic dermatitis, but also skews dendritic cell function towards a Th2 response (26). Lastly, while the role of IL-10 in food allergy has not been previously examined, Kraal and colleagues showed that neutralizing IL-10 can block allergen-induced changes in IgE and histamine production after intragastric challenge (38). Similarly, preliminary studies in our laboratory currently under investigation suggest that IL-10 neutralization may block allergic diarrhea and intestinal mast cell numbers during food allergy (unpublished data). While some of these discrepant observations may be attributed to differences in strain genetic background (39) or impairment of IL-13 function in the absence of IL-10 via the IL-13Rα2 decoy receptor (we do not observe expression of this receptor in the intestines of OVA-challenged mice, unpublished observation), it does not explain the complete range of effects mediated by IL-10, and the literature suggests a more nuanced role for IL-10 in immune regulation depending on the antigen, the target organ, and the cell type producing IL-10.
The results from this study demonstrate for the first time a proinflammatory role for IL-10 in regulating mast cell function both in cell culture and in food allergy. Our data are not only consistent with the proinflammatory effects of IL-10 described above on Th2-mediated allergic responses, but are all the more interesting in light of early observations that indicate that IL-10 can have a stimulatory effect on BMMCs (40, 41) and induce the expression of mast cell proteases such as mMCP-1 (31, 42–44). It is well established that the expansion of mucosal mast cells is Th2-dependent, and that Th2 cytokines drive the proliferation of mast cells in mucosal tissues (45–47). Our data suggest that during food allergy, IL-10 promotes mast cell activation, resulting in diminished intestinal anaphylaxis in IL-10−/− mice. While WT BALB/c mice, which are known to generate strong Th2 responses, exhibited severe disease and enhanced mastocytosis, the development of disease including allergic diarrhea was weakened in the absence of IL-10, and IL-10−/− mice failed to mount strong mast cell responses. This was also accompanied by attenuated local Th2 responses, suggesting that in the absence of IL-10, allergen-specific Th2 cells may become functionally defective and not fully participate in the allergic response. That these cells retain the capacity to produce Th2 cytokines and are not intrinsically defective is suggested by observations that the response to polyclonal T cell stimulation is intact, and that the production of OVA-IgE, which is dependent on TFH and Th2 cells, is not fundamentally impaired. Taken together, these observations suggest that IL-10 produced by pathogenic cells (potentially including Th2 cells) may enhance the allergic phenotype during food allergy. In particular, IL-10 may be required for the activation of mucosal mast cells, and exert its effects to potentiate mast cell function.
While the data from IL-10−/− mice suggest a global effect on both Th2 and mast cell responses, our cell culture data provides concrete evidence for the role of IL-10 in regulating mast cell function. In the absence of IL-10, IL-10−/− BMMCs are unable to expand to levels observed in WT BMMCs despite the presence of similar levels of IL-3 and SCF, but the addition of rIL-10 restores their proliferative capacity, suggesting that IL-10 can enhance the proliferation of mast cells. Furthermore, while activated mast cells produced elevated levels of cytokines such as TNF-α and IL-13, the production of these cytokines was suppressed in IL-10−/− BMMCs and could be restored by the addition of rIL-10. Addition of rIL-10 not only restored the functional capacity of IL-10−/− BMMCs, but also amplified the production of these cytokines by WT BMMCs when exposed to rIL-10 for one week. This may be a combination of the specific effects of IL-10 on mast cell activation as well as the increased cell number following co-culture with rIL-10.
Taken together, these data therefore suggest that IL-10 not only modulates the production of cytokines by mast cells, but can have differing effects depending on the cell culture conditions and the time of exposure. In this context, the expression and secretion of TNF-α after co-culture with rIL-10 was especially interesting with both decreased and increased secretion of the cytokine observed after co-culture for short or long periods of time. These data parallel observations reported by Marshall et al. regarding the effect of IL-10 on TNF-α production by rat peritoneal mast cells (48). These investigators found that IL-10 did not induce changes in TNF-α secretion 10 minutes after IgE activation, but induced decreases in TNF-α 18 hours later, similar to our own observations in Figs. 4C&5A. Our data further suggest that continued co-culture with rIL-10 reverses this initial inhibition resulting in increased secretion of TNF-α by day 7 of culture.
This suggests that the regulatory effects of IL-10 are conditioned by the nature of the inflammatory environment, and that different effects may be observed depending on the type of stimulus, the cell culture conditions and the genetic background of the cells used (30, 49–51). This is an especially important consideration in light of immunotherapeutic approaches that target IL-10 as the molecule of choice for circumvention of allergen sensitization. For example, Ryan and colleagues have previously demonstrated that IL-10 prevents IgE-mediated activation of mast cells, inhibits FcɛRI expression, and induces their apoptosis (51–53). However, these investigators used a different strain of mouse (C57BL/6) and different cell culture conditions to grow BMMCs (supplementation with WEHI-3 medium vs. rIL-3+rSCF). Our findings provide evidence for the direct opposite, suggesting that continuous exposure to IL-10 can induce mast cell proliferation and function, and confirms previously published reports regarding the proinflammatory role of IL-10 on mast cells and its requirement for the induction of mMCP-1 expression (31, 40, 41). Notably, these investigators also demonstrated that the effects of IL-10 were pronounced in the presence of IL-3 or IL-4, similar to our own observations, and that continuous exposure to IL-10 is required to induce mMCP-1 transcription (31). Furthermore, our data provides strong evidence that IL-10 can enhance mast cell expansion and promote allergic responses in BALB/c mice, which are known to have a strong Th2 bias. This is not only consistent with previous observations such as the ability of IL-10 to promote allergen-specific Th2 responses in BALB/c mice (26), but is also reflective of IgE-mediated allergies in atopic human patients, wherein IL-10 may exert proinflammatory effects on both mast cells and Th2 cells during allergic responses. Thus, the effects of IL-10 during inflammatory conditions in vivo may be more nuanced depending on the immune microenvironment and the host genetic background. Indeed, Ryan and colleagues recently reported that mast cell responsiveness to IL-10 may be altered by genetic background and that mast cells from BALB/c mice are resistant to IL-10 mediated apoptosis in contrast to previously reported observations (54).
The proinflammatory effects of IL-10 on mast cell homeostasis in vivo may be further confirmed from our experiments demonstrating that mast cells transferred into the peritoneal cavity of IL-10−/− mice fail to survive, suggesting that in the absence of IL-10, mast cells are unable to reach their full proliferative potential. That IL-10−/− BMMCs are able to survive in the peritoneum of naïve WT mice but not that of naïve IL-10−/− mice suggests that there is no intrinsic defect in the proliferative capacity of IL-10−/− BMMCs per se, but that the presence of IL-10 is required to promote their proliferation and/or survival in vivo, and that the reduced survival of IL-10−/− BMMCs in IL-10−/− mice is likely due to the absence of IL-10.
The data from the β-hex and passive anaphylaxis studies show that IL-10 not only promotes mast cell expansion and function, but also modulates the sensitivity of mast cells to antigen exposure. Interestingly, no differences in β-hex production were observed between both WT and IL-10−/− BMMCs prior to activation, suggesting that in the absence of IL-10, there is no intrinsic defect in the capacity of BMMCs to be activated and undergo degranulation. This is consistent with similar levels of FcɛRI expression on BMMCs from both WT and IL-10−/− mice. However, IL-10 appears to affect activation-induced events such as the production of cytokines and IgE-mediated passive anaphylaxis. Exposure to rIL-10 not only enhanced the expression of FcɛRI and the activation of mast cells in cell culture, but also restored the ability of IL-10−/− mice to undergo IgE-dependent passive anaphylaxis, suggesting that in vivo, IL-10 may prime mast cells for activation during antigen exposure and acute allergic episodes.
Finally, in light of these observations, it will be important to further elucidate the cellular sources of IL-10 and determine the cell types responsible for stimulating mast cell expansion in an IL-10-dependent manner. While Th2 cells are the prime candidate for the source of IL-10 during the intestinal allergic response, a number of other cell types can produce IL-10 including B cells, macrophages, mast cells, NKT cells, Tregs and intraepithelial lymphocytes (55). Our data suggest that the adoptive transfer of WT IL-10+ mast cells can restore allergic diarrhea as well as Th2 cytokine production, while partially restoring mMCP-1 levels, the expression of which has been shown to be IL-10-dependent. Furthermore, the adoptive transfer of WT IL-10+ CD4 T cells was also sufficient to restore allergic diarrhea and intestinal mast cell expansion, suggesting that the regulation of intestinal mast cell function by IL-10 is T cell-dependent. Future experiments aimed at assessing the contribution of IL-10-producing cells will shed further light on the interactions between various cells such as Th2 cells and mast cells during the allergic response.
Taken together, our data demonstrates that IL-10 promotes the expansion of mucosal mast cells and influences their function during food allergy, elucidating a novel role for IL-10 in the regulation of mast cell-mediated responses and suggesting that perturbations of IL-10 activity may modulate the outcome of the allergic response in susceptible individuals.
Supplementary Material
Acknowledgments
This project was supported by funds from the National Institutes of Health grants: NIAID R15AI107668 (CBM).
Abbreviations Used
- OVA
Ovalbumin
- WT
Wild-type
- BMMCs
Bone marrow derived mast cells
- MLN
Mesenteric Lymph Nodes
References
- 1.Brandt EB, Strait RT, Hershko D, Wang Q, Muntel EE, Scribner TA, Zimmermann N, Finkelman FD, Rothenberg ME. Mast cells are required for experimental oral allergen-induced diarrhea. J Clin Invest. 2003;112:1666–1677. doi: 10.1172/JCI19785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnston LK, Chien KB, Bryce PJ. The immunology of food allergy. J Immunol. 2014;192:2529–2534. doi: 10.4049/jimmunol.1303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burton OT, Darling AR, Zhou JS, Noval-Rivas M, Jones TG, Gurish MF, Chatila TA, Oettgen HC. Direct effects of IL-4 on mast cells drive their intestinal expansion and increase susceptibility to anaphylaxis in a murine model of food allergy. Mucosal immunology. 2013;6:740–750. doi: 10.1038/mi.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forbes EE, Groschwitz K, Abonia JP, Brandt EB, Cohen E, Blanchard C, Ahrens R, Seidu L, McKenzie A, Strait R, Finkelman FD, Foster PS, Matthaei KI, Rothenberg ME, Hogan SP. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen CY, Lee JB, Liu B, Ohta S, Wang PY, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, Rothenberg ME, Finkelman FD, Hogan SP, Wang YH. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity. 2015;43:788–802. doi: 10.1016/j.immuni.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mathias CB, Hobson SA, Garcia-Lloret M, Lawson G, Poddighe D, Freyschmidt EJ, Xing W, Gurish MF, Chatila TA, Oettgen HC. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J Allergy Clin Immunol. 2011;127:795–805. doi: 10.1016/j.jaci.2010.11.009. e791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rennick D, Berg D, Holland G. Interleukin 10: an overview. Prog Growth Factor Res. 1992;4:207–227. doi: 10.1016/0955-2235(92)90020-i. [DOI] [PubMed] [Google Scholar]
- 8.Moore KW, O'Garra A, de Waal Malefyt R, Vieira P, Mosmann TR. Interleukin-10. Annu Rev Immunol. 1993;11:165–190. doi: 10.1146/annurev.iy.11.040193.001121. [DOI] [PubMed] [Google Scholar]
- 9.Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 10.Li MO, Flavell RA. Contextual regulation of inflammation: a duet by transforming growth factor-beta and interleukin-10. Immunity. 2008;28:468–476. doi: 10.1016/j.immuni.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Maynard CL, Weaver CT. Diversity in the contribution of interleukin-10 to T-cell-mediated immune regulation. Immunol Rev. 2008;226:219–233. doi: 10.1111/j.1600-065X.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann TR. Role of a new cytokine, interleukin-10, in the cross-regulation of T helper cells. Ann N Y Acad Sci. 1991;628:337–344. doi: 10.1111/j.1749-6632.1991.tb17266.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohmori H, Kanda T, Takai T, Hikida M. Induction of antigen-specific IgE response in murine lymphocytes by IL-10. Immunol Lett. 1995;47:127–132. doi: 10.1016/0165-2478(95)00084-i. [DOI] [PubMed] [Google Scholar]
- 14.Jeannin P, Lecoanet S, Delneste Y, Gauchat JF, Bonnefoy JY. IgE versus IgG4 production can be differentially regulated by IL-10. J Immunol. 1998;160:3555–3561. [PubMed] [Google Scholar]
- 15.Kobayashi N, Nagumo H, Agematsu K. IL-10 enhances B-cell IgE synthesis by promoting differentiation into plasma cells, a process that is inhibited by CD27/CD70 interaction. Clin Exp Immunol. 2002;129:446–452. doi: 10.1046/j.1365-2249.2002.01932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosaka S, Tamauchi H, Terashima M, Maruyama H, Habu S, Kitasato H. IL-10 controls Th2-type cytokine production and eosinophil infiltration in a mouse model of allergic airway inflammation. Immunobiology. 216:811–820. doi: 10.1016/j.imbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 18.Makela MJ, Kanehiro A, Borish L, Dakhama A, Loader J, Joetham A, Xing Z, Jordana M, Larsen GL, Gelfand EW. IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci U S A. 2000;97:6007–6012. doi: 10.1073/pnas.100118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Scott MR, Justice JP, Bradfield JF, Enright E, Sigounas A, Sur S. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278:L667–L674. doi: 10.1152/ajplung.2000.278.4.L667. [DOI] [PubMed] [Google Scholar]
- 20.Yang X, Wang S, Fan Y, Han X. IL-10 deficiency prevents IL-5 overproduction and eosinophilic inflammation in a murine model of asthma-like reaction. Eur J Immunol. 2000;30:382–391. doi: 10.1002/1521-4141(200002)30:2<382::AID-IMMU382>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Bellinghausen I, Knop J, Saloga J. The role of interleukin 10 in the regulation of allergic immune responses. Int Arch Allergy Immunol. 2001;126:97–101. doi: 10.1159/000049499. [DOI] [PubMed] [Google Scholar]
- 22.Justice JP, Shibata Y, Sur S, Mustafa J, Fan M, Van Scott MR. IL-10 gene knockout attenuates allergen-induced airway hyperresponsiveness in C57BL/6 mice. Am J Physiol Lung Cell Mol Physiol. 2001;280:L363–L368. doi: 10.1152/ajplung.2001.280.2.L363. [DOI] [PubMed] [Google Scholar]
- 23.Lee CG, Homer RJ, Cohn L, Link H, Jung S, Craft JE, Graham BS, Johnson TR, Elias JA. Transgenic overexpression of interleukin (IL)-10 in the lung causes mucus metaplasia, tissue inflammation, and airway remodeling via IL-13-dependent and -independent pathways. J Biol Chem. 2002;277:35466–35474. doi: 10.1074/jbc.M206395200. [DOI] [PubMed] [Google Scholar]
- 24.Oh JW, Seroogy CM, Meyer EH, Akbari O, Berry G, Fathman CG, Dekruyff RH, Umetsu DT. CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J Allergy Clin Immunol. 2002;110:460–468. doi: 10.1067/mai.2002.127512. [DOI] [PubMed] [Google Scholar]
- 25.Koya T, Matsuda H, Takeda K, Matsubara S, Miyahara N, Balhorn A, Dakhama A, Gelfand EW. IL-10-treated dendritic cells decrease airway hyperresponsiveness and airway inflammation in mice. J Allergy Clin Immunol. 2007;119:1241–1250. doi: 10.1016/j.jaci.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Laouini D, Alenius H, Bryce P, Oettgen H, Tsitsikov E, Geha RS. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J Clin Invest. 2003;112:1058–1066. doi: 10.1172/JCI18246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinney SR, Carlson L, Ser-Dolansky J, Thompson C, Shah S, Gambrah A, Xing W, Schneider SS, Mathias CB. Curcumin Ingestion Inhibits Mastocytosis and Suppresses Intestinal Anaphylaxis in a Murine Model of Food Allergy. PLoS One. 2015;10:e0132467. doi: 10.1371/journal.pone.0132467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathias CB, Freyschmidt EJ, Caplan B, Jones T, Poddighe D, Xing W, Harrison KL, Gurish MF, Oettgen HC. IgE influences the number and function of mature mast cells, but not progenitor recruitment in allergic pulmonary inflammation. J Immunol. 2009;182:2416–2424. doi: 10.4049/jimmunol.0801569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalesnikoff J, Huber M, Lam V, Damen JE, Zhang J, Siraganian RP, Krystal G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity. 2001;14:801–811. doi: 10.1016/s1074-7613(01)00159-5. [DOI] [PubMed] [Google Scholar]
- 30.Gillespie SR, DeMartino RR, Zhu J, Chong HJ, Ramirez C, Shelburne CP, Bouton LA, Bailey DP, Gharse A, Mirmonsef P, Odom S, Gomez G, Rivera J, Fischer-Stenger K, Ryan JJ. IL-10 inhibits Fc epsilon RI expression in mouse mast cells. J Immunol. 2004;172:3181–3188. doi: 10.4049/jimmunol.172.5.3181. [DOI] [PubMed] [Google Scholar]
- 31.Ghildyal N, McNeil HP, Stechschulte S, Austen KF, Silberstein D, Gurish MF, Somerville LL, Stevens RL. IL-10 induces transcription of the gene for mouse mast cell protease-1, a serine protease preferentially expressed in mucosal mast cells of Trichinella spiralis-infected mice. J Immunol. 1992;149:2123–2129. [PubMed] [Google Scholar]
- 32.Gabrysova L, Howes A, Saraiva M, O'Garra A. The regulation of IL-10 expression. Curr Top Microbiol Immunol. 2014;380:157–190. doi: 10.1007/978-3-662-43492-5_8. [DOI] [PubMed] [Google Scholar]
- 33.Saraiva M, O'Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 34.Robinson DS, Tsicopoulos A, Meng Q, Durham S, Kay AB, Hamid Q. Increased interleukin-10 messenger RNA expression in atopic allergy and asthma. Am J Respir Cell Mol Biol. 1996;14:113–117. doi: 10.1165/ajrcmb.14.2.8630259. [DOI] [PubMed] [Google Scholar]
- 35.Maciorkowska E, Dzieciol J, Kemona A, Kaczmarski M. Evaluation of selected cytokines and mononuclear cell infiltration in gastric mucosa of children with food allergy. Med Sci Monit. 2000;6:567–572. [PubMed] [Google Scholar]
- 36.Lamblin C, Desreumaux P, Colombel JF, Tonnel AB, Wallaert B. Overexpression of IL-10 mRNA in gut mucosa of patients with allergic asthma. J Allergy Clin Immunol. 2001;107:739–741. doi: 10.1067/mai.2001.114111. [DOI] [PubMed] [Google Scholar]
- 37.Kabbur PM, Carson WFt, Guernsey L, Secor ER, Jr, Thrall RS, Schramm CM. Interleukin-10 does not mediate inhalational tolerance in a chronic model of ovalbumin-induced allergic airway disease. Cell Immunol. 2006;239:67–74. doi: 10.1016/j.cellimm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 38.van Halteren AG, van der Cammen MJ, Biewenga J, Savelkoul HF, Kraal G. IgE and mast cell response on intestinal allergen exposure: a murine model to study the onset of food allergy. J Allergy Clin Immunol. 1997;99:94–99. doi: 10.1016/s0091-6749(97)70305-1. [DOI] [PubMed] [Google Scholar]
- 39.Grunig G, Corry DB, Leach MW, Seymour BW, Kurup VP, Rennick DM. Interleukin-10 is a natural suppressor of cytokine production and inflammation in a murine model of allergic bronchopulmonary aspergillosis. J Exp Med. 1997;185:1089–1099. doi: 10.1084/jem.185.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson-Snipes L, Dhar V, Bond MW, Mosmann TR, Moore KW, Rennick DM. Interleukin 10: a novel stimulatory factor for mast cells and their progenitors. J Exp Med. 1991;173:507–510. doi: 10.1084/jem.173.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rennick D, Hunte B, Dang W, Thompson-Snipes L, Hudak S. Interleukin-10 promotes the growth of megakaryocyte, mast cell, and multilineage colonies: analysis with committed progenitors and Thy1loSca1+ stem cells. Exp Hematol. 1994;22:136–141. [PubMed] [Google Scholar]
- 42.Eklund KK, Ghildyal N, Austen KF, Friend DS, Schiller V, Stevens RL. Mouse bone marrow-derived mast cells (mBMMC) obtained in vitro from mice that are mast cell-deficient in vivo express the same panel of granule proteases as mBMMC and serosal mast cells from their normal littermates. J Exp Med. 1994;180:67–73. doi: 10.1084/jem.180.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghildyal N, Friend DS, Nicodemus CF, Austen KF, Stevens RL. Reversible expression of mouse mast cell protease 2 mRNA and protein in cultured mast cells exposed to IL-10. J Immunol. 1993;151:3206–3214. [PubMed] [Google Scholar]
- 44.Stevens RL, Friend DS, McNeil HP, Schiller V, Ghildyal N, Austen KF. Strain-specific and tissue-specific expression of mouse mast cell secretory granule proteases. Proc Natl Acad Sci U S A. 1994;91:128–132. doi: 10.1073/pnas.91.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee F, Yokota T, Otsuka T, Meyerson P, Villaret D, Coffman R, Mosmann T, Rennick D, Roehm N, Smith C, et al. Isolation and characterization of a mouse interleukin cDNA clone that expresses B-cell stimulatory factor 1 activities and T-cell- and mast-cell-stimulating activities. Proc Natl Acad Sci U S A. 1986;83:2061–2065. doi: 10.1073/pnas.83.7.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mosmann TR, Bond MW, Coffman RL, Ohara J, Paul WE. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986;83:5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith CA, Rennick DM. Characterization of a murine lymphokine distinct from interleukin 2 and interleukin 3 (IL-3) possessing a T-cell growth factor activity and a mast-cell growth factor activity that synergizes with IL-3. Proc Natl Acad Sci U S A. 1986;83:1857–1861. doi: 10.1073/pnas.83.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall JS, Leal-Berumen I, Nielsen L, Glibetic M, Jordana M. Interleukin (IL)-10 inhibits long-term IL-6 production but not preformed mediator release from rat peritoneal mast cells. J Clin Invest. 1996;97:1122–1128. doi: 10.1172/JCI118506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arock M, Zuany-Amorim C, Singer M, Benhamou M, Pretolani M. Interleukin-10 inhibits cytokine generation from mast cells. Eur J Immunol. 1996;26:166–170. doi: 10.1002/eji.1830260126. [DOI] [PubMed] [Google Scholar]
- 50.Kennedy Norton S, Barnstein B, Brenzovich J, Bailey DP, Kashyap M, Speiran K, Ford J, Conrad D, Watowich S, Moralle MR, Kepley CL, Murray PJ, Ryan JJ. IL-10 suppresses mast cell IgE receptor expression and signaling in vitro and in vivo. J Immunol. 2008;180:2848–2854. doi: 10.4049/jimmunol.180.5.2848. [DOI] [PubMed] [Google Scholar]
- 51.Mirmonsef P, Shelburne CP, Fitzhugh Yeatman C, 2nd, Chong HJ, Ryan JJ. Inhibition of Kit expression by IL-4 and IL-10 in murine mast cells: role of STAT6 and phosphatidylinositol 3'-kinase. J Immunol. 1999;163:2530–2539. [PubMed] [Google Scholar]
- 52.Kashyap M, Thornton AM, Norton SK, Barnstein B, Macey M, Brenzovich J, Shevach E, Leonard WJ, Ryan JJ. Cutting edge: CD4 T cell-mast cell interactions alter IgE receptor expression and signaling. J Immunol. 2008;180:2039–2043. doi: 10.4049/jimmunol.180.4.2039. [DOI] [PubMed] [Google Scholar]
- 53.Yeatman CF, 2nd, Jacobs-Helber SM, Mirmonsef P, Gillespie SR, Bouton LA, Collins HA, Sawyer ST, Shelburne CP, Ryan JJ. Combined stimulation with the T helper cell type 2 cytokines interleukin (IL)-4 and IL-10 induces mouse mast cell apoptosis. J Exp Med. 2000;192:1093–1103. doi: 10.1084/jem.192.8.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ryan MKaJ. Mast cell responsiveness to IL-10 is altered by genetic background. The Journal of Immunology. 2011;186 151.14. [Google Scholar]
- 55.Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.