Abstract
OBJECTIVES
To compare clinical and economic outcomes of early insulin initiation with those of delayed initiation in older adults with type 2 diabetes mellitus (T2DM).
DESIGN
Retrospective cohort study.
SETTING
Humana Medicare Advantage health insurance plan.
PARTICIPANTS
Older (≥65) Medicare beneficiaries with T2DM.
MEASUREMENTS
Subjects were grouped according to number of classes of oral antidiabetes drugs (OADs) they had taken before initiation of insulin: one (early insulin initiators), two, or three or more (delayed insulin initiators). One-year follow-up outcomes included change in glycosylated hemoglobin (HbA1c), percentage of older adults with HbA1c less than 8.0%, hypoglycemic events, and total healthcare costs.
RESULTS
Overall, 14,669 individuals were included in the analysis. Baseline and 1-year follow-up HbA1c levels were available for 4,028 (27.5%) individuals. Insulin was initiated early in 32% and delayed in 20%. At follow-up, unadjusted reduction in HbA1c was 0.9 ± 3.7% for the group with one OAD, 0.7 ± 2.4% for those with two, and 0.5 ± 3.6% for those with three or more. Early insulin initiation was associated with significantly greater reduction in HbA1c (0.4%; adjusted P <.001), 30% greater likelihood of achieving HbA1c less than 8.0% (adjusted odds ratio = 1.30, 95% confidence interval = 1.18–1.43), and no significant differences in total costs or hypoglycemia events (11.5% of early initiators vs 10.2% of delayed initiators; P = .32).
CONCLUSION
This study suggests beneficial effects of early insulin initiation in older adults with T2DM who do not have adequate glycemic control, without increasing the risk of hypoglycemia or greater total direct healthcare costs.
Keywords: early insulin initiation, diabetes, delayed insulin initiation, hypoglycemia
Glycemic control measured according to glycosylated hemoglobin (HbA1c) levels is the hallmark of management in all individuals with type 2 diabetes mellitus (T2DM), including those aged 65 and older.1 Despite the proven clinical benefits of early insulin initiation, oral antidiabetes drugs (OADs) remain the main treatment regimen, 2 even when HbA1c goals are not reached.3 Early insulin initiation in individuals with newly diagnosed T2DM has been found to result in long-term maintenance of glycemic control.4 A randomized trial comparing first-line insulin therapy with metformin found significant improvements in HbA1c control in individuals taking insulin, without greater risk of asymptomatic hypoglycemia.5 Other benefits of early insulin initiation include protection of pancreatic beta-cell function, better acute insulin response, alteration of disease progression,6 and prevention of damage to organs over the long term.7
Nevertheless, insulin use remains suboptimal in real-world clinical practice settings and is often considered a last resort for the management of T2DM.8 A study from the United Kingdom of insulin-naive individuals aged 40 and older who were unable to achieve glycemic control with OADs reported a delay in insulin initiation of longer than 5 years, even in those with severe diabetes mellitus.9 According to a 2007 to 2009 U.S. National Health Interview Survey of individuals diagnosed with diabetes mellitus, 12% received insulin only and 14% received insulin and OADs,10 suggesting the underuse of insulin.
Insulin initiation in older (≥65) adults can be challenging because clinical practitioners need to balance HbA1c goals with the physical, emotional, and cognitive functioning of the individual.11,12 In a Consensus Development Conference on Diabetes and Older Adults, the American Diabetes Association outlined six specific geriatric syndromes for consideration when setting targets for HbA1c control.13 Although there have been some studies on factors associated with delayed insulin initiation in individuals with T2DM,14,15 they have focused on the attitudes and preferences of healthcare providers and participants. None have examined individual-level complexities that may influence the relationship between insulin initiation and outcomes in a real-world setting. To the best of the knowledge of the authors of the current study, the association between early insulin initiation and treatment outcomes after controlling for complexities specific to older adults has not been previously evaluated.
The primary objective of this study was to investigate real-world clinical and economic outcomes resulting from early versus delayed insulin initiation in older adults with T2DM using a multivariate framework that adjusted for clinical, demographic, and insurance characteristics; healthcare use; and individual-level complexities specific to the older adults.
METHODS
Study Design and Data Source
This was a retrospective cohort study of older adults with T2DM using data derived from claims by Medicare beneficiaries enrolled in Humana Medicare Advantage Prescription Drug (MAPD) plans between January 1, 2007, and March 31, 2012. The database includes participant enrollment information and medical and pharmacy claims for more than 12 million current and previous Humana members (Medicare, commercial, and Medicaid insurance types). Laboratory results were available for approximately 30% of the members. The index date was defined as the insulin initiation date, and the baseline period was defined as the period 6 months before the index date. The follow-up period was defined as 1 year after the index date.
Study Population
The study population comprised older adults with T2DM initiated on basal insulin between July 2007 and December 2010. Participants had one or more inpatient visits or two or more physician visits that were 30 days or more apart and a primary or secondary diagnosis of T2DM (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 250.x0 or 250.x2). Additional inclusion criteria were 18 months of continuous eligibility for medical and pharmacy benefits (6 months before (baseline period) and 1 year after (follow-up period) the index date); one or more OAD prescriptions; and no prescription for pramlintide, a glucagon-like peptide-1 agonist, or any insulin during the baseline period. For the HbA1c analysis, an additional inclusion criterion was the availability of HbA1c data during the baseline and follow-up periods.
Independent Variable: Insulin Initiation Categories
Because of lack of information regarding diabetes mellitus duration in the claim data, number of OADs before insulin initiation was used as a proxy for disease progression. This was based on the previous16 and current ADA guideline on pharmacological therapy for hyperglycemia in T2DM,17 in that participants were given a recommendation to start monotherapy with OAD (metformin or sulfonylureas) first and that insulin may be initiated by adding to an OAD or subsequently combinations of OADs. Older adults were grouped into three categories based on number of OADs used during the baseline period (before insulin initiation). OAD classes such as metformin, sulfonylureas, thiazolidinediones, dipeptidyl peptidase-4 inhibitors, alpha glucosidase, and meglitinides were identified based on filled pharmacy prescription claims. Participants were grouped based on their OAD use before insulin initiation (1 (early insulin initiators), 2, ≥3 (delayed insulin initiators)).
Other Independent Variables
Clinical Characteristics
Using the consensus statement for diabetes care in older adults that the ADA published in 2012,13 complexities specific to older adults were defined as the presence or absence of five geriatric syndromes: cognitive impairment, depression screening, falls risk, polypharmacy, and urinary incontinence. These syndromes were identified using clinical diagnosis data available in the medical claims during the baseline period. Cognitive impairment was identified based on the presence of Alzheimer’s disease, dementia, Huntington’s disease, Parkinson’s disease, schizophrenia, bipolar disorder, and psychosis. Presence of major depressive disorder was used as a proxy for depression screening. Falls history, identified using ICD-9-CM E-codes18 and V-codes,19 was a proxy for falls and falls risk. Polypharmacy was based on concomitant use of multiple drugs in a 90-day period and defined as the number of drugs more than 1 standard deviation (SD) above the mean.20 The mean number of drugs prescribed ± SD was 9 ± 4 in the study population, so participants taking 13 or more drugs during a 90-day period during the baseline period were defined as having polypharmacy. Urinary incontinence was identified using ICD-9-CM diagnosis codes.21 The adapted Diabetes Complications Severity Index (aDCSI) 22 was used to measure severity of diabetes mellitus. Co-occurring conditions were defined according to the following hierarchy: dominant, concordant, and discordant conditions, using the framework specifically developed for “diabetes care within the context of comorbid chronic conditions,”23 but because of considerable overlap between the concordant and discordant conditions with the aDCSI and measures of complexities specific to older adults, only the presence of dominant conditions (cancer) was used to define co-occurring conditions. Baseline hypoglycemia was defined as any type of hypoglycemia event (inpatient, outpatient, emergency department (ED)) during the baseline period.
Demographic and Insurance Characteristics and Healthcare Use
Information was collected on baseline clinical, demographic (age, sex, race, region), and insurance (type of insurance plan, Medicare prescription drug coverage gap (doughnut hole) status) characteristics; healthcare use; and year of observation. The doughnut hole refers to the temporary limit to what amount Medicare Part D drug plans cover. Enrollees enter the coverage gap when they or their drug plan has spent more than a prespecified amount on drug reimbursements, but if the prescription drug expenses of an individual go beyond a predefined catastrophic amount in a given calendar year, that individual again qualifies for drug reimbursement through their drug plans. This Medicare prescription drug coverage or doughnut hole status was measured using three categories: index date in the doughnut hole, index date before doughnut hole in calendar year of index date, and index date after doughnut hole but before the end of calendar year of index date. Healthcare use was measured as any inpatient and ED visits and number of diabetes mellitus–related office visits.
Dependent Variables: Clinical and Economic Outcomes
Changes in HbA1c from baseline to 1-year follow-up (measured as the difference between the last observed HbA1c value at 1-year follow-up and the last observed baseline HbA1c value) and achievement of HbA1c less than 8.0% during the 1-year follow-up period were considered clinical outcomes. This HbA1c threshold was chosen because it has been suggested that an HbA1c of 8.0% or greater can trigger clinical action such as active surveillance, intensification of OADs, and insulin initiation.13,24 Because the treatment goal for T2DM is achievement of glycemic control while minimizing the risk of hypoglycemia, the likelihood of hypoglycemic events was included as an additional clinical outcome. Hypoglycemic events were identified according to ICD-9-CM codes 250.8, 251.0, 251.1, and 251.2 in inpatient, ED, and outpatient settings, based on a previously published algorithm.25 Economic outcomes comprised total direct medical care costs that the plan measured and allowable costs for adjudicated claims and included inpatient, outpatient, ED, and prescription drug services. Costs were converted to constant dollars (2011) using the consumer price index for medical care services available from the Bureau of Labor Statistics. 26
Statistical Analysis
In retrospective observational studies, there are often systematic differences in the observed characteristics between individuals being treated with different treatments. It is common to account for this kind of selection bias with techniques such as inverse probability treatment weights (IPTWs),27 which calculate the probability of individuals receiving the treatment based on their observed characteristics, and assign individual weights based on the inverse of these probabilities. These weights balance the distribution of potential confounders across treatment categories and minimize selection bias when assessing the effect of treatment on outcomes. All independent variables measured at baseline were used in a multinomial logistic regression model to predict the probabilities of insulin initiation after one, two, and three or more OADs, and the inverse of these probabilities were used as weights in all the analyses.
Bivariate and multivariate analyses using IPTWs were used to examine the relationship between insulin initiation and outcomes. Continuous outcomes (change in HbA1c from baseline to 1-year follow-up, log-transformed total costs) were assessed using Student t-tests. Chi-square tests were used for categorical outcomes such as achievement of HbA1c less than 8.0% and likelihood of hypoglycemic events at 1-year follow-up to assess unadjusted differences according to insulin initiation. Multivariable analyses were conducted using ordinary least squares (OLS) regressions for continuous outcomes and logistic regressions for categorical outcomes. Standard econometric model fitting tests were used to select the appropriate distribution family and link for cost-modeling. These tests indicated that OLS with log transformation was the best fit for the data. Plots of residuals from OLS regression with log-transformed dollars indicated normality. For changes in HbA1c levels, stratified analyses were conducted using four baseline HbA1c categories (<7.0%, 7.0–7.9%, 8.0–8.9%, ≥9.0%). All of the regressions models controlled for insulin initiation (reference delayed insulin initiation); clinical, demographic, and insurance characteristics; healthcare use variables; and year of observation. All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
Baseline Characteristics
A total of 14,669 eligible individuals were included in the study. Mean age was 74, 78% were white, 49% were female, 63% were from the southern United States, and 42% were enrolled in health maintenance organization insurance plans. Baseline and 1-year follow-up HbA1c levels were available for 4,028 (27.5%) participants; mean last observed baseline HbA1c ± SD was 8.6 ± 1.7%.
Of participants with T2DM, 32% were early insulin initiators, 48% initiated insulin after two OADs, and 20% were delayed insulin initiators (≥3 OADs). Significant differences in baseline characteristics were seen between the three groups. Older adults in the early insulin initiation (1 OAD) group were sicker than those in the delayed insulin initiation group, as shown by a higher mean aDCS I (2.79 ± 2.23 vs 2.13 ± 2.02) and higher rates of hospitalization (40.6% vs 14.8%) and ED visits (37.4% vs 16.8%). Mean baseline HbA1c values of participants with baseline and 1-year follow-up HbA1c values were 8.7 ± 2.0% for the early insulin initiator group, 8.6 ± 1.7% for the two OAD group, and 8.6 ± 1.5% for the delayed insulin initiator group (Table 1).
Table 1.
Description of Study Population According to Insulin Initiation Category in Older Medicare Beneficiaries with Type 2 Diabetes Mellitus (T2DM): Humana Medicare Advantage Prescription Drug Database (N = 14,669)
| Characteristic | Insulin Initiation Category | P-Value | ||
|---|---|---|---|---|
| 1 OAD, n = 4,702 (32.1%) | 2 OADs, n = 6,980 (47.6%) | ≥3 OADs, n = 2,987 (20.4%) | ||
| Sex, n (%) | ||||
| Female | 2,326 (32.1) | 3,505 (48.4) | 1,417 (19.6) | .04 |
| Male | 2,376 (32.0) | 3,475 (46.8) | 1,570 (21.2) | |
| Race, n (%) | ||||
| White | 3,626 (31.9) | 5,381 (47.4) | 2,357 (20.7) | .02 |
| African American | 800 (34.1) | 1,125 (47.9) | 423 (18.0) | |
| Latino | 113 (29.5) | 189 (49.3) | 81 (21.1) | |
| Other | 163 (28.4) | 285 (49.7) | 126 (22.0) | |
| Age, n (%) | ||||
| 65–74 | 2,735 (29.7) | 4,463 (48.5) | 1,997 (21.7) | ≤.001 |
| ≥75 | 1,967 (35.9) | 2,517 (46.0) | 990 (18.1) | |
| U.S. region, n (%) | ||||
| Midwest | 1,219 (33.5) | 1,730 (47.6) | 689 (18.9) | .02 |
| South | 2,881 (31.3) | 4,420 (48.0) | 1,911 (20.7) | |
| Northeast, west, other | 602 (33.1) | 830 (45.6) | 387 (21.3) | |
| Insurance type, n (%) | ||||
| Health maintenance organization | 1,880 (30.4) | 3,084 (49.8) | 1,228 (19.8) | ≤.001 |
| Preferred provider organization | 887 (33.4) | 1,221 (46.0) | 546 (20.6) | |
| Fee for service | 1,917 (33.4) | 2,634 (45.9) | 1,192 (20.8) | |
| Other | 18 (22.5) | 41 (51.3) | 21 (26.3) | |
| Doughnut hole, n (%) | ||||
| In doughnut hole | 622 (25.2) | 1,024 (41.5) | 823 (33.3) | ≤.001 |
| Before or after doughnut hole | 4,080 (33.4) | 5,956 (48.8) | 2,164 (17.7) | |
| Any inpatient visit, n (%) | ||||
| Yes | 1,272 (40.6) | 1,398 (44.6) | 462 (14.8) | ≤.001 |
| No | 3,430 (29.7) | 5,582 (48.4) | 2,525 (21.9) | |
| Any emergency department visit, n (%) | ||||
| Yes | 1,178 (37.4) | 1,440 (45.8) | 529 (16.8) | ≤.001 |
| No | 3,524 (30.6) | 5,540 (48.1) | 2,458 (21.3) | |
| Hypoglycemia, n (%) | ||||
| Yes | 314 (38.8) | 354 (43.7) | 142 (17.5) | ≤.001 |
| No | 4,388 (31.7) | 6,626 (47.8) | 2,845 (20.5) | |
| Polypharmacy, n (%) | ||||
| >13 drugs | 804 (32.6) | 1,197 (48.5) | 469 (19.0) | .18 |
| ≤13 drugs | 3,898 (32.0) | 5,783 (47.4) | 2,518 (20.6) | |
| Urinary incontinence, n (%) | ||||
| Yes | 193 (39.7) | 206 (42.4) | 87 (17.9) | .001 |
| No | 4,509 (31.8) | 6,774 (47.8) | 2,900 (20.4) | |
| Major depressive disorder, n (%) | ||||
| Yes | 503 (38.0) | 587 (44.3) | 235 (17.7) | ≤.001 |
| No | 4,199 (31.5) | 6,393 (47.9) | 2,752 (20.6) | |
| Falls risk, n (%) | ||||
| Yes | 164 (41.0) | 177 (44.3) | 59 (14.8) | ≤.001 |
| No | 4,538 (31.8) | 6,803 (47.7) | 2,928 (20.5) | |
| Cognitive impairment, n (%) | ||||
| Yes | 895 (39.1) | 1,014 (44.3) | 381 (16.6) | ≤.001 |
| No | 3,807 (30.8) | 5,966 (48.2) | 2,606 (21.1) | |
| Dominant condition (cancer), n (%) | ||||
| Yes | 491 (32.4) | 740 (48.9) | 283 (18.7) | .23 |
| No | 4,211 (32.0) | 6,240 (47.4) | 2,704 (20.6) | |
| HbA1c, mean ± SDa | ||||
| <7.0% | 188 ± 17.5 | 256 ± 12.7 | 99 ± 10.6 | ≤.001 |
| 7.0–7.9% | 294 ± 27.3 | 574 ± 28.4 | 304 ± 32.6 | |
| 8.0–8.9% | 208 ± 19.3 | 457 ± 22.6 | 223 ± 23.9 | |
| ≥9.0% | 385 ± 35.8 | 733 ± 36.3 | 307 ± 32.9 | |
| Adapted Diabetes Complications Severity Index, n, mean ± SD | 4,702, 2.8 ± 2.2 | 6,980, 2.4 ± 2.1 | 2,987, 2.1 ± 2.0 | |
| HbA1c, n, mean ± SDa | 1,075, 8.7 ± 2.0 | 2,020, 8.6 ± 1.7 | 933, 8.6 ± 1.5 | |
SD = standard deviation.
Based on older Medicare beneficiaries with T2DM continuously enrolled in Humana Medicare Advantage Prescription Drug plans for 18 months and ≥1 oral antidiabetes drugs (OADs) during the baseline period.
Significant category differences according to insulin initiation status based on chi-square tests.
Based on older adults with baseline and follow-up glycosylated hemoglobin (HbA1c) data (N = 4,028).
Clinical Outcomes
Changes in HbA1c from Baseline to 1-Year Follow-Up
For all participants with HbA1c values available for baseline and 1-year follow-up, the mean HbA1c reduction was 0.7 ± 1.8%. In this group, 26.7% of participants were early insulin initiators, and 23.2% were delayed insulin initiators. Mean HbA1c reductions were 0.9 ± 3.7% for the early insulin initiation group, 0.7 ± 2.4% for the two OAD group, and 0.5 ± 3.6% for the delayed insulin initiation group. Similar results were obtained from the OLS regressions with IPTW (Table 2). Early insulin initiation was associated with a significantly greater reduction in HbA1c at 1-year follow-up than delayed insulin initiation (−0.40%; adjusted P ≤ .001); the reduction in HbA1c for the group with two OADs was 0.18% (adjusted P = .008).
Table 2.
Parameter Estimate for Insulin Initiation Categories from Inverse Probability Treatment Weight (IPTW)-Adjusted Ordinary Least Squares Regression of Change in Glycosylated Hemoglobin (HbA1c) Values from Baseline to 1-Year Follow-Up in Older Medicare Beneficiaries with Type 2 Diabetes Mellitus: Humana Medicare Advantage Prescription Drug Database (N = 4,028)
| Insulin Initiation Category | All, N = 4,028
|
Baseline HbA1c
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | β (SE) | P-Value | <7.0%, n = 543
|
7.0–7.9%, n = 1,047
|
8.0–8.9%, n = 1,013
|
≥9.0%, n = 1,425
|
|||||||||
| n | β (SE) | P-Value | n | β (SE) | P-Value | N | β (SE) | P-Value | n | β (SE) | P-Value | ||||
| 1 OAD | 1,075 | −0.40 (0.07) | ≤.001 | 188 | −0.20 (0.11) | .06 | 258 | −0.12 (0.08) | .15 | 244 | −0.28 (0.10) | .003 | 385 | −0.70 (0.13) | ≤.001 |
|
| |||||||||||||||
| 2 OADs | 2,020 | −0.18 (0.07) | .008 | 256 | −0.04 (0.10) | .71 | 515 | −0.03 (0.08) | .67 | 516 | −0.25 (0.09) | .007 | 733 | −0.24 (0.13) | .06 |
|
| |||||||||||||||
| ≥3 OADs | 933 | Reference | 99 | Reference | 274 | Reference | 253 | Reference | 307 | Reference | |||||
SE = standard error.
Regression model was adjusted for IPTWs and controlled for clinical (complexities specific to older adults, adapted Diabetes Complications Severity Index, co-occurring conditions, baseline hypoglycemia events), demographic (sex, race, region), and insurance (Medicare prescription drug, coverage gap status, type of insurance plan) characteristics; healthcare use in baseline period (any inpatient visit, any emergency department visit, number of diabetes-related office visits); and year of observation.
Significant group differences according to insulin initiation category (1 and 2 oral antidiabetes drugs (OADs)) compared with delayed insulin initiation (≥3 OADs), based on OLS regression.
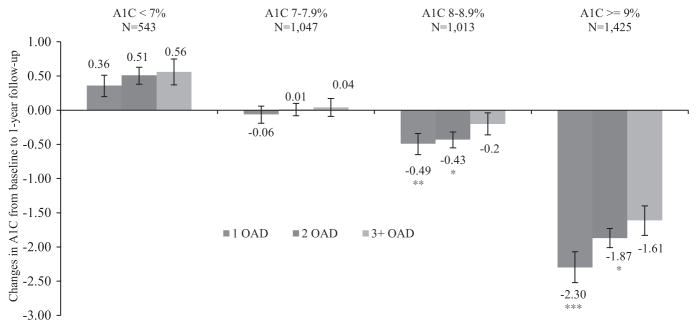
HbA1c reduction also differed between treatment categories and baseline HbA1c categories (Figure 1). When stratified according to baseline HbA1c values, the greatest reduction in HbA1c was observed in participants with early insulin initiation and baseline HbA1c of 9.0% or greater (reduction in HbA1c was 2.3% for those with early insulin initiation and 1.6% for those with delayed insulin initiation). OLS regression with IPTWs confirmed this result; a greater reduction (0.70%; adjusted P < .001) was observed in older adults with early insulin initiation and baseline HbA1c of 9.0% or greater.
Figure 1.
Mean (standard error) reduction in glycosylated hemoglobin (HbA1c) at 1-year follow-up compared with baseline, after use of 1, 2, and ≥3 oral antidiabetes drugs (OADs) according to baseline HbA1c categories. Asterisks represent significant group differences from delayed insulin initiation (≥3 OADs) in mean HbA1c reduction according to insulin initiation category (1 OAD and 2 OADs) based on unadjusted ordinary least squares regression with inverse probability treatment weights: ***P ≤ .001; **P < .01; *P < .05.
Achieving HbA1c Less Than 8.0% During the 1-Year Follow-Up Period
Early insulin initiation was associated with greater likelihood than delayed insulin initiation of achieving HbA1c less than 8.0% at 1-year follow-up (64.2% of early initiators, 62.2% of participants initiating after 2 OADs, 59.4% of delayed initiators). Results from the multivariable logistic regression with IPTW were consistent in showing a 30% higher likelihood of achieving HbA1c less than 8.0% for early insulin initiators than for delayed insulin initiators (adjusted odds ratio (aOR) = 1.30, 95% confidence interval (CI) = 1.18–1.43, P ≤ .001) (Table 3). Similarly, participants initiating insulin after two OADs were 20% more likely to achieve HbA1c less than 8.0% at 1-year follow-up (aOR = 1.20, 95% CI = 1.09–1.32, P ≤ .001) than delayed insulin initiators.
Table 3.
Inverse Probability Treatment Weight (IPTW)-Adjusted Percentages and Adjusted Odds Ratios for Insulin Initiation Categories from Logistic Regression on Achieving Glycosylated Hemoglobin (HbA1c) <8.0% at 1-Year Follow-Up (N = 4,028) and Hypoglycemia Events (N = 14,669) in Older Medicare Beneficiaries with Type 2 Diabetes Mellitus: Humana Medicare Advantage Prescription Drug Database
| Insulin Initiation Category | % | Multivariable Logistic Regression | |
|---|---|---|---|
| Adjusted Odds Ratio (95% Confidence Interval) | P-Value | ||
| Participants achieving HbA1c <8.0% | |||
| 1 OAD | 64.2 | 1.30 (1.18–1.43) | <.001 |
| 2 OADs | 62.2 | 1.20 (1.09–1.32) | <.001 |
| Participants with hypoglycemia event | |||
| 1 OAD | 11.5 | 0.95 (0.88–1.02) | .17 |
| 2 OADs | 10.4 | 0.98 (0.91–1.06) | .61 |
OAD = oral antidiabetes drug.
Reference ≥3 OADs.
Based on Medicare beneficiaries who had HbA1c values recorded at baseline and 1-year follow-up. Percentages and multivariate logistic regression estimates are adjusted for IPTWs. Multivariate logistic regression controlled for clinical (complexities specific to the older adults, adapted Diabetes Complications Severity Index, co-occurring conditions, baseline hypoglycemia events), demographic (sex, race, region), and insurance (Medicare prescription drug, coverage gap status, type of insurance plan) characteristics; healthcare use in baseline period (any inpatient visit, any emergency department visit, number of diabetes-related office visits); and year of observation.
Hypoglycemia Events During 1-Year Follow-Up
Overall, 10.7% of total participants experienced hypoglycemic events during the 1-year follow-up, which did not differ significantly according to insulin initiation category (11.5% of participants with early insulin initiation vs 10.2% of participants with delayed insulin initiation; P = .32). Multivariable logistic regression with IPTW did not show statistically significant differences in the likelihood of hypoglycemia events between the three insulin initiation categories.
Economic Outcomes at 1-Year Follow-Up
Early insulin initiators had significantly greater unadjusted average total costs ($17,511) than delayed insulin initiators ($15,427) (P < .001), although when adjusting for confounding factors, OLS regression with IPTWs showed no statistically significant differences in average total costs between the three insulin initiation groups (Table 4).
Table 4.
Inverse Probability Treatment Weight–Adjusted Average Total Direct Medical Costs and Parameter Estimates of Insulin Initiation Categories from Ordinary Least Squares (OLS) Regression of Logged Total Direct Medical Costs (2011 USD) at 1-Year Follow-Up in Older Medicare Beneficiaries with Type 2 Diabetes Mellitus: Humana Medicare Advantage Prescription Drug Database (N = 14,669)
| Insulin Initiation Categories, Number of Oral Antidiabetes Drugs | n | Costs, USD
|
OLS Regression
|
||
|---|---|---|---|---|---|
| Mean | Median (SE) | β (SE) | P-Value | ||
| 1 | 4,702 | 17,511 | 8,567 (370.38) | 0.02 (0.02) | .31 |
|
| |||||
| 2 | 6,980 | 16,125 | 8,277 (259.06) | −0.00 (0.02) | .67 |
|
| |||||
| ≥3 | 2,987 | 15,427 | 8,239 (381.25) | Reference | |
SE = standard error.
Mean and regression model are adjusted for inverse probability treatment weights (IPTWs), and regression model is controlled for clinical (complexities specific to older adults, adapted Diabetes Complications Severity Index, co-occurring conditions, any baseline hypoglycemia events), demographic (sex, race, region), and insurance (Medicare prescription drug, coverage gap status, type of insurance plan) characteristics, and year of observation.
DISCUSSION
This study examined the relationship between early insulin initiation and clinical (changes in HbA1c from baseline to 1-year follow-up, achieving HbA1c < 8.0%, risk of hypoglycemia events at 1-year follow-up) and economic (total healthcare costs) outcomes, in older Medicare beneficiaries with T2DM seeking care in real-world practice settings. After accounting for complexities specific to older adults and clinical and baseline characteristics, older Medicare beneficiaries with early insulin initiation had significantly greater reduction (0.40%) in HbA1c after 1-year follow-up than those who had delayed insulin initiation. A systematic review concluded that insulin initiation alone or insulin initiation when added to an OAD was associated with a greater than 1% reduction in HbA1c values in seven of the 14 included studies.8 However, the results of the present study may not be directly comparable with those of the studies included in the systematic review because of differences in study design, population, and length of follow-up; a majority of studies included in the systematic review were randomized clinical trials with a short follow-up period (only one study followed participants for a year).
A noteworthy finding from the current study is the relationship between baseline HbA1c categories and changes in HbA1c during 1 year of follow-up after insulin initiation. Early insulin initiation did not result in greater HbA1c reduction than delayed insulin initiation for those with baseline HbA1c less than 8.0%, although early insulin initiation resulted in significant reductions in HbA1c values for participants with HbA1c of 8.0% or greater; the greatest HbA1c reductions were observed among those with HbA1c of 9.0% or greater. These findings suggest that early initiation of insulin may be beneficial to older adults with HbA1c of 8.0% or greater, and this threshold can be used as a trigger for clinical action to initiate insulin treatment, although future research is needed to confirm this finding with additional covariates not included in the present study, for example, obesity, smoking, and health literacy.
Overall, 10.7% of the study population experienced a hypoglycemic event. Some existing studies have suggested that initiation of insulin might be associated with lower rates of hypoglycemia or fewer events than in older adults taking OADs alone.28–30 Nevertheless, insulin therapy has also been known to cause hypoglycemic events, leading to hospitalization, higher healthcare expenditures, and cardiovascular mortality.31 No statistically significant differences were found in rates of hypoglycemic events between the insulin initiation groups, although the nearly 11% rate of hypoglycemic events in the study population, as recorded in medical claims, may be a concern for providers and older adults with T2DM. Therefore, caution must be used when initiating insulin therapy in older adults with T2DM.
In physicians and patients, the fear of adverse effects such as hypoglycemia is one of the reasons for not initiating insulin therapy,32–34 but results from this study suggest that risk of hypoglycemia is not statistically different in early and delayed insulin initiators. In addition, the average total costs after 1 year of follow-up did not differ between insulin initiation categories. Taken together, the absence of significant differences in hypoglycemia events and costs and the clinically significant reduction in HbA1c highlight the beneficial effects of early insulin initiation.
The 48% of older adults who initiated insulin after use of two OADs at baseline also experienced beneficial clinical outcomes and had no statistically significant differences in risk of hypoglycemia or total cost. These results suggest that it is better to initiate insulin earlier than later.
Findings from the present study need to be interpreted in the context of its strengths and limitations. Strengths of this study include the longitudinal study design, the focus on older adults seeking care in real-world settings, and adjustments for complexities specific to older adults. Another strength of the study was the availability of HbA1c outcomes, typically unavailable in fee-for-service Medicare databases. The IPTW technique enabled the selection bias that may influence the relationship between insulin initiation and outcomes to be reduced. Nevertheless, the study had several limitations. Because it relied on observational data, causal inferences cannot be made. Only filled prescriptions and not actual use of medications could be assessed. Because HbA1c values were available for only one-third of the study population, findings regarding HbA1c reductions are only applicable to this subgroup. Furthermore, diabetes mellitus, co-occurring conditions, and hypoglycemia were identified using ICD-9-CM diagnosis codes in medical claims, which may have contained coding errors, such as under- or overcoding. The definition of insulin and OAD use had certain limitations. Types of basal insulin were not differentiated; existing literature suggests that adding insulin glargine may have modestly better glycemic benefits than adding neutral protamine Hagedorn insulin, with a low risk of hypoglycemia.35,36 Additionally, no differentiation was made between continued and one-time use of insulin. Time from T2DM diagnosis to insulin initiation could not be controlled for because T2DM diagnosis dates were unavailable. For OAD use, because of the nature of claims data and the study design, only the prescription filling records for participants within 6 months before their insulin initiation were examined, and thus it was not possible to estimate the treatment duration of each OAD class and differentiate between prevalent and incident users. This could result in a heterogeneous classification of individuals and bias the results. The study included older Medicare beneficiaries enrolled in Humana MAPD plans, so results may not be generalizable to all older Medicare beneficiaries with T2DM.
Although a “one-size fits all” HbA1c target of less than 8% was used in this manuscript, many guidelines suggest considering the age of the individual, frailty and functional status, duration of T2DM, and comorbidities in setting HbA1c targets.37–40 Nevertheless, operationalization of such personalized HbA1c targets, although ideal, has not been validated using administrative claims databases. Future studies should examine such approaches. The clinical reasons for early insulin initiation were not examined in this study. Results from this study indicated that early insulin initiators and those who initiated insulin after two OADs had higher rates of hospitalizations and ED visits than delayed insulin initiators. It is possible that those with one OAD were initiated on insulin because of high blood glucose during their encounters in acute care settings.
Using population-based retrospective data and adjusting for observed selection bias with insulin initiation, the present study evaluated the relationship between early insulin initiation and clinical and economic outcomes. Findings from the present study suggest that, in a population of older Medicare beneficiaries with T2DM in a real-world setting, early initiation of insulin is associated with clinically significantly lower HbA1c than delayed initiation of insulin. The lower HbA1c was achieved without greater risk of hypoglycemia events or higher total healthcare costs. Additionally, the present study suggests that early insulin initiation may be more effective in individuals with inadequate glycemic control. In the absence of clinical trials that include older adults with complex chronic conditions, such as those measured in the current study, these findings fill a crucial knowledge gap regarding the association between early insulin initiation and clinical and economic outcomes in older adults with T2DM.
Footnotes
Author Contributions: Bhattacharya: concept; analysis plan; input into study objectives, design, methodology; analysis; interpretation of results; preparation of study report; writing and review of manuscript drafts; comments. Zhou: proposal and development of concept; input into study objectives, design, methodology; interpretation of results; review of manuscript drafts; comments. Wei: concept; input into study objectives, design, methodology; interpretation of results; review of manuscript drafts; comments. Ajmera: input into study objectives, design, methodology; analysis; interpretation of results; preparation of report; development and review of manuscript drafts; comments. Sambamoorthi: primary investigator, concept, analysis plan, supervision of analyses, interpretation of results, preparation of study report, development and review of manuscript drafts, comments.
Any opinions, findings, and conclusions or recommendations expressed in this manuscript are those of the authors and do not necessarily reflect the views of the organizations that supported the study.
Conflicts of Interest: Wei and Zhou are employees of Sanofi US, Inc., and Wei is a shareholder of Sanofi US, Inc. The study was funded by Sanofi US, Inc.
Sponsor’s Role: The authors received writing and editorial support in the preparation of this manuscript from Pim Dekker, PhD, of Excerpta Medica, funded by Sanofi US, Inc.
References
- 1.American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krentz AJ, Bailey CJ. Oral antidiabetic agents: Current role in type 2 diabetes mellitus. Drugs. 2005;65:385–411. doi: 10.2165/00003495-200565030-00005. [DOI] [PubMed] [Google Scholar]
- 3.Calvert MJ, McManus RJ, Freemantle N. The management of people with type 2 diabetes with hypoglycaemic agents in primary care: Retrospective cohort study. Fam Pract. 2007;24:224–229. doi: 10.1093/fampra/cmm008. [DOI] [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Pistrosch F, Köhler C, Schaper F, et al. Effects of insulin glargine versus metformin on glycemic variability, microvascular and beta-cell function in early type 2 diabetes. Acta Diabetol. 2013;50:587–595. doi: 10.1007/s00592-012-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weng J, Li Y, Xu W, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: A multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 7.Owens DR. Clinical evidence for the earlier initiation of insulin therapy in type 2 diabetes. Diabetes Technol Ther. 2013;15:776–785. doi: 10.1089/dia.2013.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asche CV, Bode B, Busk AK, et al. The economic and clinical benefits of adequate insulin initiation and intensification in people with type 2 diabetes mellitus. Diabetes Obes Metab. 2012;14:47–57. doi: 10.1111/j.1463-1326.2011.01487.x. [DOI] [PubMed] [Google Scholar]
- 9.Rubino A, McQuay LJ, Gough SC, et al. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with type 2 diabetes: A population-based analysis in the UK. Diabet Med. 2007;24:1412–1418. doi: 10.1111/j.1464-5491.2007.02279.x. [DOI] [PubMed] [Google Scholar]
- 10.National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Accessed October 21, 2014]. [on-line]. Available at http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. [Google Scholar]
- 11.Ober SK, Watts S, Lawrence RH. Insulin use in elderly diabetic patients. Clin Interv Aging. 2006;1:107–113. doi: 10.2147/ciia.2006.1.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaum CS, Ofstedal MB, Langa KM, et al. Functional status and health outcomes in older Americans with diabetes mellitus. J Am Geriatr Soc. 2003;51:745–753. doi: 10.1046/j.1365-2389.2003.51256.x. [DOI] [PubMed] [Google Scholar]
- 13.Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care. 2012;35:2650–2664. doi: 10.2337/dc12-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peyrot M, Rubin RR, Lauritzen T, et al. Resistance to insulin therapy among patients and providers: Results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care. 2005;28:2673–2679. doi: 10.2337/diacare.28.11.2673. [DOI] [PubMed] [Google Scholar]
- 15.Karter AJ, Subramanian U, Saha C, et al. Barriers to insulin initiation: The Translating Research into Action for Diabetes Insulin Starts Project. Diabetes Care. 2010;33:733–735. doi: 10.2337/dc09-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM, Buse JB, Davidson MB, et al. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 17.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: A patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehta S, Chen H, Johnson ML, et al. Risk of falls and fractures in older adults using antipsychotic agents: A propensity-matched retrospective cohort study. Drugs Aging. 2010;27:815–829. doi: 10.2165/11537890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Tinetti ME, Gordon C, Sogolow E, et al. Fall-risk evaluation and management: Challenges in adopting geriatric care practices. Gerontologist. 2006;46:717–725. doi: 10.1093/geront/46.6.717. [DOI] [PubMed] [Google Scholar]
- 20.Goldberg JF, Brooks JO, III, Kurita K, et al. Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: Findings from the STEP-BD. J Clin Psychiatry. 2009;70:155–162. doi: 10.4088/jcp.08m04301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anger JT, Saigal CS, Madison R, et al. Increasing costs of urinary incontinence among female Medicare beneficiaries. J Urol. 2006;176:247–251. doi: 10.1016/S0022-5347(06)00588-X. [DOI] [PubMed] [Google Scholar]
- 22.Chang HY, Weiner JP, Richards TM, et al. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care. 2012;18:721–726. [PubMed] [Google Scholar]
- 23.Piette JD, Kerr EA. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29:725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 24.Soroka O, Tseng CL, Rajan M, et al. A clinical action measure to assess glycemic management in the 65–74 year old veteran population. J Am Geriatr Soc. 2012;60:1442–1447. doi: 10.1111/j.1532-5415.2012.04079.x. [DOI] [PubMed] [Google Scholar]
- 25.Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord. 2008;8:4. doi: 10.1186/1472-6823-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Department of Labor. Bureau of Labor Statistics. [Accessed October 21, 2014];Consumer Price Index [on-line] Available at http://www.bls.gov/cpi/
- 27.Curtis LH, Hammill BG, Eisenstein EL, et al. Using inverse probability-weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–S107. doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 28.Chen HS, Wu TE, Jap TS, et al. Beneficial effects of insulin on glycemic control and beta-cell function in newly diagnosed type 2 diabetes with severe hyperglycemia after short-term intensive insulin therapy. Diabetes Care. 2008;31:1927–1932. doi: 10.2337/dc08-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lingvay I, Legendre JL, Kaloyanova PF, et al. Insulin-based versus triple oral therapy for newly diagnosed type 2 diabetes: Which is better? Diabetes Care. 2009;32:1789–1795. doi: 10.2337/dc09-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papa G, Fedele V, Chiavetta A, et al. Therapeutic options for elderly diabetic subjects: Open label, randomized clinical trial of insulin glargine added to oral antidiabetic drugs versus increased dosage of oral antidiabetic drugs. Acta Diabetol. 2008;45:53–59. doi: 10.1007/s00592-007-0023-6. [DOI] [PubMed] [Google Scholar]
- 31.Giordano C. Insulin therapy: Unmet needs and new perspectives. Minerva Endocrinol. 2013;38:95–102. [PubMed] [Google Scholar]
- 32.Polonsky WH, Jackson RA. What’s so tough about taking insulin? Addressing the problem of psychological insulin resistance in type 2 diabetes. Clin Diabetes. 2004;22:147–150. [Google Scholar]
- 33.Korytkowski M. When oral agents fail: Practical barriers to starting insulin. Int J Obes Relat Metab Disord. 2002;26(Suppl 3):S18–S24. doi: 10.1038/sj.ijo.0802173. [DOI] [PubMed] [Google Scholar]
- 34.Nam S, Chesla C, Stotts NA, et al. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33:1747–1749. doi: 10.2337/dc10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee P, Chang A, Blaum C, et al. Comparison of safety and efficacy of insulin glargine and neutral protamine Hagedorn insulin in older adults with type 2 diabetes mellitus: Results from a pooled analysis. J Am Geriatr Soc. 2012;60:51–59. doi: 10.1111/j.1532-5415.2011.03773.x. [DOI] [PubMed] [Google Scholar]
- 36.Pandya N, DiGenio A, Gao L, et al. Efficacy and safety of insulin glargine compared to other interventions in younger and older adults: A pooled analysis of nine open-label, randomized controlled trials in patients with type 2 diabetes. Drugs Aging. 2013;30:429–438. doi: 10.1007/s40266-013-0069-9. [DOI] [PubMed] [Google Scholar]
- 37.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36:S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sue Kirkman M, Briscoe VJ, Clark N, et al. Diabetes in older adults: A consensus report. J Am Geriatr Soc. 2012;60:2342–2356. doi: 10.1111/jgs.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinclair AJ, Paolisso G, Castro M, et al. European Diabetes Working Party for Older People 2011 clinical guidelines for type 2 diabetes mellitus. Executive summary. Diabetes Metab. 2011;37:S27–S38. doi: 10.1016/S1262-3636(11)70962-4. [DOI] [PubMed] [Google Scholar]
- 40. [Accessed October 21, 2014];VA/DoD Clinical Practice Guideline for the Management of Diabetes Mellitus [on-line] Available at http://www.healthquality.va.gov/guidelines/CD/diabetes/DM2010_SUM-v4.pdf.