Abstract
Rationale
Behavioral and dopamine responses to cocaine are sexually dimorphic: Female rats exhibit higher levels of locomotor and reward-associated behaviors after cocaine administration and dopamine release than do males. Activation of the dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa (DARPP-32) intracellular cascade mediates responses to cocaine.
Objective
To examine the possibility that acute cocaine administration alters the DARPP-32 cascade in a sexually dimorphic pattern.
Materials and methods
Male and female rats received either saline or cocaine (30 mg/kg). Protein levels of DARPP-32, phosphorylation of DARPP-32 at the Thr34 site (P-Thr34-DARPP-32), protein phosphatase 1 (PP-1), and protein phosphatase 2B (PP-2B) in nucleus accumbens were measured via Western blot analysis.
Results
Females had higher protein levels of DARPP-32, P-Thr34-DARPP-32, calcineurin A (CaN-A; catalytic subunit of PP-2B), and calcineurin B (CaN-B; regulatory subunit of PP-2B) than males 5 min after saline treatment. In females, CaN-A protein levels were also higher at 15 min and PP-1 protein levels were higher 30 min after saline administration than males. In male rats, cocaine significantly increased CaN-A protein levels at 30 min and CaN-B protein levels at 15 min. In females, cocaine administration significantly decreased protein levels of DARPP-32, P-Thr34-DARPP-32, and CaN-A at 45 min but increased PP-1 protein levels at 30 min. Overall, males had higher activation of the DARPP-32 pathway after cocaine administration than did females.
Conclusion
These novel results show that basal and cocaine-induced sex differences in the DARPP-32/PP-1 cascade may be responsible for the sexual dimorphism in acute cocaine-induced behavioral responses.
Keywords: Cocaine, Sex differences, DARPP-32, P-Thr34-DARPP-32, PP-1, PP-2B, Nucleus accumbens, Fischer rats
Introduction
As more attention is paid to sex and hormonal effects on drug abuse, it is becoming apparent that men and women react differently to cocaine. More women initiate cocaine use at a younger age than men (NSDUH 2002). Women also take shorter time to develop cocaine abuse than men (Ridenour et al. 2005). Women experience more nervousness and report higher ratings of “feel good” after intermittent intranasal administration of cocaine (Kosten et al. 1996; McCance-Katz et al. 2005). Women also take longer to feel cocaine's subjective effects, report less euphoria and dysphoria than men (Lukas et al. 1996). These data suggest that women are more sensitive than men to the additive properties of cocaine. However, those sex differences are limited to women in their luteal phase, when the subjective effects of cocaine were lower compared to the follicular phase (Sofuoglu et al. 1999; Evans et al. 2002; Evans and Foltin 2006). Other clinical studies have revealed conflicting findings of no sex or menstrual cycle differences, which may result from different dose and route of cocaine administration (Mendelson et al. 1999; Collins et al. 2007).
Similar to humans, female rodents also show exaggerated and more robust locomotor responses to cocaine than do males (Van Haaren and Meyer 1991; Schindler and Carmona 2002; Harrod et al. 2005). Females also more quickly develop cocaine-induced conditioned place preference (CPP) and behavior sensitization with lower doses and more readily acquire cocaine self-administration (Russo et al. 2003; Lynch and Carroll 1999; Van Haaren and Meyer 1991; Chin et al. 2002; Hu et al. 2004; Jackson et al. 2006). Taken together, clinical and rodent studies suggest that sex-specific differences exist at all stages of the cocaine abuse process, including induction, maintenance, and relapse.
Sex differences in the mesocorticolimbic dopamine (DA) system, a regulator of cocaine's psychomotor and rewarding effects (Koob 1992; Hyman and Malenka 2001), have also been demonstrated (Festa et al. 2004, 2006; Walker et al. 2006). Cocaine reduces dopamine type 1 (D1) receptor binding levels in the striatum of male but not female rats (Festa et al. 2006). Furthermore, D1 receptor antagonists block cocaine-induced CPP and locomotor responses with different efficacies between sexes: D1 receptor antagonists inhibit cocaine's effects with a lower dose range in female than in male rats (Nazarian et al. 2004; Festa et al. 2006). After cocaine administration, accumbal DA release is higher in females than in males (Walker et al. 2006). Additionally, whereas cocaine decreases dihydroxyphenylacetic acid (DOPAC) to DA turnover ratios in the nucleus accumbens (NAc) of male rats, in female rats, it significantly reduces total levels of DA, DOPAC, and homovanillic acid metabolites (Festa et al. 2004). In female rats, the nigrostriatal DA neurotransmission is more tightly regulated by autoreceptor and transporter mechanisms than in male rats, a difference that may be related to the greater autoreceptor control of DA activity in females (Walker et al. 2006). These sexual dimorphic patterns in DA system activation after cocaine treatment strongly suggest sex difference in cocaine-induced dopamine–protein kinase A (PKA)-mediated signaling pathway responses. Indeed, two recent studies found sexual dimorphic responses to cocaine in this signaling pathway. Nazarian et al. (2008) demonstrated that PKA protein levels in the NAc were overall higher in females than males, and Lynch et al. (2007) showed that protein levels of dopamineand cAMP-regulated phosphoprotein of Mr 32 kDa (DARPP-32) phosphorylated at the PKA site were higher in females than males. Taken together, these findings suggest that sex differences in the DA-PKA signaling regulation in the NAc may contribute to sex difference in the initiation and development of rewarding properties to cocaine.
The DARPP-32 signaling pathway has been shown to mediate intracellular responses to DA. Phosphorylation of DARPP-32 at the Thr34 site (P-Thr34-DARPP-32) is required for cocaine actions in the striatum (Zachariou et al. 2006), i.e., acute cocaine administration increases phosphorylation of DARPP-32 at the Thr34 site (Nishi et al. 2000; Greengard 2001). Moreover, in DARPP-32 knockout mice and mice with site mutations of P-Thr34-DARPP-32, typical cocaine-induced locomotor activities are attenuated as compared with activities in control mice (Valjent et al. 2005; Fienberg et al. 2006; Zachariou et al. 2006). On one hand, P-Thr34-DARPP-32 is a potent inhibitor of protein phosphatase-1 (PP-1), which is a serine/threonine protein phosphatase (Svenningsson et al. 2005). On the other hand, P-Thr34-DARPP-32 is dephosphorylated by protein phosphatase-2B (PP-2B), a Ca2+/calmodulin-dependent protein phosphatase (King et al. 1984; Goto et al. 1986). Although no studies to date have determined whether PP-1 and PP-2B are altered after acute cocaine administration, in male rats, it has been shown that repeated cocaine administration decreases PP-2B but not PP-1 protein levels in the NAc (Hu et al. 2005).
It is yet to be determined whether acute cocaine administration alters the DA-mediated intracellular cascades in a sexually dimorphic pattern. Because females not only have more locomotor responses to acute cocaine treatment but also develop CPP and self-administration faster than males, we hypothesized that the cocaine-induced DARPP-32 signaling pathway responses are heightened in females as compared with males. To test this hypothesis, we made side-by-side comparisons between male and female rats of DARPP-32, P-Thr34-DARPP-32, PP-1, and PP-2B protein levels in the NAc (an area known to regulate cocaine's behavioral and reward responses (Nestler 2001)) after saline or acute cocaine treatment.
Experimental procedures
Animals
Sixty-day-old male and female Fischer rats (Charles River, Raleigh, NC, USA) were individually housed in Plexiglas chambers (20×20×41 cm) layered with beta chips. Rats were given free access to food and water and maintained on a 12-h light/dark cycle (lights on at 9:00 a.m.). All rats were weighed, handled, and intra peritoneally (i.p.) injected daily with saline for five consecutive days prior to testing. A total of four rats per group were used for each sex or time-course comparison after saline or drug treatment.
Repeated vaginal lavage attenuates cocaine-induced activity, abolishes estrous cycle effects, and establishes CPP in female rats, thus possibly increasing DA-mediated responses (Walker et al. 2002). Therefore, as noted by Walker et al. (2002), the use of lavaged female rats could result in inaccurate behavioral responses when making side-by-side comparisons with male rats. For this reason, females were randomly assigned to experimental groups without regard to their estrous cycle. Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD, USA) and approved by the Hunter Institution Animal Care Use Committee.
Materials
Cocaine hydrochloride was purchased from Sigma Chemical Co. (St. Louis, MO, USA). DARPP-32 antibody was purchased from Cell Signaling Technologies (Beverly, MA, USA). P-Thr34-DARPP-32, PP-1α (catalytic subunit of PP-1), calcineurin A (CaN-A; catalytic subunit of PP-2B), and calcineurin B (CaN-B; regulatory subunit of PP-2B) antibodies were purchased from Sigma (St Louis, MO, USA). α-Tubulin antibody was purchased from Santa Cruz Technologies (Santa Cruz, CA, USA). All appropriate secondary antibodies were purchased from Amersham Pharmacia (Piscataway, NJ, USA).
Cocaine administration and protein measurements
Cocaine solutions were prepared daily by dissolving the drug in physiological saline (0.9%). On the day of testing, rats were injected (i.p.) with saline or cocaine (30 mg/kg) and sacrificed 5, 15, 30, 45, or 90 min later. After decapitation (following a brief 20-s exposure to CO2), their brains were removed, flash frozen in 2-methylbutane (−40°C), and stored at −80°C until used.
The NAc were dissected from coronal sections (1 mm thick) using a matrix (ASI Instruments; Warren, MI, USA). Tissue was homogenized using a Polytron handheld homogenizer (Kinematica; Luzern, Switzerland) in homogenizing buffer [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid 7.9 (20 mM), KCl (10 mM), ethylenediaminetetraacetic acid (1 mM), NP40 (0.2%), glycerol (10%), NaCl (200 mM), pepstatin, leupeptin, 1,4-dithio-dl-threitol (1 M), aprotenin, phenylmethylsulphonyl fluoride (100 mM), NaF (50 mM), and Na3VO4 (1 mM)]. Total protein content was determined with use of a Bradford kit (Bio-Rad Laboratories; Hercules, CA, USA).
Western blot analysis
Protein samples (40 μg) were boiled in Lammeli buffer containing 5% β-mercaptoethanol and loaded onto 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Gels were electrophoresed, transferred to nitrocellulose membranes, and blocked for 60 min with 5% nonfat dry milk in Tris buffer saline Tween (TBST; pH= 7.4) at room temperature. Membranes were probed overnight at 4°C, with P-Thr34-DARPP-32 (1:2,000), PP-1α (1:4,000), CaN-A (1:15,000), CaN-B (1:5,000), and DARPP-32 (1:1,000). After three washes with TBST, membranes were then incubated with their appropriate secondary antibody (1:1,000) for 60 min at room temperature, followed by three more washes with TBST. Antibody binding was detected using an enhanced chemiluminescence kit (Amersham Pharmacia; Piscataway, NJ, USA). Resulting films were scanned and quantified with a computer densitometer and Image Quant Program (Molecular Dynamics; Sunnyvale, CA, USA). To compare sex differences in the protein levels, samples from saline-treated male and female rats were loaded onto the same gel. Within each sex, to determine the time course of changes in protein levels after treatment, all saline-treated samples (5–90 min), or cocaine-treated (5–90 min) samples with saline control (5 min) were run on the same gel. A total of three sets of gels were run for each determinant. To normalize band intensity to protein levels, membranes were reprobed with α-tubulin antibody (1:1,000).
Statistical analysis
All protein levels were first expressed as a ratio to α-tubulin levels. Data were presented as mean ± standard error of the mean (SEM). Student's t tests were used to determine sex differences. For comparison between sexes in cocaine-treated rats, percentage changes of protein levels between the cocaine-treated group and the average of protein levels in saline controls of the same sex were used. One-way analysis of variances followed by post hoc least significant difference analysis were used to determine differences during the time course. Statistical significance was considered to be p<0.05 for all analyses.
Results
Sex differences in protein levels in saline-treated rats
Five minutes after saline administration, females had significantly higher protein levels of DARPP-32 and P-Thr34-DARPP-32 than males (t=13.765, p<0.001; t=2.921, p<0.05, respectively; Table 1). Thirty minutes after saline administration, there were higher protein levels of PP-1α in females than males (t=2.720, p<0.05; Table 1). At both 5 and 15 min after saline treatment, female rats had significantly higher CaN-A protein levels than males (t=10.580, p<0.001; t=2.463, p<0.05, respectively; Table 1). However, CaN-B protein levels were significantly higher in females than males only at 5 min after saline treatment (t= 8.250, p<0.001; Table 1). Within each sex, no statistically significant differences were observed 5 to 90 min after saline administration (data not shown).
Table 1.
Western blot analysis of protein levels of DARPP-32 signaling proteins in NAc of saline-treated animals
| Time point | 5 min |
15 min |
30 min |
45 min |
90 min |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| DARPP-32 | 0.30±0.01 | 0.49±0.01a | 1.80±0.38 | 1.76±0.22 | 1.63±0.34 | 1.68±0.30 | 1.84±0.23 | 1.88±0.22 | 2.13±0.37 | 1.62±0.24 |
| P-Thr34-DARPP-32 | 0.43±0.04 | 0.62±0.05a | 2.18±0.43 | 2.08±0.32 | 1.86±0.14 | 2.33±0.51 | 2.00±0.25 | 1.85±0.24 | 2.30±0.43 | 1.53±0.20 |
| PP-1α | 0.83±0.04 | 0.97±0.05 | 3.27±0.75 | 4.14±0.36 | 3.00±0.30 | 3.91±0.15a | 1.94±0.15 | 1.95±0.21 | 1.94±0.25 | 1.63±0.19 |
| CaN-A | 4.38±0.10 | 7.81±0.31a | 5.90±0.75 | 8.56±0.78a | 5.16±0.65 | 7.18±0.73 | 3.51±0.14 | 4.36±0.62 | 3.60±0.23 | 3.81±0.27 |
| CaN-B | 0.68±0.03 | 0.92±0.01a | 7.28±0.76 | 9.03±1.02 | 6.17±0.52 | 8.18±1.35 | 6.36±0.86 | 6.58±1.34 | 6.69±0.96 | 6.26±1.09 |
Data are presented as the mean protein levels normalized with α-tubulin ± SEM.
Represents statistically significant differences between sexes (N=4 per group)
Cocaine's effects on the DARPP-32 pathway
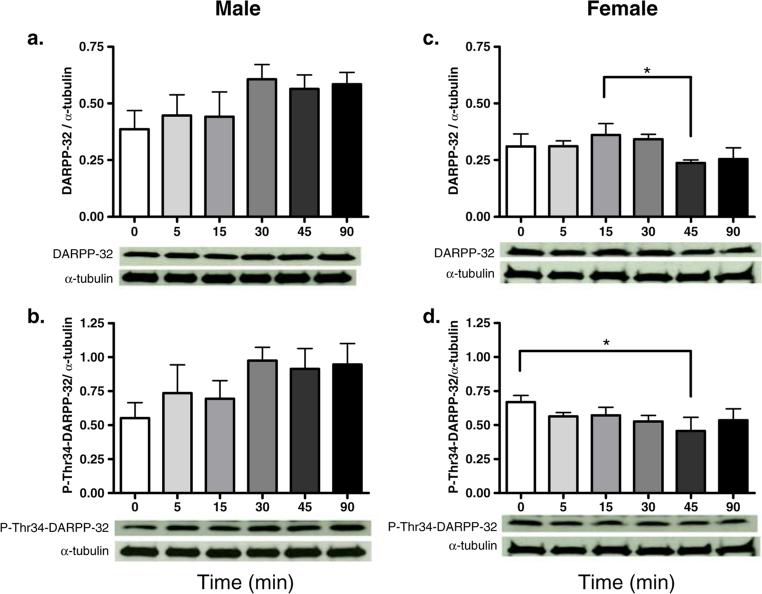
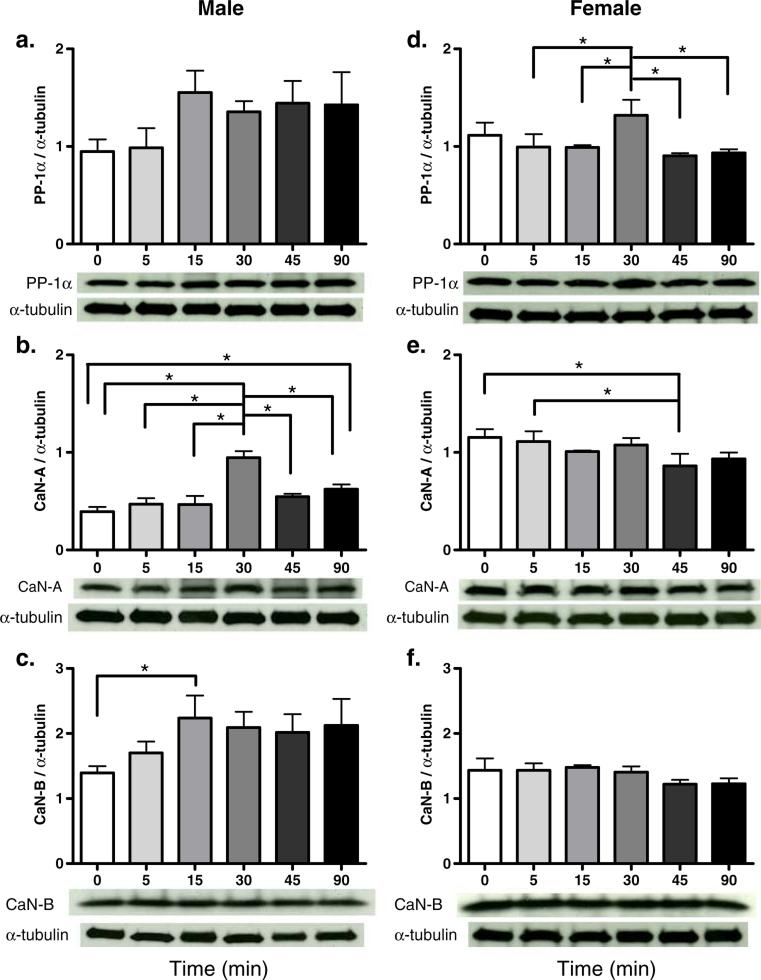
In male rats, higher P-Thr34-DARPP-32 protein levels were observed 30 min after cocaine treatment when compared with saline controls; however, the difference failed to reach statistical significance (p=0.058; Fig. 1b). In male rats, CaN-A protein levels were significantly higher at 30 min after cocaine administration than at other time points (p<0.001 for all comparisons; Fig. 2b). CaN-A protein levels were also significantly higher 90 min after cocaine treatment as compared to saline controls (p<0.02; Fig. 2b). Furthermore, CaN-B protein levels were significantly higher 15 min after drug treatment when compared with saline controls (p< 0.05; Fig. 2c).
Fig. 1.
Protein levels of DARPP-32 (a, c) or P-Thr34-DARPP-32 (b, d) after cocaine treatment in male (a, b) and female (c, d) rats. Data are presented as the mean protein levels normalized with α-tubulin ± SEM. Lower panel represents a typical Western blot for each protein. * Represents statistically significant difference (N=4 per group)
Fig. 2.
Protein levels of PP-1α (a, d), CaN-A (b, e), or CaN-B (c, f) after cocaine treatment in male (a–c) and female (d–f) rats. Data are presented as the mean protein levels normalized with α-tubulin ± SEM. Lower panel represents a typical Western blot for each protein. * Represents statistically significant difference (N=4 per group)
In female rats, 45 min after cocaine administration, DARPP-32 protein levels were lower than 15 min after cocaine administration, and P-Thr34-DARPP-32 protein levels were decreased compared to saline controls (p<0.05 for both comparisons; Fig. 1c, d). Thirty minutes after cocaine administration, PP-1α protein levels were significantly higher than at any other time point after cocaine treatment (p<0.05 for all comparisons; Fig. 2d). Forty-five minutes after cocaine administration, CaN-A protein levels were significantly lower than saline controls and significantly lower than 5 min after cocaine treatment (p<0.05 for both comparisons; Fig. 2e).
Between-sex comparisons reveal that while in male rats the percentage change of proteins in the DARPP-32 pathway increased across time, in females, it decreased. Thus, males had a significantly higher percentage change in both DARPP-32 and P-Thr34-DARPP-32 30 to 90 min after cocaine administration than female rats (p<0.05 for all comparisons; Table 2). Similarly, sex differences were observed in the magnitude and duration of PP-1α, CaN-A, and CaN-B. Specifically, male rats had higher induction of PP-1α 15 and 45 min after cocaine treatment than female rats (p<0.05 for both comparisons; Table 2). Male rats also had higher induction of CaN-A 30 to 90 min after cocaine administration than female rats (p<0.05 for all comparisons; Table 2). However, male rats had a significantly greater percentage change in CaN-B at 30 and 45 min after cocaine treatment as compared with female rats (p<0.05 for both comparisons; Table 2).
Table 2.
Percentage change of protein levels of DARPP-32 signaling proteins in NAc of cocaine-treated animals
| Time point | 5 min |
15 min |
30 min |
45 min |
90 min |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protein | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| DARPP-32 | 15.7±23.9 | 0.2±7.8 | 14.4±28.4 | 16.3±16.2 | 57.3±16.8 | 10.3±7.1a | 46.3±16.0 | –23.5±4.4a | 51.6±13.6 | –18.0±16.0a |
| P-Thr34-DARPP-32 | 33.4±37.7 | –15.8±4.2 | 25.8±24.3 | –14.5±8.9 | 76.6±18.0 | 21.3±6.5a | 65.6±27.2 | –31.8±15.1a | 71.6±28.0 | –19.9±12.5a |
| PP-1α | 3.8±21.1 | –10.7±12.0 | 63.5±23.5 | –11.0±2.0a | 42.6±11.5 | 18.5±14.3 | 52.0±24.2 | –18.8±2.4a | 50.2±35.5 | –16.1±3.4 |
| CaN-A | 19.6±15.8 | –3.5±9.0 | 18.3±22.5 | –12.5±0.9 | 140.2±17.7 | –7.7±6.1a | 38.9±7.4 | –25.3±10.9a | 58.8±12.0 | –19.0±5.7a |
| CaN-B | 22.1±12.4 | 0.0±7.5 | 60.4±25.0 | 3.0±2.6 | 50.0±17.7 | 1.9±6.2a | 44.7±19.9 | –14.9±4.7a | 52.2±29.4 | –14.4±5.9 |
Data are presented as percentage change of protein levels ± SEM as compared to 5-min saline controls of the same sex.
Represents statistically significant differences between sexes (N=4 per group)
Discussion
Results presented here are novel in that they demonstrate basal sex differences and a sexually dimorphic pattern of activation after acute cocaine administration in the DARPP-32 intracellular cascades. These results extend current knowledge by showing that the known basal and cocaine-induced sex differences at the release, receptor (number and specificity), and reuptake levels in the DA system also include differences at the DA-mediated second messenger intracellular responses.
Female rats had higher basal protein levels in most DARPP-32 pathway components. Lynch et al. (2007) have also shown that female Sprague Dawley rats have higher basal levels of phosphorylated DARPP-32. Thus, our results, which are consistent with their observations, further demonstrate that the entire DARPP-32 cascade is heightened in female rats as compared with males. Basal sex differences in the DARPP-32 cascade suggest three postulates. First, because female rats have higher accumbal dopaminergic tone than males (Becker 1999; Walker et al. 2006), PKA may be activated at higher levels by high basal DA levels, and in turn, this high level of PKA activation increases the phosphorylation of DARPP-32 at the Thr34 site (Nishi et al. 2000). Indeed, Nazarian et al. (2008) have demonstrated that in the NAc, female rats have higher basal protein levels of PKA than male rats. This finding is further supported by previous reports of higher basal dopamine release and uptake in female rats (Walker et al. 2000), which may underlie the higher basal levels of P-Thr34-DARPP-32 mediated by D1/PKA activation in females than males. The second postulate is that the differential efficiency of D1 receptors between sexes (Schindler and Carmona 2002; Festa et al. 2006) may also have an impact on the sex differences in P-Thr34-DARPP-32 protein levels. Because females are more sensitive to D1 agonists, less ligand may be needed to increase the activation of the D1/PKA/DARPP-32 cascade in females than in males. Thirdly, estrogen increases the basal extracellular concentration of DA in the striatum (Xiao and Becker 1994). Furthermore, enhanced DARPP-32 phosphorylation at the Thr-34 site after the estradiol treatment has also been observed in the medial preoptic nucleus, bed nucleus of the stria terminalis, paraventricular nucleus, and the ventromedial nucleus of the hypothalamus (Auger et al. 2001). Therefore, females’ higher estrogen levels may contribute to the basal sex differences in P-Thr34-DARPP-32 protein levels. Further experiments are necessary to determine whether one or all these postulates contribute to the sexual dimorphic pattern of DARPP-32-mediated proteins. Still unknown is the extent to which the higher basal levels of DARPP-32 phosphorylation at the Thr34 site in female rats contribute to the sexual dimorphism. However, we postulate that females’ robust and prolonged motor responses after acute cocaine administration are in part mediated by their having initial higher basal levels of P-Thr34-DARPP-32.
P-Thr34-DARPP-32 inhibits PP-1 activity (Svenningsson et al. 2005). Thus, higher basal protein levels of PP-1 in females may reflect a disinhibitory effect caused by higher P-Thr34-DARPP-32 protein levels. In addition, PP-1 protein levels are also part of PKA regulation (Surmeier et al. 1995). Thus, higher PKA activation in females (either by the higher dopamine tone (Becker 1999; Walker et al. 2006) or higher PKA protein levels (Nazarian et al. 2008)) may also counterbalance the inhibitory effect of P-Thr34-DARPP-32 in PP-1, which in turn indirectly elevates PP-1 protein levels. However, further study is needed to determine the extent to which the higher basal levels of P-Thr34-DARPP-32 in female rats contribute to the sexual dimorphism in PP-1 protein levels. To further confirm our finding and address the differences between the levels of protein expression and the levels of protein activity, future studies measuring the phosphatase activity of PP-1 in male and female rats are warranted.
PP-2B protein levels were also found to be higher in saline-treated female rats than in males. Since PP-2B dephosphorylates P-Thr34-DARPP-32 (King et al. 1984; Goto et al. 1986), the elevated PP-2B protein levels may represent a positive feedback mechanism to counteract the higher basal protein levels of P-Thr34-DARPP-32 observed in the females. Alternatively, recent evidence has also shown that the activation of PP-2B is necessary to maintain the dopamine D1-agonist-stimulated G-protein activation in the forebrain cortical tissue (Adlersberg et al. 2004). Thus, the higher PP-2B protein levels may further contribute to higher DARPP-32-mediated signaling activation in females.
In the basolateral amygdala (BLA), female rats have higher DA outflow after acute stress than do their male counterparts (Mitsushima et al. 2006). Stress-induced activation of the BLA also increases the DA extracellular level in the NAc (Floresco et al. 1998; Howland et al. 2002), possibly through BLA excitatory projections into the NAc (Kelley et al. 1982; Wright et al. 1996; Mulder et al. 1998). In addition, females have higher DA levels in the striatum than males after acute stress. Furthermore, in the prefrontal cortex (PFc; an area known to regulate cocaine reward; Kalivas et al. 2005), enhanced dopaminergic activities by acute stress were observed in male rats only (Dalla et al. 2008). It was postulated that the increased cortical DA function in turn inhibits the dopaminergic activities in the NAc (Ventura et al. 2002). However, in females, due to their higher basal dopaminergic activities in the PFc, it is possible that acute stress cannot further increase cortical DA function, resulting in failure to inhibit the high dopaminergic activities in the NAc (Dalla et al. 2008). This dysregulation of DA-mediated responses in the PFc–NAc pathway contributes to the female's liability in adapting to acute stress. Given that saline administrations may be an acute stressor, the sexual dimorphic pattern of the DARPP-32 cascade may reflect sex differences in administration-induced stress-mediated responses, such as DA releases and dopaminergic activities. Indeed, because such differences were observed for the most part soon after the saline administration, these data further support this postulate. Recently, it has been postulated that sex differences in stress-mediated responses may contribute to the known sex differences in the pattern of cocaine abuse (see review in Becker et al. 2007). Thus, stress induction of the DARPP-32 cascade may have an impact on cocaine-induced regulation in this cascade.
After cocaine treatment, sex differences in the pattern of DARPP-32 activation were observed. Although striatal P-Thr34-DARPP-32 has been reported to be increased after acute cocaine administration, most of these studies are done on male mice (Nishi et al. 2000; Valjent et al. 2005; Zachariou et al. 2006). Indeed, only Rauggi et al. (2005) reported an induction of P-Thr34-DARPP-32 occurring 30 min after cocaine treatment in the NAc of male rats. The pattern of P-Thr34-DARPP-32 protein level changes reported here is similar to that observed by Rauggi et al. (2005). Since their study and ours use different cocaine doses, a dose-response study may be needed to further clarify these differences between the two studies. In female rats, cocaine administration decreased the protein levels of DARPP-32 and P-Thr34-DARPP-32. Because females’ higher basal protein levels are already at “ceiling” levels, it is possible that cocaine can only decrease these protein levels. As a protein that dephosphorylates P-Thr34-DARPP-32, the subsequent changes in PP-2B protein levels in both males and females may counteract the changes of P-Thr34-DARPP-32 protein levels after cocaine treatment. However, in female rats, PP-1 protein levels were increased after acute cocaine administration, whereas no significant changes were observed in male rats. Unlike the other proteins, the basal protein levels of PP-1 in females did not start at high “ceiling” levels, which made possible a further increase by acute cocaine treatment.
After cocaine treatment, higher increases in protein levels were observed in males than females. Indeed, in male rats, the protein levels of DARPP-32 signaling proteins were increased after cocaine administration, but the overall levels of these proteins were decreased in females. This difference further demonstrated a sexual dimorphic pattern in the profile of this pathway after cocaine administration. However, the contribution to sex differences of short-term changes in kinase activities, which are independent of transcriptional changes, are yet to be determined.
When experience takes the form of exposure to drug abuse, alterations in DARPP-32 function and activation appear to cause tolerance and dependence, an adaptation commonly associated with the development and maintenance of rewarding behaviors (Bibb et al. 2001). Sex specific differences exist at all stages of the cocaine-abuse progress wherein females are more sensitive during different phases. Since both Lynch et al. (2007) and Zachariou et al. (2006) demonstrated that dopamine-related protein changes may not always be concordant with cocaine-reinforcing effects, a study is needed to determine to what extent heightened basal responses in females and a sexual dimorphic pattern of DARPP-32-pathway contribute to the sex differences in the regulation of central nervous system plasticity that induce drug dependence. However, taken together, our findings suggest that females may have a different profile of elevated D1-mediated intracellular second messenger transduction, which in turn may underlie their higher rewarding sensitivity and locomotor behavior. An important issue not studied here is the effect of gonadal hormones and their fluctuation on the DARPP-32 cascade. It is feasible that because DA release and reuptake is affected by estrogen and progesterone circulatory levels, the DARPP-32 pathway will be equally affected. Further studies need to be done to address this important issue, which is highly relevant to women's health.
Acknowledgments
We are grateful to Dr. Patricia Stephens for her editorial comments. This work was supported by SCORE 506-GM60654, MIDARP DA12136, RCMI RR-03037, and SNRP NS-41073. We declare that all the experiments comply with the current laws of the United States.
Contributor Information
Luyi Zhou, Department of Biological Sciences, Hunter College of the City University of New York, New York, NY 10065, USA; Biology PhD Program, Graduate School and University Center of the City University of New York, New York, NY 10016, USA.
Arbi Nazarian, Department of Psychology, Hunter College of the City University of New York, New York, NY 10065, USA; Biopsychology and Behavioral Neuroscience PhD Subprogram, Graduate School and University Center of the City University of New York, New York, NY 10016, USA.
Wei-Lun Sun, Department of Psychology, Hunter College of the City University of New York, New York, NY 10065, USA; Biopsychology and Behavioral Neuroscience PhD Subprogram, Graduate School and University Center of the City University of New York, New York, NY 10016, USA.
Shirzad Jenab, Department of Psychology, Hunter College of the City University of New York, New York, NY 10065, USA; Biopsychology and Behavioral Neuroscience PhD Subprogram, Graduate School and University Center of the City University of New York, New York, NY 10016, USA.
Vanya Quinones-Jenab, Department of Psychology, Hunter College of the City University of New York, New York, NY 10065, USA; Biopsychology and Behavioral Neuroscience PhD Subprogram, Graduate School and University Center of the City University of New York, New York, NY 10016, USA; Biology PhD Program, Graduate School and University Center of the City University of New York, New York, NY 10016, USA.
Reference
- Adlersberg M, Hsiung SC, Glickstein SB, Liu KP, Tamir H, Schmauss C. Regulation of dopamine D-receptor activation in vivo by protein phosphatase 2B (calcineurin). J Neurochem. 2004;90:865–873. doi: 10.1111/j.1471-4159.2004.02562.x. [DOI] [PubMed] [Google Scholar]
- Auger AP, Meredith JM, Snyder GL, Blaustein JD. Oestradiol increases phosphorylation of a dopamine- and cyclic AMP-regulated phosphoprotein (DARPP-32) in female rat brain. J Neuroendocrinol. 2001;13:761–768. doi: 10.1046/j.1365-2826.2001.00700.x. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol Biochem Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Becker JB, Monteggia L, Perrot-Sinal T, Romeo RD, Taylor JR, Yehuda R, Bale T. Stress and disease: is being female a predisposing factor? J Neurosci. 2007;27:11851–11855. doi: 10.1523/JNEUROSCI.3565-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. doi: 10.1038/35066591. [DOI] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol Biochem Behav. 2007;86:117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab S. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Dalla C, Antoniou K, Kokras N, Drossopoulou G, Papathanasiou G, Bekris S, Daskas S, Papadopoulou-Daifoti Z. Sex differences in the effects of two stress paradigms on dopaminergic neurotrans-mission. Physiol Behav. 2008;93(3):595–605. doi: 10.1016/j.physbeh.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin S-N, Foltz R, Jenab S, Quinones-Jenab V. Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology. 2004;46:672–687. doi: 10.1016/j.neuropharm.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Festa ED, Jenab S, Weiner J, Nazarian A, Niyomachai T, Russo SJ, Kemen LM, Akhavan A, Wu HB, Quinones-Jenab V. Cocaine-induced sex differences in D1 receptor activation and binding levels after acute cocaine administration. Brain Res Bull. 2006;68:277–284. doi: 10.1016/j.brainresbull.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi A, Mermelstein PG, Song WJ, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault JA, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 2006;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Yang CR, Phillips AG, Blaha CD. Basolateral amygdala stimulation evokes glutamate receptor-dependent dopamine efflux in the nucleus accumbens of the anaesthetized rat. Eur J Neurosci. 1998;10:1251. doi: 10.1046/j.1460-9568.1998.00133.x. [DOI] [PubMed] [Google Scholar]
- Goto S, Matsukado Y, Mihara Y, Inoue N, Miyamoto E. The distribution of calcineurin in rat brain by light and electron microscopic immunohistochemistry and enzyme-immunoassay. Brain Res. 1986;397:161–172. doi: 10.1016/0006-8993(86)91381-8. [DOI] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of dopamine signaling. Biosci Rep. 2001;21:247–269. doi: 10.1023/a:1013205230142. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol Biochem Behav. 2005;82(1):170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Howland JG, Taepavarapruk P, Phillips AG. Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats. J Neurosci. 2002;22:1145. doi: 10.1523/JNEUROSCI.22-03-01137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29(1):81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hu XT, Ford K, White FJ. Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbens neurons. Neuropsychopharmacology. 2005;30:916–926. doi: 10.1038/sj.npp.1300654. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31(1):129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45(5):647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta JH. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- King MM, Huang CY, Chock PB, Nairn AC, Hemmings HC, Chan KF, Greengard P. Mammalian brain phosphoproteins as substrates for calcineurin. J Biol Chem. 1984;259:8080–8083. [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology, and function of reward pathways. Trends Pharmacol Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology. 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144(1):77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology. 2007;191:263–271. doi: 10.1007/s00213-006-0656-0. [DOI] [PubMed] [Google Scholar]
- McCance-Katz EF, Hart CL, Boyarsky B, Kosten T, Jatlow P. Gender effects following repeated administration of cocaine and alcohol in humans. Subst Use Misuse. 2005;40:511–528. doi: 10.1081/ja-200030693. [DOI] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their response to restraint stress in rats. Eur J Neurosci. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Mulder AB, Hodenpijl MG, Lopes da Silva FH. Electrophysiology of the hippocampal and amygdaloid projections to the nucleus accumbens of the rat: convergence, segregation, and interaction of inputs. J Neurosci. 1998;18:5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarian A, Russo SJ, Festa ED, Kraish M, Quinones-Jenab V. The role of D1 and D2 receptors in the cocaine conditioned place preference of male and female rats. Brain Res Bull. 2004;63:295–299. doi: 10.1016/j.brainresbull.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Wu HBK, Sun WL, Kemen LM, Jenab S, Quinones-Jenab V. Sex differences in basal and cocaine-induced alterations in PKA protein levels in the nucleus accumbens. 2008 doi: 10.1007/s00213-008-1411-5. in press. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Natl Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NSDUH . Results from the 2001 National Survey on Drug Use and Health. Office of Applied Studies; Rockville, MD: 2002. [Google Scholar]
- Rauggi R, Scheggi S, Cassanelli A, De Montis MG, Tagliamonte A, Gambarana C. The mesolimbic dopaminergic response to novel palatable food consumption increases dopamine-D1 receptor-mediated signaling with complex modifications of the DARPP-32 phosphorylation pattern. J Neurochem. 2005;92:867–877. doi: 10.1111/j.1471-4159.2004.02920.x. [DOI] [PubMed] [Google Scholar]
- Ridenour TA, Maldonado-Molina M, Compton WM, Spitznagel EL, Cottler LB. Factors associated with the transition from abuse to dependence among substance abusers: implications for a measure of addictive liability. Drug Alcohol Depend. 2005;80:1–14. doi: 10.1016/j.drugalcdep.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Jenab S, Fabian SJ, Festa ED, Kemen LM, Quinones-Jenab V. Sex differences in the conditioned rewarding effects of cocaine. Brain Res. 2003;970:214–220. doi: 10.1016/s0006-8993(03)02346-1. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Carmona GN. Effects of dopamine agonists and antagonists on locomotor activity in male and female rats. Pharmacol Biochem Behav. 2002;72:857–863. doi: 10.1016/s0091-3057(02)00770-0. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Herve E, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol Biochem Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Puglisi-Allegra S. Genetic susceptibility of mesocortical dopamine to stress determines liability to inhibition of mesoaccumbens dopamine and to behavioral ‘despair’ in a mouse model of depression. Neuroscience. 2002;115(4):999–1007. doi: 10.1016/s0306-4522(02)00581-x. [DOI] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake in are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–1070. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–1202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- Wright CI, Beijer AVJ, Groenewegen HJ. Basal amygdaloid complex afferents to the rat accumbens are compartmentally organized. J Neurosci. 1996;16:1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentration in male and female rats: effects of estrous cycle and gonadectomy. Neurosci Lett. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]