Abstract
The water-gas shift (WGS) reaction (CO + H2O ⇆ CO2 + H2) is of major industrial significance in the production of H2 from hydrocarbon sources. High temperatures are required, typically in excess of 200 °C, using d-metal catalysts on oxide supports. In our study the WGS process is separated into two half-cell electrochemical reactions (H+ reduction and CO oxidation), catalyzed by enzymes attached to a conducting particle. The H+ reduction reaction is catalyzed by a hydrogenase, Hyd-2, from Escherichia coli, and CO oxidation is catalyzed by carbon monoxide dehydrogenase (CODH I) from Carboxydothermus hydrogenoformans. This results in a highly efficient heterogeneous catalyst with a turnover frequency, at 30 °C, of at least 2.5 s−1 per minimum functional unit (a CODH/Hyd-2 pair) which is comparable to conventional high-temperature catalysts.
Graphical Abstract
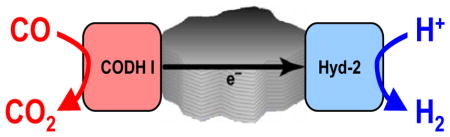
The water-gas shift (WGS) reaction is an industrially important process for converting CO into H2 (Reaction 1) and thus providing fuel-cell grade H2 from steam reforming. The standard thermodynamics are marginally favorable, ΔHө = −41.2 kJ mol−1, ΔGө298 = −28.6 kJ mol−1 and become even less favorable as the temperature is raised.1 In industry the reaction is typically carried out at high temperatures (at least 200 °C) using d-metal catalysts often on oxide supports.2–4 This is a thriving area of R&D in which various nanomaterials3, 5 are being studied as well as a range of homogeneous catalysts6 based on Fe, W and Ru carbonyl complexes.
| (1) |
Enzymes are noted for highly efficient catalysis. Although no single enzyme is known to catalyze the WGS reaction, the overall process occurs in certain bacteria using enzymes that are coupled via long-range electron transfer. These organisms include the purple photosynthetic bacterium Rubrivivax gelatinosus, which has been studied to investigate the biological WGS system,7 and Carboxydothermus hydrogenoformans (Ch), an anaerobic organism that can live on CO as sole carbon source, and as sole energy source evolving H2 as a product.8
We recently showed how attachment of complementary enzymes (one is electron-donating, the other electron accepting) to electronically conducting particles produces an unusual heterogeneous catalyst for linking separate specific oxidation and reduction reactions.9 The two enzymes are adsorbed on flake-like particles of pyrolytic graphite (platelets, several μm diameter; see Ref. 9) on which they become electrically coupled. We have now extended this concept to design and demonstrate a bespoke WGS catalyst based on two highly active Ni- and Fe-containing enzymes: a CO dehydrogenase (CODH) and a hydrogenase.
The organism Ch expresses five CODH enzyme complexes, all of which catalyze the interconversion of CO and CO2 at an unusual [Ni4Fe-5S] cluster.10 Of these, CODH I and CODH II are known to be highly efficient CO scavengers, with very low KM values (2 μM for CODH I at pH 6, 25 °C) and turnover frequencies of up to 40,000 s−1.8, 11 We previously established CODH I to be highly active in both directions when adsorbed to sub-monolayer electroactive coverage on a pyrolytic graphite ‘edge’ (PGE) electrode.11 Hydrogenases are also highly electroactive enzymes (turnover frequencies ranging between 102 and 104 s−1)12 and, as the H2 production catalyst, we used a [NiFe]-hydrogenase, Hyd-2, isolated from Escherichia coli (Ec).
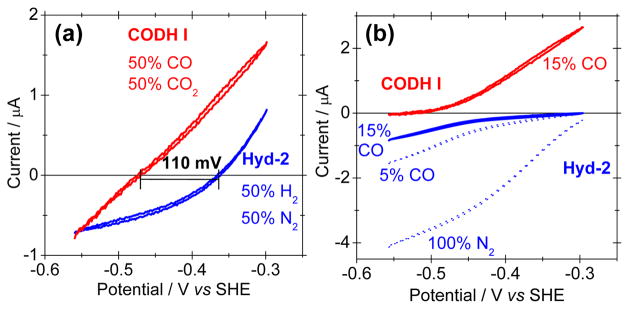
The voltammograms in Figure 1(a) show the activities of CODH I and Hyd-2 on PGE electrodes. The two voltammograms cut sharply through the zero-current axis at their respective cell potentials under experimental conditions, showing that no electrochemical overpotential is required for either system. The difference in the intersection potentials, 110 mV, marks the small Gibbs energy difference under these conditions. Figure 1(b) shows the electrocatalytic activities of CODH I and Hyd-2 under the initial conditions of the particle experiments (15% CO in N2), CO in N2 and 100% N2). The presence of 15% CO (far exceeding KMCO for CODH I11) decreases the H2 production activity of Hyd-2 to approximately 15% of its activity in 100% N2. Normally [NiFe]-hydrogenases are less CO-sensitive than their [FeFe]-counterparts, but they tend to be poor at H2 production and many are strongly inhibited by H2.12 In contrast, Hyd-2 is a proficient H2 producer even in the presence of H2 and CO (Figure 1(a) and 1(b), respectively). In turn, CODH I is not inhibited by H2, nor by CO2 to any significant degree.11
Figure 1.
Voltammograms of CODH I and Hyd-2 adsorbed on a PGE electrode under various gas atmospheres at 30 °C and pH 6, scanned across the range of interest for the WGS reaction. In all cases, the scan rate is 10 mV s−1 and electrode rotation rate is 4000 rpm. For CODH I the cell buffer was 0.20 M MES, and for Hyd-2 a mixed buffer was used (see Supporting Information).
Strict anaerobicity was maintained throughout all stages. Platelets (~15 mg) were ground from pyrolytic graphite using SiC paper and dispersed by sonication in 50 μL buffer in a glass test tube. Abrading graphite with SiC does not result in contamination by SiC debris.13 Varying amounts of CODH I and Hyd-2 (20–180 μL aliquots, stock concentrations 1–120 μM) were premixed, then added to the tube. After 30 minutes on ice (to optimize enzyme adsorption), the suspension was diluted with buffer, transferred to an ultracentrifuge tube and spun to pellet the graphite platelets. The supernatant was then removed and the platelets were resuspended in fresh enzyme-free buffer, and transferred back to the glass tube which was then sealed with a rubber septum. Further experimental details are found in Supporting Information.
The reaction was started by injecting aliquots of CO gas into the 3.5 ml headspace of the reaction vessel. Concentrations of CO and H2 were monitored by GC at regular time intervals throughout the reaction. Figure 2 shows the simultaneous H2 production and CO consumption in an experiment lasting over 55 h. Throughout this time-course, the amount of H2 produced is equal to the amount of CO consumed (within experimental error). The initial CO concentration is almost fully depleted over the first 21 h, but re-charging with further injections of CO (600 μL into the headspace) at this time, and after 52 h, fuels further H2 production. The stability of the system was assessed by injecting CO into the sealed tube after it had been stored anaerobically at 4 °C for 14 days: the activity was almost equal to that shown by the freshly prepared system in Figure 2. Results were reproducible over several preparations of functionalized platelets and using different samples of enzymes prepared according to references given in Supporting Information.
Figure 2.
H2 production and CO depletion over the course of 55 h, with fresh CO injections of 600 μL at the points indicated. Errors in H2/CO quantification are described within the Supporting Information. Quantities expressed in μmol were calculated from GC values.
Removal of free enzyme in solution (by centrifugation and buffer exchange) shows that the activity stems from the enzyme-loaded platelets, which are thus available in a form that can be separated, stored and re-used. Five control experiments were carried out, in which each of the following components were omitted in turn from the preparation described earlier: graphite platelets, both enzymes, CODH I, Hyd-2 and CO. In each of these controls, an insignificant amount of H2 (< 0.01%) was produced after 24 h. Activity increased with temperature, up to an optimum of 30 °C. Experiments carried out over the pH range 5 to 7 showed no significant variation in the amount of H2 produced by the system over 5 h (see Supporting Information).
Varying the quantities of the two enzymes showed that optimal activity was achieved with a CODH I:Hyd-2 molar ratio of approximately 20:1 (40 μL of 117 μM CODH 1 and 160 μL of 1.5 μM Hyd-2). UV absorption studies (280 nm) for individual enzymes under these conditions showed that (at most) 3.6 nmol of CODH I (77% of the amount in solution) and 0.1 nmol of Hyd-2 (50% of the amount in solution) were adsorbed onto the platelets (see Supporting Information). Although CODH I is highly active14 much is adsorbed on PGE in a non-electroactive manner.11 A key to the success of the platelet-based catalyst is that the relatively large size of the platelet (average surface area 23 μm2)9 allows thousands of enzyme molecules to adsorb, and the quantities of each enzyme can be varied to optimize activity.
Based on the estimated enzyme loadings, the information in Figure 2 corresponds to an average H2 production rate of 2.5 (mol H2) s−1 (mol adsorbed Hyd-2)−1 and a CO depletion rate of 0.07 (mol CO) s−1 (mol adsorbed CODH I)−1. The rates per enzyme molecule are lower limits because we have assumed that all adsorbed enzyme is electrocatalytically active. The empirical turnover frequency must be based on the minority enzyme i.e Hyd-2 and therefore the particles display an equivalent per-‘site’ WGS turnover frequency of at least 2.5 s−1 at 30 °C. This value is much lower than the rate at which hydrogenases can operate but it is essential to note that in this particle system, the driving force is very small and CO, albeit a weak inhibitor for Hyd-2, does attenuate activity (see Fig. 1B). Scaling this value for experiments carried out at lower temperatures (SI, Fig. S5) gives equivalent turnover frequencies of at least 1.5 s−1 at 20 °C and 0.8 s−1 at 10 °C. For comparison, a Ru3(CO)12 homogeneous catalyst is reported as having a WGS reactivity of 0.01 (mol H2) s−1 (mol catalyst)−1 at 160 °C;6 Au-CeO2 nanomaterials, regarded as being highly efficient heterogeneous catalysts, show turnover frequencies up to 3.9 (site−1) s−1 at 240 °C.3–5
In conclusion, Ec Hyd-2 and Ch CODH I co-attached to a conducting graphite platelet react in concert with such high efficiency that the industrially important WGS reaction, which has only modest thermodynamic favorability, can be carried out at room temperature at a rate at least as high as achieved by synthetic catalysts at elevated temperatures.
The experiments are unlikely to have direct relevance for energy technology as they use tiny amounts of fragile enzymes rather than robust synthetic catalysts that could be scaled up indefinitely. Even so, the study demonstrates some interesting alternative concepts for catalysis and highlights the wide gap between redox enzymes and synthetic catalysts in terms of both rates and efficiency. Coupling via electronically conducting particles enables a catalytic redox reaction to be separated into two half-reactions having lower activation energies than the entire reaction at a single site. The H+/H2 and CO2/CO redox couples are both reversible at electrodes modified with hydrogenases and CODH, respectively, whereas the best that chemistry can currently offer are Pt metal catalysts for the hydrogen system; however, Pt is incompatible with CO and could not be used here. Finally, the electrochemical reversibility of the CO2/CO couple catalyzed by CODH is not only a key requirement for the WGS particle catalyst, but is also highly inspirational in view of demonstrating the feasibility of efficient CO2/CO electrochemical cycling with CO serving as an energy store.
Supplementary Material
Acknowledgments
This research was supported by UK research councils BBSRC and EPRSC (Grant BB/D52222X and Supergen 5 to FAA, P15018 to FS), and NIH (GM39451 to SWR) and Merton College, Oxford (Junior Research Fellowship to AP).
Footnotes
Supporting Information Available: Materials, physical measurements, electrochemistry, platelets preparation technique and WGS reaction, experimental error, spectrophotometric determination of the amount of enzyme adsorbed on platelets, effect of varying the amounts of CODH I and Hyd-2, effect of temperature on H2 production rate, effect of pH on H2 production rate. This information is available free of charge via the Internet at http://pubs.acs.org/
References
- 1.Laine RM, Crawford EJ. J Mol Catal. 1988;44:357–387. [Google Scholar]
- 2.Holleman A, Wiberg N. Lehrbuch der Anorganischen Chemie Walter de Gruyter & Co. 2007. [Google Scholar]
- 3.Kim CH, Thompson LT. J Catal. 2005;230:66–74. [Google Scholar]
- 4.Burch R. Phys Chem Chem Phys. 2006;8:5483–5500. doi: 10.1039/b607837k. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez JA, Ma S, Liu P, Hrbek J, Evans J, Perez M. Science. 2007;318:1757–1759. doi: 10.1126/science.1150038. [DOI] [PubMed] [Google Scholar]
- 6.King RB. J Organomet Chem. 1999;586:2–17. [Google Scholar]
- 7.Markov SA, Weaver PF. Appl Biochem Biotechnol. 2008;145:79–86. doi: 10.1007/s12010-007-8032-z. [DOI] [PubMed] [Google Scholar]
- 8.Svetlitchnyi V, Peschel C, Acker G, Meyer O. J Bacteriol. 2001;183:5134–5144. doi: 10.1128/JB.183.17.5134-5144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vincent KA, Li X, Blanford CF, Belsey NA, Weiner JH, Armstrong FA. Nat Chem Biol. 2007;3:760–761. doi: 10.1038/nchembio.2007.47. [DOI] [PubMed] [Google Scholar]
- 10.Wu M. Public Libr Sci Genet. 2005;1:563–574. [Google Scholar]
- 11.Parkin A, Seravalli J, Vincent KA, Ragsdale SW, Armstrong FA. J Am Chem Soc. 2007;129:10328–10329. doi: 10.1021/ja073643o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent KA, Parkin A, Armstrong FA. Chem Rev. 2007;107:4366–4413. doi: 10.1021/cr050191u. [DOI] [PubMed] [Google Scholar]
- 13.Blanford CF, Armstrong FA. J Solid State Electrochem. 2006;10:826–832. [Google Scholar]
- 14.Ragsdale SW. Crit Rev Biochem Mol Biol. 2004;39:165–195. doi: 10.1080/10409230490496577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.