Abstract
Preservation of gas exchange mandates that the pulmonary alveolar surface restrain unnecessarily harmful inflammatory responses to the many challenges to which it is exposed. These responses reflect the crosstalk between alveolar epithelial cells (AECs) and resident alveolar macrophages (AMs). We recently determined that AMs can secrete suppressors of cytokine signaling (SOCS) proteins within microparticles (MPs). Uptake of these SOCS-containing vesicles by epithelial cells inhibits cytokine-induced STAT activation. However, the ability of epithelial cells to direct AM release of SOCS-containing vesicles in response to inflammatory insults has not been studied. Here we report that SOCS3 protein was elevated in bronchoalveolar lavage fluid (BALF) of both virus- and bacteria-infected mice, as well as in an in vivo LPS model of acute inflammation. In vitro studies revealed that conditioned medium from LPS-stimulated AECs (AEC-CM) enhanced AM SOCS3 secretion above basal levels. Increased amounts of PGE2 were present in AEC-CM after LPS challenge, and both pharmacologic inhibition of PGE2 synthesis in AECs and neutralization of PGE2 in AEC-CM implicated this prostanoid as the major AEC-derived factor mediating enhanced AM SOCS3 secretion. Moreover, pharmacologic blockade of PGE2 synthesis or genetic deletion of a PGE2 synthase similarly attenuated the increase in BALF SOCS3 noted in lungs of mice challenged with LPS in vivo. These results demonstrate a novel tunable form of crosstalk in which AECs utilize PGE2 as a signal to “request” SOCS3 from AMs in order to dampen their endogenous inflammatory responses during infection.
INTRODUCTION
Inflammatory responses in the alveolar space must be tightly controlled in order to ensure maintenance of gas exchange. Homeostasis in this hostile environment is facilitated by a uniquely quiescent and suppressive phenotype manifested by alveolar macrophages (AMs), the resident innate immune cells of the alveolus. For instance, these cells are known to release soluble mediators which can inhibit immune responses (1). Both older morphometric studies (2) and recent live imaging studies (3) have demonstrated approximately one AM per alveolus in the mammalian lung. Moreover, AMs have recently been reported to be primarily sessile (3), challenging classical notions. Both of these considerations support the importance of paracrine communication with other cell types in order for AMs to exert their regulatory effects.
Alveolar epithelial cells (AECs) serve as both a functional and physical barrier to invading pathogens and other insults in the lung. In conjunction with immune cells, AECs have been shown to play a role in the recognition and clearance of microbial agents (4, 5). The alveolar epithelium consists of two cell types, type I AECs and type II AECs. Type I AECs comprise approximately 95% of the alveolar surface area and function mainly in gas exchange, although they are also important participants in inflammatory responses to pathogens (6, 7). Type II AECs make up approximately 5% of the alveolar surface area and are necessary for surfactant synthesis, serve as stem cells for repair of the epithelium after injury, and can also contribute to lung defense by secreting factors such as surfactant proteins (8, 9).
Recent studies have focused on the association and crosstalk between AMs and AECs in both lung homeostasis and inflammation (4, 5, 10). In homeostasis, AMs reside in close proximity to AECs and the interaction of cell surface receptors between them aid in the suppression of resident lymphocytes (1, 10). During an inflammatory response, these interactions are inhibited, prompting the release of soluble mediators and initiation of inflammatory signaling cascades such as the JAK/STAT pathway (11, 12). Our laboratory has recently reported that AMs can secrete suppressors of cytokine signaling (SOCS) proteins within two types of extracellular vesicles, namely microparticles (MPs) (containing SOCS3) and exosomes (containing SOCS1), which can be subsequently taken up by epithelial cells to dampen their endogenous STAT activation (13).
However, the modulation of this phenomenon during innate inflammatory challenges has not been explored. Furthermore, it is not known whether AECs can direct SOCS3 release by AMs. Given the importance of JAK/STAT signaling in the AEC response to inflammatory insults (11) and the fact that AECs themselves possess low endogenous levels of SOCS3 (13, 14), we hypothesized that AECs can “request” SOCS3 from AMs when challenged with an inflammatory stimulus in order to facilitate their ability to restrain inflammation and prevent injury. Here we report that LPS challenge of AECs increases the secretion of SOCS3 by AMs, and that AEC-derived prostaglandin E2 (PGE2) was largely responsible for enhancing AM SOCS3 secretion in vitro. In an in vivo model of LPS challenge, PGE2 was also required for enhanced SOCS3 secretion in bronchoalveolar lavage fluid (BALF). Finally, AM-derived conditioned medium (AM-CM) containing SOCS3 attenuated LPS-induced STAT3 activation in AECs. These data demonstrate that AECs require AM-derived SOCS3 to inhibit their endogenous inflammatory signaling and utilize secretion of PGE2 as a signal for AMs to “send SOCS.” This bidirectional circuit of information flow enables the AEC-AM unit to calibrate its supply of anti-inflammatory signals when innate immune challenges demand it.
MATERIALS AND METHODS
Animals
Pathogen-free 125–150 g female Wistar rats were purchased from Charles River Laboratories and 6 week old male C57BL/6 wild-type mice purchased from The Jackson Laboratory. Microsomal prostaglandin E synthase-1 (mPGEs-1) knockout mice on a DBA1lac/J background (15) were originally obtained from Pfizer Inc. (Groton, CT) and backcrossed on a C57BL/6 background. Homozygous knockout mice were then bred at the University of Michigan. Animals were treated according to NIH guidelines for the use of experimental animals with the approval of the University of Michigan Committee for the Use and Care of Animals.
Cell lines
The spontaneously immortalized rat AEC cell line L2 (CCL-149) with predominant type I cell-like characteristics (16, 17) was purchased from the ATCC and cultured in F-12K medium with 10% FCS and penicillin-streptomycin.
Reagents
PGE2 from Cayman Chemical (Ann Arbor, MI) was dissolved in DMSO and stored under N2 at −80°C. Murine and rat cytokines (IL-6, MCP-1, TGFβ and GM-CSF) were purchased from Peprotech (Rocky Hill, NJ). Mouse monoclonal Ab against SOCS3 was purchased from Abcam (San Francisco, CA) and rabbit polyclonal Ab against phospho-STAT3 was purchased from Cell Signaling Technology (Danvers, MA). Mouse monoclonal Ab against β-actin and the reagents aspirin, indomethacin, LPS from Escherichia coli (clone 0111:B4) and zymosan from Saccharomyces cerevisiae were purchased from Sigma (St. Louis, MO). Neutralizing antibodies for MCP-1 and PGE2 were purchased from R&D Systems (Minneapolis, MN) and Cayman Chemical, respectively.
AM isolation and culture
Primary AMs were lavaged from rat lungs and plated in serum-free RPMI at a concentration of 1 × 106 cells/mL, as described (18). The cells were adhered for at least 1 h and then washed to remove non-adherent cells as well as all MPs and secreted substances that were activated by their adherence to plastic. The cells were then treated with appropriate compounds for indicated times.
In vitro LPS stimulation of AECs
L2 cells (0.5 × 106 cells/well in a 6-well plate) were equilibrated in F-12K medium for 24 h. They were then washed and incubated with 2 mL serum-free RPMI and cultured for 1, 8 and 24 h with or without the addition of LPS (10 ng/mL). Secreted PGE2 and cytokines were analyzed by ELISA.
Western blotting
AMs (1 × 106 cells/mL) were plated in serum-free RPMI 1640 in 6-well tissue culture dishes and incubated in the presence or absence of compounds of interest for the indicated amounts of time. The resulting supernatants were harvested and centrifuged at 500 × g (10 min) and 2,500 × g (12 min) to yield cell and apoptotic body-free CM (19). The resulting “neat” CM and secreted proteins were concentrated using 3 kDa Amicon size exclusion filters from Millipore (Billerica, MA). Protein concentrations were determined by modified Lowry protein assay from Bio-Rad (Hercules, CA). Samples containing 20–30 μg protein were separated by SDS-PAGE using 12.5% gels and then transferred overnight to nitrocellulose membranes. Membranes were blocked with 4% BSA and probed overnight with antibodies directed against SOCS3 (titer of 1:750), phospho-STAT3 (titer of 1:1000) and β-actin (titer of 1:10,000). A secondary antibody incubation with peroxidase-conjugated goat anti-rabbit (or anti-mouse) from Cell Signaling Technology was performed, and film was developed using ECL detection from Amersham Biosciences (Piscataway, NJ). Relative band densities were determined by densitometric analysis using NIH Image J software, and were expressed as described in figure legends.
In vitro co-culture experiments
To assess the ability of secretory products of rat AECs to affect SOCS3 secretion by AMs, L2 cells were equilibrated in F-12K medium for 24 h. They were washed and incubated with 2 mL serum-free RPMI and cultured for an additional 24 h with or without the addition of LPS (10 ng/mL). The resulting AEC-CM was collected and depleted of dead cells, debris and apoptotic bodies by centrifugation as previously described. Primary rat AMs were harvested as described and were cultured in serum-free RPMI for 1 h and then washed. AEC-CM (2 ml/well) was added to the AMs for 2 h, then the AMs were washed and cultured in 4 mL fresh serum-free RPMI for an additional hour. The resulting AM-CM was collected and processed as previously described, and the presence of SOCS3 was analyzed by western blot. Contributions of AEC-derived factors were investigated by the addition of neutralizing antibodies to MCP-1 (R&D Systems) and PGE2 (Cayman Chemical) or by treating AECs with pharmacologic inhibitors (aspirin).
In vivo LPS treatment
To investigate the in vivo effects of LPS treatment on SOCS3 secretion in the lung, mice were oropharyngeally administered 2.5 μg LPS in 50 μl sterile PBS or PBS alone. BALF was harvested at indicated times and analyzed for SOCS3 (Cloud-Clone Corp, Houston, TX) and PGE2 (Enzo Life Sciences, Ann Arbor, MI) by ELISA. Prior to SOCS3 ELISA analysis, BALF was concentrated using 3 kDa exclusion filters (Millipore) and briefly sonicated (30% intensity, 10 seconds 3x, on ice) to disrupt MP membranes and release SOCS3. To determine the contribution of PGE2 in LPS-induced SOCS3 secretion, mice were administered indomethacin (1.2 mg/kg, s.c.)(20) 30 min prior to and 2 h after treatment with LPS.
In vivo viral infections
Mice were infected with either mouse adenovirus type 1 (MAV-1) or murine gammaherpesvirus-68 (γHV68) as previously described (21, 22). Briefly, mice were anesthetized with ketamine and xylazine and were infected intranasally with either 1×105 pfu of MAV-1 or 5×104 pfu γHV68. Control mice were given the same volume of sterile PBS. Mice were sacrificed 7 d post-infection and BALF was collected and analyzed for SOCS3 by ELISA as described above.
In vivo bacterial infection
To determine the effects of bacterial infection on SOCS3 secretion in the lung, mice were anesthetized with ketamine and xylazine and were oropharyngeally administered 1×104 cfu of Klebsiella pneumoniae in sterile PBS or PBS alone. BALF was harvested at 24 h post infection and analyzed for SOCS3 and PGE2 by ELISA.
Zymosan-induced peritonitis model
Mice were injected intraperitoneally with 10 μg zymosan from Saccharomyces cerevisiae and sacrificed after 4 h. AMs were collected via bronchoalveolar lavage and PMNs were collected via peritoneal lavage. AMs were plated at a concentration of 1 × 106 cells/mL in serum-free RPMI and the resulting AM-CM was collected after 1 h of adherence. PMNs were plated at a concentration of 1 × 106 cells/mL in serum-free RPMI on low-binding tissue culture dishes and CM was collected after 1 h of culture. The remaining AMs and PMNs were lysed in radioimmunoprecipitation (RIPA) buffer and the resulting lysates and CM was analyzed for SOCS3 by ELISA.
RNA isolation and quantitative real-time PCR (qRT-PCR) analysis of MCP-1 mRNA in AECs
RNA was extracted from AECs using QIAGEN columns according to manufacturer’s instructions and converted to cDNA via reverse transcription. MCP-1 mRNA levels were assessed by using SYBR Green (Applied Biosystems, Foster City, CA) on an ABI Prism 7300 thermocycler (Applied Biosystems). Relative gene expression was determined by the ΔCT method, with β-actin as a reference gene. Primer sequences for MCP-1 and β-actin were 5′-AGCATCCACGTGTTGGCTC-3′ (f), 5′-CCAGCCTACTCATTGGGATCAT-3′ (r) and 5′-ACCCTAAGGCCAACCGTGA-3′ (f), 5′-CAGAGGCATACAGGGACAGCA-3′ (r), respectively.
Statistical Analysis
Data are expressed as mean ± SEM from at least 3 independent experiments unless noted in the figure legend. SEM for controls was obtained by calculating the fold change between the mean control value from replicate experiments (or animals) and individual control values. Data were analyzed using the Prism 5.0 statistical program from GraphPad software, using either ANOVA with Bonferroni’s post-hoc analysis or student’s t-test, as appropriate. Statistical significance was set at p-value <0.05.
RESULTS
SOCS3 secretion is upregulated in both viral and bacterial infections in mice
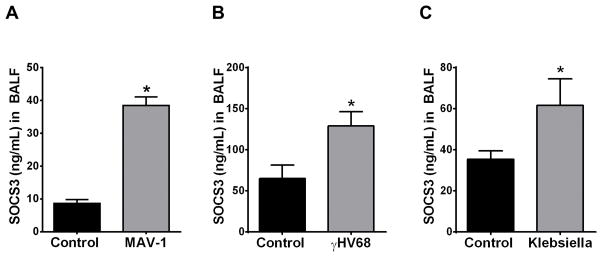
Although we previously determined that in vivo AM secretion of vesicular SOCS3 was downregulated by cigarette smoke in both mice and humans (13), it is not known if this process is altered in other inflammatory contexts. As recent studies have implicated intracellular SOCS3 in the regulation of inflammation and infection (23, 24), we sought to investigate the effects of both viral and bacterial infection on extracellular SOCS3 secretion in the lung. In these experiments, SOCS3 levels were quantified by ELISA in BALF that had been sonicated to rupture MPs. C57BL/6 mice subjected to intrapulmonary infection with MAV-1 for 7 d (Fig. 1A), γHV68 for 7 d (Fig. 1B), or the Gram-negative bacterium Klebsiella pneumoniae for 24 h (Fig. 1C) all exhibited significantly higher SOCS3 levels in BALF as compared to control mice. Interestingly, SOCS1, another SOCS family protein we previously found to be secreted by AMs (via exosomes), was not increased in any of these models of infection (data not shown).
Figure 1. SOCS3 secretion into the murine alveolar space is upregulated in both viral and bacterial infections.
SOCS3 protein levels in C57BL/6 mice that were infected with either 1×105 pfu MAV-1 (A), 5×104 pfu γHV68 (B), or 1×104 cfu Klebsiella pneumoniae (C). BALF was collected at 7 d post-viral infection and 24 h post-Klebsiella infection. After removal of cellular debris and apoptotic bodies, BALF was concentrated using a 3 kDa exclusion filter and was briefly sonicated to disrupt SOCS3-containing MPs for analysis by ELISA. Data are expressed as mean ± SEM, *= p<0.05, n=3–7 mice per group.
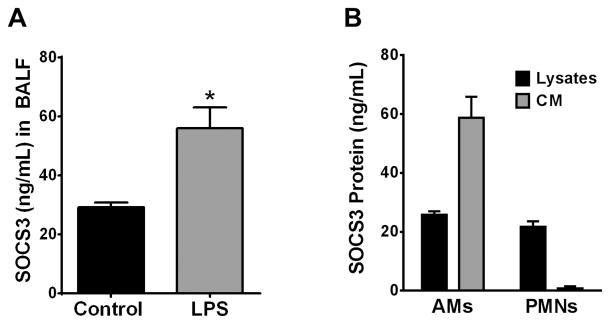
SOCS3 secretion in an in vivo LPS model of acute inflammation is mainly from resident AMs, not recruited PMNs
LPS is a prototypic Gram-negative pathogen-associated molecular pattern acting via TLR4 which is known to activate the JAK/STAT pathway (25). Moreover, intracellular SOCS3 is reported to protect myeloid cells during LPS-induced lung injury (26, 27). For these reasons, we employed LPS as a model innate inflammatory stimulus with which to elucidate the mechanisms whereby SOCS3 secretion is enhanced during infection. As was the case with microbial challenge, intrapulmonary administration of LPS (2.5 μg/mouse) resulted in a significant increase of SOCS3 in BALF after 24 h (Fig. 2A). Since LPS-induced inflammation results in recruitment of neutrophils (PMNs) to the alveolar space (28) and PMNs have the capacity to shed extracellular vesicles (29), we explored the ability of recruited PMNs to secrete SOCS3. To acquire sufficient numbers of PMNs for in vitro culture, we employed a well-characterized zymosan peritonitis model which results in recruitment of large numbers of PMNs into the peritoneal cavity within 4 h of treatment (30). PMNs (purified by their failure to adhere to low-adherence tissue culture plates) as well as lavaged AMs obtained from these same mice were cultured for 1 h and their lysates and CM analyzed by ELISA for SOCS3. Despite similar levels of SOCS3 protein in cell lysates of the two cell types, PMNs completely lacked the ability to secrete SOCS3 in comparison to AMs (Fig. 2B). These data suggest that resident AMs, but not recruited PMNs, represent the main source of the increased SOCS3 secreted in response to in vivo LPS stimulation.
Figure 2. Increased SOCS3 secretion during the host response to in vivo inflammation is mainly from resident AMs, not recruited PMNs.
(A) C57BL/6 mice were treated with 2.5 μg LPS via oropharyngeal inhalation and were sacrificed after 24 h. BALF was collected and processed as previously described and analyzed for SOCS3 by ELISA. (B) Mice were injected intraperitoneally with 10 μg zymosan and sacrificed after 4 h. The lungs and peritoneal cavity were lavaged and cells were resuspended in serum-free RPMI and cultured in low-binding tissue culture treated dishes for 1 h at a concentration of 1×106 cells/mL. The resulting cells and CM were collected, cells were lysed, and lysates and medium analyzed separately for SOCS3 by ELISA. Each value is the mean ± SEM of triplicate samples of the pooled material from 10 mice; *= p<0.05.
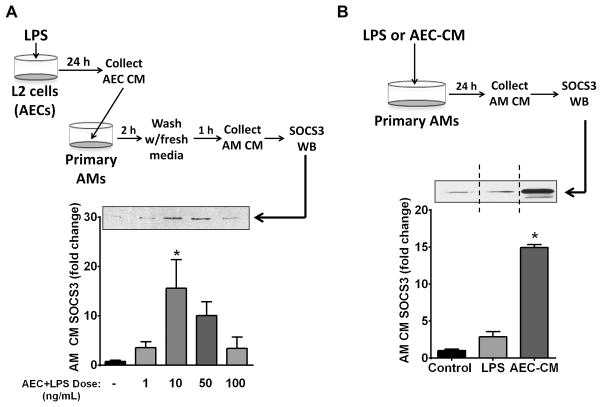
LPS elicits elaboration of AEC-derived factors that can enhance SOCS3 secretion by AMs in vitro
The ability of LPS to enhance SOCS3 secretion in vivo could reflect sensing of this pathogen-associated molecular pattern by either AECs or AMs. We therefore wished to evaluate the indirect effects via LPS potentiation of AEC-derived secretagogues as well as the direct effects of LPS on SOCS3 secretion by AMs. Cultures of rat AEC-derived L2 cells were therefore treated with varying doses of LPS for 24 h, the resulting AEC-CM was added to primary rat AMs for 2 h, and the amount of SOCS3 secreted in the subsequent 1-h was measured by western blot (Fig. 3A). LPS treatment of AECs yielded CM that dose-dependently increased the ability of AMs to secrete SOCS3, with a dose of 10 ng/mL LPS having the greatest effect with an approximately 15-fold induction (Fig. 3A). Direct addition of LPS (10 ng/mL) to primary AMs for 24 h also potentiated SOCS3 secretion, but this increase did not reach statistical significance and was approximately 5-fold less than that elicited by AM exposure to LPS-stimulated AEC-CM (Fig. 3B). In vitro exposure of macrophages to LPS has been reported to upregulate expression of SOCS3 protein within a similar time frame (31); thus, the modest increase in SOCS3 secretion by AMs directly treated with LPS may reflect potentiation of both expression as well as secretion of SOCS3. Because AM secretion of SOCS3 was much more robust in response to short-term exposure to CM from AECs treated with LPS for 24 h, we chose in subsequent experiments to focus on the identity of the AEC-derived secretagogue(s) responsible for this effect.
Figure 3. LPS induces elaboration of AEC-derived factors that can enhance SOCS3 secretion by AMs in vitro.
(A) AECs were plated at a concentration of 5 × 105 cells/well of a 6-well plate and equilibrated for 16 h. The cells were washed and cultured with the indicated doses of LPS in serum-free RPMI for 24 h and the resulting AEC-CM was collected and cleared of cellular debris and apoptotic bodies. Primary AMs were collected and adhered for 1 h. The AMs were washed and incubated with AEC-CM for 2 h. After a second wash the AMs were cultured in serum-free RPMI for an additional 1-h and the resulting AM-CM was collected and analyzed for SOCS3 protein by WB. (Top) Schematic of experiments in which CM from AECs treated with LPS is incubated with AMs for subsequent evaluation of SOCS3 secretion. (Bottom) AM SOCS3 secretion by western blot analysis; above, representative blot; below, fold increase in SOCS3 compared to control based on densitometric analysis. (B) Direct versus indirect effect of LPS on AM SOCS3 secretion. 1×106 AMs were adhered and incubated for 24 h in serum-free RPMI with either 10 ng/mL LPS or with CM from AECs that had been incubated for 24 h with 10 ng/mL LPS. The CM was collected as previously described and analyzed for SOCS3 by WB; above, representative blot; vertical dashed line indicates that the two lanes depicted were from the same blot but were not contiguous; below, fold increase in SOCS3 compared to control based on densitometric analysis. Data are expressed as mean ± SEM from three separate experiments, *= p<0.05.
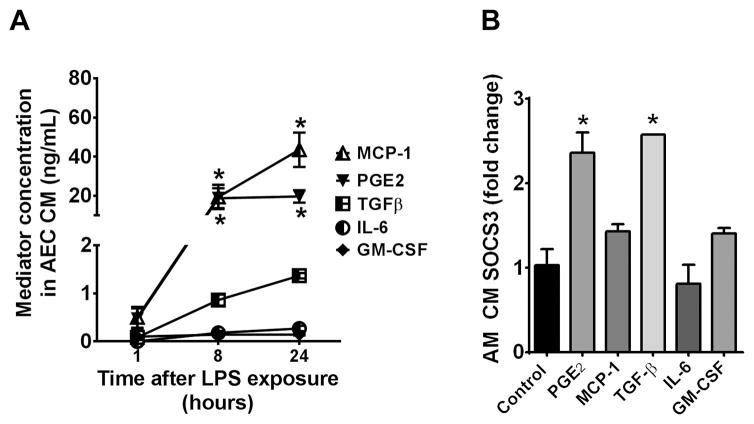
Identification of candidate mediator(s) elaborated by LPS-treated AECs that enhance SOCS3 secretion by AMs
AECs possess the ability to release a multitude of different mediators that can modulate AM activity, such as PGE2 (32, 33), MCP-1 (34), TGFβ (35), IL-6 (36) and GM-CSF (37). Generation of some of these mediators, including PGE2, has been shown to be upregulated by LPS treatment (38). To determine the effect of LPS treatment on production of these mediators in our in vitro system, AECs were stimulated with 10 ng/mL LPS for 24 h (the condition resulting in optimal stimulatory effects on AM SOCS3 secretion; Fig. 3A) and the resulting AEC-CM was analyzed by ELISA. Both PGE2 and MCP-1 were highly upregulated in response to LPS, while TGFβ was modestly increased and IL-6 and GM-CSF were not increased significantly (Fig. 4A). To determine the ability of these mediators to rapidly enhance AM SOCS3 secretion, primary AMs were directly exposed to each mediator for 1 h in vitro and the resulting AM-CM was analyzed for SOCS3 by western blot. Exposure concentrations, specified in the legend to Fig. 4B, were chosen based on the maximal levels of each mediator in AEC-CM as determined from the data presented in Fig. 4A. We previously reported that PGE2 can increase secretion of SOCS3 by AMs (13). Our current results confirmed that finding and found that TGFβ was the only other mediator tested to have a significant stimulatory effect, which was comparable to that of PGE2 (Fig. 4B). Thus, potentiation of AM SOCS3 secretion is a specific effect of only certain AEC-derived mediators. Based on both their elaboration by LPS-stimulated AECs and their effects on AM SOCS3 secretion, our results suggest that PGE2, and to a lesser extent TGFβ are leading candidates as AEC-derived secretagogues for SOCS3 secretion by AMs in the setting of LPS challenge.
Figure 4. LPS treatment of AECs in vitro results in elaboration of high levels of PGE2 and MCP-1.
(A) AECs (5×105 cells/well of a 6-well plate) were cultured in the presence of 10 ng/mL LPS for 1, 8 and 24 h in serum-free RPMI. The resulting AEC-CM was collected and analyzed for a panel of mediators by ELISA. (B) AMs were allowed to adhere for 1 h and were washed, after which they were incubated in serum-free RPMI for 1 h with the indicated mediators at the following concentrations: PGE2, 1 μM; MCP-1, 20 μg/mL; TGFβ, 2.5 μg/mL; IL-6, 100 pg/mL; and GM-CSF, 100 pg/mL. SOCS3 secretion was measured in AM-CM by WB. Data are expressed relative to the level of SOCS3 found in CM from untreated AMs, and represent the mean ± SEM from three separate experiments, *= p<0.05.
PGE2 is necessary for enhanced AM SOCS3 secretion in response to LPS-stimulated AEC-CM
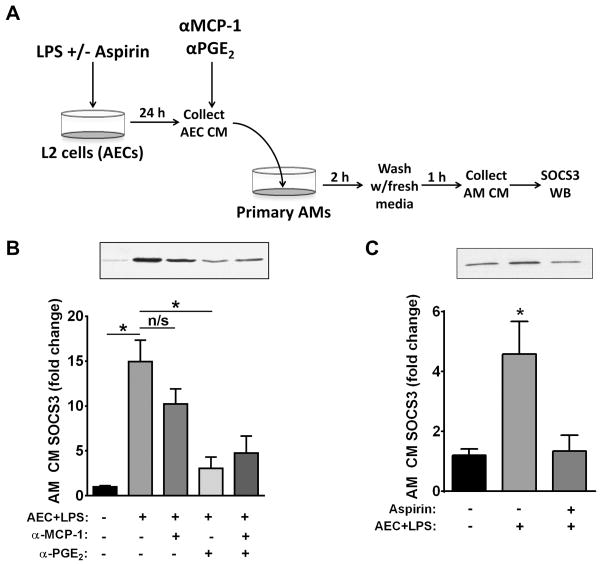
Since PGE2 levels in AEC-CM were approximately 15-fold higher than TGFβ levels after LPS treatment, we utilized antibody neutralization (Fig. 5A) to evaluate the role of this lipid mediator as an AEC-derived secretagogue acting on AMs. We chose MCP-1 as a negative control because it was elaborated by AECs at similar levels to PGE2 but failed to promote AM SOCS3 secretion. Addition to AEC-CM of a neutralizing antibody against PGE2 significantly abrogated its ability to enhance SOCS3 secretion by AMs; by contrast, a neutralizing antibody against MCP-1 failed to do so. Neutralization of MCP-1 yielded no additive blockade beyond that achieved by anti-PGE2 alone (Fig. 5B). As a confirmatory approach, we pulse-treated AECs with the irreversible cyclooxygenase (COX) and PGE2 synthesis inhibitor, aspirin, prior to LPS incubation to inhibit induction of AEC-derived PGE2, and this completely abrogated the ability of AEC-CM to promote SOCS3 secretion by AMs (Fig. 5C). Taken together, these data implicate PGE2 as a major mediator of the AM SOCS3 secretagogue activity elaborated by LPS-stimulated AECs.
Figure 5. PGE2 is necessary for enhanced AM SOCS3 secretion in response to LPS-stimulated AEC-CM.
AECs were treated for 24 h with 10 ng/mL LPS as previously described in the presence or absence of aspirin and the resulting AEC-CM was incubated with or without anti-PGE2 and/or anti-MCP-1 antibodies for 30 min at 4°C and then added to primary AMs (post-adherence) for 2 h. AM-CM was collected over a subsequent 1-h interval and SOCS3 levels analyzed by WB. (A) Schematic of experimental design. (B) Levels of SOCS3 by WB in AM-CM; top, representative blot; bottom, mean densitometric analysis of blots from multiple experiments. (C) AECs were equilibrated and treated with 100 μM aspirin for 30 min at 37°C. They were washed and stimulated with 10 ng/mL LPS in serum-free RPMI for 24 h. The resulting AEC-CM was added to AMs (post-adherence) for 2 h. AM-CM was collected as previously described and SOCS3 levels analyzed by WB; top, representative blot; bottom, mean densitometric analysis. Data are expressed as mean ± SEM from three separate experiments, *= p<0.05.
PGE2 is required for enhanced SOCS3 secretion in an acute LPS model in vivo
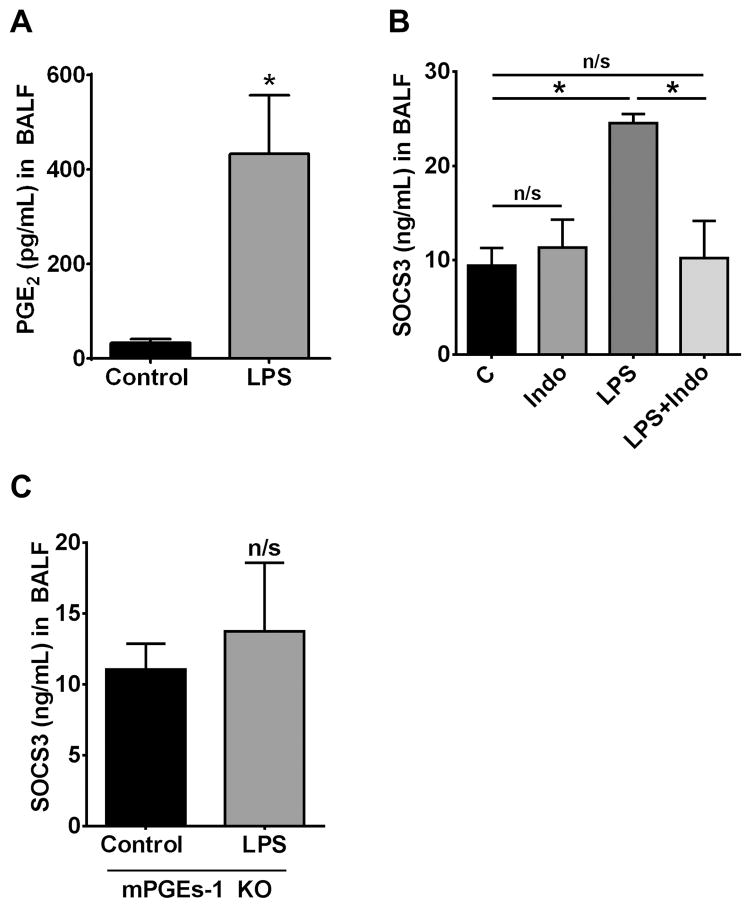
Not surprisingly, intrapulmonary challenge with LPS resulted in an increase in BALF levels of PGE2 at 24 h (Fig. 6A) that paralleled its ability to increase SOCS3 levels (Fig. 2A). Additionally, COX-2 levels in lung homogenates were increased at 8 h (data not shown), consistent with previously reported studies indicating that LPS induction of PGE2 synthesis is dependent on COX-2 upregulation (39). To evaluate the contribution of PGE2 to the LPS-stimulated increase in SOCS3 secretion in vivo, we treated mice with the COX and PGE2 synthesis inhibitor indomethacin prior to LPS stimulation. Indomethacin administration completely blocked the ability of LPS to increase AM SOCS3 secretion in the lung (Fig. 6B), paralleled with inhibition of PGE2 synthesis (control = 38.38±10.86 pg/mL; Indo = 38.30±5.4 pg/mL; LPS = 432.52±124.16 pg/mL; Indo + LPS = 56.96±16.1 pg/mL). As a final experimental approach, we determined that, unlike what was observed in wild type mice (Fig. 2A), LPS treatment was unable to augment SOCS3 levels in BALF of mice lacking microsomal PGE synthase-1 (mPGEs-1) (Fig. 6C), the enzyme downstream of COX which catalyzes the terminal step in PGE2 synthesis. Taken together, these data show that PGE2 is the major mediator responsible for increased SOCS3 secretion by AMs in vivo following intrapulmonary LPS challenge.
Figure 6. PGE2 is required for enhanced SOCS3 secretion during LPS treatment in vivo.
(A) PGE2 levels in BALF 24 h after oropharyngeal administration of LPS (2.5 μg/mouse) to wild type mice, as determined by ELISA. (B) SOCS3 levels in BALF 24 h after oropharyngeal administration of LPS in wild type mice pretreated or not with 1.5 mg/kg indomethacin. (C) SOCS3 levels in BALF 24 h after oropharyngeal administration of LPS to mPGEs-1 KO mice. Data are expressed as mean ± SEM, n= 5 mice per group, *= p<0.05.
AM-derived CM inhibits LPS-induced STAT3 activation and MCP-1 expression in AECs
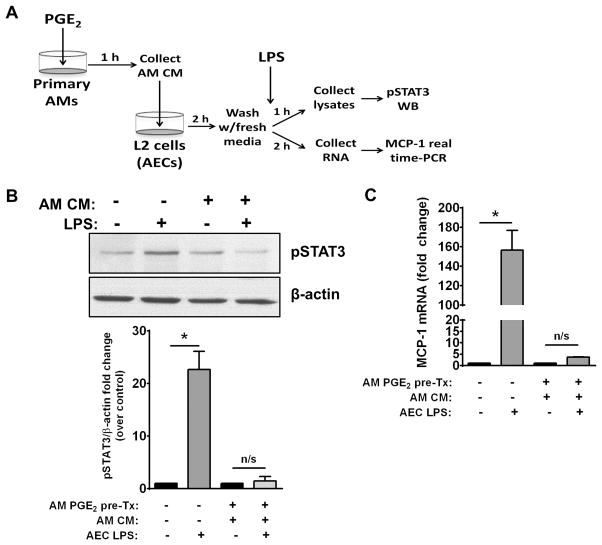
Having thus far focused on the ability of LPS-treated AECs to signal to AMs the need for increased SOCS3 secretion via release of PGE2, we sought to confirm the functional effect of delivery of AM-derived SOCS3 on inflammatory signaling in AECs. To do so, we assessed the ability of CM derived from PGE2-stimulated AMs to inhibit STAT3 activation (indicated by its phosphorylation at Tyr 705) as well as the expression of the STAT3-dependent cytokine MCP-1 in LPS-challenged AECs. Primary AMs were pre-treated with 1 μM PGE2 for 1 h and the resulting AM-CM was collected. AECs were then pretreated for 1 h with this CM or medium alone and then stimulated with LPS for 1 h prior to western blot analysis of phospho-STAT3 levels or real-time PCR analysis of MCP-1 mRNA expression (Fig. 7A). As compared to medium alone, pretreatment with PGE2-stimulated AM-CM significantly inhibited LPS-induced STAT3 activation (Fig. 7B), consistent with the inhibitory effect we observed for IL-6 or IFNγ-induced STAT activation in our previous study (13). Additionally, expression of MCP-1, a STAT3-responsive gene that we showed was enhanced by LPS treatment (Fig. 3A), was significantly inhibited by pre-treatment with PGE2-stimulated AM CM (Fig. 7C). These data support the idea that SOCS3, the secretion of which by AMs is potentiated in response to AEC-derived PGE2, serves as a functional brake on inflammatory responses by AECs during infection.
Figure 7. CM from PGE2-treated AMs inhibits LPS-induced STAT3 activation and MCP-1 expression in AECs.
AMs were treated with 1 μM PGE2 for 1 h. The resulting AM-CM was collected and cultured with AECs for 2 h. The AECs were washed and stimulated with 10 ng/mL LPS in serum-free RPMI for 1–2 h, then lysed and analyzed for phospho-STAT3 by WB or MCP-1 mRNA by real-time PCR. (A) Schematic of experimental design testing the effects of AM-CM on LPS-induced STAT3 activation in AECs. (B) Representative blot (top) and fold increase (bottom) of normalized AEC phospho-STAT3 compared to non-LPS treated control. (C) Fold increase of MCP-1 mRNA determined by qRT-PCR. Data are expressed as fold change relative to untreated control and represent mean ± SEM from three separate experiments, *= p<0.05.
DISCUSSION
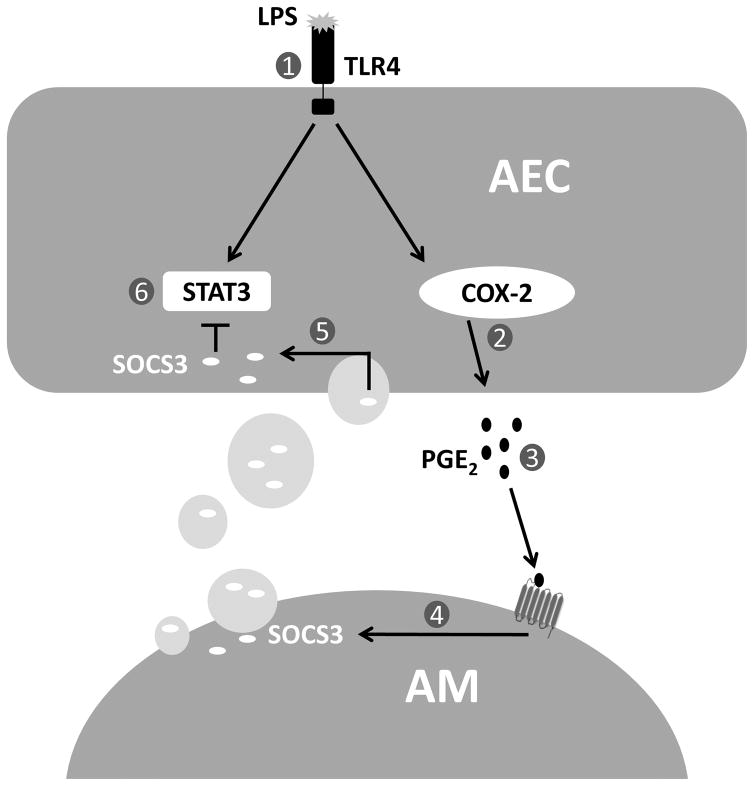
In this report, we present a new paradigm by which AECs and AMs collaborate to dampen the host response to innate inflammatory challenge, fostering the maintenance of homeostasis in the alveolar space (Fig. 8). In the face of inflammatory challenge, AECs elaborate PGE2 which serves as a signal to “request” SOCS3 secretion by neighboring AMs. Via uptake of the AM-derived MPs containing SOCS3, AECs are able to augment their own very low endogenous levels of SOCS3, which in turn allows them to restrain the activity of the pivotal inflammatory JAK-STAT signaling pathway (Fig. 8).
Figure 8. Scheme illustrating crosstalk between AMs and AECs in an LPS inflammation model.
1) LPS binds to TLR4 on AEC cell surface and 2) upregulates COX-2 and mPGES-1 expression, resulting in increased PGE2 synthesis by AECs. 3) PGE2 binds to E prostanoid receptors on neighboring AMs and 4) enhances SOCS3 packaging within secreted MPs. 5) Secreted SOCS3-containing MPs are taken up by neighboring AECs, resulting in 6) inhibition of STAT3 activation.
Both MPs and exosomes have long been recognized to exert pro-inflammatory actions in the context of vascular injury (40–42). More recently, the anti-inflammatory or immunosuppressive potential of extracellular vesicles has also been appreciated (43, 44). However, the immunosuppressive effects of vesicles have generally been limited to descriptive phenomena unattributed to the actions of specific suppressive cargo molecules within the vesicles. Tang et al. were the first to characterize a suppressive cargo molecule when they reported that platelet-derived MPs augment the production of immunosuppressive lipoxin A4 by transferring the enzyme 12-lipoxygenase to mast cells (45).
We recently for the first time reported extracellular vesicle-mediated suppression involving crosstalk between the two key cell types residing on the alveolar surface, namely, AMs and AECs (13). Additionally, the suppressive molecules transferred from AMs to AECs within vesicles were SOCS family proteins, and this represented the first time that such proteins were found to be elaborated in the extracellular space. Moreover, the levels of SOCS3 in BALF of naïve mice (~10–20 ng/mL) are remarkably high relative to those of other mediators, which tend to be in the pg/ml range. In the current report, we have extended our previous work by determining that AECs can secrete soluble mediators after infection or LPS exposure that potentiate the ability of AMs to secrete SOCS3. The conclusion that PGE2 represented the predominant AEC-derived secretagogue for AMs was based on three lines of evidence. First, LPS-challenged AECs generated large amounts of this prostanoid, consistent with our original observations that PGE2 is the predominant prostanoid synthesized by cultured AECs (46, 47). Second, the ability of AEC pretreatment with the COX inhibitor aspirin to abrogate generation of the LPS-stimulated SOCS3 secretagogue, and of indomethacin to abrogate LPS-stimulated SOCS3 secretion in the lung in vivo, indicated that the mediator was a prostanoid. Finally, the in vitro use of neutralizing antibody specific for PGE2 and the in vivo use of mice lacking PGE-specific synthase confirmed that PGE2 was the specific prostanoid responsible for enhancing AM SOCS3 secretion. This conclusion is consistent with prior observations from our laboratory that PGE2 exerts a variety of immunosuppressive effects on AMs (18, 48, 49), including the potentiation of AM secretion of SOCS3 in vitro (13). One notable feature of this modulatory effect of PGE2 was that it reflected not an increase in the number of SOCS3-containing MPs secreted, but rather an increase in the amount of SOCS3 packaged per MP (13). PGE2 can exert its effects via 4 distinct E prostanoid (EP) receptors. While a majority of its inhibitory effects on AM function have been shown to be mediated via EP2/cAMP-associated pathways (18, 50–53), the precise EP receptor and downstream pathways responsible for PGE2 modulation of SOCS3 secretion remain to be delineated.
The extracellular milieu of the alveolar space includes a diverse collection of cytokines, growth factors and other soluble mediators that are tightly regulated in order to maintain homeostasis. Of the subset of those mediators which we evaluated, only PGE2 and TGFβ were capable of enhancing SOCS3 secretion. Notably, these two substances are widely viewed as suppressive molecules, while two that did not affect SOCS3 secretion, MCP-1 and IL-6, are typically considered proinflammatory. We were surprised that GM-CSF had no effect on AM SOCS3 secretion, even though it is thought to play a prominent role in the maintenance and maturation of AMs in the lung (54–56). Although indomethacin treatment blocked the increase in SOCS3 secretion after in vivo LPS treatment, it failed to reduce SOCS3 below control levels, implying that some mediator other than PGE2 is responsible for maintaining basal SOCS3 levels. Although TGFβ did not appear to participate in the potentiation of SOCS3 secretion in response to LPS, it is a promising candidate contributor to the maintenance of basal SOCS3 secretion by AMs in the alveolar milieu. Future studies will focus on such a potential role for TGFβ.
Our current finding (Fig. 2A) that SOCS3 levels in BALF were increased above baseline 24 h after intrapulmonary administration of LPS may appear to be in conflict with our previous observation that BALF levels of SOCS3 were reduced below baseline 3 h after intrapulmonary LPS. Although we found that LPS exerts an immediate (within 1 h) direct inhibitory effect on the ability of AMs to secrete SOCS3 by an as-yet unknown mechanism (13), its subsequent ability to upregulate PGE2 synthesis as shown herein, via induction of COX-2 and mPGEs-1, is sufficient to overcome such inhibition. This upregulation of PGE2 synthesis likely occurs not only in AECs but also in AMs, as demonstrated by our data suggesting a trend towards increased SOCS3 secretion after AM exposure to LPS alone for 24 h. However, this ability of LPS to directly stimulate SOCS3 secretion by AMs pales in comparison to the stimulatory effects of AEC-CM on SOCS3 secretion, which may reflect an inherent ability of AECs to produce greater amounts PGE2 than AMs.
In addition to bacterial infection and LPS stimulation, we showed that viral infection also enhanced SOCS3 secretion in the lung. It is highly likely that this reflects the shared consequences of signaling through TLR4 in the case of LPS on the surface of Gram-negative bacteria (57) and TLR9 (and perhaps other pattern-recognition receptors) in the case of CpG DNA in adenovirus (58) and gammaherpesvirus (59). Signaling through these TLRs results in MyD88-dependent activation of NF-κB, a known inducer of COX-2 transcription and PGE2 synthesis (60–62). Indeed, both MAV-1 (21) and γHV68 (data not shown) have been shown to increase PGE2 levels in the lung, suggesting that PGE2 is responsible for increases in SOCS3 secretion in the context of viral infection as was the case with LPS treatment. TLR activation also activates the JAK/STAT pathway, and this is an integral component of the immune response to both viral and bacterial infection and has been shown to be necessary for pathogen clearance (11). In the context of microbial challenge of the lung, an initial rapid suppression of SOCS3 secretion by AMs (as demonstrated previously with LPS at 3 h (13)) may therefore facilitate STAT3-dependent microbial clearance by permitting STAT activation and downstream signaling. The subsequent induction of PGE2 synthesis at later time points, with resulting enhanced SOCS3 secretion (seen here at 24 h), may thereby suppress the JAK/STAT pathway, permitting a switch favoring the resolution of inflammation. Indeed, we demonstrated here that PGE2 stimulation of AM SOCS3 significantly inhibited LPS-induced AEC STAT3 activation as well as expression of the STAT3-dependent chemokine MCP-1.
One striking finding in this study was that, despite their similar intracellular levels of SOCS3 as AMs, PMNs recruited to an inflammatory site lacked the ability to secrete SOCS3. In our previous study, we noted a similar inability of lung fibroblasts to secrete SOCS3 despite high levels of expression (13). This inability to secrete SOCS3 is despite the fact that PMNs possess the ability to shed extracellular vesicles (63, 64), which can nevertheless exert anti-inflammatory effects (65). Such divergent patterns of expression and secretion support the conclusion that packaging of SOCS3 into MPs is an independently regulated phenomenon which is highly cell-specific. The ability of AMs to secrete such a high proportion of their intracellular SOCS3 represents yet another means by which they carry out their suppressive functions in the lung.
In contrast to secretion of SOCS3, it was surprising that secretion of SOCS1 was not potentiated in infected mice. This differential secretion of SOCS3 and SOCS1 in the setting of infection is consistent with their differential packaging within microparticles and exosomes, respectively. However, the mechanism(s) responsible for differential secretion of SOCS1 and SOCS3 in this context will require further investigation.
In conclusion, our data demonstrate that during innate inflammatory challenges to the lung, AEC-derived PGE2 potentiates AM secretion of SOCS3 within MPs. This AM-derived SOCS3 can then attenuate inflammatory JAK-STAT signaling within the AECs. This bidirectional form of cell-cell communication represents a novel means for fine tuning inflammatory responses in the alveolar space.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HL125555 (to M.P.G), R01 AI083334 (to J.B.W), R01 AI117229 (to B.B.M), and Flight Attendants Medical Research Institute Grant CIA-103071 (to P.M). J.M.S was supported by National Institutes of Health T32 training grant HL 7749-23.
Abbreviations used in this article
- AEC
alveolar epithelial cell
- AEC-CM
alveolar epithelial cell conditioned medium
- AM
alveolar macrophage
- AM-CM
alveolar macrophage conditioned medium
- BALF
bronchoalveolar lavage fluid
- COX
cyclooxygenase
- CM
conditioned medium
- γHV68
gammaherpesvirus-68
- MAV-1
mouse adenovirus type 1
- MP
microparticle
- mPGEs-1
microsomal prostaglandin E synthase-1
- PGE2
prostaglandin E2
- SOCS
suppressor of cytokine signaling
References
- 1.Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol. 2014;14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 2.Hyde DM, Tyler NK, Putney LF, Singh P, Gundersen HJ. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings. Anat Rec A Discov Mol Cell Evol Biol. 2004;277:216–226. doi: 10.1002/ar.a.20012. [DOI] [PubMed] [Google Scholar]
- 3.Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS, Bhattacharya J. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuquimia OD, Petursdottir DH, Periolo N, Fernandez C. Alveolar epithelial cells are critical in protection of the respiratory tract by secretion of factors able to modulate the activity of pulmonary macrophages and directly control bacterial growth. Infect Immun. 2013;81:381–389. doi: 10.1128/IAI.00950-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuquimia OD, Petursdottir DH, Rahman MJ, Hartl K, Singh M, Fernandez C. The role of alveolar epithelial cells in initiating and shaping pulmonary immune responses: communication between innate and adaptive immune systems. PLoS One. 2012;7:e32125. doi: 10.1371/journal.pone.0032125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fereol S, Fodil R, Pelle G, Louis B, Isabey D. Cell mechanics of alveolar epithelial cells (AECs) and macrophages (AMs) Respir Physiol Neurobiol. 2008;163:3–16. doi: 10.1016/j.resp.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 7.Williams MC. Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol. 2003;65:669–695. doi: 10.1146/annurev.physiol.65.092101.142446. [DOI] [PubMed] [Google Scholar]
- 8.Castranova V, Rabovsky J, Tucker JH, Miles PR. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. 1988;93:472–483. doi: 10.1016/0041-008x(88)90051-8. [DOI] [PubMed] [Google Scholar]
- 9.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2:33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008;8:142–152. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 11.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang S, Zheng G, Zhao L, Xu F, Qian J. Shp-2 contributes to anti-RSV activity in human pulmonary alveolar epithelial cells by interfering with the IFN-alpha-induced Jak/Stat1 pathway. J Cell Mol Med. 2015 doi: 10.1111/jcmm.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourdonnay E, Zaslona Z, Penke LR, Speth JM, Schneider DJ, Przybranowski S, Swanson JA, Mancuso P, Freeman CM, Curtis JL, Peters-Golden M. Transcellular delivery of vesicular SOCS proteins from macrophages to epithelial cells blunts inflammatory signaling. J Exp Med. 2015;212:729–742. doi: 10.1084/jem.20141675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akram KM, Lomas NJ, Forsyth NR, Spiteri MA. Alveolar epithelial cells in idiopathic pulmonary fibrosis display upregulation of TRAIL, DR4 and DR5 expression with simultaneous preferential over-expression of pro-apoptotic marker p53. Int J Clin Exp Pathol. 2014;7:552–564. [PMC free article] [PubMed] [Google Scholar]
- 15.Trebino CE, Stock JL, Gibbons CP, Naiman BM, Wachtmann TS, Umland JP, Pandher K, Lapointe JM, Saha S, Roach ML, Carter D, Thomas NA, Durtschi BA, McNeish JD, Hambor JE, Jakobsson PJ, Carty TJ, Perez JR, Audoly LP. Impaired inflammatory and pain responses in mice lacking an inducible prostaglandin E synthase. Proc Natl Acad Sci U S A. 2003;100:9044–9049. doi: 10.1073/pnas.1332766100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barth K, Reh J, Sturrock A, Kasper M. Epithelial vs myofibroblast differentiation in immortal rat lung cell lines--modulating effects of bleomycin. Histochem Cell Biol. 2005;124:453–464. doi: 10.1007/s00418-005-0048-2. [DOI] [PubMed] [Google Scholar]
- 17.Miller YE, Walker SR, Spencer JS, Kubo RT, Mason RJ. Monoclonal antibodies specific for antigens expressed by rat type II alveolar epithelial and nonciliated bronchiolar cells. Exp Lung Res. 1989;15:635–649. doi: 10.3109/01902148909069623. [DOI] [PubMed] [Google Scholar]
- 18.Aronoff DM, Canetti C, Peters-Golden M. Prostaglandin E2 inhibits alveolar macrophage phagocytosis through an E-prostanoid 2 receptor-mediated increase in intracellular cyclic AMP. J Immunol. 2004;173:559–565. doi: 10.4049/jimmunol.173.1.559. [DOI] [PubMed] [Google Scholar]
- 19.Turiak L, Misjak P, Szabo TG, Aradi B, Paloczi K, Ozohanics O, Drahos L, Kittel A, Falus A, Buzas EI, Vekey K. Proteomic characterization of thymocyte-derived microvesicles and apoptotic bodies in BALB/c mice. J Proteomics. 2011;74:2025–2033. doi: 10.1016/j.jprot.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Moore BB, Coffey MJ, Christensen P, Sitterding S, Ngan R, Wilke CA, McDonald R, Phare SM, Peters-Golden M, Paine R, 3rd, Toews GB. GM-CSF regulates bleomycin-induced pulmonary fibrosis via a prostaglandin-dependent mechanism. J Immunol. 2000;165:4032–4039. doi: 10.4049/jimmunol.165.7.4032. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy MK, Levine RE, Procario MC, McDonnell PJ, Zhu L, Mancuso P, Crofford LJ, Aronoff DM, Weinberg JB. Prostaglandin E2 induction during mouse adenovirus type 1 respiratory infection regulates inflammatory mediator generation but does not affect viral pathogenesis. PLoS One. 2013;8:e77628. doi: 10.1371/journal.pone.0077628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan TR, Moore BB, Weinberg JB, Vannella KM, Fields WB, Christensen PJ, van Dyk LF, Toews GB. Exacerbation of established pulmonary fibrosis in a murine model by gammaherpesvirus. Am J Respir Crit Care Med. 2008;177:771–780. doi: 10.1164/rccm.200708-1184OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carow B, Rottenberg ME. SOCS3, a Major Regulator of Infection and Inflammation. Front Immunol. 2014;5:58. doi: 10.3389/fimmu.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dalpke AH, Opper S, Zimmermann S, Heeg K. Suppressors of cytokine signaling (SOCS)-1 and SOCS-3 are induced by CpG-DNA and modulate cytokine responses in APCs. J Immunol. 2001;166:7082–7089. doi: 10.4049/jimmunol.166.12.7082. [DOI] [PubMed] [Google Scholar]
- 25.Severgnini M, Takahashi S, Rozo LM, Homer RJ, Kuhn C, Jhung JW, Perides G, Steer M, Hassoun PM, Fanburg BL, Cochran BH, Simon AR. Activation of the STAT pathway in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1282–1292. doi: 10.1152/ajplung.00349.2003. [DOI] [PubMed] [Google Scholar]
- 26.Hilberath JN, Carlo T, Pfeffer MA, Croze RH, Hastrup F, Levy BD. Resolution of Toll-like receptor 4-mediated acute lung injury is linked to eicosanoids and suppressor of cytokine signaling 3. FASEB J. 2011;25:1827–1835. doi: 10.1096/fj.10-169896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan C, Ward PA, Wang X, Gao H. Myeloid depletion of SOCS3 enhances LPS-induced acute lung injury through CCAAT/enhancer binding protein delta pathway. FASEB J. 2013;27:2967–2976. doi: 10.1096/fj.12-225797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harmsen AG. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect Immun. 1988;56:1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson BL, III, Kuethe JW, Caldwell CC. Neutrophil derived microvesicles: emerging role of a key mediator to the immune response. Endocr Metab Immune Disord Drug Targets. 2014;14:210–217. doi: 10.2174/1871530314666140722083717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cash JL, White GE, Greaves DR. Chapter 17. Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol. 2009;461:379–396. doi: 10.1016/S0076-6879(09)05417-2. [DOI] [PubMed] [Google Scholar]
- 31.Chaves de Souza JA, Nogueira AV, Chaves de Souza PP, Kim YJ, Silva Lobo C, Pimentel Lopes de Oliveira GJ, Cirelli JA, Garlet GP, Rossa C., Jr SOCS3 expression correlates with severity of inflammation, expression of proinflammatory cytokines, and activation of STAT3 and p38 MAPK in LPS-induced inflammation in vivo. Mediators Inflamm. 2013;2013:650812. doi: 10.1155/2013/650812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lama V, Moore BB, Christensen P, Toews GB, Peters-Golden M. Prostaglandin E2 synthesis and suppression of fibroblast proliferation by alveolar epithelial cells is cyclooxygenase-2-dependent. Am J Respir Cell Mol Biol. 2002;27:752–758. doi: 10.1165/rcmb.4857. [DOI] [PubMed] [Google Scholar]
- 33.Petrovic N, Knight DA, Bomalaski JS, Thompson PJ, Misso NL. Concomitant activation of extracellular signal-regulated kinase and induction of COX-2 stimulates maximum prostaglandin E2 synthesis in human airway epithelial cells. Prostaglandins Other Lipid Mediat. 2006;81:126–135. doi: 10.1016/j.prostaglandins.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Standiford TJ, Kunkel SL, Phan SH, Rollins BJ, Strieter RM. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–9918. [PubMed] [Google Scholar]
- 35.Kwong KY, Literat A, Zhu NL, Huang HH, Li C, Jones CA, Minoo P. Expression of transforming growth factor beta (TGF-beta1) in human epithelial alveolar cells: a pro-inflammatory mediator independent pathway. Life Sci. 2004;74:2941–2957. doi: 10.1016/j.lfs.2003.08.048. [DOI] [PubMed] [Google Scholar]
- 36.Pottratz ST, Reese S, Sheldon JL. Pneumocystis carinii induces interleukin 6 production by an alveolar epithelial cell line. Eur J Clin Invest. 1998;28:424–429. doi: 10.1046/j.1365-2362.1998.00299.x. [DOI] [PubMed] [Google Scholar]
- 37.Christensen PJ, Armstrong LR, Fak JJ, Chen GH, McDonald RA, Toews GB, Paine R., 3rd Regulation of rat pulmonary dendritic cell immunostimulatory activity by alveolar epithelial cell-derived granulocyte macrophage colony-stimulating factor. Am J Respir Cell Mol Biol. 1995;13:426–433. doi: 10.1165/ajrcmb.13.4.7546772. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JA, Belvisi MG, Akarasereenont P, Robbins RA, Kwon OJ, Croxtall J, Barnes PJ, Vane JR. Induction of cyclo-oxygenase-2 by cytokines in human pulmonary epithelial cells: regulation by dexamethasone. Br J Pharmacol. 1994;113:1008–1014. doi: 10.1111/j.1476-5381.1994.tb17093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Font-Nieves M, Sans-Fons MG, Gorina R, Bonfill-Teixidor E, Salas-Perdomo A, Marquez-Kisinousky L, Santalucia T, Planas AM. Induction of COX-2 enzyme and down-regulation of COX-1 expression by lipopolysaccharide (LPS) control prostaglandin E2 production in astrocytes. J Biol Chem. 2012;287:6454–6468. doi: 10.1074/jbc.M111.327874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McVey M, Tabuchi A, Kuebler WM. Microparticles and acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2012;303:L364–381. doi: 10.1152/ajplung.00354.2011. [DOI] [PubMed] [Google Scholar]
- 41.Sadallah S, Eken C, Schifferli JA. Ectosomes as immunomodulators. Semin Immunopathol. 2011;33:487–495. doi: 10.1007/s00281-010-0232-x. [DOI] [PubMed] [Google Scholar]
- 42.Sadallah S, Eken C, Schifferli JA. Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol. 2011;163:26–32. doi: 10.1111/j.1365-2249.2010.04271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22:369–379. doi: 10.3727/096368911X582769. [DOI] [PubMed] [Google Scholar]
- 44.Sadallah S, Eken C, Schifferli JA. Erythrocyte-derived ectosomes have immunosuppressive properties. J Leukoc Biol. 2008;84:1316–1325. doi: 10.1189/jlb.0108013. [DOI] [PubMed] [Google Scholar]
- 45.Tang K, Liu J, Yang ZS, Zhang BA, Zhang HF, Huang CM, Ma JW, Shen GX, Ye DY, Huang B. Microparticles mediate enzyme transfer from platelets to mast cells: A new pathway for lipoxin A4 biosynthesis. Biochemical and Biophysical Research Communications. 2010;400:432–436. doi: 10.1016/j.bbrc.2010.08.095. [DOI] [PubMed] [Google Scholar]
- 46.Chauncey JB, Peters-Golden M, Simon RH. Arachidonic acid metabolism by rat alveolar epithelial cells. Lab Invest. 1988;58:133–140. [PubMed] [Google Scholar]
- 47.Lipchik RJ, Chauncey JB, Paine R, Simon RH, Peters-Golden M. Arachidonate metabolism increases as rat alveolar type II cells differentiate in vitro. Am J Physiol. 1990;259:L73–80. doi: 10.1152/ajplung.1990.259.2.L73. [DOI] [PubMed] [Google Scholar]
- 48.Degraaf AJ, Zaslona Z, Bourdonnay E, Peters-Golden M. Prostaglandin E2 reduces Toll-like receptor 4 expression in alveolar macrophages by inhibition of translation. Am J Respir Cell Mol Biol. 2014;51:242–250. doi: 10.1165/rcmb.2013-0495OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Serezani CH, Chung J, Ballinger MN, Moore BB, Aronoff DM, Peters-Golden M. Prostaglandin E2 suppresses bacterial killing in alveolar macrophages by inhibiting NADPH oxidase. Am J Respir Cell Mol Biol. 2007;37:562–570. doi: 10.1165/rcmb.2007-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aronoff DM, I, Bergin L, Lewis C, Goel D, O’Brien E, Peters-Golden M, Mancuso P. E-prostanoid 2 receptor signaling suppresses lung innate immunity against Streptococcus pneumoniae. Prostaglandins Other Lipid Mediat. 2012;98:23–30. doi: 10.1016/j.prostaglandins.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M. Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292:L405–413. doi: 10.1152/ajplung.00232.2006. [DOI] [PubMed] [Google Scholar]
- 52.Zaslona Z, Okunishi K, Bourdonnay E, Domingo-Gonzalez R, Moore BB, Lukacs NW, Aronoff DM, Peters-Golden M. Prostaglandin E(2) suppresses allergic sensitization and lung inflammation by targeting the E prostanoid 2 receptor on T cells. J Allergy Clin Immunol. 2014;133:379–387. doi: 10.1016/j.jaci.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaslona Z, Serezani CH, Okunishi K, Aronoff DM, Peters-Golden M. Prostaglandin E2 restrains macrophage maturation via E prostanoid receptor 2/protein kinase A signaling. Blood. 2012;119:2358–2367. doi: 10.1182/blood-2011-08-374207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA, et al. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994;264:713–716. doi: 10.1126/science.8171324. [DOI] [PubMed] [Google Scholar]
- 55.Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA, Maher DW, Cebon J, Sinickas V, Dunn AR. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci U S A. 1994;91:5592–5596. doi: 10.1073/pnas.91.12.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bonfield TL, Raychaudhuri B, Malur A, Abraham S, Trapnell BC, Kavuru MS, Thomassen MJ. PU.1 regulation of human alveolar macrophage differentiation requires granulocyte-macrophage colony-stimulating factor. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1132–1136. doi: 10.1152/ajplung.00216.2003. [DOI] [PubMed] [Google Scholar]
- 57.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu J, Huang X, Yang Y. Innate immune response to adenoviral vectors is mediated by both Toll-like receptor-dependent and -independent pathways. J Virol. 2007;81:3170–3180. doi: 10.1128/JVI.02192-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bussey KA, Reimer E, Todt H, Denker B, Gallo A, Konrad A, Ottinger M, Adler H, Sturzl M, Brune W, Brinkmann MM. The gammaherpesviruses Kaposi’s sarcoma-associated herpesvirus and murine gammaherpesvirus 68 modulate the Toll-like receptor-induced proinflammatory cytokine response. J Virol. 2014;88:9245–9259. doi: 10.1128/JVI.00841-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–34040. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- 61.Weinlich R, Bortoluci KR, Chehab CF, Serezani CH, Ulbrich AG, Peters-Golden M, Russo M, Amarante-Mendes GP. TLR4/MYD88-dependent, LPS-induced synthesis of PGE2 by macrophages or dendritic cells prevents anti-CD3-mediated CD95L upregulation in T cells. Cell Death Differ. 2008;15:1901–1909. doi: 10.1038/cdd.2008.128. [DOI] [PubMed] [Google Scholar]
- 62.Yeo SJ, Yoon JG, Yi AK. Myeloid differentiation factor 88-dependent post-transcriptional regulation of cyclooxygenase-2 expression by CpG DNA: tumor necrosis factor-alpha receptor-associated factor 6, a diverging point in the Toll-like receptor 9-signaling. J Biol Chem. 2003;278:40590–40600. doi: 10.1074/jbc.M306280200. [DOI] [PubMed] [Google Scholar]
- 63.Gasser O, Hess C, Miot S, Deon C, Sanchez JC, Schifferli JA. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp Cell Res. 2003;285:243–257. doi: 10.1016/s0014-4827(03)00055-7. [DOI] [PubMed] [Google Scholar]
- 64.Hess C, Sadallah S, Hefti A, Landmann R, Schifferli JA. Ectosomes released by human neutrophils are specialized functional units. J Immunol. 1999;163:4564–4573. [PubMed] [Google Scholar]
- 65.Gasser O, Schifferli JA. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]