Abstract
Fragile X Mental Retardation Protein (FMRP) is a RNA-binding protein that modulates protein synthesis at the synapse and its function is regulated by glutamate. The retina is the first structure that participates in vision, and uses glutamate to transduce electromagnetic signals from light to electrochemical signals to neurons. FMRP has been previously detected in the retina, but its localization has not been studied yet. In this work, our objectives were to describe the localization of FMRP in the retina, to determine whether different exposure to dark or light stimulus alters FMRP expression in the retina, and to compare the pattern in two different species, the mouse and chick. We found that both FMRP mRNA and protein are expressed in the retina. By immunohistochemistry analysis we found that both mouse and chick present similar FMRP expression localized mainly in both plexiform layers and the inner retina. It was also observed that FMRP is down-regulated by 24h dark adaptation compared to its expression in the retina of animals that were exposed to light for 1h after 24h in the dark. We conclude that FMRP is likely to participate in retinal physiology, since its expression changes with light exposure. In addition, the expression pattern and regulation by light of FMRP seems well conserved since it was similar in both mouse and chick.
Keywords: metabotropic glutamate receptor, mGluR, FMRP, vision, plasticity
Graphical Abstract
This study describes the location of the Fragile X Mental Retardation Protein in the retina, and shows that its expression is modulated by light or dark adaptation: light adapted retinas present a higher expression of FMRP in relation to dark adapted retinas.
1-INTRODUCTION
Fragile X Syndrome is a condition caused by decreased expression of the Fragile X Mental Retardation Protein (FMRP) due to trinucleotide repeats present in the FMR1 gene of individuals with the syndrome (Hagerman, 2006). FMRP is a mRNA binding protein that is mainly responsible for regulating local rapid mRNA translation. The defective regulation of local protein synthesis in the synapse results in altered synaptic plasticity and abnormal dendrite growth (Bassell and Warren, 2008). Associated with the intellectual disability, individuals with Fragile X Syndrome present behavioral characteristics such as autism spectrum disorder, deficits in attention and hyperactivity, and hypersensitivity to sensory stimuli (Hagerman, 2006).
FMRP expression is not only altered in Fragile X syndrome, but also in conditions such as schizophrenia, major depression, and bipolar disorder (Fatemi and Folsom, 2011). Several key proteins involved in synaptic transmission have been shown to be regulated by FMRP. Glutamate receptor subunits such as GluA1, 95 kDa postsynaptic density protein (PSD-95), and amyloid-precursor protein (APP) are all upregulated in the mouse model of Fragile X Syndrome, the Fmr1 knockout mouse (Westmark and Malter, 2007; Muddashetty et al., 2007; Bassell and Warren, 2008; De Rubeis and Bagni, 2011). In addition, Fmr1 knockout mice present lower levels of GABAergic proteins, such as glutamic acid decarboxylase (GAD), GABA transporters, and potassium channels (D’Hulst et al., 2009; Adusei et al., 2010; Gross et al., 2011).
Very little is known about FMRP and its possible roles in vision. It has been shown that Fragile X premutation carriers have some visual perception impairments, which have been attributed to the lack of FMRP in the geniculo-striatal magnocellular visual pathway (M pathway) and its cortical recipients (Kéri and Benedek, 2012). It is also widely recognized that Fragile X patients present a hypersensitivity to sensory stimuli, such as sound and touch (Hagerman, 2006). One study has shown that injection of morpholinos that impair fmr1 gene expression causes malformation of the zebrafish retina (Gessert et al., 2010).
Vision is a major sense for humans, and the retina is the structure responsible for light transduction. Glutamate is the main excitatory neurotransmitter in the retina. Photoreceptors are depolarized in the dark and release glutamate onto two different types of bipolar cells: ON bipolar cells, that present mGluR6 receptors and are hyperpolarized by glutamate, and OFF bipolar cells, that present ionotropic glutamate receptors and are depolarized in the dark. Bipolar cells release glutamate onto ganglion cells, the cells responsible for transmitting the information processed in the retina to the superior visual systems in the brain. Light onset promotes the hyperpolarization of photoreceptors and decreases glutamate release from these cells which, in turn, allow ON bipolar cells to depolarize (for review, see Thoreson, 2007). Therefore, studying the glutamatergic system is important for understanding the retinal physiology.
One of the main pathways of FMRP regulation is through the activation of metabotropic glutamate receptor 5 (mGluR5) (Bear et al., 2004; Ronesi and Huber, 2008), that is expressed in the retina along with other metabotropic glutamate receptors (mGluRs) (Brandstatter et al., 1998; Sen and Gleason, 2006; Quraishi et al., 2007). All mGluRs appear to have physiological importance in the modulation of glutamatergic transmission in the retina (Guimarães-Souza et al., 2011; Guimarães-Souza and Calaza, 2012).
The mouse is the most used animal model for studying many systems, including the retina, because, among other things, 99% of mouse genes have a human homolog (Waterson et al., 2002). However, it is known that mouse vision presents a rod-dominated retina and views the world at a low resolution (Prusky and Douglas, 2004), having nocturnal habits. The parallel use of other animal models may represent a way to assess, compare and confirm neurochemical features observed in the mouse retina.
Chicken is a daily-habit animal, like humans, presenting more cones than rods and other features that appear to confer it a good vision (for review, see Hunt et al., 2009). 60% of chicken protein-coding genes have a single human orthologue (Hillier et al., 2004). Chicken embryos are widely used for retinal development studies, and many of its neurochemical features are already known (for review, see Calaza and Gardino, 2010). The chick, the newly born chicken, can also be used for studying the retina, with the advantage of presenting a mature retinal circuitry comparable to the adult animal when it hatches, only with a smaller eye (for review, see Calaza and Gardino, 2010). Therefore, chicken is also considered to be a good model for studying the retina.
In this work, our first goal was to describe the localization of FMRP in the retina. Our second objective was to determine whether different exposure to dark or light stimulus alters FMRP expression in the retina. Our third objective was to compare if the pattern observed in the mouse retina differed from the ones obtained with the chick retina, to examine whether there would be differences between a mammalian and a non-mammalian species.
2-MATERIALS AND METHODS
2.1-Animals
All animal procedures complied with the NIH Guide for the Care and Use of Laboratory Animals and the Policies for the Use of Animals of the Society for Neuroscience. Procedures carried out with mice, male and female C57Bl/6, between 4 to 6 months of age, were approved by the Institutional Animal Care and Use Committee at OHSU. Procedures carried out with chicks, male and female Gallus gallus domesticus, between post-hatch day 1 to 14 (consider that, by hatching, chicks present a mature retina and their eyes will only grow), were approved by the Commission of Animal Care (CEPA/PROPPi) at the Federal Fluminense University (UFF). Animals were housed under a 12h light/12h dark cycle.
2.2-PCR
Total retina RNA was extracted using RNeasy Miniprep Kit (Qiagen). RNA was reverse transcribed into cDNA with SuperScript III First-Strand Synthesis System (Invitrogen). PCR was performed with GoTaq Flexi Enzyme in the presence of GoTaq Flexi Buffer (Promega), MgCl2, dNTPs and the two primers. The two pairs of Fmr1 primers used were designed to detect the sequence encoding the peptide against which the FMRP ab17722 antibody was made (Abcam). The primer sequences, derived from exons 14, 16, and 17, are the following (5′ to 3′):
Primer 14s: AGTAGACCAGTTGCGTTTGGAG
Primer 16as: GCTGTTCTTCCTTTAGCCTCTC
Primer 16s: AACGACGATCATTCCCGAAC
Primer 17as: TCCTGCCCTGAAGTGTTAAG
2.3-Dark adaptation
24h prior to the experiment, animals were kept in absolute darkness. Mice and chicks were euthanized with isoflurane inhalation or decapitation respectively, eyes were enucleated, and retinas were removed from the eyecup under a red light (referred to as “dark”). Part of the group (referred to as “light”) was allowed to recover from the 24h period of dark adaptation for 1h in room lights before the same procedure was performed. The experiments were always performed at the same time of the day (early afternoon).
2.4-Antibody characterization
All antibodies are described in Table 1. We used rabbit anti-FMRP polyclonal antibody (ab17722, Abcam, validated in the Fmr1 knockout mouse by Rossignol et al., 2014), 2F5 mouse anti-FMRP monoclonal antibody (a kind gift from Dr. Kimberly Huber, validated in the Fmr1 knockout mouse by Gabel et al., 2004), and rabbit phospho-FMRP polyclonal antibody (p1125-499, Phosphosolutions). Phospho-FMRP (or pFMRP) antibody was validated by Western blot and immunohistochemistry in wildtype and Fmr1 knockout mice (Supplementary material, Figures 1S and 2S). Mouse PKCα monoclonal antibody (NB600-201, Novus Biologicals) was used as a bipolar cell marker (Quraishi et al., 2007), and mouse Brn3a monoclonal antibody (MAB1585, Millipore) was used as a ganglion cell marker (Schitine et al., 2015). Specificity of the ab17722 FMRP antibody was further validated by preadsorption of the antibody with a human FMRP peptide (ab19074, Abcam) at a 4:1 molar dilution (peptide: ab17722 primary antibody).
Table 1.
Information about antibodies used in the present study.
| Antigen | Immunogen | Manufacturer Catalog number / RRID / Species | Concentration used |
|---|---|---|---|
| FMRP | C-terminal of human FMRP (aa 549–569) | Abcam Cat# ab17722 RRID:AB_2278530 Rabbit Polyclonal Antibody |
1:5000 (WB) 1:2000 (immuno mouse) 1:500 (immuno chick) |
| FMRP | N-terminal of FMRP (aa 1–200) | 2F5; Gabel et al., 2004 Cat# FMRP, RRID:AB_2314420 | 1:2000 (WB) 1:800 (immuno) |
| FMRP (phospho S499) | Phosphopeptide corresponding to amino acid residues surrounding the phospho-Ser499 (aa 450–550) | PhosphoSolutions Cat# p1125-499 RRID: AB_2341542 Rabbit Polyclonal Antibody |
1:2000 (WB) 1:1000 (immuno) |
| PKCα | Purified bovine brain Protein Kinase C | Novus Biologicals Cat# NB600-201, RRID:AB_10003372 Mouse Monoclonal Antibody |
1:5000 (immuno) |
| Brn3a | Brn3a fused to the T7 gene 10 protein (aa 186–224) | Millipore Cat# MAB1585, RRID:AB_94166 Mouse Monoclonal Antibody |
1:200 (immuno) |
2.5-Western Blot
Tissue was collected in RIPA buffer: 150 mM NaCl, 50 mM Tris-HCl, 5 mM EGTA, 1% Triton, 0.5% deoxycholate, 0.1% SDS, 100 mM DTT, and Complete Mini protease inhibitor (Roche), at a pH=7.4. Samples were homogenized and centrifuged at 25,000× g, and only the supernatant was used for the next steps. Bicinchoninic acid (BCA) protein assay (Thermo Fisher Scientific) was performed. Alkaline denaturing buffer was added, and 60 μg of each sample were run in pre-cast bis-tris gels (4–12%, Invitrogen), and transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). Membranes were incubated with the primary antibodies (Table 1, referenced as “WB”) overnight at 4°C. Membranes were then rinsed in TBS + 0.1% Tween and incubated with infrared fluorescent dye (IRDye)-conjugated anti-rabbit or anti-mouse secondary antibodies (1:10,000, Licor) for 1h, rinsed and imaged with an Odyssey Infrared Reader (Licor). Optical density was analyzed with ImageJ software; the statistical analysis was performed using GraphPad Prism 5 (Student’s t test, CI>95%). Since the 75 kDa FMRP sometimes comprised a triple band, we analyzed the whole area containing all bands at the same time.
2.6-Immunofluorescence
Eyecups were fixed in 4% paraformaldehyde for 30 min, rinsed in phosphate buffer, and cryoprotected with sucrose. Eyecups were then frozen and sectioned in a cryostat and stored frozen at −20 °C. Light and dark-adapted retinas were collected on the same slide so they would be processed together. Sections were thawed, rinsed in PBS, blocked with 3% (v/v) horse serum in PBS for 1h and incubated with primary antibodies (Table 1, referenced as “immuno”) overnight. Eyecups were washed and incubated with Cy3- or Alexa 594-labeled secondary anti-rabbit antibodies (1:1000) for 2h, rinsed and incubated with the other primary antibody overnight for double labeling. After incubation with an Alexa 488-labeled secondary anti-mouse antibody (1:1000) for 2h, retinas were mounted with Vectashield (Vector), ProLong Gold (Life Technologies) or Glycergel (Dako). Images were acquired with an Olympus FluoView FV1000 or Leica confocal microscope. Immunofluorescence intensity from 6 to 12 pictures from each animal (24 to 48 images per experimental group) was analyzed with the RGB value plugin available in ImageJ software. Statistical analysis was performed using GraphPad Prism 5 (Student’s t test, CI>95%).
3-RESULTS
3.1-FMRP expression in the mouse retina
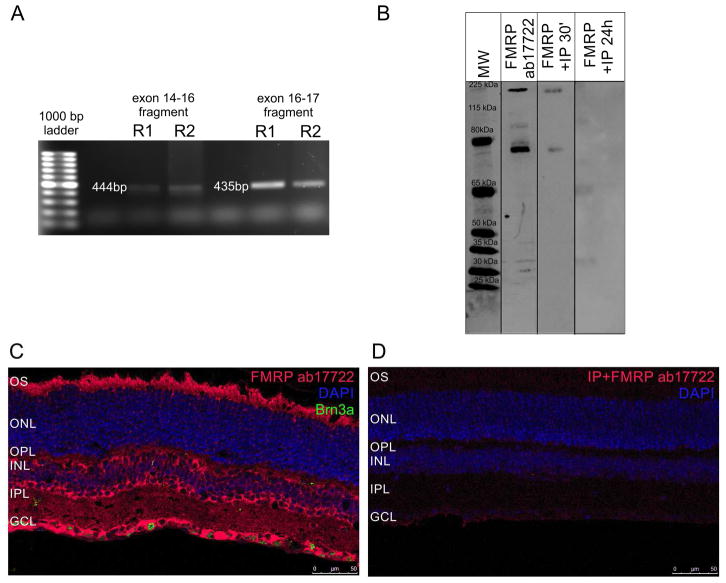
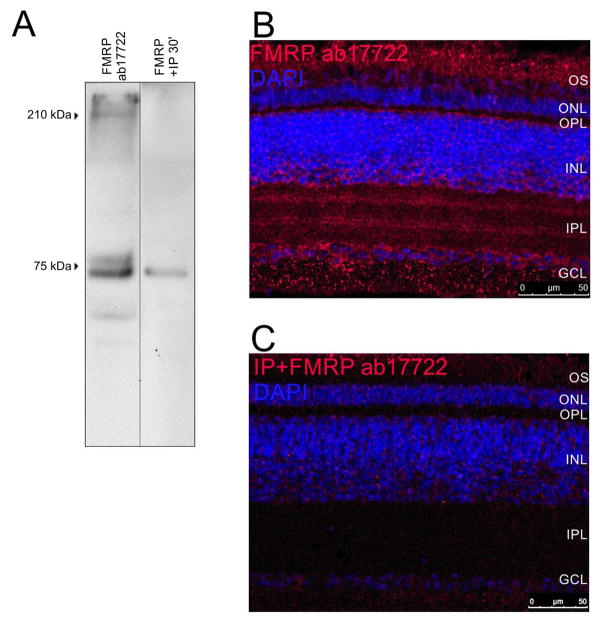
We first determined whether FMRP mRNA was expressed in the mouse retina. Reverse-transcription PCR assays with two different primer pairs detected fragments that corresponded to regions between exon 14 and exon 16 (444 bp), and between exon 16 and exon 17 (435 bp) (Figure 1A). Western blot assays using a polyclonal antibody directed against the C-terminal domain of FMRP (ab17722) strongly labeled a double band at approximately 80 kDa, which is close to the FMRP predicted molecular weight of 75 kDa, result that is consistent with previous reports, and a 210 kDa band (Figure 1B, “FMRP ab17722”) (Hurema and Oostra, 2013). When the antibody was preincubated with FMRP peptide for 30 min (Figure 1B, “FMRP+IP 30’”) or 24h (Figure 1B, “FMRP+IP 24h”), labeling was blocked. Using immunohistochemical techniques, we observed that FMRP was largely expressed in the photoreceptor outer segments (OS), in cells of the INL that appear to be amacrine and bipolar cells, in both synaptic layers (OPL and IPL), and in cells in the GCL, some co-localizing with Brn3a while others not (Figure 1C). The immunostaining specificity was corroborated by inhibitory peptide assay in which incubation of the primary antibody with FMRP peptide for 24h resulted in no staining in retinal sections (Figure 1D). However, OS staining was considered unspecific, since Fmr1 knockout retinas also presented staining in the OS (Supplementary material, Figure 2S).
Figure 1. FMRP is expressed in the mouse retina under a 12h light/12h dark cycle.
A) Ethidium bromide-stained agarose gel with PCR reactions from two different mouse retinas (R1 and R2). Bands represent FMRP mRNA fragments from exon 14 to exon 16 (444 bp) and from exon 16 to exon 17 (435 bp). Bolder band in the ladder is 500 bp. B) Western blots of mouse retina protein samples with the ab17722 FMRP antibody. It labels bands at the expected 75 kDa size, but also one band at approximately 210 kDa (FMRP ab17722). Pre-adsorbing the antibody with an inhibitory peptide for 30 min (FMRP +IP 30′) or 24h (FMRP +IP 24h) blocks the labeling. C) Immunostaining of the mouse retina with the ab17722 FMRP antibody (red), cell nuclei are labeled with DAPI (blue). Labeling appears mostly in the OS, OPL, INL, IPL and GCL (two independent experiments from three animals). D) Pre-adsorbing the antibody with an inhibitory peptide makes the staining almost disappear (two independent experiments from three animals). OS: photoreceptor outer segments, OPL: outer plexiform layer, INL: inner nuclear layer, IPL, inner plexiform layer, GCL: ganglion cell layer.
3.2-Modulation of FMRP expression by light or dark adaptation in the mouse retina
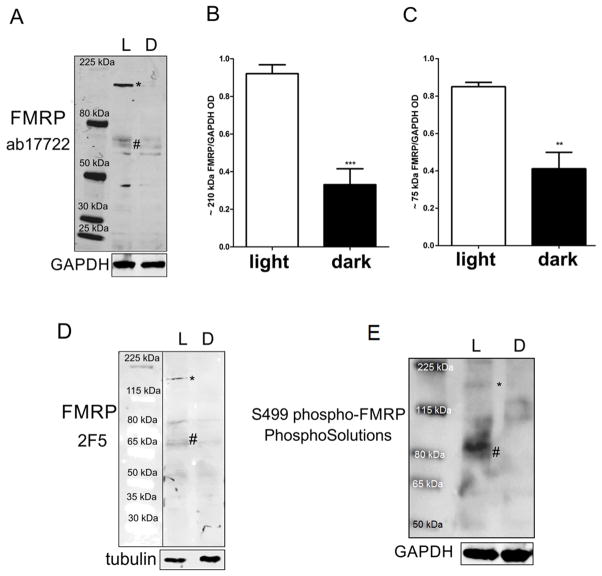
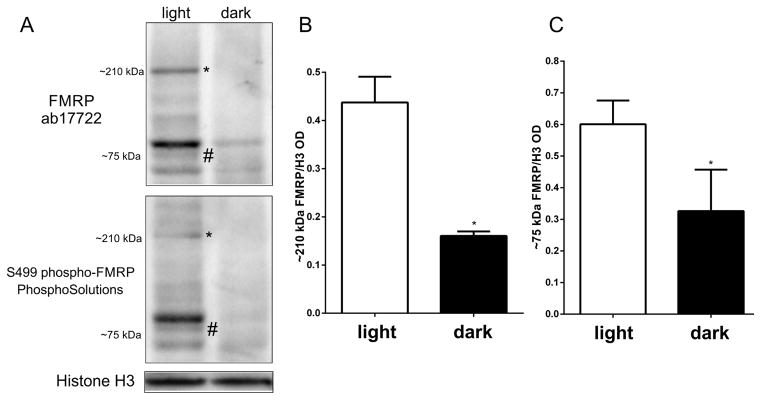
Since glutamate is the main neurotransmitter responsible for regulating the local protein synthesis function of FMRP (Bear et al., 2004; Ronesi and Huber, 2008), and that glutamate release from photoreceptors is increased in the dark, we examined by western blotting whether retinal FMRP expression was regulated by light stimulation. Two experimental conditions were used: 1) a 24h-dark-adapted group; 2) a group exposed to light for 1h after the 24h-period in the dark. We found that FMRP expression was higher in the animals that had been exposed to light after the 24h period in the dark (Figure 2A, “L”). We quantified the data obtained with the polyclonal antibody and determined that the expression of the 210 kDa (asterisk) and 75kDa FMRP bands (hash symbol) was decreased in the retinas of animals that were kept in the dark for 24h (Figure 2B and C, “L” versus “D”).
Figure 2. FMRP expression in the mouse retina is modulated by light.
A) Western blot of light adapted (L) and dark adapted (D) retinas with ab17722 FMRP antibody labeling bands at 75 kD (hash symbol), corresponding to the predicted molecular weight of FMRP, as well as a higher molecular weight band of approximately 210 kDa (asterisk). The epitope recognized by ab17722 appears mostly in the light adapted retina sample. B) Quantification of the 210 kDa band optical density in relation to loading control (ab17722 FMRP/GAPDH). Results are expressed as mean±SEM: 0.92±0.04 in light adapted vs 0.33±0.08 in dark adapted; ***P=0.0003. C) Quantification of the 75 kDa band optical density in relation to loading control (0.85±0.02 vs 0.41±0.08; **P=0.008). D) Western blot using monoclonal 2F5 FMRP antibody showing a staining similar to that found previously, with both 210 kDa (asterisk) and 75 kDa (hash symbol) bands. E) Western blot using phosphorylated FMRP antibody also showing 210 kDa (asterisk) and 75 kDa (hash symbol) bands. Three independent experiments were performed from four animals in each group.
In order to confirm our results, we used a different anti-FMRP antibody, the 2F5 monoclonal antibody, which reacts with the N-terminus of FMRP and has been validated by confirming the absence of staining in tissue from the Fmr1 knockout mouse (Gabel et al., 2004). Expression of the 75 kDa (hash symbol) and 210 kDa (asterisk) bands was also shown to be increased following light exposure when detected with the 2F5 monoclonal antibody, although the staining was fainter than with the ab17722 antibody (Figure 2D).
In the absence of mGluR5 activation induced by glutamate, FMRP is normally phosphorylated at serine 499 (S499) and bound to mRNA (Bassell and Warren, 2008). Therefore, we were interested in evaluating phosphorylated FMRP (pFMRP) content in retinas kept in the dark and the ones that were later exposed to light. The antibody against S499 pFMRP also labeled a broad band at about 80 kDa in retinas exposed to light (hash symbol) and a thin band at 210 kDa (asterisk), but not in the retinas of animals kept in the dark (Figure 2E).
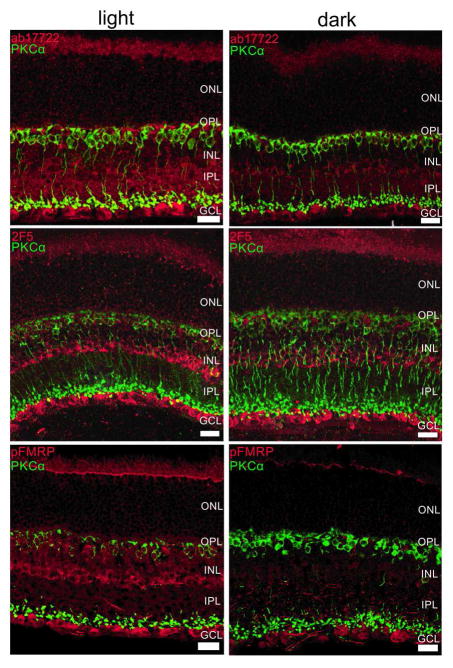
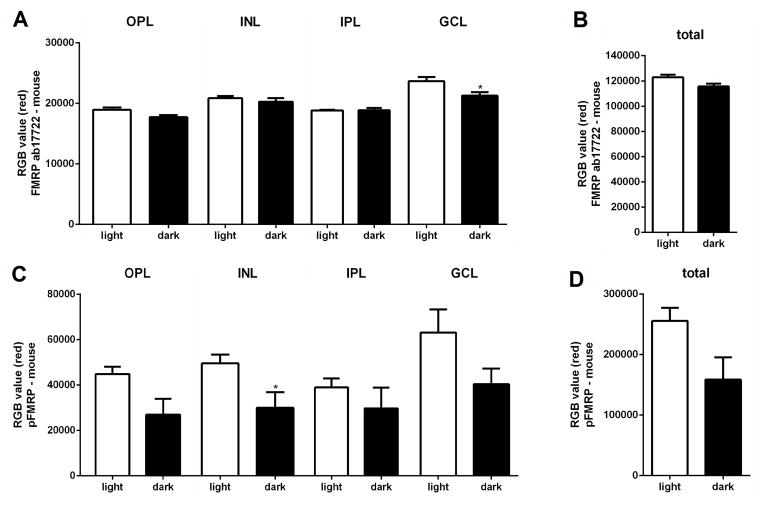
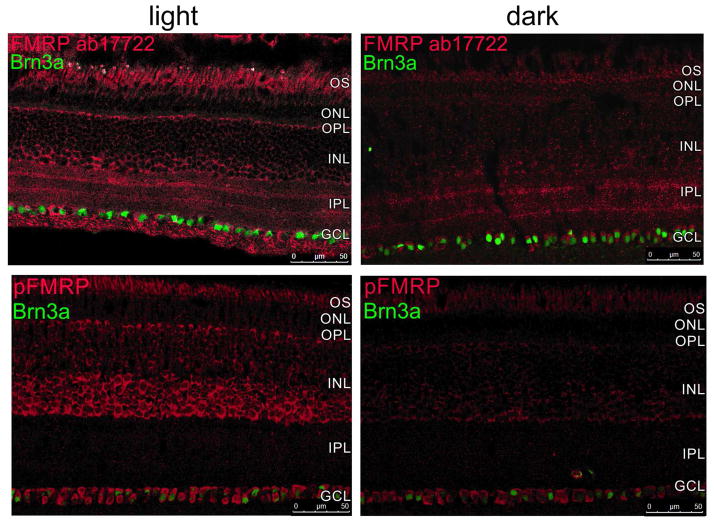
Next, we investigated if the differences in light and dark-adapted FMRP expression were specific to a retinal cell type or layer using immunohistochemical techniques (Figure 3). Our first observation was that both FMRP and pFMRP antibodies stained the OS, cells in the inner nuclear layer (INL) that appear to be amacrine cells, and cells in the ganglion cell layer (Figure 3). OS staining was considered unspecific, since Fmr1 knockout retinas also presented staining in the OS (Supplementary material, Figure 2S). FMRP labeling was also observed in the synaptic layers in the light adapted retinas when using the polyclonal antibody (ab17722) (Figure 3, top panels). Staining with the ab17722, 2F5 and phosphorylated FMRP antibodies show increased immunoreactivity in the retinas exposed to light in comparison to the retinas kept in the dark for 24h (Figure 3, comparing between left, light, and right, dark). Quantification of the immunofluorescence measures showed significantly decreased intensities in the INL (for pFMRP) and the GCL (for FMRP) in mice kept in the dark (Figure 4).
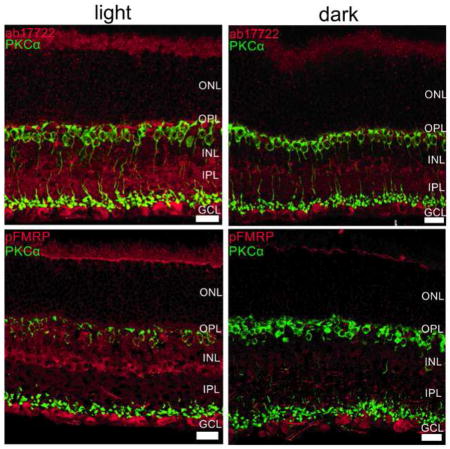
Figure 3. FMRP expression is modulated mostly in the OPL, INL and IPL in the mouse retina.
Double-labeling of the rod bipolar cell marker, PKCα (green) and FMRP (ab17722, red, top; 2F5, red, middle) and phospho-FMRP (pFMRP, red, bottom panels) antibodies show that FMRP is located in cell bodies in the INL that appear to be amacrine cells, and also in cells in the GCL. OS staining was considered unspecific because Fmr1 knockout retinas presented OS staining as well. It is noticeable that FMRP and pFMRP staining is stronger in light-adapted compared to dark-adapted retinas. Three independent experiments were performed from four animals in each group. Scale bar=20 μm.
Figure 4. FMRP immunofluorescence intensity in the mouse retina layers.
A) Quantification of FMRP immunofluorescence in the retinal layers of mice exposed to light for 1h after 24h in the dark versus mice kept in the dark (mean of red value±SEM, light vs dark); OPL=18912±374.9 vs 17678±342.9; INL=20871±350.1 vs 20269±600.6; IPL=18833±107.4 vs 18867±371.0; GCL=23691±674.8 vs 21279±560.4, *P=0.03). B) Quantification of total FMRP immunofluorescence in the mouse retina, light vs dark; 122895±2117 vs 115624±2272. C) Quantification of pFMRP immunofluorescence in the retinal layers of mice, light vs dark; OPL=44851±3200 vs 26957±7019; INL=49591±3843 vs 30047±6796, *P=0.04; IPL=38974±3922 vs 29709±9177; GCL=63177±10129 vs 40356±6901. D) Quantification of total pFMRP immunofluorescence in the mouse retina, light vs dark; 255660±21742 vs 158689±36775.
Since PKCα expression is modulated by light, we used it as a control for the light/dark effect. As expected, PKCα was observed in ON bipolar cells with cell bodies in the outer part of the INL with processes extending from OPL to IPL. Some colocalization with FMRP and pFMRP in the inner portion of IPL was visualized. Accordingly, PKCα staining reinforced the state of light and dark adaptation, since bipolar cell lower dendrites are more strongly labeled in the light adapted versus the dark adapted as previously shown by Gabriel and co-workers (2001).
3.3-FMRP expression in the chick retina
In order to evaluate the expression and regulation of FMRP in a non-mammalian animal model, we performed the same experiments in the chick retina. Western blotting for FMRP using the ab17722 antiserum detected the 75 kDa double band similar to the findings using mouse retina. Preadsorption of the antibody with the FMRP peptide for 30 min significantly decrease the labelling (Figure 5A). Immunohistochemical labeling of the chick retina revealed FMRP expression in the OPL, in the inner portion of the INL where amacrine cells are located, IPL, and GCL (Figure 5B). Pre-incubation of the primary antibody with the FMRP peptide blocked the staining (Figure 5C).
Figure 5. FMRP is expressed in the chick retina under a 12h light/12h dark cycle.
A) Western blots of chick retina protein samples with the ab17722 FMRP antibody. Bands are visible at the expected 75 kDa size and one band at approximately 210 kDa. Pre-adsorbing the primary antibody with an inhibitory peptide for 30 min blocks most of the signal. B) Immunostaining of chick retina with the ab17722 FMRP antibody (red) with cell nuclei revealed with DAPI (blue). Labeling appears mostly in OS, OPL, INL, IPL and GCL. C) Pre-adsorbing the primary antibody with an inhibitory peptide for 30 min blocks the staining. Three independent experiments were performed from three animals each.
3.4-The modulation of FMRP content by light or dark in the chick retina
In the mouse retina, we have observed that dark adaptation decreased expression of FMRP (Figures 2 and 3). To test if these findings were unique to the mouse, we performed the same experiments with chicks. Western blotting retina samples of chicks that were kept in the dark for 24h and either left in the dark, or exposed to light for 1h showed that FMRP expression is higher in retinas exposed to light (Figure 6A). As in mice, the 75 kDa FMRP bands appeared to be modulated by light with both ab17722 antibody (Figure 6A, top panel, and 6B) and the S499 phospho-FMRP (Figure 6A, bottom panel).
Figure 6. FMRP expression in the chick retina is modulated by light.
A) Western blots of protein extracts from light and dark adapted retinas with FMRP antibodies (top: FMRP ab17722; bottom: S499 phospho-FMRP) showing both 75 kDa (hash symbol) and 210 kDa (asterisk) bands. FMRP expression appears mostly in the light adapted retina sample. B) Quantification of the 210 kDa band optical density in relation to loading control (ab17722 FMRP/H3; 0.44±0.09 in light adapted vs 0.15±0.02 in dark adapted; *P=0.0107). C) Quantification of the 75 kDa band optical density in relation to loading control (ab17722 FMRP/H3: 0.55±0.15 in light adapted vs 0.18±0.01 in dark adapted; *P=0.0403). Three independent experiments were performed from three animals in each group.
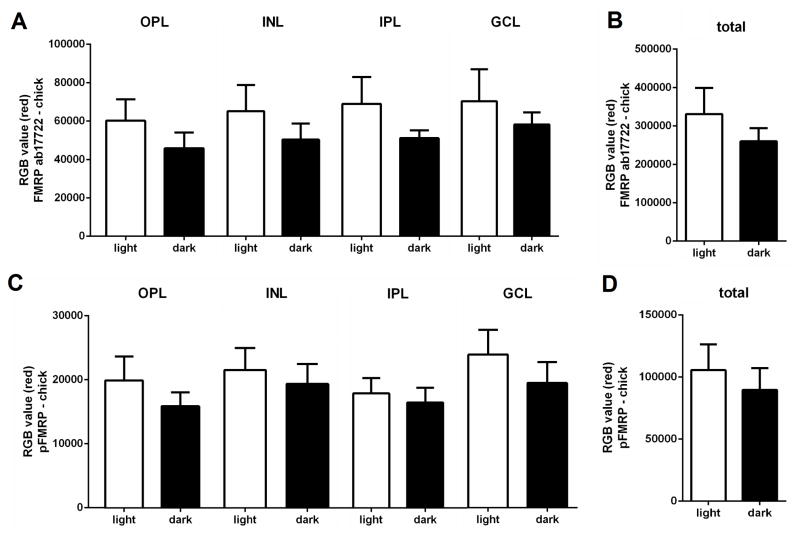
Immunohistochemical localization of FMRP in the chick retinas revealed the same pattern of expression changes as in the mouse retina: less FMRP immunofluorescence in the retina of animals kept in the dark for 24h when compared to retinas exposed to light for 1h after 24h in the dark, especially in the INL (Figure 7). It is also possible to observe Brn3a immunolabelling, which is specific to ganglion cells, co-localizing with some FMRP-positive cells in the GCL. Some FMRP-positive cells in the ganglion cell layer that did not present Brn3a labelling were probably displaced amacrine cells. It was also interesting to note that Brn3a expression is not modified by either light exposure or dark adaptation (Figure 7) indicating that changes in FMRP expression by light were not an artifact. Quantification of the immunofluorescence measures showed similar trends as in the mice with decreased intensities in most layers in chicks kept in the dark, for both FMRP and pFMRP (Figure 8).
Figure 7. FMRP expression is modulated mostly in the OPL, INL and IPL in the chick retina.
Double-labeling immunohistochemistry of the ganglion cell marker, Brn3a (green) and FMRP (ab17722, red, top) and S499 phosphorylated FMRP (pFMRP, red, bottom panels) antibodies show that FMRP is located in the OS, OPL, INL, IPL and GCL (both in ganglion cells labelled with Brn3a and displaced amacrine cells negative for Brn3a ganglion cell marker). OS staining was considered unspecific because Fmr1 knockout retinas presented OS staining as well. It is noticeable that FMRP and pFMRP staining is stronger in light adapted compared to dark adapted retinas, except for the cells in the GCL. Three independent experiments were performed from three animals in each group.
Figure 8. FMRP immunofluorescence measures in the chick retina layers.
A) Quantification of FMRP immunofluorescence in the retinal layers of chicks exposed to light for 1h after 24h in the dark versus chicks kept in the dark (mean of red value±SEM, light vs dark); OPL=60168±11190 vs 45791±8293; INL=65192±13652 vs 50324±8356; IPL=68965±13939 vs 51119±4018; GCL=70356±16586 vs 58239±6285). B) Quantification of total FMRP immunofluorescence in the chick retina, light vs dark; 330977±68067 vs 260017±34414. C) Quantification of pFMRP immunofluorescence in the retinal layers of chicks, light vs dark; OPL=19936±3754 vs 15899±2164; INL=21567±3417 vs 19376±3136; IPL=17905±2383 vs 16490±2281; GCL=23931±3888 vs 19499±3278. D) Quantification of total pFMRP immunofluorescence in the chick retina, light vs dark; 105579±20669 vs 89730±17405.
4-DISCUSSION
4.1-FMRP in the retina and its regulation by light
FMRP has been shown to be necessary for the correct establishment of circadian rhythms (Zhang et al., 2008). Also, expression of FMRP in the visual cortex has been shown to be regulated by light with expression elevated in rats exposed to light, compared to those that were dark-adapted (Gabel et al., 2004). In this study, we show that FMRP is expressed in the retina and that its content changes depending on light exposure. Both western blotting and immunohistochemistry show higher levels of FMRP expression in retinas exposed to light when compared to 24h-dark-adapted retinas. The slight difference between the immunostaining detected with ab17722 (polyclonal) compared to 2F5 (monoclonal) and pFMRP (p1125-499, polyclonal) might be due to different reactivity to distinct FMRP epitopes (see table 1). Also, it is likely that the 210 kDa band found in our study represents the previously described heteromer composed of two FMRP proteins and one nucleolin (Ceman et al., 1999; Taha et al., 2014), since nucleolin expression is also found in INL cell bodies in the retina (Hollander et al., 1999).
Considering that FMRP phosphorylation and degradation are mostly regulated by glutamate (Bassell and Warren, 2008), and that photoreceptors release glutamate in the dark (Thoreson, 2007), it is possible that different light intensity ranges may modulate FMRP content in the retina. It is likely that, in the light, little glutamate is being released by photoreceptors, FMRP is phosphorylated, bound to mRNA, and located near the synapse. In the dark, glutamate is released by photoreceptors (Thoreson, 2007). The retina presents mGluR5 (Brandstätter et al., 1998), and this receptor activates PP2A (Bassell and Warren, 2008), a phosphatase strongly expressed in the retina (Liu et al., 2008). Therefore, the increased release of glutamate in the dark may promote the dephosphorylation of FMRP, leading to its degradation.
On the other hand, activity has been shown to increase both FMRP and Fmr1 mRNA levels in cultured hippocampal neurons treated with KCl (Antar et al., 2004). In the retina, the ON and OFF pathways respond in opposite ways to glutamate released by photoreceptors: in the dark, mGluR6 promotes hyperpolarization of ON bipolar cells, while ionotropic glutamate receptors promote depolarization of OFF bipolar cells in response to glutamate. Light decreases glutamate release from photoreceptors, allowing ON bipolar cells to depolarize, while OFF bipolar cells hyperpolarize (Thoreson, 2007). Therefore, the increase in FMRP accumulation could represent an effect that is specific to ON pathway cells activation that happens in the light, while OFF pathway cells might simply not present similar mechanisms when activated in the dark. Also, FMRP immunolabeling in the retina of animals exposed to light for 1h following 24 h darkness (Figures 3 and 5, “light”) were similar to those exposed to light (during light phase/daytime), that were under regular 12/12-light-dark cycles (Figures 1 and 4).
However, in addition to glutamate, other neurotransmitters are differentially released when the retina is exposed to light or dark. Dopamine release is increased in the light (Doyle et al., 2002), while adenosine release is increased in the dark (Ribelayga and Mangel, 2005). Therefore, our results open many pathways to be explored regarding the regulation of FMRP expression and its function in the retina, and how glutamate, dopamine and adenosine might interact and differently modulate FMRP in light or dark.
4.2-FMRP in mouse versus chick and other species
In this study, we compared results obtained with two species: the mouse, which is considered to have a nocturnal behavior, and the chick, which has a diurnal behavior. Our intention was to unravel if their different retinal features present mainly due to visual adaptation for nocturnal or diurnal behavior or even some neurochemical specificities would lead to distinct patterns of FMRP expression or modulation. However, there was no difference in the results observed, and light yielded the same effect on FMRP in comparison to dark in both species. Therefore, FMRP modulation by light probably happens through a mechanism that is very well conserved.
One study has recently evaluated the behavior of wild type and fmr1 knockout zebrafish in a test that uses swimming in light or dark water tanks as a measure of stress (Ng et al., 2013). They found that fmr1 knockout zebrafish tend to swim for longer periods in the light tank, which is interpreted as an anxiolytic-like behavior (Ng et al., 2013). Nonetheless, concerning the results obtained in our work, these results could be related to a poorer visual capability of the fmr1 knockout to distinguish the luminance differences (its FMRP absence relating to our “dark” condition), not being so disturbed by the light and therefore swimming more interchangeably between the tanks in comparison to the wild type. This example shows how important it is to observe and study FMRP in the visual system, including the retina of closer species, like primates. It would probably help us understand some distinguished behaviors that Fragile X Syndrome individuals present that may be caused by vision rather than cognition-related problems. It should also reinforce the hypothesis (Rossignol et al., 2014) that the overall “sensorial hypersensitivity” phenotype of Fragile X Syndrome is not only due to the well-known cerebral defects, but also to sensorial perception defects through the peripheral sensorial tissue.
4.3-FMRP and its possible functions in retinal physiology
The purpose of FMRP signaling in the CNS is generally related to local protein translation during plasticity events. In particular, FMRP appears to regulate the expression of Arc, a protein that is important for AMPA receptor internalization and LTD in the hippocampus (Park et al., 2008; Waung et al., 2008). Such plasticity events have been described in the retina as well, and could account for modifications in retinal ganglion cell rectification currents (Jones et al., 2012). One recent paper has shown that FMRP is expressed in the retina and that its absence correlates with increases in the electroretinogram b-wave, which mostly reflects ON-bipolar cell depolarization to light (Rossignol et al., 2014). FMRP thus appears to influence retinal physiology. Testing vision performance in patients with a disorder in which there is lack of FMRP, i.e. Fragile X, schizophrenia, major depression or bipolar disorder (Fatemi and Folsom, 2011) could clarify which visual pathways might be affected by its absence.
4.4-Conclusions
FMRP is expressed in both mouse and chick retinas and it is modulated in the same way by light stimulus. Thus, FMRP might have a role in retinal physiology.
Supplementary Material
Highlights.
FMRP mRNA is expressed in the mouse retina
FMRP expression is decreased by dark adaptation
FMRP is located mostly in the inner retina
Acknowledgments
We thank Dr. Kimberly Huber for the 2F5 antibody and helpful discussions. We thank Tammie Haley and Sarah Rodrigues for the technical support. E. M. Guimarães-Souza received a scholarship from the program “Ciência sem Fronteiras”, by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, protocol number 245861/2012-2) to perform experiments abroad, and a regular PhD scholarship in Brazil by Coordenação de Aperfeiçoamento de Pessoal de Ensino Superior (CAPES). KCC is a research fellow of CNPq. This research was supported by NIH grants EY09534 (RMD), EY022369 (CWM), CNPq, CAPES, FAPERJ and PRONEX/MCT. Fmr1 KO investigation was supported by CNRS, Regional Hospital of Orléans, University of Orléans, FEDER 35106 grant, and FRAXA Research Foundation (USA) grant (2015–2016).
Footnotes
DISCLOSURE: All authors have approved the final version of the paper, and none of the authors have any conflicts of interest. Elisa M. Guimarães-Souza designed and conducted experiments and analysis, discussed data and wrote the paper. Olivier Perche conducted experiments and analysis on the Fmr1 Knockout mouse model. Catherine W. Morgans provided reagents, obtained the confocal images, discussed data and wrote the paper. Robert M. Duvoisin designed experiments, provided reagents and animals, discussed data and wrote the paper. Karin C. Calaza designed experiments, provided reagents, discussed data and wrote the paper. We also state that none of this material has been submitted elsewhere, and that we have read and have abided by the statement of ethical standards for manuscripts submitted to Experimental Eye Research.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adusei DC, Pacey LK, Chen D, Hampson DR. Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology. 2010;59(3):167–171. doi: 10.1016/j.neuropharm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Antar LN, Afroz R, Dictenberg JB, Carroll RC, Bassell GJ. Metabotropic glutamate receptor activation regulates fragile x mental retardation protein and FMR1 mRNA localization differentially in dendrites and at synapses. J Neurosci. 2004;24(11):2648–2655. doi: 10.1523/JNEUROSCI.0099-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60(2):201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear MF, Huber KM, Warren ST. The mGluR theory of fragile X mental retardation. Trends Neurosci. 2004;27(7):370–377. doi: 10.1016/j.tins.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Brandstätter JH, Koulen P, Wässle H. Diversity of glutamate receptors in the mammalian retina. Vis Res. 1998;38(10):1385–1397. doi: 10.1016/s0042-6989(97)00176-4. [DOI] [PubMed] [Google Scholar]
- Calaza KC, Gardino PF. Neurochemical phenotype and birthdating of specific cell populations in the chick retina. An Acad Bras Ciênc. 2010;82(3):595–608. doi: 10.1590/s0001-37652010000300007. [DOI] [PubMed] [Google Scholar]
- Ceman S, Brown V, Warren ST. Isolation of an FMRP-associated messenger ribonucleoprotein particle and identification of nucleolin and the fragile X-related proteins as components of the complex. Mol Cell Biol. 1999;19(12):7925–7932. doi: 10.1128/mcb.19.12.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, Bagni C. Regulation of molecular pathways in the Fragile X Syndrome: insights into Autism Spectrum Disorders. J Neurodev Disord. 2011;3(3):257–269. doi: 10.1007/s11689-011-9087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker CJ, Parker R. P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol. 2012;4(9):a012286. doi: 10.1101/cshperspect.a012286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst C, Heulens I, Brouwer JR, Willemsen R, De Geest N, Reeve SP, De Deyn PP, Hassan BA, Kooy RF. Expression of the GABAergic system in animal models for fragile X syndrome and fragile X associated tremor/ataxia syndrome (FXTAS) Brain Res. 2009;1253:176–183. doi: 10.1016/j.brainres.2008.11.075. [DOI] [PubMed] [Google Scholar]
- Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19(5):593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The role of fragile X mental retardation protein in major mental disorders. Neuropharmacology. 2011;60:1221–1226. doi: 10.1016/j.neuropharm.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel LA, Won S, Kawai H, McKinney M, Tartakoff AM, Fallon JR. Visual experience regulates transient expression and dendritic localization of fragile X mental retardation protein. J Neurosci. 2004;24(47):10579–10583. doi: 10.1523/JNEUROSCI.2185-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel R, Lesauter J, Silver R, Garcia-España A, Witkovsky P. Diurnal and circadian variation of protein kinase C immunoreactivity in the rat retina. J Comp Neurol. 2001;439(2):140–150. doi: 10.1002/cne.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gessert S, Bugner V, Tecza A, Pinker M, Kühl M. FMR1/FXR1 and the miRNA pathway are required for eye and neural crest development. Dev Biol. 2010;341(1):222–235. doi: 10.1016/j.ydbio.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Gross C, Yao X, Pong DL, Jeromin A, Bassell GJ. Fragile X mental retardation protein regulates protein expression and mRNA translation of the potassium channel Kv4.2. J Neurosci. 2011;31(15):5693–5698. doi: 10.1523/JNEUROSCI.6661-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães-Souza EM, Calaza KC. Selective activation of group III metabotropic glutamate receptor subtypes produces different patterns of γ-aminobutyric acid immunoreactivity and glutamate release in the retina. J Neurosci Res. 2012;90(12):2349–2361. doi: 10.1002/jnr.23123. [DOI] [PubMed] [Google Scholar]
- Guimarães-Souza EM, Gardino PF, De Mello FG, Calaza KC. A calcium-dependent glutamate release induced by metabotropic glutamate receptors I/II promotes GABA efflux from amacrine cells via a transporter-mediated process. Neuroscience. 2011;179:23–31. doi: 10.1016/j.neuroscience.2011.01.035. [DOI] [PubMed] [Google Scholar]
- Hagerman RJ. Lessons from Fragile X Regarding Neurobiology, Autism and Neurodegeneration. J Dev Behav Pediatr. 2006;27:63–75. doi: 10.1097/00004703-200602000-00012. [DOI] [PubMed] [Google Scholar]
- Hillier LDW, et al. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature. 2004;432(7018):695–716. doi: 10.1038/nature03154. [DOI] [PubMed] [Google Scholar]
- Hollander BA, Liang MY, Besharse JC. Linkage of a nucleolin-related protein and casein kinase II with the detergent-stable photoreceptor cytoskeleton. Cell Motil Cytoskeleton. 1999;43(2):114–127. doi: 10.1002/(SICI)1097-0169(1999)43:2<114::AID-CM3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Hunt DM, Carvalho LS, Cowing JA, Davies WL. Evolution and spectral tuning of visual pigments in birds and mammals. Philos Trans R Soc Lond B Biol Sci. 2009;364:2941–2955. doi: 10.1098/rstb.2009.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurema RK, Oostra BA. The CCG repeat and the Fmr1 gene. In: Kowhi Y, McMurray CT, editors. Methods Mol Biol. Chapter 11. Vol. 1010. 2013. pp. 155–176. [DOI] [PubMed] [Google Scholar]
- Jones RS, Carroll RC, Nawy S. Light-induced plasticity of synaptic AMPA receptor composition in retinal ganglion cells. Neuron. 2012;75(3):467–478. doi: 10.1016/j.neuron.2012.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kéri S, Benedek G. Why is vision impaired in fragile X premutation carriers? The role of fragile X mental retardation protein and potential FMR1 mRNA toxicity. Neuroscience. 2012;206:183–189. doi: 10.1016/j.neuroscience.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Liu WB, Li Y, Zhang L, Chen HG, Sun S, Liu JP, Liu Y, Li DW. Differential expression of the catalytic subunits for PP-1 and PP-2A and the regulatory subunits for PP-2A in mouse eye. Mol Vis. 2008;14:762–773. [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Keli3 S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27(20):5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng MC, Yang YL, Lu KT. Behavioral and synaptic circuit features in a zebrafish model of fragile X syndrome. PLoS One. 2013;8(3):e51456. doi: 10.1371/journal.pone.0051456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59(1):70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Douglas RM. Characterization of mouse cortical spatial vision. Vis Res. 2004;44:3411–3418. doi: 10.1016/j.visres.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Quraishi S, Gayet J, Morgans CW, Duvoisin RM. Distribution of group-III metabotropic glutamate receptors in the retina. J Comp Neurol. 2007;501(6):931–943. doi: 10.1002/cne.21274. [DOI] [PubMed] [Google Scholar]
- Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25(1):215–22. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Metabotropic glutamate receptors and fragile x mental retardation protein: partners in translational regulation at the synapse. Sci Signal. 2008;1(5):pe6. doi: 10.1126/stke.15pe6. [DOI] [PubMed] [Google Scholar]
- Rossignol R, Ranchon-Cole I, Pâris A, Herzine A, Perche A, Laurenceau D, Bertrand P, Cercy C, Pichon J, Mortaud S, Briault S, Menuet A, Perche O. Visual sensorial impairments in neurodevelopmental disorders: evidence for a retinal phenotype in Fragile X Syndrome. PLoS One. 2014;9(8):e105996. doi: 10.1371/journal.pone.0105996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schitine CS, Mendez-Flores OG, Santos LE, Ornelas I, Calaza KC, Pérez-Toledo K, López-Bayghen E, Ortega A, Gardino PF, de Mello FG, Reis RA. Functional plasticity of GAT-3 in avian Müller cells is regulated by neurons via a glutamatergic input. Neurochem Int. 2015;82:42–51. doi: 10.1016/j.neuint.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Sen M, Gleason E. Immunolocalization of metabotropic glutamate receptors 1 and 5 in the synaptic layers of the chicken retina. Vis Neurosci. 2006;23(2):221–231. doi: 10.1017/S0952523806232073. [DOI] [PubMed] [Google Scholar]
- Taha MS, Nouri K, Milroy LG, Moll JM, Herrmann C, Brunsveld L, Piekorz RP, Ahmadian MR. Subcellular fractionation and localization studies reveal a direct interaction of the fragile X mental retardation protein (FMRP) with nucleolin. PLoS One. 2014;9(3):e91465. doi: 10.1371/journal.pone.0091465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreson WB. Kinetics of Synaptic Transmission at Ribbon Synapses of Rods and Cones. Mol Neurobiol. 2007;36(3):205–223. doi: 10.1007/s12035-007-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59(1):84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterson RH, et al. Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420(6915):520–562. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]
- Westmark CJ, Malter JS. FMRP mediates mGluR5-dependent translation of amyloid precursor protein. PLoS Biol. 2007;5(3):e52. doi: 10.1371/journal.pbio.0050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Fang Z, Jud C, Vansteensel MJ, Kaasik K, Lee CC, Albrecht U, Tamanini F, Meijer JH, Oostra BA, Nelson DL. Fragile X-related proteins regulate mammalian circadian behavioral rhythms. Am J Hum Genet. 83(1):43–52. doi: 10.1016/j.ajhg.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.