Abstract
Objective
To elucidate the prevalence and role of β1 adrenergic receptor autoantibodies (β1AR-AAb) belonging to the immunoglobulin (Ig)G3 subclass in patients with heart failure (HF) treated with β-adrenergic blockers.
Background
Several cardiac AAbs have been reported to be present in sera from patients with dilated cardiomyopathy and other etiologies. Among AAbs, those recognizing β1AR-AAbs show agonist-like effects, have detrimental effects on cardiomyocytes, and may induce persistent myocardial damage.
Methods
We quantify total IgG and IgG3 subclass β1AR-AAb in subjects with chronic stable HF with long-term follow-up.
Results
In our study cohort of 121 subjects, non-IgG3-β1AR-AAb and IgG3-β1AR-AAb were found to be positive in 20 (17%) and 26 patients (21%), respectively. The positive rate of IgG3-β1AR-AAb was significantly higher for those with nonischemic compared with ischemic HF etiology (27% vs 8%, P = .01), but the positive rate for non-IgG3-β1AR-AAb was similar between the 2 groups (18% vs 16%, respectively, P = NS). There were no significant differences in clinical and echocardiographic measures among total β1AR-AAb negative, non-IgG3-β1AR-AAb positive, and IgG3-β1AR-AAb positive groups at baseline. During 2.2 ± 1.2 years of follow-up, we observed similar rates of the composite endpoint of all-cause mortality, cardiac transplantation, or hospitalization resulting from HF between total IgG-β1AR-AAb negative and positive patients. However, the composite endpoint events were significantly more common in the patients without than in those with IgG3-β1AR-AAb (P = .048, log-rank test).
Conclusions
Presence of IgG3-β1AR-AAb, not total IgG, was associated with paradoxically more favorable outcomes in our cohort of patients with chronic systolic HF largely treated by β-blockers.
Keywords: Autoantibody, IgG3, β1-adrenergic receptor, β-blocker
There has been a longstanding belief that dysregulated autoimmune processes may lead to disease progression in heart failure (HF). Specifically, several cardiac autoantibodies (AAbs) against specific cardiac antigens have been detected in sera from patients with dilated cardiomyopathy (DCM).1,2 In addition, immunization against the second extracellular loop peptide of the β1-adrenergic receptor (β1AR),3 muscarinic M2 receptor peptide,4 or troponin I peptide5 can generate AAbs and can lead to the development of DCM-like phenotypes in experimental models. Among the various anti-cardiac AAbs, autoantibodies against β1AR (β1AR-AAb) have been detected in 30–40% of DCM patients.6–10 Clinical studies conducted in the 1980s and 1990s (before the broad adoption of β-adrenergic blockers) demonstrated the associations between detectable β1AR-AAb and increased rates of mortality,8 fatal ventricular arrhythmias, and sudden death7,11 in patients with DCM. This finding suggests that certain β1AR-AAbs can be generated, at least in part, by cardiac loading or damage. Mechanistic studies have also demonstrated that β1AR-AAb may possess agonist-like properties,12–15 inducing receptor uncoupling,3 myocyte apoptosis,16 sustained calcium influx resulting in electric instability of the heart,17 and persistent myocardial damage.18 These potentially detrimental effects by the β1AR-AAb can be abolished by β-blockers in in vitro19 and in vivo3 experiments. Indeed, β1AR-AAb-positive HF patients have demonstrated a more favorable recovery of cardiac performance than β1AR-AAb-negative patients in response to β-adrenergic blocker therapy.9,20 Furthermore, immunoadsorption (IA) using columns specific for β1AR-AAb was effective in alleviating the cardiac dysfunction of an observational series of patients with DCM.21 Interestingly, in the analysis of weaned DCM patients who tested positive for β1AR-AAb before left ventricular assist device implantation, β1AR-AAb became undetectable after left ventricular unloading by mechanical circulatory assist support.22 Herein, the objective of our study is to determine the prevalence and clinical significance of specific β1AR-AAb in contemporary patients with chronic systolic HF predominantly treated with β-blockers.
Material and Methods
Study Population
One hundred and twenty one consecutive ambulatory and stable adult subjects (>18 years of age) with a clinical diagnosis of chronic systolic HF (left ventricular ejection fraction [LVEF] < 40%) who received longitudinal care at the Cleveland Clinic outpatient HF clinic were prospectively enrolled. Exclusion criteria consisted of a major cardiovascular event (myocardial infarction, unstable angina, stroke, transient ischemic attack, pulmonary embolism), having undergone major surgery, hospitalization, or emergency room visits for HF exacerbation, or the use of inotropic agents within the preceding 30 days. Written informed consent was obtained from each of the patients before participation in the study, and the protocol was approved by the Cleveland Clinic Institutional Review Board.
Study Design
After informed consent and data collection, all patients underwent a blood draw and comprehensive transthoracic echocardiography evaluation using a Vivid 7 system (GE Vingmed Ultrasound, Horten, Norway). Two-dimensional grayscale and Doppler imaging were performed in standard parasternal and apical views. All subjects received guideline-directed medical therapy as tolerated, and their long-term outcomes (including all-cause mortality, cardiac transplantation, or hospitalization resulting from HF) were tracked by electronic medical record and determined by manual chart review. Ischemic cardiomyopathy (ICM) was defined as the presence of HF and angiographic evidence of coronary artery obstruction as contributing etiology.
β1AR-AAb Immunoassays
The presence of β1AR-AAb was determined by enzyme-linked immunoabsorbent assay using a synthetic peptide corresponding to the putative sequence of the second extracellular loop of human β1AR (amino acid sequence number, 197 to 222; H-W-W-R-A-E-S-D-E-A-R-R-C-Y-N-D-P-K-C-C-D-F-V-T-N-R) as an epitope peptide, as previously described.7,9,23 Anti-human immunoglobulin G (IgG) antibody or IgG3 antibody was used as a secondary antibody to detect β1AR-AAb belonging to IgG or IgG3 subclass. Positivity was defined as 2.5 times the background density as consistent with prior reports.7,9,23 IgG β1AR-AAb positive but IgG3 β1AR-AAb negative subjects were classified as “non-IgG3 β1AR-AAb positive” group.
Statistical Analysis
All values were expressed as the mean value ± SD. Differences between groups were compared using the non-paired t test or Mann-Whitney U rank-sum test for unpaired data and by the chi-square test for discrete variables. Kaplan-Meier survival curves for the composite endpoint of all-cause death, cardiac transplantation, or hospitalization due to the exacerbation of HF were calculated according to the presence or absence of IgG β1AR-AAb and/or IgG3 β1AR-AAb. Sensitivity analysis was performed by dividing the study cohort into 3 groups: (1) IgG3 β1AR-AAb positive; (2) non-IgG3 β1AR-AAb positive; and (3) β1AR-AAb negative groups. The differences among groups were analyzed by the log-rank test. Cox proportional hazards model was used to identify independent predictors of the composite endpoint of all-cause death, cardiac transplantation, and hospitalization resulting from exacerbation of HF. Heart rate (HR) at baseline, LVEF, estimated glomerular filtration rate calculated by the Modification of Diet in Renal Disease formula, and β1AR-AAb status were included in this model. A P value <.05 was considered statistically significant. All statistical analyses were performed in JMP 10.0.2 (SAS, Cary NC).
Results
Patient Characteristics
Baseline characteristics of the study population based on the β1AR-AAb status is shown in Table 1. There were no significant differences in age, gender, heart rate, LVEF, or plasma B-type natriuretic peptide levels among the 3 groups. In addition, there were no significant differences in medication or in the proportion of patients who underwent implantable cardioverter defibrillator implantation among the 3 groups. Of note, the large majority of patients (97%) were treated with β-adrenergic blockers in this ambulatory, stable HF population.
Table 1.
Baseline Characteristics Based on the β1AR-AAb Status
| β1AR-AAb | |||
|---|---|---|---|
| (−) (n = 75) |
Non-IgG3 (+) (n = 20) |
IgG3 (+) (n = 26) |
|
| Age | 56 ± 10 | 53 ± 9 | 55 ± 15 |
| Male sex | 71% (53) | 60% (12) | 62% (16) |
| Systolic blood pressure (mmHg) |
114 ± 17 | 119 ± 22 | 119 ± 21 |
| Heart rate (bpm) | 73 ± 12 | 75 ± 13 | 78 ± 16 |
| LVEF (%) | 31 ± 12 | 31 ± 15 | 34 ± 14 |
| BNP (pg/mL) | 262.8 ± 362.8 | 174.4 ± 164.2 | 269.2 ± 426.4 |
| eGFR (mL/min) | 78 ± 23 | 78 ± 28 | 82 ± 26 |
| Sodium (mEq/L) | 139 ± 3 | 139 ± 3 | 138 ± 3 |
| Hemoglobin (g/dL) | 13.6 ± 1.8 | 13.1 ± 1.5 | 13.3 ± 1.7 |
| Medication | |||
| ACEi or ARB | 89% (65/73) | 75% (15/20) | 92% (22/24) |
| β blocker | 96% (70/73) | 100% (19/19) | 96% (23/24) |
| Digoxin | 40% (29/73) | 40% (8/20) | 17% (4/24) |
| Loop diuretics | 67% (49/73) | 60% (12/20) | 67% (16/24) |
| Aldosterone antagonist |
45% (33/73) | 70% (14/20) | 42% (10/24) |
| ICD | 64% (47/73) | 65% (13/20) | 54% (13/24) |
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; β1AR-AAb, autoantibody against β1-adrenergic receptor; eGFR, estimated glomerular filtration rate calculated by Modification of Diet in Renal Disease formula; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction.
Prevalence of Detectable β1AR-AAb in Study Cohort
Among 121 subjects, we observed 46 patients with detectable β1AR-AAb (38%); 26 patients (21%) tested positive for belonging to the IgG3 subclass (IgG3-β1AR-AAb) and 20 patients (17%) tested positive for β1AR-AAb belonging to other IgG subclasses. The remaining 75 patients (62%) were deemed negative. The positive rate of IgG3 β1AR-AAb was significantly higher in patients with DCM vs ICM (27% vs 8%, P = .01), though the positive rates of non-IgG3 β1AR-AAb did not differ between the different HF etiologies (18% vs 16% respectively, P = NS).
Clinical Events Based on the β1AR-AAb Status
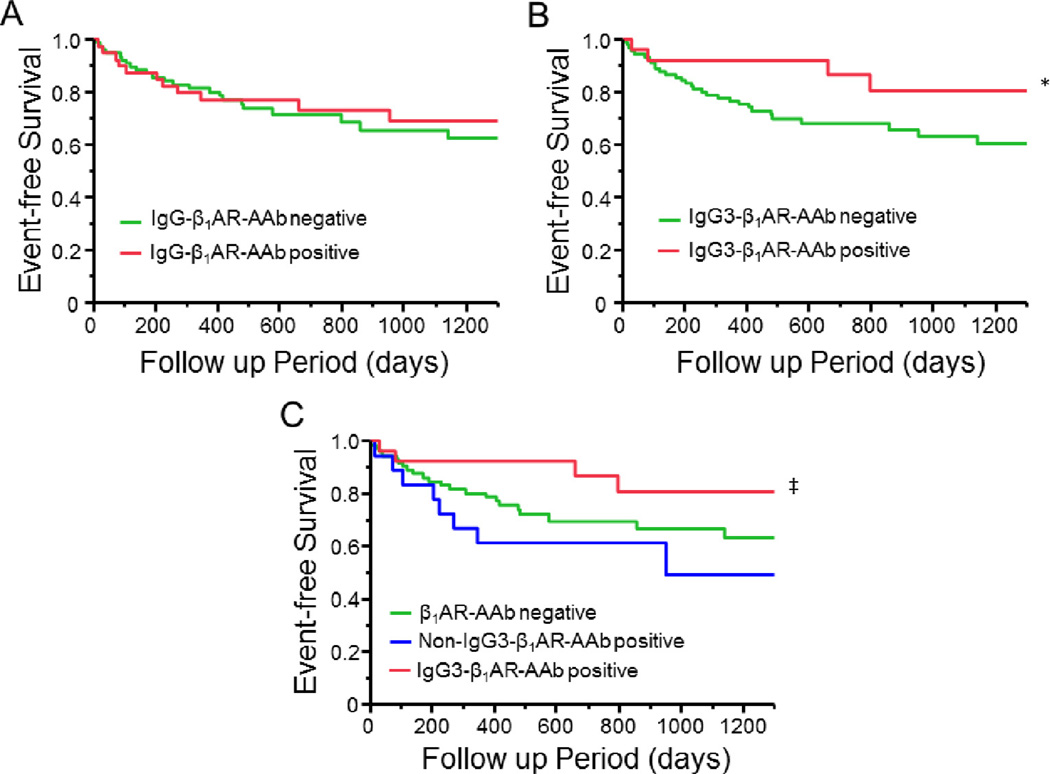
Figure 1 shows the Kaplan-Meier curves based on β1AR-AAb status. During 2.2 ± 1.2 years of follow-up, there was no significant difference in the composite endpoint of all-cause death, cardiac transplantation, and hospitalization resulting from HF between total IgG β1AR-AAb positive and negative groups (Fig. 1A). However, when the study population was divided into 2 groups based on IgG3 β1AR-AAb positivity, we observed that the composite endpoint was more common in the IgG3 negative group than in the positive group (P = .048, log-rank test, Fig. 1B). Furthermore, when study subjects were divided into 3 groups based on the presence or absence of both β1AR-AAb and IgG3 subclass (β1 AR-AAb negative, non-IgG3 β1AR-AAb positive, and IgG3 β1AR-AAb positive), subjects with IgG3 β1AR-AAb had the lowest rate of adverse clinical events compared with the other 2 groups (Fig. 1C). Specifically within the β1AR-AAb positive cohort, the difference in adverse event rates between IgG3 positive and negative groups was statistically significant (P < .01, log-rank test). As seen in Table 2, univariate analysis showed that HR at baseline, LVEF, and β1AR-AAb status were associated with the occurrence of the composite endpoint. Multivariate analysis demonstrated that both HR at baseline and β1AR-AAb status were independent predictors of the composite endpoint.
Fig. 1.
Kaplan-Meier survival curves for the composite endpoint of all-cause death, cardiac transplantation, or hospitalization resulting from the exacerbation of heart failure. Kaplan-Meier curve based on immunoglobulin (Ig)G-β1 adrenergic receptor autoantibody (β1AR-AAb) (A) and IgG3-β1AR-AAb status (B) and Kaplan-Meier curve of the 3 groups β1AR-AAb negative, non-IgG3-β1AR-AAb positive and IgG3-β1AR-AAb (C). *P < .05 vs β1AR-AAb negative group; ‡P < .01 vs non-IgG3-β1AR-AAb positive group.
Table 2.
Cox Proportional Hazards Model for Composite Endpoint
| Univariate | Multivariate | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n/N | Hazard Ratio (Unit) |
Lower 95% |
Higher 95% |
P value | n/N | Hazard Ratio (Unit) |
Lower 95% |
Higher 95% |
P value | |
| eGFR (mL/min) | 34/113 | 0.990 | 0.976 | 1.004 | .171 | 29/104 | 0.997 | 0.981 | 1.013 | .726 |
| HR (bpm) | 34/119 | 1.029 | 1.003 | 1.053 | .030 | 29/104 | 1.042 | 1.010 | 1.074 | .011 |
| LVEF (%) | 31/114 | 0.943 | 0.905 | 0.980 | .002 | 29/104 | 0.969 | 0.930 | 1.004 | .083 |
| β1AR-AAb status (neg./non-IgG3/IgG3) | 35/120 | .038 | 29/104 | .021 | ||||||
| Non-IgG3(+) vs neg. | 1.751 | 0.764 | 3.682 | .175 | 2.180 | 0.913 | 4.873 | .078 | ||
| IgG3(+) vs neg. | 0.412 | 0.120 | 1.080 | .07 | 0.358 | 0.080 | 1.123 | .081 | ||
| IgG3(+) vs non-IgG3(+) | 0.236 | 0.064 | 0.725 | .011 | 0.164 | 0.034 | 0.597 | .005 | ||
β1AR-AAb, autoantibody against β1-adrenergic receptor; eGFR, estimated glomerular filtration rate calculated by Modification of Diet in Renal Disease formula; HR, heart rate; LVEF, left ventricular ejection fraction; n, number of patients with endpoints; N, number patients with baseline condition.
Discussion
In our single-center study cohort with contemporary HF therapy including broad β-blocker use, we report that β1AR-AAb is detectable in excess of 1 of 3 patients, which is consistent with previous reports from Germany6,8,24–26 and Japan.7,9,10,20 Interestingly, the positive rate of the IgG3 subclass of β1AR-AAb was significantly higher in DCM compared with ICM patients, whereas the positive rate of non-IgG3 β1AR-AAb was similar between different HF etiologies. Contrary to prior reports, we observed significantly lower adverse clinical event rates in those with detectable IgG3 β1AR-AAb positive vs negative patients in this patient cohort with 97% β-blocker use. Taken together, these hypothesis-generating findings imply the possibility that β1AR-AAb IgG subclasses might play differential roles in the pathophysiology of cardiomyopathies. Specifically, it is conceivable that IgG3 β1AR-AAb may exert a more direct pathologic effect related to a primary autoimmune process, such as failure of self-tolerance, than other non-IgG3 β1AR-AAbs that are more dependent on secondary autoimmune responses to self-antigens released as a result of cardiac damage.
Often referred to as “cardiodepressant” AAbs, certain types of AAbs purified from patients with DCM have been found to induce a negative inotropy and reduction of calcium transients in vitro27–29 and ex vivo. Interestingly, patients with cardiodepressant AAbs demonstrate an acute increase in cardiac index and LVEF after immunoadsorption (IA) therapy, whereas those without cardio-depressant AAbs do not show significant changes.27,29 Staudt and colleagues have reported that the cardio-depressant effects of these AAbs are unlikely to be induced by either the F(ab′)2 or Fc fragment alone. They showed that reconstitution of the antibody Fc portion by incubating cardiomyocytes with DCM-F(ab′)2 fragments followed by goat-anti-human-F(ab′)-IgG can still induce reduction of cell shortening and calcium transients.30 Therefore, AAb requires Fc fragment binding to Fc receptors as well as F(ab′)2 fragments binding to their epitope peptide to exert its pathological effect, and therefore the effects of the AAb may vary depending on the structure of the Fc fragment.
Immunoglobulin G has 4 subclasses (IgG1, 2, 3, and 4) according to the structure of the Fc fragment. IgG antibody subclasses 1 and 3 are most likely to trigger effector function and be involved in immunoregulatory activities. IgG1 and IgG3 both act by binding to and activating the Fcγ receptor and complement.28,31 IgG3 AAbs against some cardiac proteins can be detected in patients with DCM, and their presence has been shown to be an independent predictor of the presence of cardiodepressant AAb.10 Also, indices of hemodynamic dysfunction were correlated with anti-myosin AAb titer belonging to IgG3, but not total IgG, in patients with DCM.32 The importance of IgG3 AAbs was further supported when IA via anti-human IgG columns (high affinity for all IgG subclasses) resulted in additional improvement of cardiac function compared with using protein A (high affinity for IgG1, 2, and 4, but low affinity for IgG3).33 Similarly, anti-IgG column eluent of DCM patients, but not protein A column eluent, has been observed to exert cardiodepressant effects on rat cardiomyocytes that were abolished after subsequent removal of IgG3 subclass from the eluent of the anti-IgG column.34 In humans, IA using protein A columns with a modified protocol to effectively eliminate IgG3 subclass has also demonstrated better hemodynamic improvement in patients with DCM.35 Alternatively, using the tryptophan column, IgG3 subclass was eliminated effectively by IA and to a greater extent than other subclasses.23 The increase in LVEF after IA was better correlated with AAb titers belonging to the IgG3 subclass than total IgG, which also suggests that the removal of IgG3-AAb is important to maximize the effect of IA for patients with DCM.29
There is an emerging appreciation that only a subset of β1AR-AAbs may be functionally active.36,37 The notion that IgG3 β1AR-AAbs may serve as a potential pathogenic factor that can be counteracted with β-blockers raises an exciting possibility that their detection in patients who are at risk for developing cardiomyopathies may serve as potential indication for preventive β-blocker therapy. Further investigations into the presence of IgG3 β1AR-AAbs in at-risk patients are warranted.
Limitations
We note the following limitations in the present study. Although we evaluated β1AR-AAb status at baseline by enzyme-linked immunoabsorbent assay and examined clinical endpoints in our cohort, the mechanism that differentiated IgG3 and non-IgG3 β1AR-AAb was beyond our scope of investigation. In addition, although the administration of β-blockers is speculated as a mechanism that might have yielded more favorable outcomes in patients with IgG3 β1AR-AAb, the observational nature of our study did not allow further clarifications regarding the interrelationship between β-blockers and IgG3 β1AR-AAb.
Conclusions
The presence of IgG3 subclass of β1AR-AAb was paradoxically associated with favorable long-term outcomes in our HF patients compared with those without detectable IgG3 β1AR-AAb. Future investigations will be necessary to better elucidate the detailed mechanisms that differentiate the effects of IgG3 and non-IgG3 β1AR-AAb.
Acknowledgments
Funding: This work has been supported by a grant from the National Institutes of Health (1R01HL103931) as well as support from the Cleveland Clinic Clinical Research Unit of the Case Western Reserve University CTSA (UL1TR 000439) and funding from the Cleveland Clinic Research Programs Committee (#2013-1059). Dr. Nagatomo is the recipient of the Postdoctoral Fellowship award from the Myocarditis Foundation (#MYF1401MF).
Footnotes
Disclosure
There are no relationships to disclose.
References
- 1.Caforio AL, Grazzini M, Mann JM, Keeling PJ, Bottazzo GF, McKenna WJ, et al. Identification of alpha- and beta-cardiac myosin heavy chain isoforms as major autoantigens in dilated cardiomyopathy. Circulation. 1992;85:1734–1742. doi: 10.1161/01.cir.85.5.1734. [DOI] [PubMed] [Google Scholar]
- 2.Limas CJ, Goldenberg IF, Limas C. Autoantibodies against beta-adrenoceptors in human idiopathic dilated cardiomyopathy. Circ Res. 1989;64:97–103. doi: 10.1161/01.res.64.1.97. [DOI] [PubMed] [Google Scholar]
- 3.Iwata M, Yoshikawa T, Baba A, Anzai T, Nakamura I, Wainai Y, et al. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces beta-adrenergic receptor desensitization and myocardial hypertrophy in vivo. Circ Res. 2001;88:578–586. doi: 10.1161/01.res.88.6.578. [DOI] [PubMed] [Google Scholar]
- 4.Matsui S, Fu ML, Hayase M, Katsuda S, Yamaguchi N, Teraoka K, et al. Beneficial effect of muscarinic-2 antagonist on dilated cardiomyopathy induced by autoimmune mechanism against muscarinic-2 receptor. J Cardiovasc Pharmacol. 2001;38(Suppl 1):S43–S49. doi: 10.1097/00005344-200110001-00010. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T, Tanaka Y, Nishio R, Mitsuiye T, Mizoguchi A, Wang J, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 6.Magnusson Y, Marullo S, Hoyer S, Waagstein F, Andersson B, Vahlne A, et al. Mapping of a functional autoimmune epitope on the beta 1-adrenergic receptor in patients with idiopathic dilated cardiomyopathy. J Clin Invest. 1990;86:1658–1663. doi: 10.1172/JCI114888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata M, Yoshikawa T, Baba A, Anzai T, Mitamura H, Ogawa S. Autoantibodies against the second extracellular loop of beta1-adrenergic receptors predict ventricular tachycardia and sudden death in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:418–424. doi: 10.1016/s0735-1097(00)01109-8. [DOI] [PubMed] [Google Scholar]
- 8.Stork S, Boivin V, Horf R, Hein L, Lohse MJ, Angermann CE, et al. Stimulating autoantibodies directed against the cardiac beta1-adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J. 2006;152:697–704. doi: 10.1016/j.ahj.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Nagatomo Y, Yoshikawa T, Kohno T, Yoshizawa A, Baba A, Anzai T, et al. A pilot study on the role of autoantibody targeting the beta1-adrenergic receptor in the response to beta-blocker therapy for congestive heart failure. J Card Fail. 2009;15:224–232. doi: 10.1016/j.cardfail.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Baba A. Targeted autoantibodies in apheresis treatment against severe heart failure. Jap J Apheresis. 2010;29:187–193. [Google Scholar]
- 11.Fukuda Y, Miyoshi S, Tanimoto K, Oota K, Fujikura K, Iwata M, et al. Autoimmunity against the second extracellular loop of beta(1)-adrenergic receptors induces early afterdepolarization and decreases in K-channel density in rabbits. J Am Coll Cardiol. 2004;43:1090–1100. doi: 10.1016/j.jacc.2003.09.057. [DOI] [PubMed] [Google Scholar]
- 12.Staudt A, Mobini R, Fu M, Grosse Y, Stangl V, Stangl K, et al. beta(1)-Adrenoceptor antibodies induce positive inotropic response in isolated cardiomyocytes. Eur J Pharmacol. 2001;423:115–119. doi: 10.1016/s0014-2999(01)01113-x. [DOI] [PubMed] [Google Scholar]
- 13.Wallukat G, Wollenberger A, Morwinski R, Pitschner HF. Anti-beta 1-adrenoceptor autoantibodies with chronotropic activity from the serum of patients with dilated cardiomyopathy: mapping of epitopes in the first and second extracellular loops. J Mol Cell Cardiol. 1995;27:397–406. doi: 10.1016/s0022-2828(08)80036-3. [DOI] [PubMed] [Google Scholar]
- 14.Mobini R, Magnusson Y, Wallukat G, Viguier M, Hjalmarson A, Hoebeke J. Probing the immunological properties of the extracellular domains of the human beta(1)-adrenoceptor. J Autoimmun. 1999;13:179–186. doi: 10.1006/jaut.1999.0310. [DOI] [PubMed] [Google Scholar]
- 15.Mobini R, Fu M, Wallukat G, Magnusson Y, Hjalmarson A, Hoebeke J. A monoclonal antibody directed against an autoimmune epitope on the human beta1-adrenergic receptor recognized in idiopathic dilated cardiomyopathy. Hybridoma. 2000;19:135–142. doi: 10.1089/02724570050031176. [DOI] [PubMed] [Google Scholar]
- 16.Staudt Y, Mobini R, Fu M, Felix SB, Kuhn JP, Staudt A. Beta1-adrenoceptor antibodies induce apoptosis in adult isolated cardiomyocytes. Eur J Pharmacol. 2003;466:1–6. doi: 10.1016/s0014-2999(03)01431-6. [DOI] [PubMed] [Google Scholar]
- 17.Christ T, Wettwer E, Dobrev D, Adolph E, Knaut M, Wallukat G, et al. Autoantibodies against the beta1 adrenoceptor from patients with dilated cardiomyopathy prolong action potential duration and enhance contractility in isolated cardiomyocytes. J Mol Cell Cardiol. 2001;33:1515–1525. doi: 10.1006/jmcc.2001.1414. [DOI] [PubMed] [Google Scholar]
- 18.Jahns R, Boivin V, Hein L, Triebel S, Angermann CE, Ertl G, et al. Direct evidence for a beta 1-adrenergic receptor-directed autoimmune attack as a cause of idiopathic dilated cardiomyopathy. J Clin Invest. 2004;113:1419–1429. doi: 10.1172/JCI20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Podlowski S, Luther HP, Morwinski R, Muller J, Wallukat G. Agonistic anti-beta1-adrenergic receptor autoantibodies from cardiomyopathy patients reduce the beta1-adrenergic receptor expression in neonatal rat cardiomyocytes. Circulation. 1998;98:2470–2476. doi: 10.1161/01.cir.98.22.2470. [DOI] [PubMed] [Google Scholar]
- 20.Nagatomo Y, Yoshikawa T, Okamoto H, Kitabatake A, Hori M. Presence of autoantibody directed against beta1-adrenergic receptors is associated with amelioration of cardiac function in response to carvedilol: Japanese Chronic Heart Failure (J-CHF) Study. J Card Fail. 2015;21:198–207. doi: 10.1016/j.cardfail.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Wallukat G, Muller J, Hetzer R. Specific removal of beta1-adrenergic autoantibodies from patients with idiopathic dilated cardiomyopathy. N Engl J Med. 2002;347:1806. doi: 10.1056/NEJM200211283472220. [DOI] [PubMed] [Google Scholar]
- 22.Dandel M, Weng Y, Siniawski H, Potapov E, Drews T, Lehmkuhl HB, et al. Prediction of cardiac stability after weaning from left ventricular assist devices in patients with idiopathic dilated cardiomyopathy. Circulation. 2008;118:S94–S105. doi: 10.1161/CIRCULATIONAHA.107.755983. [DOI] [PubMed] [Google Scholar]
- 23.Nagatomo Y, Baba A, Ito H, Naito K, Yoshizawa A, Kurita Y, et al. Specific immunoadsorption therapy using a tryptophan column in patients with refractory heart failure due to dilated cardiomyopathy. J Clin Apher. 2011;26:1–8. doi: 10.1002/jca.20268. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson Y, Wallukat G, Waagstein F, Hjalmarson A, Hoebeke J. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89:2760–2767. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- 25.Magnusson Y, Hjalmarson A, Hoebeke J. Beta 1-adrenoceptor autoimmunity in cardiomyopathy. Int J Cardiol. 1996;54:137–141. doi: 10.1016/0167-5273(96)02590-9. [DOI] [PubMed] [Google Scholar]
- 26.Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99:649–654. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- 27.Staudt A, Staudt Y, Dorr M, Bohm M, Knebel F, Hummel A, et al. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J Am Coll Cardiol. 2004;44:829–836. doi: 10.1016/j.jacc.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 28.Baba A. Autoantigen estimation and simple screening assay against cardiodepressant autoantibodies in patients with dilated cardiomyopathy. Ther Apher Dial. 2008;12:109–116. doi: 10.1111/j.1744-9987.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 29.Baba A, Akaishi M, Shimada M, Monkawa T, Wakabayashi Y, Takahashi M, et al. Complete elimination of cardiodepressant IgG3 autoantibodies by immunoadsorption in patients with severe heart failure. Circ J. 2010;74:1372–1378. doi: 10.1253/circj.cj-09-0748. [DOI] [PubMed] [Google Scholar]
- 30.Staudt A, Eichler P, Trimpert C, Felix SB, Greinacher A. Fc(gamma) receptors IIa on cardiomyocytes and their potential functional relevance in dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:1684–1692. doi: 10.1016/j.jacc.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 31.Bruggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, et al. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med. 1987;166:1351–1361. doi: 10.1084/jem.166.5.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warraich RS, Noutsias M, Kazak I, Seeberg B, Dunn MJ, Schultheiss HP, et al. Immunoglobulin G3 cardiac myosin autoantibodies correlate with left ventricular dysfunction in patients with dilated cardiomyopathy: immunoglobulin G3 and clinical correlates. Am Heart J. 2002;143:1076–1084. doi: 10.1067/mhj.2002.124406. [DOI] [PubMed] [Google Scholar]
- 33.Braun N, Gutenberger S, Erley CM, Risler T. Immunoglobulin and circulating immune complex kinetics during immunoadsorption onto protein A sepharose. Transfus Sci. 1998;19(Suppl):25–31. doi: 10.1016/s0955-3886(97)00099-4. [DOI] [PubMed] [Google Scholar]
- 34.Staudt A, Bohm M, Knebel F, Grosse Y, Bischoff C, Hummel A, et al. Potential role of autoantibodies belonging to the immunoglobulin G-3 subclass in cardiac dysfunction among patients with dilated cardiomyopathy. Circulation. 2002;106:2448–2453. doi: 10.1161/01.cir.0000036746.49449.64. [DOI] [PubMed] [Google Scholar]
- 35.Staudt A, Dorr M, Staudt Y, Bohm M, Probst M, Empen K, et al. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: results from protein A immunoadsorption. Am Heart J. 2005;150:729–736. doi: 10.1016/j.ahj.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Jahns R, Boivin V, Siegmund C, Boege F, Lohse MJ, Inselmann G. Activating beta-1-adrenoceptor antibodies are not associated with cardiomyopathies secondary to valvular or hypertensive heart disease. J Am Coll Cardiol. 1999;34:1545–1551. doi: 10.1016/s0735-1097(99)00381-2. [DOI] [PubMed] [Google Scholar]
- 37.Bornholz B, Weidtkamp-Peters S, Schmitmeier S, Seidel CA, Herda LR, Felix SB, et al. Impact of human autoantibodies on beta1-adrenergic receptor conformation, activity, and internalization. Cardiovasc Res. 2013;97:472–480. doi: 10.1093/cvr/cvs350. [DOI] [PMC free article] [PubMed] [Google Scholar]