Abstract
Research using low-density polyethylene (LDPE) passive samplers has steadily increased over the past two decades. However such research efforts remain hampered because of strict guidelines, requiring that these samplers be quickly transported in airtight metal or glass containers, or foil-wrapped on ice. We investigate the transport stability of model pesticides and polycyclic aromatic hydrocarbons (PAHs) with varying physicochemical properties using polytetrafluoroethylene (PTFE) bags instead. Transport scenarios were simulated with transport times up to 14 days with temperatures ranging between -20 and 35 degrees Celsius. Our findings show that concentrations of all model compounds examined were stable for all transport conditions tested, with mean recoveries ranging from 88% to 113%. Furthermore, PTFE bags proved beneficial as reusable, lightweight, low-volume, low-cost alternatives to conventional containers. This documentation of stability will allow for more flexible transportation of LDPE passive samplers in an expanding range of research applications while maintaining experimental rigor.
Keywords: PAH, Pesticide, LDPE, Passive sampling device, Transport stability, Storage
Introduction
Passive sampling devices made from low-density polyethylene (LDPE) or other polymers have been used for over two decades to sample the freely-dissolved fraction of organic contaminants in numerous environmental media (Huckins et al. 2006; Mills et al. 2013). LDPE passive samplers have been used to sample non-polar and semi-polar compounds in air (Paulik et al. 2015; Tidwell et al. 2015), water (Allan et al. 2012; McDonough et al. 2014), and sediment porewater (Fernandez et al. 2014; Liu et al. 2013). Contaminants diffuse into passive samplers, and concentrations increase until equilibrium is reached with the sampled matrix. The first generation of samplers, called semipermeable membrane devices (SPMDs), consisted of LDPE strips containing a volume of triolein to retain sequestered hydrophobic contaminants (Huckins et al. 1990). Recent single-phase variations without triolein afford simpler extraction and analytical clean-up (Adams et al. 2007; Anderson et al. 2008; Mills et al. 2013). LDPE passive samplers are constructed from low-cost materials and are often more cost effective compared to active sampling methods (Melymuk et al. 2014; U.S. Environmental Protection Agency 2012). Additionally, performance reference compounds (PRCs), also called depuration compounds, are infused into the passive sampler material before deployment The rate at which PRCs diffuse from the material into the surrounding environment, either air or aqueous, corresponds to the rate at which compounds are sequestered from that surrounding environment (Huckins et al. 2002; Melymuk et al. 2014). The use of these PRCs, along with solvent extraction and instrumental analysis allows for determination of time-weighted averages of bioavailable freely-dissolved or vapor-phase environmental concentrations.
Guidelines proposed by the U.S. Environmental Protection Agency (2012) and the US Geological Society (Alvarez 2010) indicate that field-deployed LDPE passive samplers or SPMDs should be stored immediately in airtight cans or jars and transported frozen or near frozen via overnight courier, or as soon as possible. Overnight frozen shipping can be expensive or logistically unattainable from some locations (Anderson et al. 2014). Moreover, airtight canisters are suggested for passive sampler transport to and from the study site as a means to suspend sampling and to prevent loss of compounds by volatilization. Recommended canister materials are either glass or metal to limit compound absorption to canister surfaces (Huckins et al. 2006). Rigid canisters add volume and weight that may increase shipping costs. Other transportation guidelines propose wrapping passive samplers in clean aluminum foil and subsequently placing them in plastic bags (U.S. Environmental Protection Agency 2012). While plastic bags are more amenable to shipping, a barrier of pre-cleaned aluminum foil is needed to prevent direct exchange of compound between the passive sampling material and the polymer of the transport bag, often polyethylene. Additionally, polyethylene bags are neither airtight nor chemically impervious, and vapor-phase chemicals can potentially diffuse through the polyethylene bag and be captured by the LDPE passive sampler during transport. The polyethylene bag itself may also sequester contaminants that volatilize from the passive sampler. Alternatively, bags made of polytetrafluoroethylene (PTFE) would provide an airtight, lightweight, low-volume, and chemically inert solution for cost-effective shipping. The use of such PTFE bags is only supported by limited data regarding silicone, rather than LDPE passive samplers (O’Connell et al. 2013). To the authors’ knowledge, there are no studies of transport of LDPE passive samplers in PTFE bags. Data-based criteria for transport conditions will increase the utility of passive sampling techniques in an expanding range of applications.
Transportation at ambient temperatures in lightweight, durable bags would allow more cost-effective shipping or transport compared to airtight metal cans or glass jars shipped overnight on ice. In contrast to samples wrapped in aluminum foil and enclosed in polyethylene bags, the PTFE bags are air-tight and chemically inert, eliminating the need for foil. We hypothesize that less stringent transport conditions will have no effect on concentrations of commonly studied contaminants sequestered in LDPE passive samplers. The aim of this work is to demonstrate the stability of model pollutants in LDPE passive samplers under simulated transport in PTFE bags, with temperatures between -20° and 35°C and for durations between 10 hours and 14 days. These conditions were chosen to mimic a worst-case scenario of a 14-day transport from a hot climate. Model compounds include organochlorine and organophosphate pesticides and polycyclic aromatic hydrocarbons (PAHs).
Materials and methods
Standards, solvents, and materials
Pesticide (alachlor, alpha-BHC, chlorpyrifos, and endrin ketone) and PAH (anthracene, benzo[ghi]perylene, chrysene, and fluoranthene) compounds were selected to represent a range physicochemical properties (Table 1). All were of purity ≥ 98% (Accustandard, USA). Tetrachloro-meta-xylene and PCB-209 (Accustandard, USA) were used as extraction surrogate standards for pesticides, and phenanthrene-d10, fluoranthene-d10, chrysene-d12, and benzo[ghi]perylene-d12 were used for PAHs (CDN Isotopes, Canada). Internal standards 4,4′-dibromooctafluorobiphenyl (Supelco Analytical, USA) and perylene-d12 (Chemservice, USA) were added immediately before instrumental analysis to correct for instrument variation (Table 2). Hexane solvents were Optima™ grade or better (Fisher Chemical, USA). PTFE transport/storage bags and Clip N Seal closures were purchased from Welch Fluorocarbon, Inc. (USA). LDPE lay-flat tubing used to make passive samplers was purchased from Brentwood Plastics, Inc. (USA). Average width of tubing is 2.7 cm, average membrane thickness is 75–95 μm, and average transient polymer cavity size is 10 Å (Anderson et al. 2008).
Table 1.
Model compounds used in transport stability analysis
| CAS number | Molecular weight (g mol−1)a | log KOWa | log KOAa | IDL (ng/ml)c | ||
|---|---|---|---|---|---|---|
| Pesticide | alachlor | 15972-60-8 | 269.77 | 3.52 | 10.0b | 0.5 |
| alpha-BHC | 319-84-6 | 290.83 | 3.72 | 8.84 | 2.0 | |
| chlorpyrifos | 2921-88-2 | 350.59 | 4.66 | 8.88 | 0.5 | |
| endrin ketone | 53494-70-5 | 380.91 | 4.99b | 11.1b | 1.0 | |
|
|
||||||
| PAH | anthracene | 1719-06-8 | 178.23 | 4.45 | 7.55 | 1.7 |
| benzo[ghi]perylene | 191-24-2 | 276.33 | 6.70b | 12.0 | 1.7 | |
| chrysene | 218-01-9 | 228.29 | 5.81 | 9.48b | 1.7 | |
| fluoranthene | 206-44-0 | 202.25 | 5.16 | 8.88 | 1.7 |
estimated value
Instrument detection limits (IDL) for extracts of LDPE are determined as 3 times the standard deviation of 7 runs of the lowest standard, expressed as concentration.
Table 2.
Analytical parameters
| Pesticide method | PAH method | |
|---|---|---|
| Extraction Surrogate Standards | tetrachloro-meta-xylene, PCB-209 | phenanthrene-d10, fluoranthene-d10, chrysene-d12, benzo[a]pyrene-d12 |
| Internal Standard | 4,4′-dibromooctafluorobiphenyl | perylene-d12 |
| Gas Chromatograph | 6890N (Agilent) | 7890A (Agilent) |
| Detector(s) | 2x micro-electron capture detectors | 5975C mass spectrometer (Agilent) |
| Column(s) | DB-XLB and DB-17MS (both Agilent) | DB5-MS (Agilent) |
| No. of calibration points (R2>0.98) | 5 | 6 or 7 |
| Temperature program | 110°C, 1 min. 4°C/min to 300°C, hold 10 min. |
60°C, 1 min. 30°C/min. to 180°C 3°C/min. to 230°C, hold 5 min. 28°C/min. to 280°C, hold 10 min. 8°C/min. to 310°C 16°C/min. to 350°C, hold 5 min. |
| Reference | Anderson et al. (2014) | Allan et al. (2012) |
Sample Preparation
Passive samplers were constructed from LDPE tubing cut into 100 cm lengths. Each LDPE strip was pre-cleaned to remove potential chemical interferences with three successive conditioning washes in 100 mL of hexanes, each for 24 hours. After drying, each strip of tubing was heat-sealed at one end, infused with <100 μL of target compound solution in n-hexane (200–600 ng of each compound per strip), and then heat-sealed at the remaining end. Pressure was applied lengthwise between (gloved) thumb and index finger to uniformly disperse target compound solution throughout the sealed LDPE sampler. The same target compound solution was used in all LDPE strips, and all were constructed in one batch. This method of infusion and heat-sealing was chosen because it requires less solvent than equilibration techniques as in Booij et al. (2002). Unlike SPMDs which can contain 1 mL of triolein in each strip of tubing (Huckins et al. 1990), the constructed strips contained only a small volume and are considered single-phase samplers. Each passive sampler strip was placed in an individual PTFE bag (Fig. 1). Samples were immediately moved to dark, temperature-controlled environments at -20, 4, 20, or 35°C. Ambient light was minimized during laboratory preparation steps. The PTFE bags used in this study are translucent, and attenuate UVA and UVB transmittance by 49% (Supplementary Information Fig S1). UV degradation of chemicals was not examined in this study, but is expected to be minimal based on previous findings of reduced rates of photodecomposition of PAHs when adsorbed to coal ash (Korfmacher et al. 1980) and silicone passive sampling devices (O’Connell et al. 2014).
Fig. 1.

LDPE passive sampling strip in PTFE bag
Eight samplers were extracted immediately following preparation to represent the t=0 treatment. Four samplers from each temperature treatment were extracted at 10 h, 1.5 days, 3 days, and 7 days. An additional 4 samplers at 35°C were extracted after 14 days. Passive samplers were extracted with two 40 mL n-hexane dialyses following the addition of extraction surrogate standards. Dialysates were combined and quantitatively reduced to a volume of 1 mL. Extracts were stored in the dark in amber glass vials at -20°C until analyzed.
Instrumental Analysis
Instrumental analysis for each of the model compounds was performed on two methods (Table 2). Pesticides were quantified with gas chromatography with electron capture detectors (GC-ECD). PAHs were quantified with gas chromatography with mass spectrometry (GC-MS). All concentrations were quantified by the relative response of the internal standard to target compounds in a 5–8 point calibration curve (all R2> 0.99). Instrument detection limits are given in Table 1, and analytical parameters are given in Table 2.
Statistical Analysis
Treatment recoveries were scaled as a percentage of the mean control (t=0) treatments. Mean percent recoveries were analyzed by one-sided Dunnett’s tests. Significance for all tests was set at α = 5%. Statistical analyses were performed with JMP Pro 11.2.0 and Microsoft Excel 2013.
Quality Control
Over 30% of the samples analyzed were quality control samples. Blank LDPE samples were pulled during the pre-cleaning and construction phases and retained as quality control samples. The extraction process was performed without LDPE for a solvent extraction blank. Injections of n-hexane solvent, instrument reagent blanks, were included in all analytical batches, and were used to demonstrate that the instruments had low background responses. All target compounds were below detection limits in all blank quality control samples. Continuing calibration verifications consist of a solution of known concentration of all target compounds to monitor instrument performance and were within 20% of known value for all target compounds. Extraction surrogate standards were added to passive samplers prior to extraction in order to quantify procedural recoveries. Pesticide surrogate recoveries averaged 92% (standard deviation = 10%) and concentrations were not corrected for procedural losses. Recoveries of PAH surrogate standards averaged 65% (standard deviation = 11%), and PAH concentrations were corrected for losses.
Results and Discussion
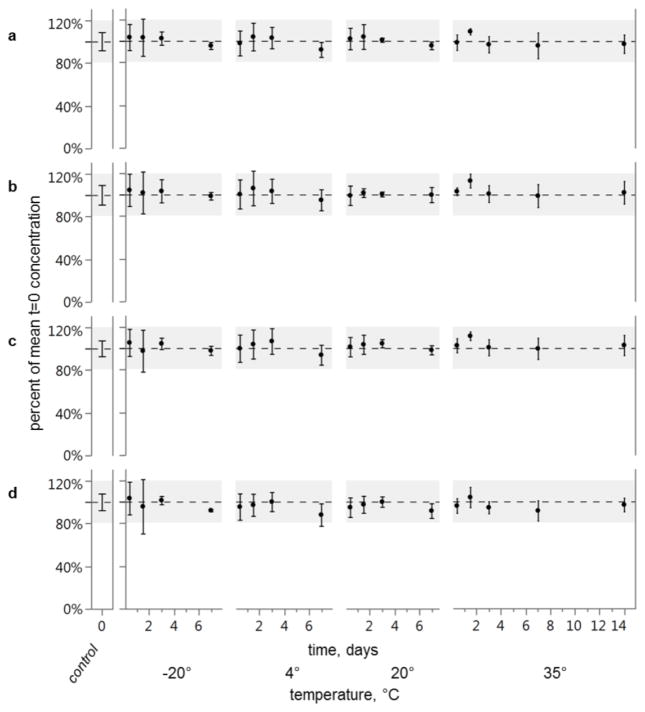
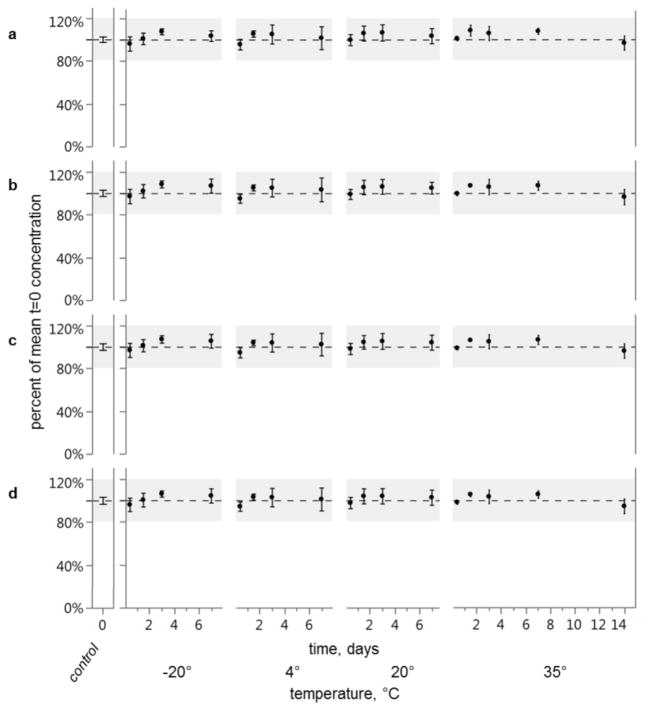
Overall mean recovery was 101% (standard deviation = 6%) of t=0 across all time and temperature treatments for all pesticides (Fig. 2) and PAHs (Fig. 3). The lowest mean recovery among all time/temperature treatment groups was endrin ketone at 88% (95% confidence interval (CI) = 77–98) for the 7 day, 4°C treatment, and the highest mean recovery was for alpha-BHC at 113% (95% CI = 106–119) for the 1.5 day, 35°C treatment. Mean recoveries and standard deviations for these and other compounds and treatment conditions and are given in Supplementary Information Table S1. Average relative standard deviation (RSD) for pesticides was 7.4%. Average RSD was lower for PAHs at 4.9%, likely because PAH concentrations were corrected for sample preparation losses while pesticide concentrations were not. No mean recovery was less than mean t=0 treatment (one-sided Dunnett’s test, all p-values < 0.05) and therefore, there was no effect of transport on target compound concentrations for any condition tested.
Fig. 2.
Mean recoveries of pesticides a alachlor, b alpha-BHC, c chlorpyrifos, and d endrin ketone. Concentrations are represented as a percent of control treatment (t=0). No recovery is less than control (one-sided Dunnett’s test). Grey area highlights ±20% of control. Error bars represent 95% confidence intervals of the means (n=8 for t=0 control, and n=4 for all other treatments)
Fig. 3.
Mean recoveries of PAHs a anthracene, b benzo[ghi]perylene, c chrysene, d fluoranthene. Concentrations are represented as a percent of control treatment (t=0). No recovery is less than control (one-sided Dunnett’s test). Grey area highlights ±20% of control. Error bars represent 95% confidence intervals of the means (n=8 for t=0 control, and n=4 for all other treatments)
The model pesticides and PAHs in this transport study exhibited no decrease in recovery after 14 days of simulated transport conditions in temperatures as high as 35°C. As the selected model compounds span a range of physicochemical properties, these data suggest that similar compounds would also exhibit no decrease in concentration. Care should be taken in extending the inferences to more extreme conditions, as effects may exist that were not detectable within the given experimental design. The transport stability findings presented here suggest that researchers performing targeted analysis on PAHs and pesticides can do so using more flexible transport conditions. However, if the intended chemical analysis is non-targeted, then expedient transport at or near freezing is a conservative approach to ensure recovery. Huckins et al. (2006) caution that in SPMDs, high-fugacity compounds such as naphthalene can be lost if samplers are not kept under freezing conditions within hours of retrieval. The compounds selected for this study (log Koa range: 7.55–12.0, Table 1) are comparatively less volatile than naphthalene (log Koa: 5.19) (U.S. Environmental Protection Agency 2015). We did not observe any trend between compound volatility and recovery loss, because no recovery loss was observed for any compound in any treatment. If compound loss were to occur under the conditions mimicked in this study, it would be limited to compounds more volatile than the pesticide alpha-BHC, the three-ringed PAH anthracene, or compounds that have lower thermal stability, a chemical characteristic not examined in this study. Biodegradation was also not examined in the present study. LDPE that are deployed in water can develop a biofilm (Anderson et al. 2014; Huckins et al. 2006) that might favor biodegradation. Passive samplers deployed in air are unlikely to develop a biofilm. Booij et al. (2006) demonstrated that biofouling does not drastically affect target compound uptake while the passive samplers are deployed in water, but biodegradation resulting from biofouling is not well described. Careful selection of PRCs allows researchers to estimate potential effects from biofilms, including biodegradation (Ghosh et al. 2014). During retrieval, the LDPE passive samplers can be cleaned in water from the sampling location to remove biofouling and limit biodegradation during transport. In addition to thermal stability and biodegradation, the effects of more extreme transport durations or temperatures for other classes of semi-volatile organic compounds in passive samplers are also worthy of future study.
The LDPE tubing strips selected for this study have an average thickness of 75–95 μm, a thickness that has been used previously in passive sampling techniques (Adams et al. 2007; Anderson et al. 2008; Booij et al. 2002; Rusina et al. 2007). However, LDPE sheets nominally 50 μm (Khairy et al. 2014; Liu et al. 2013; Oen et al. 2011) or 20–30 μm (Alvarez et al. 2014; Fernandez and Gschwend 2015) are also used. Equilibrium partition coefficients are not affected by LDPE polymer thickness (Lohmann 2012), but it is expected that thinner polymers reach equilibrium faster. We conclude that compounds in the present study reached equilibrium quickly with the small volume of air in the airtight PTFE bag because concentrations did not change across temperature or time. Similarly, we hypothesize concentrations of compounds sequestered in thinner LDPE to also exhibit stability, because equilibrium is expected to be reached quickly.
Accelerated stability tests have been used in chemical standard and pharmaceutical industries as a means to estimate long-term storage stability albeit on a shorter time scale. In such studies, the storage temperature is increased by at least 20°C and recoveries are evaluated at standard time intervals (Rueck and Hellriegel 2014). Deviations from acceptable stability in accelerated tests give an early indication of shorter shelf life and inform study design in subsequent long-term studies (Bajaj et al. 2012). Typically, for every 10°C increase, the rate of degradation doubles (International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use 2003; Rueck and Hellriegel 2014). The design of the present study represents accelerated stability tests across a temperature range of 55 degrees Celsius, or the equivalent of about 634 days (14 days × 25.5). Using the principles of accelerated stability tests, the present study suggests that these compound concentrations are expected to be stable in cold storage for about two years.
The compound stabilities tested herein support the use of PTFE bags as a reliable alternative to glass jars, metal canisters, or aluminum foil and plastic bags when transporting LDPE passive samplers. The burden of cost in passive sampling campaigns is in extraction and analysis, while the materials and preparation of an LDPE passive sampler is comparatively inexpensive. In one cost analysis for polychlorinated biphenyl analysis, the U.S. Environmental Protection Agency (2012) reported that an LDPE passive sampler costs only $5 USD to prepare, but costs about $375 USD for extraction and analysis. The PTFE bags used in the present study cost approximately $5 USD each. Similarly, pre-cleaned glass jars with PTFE liners cost $3–8 USD each, depending on the volume. Both PTFE bags and glass jars may be solvent-cleaned and re-used, and therefore have similar costs for repeated uses. The PTFE bags have lower risk of breakage during transport or shipment and cost less to ship because they weigh less. Another transport option is to wrap the passive sampler in aluminum foil and transport on ice, optionally stored in a plastic bag. While this method is more cost-effective than jars or PTFE bags, it does not prevent analytes from partitioning out of the sampling material into or through the plastic bag, if used. As demonstrated in this work, PTFE bags allow for lower cost, chemically-inert transport at ambient temperature without increasing material costs.
Passive samplers have been gaining utility in recent decades as a cost-effective means of detecting low concentrations of hydrophobic contaminants in a variety of environments. The present study documents an additional benefit of LDPE passive samplers when studying environmental contaminants represented by the chosen model pesticides and PAHs—that they may be transported in the dark in lightweight PTFE bags at ambient temperature up to 14 days at 35°C.
Supplementary Material
Acknowledgments
This project was supported in part by award number P42 ES016465 and the associated Chemistry Facility Core, P30 ES000210 from the National Institute of Environmental Health Sciences (NIEHS). Carey E. Donald and Marc R. Elie were supported in part by NIEHS Training Grant Fellowship T32ES007060 from the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS or NIH. The authors wish to thank Ricky Scott, Glenn Wilson, Melissa McCartney, and Kristin Kamerud for help in chemical analysis and instrumentation.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Adams RG, Lohmann R, MacFarlane LK, Gschwend PM. Polyethylene devices: passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments. Environ Sci Technol. 2007;41:1317–1323. doi: 10.1021/es0621593. [DOI] [PubMed] [Google Scholar]
- Allan SE, Smith BW, Anderson KA. Impact of the Deepwater Horizon Oil Spill on bioavailable polycyclic aromatic hydrocarbons in Gulf of Mexico coastal waters. Environ Sci Technol. 2012;46:2033–2039. doi: 10.1021/es202942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez DA. Guidelines for the Use of the Semipermeable Membrane Device (SPMD) and the Polar Organic Chemical Integrative Sampler (POCIS) in Environmental Monitoring Studies. US Geological Survey 2010 [Google Scholar]
- Alvarez DA, Maruya KA, Dodder NG, Lao W, Furlong ET, Smalling KL. Occurrence of contaminants of emerging concern along the California coast (2009–10) using passive sampling devices. Mar Pollut Bull. 2014;81:347–354. doi: 10.1016/j.marpolbul.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Seck D, Hobbie KA, Traore AN, McCartney MA, Ndaye A, Forsberg ND, Haigh TA, Sower GJ. Passive sampling devices enable capacity building and characterization of bioavailable pesticide along the Niger, Senegal and Bani Rivers of Africa. Philos T R Soc B. 2014;369:20130110. doi: 10.1098/rstb.2013.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Sethajintanin D, Sower GJ, Quarles L. Field trial and modeling of uptake rates of in situ lipid-free polyethylene membrane passive sampler. Environ Sci Technol. 2008;42:4486–4493. doi: 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- Bajaj S, Singla D, Sakhuja N. Stability testing of pharmaceutical products. J App Pharm. 2012;02:129–138. doi: 10.7324/JAPS.2012.2322. [DOI] [Google Scholar]
- Booij K, Smedes F, Weerlee EMv. Spiking of performance reference compounds in low density polyethylene and silicone passive water samplers. Chemosphere. 2002;46:1157–1161. doi: 10.1016/S0045-6535(01)00200-4. [DOI] [PubMed] [Google Scholar]
- Booij K, van Bommel R, Mets A, Dekker R. Little effect of excessive biofouling on the uptake of organic contaminants by semipermeable membrane devices. Chemosphere. 2006;65:2485–2492. doi: 10.1016/j.chemosphere.2006.04.033. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Gschwend PM. Predicting bioaccumulation of polycyclic aromatic hydrocarbons in soft-shelled clams (Mya arenaria) using field deployments of polyethylene passive samplers. Environ Toxicol Chem. 2015;34:993–1000. doi: 10.1002/etc.2892. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Lao W, Maruya KA, Burgess RM. Calculating the diffusive flux of persistent organic pollutants between sediments and the water column on the Palos Verdes Shelf Superfund Site using polymeric passive samplers. Environ Sci Technol. 2014;48:3925–3934. doi: 10.1021/es404475c. [DOI] [PubMed] [Google Scholar]
- Ghosh U, et al. Passive sampling methods for contaminated sediments: practical guidance for selection, calibration, and implementation. Integr Environ Assess Manag. 2014;10:210–223. doi: 10.1002/ieam.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huckins J, Petty J, Booij K. Monitors of Organic Chemicals in the Environment: Semipermeable Membrane Devices. Springer; New York, USA: 2006. [DOI] [Google Scholar]
- Huckins JN, Petty JD, Lebo JA, Almeida FV, Booij K, Alvarez DA, Cranor WL, Clark RC, Mogensen BB. Development of the permeability/performance reference compound approach for in situ calibration of semipermeable membrane devices. Environ Sci Technol. 2002;36:85–91. doi: 10.1021/es010991w. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Tubergen MW, Manuweera GK. Semipermeable membreane devices containing model lipid: a new approach to monitoring the bioavailability of liphphilic contaminants and estimating their bioconcentration potential. Chemosphere. 1990;20:533–552. doi: 10.1016/0045-6535(90)90110-F. [DOI] [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Stability Testing of New Drug Substances and Products Q1A(R2) 2003. [Google Scholar]
- Khairy M, Muir D, Teixeira C, Lohmann R. Spatial trends, sources, and air-water exchange of organochlorine pesticides in the Great Lakes basin using low density polyethylene passive samplers. Environ Sci Technol. 2014;48:9315–9324. doi: 10.1021/es501686a. [DOI] [PubMed] [Google Scholar]
- Korfmacher WA, Wehry EL, Mamantov G, Natusch DFS. Resistance to photochemical decomposition of polycyclic aromatic hydrocarbons vapor-adsorbed on coal fly ash. Environ Sci Technol. 1980;14:1094–1099. doi: 10.1021/es60169a019. [DOI] [PubMed] [Google Scholar]
- Liu H-H, Bao L-J, Feng W-H, Xu S-P, Wu F-C, Zeng EY. A multisection passive sampler for measuring sediment porewater profile of dichlorodiphenyltrichloroethane and its metabolites. Anal Chem. 2013;85:7117–7124. doi: 10.1021/ac400589a. [DOI] [PubMed] [Google Scholar]
- Lohmann R. Critical review of low-density polyethylene’s partitioning and diffusion coefficients for trace organic contaminants and implications for its use as a passive sampler. Environ Sci Technol. 2012;46:606–618. doi: 10.1021/es202702y. [DOI] [PubMed] [Google Scholar]
- McDonough CA, Khairy MA, Muir DC, Lohmann R. Significance of population centers as sources of gaseous and dissolved PAHs in the lower Great Lakes. Environ Sci Technol. 2014;48:7789–7797. doi: 10.1021/es501074r. [DOI] [PubMed] [Google Scholar]
- Melymuk L, Bohlin P, Sanka O, Pozo K, Klanova J. Current challenges in air sampling of semivolatile organic contaminants: sampling artifacts and their influence on data comparability. Environ Sci Technol. 2014;48:14077–14091. doi: 10.1021/es502164r. [DOI] [PubMed] [Google Scholar]
- Mills GA, Gravell A, Vrana B, Harman C, Budzinski H, Mazzella N, Ocelka T. Measurement of environmental pollutants using passive sampling devices – an updated commentary on the current state of the art. Env Sci Process Impact. 2013;16:369–373. doi: 10.1039/c3em00585b. [DOI] [PubMed] [Google Scholar]
- O’Connell SG, Haigh T, Wilson G, Anderson KA. An analytical investigation of 24 oxygenated-PAHs (OPAHs) using liquid and gas chromatography–mass spectrometry. Anal Bioanal Chem. 2013;405:8885–8896. doi: 10.1007/s00216-013-7319-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell SG, Kincl LD, Anderson KA. Silicone Wristbands as Personal Passive Samplers. Environ Sci Technol. 2014;48:3327–3335. doi: 10.1021/es405022f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oen AM, Janssen EM, Cornelissen G, Breedveld GD, Eek E, Luthy RG. In situ measurement of PCB pore water concentration profiles in activated carbon-amended sediment using passive samplers. Environ Sci Technol. 2011;45:4053–4059. doi: 10.1021/es200174v. [DOI] [PubMed] [Google Scholar]
- Paulik LB, Donald CE, Smith BW, Tidwell LG, Hobbie KA, Kincl L, Haynes EN, Anderson KA. Impact of natural gas extraction on PAH levels in ambient air. Environ Sci Technol. 2015;49:5203–5210. doi: 10.1021/es506095e. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Rueck A, Hellriegel C. The importance of accelerated stability tests for the development of certified reference materials (CRM) Sigma-Aldrich Marketing Communications Europe; Buchs SG, Switzerland: 2014. [Google Scholar]
- Rusina TP, Smedes F, Klanova J, Booij K, Holoubek I. Polymer selection for passive sampling: A comparison of critical properties. Chemosphere. 2007;68:1344–1351. doi: 10.1016/j.chemosphere.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Tidwell LG, Allan SE, O’Connell SG, Hobbie KA, Smith BW, Anderson KA. Polycyclic aromatic hydrocarbon (PAH) and oxygenated PAH (OPAH) air-water exchange during the Deepwater Horizon oil spill. Environ Sci Technol. 2015;49:141–149. doi: 10.1021/es503827y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- U.S. Environmental Protection Agency. Guidelines for Using Passive Samplers to Monitor Organic Contaminants at Superfund Sediment Sites. Office of Superfund Remediation and Technology Innovation, Office of Research and Development; 2012. [Google Scholar]
- U.S. Environmental Protection Agency. Estimation Programs Interface Suite™ for Microsoft® Windows, v 4.1.25. United States Environmental Protection Agency; Washington, DC, USA: 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.