Abstract
Increased histone deacetylase (HDAC) activity and the resulting dysregulation of protein acetylation is an integral event in retinal degenerations associated with ischemia and ocular hypertension. This study investigates the role of preconditioning on the process of acetylation in ischemic retinal injury. Rat eyes were unilaterally subjected to retinal injury by 45 minutes of acute ischemia, and retinal neuroprotection induced by 5 minutes of an ischemic preconditioning (IPC) event. HDAC activity was evaluated by a fluorometric enzymatic assay with selective isoform inhibitors. Retinal localization of acetylated histone-H3 was determined by immunohistochemistry on retina cross sections. Cleaved caspase-3 level was evaluated by Western blots. Electroretinogram (ERG) analyses were used to assess differences in retinal function seven days following ischemic injury. In control eyes, analysis of HDAC isoforms demonstrated that HDAC1/2 accounted for 28.4 ± 1.6%, HDAC3 for 42.4 ± 1.5% and HDAC6 activity 27.3 ± 3.5% of total activity. Following ischemia, total Class-I HDAC activity increased by 21.2 ± 6.2%, and this increase resulted solely from a rise in HDAC1/2 activity. No change in HDAC3 activity was measured. Activity of Class-II HDACs and HDAC8 was negligible. IPC stimulus prior to ischemic injury also suppressed the rise in Class-I HDAC activity, cleaved caspase-3 levels, and increased acetylated histone-H3 in the retina. In control animals 7 days post ischemia, ERG a- and b-wave amplitudes were significantly reduced by 34.9 ± 3.1% and 42.4 ± 6.3%, respectively. In rats receiving an IPC stimulus, the ischemia-induced decline in ERG a- and b-wave amplitudes was blocked. Although multiple HDACs were detected in the retina, these studies provide evidence that hypoacetylation associated with ischemic injury results from the selective rise in HDAC1/2 activity and that neuroprotection induced by IPC is mediated in part by suppressing HDAC activity.
Keywords: retina, neuroprotection, protein acetylation, ischemia, preconditioning, HDAC
1. INTRODUCTION
Preconditioning is the ability of a transient non-damaging stimulus to protect an organ or tissue from a subsequent normally damaging insult. Preconditioning was first described in 1986 by Murry and colleagues where they demonstrated that brief periods of ischemia could protect the heart from subsequent prolonged ischemic events (Murry et al., 1986). Since this original work, preconditioning has been identified in the brain, kidney, liver and the retina. In the retina, preconditioning stimuli have been shown to protect the retina from acute light, ischemic injury, as well as chronic ocular hypertensive and diabetic stress (Fernandez et al., 2011; Grimm et al., 2002; Li et al., 2003; Roth et al., 1998; Zhu et al., 2012). Initial studies described the protective periods induced by preconditioning as transient (i.e., hours to days); however, recent studies in the brain and the retina have shown that periods of protection can be extended up to four weeks using repetitive hypoxic stimuli (Stowe et al., 2011; Zhu et al., 2007). These long-term stress-resistant phenotypes provide evidence that epigenetics can play a role in the preconditioning process (Gidday, 2015).
Histone acetylation represents one of the principal mechanisms involved in epigenetic responses (Choudhuri, 2011). Protein acetylation occurs in all cells, and plays a major role in regulating physiological and pathophysiological processes. Protein acetylation is controlled by opposing activities of histone acetyltransferases and histone deacetylases (HDACs). Although protein acetylation is best known for its role in transcriptional regulation (Jenuwein and Allis, 2001), studies have identified over 3,500 acetylation sites on approximately 1,700 proteins (Choudhary et al., 2009). In the central nervous system, dysregulation of protein acetylation has been shown to contribute to the pathogenesis of numerous diseases including stroke, multiple sclerosis, Alzheimer’s and Parkinson’s diseases (Beharry et al., 2014; Harrison and Dexter, 2013; Lazo-Gomez et al., 2013; Murphy et al., 2014). Recent studies from our laboratory have shown that increased retinal HDAC activity and the associated hypoacetylation of proteins contribute to the injury response induced by ischemia and ocular hypertension (Alsarraf et al., 2014a; Alsarraf et al., 2014b; Crosson et al., 2010). Additional studies, have shown that the administration of HDAC inhibitors can protect the neural retina from ischemic, optic nerve injury, and ocular hypertensive injury (Alsarraf et al., 2014b; Crosson et al., 2010; Lebrun-Julien and Suter, 2015; Zhu et al., 2012).
In the current study, we evaluated the effects of an ischemic preconditioning and ischemic injury on retinal HDAC activity, retinal histone-H3 acetylation, caspase-3 activation, and retinal function. This study provides the first evidence linking the retinal neuroprotective actions of ischemic preconditioning to reduced HDAC activity and retinal hyperacetylation.
2. MATERIAL and METHODS
2.1 Animals
Adult brown Norway rats (3–5 months of age, 150–200 grams; Charles River Laboratories, Inc., Wilmington, MA) were used in this study. Rats were maintained in an environmental cycle of 12-hours light and 12-hours dark. Animal handling was performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research; and the Animal Care and Use Committee at the Medical University of South Carolina approved all study protocols.
2.2 Retinal Ischemia and Ischemic Preconditioning
Prior to the induction of retinal ischemia, rats were anesthetized by i.p. injection of ketamine (75 mg/kg) and xylazine (8 mg/kg) (Ben Venue Laboratories, Bedford, OH, USA), and corneal analgesia created by the application of proparacaine (0.5%; 5 µL; Akorn, Inc., Buffalo Grove, IL, USA). Body temperature was maintained at 37°C by means of a heating pad (Harvard Apparatus; Holliston, MA, USA). Retinal ischemia was created using methods previously described (Whitlock et al., 2005). Briefly, the anterior chamber was cannulated with a 30-G needle that was connected to a container of sterile normal saline via polyethylene tubing (PE-50; Fischer, Atlanta, GA, USA). To induce retina ischemia, the reservoir was elevated to raise the intraocular pressure (IOP) above systolic blood pressure to 160 mmHg for 45 minutes. The IOP was monitored by an in-line pressure transducer connected to a computer. Each pressure then returned to normal and the eye was examined to ensure that retinal blood flow was reestablished.
For ischemic preconditioning (IPC), the IOP was elevated to 160 mmHg for 5 minutes, the needle removed from the anterior chamber, and the eye allowed to reperfuse normally. In eyes that received IPC and ischemia, 5 minutes of IPC was administered in an identical fashion and was followed 24 hours later with ischemic retinal injury, whereby the IOP was elevated to 160 mmHg for 45 minutes. The contralateral eye was left untreated, serving as a control.
2.3 HDAC Activity Assay
The deacetylase activities of HDACs were measured by assaying enzyme activity using the peptidase, trypsin, and the fluorophore-conjugated synthetic substrates, t-butoxyacetyllysine aminomethoxy-cumarin (Boc-Lys(Ac)-AMC) and t-butoxytrifluoroacetyl-lysine aminomethoxy-cumarin (Boc-Lys(TFA)-AMC), respectively, as previously described (Bradner et al., 2010; Wegener et al., 2003). Briefly, the retinal lysates were prepared in lysis buffer including 50 mM Tris-base; 10 mM EDTA; 0.5 mM sodium orthovanadate; 0.5% sodium deoxycholic acid; 1% Triton X-100 and protease inhibitors. After brief sonication and 5 minutes centrifugation at 1000 × g, the supernatant was used for HDAC activity assay. Lysates were diluted to a concentration of 1.0 µg/µL using standard HDAC buffer (50 mM Tris-Cl pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2 and 0.1 mg/mL bovine serum albumin) and incubated with the appropriate substrate in 96-well non-binding plates (Greiner Bio-one, NC) at room temperature for 2 hours. The Boc-Lys(Ac)-AMC substrate in this assay is specific for HDAC1, 2, 3 and 6; and Boc-Lys(TFA)-AMC substrate is specific for HDAC4, 5, 7, 8, 9, 10 and 11.
Baseline fluorescence was measured followed by treatment with the peptidase enzyme trypsin, freeing the fluorogenic 4-methylcoumarin-7-amide (AMC). The amount of fluorogenic AMC generated was then measured using an excitation wavelength of 355 nm and emission wavelength of 460 nm with a standard fluorospectrometer. To evaluate individual isoform activities, lysates were incubated with 100nM Tubastatin-A (HDAC6 inhibitor) alone or in combination with 1 µM 2- thiophenyl biaryl (HDAC1/2 inhibitor; supplied by Lexicon Pharmaceutical, Inc., The Woodlands, TX, USA) (Methot et al., 2008). The activities of HDAC6, HDAC1/2 and HDAC3 were calculated by measuring the suppression induced by each inhibitor.
2.4 Western Blot Analyses
To assess the changes in activated caspase-3, retinal lysates were obtained at 24 hours after ischemia. Western blot analysis was performed after homogenization of the whole retina in lysis buffer (50 mM Tris-base; 10 mM EDTA; 0.5 mM sodium orthovanadate; 0.5% sodium deoxycholic acid; 1% Triton X-100) and protease inhibitors (Roche). After brief sonication and 5 minutes centrifugation at 1000 × g, the supernatant was used for Western blots. Equivalent amounts of protein were loaded onto 4–12% SDS polyacrylamide gels; proteins were separated by PAGE and transferred to nitrocellulose paper. The membranes were blocked in 5% nonfat dry milk followed by incubation for 24 hours at 4°C with appropriate primary antibodies selective for cleaved caspase-3 (1:500 dilution, Cell-Signaling Technologies, Danvers, MA) and β-actin (1:2000 dilution, Cell-Signaling Technologies, Danvers, MA). After washing, membranes were incubated for 1 hour at room temperature with appropriate secondary antibodies (horseradish peroxidase [HRP]-conjugated; 1:1000 dilution, Sigma-Aldrich, St. Louis, MO). For chemiluminescent detection, the membranes were treated with enhanced chemiluminescence reagents, and the densitometric signal monitored using a Biorad Versadoc imaging system (Biorad, Hercules, CA). Beta actin was used to normalize protein levels on Western blots for protein quantitation.
2.5 Immunohistochemical Analyses
For immunohistochemical analysis, selected eyes were fixed in 4% paraformaldehyde for 2 hours at 4°C. Globes were washed in phosphate-buffered saline and transferred into 15% sucrose solution for 1 hour, followed by 30% sucrose solution overnight at 4°C. Tissues were embedded in optimal cutting temperature (OCT) compound (Tissue Tek; Sakura Finetech, Torrance, CA) and 10 µm slices sectioned at −26°C. The sections were washed in PBS to remove OCT and blocked with 5% normal donkey serum, 3% bovine serum albumin, and 0.1% Triton X-100 in PBS for 1 hour at room temperature. Sections were incubated in primary antibody specific to acetyl histone-H3 (1:200 dilution, Cell-Signaling Technologies, Danvers, MA) at 4°C overnight. Sections were then washed and incubated for 2 hours at room temperature with Alexa 488 secondary antibody (Invitrogen, Grand Island, NY, USA). Nuclei were stained with propidium iodide at 1:1000 (Sigma-Aldrich, St Louis, MO, USA). Retina sections were imaged by means of a Leica confocal microscope (Leica, Wetzlar, Germany). The fluorescence pixel intensities for the entire area of the photomicrograph (0.04 mm2) were measured using Adobe Photoshop CS2. The same microscope acquisition settings (image exposure time and gain) were used for all these images.
2.6 Electroretinograms
To quantitate baseline and post-treatment neuroretinal function, electroretinograms (ERGs) were performed one day prior to IPC or ischemia and 7 days post-ischemic injury. For these studies, rats were dark-adapted overnight. On the following day, rats were anesthetized with intraperitoneal ketamine and xylazine administration as described above, and pupils dilated with a 10 µL drop of a solution containing phenylephrine HCl (2.5%) and tropicamide (1%) (Akorn, Inc., Buffalo Grove, IL, USA). A needle ground-electrode was placed subcutaneously in the back of the animal and a reference electrode on the tongue. A contact lens gold-ring electrode was held in place on the cornea with a drop of methylcellulose. A stimulus-intensity series of ERGs was recorded in response to the 10 msec single-flash with intensity of 2.48 cd·s/m2. Responses were an average of 3 flashes with an inter-stimulus interval of 2 minutes. Electroretinograms were recorded by means of an UTAS-2000 system (LKC Technologies, Gaithersburg, MD).
2.7 Statistical Analysis
For all experiments, data were expressed as mean ± SE. Statistical comparisons for two groups were made with the Student t test for unpaired data. For comparing multiple treatment groups, analysis of variance (ANOVA) using the Dunnett posttest (GraphPad Software, Inc., San Diego, CA) was utilized. A P value of less than 0.05 was considered significant.
3. RESULTS
3.1 HDAC Activity in the Retina
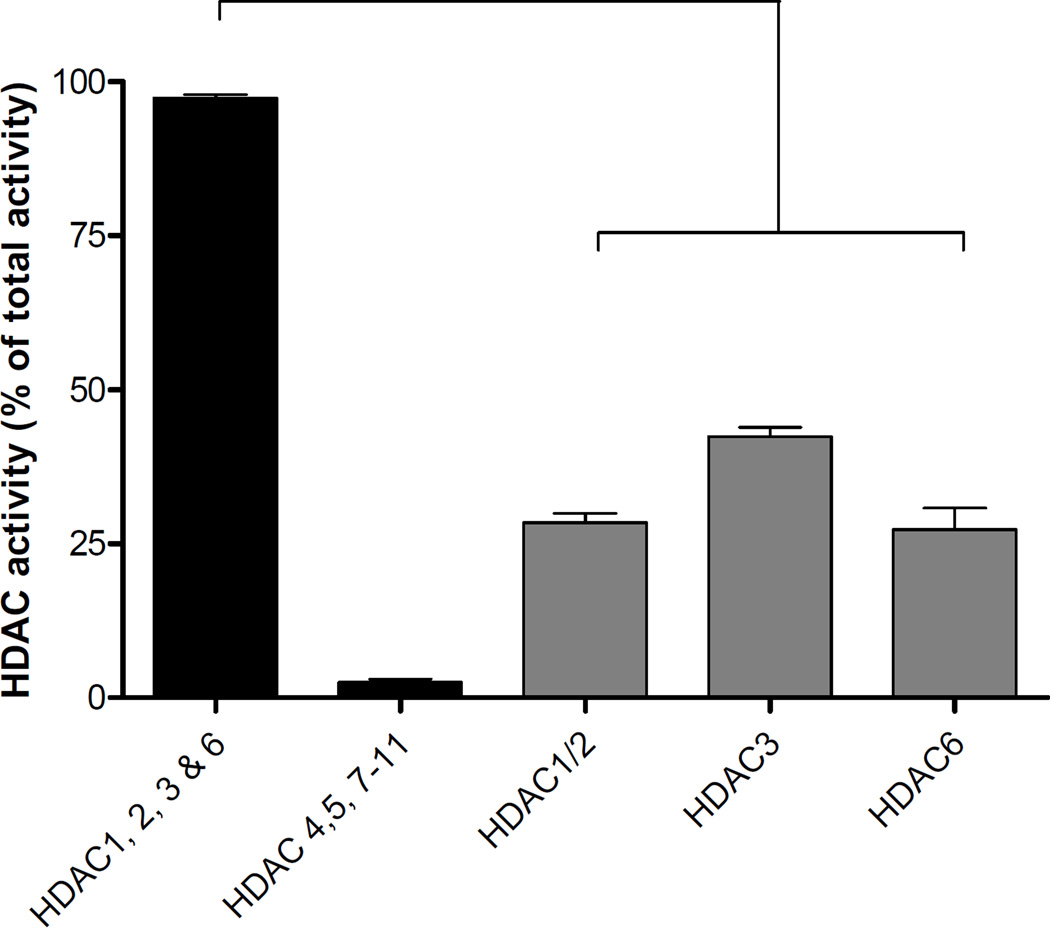
Total HDAC activity is summarized in Figure 1. In control retinas, the activity of HDAC1, 2, 3, and 6 represented 97.4 ± 0.5 % of the total HDAC activity. The combined activity of HDAC4, 5, 7, 8, 9, 10, and 11 accounted for the remaining 2.5 ± 0.5% of total activity. Using HDAC6 inhibitor, Tubastatin-A, alone and in combination with the HDAC1/2 inhibitor, the following estimates of HDAC activity were determined: HDAC1/2 = 28.4 ± 1.6%, HDAC3 = 42.4 ± 1.5%, and HDAC6 = 27.3 ± 3.5%.
Figure 1.
Retinal HDAC enzymatic activity: Total activity of HDAC1, 2, 3 and 6; and total activity of HDAC4, 5, 7, 8, 9, 10, 11; and activities of HDAC1/2, HDAC3 and HDAC6. Extent of HDAC activity was examined by fluorescent detection of aminomethoxy-Cumarin (AMC) following cleavage from enzymatically-deacetylated lysines. Data are presented as mean ± SE, n≥4.
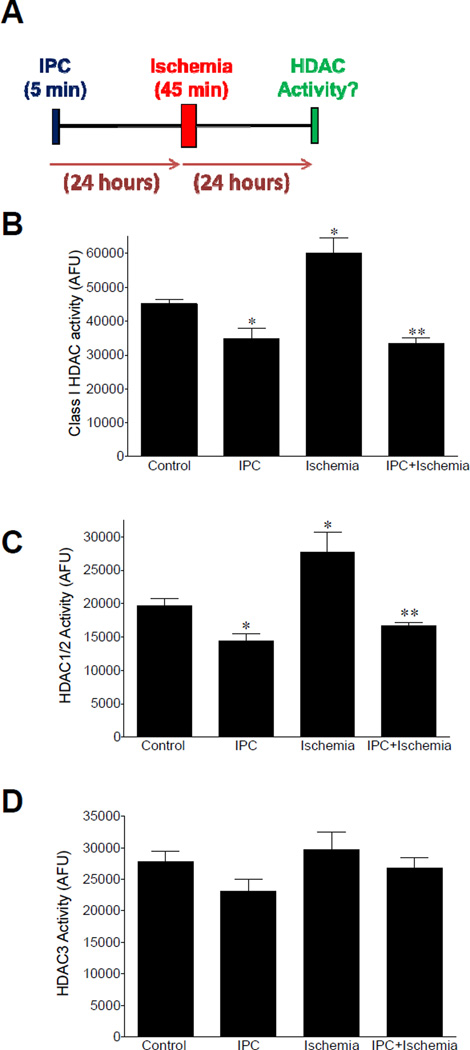
Consistent with our previous findings, Figure 2B shows that 24 hours post ischemic injury a significant increase (P <0.05) in HDAC1, 2 and 3 was measured (Alsarraf et al., 2014a). This increase in activity was associated with an elevation in HDAC1/2 activity (Fig. 2C). Although HDAC3 activity represented the largest percentage of Class I activity, we were not able to detect any significant change in activity of this isozyme following ischemia (Fig. 2D). Neither change in HDAC6 activity nor protein expression was detected by Western blot analyses 24 hours post ischemia (data not shown).
Figure 2.
Effect of IPC on retinal HDAC enzymatic activity. (A) Schematic representation of experimental procedure. (B) Effect of IPC on class I HDAC (HDAC1/2 and HDAC3) activity in control, IPC, ischemia and IPC plus ischemia retinas. Effect of IPC on (C) HDAC1/2 activity and (D) HDAC3 activity in control, IPC, ischemia and IPC plus ischemia retinas. Data are expressed as mean ± SE. *Indicates significant difference from control values (P < 0.05), **indicates significant difference from control and ischemic values, n≥4.
Retinas from animals 48 hours post IPC stimulus showed a significant reduction in combined HDAC1, 2, and 3 activities when compared to control eyes. Using HDAC inhibitors revealed that IPC induced a significant reduction in HDAC1/2 activity levels (Fig. 2C). While a trend toward lower HDAC3 activity was observed 48 hours post-IPC, this change was not significant (Fig. 2D). In animals receiving both IPC and 45 minute ischemic insult and evaluated 24 hours post ischemia, significant reductions in retinal HDAC1, 2, and 3 activities were measured when compared to retinas from control eyes and ischemic eyes (Fig. 2C). The activity of HDAC1/2 from retinas receiving both IPC and ischemia exhibited significant reduction when compared to the activity measured in control and ischemic retinas. The activity of HDAC3 from retinas receiving both IPC and ischemia were not significantly different from activity measured in control, IPC alone or ischemic retinas.
3.2 Ischemic Preconditioning and Histone H3-Acetylation
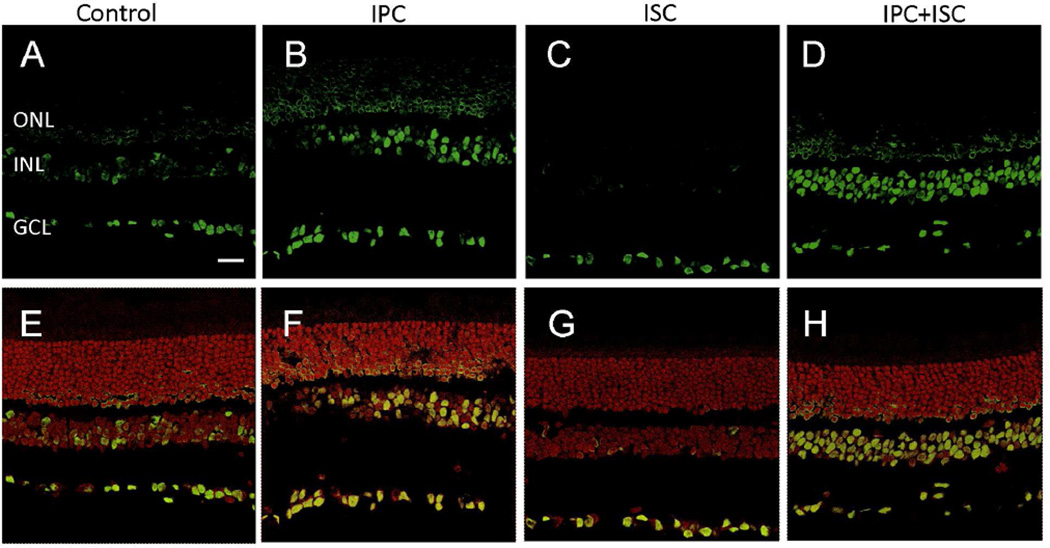
To assess if IPC can alter retinal acetylation, rats received an IPC stimulus alone or in combination with ischemic insult. Retinal acetylated histone-H3 was evaluated by immunohistochemistry at times corresponding to 24 hours post ischemic insult. In control animals, acetylated histone-H3 staining was mainly observed in the retinal ganglion cell layer (GCL) with light staining detected in the inner nuclear layer (INL) (Fig. 3A & E). However, 48 hours after IPC alone, immunofluorescence-labeling of acetylated histone-H3 increased in the INL and GCL and was also visible in the outer nuclear layer (Fig. 3B & F). In ischemic animals, acetylated H3 staining is only observed in GCL and very weak in INL (Fig. 3C & G). Ischemic injury 24 hours after IPC did not alter acetylation levels with respect to IPC alone (Fig. 3D & H). Analysis of fluorescence intensity demonstrated that IPC alone increased total retinal acetyl histone-H3 labeling by 177% when compared to sections from control retinas, while ischemic injury alone reduced total retinal labeling by 55.4% compared to control retinas. In animals that received IPC followed by ischemic insult, total retinal acetyl histone-H3 labeling was increased by 161% when compared to sections from control retinas.
Figure 3.
Effect of IPC on acetylated histone-H3 immunostaining in rat retina cross sections. Immunohistochemical staining of acetylated histone-H3 (green) in (A) control, (B) IPC, (C) ischemia, and (D) IPC plus ischemia retinas; and sections overlaid with propidium iodide, nuclei staining (red) in (E) control, (F) IPC, (G) ischemia, and (H) IPC plus ischemia retinas. ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar: 20 µm.
3.3 Ischemic Preconditioning and Neuroprotection
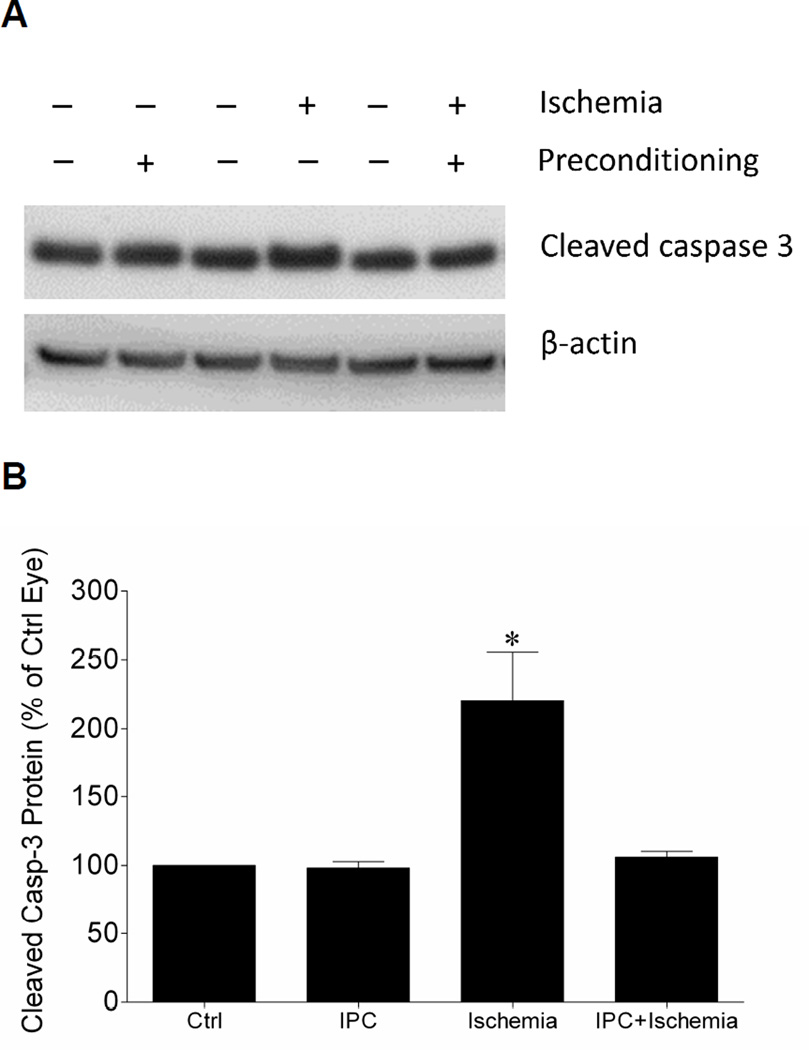
To estimate apoptotic activity the level of cleaved caspase-3 in retinas from animals receiving IPC, ischemic insults or both were measure by Western blot analyses. As shown in Figure 4, ischemia resulted in a significant increase in the level of cleaved caspase-3. No significant change in cleaved caspase-3 was measured in retinas from eyes that received IPC or IPC followed by ischemia.
Figure 4.
Effect of IPC on retinal cleaved caspase-3 levels (A) Representative Western blot of retinal lysates for cleaved caspase-3 and β-actin at 24 hours after initiation of ischemic injury. (B) Levels are expressed as a mean percentage of the IPC or ischemic eyes relative to the control eyes. Data are expressed as mean ± SE, n=4. *Indicates significant difference (P <0.05), n=4.
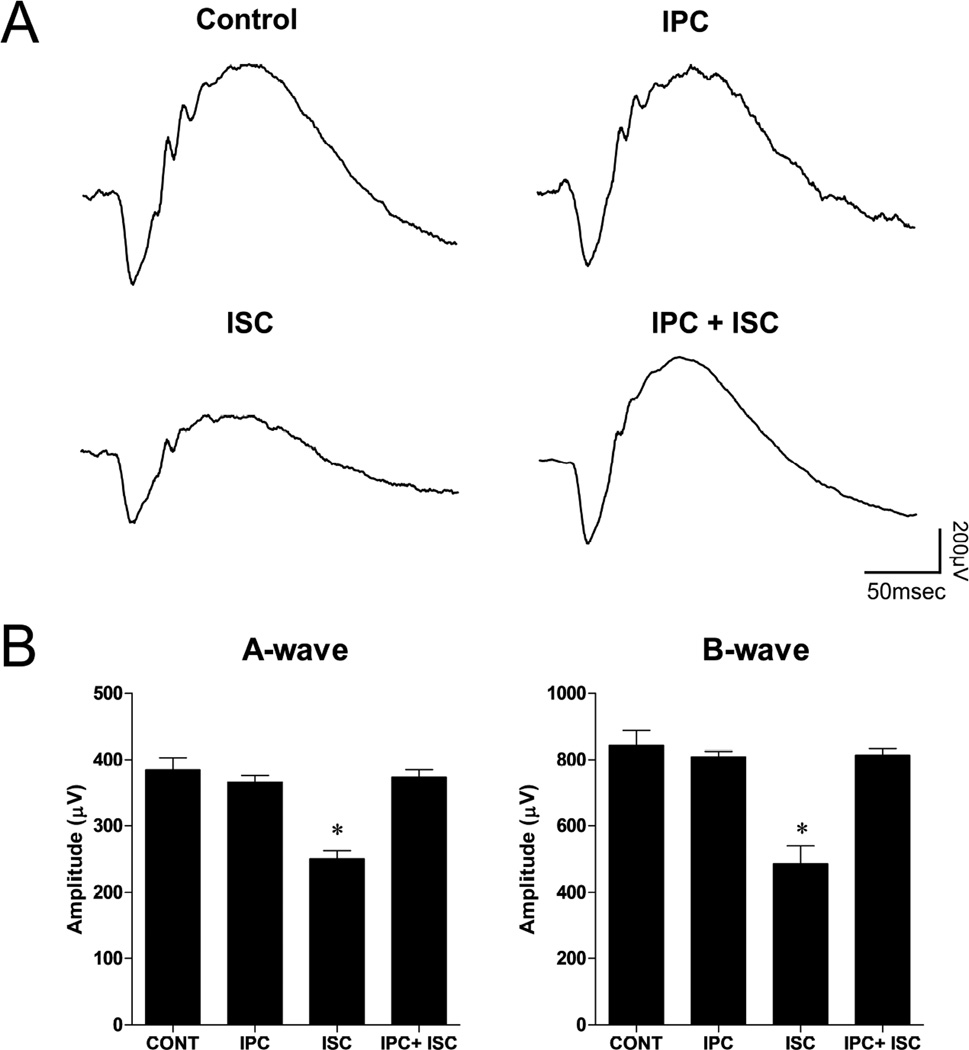
Figure 5 summarizes the electroretinogram (ERG) analyses from animals receiving IPC, ischemic insult or IPC and ischemic insult. All animals were evaluated at times corresponding to 7 days post ischemic insult. In control and IPC groups, no change in a- and b-wave amplitudes was measured. In ischemic eyes, ERG a- and b-wave amplitudes were significantly reduced by 34.9 ± 3.1% and 42.4 ± 6.3%, respectively. Although rats receiving IPC plus ischemia revealed small reductions in a- and b-wave amplitudes, these changes were not significant.
Figure 5.
Effect of IPC on functional neuroprotection using electroretinograms (ERGs). ERGs were obtained by averaging three responses to full-intensity flashes with an inter-stimulus interval of 2 minutes. (A) Representative ERG waveforms and (B) a- and b-wave amplitudes 7 days following unilateral ischemic retinal injury. Data are expressed as mean ± SE, n=6. Asterisks denote a significant difference (* P <0.05) from contralateral, IPC alone and IPC plus ischemia eyes. No significant differences among contralateral, IPC alone and IPC plus ischemia were measured.
4. DISCUSSION
Preconditioning is defined as the ability of a stressful, but non-damaging stimulus to cells or tissues to promote an adaptive response, so that injury resulting from subsequent exposure to a harmful stimulus is reduced (Gidday, 2006). Preconditioning has been studied in numerous tissues, and multiple autacoids and signaling pathways have been proposed to contribute to these protective responses (Murry et al., 1986; Neely and Keith, 1995; Schultz and Gross, 2001; Tang et al., 2011). However, the underlying mechanisms are still not understood. Although early studies described preconditioning periods as transient, recent studies in the brain and the retina have shown that periods of protection can be extended up to four weeks using repetitive hypoxic stimuli (Stowe et al., 2011; Zhu et al., 2007). These long-term stress-resistant phenotypes provide evidence that epigenetics mechanisms play a role in the preconditioning response elicited in the central nervous system.
The term epigenetics has evolved to refer to changes in gene expression that do not involve alterations in an organism’s primary DNA sequence. The molecular basis of epigenetic regulation of gene expression includes DNA methylation, histone modifications, and noncoding RNAs (Choudhuri, 2011). Histone acetylation represents one of the principal histone modifications regulating gene expression and is controlled by two competing enzymes, histone acetyltransferases (HATs) and histone deacetylases (HDACs). Previous studies from our laboratory have shown that increased HDAC activity is associated with retinal hypoacetylation and these changes are early events in retinal ischemic and ocular hypertensive injury (Alsarraf et al., 2014a). These studies also demonstrate that treatment with HDAC inhibitors provides robust structural and functional neuroprotection of the retina from ischemic and ocular hypertensive injury (Alsarraf et al., 2014a; Alsarraf et al., 2014b; Crosson et al., 2010; Fan et al., 2013). The tumor suppressor, P53, plays a central role in the initiation of apoptotic insults in many neurodegenerative disorders and is a non-histone target for HATs and HDACs (Brooks and Gu, 2011; Gu and Roeder, 1997; Uo et al., 2009). Recent work has provided evidence that altering the acetylation status of P53 by HDAC depletion protects retinal ganglion cells by suppressing apoptosis induced by optic nerve transection (Lebrun-Julien and Suter, 2015). In the current study, we investigated if IPC modulates HDAC activity, retinal protein acetylation, and activation of caspase-3, to produce an ischemic resistant phenotype.
Results from this study demonstrate that in the normal retina, the majority of HDAC deacetylase activity is confined to four isoforms: HDAC1, 2, 3 and 6. Although HDAC6, a class IIb HDAC, represented approximately 27.3 ± 3.5% of the total HDAC activity in the normal retina, we did not detect any significant change in the activity of HDACs following IPC or ischemic injury. As shown in Figure 2, the remaining deacetylase activity is confined to the class I HDAC1, 2 and 3 with HDAC1/2 and HDAC3 accounting for 28% and 42%, respectively. The percent of total HDAC activity measured for HDAC1/2 in this study is slightly less than the 35% measured for HDAC2 in mice (Fan et al., 2013). This difference may reflect efficacy of the methods used to selectively suppress HDAC isoform activity (i.e., genetic deletion and pharmacological inhibition) or species difference. However, these studies confirm previous studies that the majority of deacetylase activity is confined to these isoforms.
As we have shown previously, ischemic retinal injury significantly increased class I HDAC activity (Alsarraf et al., 2014a). In eyes that received IPC alone or in combination with ischemia, class I HDAC activity was significantly reduced when compared to control or ischemic retinas. In assays utilizing isoform selective inhibitors, the ischemic-induced increase and IPC-induced reduction was confined to HDAC1/2. This selective reduction in HDAC1/2 activity and the associated IPC-induced neuroprotection (see below) is consistent with recent studies demonstrating that genetic deletion of HDAC1/2 is neuroprotective in retinal ganglion cell degeneration induced by optic nerve transection (Lebrun-Julien and Suter, 2015).
Previous studies have shown that the pharmacologic inhibiting HDACs, or suppressing HDAC2 expression, increases retinal protein acetylation (Alsarraf et al., 2014a; Alsarraf et al., 2014b; Crosson et al., 2010; Fan et al., 2013). To determine if IPC-induced reduction in class I HDAC activity altered retinal protein acetylation, we utilized immunohistochemistry to examine the location and relative abundance of acetyl histone-H3 in retinas from control eyes, eyes that received ischemic injury, and eyes that received IPC alone and combined with ischemic injury. Consistent with previous studies, acetyl histone-H3 staining in normal eyes was confined to the ganglion cell layer and light staining of the inner plexiform layer (Fan et al., 2013). Twenty-four hours following ischemic injury an overall reduction of acetyl histone-H3 staining was observed. In eyes that received IPC alone or in combination with ischemic injury, an increase in staining in all nuclear layers was observed when compared to control and ischemic retinas (Fig. 4). This IPC-induced increase in acetyl histone-H3 staining is consistent with the reduction in class I HDAC activity measured in these animals. In addition, the pattern of increased staining in retinas that received IPC was similar to that observed following the administration of HDAC inhibitor or suppressing HDAC 2 expression (Alsarraf et al., 2014b; Fan et al., 2013). Taken together these studies provide the first evidence that IPC suppresses class I HDAC activity resulting in the hyperacetylation of retinal proteins.
Several studies have concluded that IPC activates endogenous mechanisms to protect the retina from subsequent injury (Belforte et al., 2011; Roth et al., 1998; Zhu et al., 2012). There is also increasing evidence that hypoacetylation of retinal proteins is associated with the sequel of events leading to retinal degeneration, while hyperacetylation of retinal proteins can protect the retina from ischemic and optic nerve injury (Biermann et al., 2010; Crosson et al., 2010; Fan et al., 2013; Petri et al., 2006). As HDAC inhibitors and genetic deletion of HDACs have been shown to suppress apoptotic pathway activity (Alsarraf et al., 2014a; Lebrun-Julien and Suter, 2015; Zhang et al., 2012), we evaluated if the activation of caspase-3 is reduced by IPC at a time consistent with reduced HDAC activity and hyperacetylation of retinal proteins. As shown in Figure 4, IPC blocked ischemia-induced increases in cleaved caspase-3 at a time corresponding to reduced HDAC activity. These results are consistent with the paradigm that HDACs play a significant role in suppressing retinal apoptosis in eyes receiving IPC stimuli.
To confirm that IPC in the current study produced functional neuroprotection, ERG a- and b-wave amplitudes from animals that received IPC alone or in combination with ischemic injury were compared to ERGs from control eyes and eyes that received ischemic injury alone. As shown in Figure 5, 7 days post ischemic injury, eyes that received ischemic injury alone exhibited mean deficits in ERG a- and b-wave amplitudes of 34.9 ± 3.1% and 42.4 ± 6.3%, respectively. In eyes that received IPC 24 hours prior to the ischemic event, ERG a- and b- wave amplitudes were not significantly different from control (non-ischemic) eyes. These results are consistent with previous studies that IPC produces robust neuroprotection from ischemic injury.
5. CONCLUSIONS
This study expands on previous studies from our laboratory linking ischemic retinal injury with changes in HDAC activity. Results presented in this study provide evidence that HDAC1/2, HDAC3 and HDAC6 isoforms are the major active HDACs in control retinas. However, only the HDAC1/2 isoforms revealing deacetylase activity are modulated by ischemia. Although other factors, such as nuclear translocation can influence the functional response to HDACs, our results support the idea that the hypoacetylation induced by HDAC1/2 during ischemic injury plays a central role in the sequelae of events leading to retinal degeneration. Our results also provide the first evidence that the protective effects of IPC in the retina are at least in part due to the suppression of HDAC1/2 activity and the resulting hyperacetylated state of the retinal proteins. Understanding the endogenous pathways that link IPC and HDAC activity should provide new therapeutic opportunities to treat a variety of retinal degenerative disorders.
HIGHLIGHTS.
HDAC1/2, HDAC3 and HDAC6 isoforms are the major active HDACs in retinas.
Only HDAC1/2 isoforms revealing deacetylase activity are modulated by ischemia.
Neuroprotection induced by IPC is mediated in part by suppressing HDAC1/2 activity.
Linking the neuroprotection by IPC to reduced HDAC activity.
Acknowledgments
Supported in part by National Institutes of Health grants NEI 5R01EY021368 (C.E.C.); and an unrestricted grant to Storm Eye Institute, Medical University of South Carolina, from Research to Prevent Blindness, New York, N.Y. Special appreciation to Luanna Bartholomew, PhD, for critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alsarraf O, Fan J, Dahrouj M, Chou CJ, Menick DR, Crosson CE. Acetylation: a lysine modification with neuroprotective effects in ischemic retinal degeneration. Exp Eye Res. 2014a;127:124–131. doi: 10.1016/j.exer.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsarraf O, Fan J, Dahrouj M, Chou CJ, Yates PW, Crosson CE. Acetylation preserves retinal ganglion cell structure and function in a chronic model of ocular hypertension. Invest Ophthalmol Vis Sci. 2014b;55:7486–7493. doi: 10.1167/iovs.14-14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beharry C, Cohen LS, Di J, Ibrahim K, Briffa-Mirabella S, Alonso Adel C. Tau-induced neurodegeneration: mechanisms and targets. Neurosci Bull. 2014;30:346–358. doi: 10.1007/s12264-013-1414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belforte N, Sande PH, de Zavalia N, Fernandez DC, Silberman DM, Chianelli MS, Rosenstein RE. Ischemic tolerance protects the rat retina from glaucomatous damage. PLoS One. 2011;6:e23763. doi: 10.1371/journal.pone.0023763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biermann J, Grieshaber P, Goebel U, Martin G, Thanos S, Di Giovanni S, Lagreze WA. Valproic acid-mediated neuroprotection and regeneration in injured retinal ganglion cells. Invest Ophthalmol Vis Sci. 2010;51:526–534. doi: 10.1167/iovs.09-3903. [DOI] [PubMed] [Google Scholar]
- Bradner JE, West N, Grachan ML, Greenberg EF, Haggarty SJ, Warnow T, Mazitschek R. Chemical phylogenetics of histone deacetylases. Nat Chem Biol. 2010;6:238–243. doi: 10.1038/nchembio.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CL, Gu W. The impact of acetylation and deacetylation on the p53 pathway. Protein Cell. 2011;2:456–462. doi: 10.1007/s13238-011-1063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- Choudhuri S. From Waddington's epigenetic landscape to small noncoding RNA: some important milestones in the history of epigenetics research. Toxicol Mech Methods. 2011;21:252–274. doi: 10.3109/15376516.2011.559695. [DOI] [PubMed] [Google Scholar]
- Crosson CE, Mani SK, Husain S, Alsarraf O, Menick DR. Inhibition of histone deacetylase protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2010;51:3639–3645. doi: 10.1167/iovs.09-4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Alsarraf O, Dahrouj M, Platt KA, Chou CJ, Rice DS, Crosson CE. Inhibition of HDAC2 protects the retina from ischemic injury. Invest Ophthalmol Vis Sci. 2013;54:4072–4080. doi: 10.1167/iovs.12-11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez DC, Sande PH, Chianelli MS, Aldana Marcos HJ, Rosenstein RE. Induction of ischemic tolerance protects the retina from diabetic retinopathy. Am J Pathol. 2011;178:2264–2274. doi: 10.1016/j.ajpath.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gidday JM. Extending injury- and disease-resistant CNS phenotypes by repetitive epigenetic conditioning. Front Neurol. 2015;6:42. doi: 10.3389/fneur.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Wenzel A, Groszer M, Mayser H, Seeliger M, Samardzija M, Bauer C, Gassmann M, Reme CE. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002;8:718–724. doi: 10.1038/nm723. [DOI] [PubMed] [Google Scholar]
- Gu W, Roeder RG. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- Harrison IF, Dexter DT. Epigenetic targeting of histone deacetylase: therapeutic potential in Parkinson's disease? Pharmacol Ther. 2013;140:34–52. doi: 10.1016/j.pharmthera.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Lazo-Gomez R, Ramirez-Jarquin UN, Tovar YRLB, Tapia R. Histone deacetylases and their role in motor neuron degeneration. Front Cell Neurosci. 2013;7:243. doi: 10.3389/fncel.2013.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebrun-Julien F, Suter U. Combined HDAC1 and HDAC2 depletion promotes retinal ganglion cell survival after injury through reduction of p53 target gene expression. ASN Neuro. 2015;7 doi: 10.1177/1759091415593066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Roth S, Laser M, Ma JX, Crosson CE. Retinal preconditioning and the induction of heat-shock protein 27. Invest Ophthalmol Vis Sci. 2003;44:1299–1304. doi: 10.1167/iovs.02-0235. [DOI] [PubMed] [Google Scholar]
- Methot JL, Chakravarty PK, Chenard M, Close J, Cruz JC, Dahlberg WK, Fleming J, Hamblett CL, Hamill JE, Harrington P, Harsch A, Heidebrecht R, Hughes B, Jung J, Kenific CM, Kral AM, Meinke PT, Middleton RE, Ozerova N, Sloman DL, Stanton MG, Szewczak AA, Tyagarajan S, Witter DJ, Secrist JP, Miller TA. Exploration of the internal cavity of histone deacetylase (HDAC) with selective HDAC1/HDAC2 inhibitors (SHI-1:2) Bioorg Med Chem Lett. 2008;18:973–978. doi: 10.1016/j.bmcl.2007.12.031. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Lee RJ, McClean ME, Pemberton HE, Uo T, Morrison RS, Bastian C, Baltan S. MS-275, a class I histone deacetylase inhibitor, protects the p53-deficient mouse against ischemic injury. J Neurochem. 2014;129:509–515. doi: 10.1111/jnc.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Neely CF, Keith IM. A1 adenosine receptor antagonists block ischemia-reperfusion injury of the lung. Am J Physiol. 1995;268:L1036–L1046. doi: 10.1152/ajplung.1995.268.6.L1036. [DOI] [PubMed] [Google Scholar]
- Petri S, Kiaei M, Kipiani K, Chen J, Calingasan NY, Crow JP, Beal MF. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiology of disease. 2006;22:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Roth S, Li B, Rosenbaum PS, Gupta H, Goldstein IM, Maxwell KM, Gidday JM. Preconditioning provides complete protection against retinal ischemic injury in rats. Invest Ophthalmol Vis Sci. 1998;39:777–785. [PubMed] [Google Scholar]
- Schultz JE, Gross GJ. Opioids and cardioprotection. Pharmacol Ther. 2001;89:123–137. doi: 10.1016/s0163-7258(00)00106-6. [DOI] [PubMed] [Google Scholar]
- Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol. 2011;69:975–985. doi: 10.1002/ana.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L, Jiang R, Zheng K, Zhu X. Enhancing the recombinant protein expression of halohydrin dehalogenase HheA in Escherichia coli by applying a codon optimization strategy. Enzyme Microb Technol. 2011;49:395–401. doi: 10.1016/j.enzmictec.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Uo T, Veenstra TD, Morrison RS. Histone deacetylase inhibitors prevent p53-dependent and p53-independent Bax-mediated neuronal apoptosis through two distinct mechanisms. J Neurosci. 2009;29:2824–2832. doi: 10.1523/JNEUROSCI.6186-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener D, Hildmann C, Riester D, Schwienhorst A. Improved fluorogenic histone deacetylase assay for high-throughput-screening applications. Anal Biochem. 2003;321:202–208. doi: 10.1016/s0003-2697(03)00426-3. [DOI] [PubMed] [Google Scholar]
- Whitlock NA, Agarwal N, Ma JX, Crosson CE. Hsp27 upregulation by HIF-1 signaling offers protection against retinal ischemia in rats. Invest Ophthalmol Vis Sci. 2005;46:1092–1098. doi: 10.1167/iovs.04-0043. [DOI] [PubMed] [Google Scholar]
- Zhang ZZ, Gong YY, Shi YH, Zhang W, Qin XH, Wu XW. Valproate promotes survival of retinal ganglion cells in a rat model of optic nerve crush. Neuroscience. 2012;224:282–293. doi: 10.1016/j.neuroscience.2012.07.056. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Schmidt JF, Gidday JM. Glaucoma-induced degeneration of retinal ganglion cells prevented by hypoxic preconditioning: a model of glaucoma tolerance. Mol Med. 2012;18:697–706. doi: 10.2119/molmed.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Zhang Y, Ojwang BA, Brantley MA, Jr, Gidday JM. Long-term tolerance to retinal ischemia by repetitive hypoxic preconditioning: role of HIF-1alpha and heme oxygenase-1. Invest Ophthalmol Vis Sci. 2007;48:1735–1743. doi: 10.1167/iovs.06-1037. [DOI] [PubMed] [Google Scholar]