Abstract
Objective
To evaluate the relationship between pre-treatment intake of whole grains and outcomes of in vitro fertilization (IVF).
Design
Prospective cohort study.
Setting
Academic medical center.
Patient(s)
273 women who collectively underwent 427 IVF cycles.
Intervention(s)
Whole grain intake was assessed with a validated food frequency questionnaire at enrollment.
Main Outcome Measures
Intermediate and clinical endpoints of IVF were abstracted from medical records.
Results
Women had a median whole grain intake of 34.2 g/day (~1.2 servings/day). Higher pre-treatment whole grain intake was associated with higher probability of implantation and live birth (p-trend=0.02 and 0.03, respectively). The adjusted percentage of cycles resulting in live birth for women in the highest quartile of whole grain intake (>52.4 g/day) was 53% (95% CI 41, 65) compared to 35% (95% CI 25, 46%) for women in the lowest quartile (<21.4 g/day). This association was largely driven by intake of bran as opposed to germ. When intermediate endpoints of IVF were examined, only endometrial thickness on the day of embryo transfer was associated with whole grain intake. A 28 g/day (~1 serving/day) increase in whole grain intake was associated with a 0.4 mm (95% CI 0.1, 0.7 mm) increase in endometrial thickness.
Conclusions
Higher pre-treatment whole grain intake was related to higher probability of live birth among women undergoing IVF. The higher probability of live birth may result from increased endometrial thickness on the day of embryo transfer and improved embryo receptivity manifested in a higher probability of implantation.
Keywords: whole grains, in vitro fertilization, female infertility, assisted reproductive technology
Introduction
Whole grains are rich in many components, including dietary fiber, antioxidant nutrients, vitamins, minerals, lignans, and phenolic compounds, that have been linked to reduced risk of coronary heart disease (1), cancer (2), diabetes (3), obesity (4), and all-cause mortality (5). While it is generally accepted that whole grains are beneficial in preventing most chronic diseases, less is known about their impact on reproductive outcomes such as fertility and fecundity.
Whole grain foods and its components have physiologic properties that support a link between whole grain intake and fertility. Whole grain intake has been linked to lower levels of systematic inflammation (6, 7) and specific nutrients found in wholes grains such as phytic acid, vitamin E, and selenium are known to support antioxidant defense (8, 9). As an imbalance between pro-oxidants and antioxidants has been identified to play a key role in the pathogenesis of female subfertility (10), the anti-inflammatory actions of whole grains could promote fertility through their capacity to control the production of reactive oxygen species. Whole grain intake has also been shown to help regulate glucose metabolism and decrease insulin resistance (11), Since insulin resistance has been implicated in the development of polycystic ovarian syndrome (12) and recurrent pregnancy loss (13), this could also be a mechanism through which whole grain could exert potentially beneficial reproductive effects. Finally, although less researched, the hormonally active compounds in whole grains, called lignans, may also exert proestrogenic and antiestrogenic effects that could have reproductive benefits (14).
Based on these biological hypotheses, we sought to determine the extent to which habitual whole grain intake in the year prior to infertility treatment initiation influences both intermediate and clinical outcomes of in vitro fertilization (IVF).
Materials and Methods
Study Population
Participants were women enrolled in the Environment and Reproductive Health (EARTH) Study, an ongoing prospective cohort started in 2006 aimed at identifying determinants of fertility among couples presenting to the Massachusetts General Hospital Fertility Center (Boston, MA, USA). All women who meet eligibility requirements (age 18–46 years and no planned use of donor gametes at enrollment) are invited to participate in the study. Approximately 55% of those referred by physicians ultimately enroll in the study; however, among referred women who research nurses are able to contact, 78% enroll in the study. A food frequency questionnaire (FFQ) was introduced in 2007. For this analysis, women were eligible if they had completed at least 1 IVF cycle between February 2007 and August 2014 (n=370). Of these, 94 women (25%) were excluded because they had not completed the FFQ and 12 women (3%) were excluded because they had started their IVF cycle prior to completion of the FFQ. Women missing diet were more likely to be diagnosed with diminished ovarian reserve (17.0% vs. 7.3%) or endometriosis (13.8% vs. 4.0%) and more likely to have IVF cycles that failed prior to embryo transfer (16.0% vs. 8.0%). All other characteristics were similar to the women included in our analysis. Some women re-enrolled in the study years after their initial entry and filled out a second FFQ (n=10). In these rare instances, the cycles initiated subsequent to the receipt of the second FFQ were assigned to this FFQ. The study was approved by the Institutional Review Boards of the Massachusetts General Hospital and the Harvard T. H. Chan School of Public Health. All participants provided written informed consent after study procedures were explained by a research nurse.
Diet Assessment
Diet was assessed before IVF treatment initiation using a validated food frequency questionnaire (15). Participants were asked to report how often, on average, they consumed 131 foods (with a prespecified portion size) during the previous year using 9 categories of intake frequency, ranging from less than 1 per month to 6 or more per day. Open-ended questions were included for breakfast cereal brand names and foods that were not listed on the FFQ. Multivitamin and supplement users were asked to specify the brand of the multivitamin or supplement, the dose, and frequency of use. Nutrient intakes were estimated by summing the nutrient contribution of all food and supplement items. Nutrient contents were obtained from the nutrient database of the US Department of Agriculture with additional information from manufacturers (16). Previous validation studies have indicated good correlations between foods and nutrients assessed by this dietary questionnaire and multiple weeks of food records completed over the previous year such as 0.75 for ready-to-eat cereals and 0.77 for dark breads (15, 17).
Intakes of whole grain were estimated from all grain-containing foods (rice, bread, pasta, and breakfast cereals) according to the dry weight of the whole grain ingredients in each food (18). Whole grain consumption from breakfast cereal was derived from more than 250 brand name cereals based on information provided by product labels and breakfast cereal manufacturers. In our study, whole grains included intact and pulverized forms that contained the expected proportion of bran, germ, and endosperm for the specific grain types. By definition, the following foods and ingredients were considered whole grains: whole wheat and whole wheat flour, whole oats and whole oat flour, whole cornmeal and whole corn flour, whole rye and whole rye flour, whole barley, bulgur, buckwheat, brown rice and brown rice flour, popcorn, amaranth, and psyllium. In the FFQ, we also asked the frequency of consuming added bran (oat bran and other bran) and added wheat germ. Intakes of bran and germ were derived directly from whole grain foods and those added to foods. Total bran and total germ are the sum of intakes from both sources.
We used two data-derived dietary patterns, calculated using factor analysis based on 40 pre-defined food groups, to describe general patterns of food consumption (19). Orthogonal transformations were used to achieve uncorrelated factors (dietary patterns) with simpler structures with greater interpretability. In brief, two dietary patterns were identified using factor analysis: the Prudent pattern, characterized by intakes of fish, fruits, vegetables, nuts, and legumes; and the Western pattern, characterized by high intakes of red and processed meat, butter, refined grains, and sweets. For every subject we calculated factor scores for each of the factors by summing the frequency of consumption for each of the 40 food groups multiplied by factor loadings across all food items. Scores on the ‘Prudent’ and ‘Western’ dietary patterns have a mean of 0 and a standard deviation of 1. Women received a score on each of these patterns with higher scores indicating higher adherence. These dietary pattern scores were used to account for potential confounding by overall diet quality in the multivariable models.
Outcome Assessment
Patients underwent one of three stimulation protocols as clinically indicated: 1) luteal-phase GnRH agonist protocol; 2) follicular-phase GnRH-agonist/Flare protocol; or 3) GnRH-antagonist protocol. Patients were monitored during gonadotropin stimulation for serum estradiol, follicle size measurements and counts, and endometrial thickness. Human chorionic gonadotropin (hCG) was administered approximately 36 hours before the scheduled egg-retrieval procedure to induce ovulation. Details of egg retrieval have been previously described. (20)
Couples underwent IVF with conventional insemination or intra-cytoplasmatic sperm injection (ICSI) as clinically indicated. Embryologists classified oocytes as germinal vesicle, metaphase I, metaphase II (MII) or degenerated. Embryologists determined fertilization 17–20 hours after insemination as the number of oocytes with two pronuclei. Fertilization rate was defined as the total number of fertilized embryos divided by the number of MII oocytes. The resulting embryos were monitored for cell number and morphological quality (1 (best) to 5 (worst)) on day 2 and 3. For analysis we classified embryos as best quality if they had 4 cells on day 2, 8 cells on day 3, and a morphologic quality score of 1 or 2 on days 2 and 3. We defined implantation as a serum β-hCG level >6 mIU/mL typically measured 17 days (range 15–20 days) after egg retrieval, clinical pregnancy as the presence of an intrauterine pregnancy confirmed by ultrasound, and live birth as the birth of a neonate on or after 24 weeks gestation.
Covariate Assessment
At enrollment, height and weight were measured by a trained research nurse to calculate body mass index (BMI) (kg/m2) and a brief, nurse-administered questionnaire was used to collect data on demographics, medical history, and lifestyle. Participants also completed a detailed take-home questionnaire with additional questions on lifestyle factors, reproductive health, and medical history. Clinical information including infertility diagnosis and protocol type was abstracted from electronic medical records.
Statistical Analysis
Women were classified into quartiles based on whole grain intake and descriptive statistics were calculated across quartiles for demographic characteristics, dietary nutrients, and initial cycle characteristics. Multivariate generalized linear mixed models with random intercepts were used to evaluate the association between whole grain intake and IVF outcomes while accounting for within-person correlations in outcomes. These models generate unbiased estimates in the presence of an unbalanced design (e.g. different number of cycles per woman) when data is not missing completely at random and the model is correctly specified. Normal distribution and identity link function were specified for peak estradiol and endometrial thickness (both normally distributed), Poisson distribution and log link function were specified for oocyte counts, and binomial distribution and logit link function were specified for fertilization rate, embryo quality, and clinical outcomes. Tests for trend across quartiles were conducted using a variable with the median whole grain intake in each quartile. Whole grain intake was also evaluated as continuous linear and quadratic variable. All results are presented as population marginal means, adjusted for covariates (21).
Confounding was evaluated using prior knowledge and descriptive statistics from our cohort through the use of directed acyclic graphs. Variables retained in the final multivariable models were calorie intake, age, BMI, race, dietary patterns, and folate and alcohol intake. Nutrients highly correlated with whole grain intake were analyzed as collinear variables and potential confounders by adding the nutrients to the fully adjusted model separately and then in combination to see if it affected the magnitude or significance of the effect estimate for whole grain. Effect modification by demographic and cycle characteristics were tested using cross-product terms in the final multivariate models. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all statistical analyses.
Results
Our analysis included 273 women who had an average age of 35.4 years and BMI of 24.2 kg/m2. Seventy-one percent of our women had never smoked tobacco and 84% were Caucasian. The primary infertility diagnosis was 36% unexplained, followed by 34% male factor and 30% female factor. The median whole grain intake was 34.2 g/day (range 0.6–195.6 g/day) which corresponds to approximately 1.2 whole grain servings/day (range 0–7 servings/day) (Supplemental Table 1). Women with higher whole grain intake tended to have higher intake of total calories, carbohydrates, dietary fiber, folate, and alcohol (Table 1). They also had higher adherence to a Prudent dietary pattern and lower adherence to a Western dietary pattern. Women in our cohort underwent a total of 438 IVF cycles with 166 women (61%) followed for 1 cycle, 66 women (24%) followed for 2 cycles, 30 women (11%) followed for 3 cycles, and 11 women (4%) followed for 4–6 cycles. Supplemental Figure 1 gives an overview of the 438 IVF cycles.
Table 1.
Baseline characteristics of 273 women (283 unique FFQs) in Environment and Reproductive Health (EARTH) Study (2007–2014) by quartile of whole grain intake.
| Quartiles of Whole Grain Intake | |||||
|---|---|---|---|---|---|
| Quartile (Range, g/day) |
Q1 (<21.4) |
Q2 (21.5–34.2) |
Q3 (34.3–52.4) |
Q4 (>52.4) |
|
| Median Intake (g/day) | 15.4 | 26.4 | 41.9 | 70.8 | |
| N | 70 | 71 | 71 | 71 | p-valuea |
| Personal Characteristics | |||||
| Age, years | 35.0 (6.0) | 35.5 (4.0) | 35.5 (3.6) | 35.7 (4.1) | 0.96 |
| BMI, kg/m2 | 23.9 (3.8) | 24.1 (3.6) | 24.6 (4.8) | 24.0 (4.4) | 0.91 |
| Ever smoker, n (%) | 21 (30.0) | 22 (31.0) | 20 (28.2) | 18 (25.4) | 0.89 |
| White/Caucasian, n (%) | 53 (75.7) | 64 (90.1) | 62 (87.3) | 60 (84.5) | 0.10 |
| Baseline Reproductive Characteristics | |||||
| Infertility diagnosis, n (%) | |||||
| Female factor | 22 (31.4) | 21 (29.6) | 20 (28.2) | 21 (29.6) | 0.93 |
| Ovulation Disorders | 4 (5.7) | 5 (7.0) | 7 (9.9) | 10 (14.1) | |
| Diminished Ovarian Reserve | 5 (7.1) | 7 (9.9) | 3 (4.2) | 5 (7.0) | |
| Tubal | 8 (11.4) | 5 (7.0) | 5 (7.0) | 3 (4.2) | |
| Endometriosis | 3 (4.3) | 3 (4.3) | 4 (5.6) | 2 (2.8) | |
| Uterine | 2 (2.9) | 1 (1.4) | 1 (1.4) | 1 (1.4) | |
| Male factor | 25 (35.7) | 27 (38.0) | 23 (32.4) | 22 (31.0) | |
| Unexplained | 23 (32.9) | 23 (32.4) | 28 (39.4) | 28 (39.4) | |
| Treatment protocol, n (%) | 0.16 | ||||
| Antagonist or Flare | 13 (18.6) | 23 (32.4) | 13 (18.3) | 16 (22.5) | |
| Luteal phase agonist | 57 (81.4) | 48 (67.6) | 58 (81.7) | 55 (77.5) | |
| Embryo Transfer Day, n (%) | 0.20 | ||||
| No embryos transferred | 8 (11.4) | 5 (7.0) | 5 (7.0) | 6 (8.5) | |
| Day 2 | 3 (4.3) | 4 (5.6) | 1 (1.4) | 5 (7.0) | |
| Day 3 | 27 (38.6) | 25 (35.2) | 43 (60.6) | 36 (50.7) | |
| Day 5 | 26 (37.1) | 29 (40.9) | 19 (26.8) | 20 (28.2) | |
| Egg Donor or Cryo Cycle | 6 (8.6) | 8 (11.3) | 3 (4.2) | 4 (5.6) | |
| Number of Embryos Transferred, n (%) | 0.96 | ||||
| No embryos transferred | 8 (11.4) | 5 (7.0) | 5 (7.0) | 6 (8.5) | |
| 1 embryo | 9 (12.9) | 9 (12.7) | 9 (12.7) | 10 (14.1) | |
| 2 embryos | 35 (50.0) | 36 (50.7) | 40 (56.3) | 40 (56.3) | |
| 3+ embryos | 12 (17.1) | 13 (18.3) | 14 (19.7) | 11 (15.5) | |
| Egg Donor or Cryo Cycle | 6 (8.6) | 8 (11.3) | 3 (4.2) | 4 (5.6) | |
| Dietary Characteristics | |||||
| Total Calories, kcal/day | 1457 (452) | 1568 (532) | 1905 (484) | 2237 (591) | <0.001 |
| Carbohydrates, % of kcal/day | 47.6 (8.7) | 48.1 (7.5) | 50.4 (6.7) | 52.3 (6.5) | 0.001 |
| Protein, % of kcal/day | 17.2 (3.2) | 17.0 (2.8) | 16.2 (2.5) | 16.2 (2.2) | 0.05 |
| Fat, % of kcal/day | 33.4 (6.9) | 33.2 (6.1) | 32.8 (6.3) | 30.9 (5.7) | 0.10 |
| Total fiber, g/day | 15.2 (6.7) | 16.9 (4.6) | 23.0 (6.0) | 31.2 (11.8) | <0.001 |
| Folate intake, DFE µg/day | 1564 (881) | 1784 (816) | 1959 (796) | 1970 (792) | 0.001 |
| Alcohol, g/day | 8.0 (13.5) | 8.3 (8.0) | 8.1 (7.9) | 10.8 (12.3) | 0.05 |
| Caffeine, mg/day | 111 (107) | 124 (106) | 125 (98) | 127 (124) | 0.66 |
| Prudent pattern | −0.39 (0.9) | −0.39 (0.6) | 0.04 (0.9) | 0.44 (1.02) | <0.001 |
| Western pattern | −0.28 (0.8) | −0.10 (1.0) | 0.09 (0.9) | 0.20 (0.9) | 0.003 |
Abbreviations: BMI, body mass index; DFE, dietary folate equivalents.
For continuous variables, Kruskal Wallis tests were used to test for associations across quartiles of whole grain intake. For categorical variables, chi-squared tests were used to test the associations across quartiles of whole grain intake.
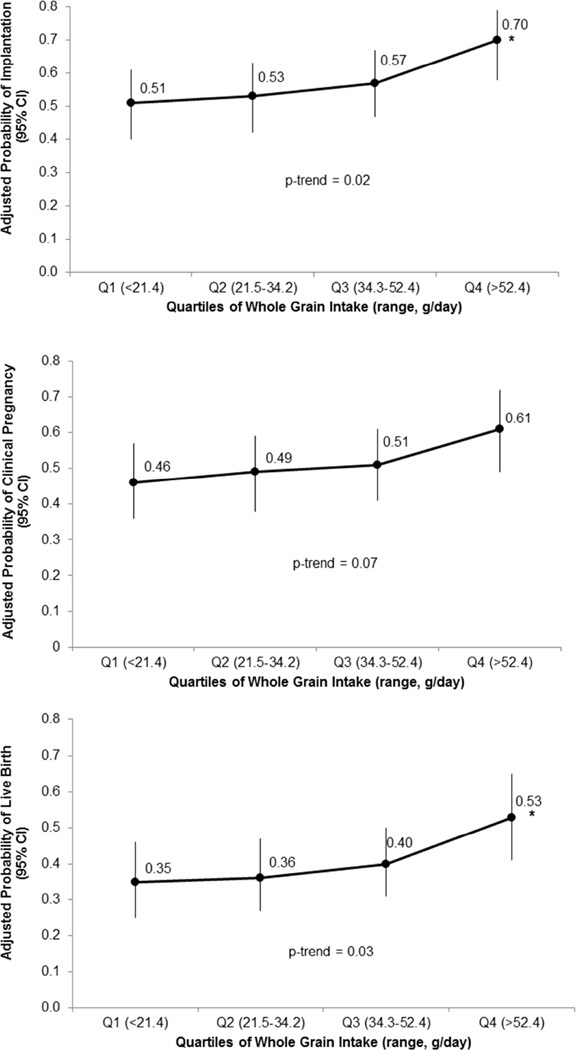
Whole grain intake was positively associated with probability of implantation and live birth per initiated cycle in models adjusted for age, BMI, race, dietary patterns, and calorie, folate, and alcohol intake (Figure 1). Specifically, the adjusted percentage of cycles with implantation for women in the lowest quartile of whole grain intake (>52.4 g/day) was 70% (95% CI 58%, 79%) compared to 51% (95% CI 40, 61%) for women in the lowest quartile of whole grain intake (<21.4 g/day) (p-trend=0.02). The adjusted absolute difference (95% CI) in live birth percentages between women in the highest versus lowest quartile of whole grain intake was 18% (6, 30%), representing a decrease from 53% to 35% (p-trend=0.03). There was a similar pattern of increased clinical pregnancy with increasing quartiles of whole grain consumption; however this association did not attain statistical significance (p-trend=0.08). When evaluated as a continuous measure, a one serving per day increase in whole grain intake was associated with a 33% (95% CI 1, 75%) and 27% (95% CI −2, 65%) higher odds of implantation and live birth, respectively. There was no evidence of a non-linear relationship. The association between whole grain intake and implantation, clinical pregnancy, and live birth was similar when the analyses were restricted to cycles with embryo transfer (Supplemental Figure 2). The adjusted absolute difference (95% CI) in live birth percentages per embryo transfer between women in the highest and lowest quartile of whole grain intake was 18% (5%, 29%) (p-trend=0.04).
Figure 1.
Association between whole grain intake and in vitro fertilization (IVF) outcomes per initiated cycle in 273 women (438 IVF cycles) in the Environment and Reproductive Health (EARTH) Study. All analyses were conducted using generalized linear mixed models with random intercepts, binomial distribution, and logit link function. Data are presented as marginal mean probabilities adjusted for calorie intake, age, BMI, race, dietary patterns, and folate and alcohol intake. *indicates that the p-value for the comparison vs. Q1 was <0.05.
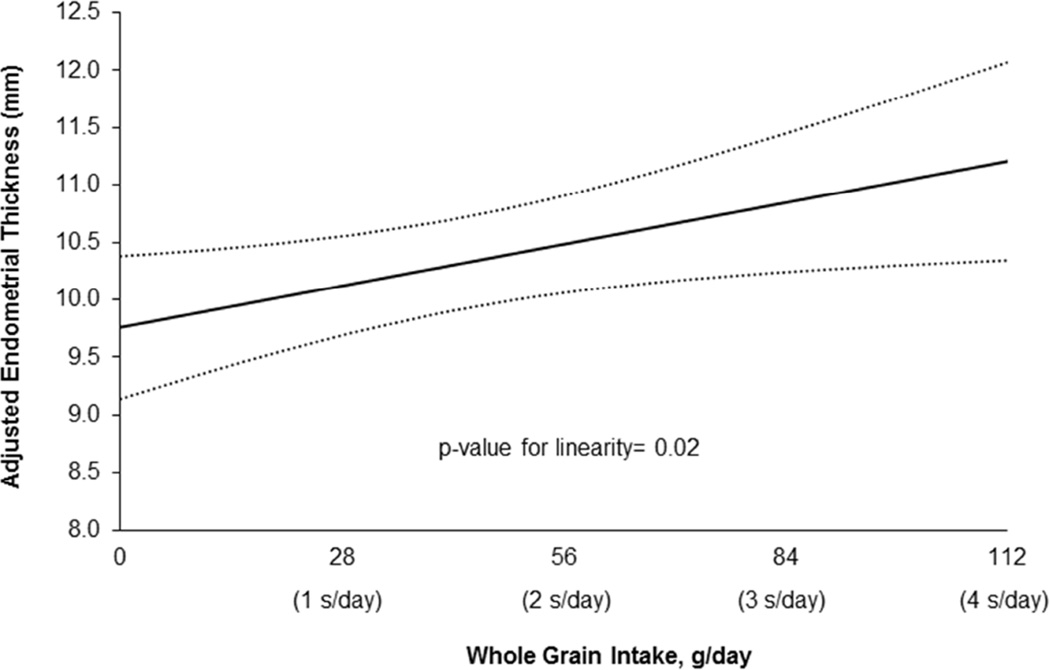
In regards to intermediate endpoints of IVF, whole grain intake was not associated with peak estradiol levels, total or mature oocyte yield, fertilization rate, number of fertilized embryos, or embryo quality (Table 2). However, whole grain intake was positively associated with endometrial thickness on the day of embryo transfer (Figure 2). A 28 g/day (~1 serving/day) increase in whole grain intake was associated with a 0.4 mm (95% CI 0.1, 0.7 mm) increase in endometrial thickness (p-value for linearity=0.02).
Table 2.
Associations between whole grain intake and early in vitro fertilization (IVF) outcomes in 255 women (352 fresh IVF cycles with egg retrieval) from the Environment and Reproductive Health Study.
| Adjusted Means (95% CI)a | Whole Grain Intake Quartile (range, g/day) | ||||
|---|---|---|---|---|---|
| Q1 (<21.4) | Q2 (21.5–34.2) | Q3 (34.3–52.4) | Q4 (>52.4) | P trend | |
| Estradiol Trigger Levels, pmol/L | 2026 (1790, 2262) | 2124 (1899, 2349) | 2214 (2000, 2429) | 2194 (1954, 2434) | 0.41 |
| Total Oocyte Yield, number | 10.6 (9.4, 12.0) | 10.8 (9.6, 12.1) | 11.8 (10.5, 13.1) | 11.8 (10.4, 13.3) | 0.26 |
| Mature Oocytes, number | 8.9 (7.9, 10.1) | 9.1 (8.1, 10.3) | 9.7 (8.7, 10.8) | 9.9 (8.8, 11.2) | 0.24 |
| Fertilization, proportion | 0.70 (0.64, a0.75) | 0.73 (0.68, 0.78) | 0.73 (0.68, 0.78) | 0.71 (0.65, 0.76) | 1.00 |
| Fertilized Embryos, number | 6.1 (5.3, 7.0) | 6.5 (5.7, 7.5) | 6.9 (6.1, 7.9) | 6.8 (5.9, 7.8) | 0.39 |
| ≥ 1 Best Quality Embryo, proportion | 0.55 (0.42, 0.67) | 0.60 (0.48, 0.72) | 0.67 (0.56, 0.77) | 0.52 (0.39, 0.65) | 0.94 |
All analyses were conducted using generalized linear mixed models with random intercepts, normal (for endometrial thickness and estradiol levels), Poisson (for oocyte counts) or binomial (for fertilization and embryo quality) distribution and identity (for endometrial thickness and E2 levels), log (for oocyte counts) or logit (for fertilization and embryo quality) link function. Data are presented as predicted marginal means adjusted for calorie intake, age, BMI, race, dietary patterns, and folate and alcohol intake.
Figure 2.
Predicted mean endometrial thickness with increasing whole grain intake in 255 women (352 fresh IVF cycles with egg retrieval) from the Environment and Reproductive Health Study. All analyses were conducted using mixed models with random intercepts, normal distribution, and identity link function. Data are presented as marginal means adjusted for calorie intake, age, BMI, race, dietary patterns, and folate and alcohol intake.
We also investigated whether total bran and germ intake (components of whole grains) were associated with clinical outcomes of IVF (Supplemental Table 2). There was a strong positive association between total bran intake and implantation, clinical pregnancy, and live birth (p-trend=0.003, 0.007, and 0.005, respectively) and the relationship was similar comparing bran in food and bran added to food. There were no associations between germ intake and implantation, clinical pregnancy, or live birth. Similarly, there was no significant relationship between total fiber intake and clinical outcomes of IVF. When we further adjusted models of whole grain intake and clinical outcomes of IVF for other nutrients strongly correlated with whole grain intake such as fiber, iron, magnesium, manganese, selenium, thiamin, riboflavin, and niacin the associations remained similar.
There was no modification of the effect of whole grains on clinical outcomes of IVF by age (<37, ≥37 years), BMI (<25, ≥25 kg/m2), initial infertility diagnosis (male, female, unexplained), or fertilization type (conventional, ICSI). Effect estimates were also similar when analyses were restricted to the first cycle per woman and to fresh IVF cycles (i.e. no egg donor or cryo-thawed cycles).
Discussion
In a prospective cohort of women undergoing in vitro fertilization in the United States we found that higher habitual whole grain consumption in the year prior to infertility treatment was associated with increased endometrial thickness on the day of embryo transfer and higher probability of implantation and live birth. The adjusted percentage of cycles resulting in live birth comparing women in the highest versus lowest quartile of whole grain intake was 53% versus 35% which corresponded to a difference in median whole grain intake of ~2 servings per day. Higher intake of bran, whether naturally occurring in foods or added to foods, appeared to be the component of whole grains that was driving this benefit as germ intake was not related to outcomes of IVF.
To our knowledge, no other studies have specifically evaluated the relationship between whole grain intake and outcomes of IVF. However, studies on dietary patterns which prioritize whole grain intake and outcomes of IVF present heterogeneous results. In a subfertile Dutch population, higher adherence to a “health conscious-low processed” dietary pattern which was rich in whole grains did not affect the probability of achieving pregnancy after IVF/ICSI (22). However, a cohort study of women from the Netherlands found that higher adherence to a Preconception Dietary Risk Score which prioritizes at least 4 servings of whole grains per day was associated with increased chance of pregnancy following IVF/ICSI treatment (23). While the results of this latter study supports our findings, the authors did not evaluate specific components of the Preconception Dietary Risk Score and thus it is hard to ascertain whether whole grain intake, one of the other 5 dietary components (i.e. monounsaturated and polyunsaturated fats, vegetables, fruits, meat, or fish), or a combination of these components was driving this association. Interestingly, a recent study from an US population of women found that higher urinary lignan concentrations (which are highly correlated with whole grain intake (24)) were associated with shorter times to pregnancy (25). While urinary lignan concentrations may or may not adequately reflect whole grain intake in that population, it is in line with our findings of a beneficial effect of whole grains on fertility and points to a possible mechanism of effect.
Our data suggests that whole grain intake has a beneficial effect on endometrial thickness, a marker of endometrial receptivity, which could be one mechanism through which whole grain consumption leads to higher probability of implantation and live birth. There are several biological mechanisms which could explain this beneficial effect. First, whole grains contain many antioxidants, including vitamins, trace minerals, nonnutrients (e.g. phenolic acids, lignans, and phytoestrogens), and antinutrients (e.g. phytic acid) which could work synergistically to dispose, scavenge, or suppress the formation of reactive oxygen species (8, 9). A pro-oxidant status has been shown to negatively influence fertilization, early embryo development, and implantation possibly through mechanisms involving mitochondrial alterations, peroxidative DNA damage, protein modifications, and lipid peroxidation (26, 27). Second, whole grains, and specifically the bran layer of grains, are concentrated sources of lignans, the principal source of dietary phytoestrogens in a typical Western diet. (28) As phytoestrogens are known to have estrogen-like activity through their ability to bind and antagonize the activity of estrogen receptors they could act directly on the endometrium to enhance endometrial thickness and receptivity (29). In support of this hypothesis is a randomized controlled trial of high dose phytoestrogens among women undergoing intrauterine insemination which found a positive effect of phytoestrogen supplementation on endometrial thickness (30). Finally, whole grain intake may exert its beneficial effects through its ability to regulate glucose and insulin metabolism (11). Hyperinsulinemia has been shown to increase ovarian androgen production (31) and decrease serum sex hormone-binding globulin concentrations (32). When insulin secretion is reduced by diet (33) or drugs (34), free testosterone concentrations decline. As testosterone and SHBG have been shown to have both direct and indirect effects on endometrial receptivity (35, 36), this could be one mechanism through which whole grain intake is impacting clinical outcomes of IVF. Alternatively, high levels of insulin could be directly inhibiting production of the endometrial stromal product IGF binding protein 1 (37), a biomarker of decidualization, which is needed for successful endometrial proliferation, development, and implantation (38). Unfortunately, at present, all of these mechanisms remain speculative as we were unable to assess urinary lignan concentrations or serum biomarkers of sex steroids, insulin resistance, or antioxidant capacity in our study.
The limitations of our study are worth considering in light of our novel findings. Despite use of a validated questionnaire, self-report of diet by FFQ is subject to measurement error. Due to the prospective nature of our study, however, any measurement error would likely be non-differential with respect to IVF outcomes and result in an attenuation of the observed associations. This suggests that the relation between whole grains and IVF outcomes might actually be stronger than that reported here. As this was an observational study, there also remains the possibility of residual confounding by lifestyle factors that were not measured or were poorly measured in our study. The generalizability of our results to women presenting at infertility clinics worldwide and to women conceiving naturally is also unclear. The median whole grain intake in our population was approximately 1.2 servings/day which is higher than the average in the US population (0.6 servings/day (39)) but less than that reported in a cohort of women undergoing IVF/ICSI in the Netherlands where ~40% of women reported consuming at least 4 servings per day (23).
Despite these limitations, the strengths of our study include the prospective design and the standardized assessment of a wide variety of participant characteristics including a comprehensive dietary assessment which increased our ability to adjust for confounding. By studying a fertility clinic population we were also able to utilize an efficient study design with sufficient power to investigate dietary influences on clinically relevant, yet previously unobservable, outcomes (e.g. endometrial thickness and implantation) in a potentially vulnerable sub-population.
In conclusion, higher whole grain intake was related to a higher probability of live birth among women undergoing IVF in the US. Analysis of intermediate IVF endpoints suggest that the higher probability of live birth may result from increased endometrial thickness on the day of embryo transfer and improved embryo receptivity manifested in higher probability of implantation. These results highlight the importance of dietary influences on fertility and suggest the need for additional research on the effects of whole grain intake on reproductive endpoints in women conceiving naturally and through infertility treatments.
Supplementary Material
Acknowledgments
Study funding: This work was supported by NIH Grants R01-ES009718 from NIEHS, P30-DK046200 and T32-DK007703-16 from NIDDK, and L50-HD085359 from the NICHD. The funding sources had no involvement in the study design, collection, analysis, or interpretation of the data; in the writing of the report; and in the decision to submit the article for publication.
The authors would like to especially thank the patients from the Massachusetts General Hospital Fertility Center who participated in the study and Jennifer Ford, Ramace Dadd, and Patricia Morey, Harvard T.H. Chan School of Public Health research staff.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
competing interest(s): The authors report no conflict of interest.
References
- 1.Tang G, Wang D, Long J, Yang F, Si L. Meta-analysis of the association between whole grain intake and coronary heart disease risk. Am J Cardiol. 2015;115:625–629. doi: 10.1016/j.amjcard.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 2.Jacobs DR, Jr, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: an expanded review and meta-analysis. Nutr Cancer. 1998;30:85–96. doi: 10.1080/01635589809514647. [DOI] [PubMed] [Google Scholar]
- 3.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28:845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr. 2003;78:920–927. doi: 10.1093/ajcn/78.5.920. [DOI] [PubMed] [Google Scholar]
- 5.Huang T, Xu M, Lee A, Cho S, Qi L. Consumption of whole grains and cereal fiber and total and cause-specific mortality: prospective analysis of 367,442 individuals. BMC Med. 2015;13:59. doi: 10.1186/s12916-015-0294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buyken AE, Goletzke J, Joslowski G, Felbick A, Cheng G, Herder C, et al. Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. Am J Clin Nutr. 2014;99:813–833. doi: 10.3945/ajcn.113.074252. [DOI] [PubMed] [Google Scholar]
- 7.Gaskins AJ, Mumford SL, Rovner AJ, Zhang C, Chen L, Wactawski-Wende J, et al. Whole grains are associated with serum concentrations of high sensitivity C-reactive protein among premenopausal women. J Nutr. 2010;140:1669–1676. doi: 10.3945/jn.110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomhoff R. Dietary antioxidants and cardiovascular disease. Curr Opin Lipidol. 2005;16:47–54. doi: 10.1097/00041433-200502000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Gutteridge JM, Halliwell B. Free radicals and antioxidants in the year 2000. A historical look to the future. Ann N Y Acad Sci. 2000;899:136–147. doi: 10.1111/j.1749-6632.2000.tb06182.x. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. doi: 10.1186/1477-7827-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liese AD, Roach AK, Sparks KC, Marquart L, D'Agostino RB, Jr, Mayer-Davis EJ. Whole-grain intake and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Am J Clin Nutr. 2003;78:965–971. doi: 10.1093/ajcn/78.5.965. [DOI] [PubMed] [Google Scholar]
- 12.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 13.Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. 2002;78:487–490. doi: 10.1016/s0015-0282(02)03247-8. [DOI] [PubMed] [Google Scholar]
- 14.Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 15.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 16.Agricultural Research Service U.S. Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 25. Nutrient Data Laboratory Home Page. 2012 [Google Scholar]
- 17.Salvini S, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner B, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 18.Franz M, Sampson L. Challenges in developing a whole grain database: definitions, methods and quantification. J Food Compos Anal. 2006;19:S38–S44. [Google Scholar]
- 19.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary Patterns and Semen Quality in Young Men. Hum Reprod. 2012;27(10):2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–221. [Google Scholar]
- 22.Vujkovic M, de Vries JH, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, et al. The preconception Mediterranean dietary pattern in couples undergoing in vitro fertilization/intracytoplasmic sperm injection treatment increases the chance of pregnancy. Fertil Steril. 2010;94:2096–2101. doi: 10.1016/j.fertnstert.2009.12.079. [DOI] [PubMed] [Google Scholar]
- 23.Twigt JM, Bolhuis ME, Steegers EA, Hammiche F, van Inzen WG, Laven JS, et al. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum Reprod. 2012;27:2526–2531. doi: 10.1093/humrep/des157. [DOI] [PubMed] [Google Scholar]
- 24.Adlercreutz H, Fotsis T, Bannwart C, Wahala K, Makela T, Brunow G, et al. Determination of urinary lignans and phytoestrogen metabolites, potential antiestrogens and anticarcinogens, in urine of women on various habitual diets. J Steroid Biochem. 1986;25:791–797. doi: 10.1016/0022-4731(86)90310-9. [DOI] [PubMed] [Google Scholar]
- 25.Mumford SL, Sundaram R, Schisterman EF, Sweeney AM, Barr DB, Rybak ME, et al. Higher urinary lignan concentrations in women but not men are positively associated with shorter time to pregnancy. J Nutr. 2014;144:352–358. doi: 10.3945/jn.113.184820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal A, Allamaneni SS. Role of free radicals in female reproductive diseases and assisted reproduction. Reprod Biomed Online. 2004;9:338–347. doi: 10.1016/s1472-6483(10)62151-7. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Kleijn MJ, van der Schouw YT, Wilson PW, Grobbee DE, Jacques PF. Dietary intake of phytoestrogens is associated with a favorable metabolic cardiovascular risk profile in postmenopausal U.S. women: the Framingham study. J Nutr. 2002;132:276–282. doi: 10.1093/jn/132.2.276. [DOI] [PubMed] [Google Scholar]
- 29.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 30.Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. High dose of phytoestrogens can reverse the antiestrogenic effects of clomiphene citrate on the endometrium in patients undergoing intrauterine insemination: a randomized trial. J Soc Gynecol Investig. 2004;11:323–328. doi: 10.1016/j.jsgi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Barbieri RL, Makris A, Randall RW, Daniels G, Kistner RW, Ryan KJ. Insulin stimulates androgen accumulation in incubations of ovarian stroma obtained from women with hyperandrogenism. J Clin Endocrinol Metab. 1986;62:904–910. doi: 10.1210/jcem-62-5-904. [DOI] [PubMed] [Google Scholar]
- 32.Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster RS, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding globulin levels in obese women with the polycystic ovary syndrome. J Clin Endocrinol Metab. 1991;72:83–89. doi: 10.1210/jcem-72-1-83. [DOI] [PubMed] [Google Scholar]
- 33.Kiddy DS, Hamilton-Fairley D, Seppala M, Koistinen R, James VH, Reed MJ, et al. Diet-induced changes in sex hormone binding globulin and free testosterone in women with normal or polycystic ovaries: correlation with serum insulin and insulin-like growth factor-I. Clin Endocrinol (Oxf) 1989;31:757–763. doi: 10.1111/j.1365-2265.1989.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 34.Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resistance, hyperandrogenemia, and systolic blood pressure, while facilitating normal menses and pregnancy. Metabolism. 1994;43:647–654. doi: 10.1016/0026-0495(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 35.Mokhtar HM, Giribabu N, Muniandy S, Salleh N. Testosterone decreases the expression of endometrial pinopode and L-selectin ligand (MECA-79) in adult female rats during uterine receptivity period. Int J Clin Exp Pathol. 2014;7:1967–1976. [PMC free article] [PubMed] [Google Scholar]
- 36.Misao R, Itoh N, Mori H, Fujimoto J, Tamaya T. Sex hormone-binding globulin mRNA levels in human uterine endometrium. Eur J Endocrinol. 1994;131:623–629. doi: 10.1530/eje.0.1310623. [DOI] [PubMed] [Google Scholar]
- 37.Lathi RB, Hess AP, Tulac S, Nayak NR, Conti M, Giudice LC. Dose-dependent insulin regulation of insulin-like growth factor binding protein-1 in human endometrial stromal cells is mediated by distinct signaling pathways. J Clin Endocrinol Metab. 2005;90:1599–1606. doi: 10.1210/jc.2004-1676. [DOI] [PubMed] [Google Scholar]
- 38.Nayak NR, Giudice LC. Comparative biology of the IGF system in endometrium, decidua, and placenta, and clinical implications for foetal growth and implantation disorders. Placenta. 2003;24:281–296. doi: 10.1053/plac.2002.0906. [DOI] [PubMed] [Google Scholar]
- 39.McGill CR, Fulgoni VL, 3rd, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001–2010. Nutrients. 2015;7:1119–1130. doi: 10.3390/nu7021119. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.