Abstract
Transcription by RNA polymerase II (Pol II) is required to produce mRNAs and some noncoding RNAs (ncRNAs) within mammalian cells. This coordinated process is precisely regulated by multiple factors, including many recently discovered ncRNAs. In this perspective, we will discuss newly identified ncRNAs that facilitate DNA looping, regulate transcription factor binding, mediate promoter-proximal pausing of Pol II, and/or interact with Pol II to modulate transcription. Moreover, we will discuss new roles for ncRNAs as well as a novel Pol II RNA-dependent RNA polymerase (RdRP) activity that regulates an ncRNA inhibitor of transcription. As the multifaceted nature of ncRNAs continues to be revealed, we believe that many more ncRNA species and functions will be discovered.
Keywords: RNA polymerase II, circular RNAs, enhancer RNAs, tethered RNAs, divergent RNAs
Graphical Abstract
Introduction
Investigators have been on a quest to understand how mammalian cells manage gene expression since the inception of the central dogma, but noncoding RNA (ncRNA) molecules were largely overlooked players in this process. Those ncRNAs known to be involved in gene expression were thought to participate indirectly, by acting as scaffolds upon which the functional proteins could assemble and carry out their activities. Thus, decades of research focused on protein regulators of gene expression and largely ignored the notion of regulatory ncRNAs. Over time, evidence emerged that the genome is widely transcribed to generate a diversity of ncRNAs; moreover, ncRNAs are not merely “transcriptional noise” but can carry out many unique functions within the cell. What started as a trickle of evidence became a steady stream, and thanks to the development of advanced RNA-related technologies, the last 5–10 years have produced a cascade of new classes of ncRNAs with novel functions. Although many recently identified ncRNAs have not been functionally characterized, the evidence is still overwhelming: ncRNAs govern many aspects of gene expression. These molecules range from small microRNAs (miRNAs; ~22 nt) to long noncoding RNAs (lncRNAs; > 200 nt), which can be many kilobases in length (as reviewed in [1–4]).
Several ncRNAs have been shown to directly or indirectly influence transcription by RNA polymerase II (Pol II), the multi-protein complex responsible for transcribing all of the mRNAs and some ncRNAs within mammalian cells. These ncRNAs have been shown to act both in cis to influence the expression of local alleles near the site of ncRNA transcription, as well as in trans to modulate the expression of distant genetic loci, oftentimes on different chromosomes. In order to regulate Pol II activity, ncRNAs act through a variety of mechanisms, which can be loosely separated into two functional categories. Perhaps the largest and most studied group are those that influence the state of chromatin. This category of ncRNAs controls either the methylation state of DNA or the post-translational modifications of histones to ultimately make the DNA more or less accessible for transcription (as reviewed in [5–7]). The prototypical example in this category is the X-inactive specific transcript (Xist) ncRNA, which acts to silence one of the X chromosomes in female mammalian cells. Xist coats the inactive X chromosome and recruits chromatin-modifying enzymes, rendering the chromosome transcriptionally inactive [6]. Although these chromatin-modifying ncRNAs have built much of our knowledge of ncRNA transcriptional regulators, they will not be covered in detail in this perspective, as they are reviewed elsewhere [5,6,8].
This perspective will focus on several newly identified mammalian ncRNAs that directly control Pol II transcription by interacting with the transcription machinery and/or impacting the assembly of transcriptional complexes at gene promoters. Specifically, the ncRNAs discussed here have been shown to facilitate DNA looping, modulate the promoter-proximal pausing step during early transcription, alter the recruitment of transcription factors (TFs), and/or interact with Pol II to regulate transcription. Moreover, we will describe recent studies unraveling novel roles for known ncRNAs, examine a new method used by Pol II to regulate an inhibitory ncRNA, and briefly speculate on where the growing field of ncRNA biology will lead investigators in the years to come.
Enhancer RNAs contribute to DNA looping and gene-specific activation
Enhancer elements are regions of DNA found upstream or downstream a gene’s transcription start site that bind TFs responsible for regulating transcription of the gene. The term enhancer RNA (eRNA) refers to a broad class of ncRNAs that are generated by Pol II-dependent transcription of enhancer DNA elements. eRNAs are categorized by the methylation state of the enhancer DNA from which they are derived, the direction of transcription across the enhancer element (i.e. unidirectional or bidirectional), and the processing of the eRNA (i.e. whether polyadenylated, spliced, or capped). Thousands of putative eRNAs have been identified and are indicative of actively transcribed regions of the genome, yet the cellular functions of only a handful of eRNAs have been characterized (as reviewed in [9]). Like many ncRNAs, some eRNAs act in cis to regulate chromatin modifications near the enhancer element from which the eRNA was transcribed, hence altering the accessibility of nearby mRNA loci and regulating Pol II transcription in this region [10]. However, new mechanisms of eRNA regulation have recently been identified, which do not involve modulating the state of chromatin.
Looping of DNA enables the Pol II transcriptional complex at core promoters to interact and communicate with factors bound at distant enhancer sequences. DNA looping is facilitated by the multi-protein complex Mediator, which coordinates interactions between enhancer-bound transcriptional regulatory proteins and Pol II (as reviewed in [11]). Cohesin also associates with Mediator and aids in gene looping by acting as a ring to connect two regions of DNA, ultimately contributing to transcriptional activation [12,13]. A subset of eRNAs called activating RNAs (ncRNA-a; ~800 nt) were initially shown to regulate the expression of nearby genes in several human cell lines, suggesting a model in which ncRNA-a molecules regulate transcription in cis [14]. More recently, several ncRNA-a species were shown to interact with the transcriptional machinery within human cells to facilitate promoter-enhancer looping (Figure 1A; [15]).
Figure 1.
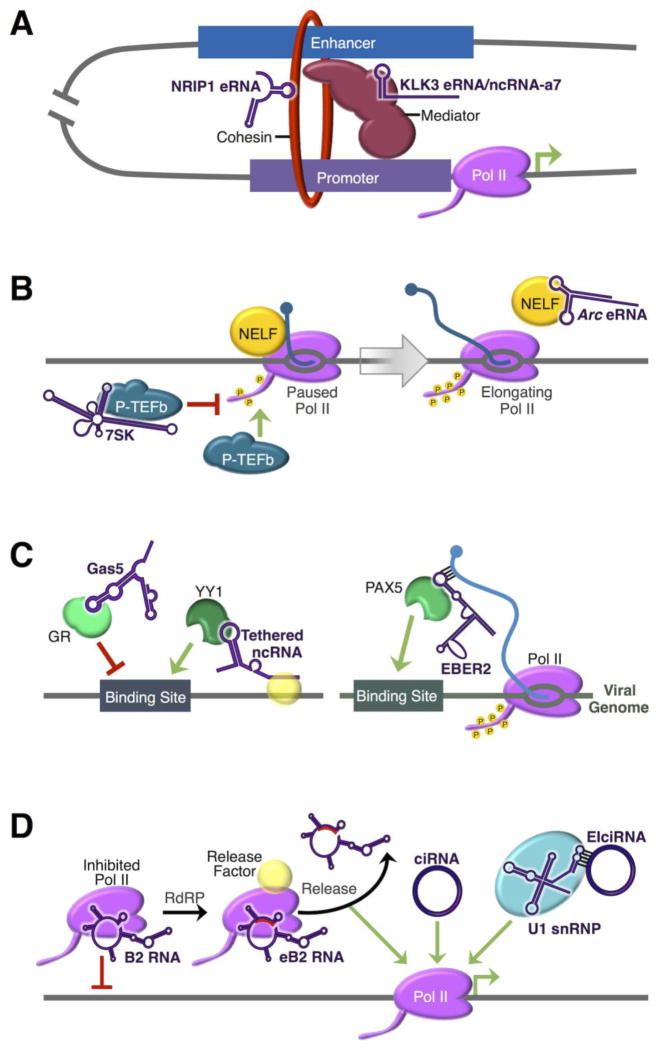
Noncoding RNAs (ncRNAs) influence multiple steps in mammalian RNA polymerase II (Pol II) transcription. A) Enhancer RNAs (eRNAs) facilitate gene looping in activate transcription of nearby genes. eRNAs enhance gene looping by interacting with Mediator, such as Kallikrein-related peptidase 3 (KLK3) eRNA and activating ncRNA-7 (ncRNA-a7). Alternatively, eRNAs like NRIP1 eRNA can also interact with cohesin. B) ncRNAs regulate the transition of promoter-proximal paused Pol II to elongating Pol II. 7SK RNA sequesters the positive transcription elongation factor b (P-TEFb) to inhibit phosphorylation of the Pol II C-terminal domain (CTD; phosphorylation is indicated by yellow circles) and subsequent transition into the elongation phase of transcription. The negative elongation factor (NELF) is recruited to Pol II, possibly through interactions with nascent RNA, and inhibits Pol II elongation. The Activity-regulated cytoskeletal protein (Arc) eRNA acts as an RNA decoy to bind NELF and prevent NELF-mediated Pol II pausing. C) ncRNAs influence the association of transcription factors (TFs) with DNA. Gas5 ncRNA binds to the glucocorticoid receptor (GR) DNA-binding domain to block GR from interacting with DNA. Tethered ncRNAs, possibly bound to Pol II, interact with Yin-Yang 1 (YY1) and facilitate recruitment to its DNA-binding site. The viral EBV-encoded RNA 2 (EBER2) ncRNA interacts with nascent viral RNA to recruit PAX5 to viral chromatin. D) ncRNAs directly interact with Pol II to inhibit or enhance transcription. B2 RNA prevents Pol II from making important contacts with DNA, inhibiting transcription. Pol II RNA-dependent RNA polymerase (RdRP) activity produces extended B2 RNA (eB2 RNA), which is then released by an unknown cellular factor, possibly allowing freed Pol II to resume transcription. Circular RNAs (circRNAs), including circular intronic RNAs (ciRNAs) and exon intron circular RNAs (EIciRNAs), associate with Pol II to facilitate transcription of their parental mRNAs. EIciRNAs interact with Pol II through the U1 small nuclear ribonucleoprotein (snRNP) complex to enhance transcription.
Chromosome conformation capture assays detected DNA looping between the ncRNA-a loci and their target mRNA genes. Specifically, ncRNA-a7 (also known as LINC00651) increased transcription of proximal genes, showed a specific association with the Mediator complex, and facilitated looping between the enhancer locus and the core promoters of neighboring genes (Figure 1A). Importantly, knockdown of the ncRNA-a or Mediator subunits reduced the chromatin looping and decreased expression of nearby mRNAs [15]. These experiments support a model in which ncRNA-a interact with Mediator to promote gene looping and transcription of nearby genes. Similarly, eRNA transcribed from the androgen receptor-responsive enhancer of the Kallikrein-related peptidase 3 (KLK3) gene was shown to cooperate with Med1, a member of the Mediator complex, to facilitate chromosomal looping in human prostate cancer cells. These investigators proposed that eRNAs may tether to their own transcriptional modules, acting as a scaffold to enhance transcription; however, this mechanism was not elucidated [16]. Together, these results suggest that the Mediator complex and eRNAs interact to facilitate DNA looping between enhancers and core promoters, thereby activating transcription.
eRNAs produced from several estrogen-responsive enhancers may also facilitate promoter-enhancer looping through interactions with cohesin (Figure 1A; [17]). Knockdown of eRNAs transcribed from the NRIP1 and GREB1 enhancers in human breast cancer cells decreased promoter-enhancer gene looping at their corresponding loci, as measured by three-dimensional DNA selection and ligation, a technique similar to chromosome conformation capture. Further experiments suggested that eRNAs interact with cohesin near enhancer elements, as chromatin immunoprecipitation followed by deep sequencing (ChIP-seq) localized cohesin to eRNA-producing enhancers, and RNA-immunoprecipitation experiments showed association between eRNAs and the cohesin complex. Knockdown of NRIP1 eRNA or cohesin reduced DNA looping, cohesin localization, and transcription of the NRIP1 mRNA, providing evidence that eRNAs can facilitate DNA looping and increase transcription of nearby genes through interactions with cohesin [17]. Together, these studies identify a new function for eRNAs; however, the underlying mechanism(s) by which eRNAs act to promote enhancer-promoter looping via interactions with cohesin, Mediator, and/or their own transcriptional complex is still unclear.
Progression of Pol II transcription is regulated by ncRNAs
During early transcription, Pol II undergoes a promoter-proximal pausing event after synthesis of ~30–100 nt of RNA, which is tightly regulated by several factors. The positive transcription elongation factor b (P-TEFb) promotes Pol II elongation by phosphorylating the C-terminal domain (CTD) of Pol II to alleviate pausing and facilitate productive RNA synthesis (as reviewed in [18]). The well-characterized 7SK small nuclear RNA (snRNA) binds to and sequesters P-TEFb, thereby inhibiting the ability of P-TEFb to phosphorylate the Pol II CTD and preventing the transition from the paused to the elongation phase of transcription (Figure 1B). The trans-acting regulation of Pol II transcription by 7SK snRNA is discussed in the following reviews [19,20].
Recent studies have shown that Pol II transcriptional progression can also be regulated by eRNAs [21]. Depletion of an eRNA synthesized from the enhancer of the Activity-regulated cytoskeletal protein (Arc) gene in mouse neurons resulted in decreased Arc mRNA production, suggesting that Arc eRNA activates transcription [21]. Further experiments revealed Arc eRNA interacted with the negative elongation factor (NELF), which binds Pol II to induce promoter-proximal pausing (Figure 1B). Knockdown of Arc eRNA increased NELF association with the Arc promoter and decreased Arc mRNA expression, suggesting Arc eRNA facilitated NELF release [21]. Evidence has suggested that NELF is recruited to early transcribing Pol II by interacting with the Pol II-bound nascent RNA [22–24]. Thus, these data support a model in which Arc eRNA acts as a decoy RNA to release NELF from the paused Pol II complex [21]. This example illustrates that additional regulatory mechanisms are likely to be identified as eRNAs are further investigated.
ncRNAs alter transcription factor recruitment
It is not a new concept that TFs, which bind to DNA in a sequence-specific manner to regulate transcription, can also bind to RNA [25]. A characterized example of this regulatory mechanism is the Gas5 ncRNA binding to the glucocorticoid receptor (GR) TF (Figure 1C; [26]). Acting as a decoy RNA, Gas5 ncRNA binds to the DNA-binding domain of GR to prevent it from interacting with its DNA-binding site [26]. As such, Gas5 is a transcriptional inhibitor of GR-activated genes. In contrast, a recent study revealed that ncRNAs can bind a TF outside of its DNA-binding domain to enhance Pol II transcription [27].
At many active promoters, Pol II transcribes in the opposite direction of the canonical transcription start site, producing divergent ncRNAs transcribed from core promoter DNA elements [28,29]. Until recently, most data supported a model in which these divergent ncRNAs were terminated early and targeted for degradation within the cell [30,31]. However, a new study found that promoter-proximal divergent ncRNAs, as well as eRNAs, bind to the TF Yin-Yang 1 (YY1), resulting in transcriptional activation (Figure 1C; [27]). Using a combination of ChIP-seq and CLIP-seq (cross-linking and immunoprecipitation followed by deep sequencing) in mouse embryonic stem cells, YY1 was shown to bind DNA, as well as several ncRNAs, at enhancer and promoter elements. Further in vitro experiments demonstrated that recombinant YY1 binds DNA via its zinc-finger domain but binds RNA through a different region near its N-terminus, suggesting regulatory RNAs may bind YY1 to recruit the TF to sites of transcription. These investigators proposed eRNAs and divergent ncRNAs may remain bound to Pol II and tethered to DNA, stabilizing YY1 binding, and increasing transcription. To test this hypothesis, several experiments were performed to inhibit transcription of tethered RNAs, degrade the tethered RNAs with ribonuclease A, or prevent tethered RNA degradation by depleting the exosome; these experiments provided evidence that promoter- and enhancer-derived ncRNAs remain tethered near their sites of transcription where they recruit YY1 to promoters and enhancers and increase transcription [27].
Perhaps most notably, these investigators tested their model using a modified CRISPR/Cas9/fusion RNA system within cells in order to artificially tether a promoter-derived ncRNA near several YY1 binding sites in an effort to determine if this ncRNA can alter YY1 binding. This system was comprised of an inactive Cas9 and an RNA construct containing one of six different guide RNAs corresponding to six different enhancer loci, each in the vicinity of a YY1 binding site. The guideRNA was fused to a 60-nt sequence derived from the Arid1a promoter ncRNA, which was shown to bind YY1 in vitro. Using this system, investigators demonstrated that the tethered Arid1a promoter ncRNA increased the binding of YY1 by ChIP-qPCR in mouse embryonic stem cells. Although more experiments are needed to investigate the breadth of divergent ncRNA and eRNA function, as well as their association with Pol II to “tether” them to DNA, this study provides evidence that a tethered ncRNA can recruit a TF to specific sites in the genome [27].
Although not mammalian in origin, a recent study has identified a viral ncRNA with the ability to mobilize a mammalian host cell TF to viral chromatin [32]. In these experiments, the Epstein-Barr virus-encoded ncRNA, EBV-encoded RNA 2 (EBER2; 173 nt), was shown to associate with the B cell TF PAX5 and recruit PAX5 to the viral DNA in infected human cells (Figure 1C). Interestingly, this recruitment required RNA-RNA interactions between the EBER2 and nascent viral transcripts, likely tethered to a transcribing Pol II. The interaction between EBER2 and nascent viral RNA allowed the EBER2-associated PAX5 to make important contacts with viral DNA near the site of Pol II transcription [32]. The observation that a TF can be recruited to its DNA-binding site through RNA-RNA interactions between ncRNA and nascent RNA is unique. Furthermore, it is possible that mammalian examples of this regulatory mechanism exist but have not yet been observed.
Circular RNAs activate transcription of their parental mRNA genes
Circular RNAs (circRNAs) are a newly recognized class of mammalian ncRNAs with the ability to regulate Pol II transcription. Tens of thousands of these covalently closed RNA loops have been detected within mammalian cells; they are abundant, conserved, and stable, suggesting circRNAs have important, yet generally uncharacterized, functions [33,34]. Several models have been proposed for how circRNAs are produced within the mammalian cell; all circRNAs appear to be the product of various splicing events resulting in different types of closed RNA circles (as reviewed in [35]). While some circRNAs may still serve as templates for translation [36], it is thought the majority of circRNAs are not translated, as representative cellular circRNAs are not bound to ribosomes [33]. There are at least three types of circRNAs with distinct regulatory functions in the mammalian cell: circular exonic RNAs, circular intronic RNAs (ciRNAs), and exon-intron circular RNAs (EIciRNAs). The current understanding of circular exonic RNAs is that they primarily reside in the cytoplasm, where they have been found to act as miRNA sponges, while ciRNAs and EIciRNAs appear to primarily mediate their effects in the nucleus by modulating transcription, as described below [33,34,37,38].
A class of abundant circRNAs derived entirely from introns (ciRNAs) were identified in HeLa cells and human embryonic stem cells (Figure 1D; [38]). Further investigation showed ciRNAs are retained in the nucleus, and several localized to their own sites of transcription as determined by double fluorescence in situ hybridization (FISH) colocalization experiments using probes that target the ciRNA or the adjacent DNA region. Antisense oligos directed against ciRNA-producing introns reduced the parent mRNA levels, while antisense oligos against other introns within the parent mRNA did not. In addition, nuclear run-on experiments demonstrated that depletion of ciRNAs decreased transcription of the parental mRNA, and Pol II immunoprecipitation experiments showed several ciRNAs were associated with Pol II [38]. These results suggest ciRNAs act in cis to increase transcription of the ciRNA parent mRNA by associating with the Pol II complex through a yet unknown mechanism.
A different class of circRNAs has also been found to regulate transcription of their parental mRNAs. RNAs associated with Pol II in human cells were identified using CLIP-seq [39]. Fifteen abundant circRNAs that co-immunoprecipitated with Pol II were validated in follow-up RNA-immunoprecipitation experiments, with some demonstrating cell-specific expression in HeLa and HEK293 cell lines. Analysis of two circRNAs, circEIF3J and circPAIP2, demonstrated that each contained exons and introns (EIciRNAs) from the parental genes EIF3J and PAIP2, respectively, and likely formed as a result of complementary sequence-pairing within introns. These two EIciRNAs colocalized with their parental loci by FISH, suggesting they influence the expression of their parental genes in cis. Further experiments showed each of these EIciRNAs interacted with Pol II to increase the transcription of their respective parental gene (Figure 1D), and this effect was mediated through contacts with the spliceosomal U1 snRNP, as discussed in greater detail below. These investigators noted that their initial CLIP-seq method was not optimized for identifying circRNAs [39]; therefore, it is possible many more circRNAs will be found to interact with and regulate Pol II.
As a key player in the first step of splicing, the U1 small nuclear ribonucleoprotein (snRNP) complex includes the U1 snRNA and the U1A and U1C proteins. The U1 snRNP interacts with the 5′ splice site of introns to enable spliceosome assembly and has also been implicated in polyA site selection, exemplifying how one ncRNA can play several roles within the cell [40–42]. U1 snRNP continues to surprise investigators, as it has recently been shown to interact with EIciRNAs and Pol II to enhance transcription [39].
During the functional characterization of circEIF3J and circPAIP2 (described above), investigators found that the 5′ splice site of the retained intron within circEIF3J and circPAIP2 remained bound to U1 snRNP. Data from pulldown experiments, FISH colocalization, and antisense experiments, support a model in which U1 snRNP interacts with an EIciRNA to form a complex with Pol II that is present at promoter DNA and functions to increase transcription of the EIciRNA parental gene (Figure 1D). Moreover, investigators used antisense morpholino oligonucleotides to sterically block the RNA-RNA interactions between U1 snRNA and the intron within circEIF3J or circPAIP2, which resulted in decreased Pol II association with the promoter of parental genes and reduced transcription in nuclear run-on experiments [39]. These studies suggest that U1 snRNA acts as a lynchpin for the EIciRNA-mediated enhancement of Pol II transcription. Interestingly, EIciRNAs and ciRNAs both retain introns, which may associate with U1 snRNP, presenting the possibility that additional ciRNAs and EIciRNAs may activate transcription of their own parental mRNAs through a U1 snRNP-mediated mechanism.
Pol II can regulate the function of ncRNAs
As described here and elsewhere, ncRNAs can bind directly to Pol II and/or other members of the transcriptional machinery to modulate transcription. Recent evidence suggests Pol II has the capacity to override the inhibitory effects of one such ncRNA by modifying the ncRNA [43]. The mouse short interspersed element (SINE)-derived B2 RNA directly binds to Pol II and prevents transcription initiation in trans by inhibiting important contacts between Pol II and promoter DNA [44]. Biochemical experiments revealed that Pol II can use a unique RNA-dependent RNA polymerase (RdRP) activity to add an internally templated, 18-nt extension to the 3′ end of B2 RNA, producing an extended B2 RNA (eB2 RNA; Figure 1D). As a result of this extension, eB2 RNA is a less potent inhibitor of Pol II transcription and less stable within cells. Moreover, only after extension can an unidentified cellular factor release eB2 RNA from Pol II, resulting in subsequent eB2 RNA degradation within mouse cells [43]. This mechanism of RdRP extension, release, and degradation may allow the once-inhibited Pol II to resume its task of transcribing genes. As ncRNA transcriptional regulators continue to be characterized, we anticipate other mechanisms that control ncRNA function will be discovered—perhaps even other targets of mammalian Pol II RdRP activity.
Conclusions and thoughts about the future of ncRNA transcriptional regulators
The ncRNAs described here have revealed new and unanticipated mechanisms of Pol II transcriptional regulation. We expect more novel mechanisms, as well as common regulatory themes, to emerge in the years to come. Indeed, modern sequencing and bioinformatics have not left us wanting of ncRNAs to study, with ncRNA discovery currently outpacing functional characterization [3,4]. For example, thousands of chromatin-tethered ncRNAs have recently been identified near actively transcribing genes in human cells, the majority of which are tethered by Pol II [45]. It is possible that members of these genome-tethered ncRNAs can “trap” TFs near their active promoters and/or act as scaffolds for Mediator, cohesin, or other regulators. Additionally, a catalog of divergently transcribed ncRNAs has been recently identified in human and murine embryonic stem cells that appear to control expression of nearby mRNA molecules [46]. The novel functions and sheer numbers of mammalian ncRNAs continue to increase, hence it is likely we have only just begun to realize their biological impact. Although this perspective focuses on ncRNA regulators of mammalian Pol II transcription, there is evidence that ncRNAs control transcription by RNA polymerase I and mitochondrial RNA polymerase [47,48]. Overall, ncRNA regulation of many cellular processes, including transcription, is sure to surprise and intrigue investigators in the years to come.
Highlights.
RNA polymerase II (Pol II) transcription is regulated by noncoding RNAs (ncRNAs).
ncRNAs facilitate DNA looping and transcription factor recruitment.
Promoter-proximal pausing by Pol II is influenced by ncRNAs.
ncRNAs interact with Pol II to activate or inhibit transcription.
Pol II uses its RNA-dependent RNA polymerase (RdRP) activity to regulate an ncRNA.
Acknowledgments
This work was supported by a Public Health Service Grant (R01 GM068414) from the National Institute of General Medical Sciences.
Abbreviations
- Arc
Activity-regulated cytoskeletal protein
- ChIP-seq
chromatin immunoprecipitation followed by deep sequencing
- circRNA
circular RNA
- ciRNA
circular intronic RNA
- CLIP-seq
cross-linking and immunoprecipitation followed by deep sequencing
- eB2 RNA
extended B2 RNA
- EBER2
EBV-encoded RNA 2
- EIciRNA
exon-intron circular RNA
- eRNA
enhancer RNA
- FISH
fluorescence in situ hybridization
- GR
glucocorticoid receptor
- KLK3
Kallikrein-related peptidase 3
- lncRNA
long noncoding RNA
- miRNA
microRNA
- ncRNA
noncoding RNA
- ncRNA-a
activating noncoding RNA
- NELF
negative elongation factor
- P-TEFb
positive transcription elongation factor b
- Pol II
RNA polymerase II
- RdRP
RNA-dependent RNA polymerase
- SINE
short interspersed element
- snRNA
small nuclear RNA
- snRNP
small nuclear ribonucleoprotein
- TF
transcription factor
- Xist
X-inactive specific transcript
- YY1
Yin-Yang 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–24. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 2.Yang L, Froberg JE, Lee JT. Long noncoding RNAs: fresh perspectives into the RNA world. Trends Biochem Sci. 2014;39:35–43. doi: 10.1016/j.tibs.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark MB, Choudhary A, Smith MA, Taft RJ, Mattick JS. The dark matter rises: the expanding world of regulatory RNAs. Essays Biochem. 2013;54:1–16. doi: 10.1042/bse0540001. [DOI] [PubMed] [Google Scholar]
- 4.Cech TR, Steitz JA. The Noncoding RNA Revolution— Trashing Old Rules to Forge New Ones. Cell. 2014;157:77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20:300–7. doi: 10.1038/nsmb.2480. [DOI] [PubMed] [Google Scholar]
- 6.Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152:1308–23. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann JH, Spector DL. Long non-coding RNAs: modulators of nuclear structure and function. Curr Opin Cell Biol. 2014;26:10–18. doi: 10.1016/j.ceb.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–93. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 9.Lam MTY, Li W, Rosenfeld MG, Glass CK. Enhancer RNAs and regulated transcriptional programs. Trends Biochem Sci. 2014;39:170–82. doi: 10.1016/j.tibs.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mousavi K, Zare H, Dell’orso S, Grontved L, Gutierrez-Cruz G, Derfoul A, et al. eRNAs promote transcription by establishing chromatin accessibility at defined genomic loci. Mol Cell. 2013;51:606–17. doi: 10.1016/j.molcel.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen BL, Taatjes DJ. The Mediator complex: a central integrator of transcription. Nat Rev Mol Cell Biol. 2015;16:155–66. doi: 10.1038/nrm3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kagey MH, Newman JJ, Bilodeau S, Zhan Y, Orlando DA, van Berkum NL, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467:430–5. doi: 10.1038/nature09380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowen JM, Fan ZP, Hnisz D, Ren G, Abraham BJ, Zhang LN, et al. Control of Cell Identity Genes Occurs in Insulated Neighborhoods in Mammalian Chromosomes. Cell. 2014;159:374–87. doi: 10.1016/j.cell.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ørom UA, Derrien T, Beringer M, Gumireddy K, Gardini A, Bussotti G, et al. Long noncoding RNAs with enhancer-like function in human cells. Cell. 2010;143:46–58. doi: 10.1016/j.cell.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai F, Orom UA, Cesaroni M, Beringer M, Taatjes DJ, Blobel GA, et al. Activating RNAs associate with Mediator to enhance chromatin architecture and transcription. Nature. 2013;494:497–501. doi: 10.1038/nature11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh C, Fei T, Chen Y, Li T, Gao Y, Wang X, et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc Natl Acad Sci U S A. 2014;111:7319–24. doi: 10.1073/pnas.1324151111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Notani D, Ma Q, Tanasa B, Nunez E, Chen AY, et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 2013;498:516–20. doi: 10.1038/nature12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adelman K, Lis JT. Promoter-proximal pausing of RNA polymerase II: emerging roles in metazoans. Nat Rev Genet. 2012;13:720–31. doi: 10.1038/nrg3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterlin BM, Brogie JE, Price DH. 7SK snRNA: a noncoding RNA that plays a major role in regulating eukaryotic transcription. Wiley Interdiscip Rev RNA. 2012;3:92–103. doi: 10.1002/wrna.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diribarne G, Bensaude O. 7SK RNA, a non-coding RNA regulating P-TEFb, a general transcription factor. RNA Biol. 2009;6:122–8. doi: 10.4161/rna.6.2.8115. [DOI] [PubMed] [Google Scholar]
- 21.Schaukowitch K, Joo J, Liu X, Watts JK, Martinez C, Kim T. Enhancer RNA Facilitates NELF Release from Immediate Early Genes. Mol Cell. 2014;56:29–42. doi: 10.1016/j.molcel.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Missra A, Gilmour DS. Interactions between DSIF (DRB sensitivity inducing factor), NELF (negative elongation factor), and the Drosophila RNA polymerase II transcription elongation complex. Proc Natl Acad Sci. 2010;107:11301–06. doi: 10.1073/pnas.1000681107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao JN, Schweimer K, Wenzel S, Wöhrl BM, Rösch P. NELF-E RRM undergoes major structural changes in flexible protein regions on target RNA binding. Biochemistry. 2008;47:3756–61. doi: 10.1021/bi702429m. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi Y, Inukai N, Narita T, Wada T, Handa H. Evidence that Negative Elongation Factor Represses Transcription Elongation through Binding to a DRB Sensitivity-Inducing Factor/RNA Polymerase II Complex and RNA. Mol Cell Biol. 2002;22:2918–27. doi: 10.1128/MCB.22.9.2918-2927.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cassiday LA, Maher LJ. Having it both ways: transcription factors that bind DNA and RNA. Nucleic Acids Res. 2002;30:4118–26. doi: 10.1093/nar/gkf512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kino T, Hurt DE, Ichijo T, Nader N, Chrousos GP. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sigova AA, Abraham BJ, Ji X, Molinie B, Hannett NM, Guo YE, et al. Transcription factor trapping by RNA in gene regulatory elements. Science. 2015;350:978–81. doi: 10.1126/science.aad3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Core LJ, Waterfall JJ, Lis JT. Nascent RNA sequencing reveals widespread pausing and divergent initiation at human promoters. Science. 2008;322:1845–8. doi: 10.1126/science.1162228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seila AC, Calabrese JM, Levine SS, Yeo GW, Rahl PB, Flynn RA, et al. Divergent transcription from active promoters. Science. 2008;322:1849–51. doi: 10.1126/science.1162253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almada AE, Wu X, Kriz AJ, Burge CB, Sharp PA. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature. 2013;499:360–3. doi: 10.1038/nature12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flynn RA, Almada AE, Zamudio JR, Sharp PA. Antisense RNA polymerase II divergent transcripts are P-TEFb dependent and substrates for the RNA exosome. Proc Natl Acad Sci U S A. 2011;108:10460–5. doi: 10.1073/pnas.1106630108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee N, Moss WN, Yario TA, Steitz JA. EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell. 2015;160:607–18. doi: 10.1016/j.cell.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, et al. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2012;19:141–57. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–8. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 35.Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, et al. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015;365:141–8. doi: 10.1016/j.canlet.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang Z. Efficient backsplicing produces translatable circular mRNAs. RNA. 2015;21:172–9. doi: 10.1261/rna.048272.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y, Zhang X, Chen T, Xiang J, Yin Q, Xing Y, et al. Circular Intronic Long Noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–64. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 40.Berg MG, Singh LN, Younis I, Liu Q, Pinto AM, Kaida D, et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell. 2012;150:53–64. doi: 10.1016/j.cell.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merkhofer EC, Johnson TL. U1 snRNA rewrites the “script”. Cell. 2012;150:9–11. doi: 10.1016/j.cell.2012.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiluttini B, Gu B, Belagal P, Smirnova AS, Nguyen VT, Hébert C, et al. Splicing-independent recruitment of U1 snRNP to a transcription unit in living cells. J Cell Sci. 2010;123:2085–93. doi: 10.1242/jcs.061358. [DOI] [PubMed] [Google Scholar]
- 43.Wagner SD, Yakovchuk P, Gilman B, Ponicsan SL, Drullinger LF, Kugel JF, et al. RNA polymerase II acts as an RNA-dependent RNA polymerase to extend and destabilize a non-coding RNA. EMBO J. 2013;32:781–90. doi: 10.1038/emboj.2013.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakovchuk P, Goodrich JA, Kugel JF. B2 RNA and Alu RNA repress transcription by disrupting contacts between RNA polymerase II and promoter DNA within assembled complexes. Proc Natl Acad Sci U S A. 2009;106:5569–74. doi: 10.1073/pnas.0810738106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Werner MS, Ruthenburg AJ. Nuclear Fractionation Reveals Thousands of Chromatin-Tethered Noncoding RNAs Adjacent to Active Genes. Cell Rep. 2015;12:1089–98. doi: 10.1016/j.celrep.2015.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sigova AA, Mullen AC, Molinie B, Gupta S, Orlando DA, Guenther MG, et al. Divergent transcription of long noncoding RNA/mRNA gene pairs in embryonic stem cells. Proc Natl Acad Sci U S A. 2013;110:2876–81. doi: 10.1073/pnas.1221904110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grummt I, Längst G. Epigenetic control of RNA polymerase I transcription in mammalian cells. Biochim Biophys Acta. 2013;1829:393–404. doi: 10.1016/j.bbagrm.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 48.Dietrich A, Wallet C, Khalid Iqbal R, Gualberto JM, Lotfi F. Organellar non-coding RNAs: Emerging regulation mechanisms. Biochimie. 2015;117:48–62. doi: 10.1016/j.biochi.2015.06.027. [DOI] [PubMed] [Google Scholar]