Abstract
Background: The effectiveness of inhaled aerosolized antibiotics is limited by poor ventilation of infected airways. Pulmonary delivery of antibiotics emulsified within liquid perfluorocarbon [antibacterial perfluorocarbon ventilation (APV)] may solve this problem through better airway penetration and improved spatial uniformity. However, little work has been done to explore emulsion formulation and the corresponding effects on drug delivery during APV. This study investigated the effects of emulsion formulation on emulsion stability and the pharmacokinetics of antibiotic delivery via APV.
Methods: Gravity-driven phase separation was examined in vitro by measuring emulsion tobramycin concentrations at varying heights within a column of emulsion over 4 hours for varying values of fluorosurfactant concentration (Cfs = 5–48 mg/mL H2O). Serum and pulmonary tobramycin concentrations in rats were then evaluated following pulmonary tobramycin delivery via aerosol or APV utilizing sufficiently stable emulsions of varying aqueous volume percentage (Vaq = 1%–5%), aqueous tobramycin concentration (Ct = 20–100 mg/mL), and Cfs (15 and 48 mg/mL H2O).
Results: In vitro assessment showed sufficient spatial and temporal uniformity of tobramycin dispersion within emulsion for Cfs ≥15 mg/mL H2O, while lower Cfs values showed insufficient emulsification even immediately following preparation. APV with stable emulsion formulations resulted in 5–22 times greater pulmonary tobramycin concentrations at 4 hours post-delivery relative to aerosolized delivery. Concentrations increased with emulsion formulations utilizing increased Vaq (with decreased Ct) and, to a lesser extent, increased Cfs.
Conclusions: The emulsion stability necessary for effective delivery is retained at Cfs values as low as 15 mg/mL H2O. Additionally, the pulmonary retention of antibiotic delivered via APV is significantly greater than that of aerosolized delivery and can be most effectively increased by increasing Vaq and decreasing Ct. APV has been further proven as an effective means of pulmonary drug delivery with the potential to significantly improve antibiotic therapy for lung disease patients.
Key words: : emulsion, perfluorocarbon, respiratory infection, ventilation
Introduction
Chronic bacterial respiratory infections are the primary cause of morbidity and mortality in patients with cystic fibrosis (CF),(1,2) as well as the main cause of exacerbation in chronic obstructive pulmonary disease (COPD).(3,4) Conditions such as CF and COPD feature changes in mucus rheology and mucociliary clearance that can make bacterial elimination exceedingly difficult.(5,6) Antibacterial perfluorocarbon ventilation (APV) has been previously presented as a possible means of pulmonary antibiotic delivery in which the lungs are filled with aqueous antibiotics emulsified in perfluorocarbon (PFC) (i.e., water-in-PFC).(7)
During APV, the lungs are either partially filled with emulsion and ventilated with gas (i.e., partial APV) or fully filled and ventilated with emulsion (i.e., total APV). APV is proposed as a short-term (≤ 2 hours) adjunct therapy to systemic or inhaled antibiotics that could wash infected mucus from the lungs while delivering antibiotic to poorly ventilated regions. Mucus removal during ventilation with PFCs has been previously noted(8,9) and occurs due to both buoyant forces and flow-induced shear stress acting on the mucus.
The water-in-PFC emulsions used during APV consist of small droplets of aqueous antibiotic (diameter previously shown to be 1–4 μm)(7) suspended within a continuous PFC phase (≥ 95% by volume). Due to the immiscibility of water and PFC, a small amount of fluorosurfactant is used to stabilize the droplets and delay their coalescence and creaming by lowering the aqueous–PFC interfacial tension. Initial studies of APV have shown its ability to achieve increased pulmonary retention of delivered antibiotics while producing lower and delayed peak serum concentrations relative to aerosolized delivery.(7) This presents the potential for not only increased efficacy in eradicating infection but also the opportunity for the administration of larger antibiotic doses without increased risk of systemic toxicity.
APV should also result in more spatially uniform drug delivery throughout the lung relative to aerosolized delivery. The effectiveness of aerosolized antibiotic delivery is limited by its inherent dependence on airflow within the lung. Poor ventilation during disease due to lung damage or mucus plugging can result in significantly decreased aerosolized delivery to the most burdened regions of the lung.(10–12) Furthermore, the significant effects of aerosolized particle size on site of deposition often results in substantial deposition in the oropharynx and delivery device, typically resulting in 25–90% of delivered drug never reaching the lung.(13)
Delivery is further impaired for patients on a mechanical ventilator in which approximately 20% or less of the delivered drug reaches the lung with the majority deposited in the delivery device and ventilator tubing.(14,15) PFC, on the other hand, has shown uniform spatial distribution in the injured lung during liquid ventilation.(16) Additionally, the physical characteristics that determine this distribution (viscosity and surface tension) are retained, and possibly enhanced, following the emulsification process.(7)
During total APV, the lungs are completely filled with emulsion and thus the airway surfaces throughout the entire lung are in constant contact with the emulsion. During partial APV, the lung is filled to a lesser degree, typically to end-expiratory volume. In this setting, the entire lung would experience contact with the emulsion at the end of each expiration while some of the larger conducting airways would be gas filled during the rest of the respiratory cycle. Ultimately, by filling the lungs with a liquid and removing dependence on airflow, APV should result in more efficient delivery and spatially uniform drug distribution within the lung relative to currently used treatments.
Optimization of APV requires an understanding of the temporal patterns of drug delivery and removal from the lung. However, the pharmacokinetics of these emulsions are complicated and not yet fully understood. We hypothesize that the pharmacokinetics during APV are a function of the aqueous volume percentage (Vaq), aqueous antibiotic concentration (Ct), and fluorosurfactant concentration (Cfs). The Vaq and Ct are defined as the percentage of aqueous phase in the emulsion and the antibiotic concentration within that aqueous phase, respectively. Together they define the total amount of drug delivered to the lungs upon initiating APV.
The fluorosurfactant is responsible for maintaining emulsion stability and avoiding droplet coalescence and creaming. Cfs, therefore, is likely to affect the transport of antibiotic to the lung parenchyma and pulmonary capillaries. Throughout this work, Cfs values are specified as a ratio of fluorosurfactant mass to aqueous volume (i.e., mg per mL H2O). Previous studies indicate that in vitro bacterial killing of Pseudomonas aeruginosa biofilms is optimized by maximizing Ct with a far smaller increase in killing with increasing Vaq.7 However, the in vivo setting is a vastly different scenario with a more complex geometry and multiple transport processes continually affecting antibiotic presence and availability.
Therefore, this study sought to determine the effect of Vaq, Ct, and Cfs on antibiotic delivery to the lung. Because the fluorosurfactant enables the emulsification of the antibiotics within the PFC phase, there is a minimum Cfs value below which the dispersion of drug is not maintained following sonication and the potential for improved pulmonary distribution is lost. In order to establish an appropriate range of Cfs values to be examined in vivo, an additional in vitro study was performed to determine the minimum value of Cfs that exhibited sufficiently stable drug dispersion.
Materials and Methods
Tobramycin–PFC emulsion preparation
Tobramycin–PFC emulsions were prepared similar to methods described elsewhere using the same combination of fluorosurfactants.(7) Briefly, Krytox 157 FSL (DuPont, Wilmington, DE, USA) and Krytox-PEG copolymer (synthesized as previously described)(17) were first dissolved in PFC liquid. The PFC used in all experiments was perfluorocycloether/perfluorooctane (FC-770; 3M Inc., St. Paul, MN, USA), although a PFC with a larger amount of documented in vivo safety would likely be used for eventual clinical translation. Next, tobramycin (X-Gen Pharmaceuticals Inc, Horseheads, NY, USA) dissolved in sterile water was added to the PFC solution. The mixture was then emulsified via sonication (Model S-450D, 3.2 mm diameter microtip; Branson Ultrasonics, Danbury, CT, USA) at 200 W/cm2 for 60 seconds. If the emulsion was not used immediately following preparation, it was sonicated a final time at the same settings immediately prior to experimentation.
In vitro assessment of emulsion drug dispersion
The spatial distribution of tobramycin-containing aqueous droplets within a column of emulsion was evaluated over a period of 4 h following emulsion preparation in order to evaluate the effect of Cfs on emulsion stability. Emulsions with fixed Vaq (2.5%) and Ct (40 mg/mL) were examined with varying Cfs (5, 15, or 48 mg/mL H2O). A graduated cylinder (polystyrene, 12.7 mm inner diameter) was filled with 30 mL of freshly sonicated emulsion to a height of approximately 24 cm. The graduated cylinder was modified to feature access ports with a needle (18 gauge) protruding through the cylinder wall into the center of the column lumen at heights of 4, 10, and 16 cm from the base. A schematic of the graduated cylinder can be seen in Figure 1.
FIG. 1.

A schematic of the graduated cylinder used during in vitro assessment of emulsion drug dispersion. Access ports to the cylinder lumen are featured at heights of 4, 10, and 16 cm from the base.
At 0, 30, 120, and 240 min following emulsion preparation, a 200 μL sample of emulsion was drawn from each height. In order to quantify the presence of active tobramycin in each sample of emulsion, the samples were diluted with 4 mL of sterile water and re-sonicated at 200 W/cm2 for 2 min, resulting in an inverted emulsion (continuous aqueous phase with dispersed PFC phase). The phase inversion was visually confirmed by the observation of PFC droplets accumulating at the bottom of the continuous aqueous phase. The inverted emulsion was then centrifuged at 3000 g for 20 min in order to separate the PFC and aqueous phase.
The presence of active tobramycin in the separated aqueous phase was then quantified via a microbiological assay similar to that described elsewhere.(18) Briefly, the surface of LB agar plates was inoculated with 500 μL of mid-log growth Pseudomonas aeruginosa in tryptic soy broth. Aqueous samples were then diluted and loaded into wells (75 μL/well) within the inoculated agar along with standard solutions of known tobramycin concentration. The plates were then incubated at 37o C for 24 hours before circular inhibition zones were imaged and measured. The concentration of the unknown samples was then determined by interpolation from the inhibition zones of the standard solutions.
Each sample was measured in triplicate and the resulting values averaged to produce a single concentration. The lower limit of detection using these methods was 5 μg/mL, although all experimental values were well above this limit. Three separate trials were performed for each emulsion formulation. All emulsion formulations evaluated had a total emulsion tobramycin concentration of 1 mg/mL. In theory, if the tobramycin were homogenously distributed throughout the emulsion and all tobramycin was recovered during the emulsion inversion process, the assayed aqueous phase would have a tobramycin concentration of 50 μg/mL. Experimental values from this study were normalized by this value and are thus reported as a percentage of the theoretical tobramycin content.
Pharmacokinetic evaluation
Pulmonary delivery of tobramycin (15 mg/kg) was achieved in specific pathogen-free, male Sprague Dawley rats [n = 40, weight 416 ± 16 g (mean ± standard deviation); Taconic, Hudson, NY, USA] via either intratracheal aerosolized delivery or partial APV with various emulsion formulations. All rats were initially anesthetized with a mixture of ketamine hydrochloride [50 mg/kg, intraperitoneal (IP); Hospira Inc., Lake Forest, IL, USA] and xylazine hydrochloride (5 mg/kg, IP; Lloyd Laboratories, Shenandoah, IA, USA).
Following initial anesthesia, intravenous (IV) access was acquired via the lateral tail vein, and a constant IV infusion of ketamine hydrochloride (1 mg/kg/min) was used to maintain sedation for the remainder of the experiment. Heart rate and arterial oxygen saturation were monitored via a pulse oximeter (VetOx Plus 4800; Heska, Loveland, CO, USA). Rat body temperature was monitored and maintained with a homeothermic blanket system (Model 507220F; Harvard Apparatus, Holliston, MA, USA). Rats receiving aerosolized treatment were then orally intubated with a 16 gauge angiocatheter and placed in a supine position. Aerosolized delivery was accomplished via a Microsprayer Aerosolizer (Model IA-1B; Penn-Century, Wyndmoor, PA, USA).
Prior to intubation, the length of the angiocatheter was trimmed to ensure that the Microsprayer nozzle was sufficiently exposed when the Microsprayer was fully inserted into the angiocatheter. Following intubation with the trimmed angiocatheter, the Microsprayer was fully inserted into the angiocatheter and tobramycin (40 mg/mL tobramycin concentration, 15 mg/kg bodyweight) was delivered intratracheally. Although efforts were made to synchronize actuation of the Microsprayer to inspiration, the rats often became temporarily apneic following intubation and thus the aerosol was sometimes delivered in the absence of inspiratory or expiratory flow.
Following delivery, rats were extubated and provided supplemental oxygen via a nose cone until euthanasia. For rats receiving partial APV, the neck was shaved and cleaned with alcohol and a 1 cm midline incision made. The trachea was isolated and a tie positioned loosely around it. The rat was then orally intubated with a 16 gauge angiocatheter and connected to a ventilator (Model 683; Harvard Apparatus) in a supine position. Immediately after beginning ventilation the tie was tightened around the trachea, creating a seal on the angiocatheter already within the trachea.
Ventilation was carried out with a tidal volume of 9 mL/kg bodyweight, a respiratory rate of 50–80 breaths/min, a positive end-expiratory pressure of 3 cmH2O, and a fraction of inspired oxygen of 1. Respiratory rate was adjusted to maintain peak inspiratory pressures less than 30 cmH2O. Preoxygenated emulsion was instilled (15 mL/kg bodyweight) via a port connected to the angiocatheter, resulting in a delivered dose of 15 mg/kg bodyweight of tobramycin. The emulsion was prepared no more than 5 min before use and was instilled during gas ventilation in successive aliquots (2–3 mL/aliquot) with each aliquot instilled over a period of approximately 60 s. Gas ventilation was continued following delivery of the emulsion until euthanasia.
The delivery methods and emulsion formulations used are summarized in Table 1. In order to evaluate the isolated effect of Vaq and Ct, emulsions with constant Cfs (15 mg/mL H2O) and varying Vaq (1%, 2.5%, and 5%) were assessed. Ct was varied inversely with Vaq in order to maintain a constant delivered dose of tobramycin. Similarly, in order to evaluate the effect of Cfs, emulsions with constant Vaq (2.5%) and Ct (40 mg/mL) and varying Cfs (15 or 48 mg/mL H2O) were assessed.
Table 1.
Delivery Methods and Corresponding Formulations of All Treatments Evaluated
| Delivery method [-] | Aqueous volume percent, Vaq [%] | Aqueous tobramycin concentration, Ct [mg/mL] | Emulsion tobramycin concentration [mg/mL emulsion] | Ratio of each Krytox and Krytox-PEG mass to aqueous volume, Cfs [mg/mL H2O] | Total concentration of each Krytox and Krytox-PEG [mg/mL emulsion] |
|---|---|---|---|---|---|
| APV | 1.0 | 100 | 1 | 15 | 0.15 |
| APV | 2.5 | 40 | 1 | 15 | 0.375 |
| APV | 5.0 | 20 | 1 | 15 | 0.75 |
| APV | 2.5 | 40 | 1 | 48 | 1.2 |
| Aerosol + PFC | 2.5 | 40 | N/A | 0 | 0 |
| Aerosol | 100.0 | 40 | N/A | N/A | N/A |
Additionally, tobramycin delivery via a combination of PFC and aqueous tobramycin without fluorosurfactant was evaluated using a modified partial APV procedure. Due to the immiscibility of the two phases in the absence of fluorosurfactant, the emulsion delivery procedure previously described was slightly modified in order to achieve accurate and consistent delivery. For this group, neat PFC was instilled in an identical fashion to that described for emulsion delivery during partial APV. Next, the angiocatheter was temporarily disconnected from the ventilator and the aqueous tobramycin phase intratracheally delivered via the Microsprayer. As with the aerosolized delivery group, the angiocatheters used in this treatment were appropriately trimmed in order to ensure uninhibited aerosol delivery. The rats were then reconnected to the ventilator and the remainder of the experiment carried out as described above.
In all experiments, blood samples were drawn via the lateral tail vein (opposite side of the tail relative to IV infusion) at 30, 60, 120, and 240 min following delivery of tobramycin. In treatment groups showing peak serum concentrations 30 min post-delivery, treatment was repeated with blood samples drawn at 10, 20, and 30 min post-delivery in order to better determine the time and magnitude of peak serum concentrations. A hematocrit measurement was taken at the time of each blood sampling and was used to normalize serum tobramycin measurements to account for hemodilution due to IV fluid delivery. Five rats were analyzed for each set of conditions evaluated.
Rats were euthanized with pentobarbital sodium (175 mg/kg, IP, Fatal Plus; Vortech Pharmaceuticals, Dearborn, MI, USA), the trachea immediately tied off with suture, and the lungs and trachea removed intact. The lungs were then thoroughly homogenized with sterile saline (25 mL/kg bodyweight). After centrifugation, supernatant from homogenized lung tissue samples was collected, serially diluted, and the presence of active tobramycin measured via the previously described microbiological assay. Any result below the lower limit of detection (5 μg/mL) was assigned a value of 2.5 μg/mL. Serum tobramycin concentration measurements were performed via an immunoassay by the Laboratory Medicine Department within Allegheny General Hospital (Pittsburgh, PA).
Statistical analysis
SPSS 22 (IBM Corporation, Armonk, NY) was used to perform all statistical analysis. A one-way ANOVA and post-hoc analysis was performed on the peak serum tobramycin concentrations and lung tissue tobramycin concentrations. Due to unequal variances between groups, the Games-Howell method was used to determine significant differences. A mixed model analysis was performed to examine differences in the serum tobramycin concentrations with time post-delivery used as the repeated-measure variable. A similar analysis was also used in the in vitro stability studies to examine differences in tobramycin content, using time post-preparation and height as the repeated-measure variables.
Results
In vitro assessment of emulsion drug dispersion
The formation of a distinct aqueous layer of varying sizes was observed at the top of the emulsion column between 0 and 30 minutes post-preparation for all trials performed. Figure 2 shows tobramycin content for emulsions (Vaq = 2.5% and Ct = 40 mg/mL) with Cfs values of 5, 15, and 48 mg/mL H2O. In Figure 2A, tobramycin content is shown as a function of height for each Cfs value and time point examined. Figure 2B shows the same data as a function of time for each Cfs value and height examined. Results showed a significant (p < 0.01) effect of Cfs on tobramycin content.
FIG. 2.
Tobramycin content within the emulsion (normalized by theoretical value) as a function of height from the base of the column for varying Cfs values and times post-preparation (A) and as a function of time post-preparation for varying Cfs values and heights from the base of the column (B). Error bars represent standard deviations and n = 3 for each condition evaluated.
Tobramycin content resulting from a Cfs of 5 mg/mL H2O was significantly (p < 0.01) lower than that for Cfs values of both 15 and 48 mg/mL H2O. Emulsion utilizing a Cfs value of 5 mg/mL H2O showed insufficient dispersion of antibiotic, even immediately following preparation and was thus not evaluated during the in vivo pharmacokinetic experiments. Tobramycin content for Cfs of 15 and 48 mg/mL H2O did not significantly differ from each other. No significant effect was shown for time post-preparation or height on tobramycin content.
Pharmacokinetic evaluation
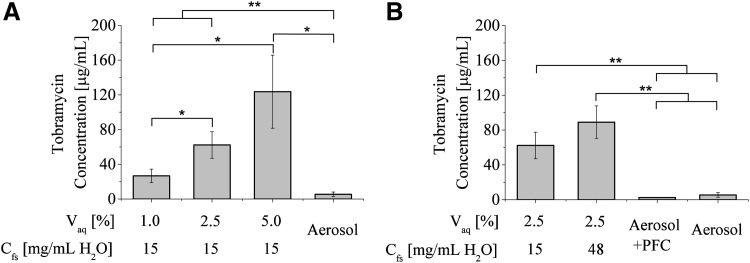
Lung tissue homogenate tobramycin concentrations at 4 hours post-delivery for all treatments evaluated are shown in Figure 3. Two of the five aerosolized delivery trials and all five of the aerosolized delivery in combination with PFC trials resulted in concentrations below the lower limit of detection (5 μg/mL). All APV treatments resulted in significantly (either p < 0.01 or p < 0.05, see Fig. 3 for distinction) greater pulmonary tobramycin concentrations relative to aerosolized delivery at 4 hours post-delivery. As shown in Figure 3A, pulmonary tobramycin concentrations increased with increasing Vaq (decreasing Ct), with the largest Vaq (5%) resulting in 22 times greater concentration than aerosolized delivery. As shown in Figure 3B, increased Cfs also resulted in larger pulmonary tobramycin concentrations, although the difference between the two Cfs values examined was not significant (p = 0.14).
FIG. 3.
(A) Tobramycin concentration of lung tissue homogenate at 4 hours following tobramycin delivery via aerosolized delivery and partial APV with varying values of Vaq. (B) Tobramycin concentration of lung tissue homogenate at 4 hours following tobramycin delivery via aerosolized delivery, aerosolized delivery in combination with PFC, and partial APV with varying Cfs values. Error bars represent standard deviations, n = 5 for each condition evaluated, and statistically significant differences between groups are denoted by an asterisk (*p < 0.05, **p < 0.01).
Serum tobramycin concentrations for all treatments are shown in Figure 4. A Vaq of 5% resulted in significantly (p < 0.01) lower serum tobramycin concentrations relative to all other groups shown in Figure 4A. Similarly, the largest value of Cfs (48 mg/mL H2O) had significantly (p < 0.01) lower serum tobramycin concentrations than all other groups shown in Figure 4B. The time and magnitude of peak serum concentrations for all treatments evaluated are shown in Table 2. Peak serum concentrations in all APV groups occurred at a later time point than both aerosolized delivery alone and in combination with PFC (60 min post-delivery for APV groups vs. 10 min post-delivery for aerosol groups). Peak serum concentrations resulting from aerosolized delivery or aerosolized delivery in combination with PFC were greater than all those produced by APV, although not significantly different in all cases.
FIG. 4.
(A) Serum tobramycin concentration out to 240 min following tobramycin delivery via aerosolized delivery and partial APV with Cfs = 15 mg/mL H2O and varying Vaq values. (B) Serum tobramycin concentration out to 240 min following tobramycin delivery via aerosolized delivery, aerosolized delivery in combination with PFC, and partial APV with Vaq = 2.5% and varying Cfs values. Error bars represent standard deviations, and n = 5 for each condition evaluated.
Table 2.
Peak Serum Concentrations After Tobramycin Delivery
| Delivery method [-] | Aqueous volume percent, Vaq [%] | Ratio of each Krytox and Krytox-PEG mass to aqueous volume, Cfs [mg/mL H2O] | Time to peak serum concentration [min] | Peak serum concentration [μg/mL] |
|---|---|---|---|---|
| APV | 1.0 | 15 | 60 | 8.7 ± 0.9 |
| APV | 2.5 | 15 | 60 | 9.4 ± 1.2 |
| APV | 5.0 | 15 | 60 | 4.7 ± 1.6 |
| APV | 2.5 | 48 | 60 | 2.3 ± 0.7 |
| Aerosol + PFC | 2.5 | 0 | 10 | 16.1 ± 3.9 |
| Aerosol | 100.0 | N/A | 10 | 10.8 ± 2.7 |
Concentration values are reported as mean ± standard deviation.
At the same Cfs value (15 mg/mL H2O), a Vaq of 5% produced significantly (p < 0.01) lower peak serum concentrations than the 1% and 2.5% Vaq groups and aerosolized delivery. Similarly, a Cfs of 48 mg/mL H2O produced significantly (p < 0.01) lower peak serum concentrations relative to a Cfs of 15 mg/mL H2O, aerosolized delivery, and aerosolized delivery in combination with PFC.
Discussion
In order to achieve effective antibiotic therapy, the antimicrobial agent must reach the site of infection and remain in the vicinity for an adequate length of time.(19) In the case of pulmonary treatment in the setting of lung disease, delivery to the site of infection is challenging. Inhaled aerosolized treatment is currently the most widely used and successful means of achieving such delivery. However, lung disease can result in poor ventilation in many of the airways, rendering a delivery method relying on airflow less than ideal. APV provides a means of pulmonary drug delivery with no dependence on airflow and thus increased ability to achieve effective delivery throughout the lung.
Previous work has proven APV to be a viable means of pulmonary antibiotic delivery, as well as shown the bactericidal ability of emulsions against CF-derived Pseudomonas aeruginosa biofilms.(7) However, the effects of emulsion formulation on the resulting antibiotic delivery have yet to be fully investigated. Understanding these effects is a vital step in optimizing the emulsion formulation for safe and effective treatment. In this study we sought to evaluate the effects of emulsion formulation (Vaq, Ct and Cfs) on emulsion stability as well as the pharmacokinetics of antibiotic delivery via APV.
As the long-term biocompatibility of the fluorosurfactants used (or rather any available fluorosurfactants) has not been evaluated, there is warranted caution regarding their use in the lung. This only highlights the importance of understanding the effects of the amount of fluorosurfactant used in order to determine the degree to which it can be minimized without impairing effective drug delivery. Results from this work suggest that a Cfs value as low as 15 mg/mL H2O (a nearly 70% reduction from previous APV in vivo trials)(7) still exhibits uniform drug dispersion up to 4 hours following preparation.
As noted in previous work with APV, the emulsion preparation is straightforward and could be performed immediately prior to administration, similar to processes for drug-laden emulsions used in chemoembolization.(20) Thus, even the reduced value of Cfs (15 mg/mL H2O) evaluated should be able to maintain uniform drug dispersion for the entirety of the intended treatment duration (≤2 hours). This reduction of Cfs also caused a slight decrease in pulmonary antibiotic retention, but the effect of this retention on the anti-biofilm activity of the emulsion is not yet known.
The presence of antibiotics within the lung following treatment was evaluated by measuring the tobramycin concentration of homogenized lung tissue via a microbiological assay. A significant advantage of this detection method is the assurance that the measured tobramycin content is still active and able to exert its intended bactericidal effect. The most clinically relevant measure of antibiotic presence in the lung is likely the aqueous concentration at the airway surfaces, however, such a measurement is difficult to perform in rats.
Due to the complex environment present within the airways during APV, it is difficult to speculate how the tobramycin concentrations of lung tissue homogenate relate to the concentrations encountered at the aqueous surfaces of the lung. A greater understanding of the physical transfer of drug from the emulsion to the aqueous surfaces which it encounters would likely best be accomplished in a more controlled in vitro setting and should be a primary aim of future efforts towards advancing APV.
When interpreting the pharmacokinetic results of this work, it is also important to note that emulsion was not suctioned from the lungs prior to euthanasia and subsequent tissue tobramycin measurements. This differs from the clinical scenario in which some portion of the emulsion, along with dislodged mucus and biofilm, would be suctioned from the lungs within 2 hours of delivery.
From the authors' previous experience performing APV in rats, suctioning emulsion from the airways of a rat has proven to be technically difficult and highly inconsistent. Thus, during APV treatment in this work, the emulsion was allowed to remain in the lungs in order to reduce treatment variability. The volume of emulsion remaining in the lungs at the time of euthanasia was not measured in this work. However, a previous study evaluating liquid ventilation with neat PFC in similarly sized rats has shown an evaporative loss rate of 1.6 mL/hour.(21) The average amount of emulsion instilled during this study was 6.2 mL. Although the evaporative loss rate likely decreases with time, one would expect very little, if any, emulsion to remain in the lungs after 4 hours of ventilation at the time of euthanasia and tissue tobramycin measurement.
Similar to a previous study,(7) increased pulmonary antibiotic concentrations were measured following APV (all emulsion formulations) relative to aerosolized delivery. Aerosolized delivery in combination with PFC showed low retention similar to aerosolized delivery alone. During such delivery, the aerosol droplets likely initially deposited on the epithelium or PFC surface. Any further dispersion of the aqueous drug would have relied solely on the mixing induced by tidal gas flow once ventilation was resumed. These results suggest that the increased retention of delivered drug observed during APV is likely a result of the initial dispersion of the aqueous drug throughout the PFC and the presence of fluorosurfactant. Although ventilation with PFC has been shown to alter the distribution of pulmonary perfusion,(22) the similar lack of retention between aerosolized delivery alone and in the presence of PFC would suggest that PFC-induced differences in pulmonary blood flow are likely not the primary cause of the retention associated with APV.
Regarding effects of emulsion formulation, increasing either Vaq or Cfs was shown to increase pulmonary retention, although the effect of Vaq was much stronger. A five-fold increase in Vaq (and corresponding five-fold decrease in Ct) resulted in a nearly identical increase in pulmonary tobramycin content 4 hours post-delivery. In the case of Cfs, an approximately three-fold increase resulted in a less than 50% increase in pulmonary retention. These results indicate that increased Vaq in combination with decreased Ct slows diffusion of tobramycin from the airways into the pulmonary circulation.
Antibiotics are believed to move between the bronchial space and blood at a rate dependent on the concentration gradient between the two compartments.(23) During APV, as aqueous droplets come in to contact and combine with the aqueous lining of the airways, the local antibiotic concentration near the epithelium increases thus increasing diffusion into the blood. The rate at which this occurs is determined by both the frequency of droplet deposition as well as the antibiotic concentration within each droplet (i.e., Ct). An increase in Vaq increases the number of droplets and as such could increase the frequency of droplet deposition. However, these results suggest that increased Vaq does not increase droplet deposition enough to compensate for the decreased antibiotic mass within each droplet, leading to an overall slowed diffusion for increased Vaq and decreased Ct.
Although not as profound as with Vaq, increases in Cfs also showed increased pulmonary antibiotic retention at 4 hours post-delivery. Additionally, serum tobramycin concentrations resulting from the largest Cfs value (48 mg/mL H2O) were significantly lower than those at 15 mg/mL H2O, further suggesting delayed antibiotic absorption into the pulmonary circulation for increased Cfs. The cause of this diminished absorption at increased values of Cfs is not yet fully understood.
In vitro assessment of emulsion stability showed no difference between Cfs values of 15 and 48 mg/mL H2O (both emulsions exhibited uniform antibiotic distribution) and thus suggests that the observed pharmacokinetic effect of Cfs is likely not due to gravity-driven phase separation. However, it is possible that coalescence and creaming is altered for emulsion in the airways at physiological temperatures under constant ventilation as compared to our in vitro experiment. Increased phase separation within emulsion in the airways for the 15 mg/mL H2O group could enhance and accelerate contact between tobramycin within the bulk of the emulsion and epithelial surfaces through which it may ultimately leave the lungs.
Alternatively, the diminished absorption effects could be due to the accumulation of fluorosurfactant at the interface between the emulsion and airway aqueous lining. The fluorosurfactant molecules possess both hydrophilic and fluorophilic moieties and thus aggregate at aqueous–PFC interfaces. It is possible that some amount of fluorosurfactant accumulates at the boundary of the lung aqueous lining and impairs either the deposition of droplets or the diffusion of tobramycin following deposition. Further in-depth studies are needed to fully understand the effects of fluorosurfactant on drug transport from the emulsion in a physiological setting.
Considering the results of this study, the optimal emulsion formulation for APV may utilize a significantly reduced Cfs value as compared to previous work (Cfs = 48 mg/mL H2O in previous studies).(7) Additionally, pulmonary retention of the delivered antibiotic can be most effectively increased by increasing Vaq and decreasing Ct to achieve the desired delivery dose. However, it is unknown whether the rate of tobramycin delivery from the airway to the tissue has a significant effect on anti-biofilm activity and thus treatment efficacy. Studies comparing rapid, nearly complete delivery of the antibiotic must be compared to slower, controlled delivery. Whatever the outcome, the ability to reliably control such kinetics represents an attractive quality of pulmonary drug delivery via PFC emulsions.
There are multiple factors to consider when choosing an optimal Vaq and Ct for treatment. As Ct is decreased, a scenario could be approached in which the aqueous concentration of antibiotics in the lung during APV is less than that required for bacterial killing. A previous study with APV has shown that emulsions utilizing Ct values as low as 4 mg/mL (five-fold smaller than lowest Ct value used in present study) were still able to reduce proliferation of biofilm-derived bacteria following exposure.(7)
Additionally, as Vaq is increased, larger aqueous volumes will be delivered to the lung during APV. Therefore caution should be used when determining Vaq as to not increase this value to the point of inhibiting normal respiratory function. If the Vaq values evaluated in this study (1%–5%) were to be used clinically during partial APV with a 15 mL/kg dose of emulsion (dose used in previous clinical trials of partial liquid ventilation), a 70-kg patient would receive approximately 10–50 mL of aqueous antibiotic delivered to the lung. However, at the conclusion of treatment, some portion of the emulsion would be suctioned from the lung. Conservatively, estimating that only one-third of the emulsion volume would be removed from the lung, 7–35 mL of aqueous antibiotic would ultimately remain in the lung. During currently used nebulized tobramycin treatment for CF patients, approximately 10 mL of aqueous antibiotics are delivered to the lung on a daily basis.(24) Considering that such treatment is generally well tolerated without supplemental oxygen or ventilatory support, the range of Vaq values used in this work are likely within reason for a patient on mechanical ventilation.
The current study has confirmed the use of APV as a viable and effective means of pulmonary antibiotic delivery and taken critical steps in further developing this emerging technology. APV utilizing an optimized emulsion formulation should result in increased pulmonary retention, decreased and delayed peak serum concentrations, as well as improved dispersion of delivered antibiotic relative to aerosolized delivery. Future studies should evaluate the efficacy of an optimized emulsion during APV in an animal model of lower respiratory infection, as well as the biocompatibility of the used fluorosurfactants and any others available.
Acknowledgments
This work was funded by a Ford Foundation predoctoral fellowship and National Institutes of Health Grant R03AI096029.
Author Disclosure Statement
There are no financial conflicts of interest for any authors of this work.
References
- 1.Geller DE: Aerosol antibiotics in cystic fibrosis. Respir Care. 2009;54:658–670 [DOI] [PubMed] [Google Scholar]
- 2.Ciofu O, Hansen CR, and Hoiby N: Respiratory bacterial infections in cystic fibrosis. Curr Opin Pulm Med. 2013;19:251–258 [DOI] [PubMed] [Google Scholar]
- 3.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, and Johnston SL: Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121 [DOI] [PubMed] [Google Scholar]
- 4.Sethi S, and Murphy TF: Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365 [DOI] [PubMed] [Google Scholar]
- 5.Puchelle E, de Bentzmann S, and Zahm JM: Physical and functional properties of airway secretions in cystic fibrosis—Therapeutic approaches. Respiration. 1995;62:2–12 [DOI] [PubMed] [Google Scholar]
- 6.Donaldson SH, and Boucher RC: Update on pathogenesis of cystic fibrosis lung disease. Curr Opin Pulm Med. 2003;9:486–491 [DOI] [PubMed] [Google Scholar]
- 7.Orizondo RA, Babcock CI, Fabiilli ML, Pavlovsky L, Fowlkes JB, Younger JG, and Cook KE: Characterization of a reverse-phase perfluorocarbon emulsion for the pulmonary delivery of tobramycin. J Aerosol Med Pulm Drug Deliv. 2014;27:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirschl RB, Pranikoff T, Wise C, Overbeck MC, Gauger P, Schreiner RJ, Dechert R, and Bartlett RH: Initial experience with partial liquid ventilation in adult patients with the acute respiratory distress syndrome. JAMA. 1996;275:383–389 [PubMed] [Google Scholar]
- 9.Pohlmann JR, Brant DO, Daul MA, Reoma JL, Kim AC, Osterholzer KR, Johnson KJ, Bartlett RH, Cook KE, and Hirschl RB: Total liquid ventilation provides superior respiratory support to conventional mechanical ventilation in a large animal model of severe respiratory failure. ASAIO J. 2011;57:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay S, Staddon GE, Eastman C, Palmer M, Davies ER, and Carswell F: The quantitative distribution of nebulized antibiotic in the lung in cystic fibrosis. Resp Med. 1994;88:203–211 [DOI] [PubMed] [Google Scholar]
- 11.Labiris NR. and Dolovich MB: Pulmonary drug delivery. Part I: Physiological factors affecting therapeutic effectiveness of aerosolized medications. J Clin Pharmacol. 2003;56:588–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin MH, Le Naour G, Marquette CH, and Rouby JJ: Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 2010;36:1147–1155 [DOI] [PubMed] [Google Scholar]
- 13.Dolovich M: New propellant-free technologies under investigation. J Aerosol Med. 1999;12:S9–17 [DOI] [PubMed] [Google Scholar]
- 14.Palmer LB, Smaldone GC, Simon SR, O'Riordan TG, and Cuccia A: Aerosolized antibiotics in mechanically ventilated patients: Delivery and response. Crit Care Med. 1998;26:31–39 [DOI] [PubMed] [Google Scholar]
- 15.Miller DD, Amin MM, Palmer LB, Shah AR, and Smaldone GC: Aerosol delivery and modern mechanical ventilation: In vitro/in vivo evaluation. Am J Respir Crit Care Med. 2003;168:1205–1209 [DOI] [PubMed] [Google Scholar]
- 16.Hirschl RB, Overbeck MC, Parent A, Hernandez R, Schwartz S, Dosanjh A, Johnson K, and Bartlett RH: Liquid ventilation provides uniform distribution of perfluorocarbon in the setting of respiratory failure. Surgery. 1994;116:159–167 [PubMed] [Google Scholar]
- 17.Fabiilli ML, Lee JA, Kripfgans OD, Carson PL, and Fowlkes JB: Delivery of water-soluble drugs using acoustically triggered perfluorocarbon double emulsions. Pharm Res. 2010;27:2753–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Omri A, Beaulac C, Bouhajib M, Montplaisir S, Sharkawi M, and Lagace J: Pulmonary retention of free and liposome-encapsulated tobramycin after intratracheal administration in uninfected rats and rats infected with Pseudomonas aeruginosa. Antimicrob Agents Chemotherapy. 1994;38:1090–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flume P, and Klepser ME: The rationale for aerosolized antibiotics. Pharmacotherapy. 2002;22:71S–79S [DOI] [PubMed] [Google Scholar]
- 20.Hino T, Kawashima Y, and Shimabayashi S: Basic study for stabilization of w/o/w emulsion and its application to transcatheter arterial embolization therapy. Adv Drug Deliv Rev. 2000;45:27–45 [DOI] [PubMed] [Google Scholar]
- 21.Hirayama Y, Hirasawa H, Oda S, Shiga H, Matsuda K, Ueno H, and Nakamura M: Partial liquid ventilation with FC-77 suppresses the release of lipid mediators in rat acute lung injury model. Crit Care Med. 2004;32:2085–2089 [DOI] [PubMed] [Google Scholar]
- 22.Gauger PG, Overbeck MC, Koeppe RA, Shulkin BL, Hrycko JN, Weber ED, and Hirschl RB: Distribution of pulmonary blood flow and total lung water during partial liquid ventilation in acute lung injury. Surgery. 1997;122:313–323 [DOI] [PubMed] [Google Scholar]
- 23.Pennington JE: Penetration of antibiotics into respiratory secretions. Rev Infect Dis. 1981;3:67–73 [DOI] [PubMed] [Google Scholar]
- 24.Novartis Pharmaceuticals Corporation. TOBI, Tobramycin Inhalation Solution: Prescribing Information, 2014 [Google Scholar]