Abstract
We recently encountered a patient with acute inflammatory demyelinating polyneuropathy (AIDP) that was associated with systemic lupus erythematosus (SLE). A 34-year-old Chinese female with a 3-year history of SLE presented with acute bilateral leg weakness and paraparesis, and lost the ability to walk 1 day after noticing bilateral leg numbness and pain for 12 days. Physical examination revealed bilateral facial muscle paralysis, muscle strength in the legs with graded 1/5 proximally and 2/5 distally bilaterally and absence of deep tendon reflex in both knees and ankles. Paresthesia was observed in distal limbs with glove and stocking distribution. Cerebrospinal fluid analysis demonstrated albuminocytologic dissociation. Electrophysiologic survey also indicated sensory-motor demyelinating polyneuropathy. The diagnosis of SLE was established based on her initial symptoms including intermittent fevers, hair loss, oral ulcers, malar rash and arthritis affecting the elbow, wrist and hand joints; positive immunologic findings for antinuclear antibody (ANA), anti-DNA antibody, anti-Smith (anti-Sm) antibody, low serum complement levels, and the kidney biopsy specimen showed glomerular mesangial proliferation with focal endothelial cell proliferation (ISN/PPS 2004 classification lupus nephritis, class III). Treatment with intravenous immunoglobulin, methylprednisolone and cyclophosphamide resulted in clinical and electrophysiological improvement.
Keywords: Systemic lupus erythematosus, Acute inflammatory demyelinating polyneuropathy, Guillain-Barre syndrome
Introduction
Systemic lupus erythematosus (SLE) is a chronic, inflammatory, relapsing-remitting, autoimmune disease characterized by multisystemic involvement with diverse clinical presentations. Neurologic complications are common and frequent in SLE. Central nervous system (CNS) involvement is one of the more common complications that can occur at any stage of the SLE. However, peripheral nervous system involvement in SLE is rare and dominated by distal symmetric axonal polyneuropathy and multiple mononeuropathy [1]. Acute inflammatory demyelinating polyneuropathy (AIDP) or the classic type of Guillain-Barre syndrome (GBS) is very uncommon. Here we report a patient with AIDP that was associated with SLE.
Case Report
A 34-year-old Chinese female presented with a 3-year history of SLE presented with acute bilateral leg weakness and paraparesis, and lost the ability to walk 1 day after noticing bilateral leg numbness and pain for 12 days, accompanied by fever, fatigue, incomplete closure of the eyelids (lagophthalmos) and dysphagia. Three weeks before admission, she had intermittent abdominal pain and watery diarrhea. Her initial symptoms 3 years before her visit had intermittent fevers, hair loss, oral ulcers, malar rash and arthritis affecting the elbow, wrist and hand joints. The laboratory test results at that time were as follows: antinuclear antibody (ANA) titer: 1:320 (+); anti-DNA antibody: (+); anti-Smith (anti-Sm) antibody: (+); serum complement (CH50): 17 (26 - 48) units/mL; C3: 53 (86 - 160) mg/dL; C4: 11 (17 - 45) mg/dL; urinary protein: 1+; 24-h urinary protein (UP): 1.65 g/day and hematuria: -. Her renal function test and hematologic evaluation results were within normal ranges. Renal biopsy was not conducted.
Physical examination at admission revealed that she had a temperature of 38.2 °C, a heart rate of 115 bpm, a respiratory rate of 20 breaths/min, blood pressure of 135/90 mm Hg and an oxygen saturation of 97% on room air. She had malar rash, but there was no clinical evidence of arthritis or muscle inflammation. Neurologic examination indicated she had bilateral facial muscle paralysis, and motor examination revealed muscle strength in the legs with graded 2/5 proximally and distally bilaterally and absence of deep tendon reflex in both knees and ankles. Paresthesia was observed in distal limbs with glove and stocking distribution. The deep tendon reflexes were absent. The bilateral Babinski test was unremarkable. Cardiovascular, respiratory and abdominal examinations were normal. The autonomic and sphincter functions related to urination and defecation were preserved.
This time, abnormal laboratory findings included ESR 46 mm, CRP 8.5 mg/L, positive ANA +1:640 (< 1:160), anti-SSA, anti-SSB antibody and low levels of serum complement components (CH50, C3, C4). Anti-dsDNA and anticardiolipin antibodies were negative or within the normal range. Anti-ganglioside antibodies were negative. Viral and bacterial serology and antiganglioside antibodies were negative. Serologic tests for HIV, hepatitis B/C and cytomegalovirus were all negative. Cerebrospinal fluid examination revealed albumino-cytological dissociation (total protein, 154.3 mg/dL and white blood cell, 3/mm3, respectively). Abdominal ultrasound exam, chest radiograph and ECG revealed no obvious abnormalities. Brain magnetic resonance imaging did not show any pathologic lesions. Electroneuromyography (ENMG) was highly suggestive of demyelinating polyradiculoneuropathy with prolonged distal motor latencies, decreased amplitudes of compound muscle action potential, slow nerve conduction velocities, absence of F waves and delayed M-wave, without acute denervation (Table 1). A percutaneous renal biopsy was performed on the patient after hospitalization. The kidney biopsy specimen showed glomerular mesangial proliferation with focal endothelial cell proliferation (ISN/PPS 2004 classification lupus nephritis, class III). Immunofluorescent study findings for IgA, C1q, C3 (diffuse capillary loops, mesangial area, 2+), IgM (diffuse capillary loops, mesangial area, +), and IgG (segmental capillary loops, mesangial area, +) were positive. Light microscopy revealed segmental glomerular sclerosis, mild to moderate glomerular mesangial expansion, mesangial cells and matrix hyperplasia, infiltration of mononuclear cells and neutrophils, capillary endothelial cells proliferation, capillary loops wall thickening, and lumens stenosis. Masson trichrome (Masson) staining and periodic Schiff methenamine (PASM) staining showed the deposition of complex red deposits in the glomerular endothelial cells mesangial region, mild renal tubular interstitial lesions and mononuclear cell infiltration, segmental degeneration of small arteries (Fig. 1). There was no obvious tubular atrophy and interstitial fibrosis in the kidney. Timely management involved plasma exchange followed by intravenous gammaglobulin infusion (0.4 g/kg daily for 5 days), and continuation of high-dose systemic corticosteroids, two 1.0 g pulses of methylprednisolone. Cyclophosphamide therapy was continued with 400 mg intravenously every second week for 2 months.
Table 1. Electrophysiological Findings.
| Lat (SD), ms | Amp (SD), mV | CV (SD), m/s | Amp (SD), % | |
|---|---|---|---|---|
| Motor nerve | ||||
| Right medianus | ||||
| Wrist-APB | 4.5 (3.9) | 0.2 (-2.7) | ||
| Elbow-wrist | 9.5 | 0.2 | 35.4 | 34 |
| Left ulnaris | ||||
| Wrist-ADM | 3.8 (-6.2) | 0.1 (-4.5) | ||
| Ab elbow-wrist | 8.5 | 0.6 | 47.8 (-1.7) | 435 (52) |
| Right tibialis | ||||
| Ankle-AHB | 9.4 (15.7 | 0.1 | ||
| PF-ankle | 25.7 | 0.1 | 23.4 | 351 |
| Left tibialis | ||||
| Ankle-AHB | 9.0 (14.6) | 0.0 | ||
| PF-ankle | 24.0 | 0.1 | 27.3 | 352 |
| Right peroneus | ||||
| Ankle-EDB | 7.9 (8.3) | 0.2 (-2.1) | ||
| CF down-ankle | 17.0 | 0.2 | 34.2 | 3 |
| Left peroneus | ||||
| Ankle-EDB | 9.0 (9.8) | 0.1 (-2.1) | ||
| CF down-ankle | 18.7 | 0.3 | 32.4 | 415 |
| Sensory nerve | ||||
| Right medianus | ||||
| Dig II-wrist | 2.6 (-0.7) | 16 | 53.7 | |
| Left ulnaris | ||||
| Dig V-wrist | 2.8 (0.1) | 12 | 46.5 | |
| Right peroneus super | ||||
| Ankle-foreleg | 4.1 | 4.0 | 35.0 (-3.2) | |
| Left peroneus super | ||||
| Ankle-foreleg | 3.9 | 5.9 | 33.4 (-3.6) |
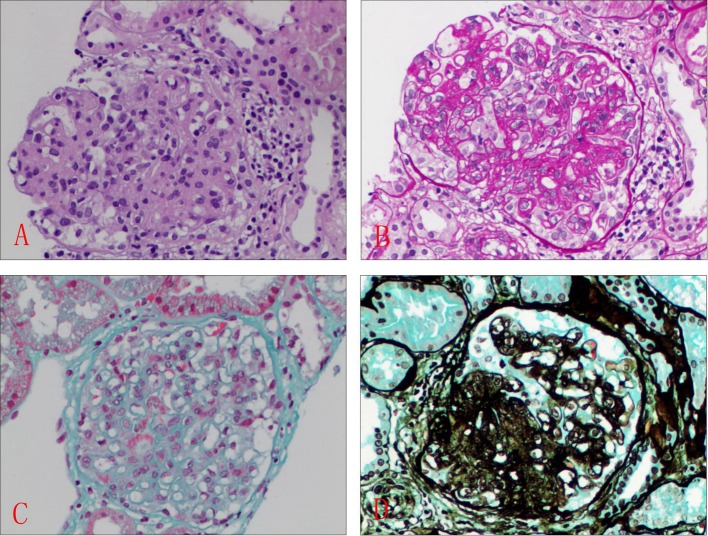
Figure 1.
Histologic features of section of kidney biopsy. Glomerular mesangial proliferation with focal endothelial cell proliferation. (A) H&E, × 400; (B) PASM, × 400; (C) Masson, × 400; (D) Gomori methenamine silver (GMS), × 400.
Discussion
Peripheral neuropathy (NP) is a known and underestimated complication in SLE. Only a few previous studies have reported frequent occurrence of NP in SLE, with incidences from 1.5% to 27.8% [2-7]. NP usually develops during the course of the SLE, especially in the advanced stage of the SLE, and very rarely from the outset of the SLE [8, 9]. AIDP is the most prevalent form of GBS. The prevalence of SLE in AIDP or GBS has been reported to be 0.6-1.7% [2, 10]. Our review of the literature finds that AIDP or GBS with SLE was more common in females (73.3%, 11/15) than males (26.7%, 4/15), and cerebrospinal fluid albuminocytological dissociation was present in 66.7 % (9/15) of cases, and more importantly, usually occurred in early course of the SLE (Table 2) [8, 9, 11-23], even as the first manifestation of SLE [8, 16-21]. We reported the AIDP patient with SLE occurring early in the course of lupus, which was consistent with the previously reported ones [16].
Table 2. Previous Reports About GBS or AIDP With SLE.
| Author | Age/sex | GBS and SLE | Organ involvement | Auto-Ab(+) | Treatment (effect +, -) |
|---|---|---|---|---|---|
| Hsu et al [18] | 28/F | Early | PNS, pleurisy, lymphopenia | ANA, anti-dsDNA, anti-Ro/SS-A, anti-La/SS-B | Prednisolone, PE, (+) |
| Chaudhuri et al [20] | 40/M | Early | PNA, lymphopenia, seizure, membranous glomerulonephritis | ANA, anti-dsDNA | Prednisolone, cyclophosphamide, PE, (+) |
| Santiago-Casas et al [22] | 20/F | Early | PNA, lymphopenia, proteinuria | ANA, anti-dsDNA | Cyclophosphamide, glucocorticoids, PE, IVIG, (+) |
| Santiago-Casas et al [22] | 34/F | Early | PNS, arthritis, lymphopenia | ANA, anti-dsDNA | Glucocorticoids, IVIG, cyclophosphamide, (+) |
| Miyagawa et al [13] | 9/F | Late | PNS, chilblain or erythema multiforme, oral ulcers | ANA, anti-dsDNA, anti-Ro/SS-A, anti-La/SS-B | Corticosteroids, PE, IVIG, (+) |
| Robson et al [23] | 33/F | Early | Hemolytic anemia, thrombocytopenia, retinal vasculitis, PNS, CNS | ANA, anti-dsDNA | Glucocorticoids, cyclophosphamide, (+) |
| Vaidya et al [16] | 23/M | Early | PNS, WHO class V lupus nephropathy | ANA, anti-dsDNA | Glucocorticoids, cyclophosphamide, PE, (+) |
| Millette et al [21] | 23/F | Early | PNS, cranial nerve | ANA, anti-dsDNA, anti-Smith, anti-RNP | Glucocorticoids, (+) |
| Okoh et al [8] | 41/F | Early | Leukopenia, pericardial and pleural effusions, proteinuria, and hematuria | ANA, anti-dsDNA, anti-Smith | Cyclophosphamide, glucocorticoids, PE, IVIG, mycophenolate mofetil, (+) |
| van Laarhoven et al [19] | 20/F | Early | Facial exanthema pleural and pericardial, proteinuria, autoimmune hemolytic anemia | ANA, anti-Smith, anti-RNP | IVIG, cyclophosphamide, glucocorticoids, (+) |
| Rajadhyaksha et al [17] | 30/F | Early | Oral ulcers, arthralgias, bulbar palsy, cranial nerve, class II mesangio proliferative lupus nephritis nerve | ANA, anti-dsDNA, anti-Smith, GT1a, GQ1b, GM1b | IVIG, glucocorticoids, (+) |
| Ha-Ou-Nou et al [11] | 44/M | Early | Lymphopenia, proteinuria, thrombocytopenia | anti-dsDNA | Corticosteroids, IVIG, (-) |
| Stahl et al [14] | 56/F | Early | Thrombocytopenia, stomatitis, hair loss, arthritis | ANA, AHA | IVIG, cyclophosphamide, corticosteroids, (-) |
| Lewis and Gibson [12] | 21/M | Late | Arthritis, pleuro-pericarditis, proteinuria | ANA, anti-dsDNA, anti-RNP, ACA | Cyclophosphamide, IVIG, prednisolone, (+) |
| Yildiz et al [9] | 47/F | Late | Discoid rash, photosensitivity, oral ulcers, leucopenia, lymphopenia | ANA | Corticosteroid, cyclophosphamide, (+) |
| Matsuki et al [15] | 61/F | Early | Arthritis, oral ulcers, pericarditis, lymphopenia | ANA, anti-dsDNA, anti-Ro/SS-A, anti-GMl | Methylprednisolone, mizoribine, immunoadsorption, PE, (+) |
PE: plasma exchange; IVIG: intravenous immunoglobulin; ANA: antinuclear antibody; anti-dsDNA: anti-double-stranded DNA; anti-SS-A: anti-Ro antibodies; anti-SS-B: anti-La antibodies; AHA: anti-histone antibodies; ACA: anti-cardiolipin antibodies; PNS: peripheral nervous system; anti-Smith: anti-Smith antibodies.
The pathogenesis of AIDP or GBS with SLE is not clearly understood [22, 23]. There is some considerable speculation that cell-mediated and humoral processes, or immunological cross-reactivity, or the presence of auto-reactive antibodies resulted in subsequent damage to the peripheral nervous system [22, 23]. Further studies are needed to elucidate the underlying reasons for AIDP or GBS with SLE.
The previous literature reported four treatment options have been used in AIDP or GBS with SLE, including corticosteroids, cyclophosphamide, plasmapheresis and immunoglobulin. Although clinical trials have demonstrated that corticosteroids treatment has no beneficial effect on GBS during the past decades [24, 25], steroid therapy is still the more frequently used treatment modality for SLE with neuropsychiatric manifestations. The combination of corticosteroids and cyclophosphamide was considered the first-line treatment option in a review of the literatures (Table 2) [19]. The efficacy of plasmapheresis and immunoglobulin for AIDP or GBS with nephritis lupus is controversial [19]. Varying responses have been noted with each patient encountered and no consensus guidelines have yet been established.
AIDP or GBS with SLE is rare in SLE patients. Controlled clinical trials are largely lacking which results in various non-standardized treatment regimens. However, the association of them has been strongly evidenced by reported literatures, which translates into significant implications for their management and long-term outcomes. Our findings suggest prompt diagnosis and treatment, early in the course of illness, is of critical importance for a positive clinical outcome.
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Ait Benhaddou E, Birouk N, El Alaoui-Faris M, Mzalek-Tazi Z, Aidi S, Belaidi H, Kably B. et al. [Acute Guillain-Barre-like polyradiculoneuritis revealing acute systemic lupus erythematosus: two case studies and review of the literature] Rev Neurol (Paris) 2003;159(3):300–306. [PubMed] [Google Scholar]
- 2.Xianbin W, Mingyu W, Dong X, Huiying L, Yan X, Fengchun Z, Xiaofeng Z. Peripheral neuropathies due to systemic lupus erythematosus in China. Medicine (Baltimore) 2015;94(11):e625. doi: 10.1097/MD.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navinan MR, Piranavan P, Akram AU, Yudhishdran J, Kandeepan T, Kulatunga A. Sensory neuronopathy complicating systemic lupus erythematosus: a case report. J Med Case Rep. 2014;8:141. doi: 10.1186/1752-1947-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su YJ, Huang CR, Chang WN, Tsai NW, Kung CT, Lin WC, Huang CC. et al. The association between autoantibodies and peripheral neuropathy in lupus nephritis. Biomed Res Int. 2014;2014:524940. doi: 10.1155/2014/524940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oomatia A, Fang H, Petri M, Birnbaum J. Peripheral neuropathies in systemic lupus erythematosus: clinical features, disease associations, and immunologic characteristics evaluated over a twenty-five-year study period. Arthritis Rheumatol. 2014;66(4):1000–1009. doi: 10.1002/art.38302. [DOI] [PubMed] [Google Scholar]
- 6.Shoshtary J, Adib M. Peripheral neuropathy in systemic lupus erythematosus in Southern Iran. Electromyogr Clin Neurophysiol. 2005;45(3):145–148. [PubMed] [Google Scholar]
- 7.Huynh C, Ho SL, Fong KY, Cheung RT, Mok CC, Lau CS. Peripheral neuropathy in systemic lupus erythematosus. J Clin Neurophysiol. 1999;16(2):164–168. doi: 10.1097/00004691-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Okoh HC, Lubana SS, Langevin S, Sanelli-Russo S, Abrudescu A. A Case of Systemic Lupus Erythematosus Presenting as Guillain-Barre Syndrome. Case Rep Rheumatol. 2015;2015:528026. doi: 10.1155/2015/528026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yildiz OK, Balaban H, Senel S, Cevik S. Acute lumbosacral polyradiculoneuropathy heralding transformation to systemic lupus erythematosus in a patient with discoid lupus. Lupus. 2011;20(9):972–974. doi: 10.1177/0961203310392427. [DOI] [PubMed] [Google Scholar]
- 10.Korn-Lubetzki I, Abramsky O. Acute and chronic demyelinating inflammatory polyradiculoneuropathy. Association with autoimmune diseases and lymphocyte response to human neuritogenic protein. Arch Neurol. 1986;43(6):604–608. doi: 10.1001/archneur.1986.00520060066020. [DOI] [PubMed] [Google Scholar]
- 11.Ha-ou-nou FZ, Dehbi S, Zahlane M, Kissani N, Essaadouni L. [Acute polyradiculoneuropathy revealing systemic lupus erythematosus: an unusual presentation with fatal outcome] Rev Med Interne. 2014;35(1):65–67. doi: 10.1016/j.revmed.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Lewis M, Gibson T. Systemic lupus erythematous with recurrent Guillain-Barre-like syndrome treated with intravenous immunoglobulins. Lupus. 2003;12(11):857–859. doi: 10.1191/0961203303lu464cr. [DOI] [PubMed] [Google Scholar]
- 13.Miyagawa S, Nakajima M, Nishio K, Sogami J, Tsubakimoto A, Yoshioka A, Shirai T. Guillain-Barre syndrome in a child with systemic lupus erythematosus and anti-Ro/SSA and anti-La/SSB autoantibodies. Br J Dermatol. 2000;143(5):1050–1054. doi: 10.1046/j.1365-2133.2000.03842.x. [DOI] [PubMed] [Google Scholar]
- 14.Stahl HD, Kalischewski P, Orda C, Baum P, Grahmann F, Emmrich F. Filtration of cerebrospinal fluid for acute demyelinating neuropathy in systemic lupus erythematosus. Clin Rheumatol. 2000;19(1):61–63. doi: 10.1007/s100670050013. [DOI] [PubMed] [Google Scholar]
- 15.Matsuki Y, Hidaka T, Matsumoto M, Fukushima K, Suzuki K. Systemic lupus erythematosus demonstrating serum anti-GM1 antibody, with sudden onset of drop foot as the initial presentation. Intern Med. 1999;38(9):729–732. doi: 10.2169/internalmedicine.38.729. [DOI] [PubMed] [Google Scholar]
- 16.Vaidya S, Jasin HE, Logan J. Systemic lupus erythematosus and guillain-barre syndrome. J Clin Rheumatol. 1999;5(6):349–353. doi: 10.1097/00124743-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Rajadhyaksha A, Mehra S. Pharyngeal-cervical-brachial variant of Guillain-Barre syndrome with predominant bulbar palsy as the initial presentation of systemic lupus erythematosus and lupus nephritis: a case report. Int J Rheum Dis. 2012;15(6):e162–164. doi: 10.1111/j.1756-185X.2012.01761.x. [DOI] [PubMed] [Google Scholar]
- 18.Hsu TY, Wang SH, Kuo CF, Chiu TF, Chang YC. Acute inflammatory demyelinating polyneuropathy as the initial presentation of lupus. Am J Emerg Med. 2009;27(7):900. doi: 10.1016/j.ajem.2008.11.006. e903-905. [DOI] [PubMed] [Google Scholar]
- 19.van Laarhoven HW, Rooyer FA, van Engelen BG, van Dalen R, Berden JH. Guillain-Barre syndrome as presenting feature in a patient with lupus nephritis, with complete resolution after cyclophosphamide treatment. Nephrol Dial Transplant. 2001;16(4):840–842. doi: 10.1093/ndt/16.4.840. [DOI] [PubMed] [Google Scholar]
- 20.Chaudhuri KR, Taylor IK, Niven RM, Abbott RJ. A case of systemic lupus erythematosus presenting as Guillain-Barre syndrome. Br J Rheumatol. 1989;28(5):440–442. doi: 10.1093/rheumatology/28.5.440. [DOI] [PubMed] [Google Scholar]
- 21.Millette TJ, Subramony SH, Wee AS, Harisdangkul V. Systemic lupus erythematosus presenting with recurrent acute demyelinating polyneuropathy. Eur Neurol. 1986;25(6):397–402. doi: 10.1159/000116042. [DOI] [PubMed] [Google Scholar]
- 22.Santiago-Casas Y, Peredo RA, Vila LM. Efficacy of low-dose intravenous cyclophosphamide in systemic lupus erythematosus presenting with Guillain-Barre syndrome-like acute axonal neuropathies: report of two cases. Lupus. 2013;22(3):324–327. doi: 10.1177/0961203313476358. [DOI] [PubMed] [Google Scholar]
- 23.Robson MG, Walport MJ, Davies KA. Systemic lupus erythematosus and acute demyelinating polyneuropathy. Br J Rheumatol. 1994;33(11):1074–1077. doi: 10.1093/rheumatology/33.11.1074. [DOI] [PubMed] [Google Scholar]
- 24.Wang YZ, Lv H, Shi QG, Fan XT, Li L, Yi Wong AH, Hao YL. et al. Action mechanism of corticosteroids to aggravate Guillain-Barre syndrome. Sci Rep. 2015;5:13931. doi: 10.1038/srep13931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hughes RA, Wijdicks EF, Barohn R, Benson E, Cornblath DR, Hahn AF, Meythaler JM. et al. Practice parameter: immunotherapy for Guillain-Barre syndrome: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2003;61(6):736–740. doi: 10.1212/WNL.61.6.736. [DOI] [PubMed] [Google Scholar]