Abstract
CXCR4 inhibitors are promising agents for the treatment of cancer metastasis and inflammation. A series of novel tertiary amine derivatives targeting CXCR4 were designed, synthesized, and evaluated. The central benzene ring linker and side chains were modified and optimized to study the structure-activity relationship. Seven compounds displayed much more potent activity than the reference drug, AMD3100, in both the binding affinity assay and the blocking of Matrigel invasion functional assay. These compounds exhibited effective concentration ranging from 1 to 100 nM in the binding affinity assay and inhibited invasion from 65.3% to 100% compared to AMD3100 at 100 nM. Compound IIn showed a 50% suppressive effect against carrageenan-induced paw inflammation in a mouse model, which was as effective as the peptidic antagonist, TN14003 (48%). These data demonstrate that symmetrical bis-tertiary amines are unique CXCR4 inhibitors with high potency.
Keywords: Tertiary amines, CXCR4 inhibitors, Binding affinity, Matrigel invasion, Anti-inflammatory activity
Graphical Abstract
1. Introduction
C-X-C chemokine receptor type 4 (CXCR4), also known as fusin or cluster of differentiation 184 (CD184), is a seven transmembrane G-protein coupled receptor (GPCR) belonging to the Class I GPCR or rhodopsin-like GPCR family [1, 2]. Stromal-derived-factor-1 (SDF-1) or C-X-C chemokine ligand 12 (CXCL12) is the major ligand of CXCR4 and the interaction recognition between CXCL12 and CXCR4 recruits cells to the organ sites with high levels of CXCL12 expression [3, 4]. The CXCL12/CXCR4 axis has been shown to be involved in a number of pathological conditions, including cancer and inflammation [5, 6]. CXCR4 plays a key role as a homing receptor to the lymph nodes, lung, liver, and bone. Homing, the mechanism that allows foreign tissue-origin cells to reside and proliferate, is believed to be the rate-limiting step of the multi-step metastatic process [7, 8]. These suggest that the interaction between CXCL12 and CXCR4 plays a key role in the chemotaxis and homing of metastatic cells [9]. CXCR4 is overexpressed in more than 23 human cancer types [10, 11], such as breast [12–14], leukemia [9], lung [15], and prostate cancers [16].
Inflammation is inextricably associated with primary tumor progression and contributes to metastatic outgrowth in distant organs [17]. The COX pathway has long been used as the major target for anti-inflammatory drugs [18]. Both traditional non-steroidal anti-inflammatory drugs (NSAIDs) and selective COX–2 inhibitors exhibit their anti-inflammatory activity by inhibiting COX-2 [18]. Nevertheless, the prolonged use of traditional anti-inflammatory drugs is associated with potential serious side effects such as kidney failure, ulcers and prolonged bleeding after an injury or surgery [19–21]. Furthermore, rofecoxib and valdecoxib were withdrawn from the market due to an increased risk of cardiovascular complications [22, 23].
Accumulating evidence suggests the involvement of CXCR4–CXCL12 interaction in various inflammatory diseases, including rheumatoid arthritis, autoimmune diseases, ischemic injuries, inflammatory bowel disease, and pneumonia [24, 25]. Based on these findings, development of inhibitors blocking CXCR4 presents a new avenue for complementary therapeutic strategy in inflammatory diseases as well as cancer metastasis.
The first CXCR4 inhibitor to enter clinical trials for treatment of HIV infection is AMD3100 [25]. The development of AMD3100 has followed a meandering course, starting with JM1657, an impurity in commercial cyclam preparation with anti-HIV activity, and finally leading to AMD3100 [26]. Because of the preorganisation and the flexibility of the AMD3100 macrocyclic cyclam, it can bind to metal ions and form highly stable metal complexes [3]. These chelating properties are likely related to the cardiotoxicity reported in its clinical trial against HIV [26–29]. In 2008, AMD3100 was approved by FDA for stem cell mobilization [25]. Although it benefits patients with certain diseases, long term treatment can introduce lung and liver fibrosis [27]. Hence there is an urgent need to develop safe CXCR4 inhibitors that can be used in long-term treatment.
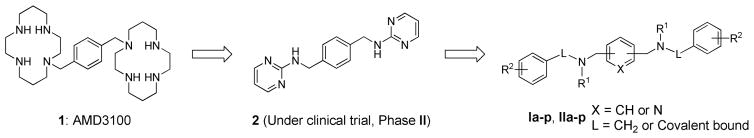
Taking AMD3100 as the lead compound, we simplified and optimized its bicyclam rings, leading to the design and synthesis of a series of novel and potent CXCR4 inhibitors [30, 31]. Previously, we reported a novel small molecule, compound 2 (Figure 1), which is now under clinical trial (Phase II). Compound 2 is a partial CXCR4 inhibitor with properties quite unlike that of any other reported CXCR4 antagonists. Compound 2 does not have stem cell mobilizing capability nor calcium flux modulating activity, which can be safer for long-term blockade of metastasis than other reported CXCR4 antagonists [27].
Figure 1.
Strategy for the design and optimization of anti-CXCR4 compounds.
Most of our reported CXCR4 inhibitors are secondary amines. Here we attempted to design and synthesize a new series of tertiary amine compounds to explore whether these new structures will maintain the CXCR4 inhibitory effect. Meanwhile, an extensive structure-activity profile indicates that the central benzene ring is critical for high CXCR4 affinity [31]. Since pyridine derivatives have been explored for their potential as pharmaceutical agents [32], we also substituted the middle benzene ring with a pyridine framework. The structural formula was shown in Figure 1.
2. Results and discussion
2.1. Chemistry
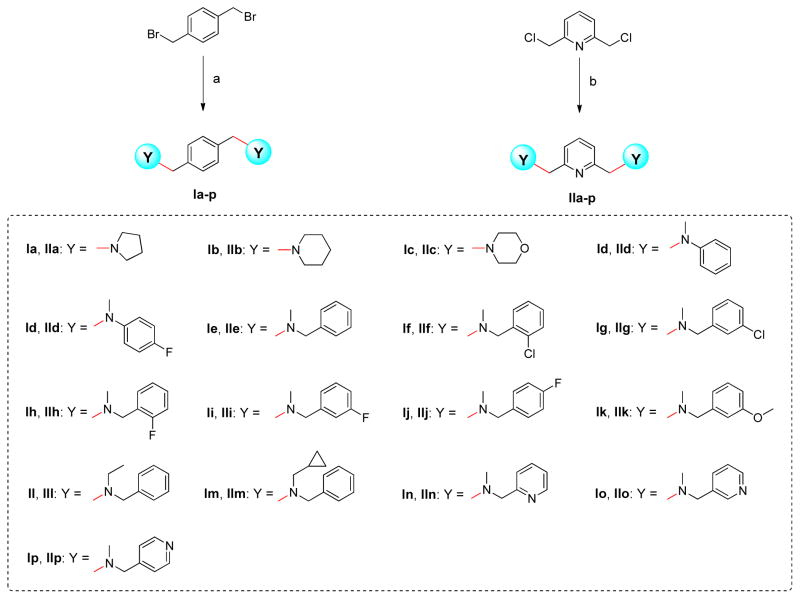
The synthetic route chosen to synthesize the title compounds was outlined in Scheme 1. The previous method to efficiently prepare symmetrical bis-secondary amine compounds was reductive amination of 1,4-benzenedialdehyde with phenylamine derivatives [30–32]. Since the reagents used in this preparation are secondary amines like N-alkyl anilines or cyclic amines, the yield is no longer satisfying. Herein, 1,4-bis(bromomethyl) benzene was used as the starting material, which reacted with corresponding secondary amines in anhydrous acetonitrile in the presence of potassium carbonate, and afforded the target compounds Ia-p [33]. Similarly, 2,6-bis(chloromethyl)pyridine reacted with amine derivatives in acetonitrile provided the title compounds IIa-p. Considering benzyl chloride is less active than benzyl bromide, cesium carbonate was used to shorten the reaction time.
Scheme 1.
Reagents and conditions to make our compounds: (a) CH3CN, K2CO3, reflux, 3 h, 78–95%; (b) CH3CN, Cs2CO3, reflux, 5 h, 70–90%.
2.2. Primary binding affinity screening
All the compounds were initially screened with a binding affinity assay as described in our previous publications [31, 34]. This assay involves competition of a potent CXCR4 peptidic inhibitor, TN14003, with the target compounds Ia-p and IIa-p. MDA-MB-231 cells were pre-incubated with compounds at concentrations of 1, 10, 100, and 1000 nM, then incubated with biotinylated TN14003 and streptavidin-conjugated rhodamine to determine the binding efficiency of the new compounds to the CXCL12 binding domain of CXCR4. The effective concentration (EC) is defined as the lowest concentration at which a significant reduction in the rhodamine fluorescent color is observed as compared to the control reflecting the competitive displacement by peptide TN14003. Thus, this screening is a semi-quantitative, first pass measure of the level of activity.
Just like the reported secondary amine derivatives, the symmetrical bis-tertiary amine framework also successfully retained the significant CXCR4 antagonistic effect. Nineteen of the 32 synthesized compounds exhibited moderate to good activity (≤ 1000 nM) in blocking the binding of peptide TN14003 to CXCR4 (Table 1 and Figure 2). Ten substances displayed potent binding affinity, with EC values from 1 to 100 nM. They were all comparatively more effective than the reference drug, AMD3100 (1000 nM).
Table 1.
Preliminary effective concentration (EC) of anti-CXCR4 compounds in our binding affinity assay.
| Compd | EC (nM) | Compd | EC (nM) | Compd | EC (nM) | Compd | EC (nM) |
|---|---|---|---|---|---|---|---|
| Ia | 1000 | Ii | >1000 | IIa | 1 | IIi | >1000 |
| Ib | 10 | Ij | >1000 | IIb | 1 | IIj | 1000 |
| Ic | >1000 | Ik | 10 | IIc | 100 | IIk | 10 |
| Id | 1000 | Il | 1000 | IId | 1000 | IIl | >1000 |
| Ie | 100 | Im | 1000 | IIe | 1000 | IIm | 1 |
| If | >1000 | In | >1000 | IIf | >1000 | IIn | 10 |
| Ig | 1000 | Io | >1000 | IIg | 10 | IIo | >1000 |
| Ih | >1000 | Ip | >1000 | IIh | 1000 | IIp | >1000 |
| AMD3100 | 1000 |
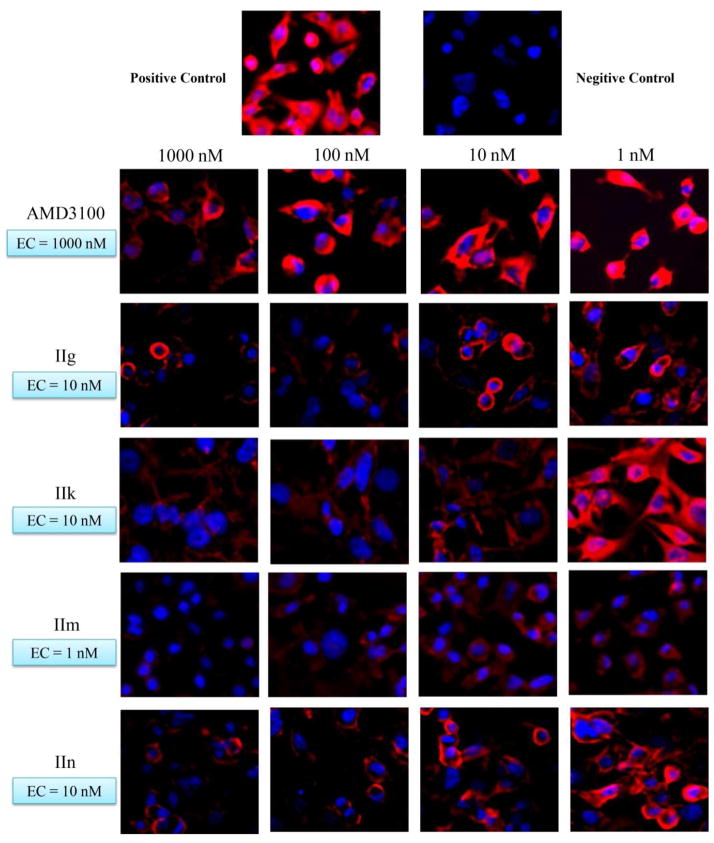
Figure 2.
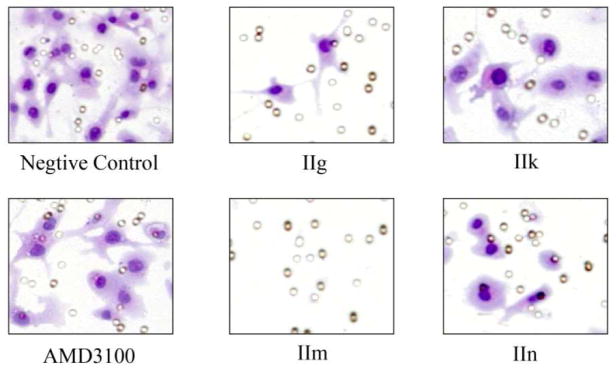
Fluorescence micrographs of binding affinity assay results of four selected compounds compared to AMD3100. The effective concentration (EC) of AMD3100 is 1000 nM, while those of the compounds IIg, IIk, IIm and IIn are better, EC of 10, 10, 1, and 10 nM, respectively.
When only the benzene central ring was substituted with a pyridine ring and the structure of side chains remained the same, most of the compounds demonstrated better activity. This result indicates that the central pyridine linker is well tolerated and further modification of the middle ring functionality is a reasonable strategy.
Surprisingly, if the terminal rings of the side chains were non-aromatic instead of aromatic amines (for example, a pyrrolidine, piperidine, or morpholine ring), the target compounds showed improved binding affinity to the compounds possessing aromatic side chains. Compounds IIa and IIb showed an EC of 1 nM, and experienced 1000-fold improvement compared with AMD3100. In addition, the substituent group introduced onto the 3′ position of the terminal ring may enhance the binding effect. By contrast, most compounds substituted into the 2′ or 4′ positions are less active or even inactive. For example, Ik, IIk and IIg all showed an EC of 10 nM, while compounds If, Ih, Ij, IIf, and IIk were not effective even at 1000 nM.
2.3. Matrigel invasion assay
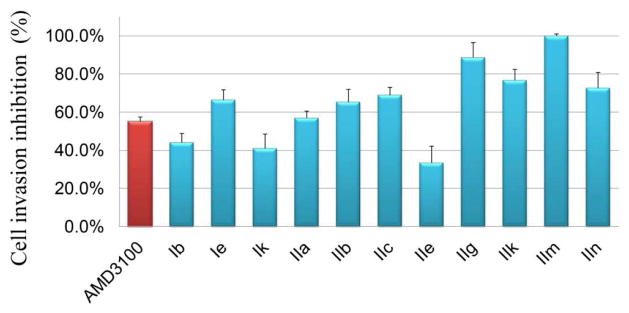
To model in vitro metastasis, a Matrigel invasion assay was used as the functional assay [31, 34]. This assay was performed for those compounds with an EC value lower than 1000 nM in the binding affinity assay to test whether they could block the CXCR4/CXCL12-mediated chemotaxis and invasion at a single concentration of 100 nM. The compounds and cells were added to the upper chamber and CXCL12 was added to the lower chamber as a chemoattractant. A layer of Matrigel matrix-coated permeable support (8 μm pore diameter) separates the upper and lower chambers. If the compounds demonstrate a strong CXCR4 inhibitory effect, fewer cells are able to move through the Matrigel. The results of Matrigel invasion were summarized in Figure 3 and Figure 4.
Figure 3.
Matrigel invasion inhibition of AMD3100 and anti-CXCR4 compounds
Figure 4.
Anti-Matrigel invasion effect of AMD3100 and four selected compounds. Fewer MDA-MB-231 cells are able to invade through the Matrigel after treatment with compounds IIg, IIk, IIm and IIn than AMD3100.
The invasion evaluation proved that most of the selected compounds performed well in both the binding affinity assay and blocking of Matrigel invasion assay. Only Ib, Ik and IIe exhibited an inhibitory rate below 50%. The potency of Ie (66.4%), IIb (65.3%), IIc (68.9%), IIg (88.6%), IIk (76.7%), IIm (100%) and IIn (72.6%) was superior to the reference drug AMD3100 (62.0%).
2.4. In vivo suppression against carrageenan-induced paw edema
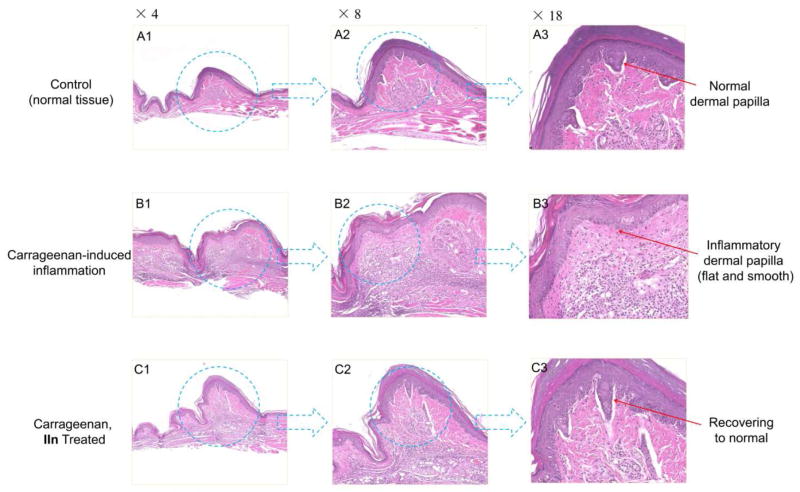
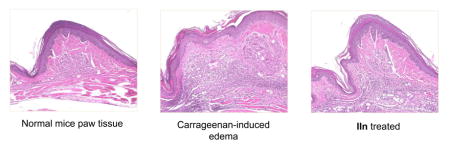
CXCR4 plays a key role in the recruitment of inflammatory cells to sites of inflammation. Blocking CXCR4 is a therapeutic strategy in inflammatory diseases [24]. Previously, we reported utilizing a carrageenan-induced mouse paw edema model as an efficient model to evaluate the in vivo CXCR4 antagonistic activity for CXCR4 inhibitors [27, 31]. It is a widely used test to assess anti-inflammatory activity in vivo. On the basis of the in vitro assay results, four of the best compounds (IIg, IIk, IIm and IIn) were investigated. Because of the in vivo toxicity of AMD3100, we have been using TN14003 as the reference drug for our animal experiments [27, 35]. As illustrated in Figure 5, although substances IIg and IIm demonstrated excellent CXCR4 antagonistic activity, they showed very weak in vivo potency (< 20%), which may be attributed to their metabolism or poor pharmacokinetic profile. Compound IIk exhibited moderate inhibition (31.0%). IIn showed a 50% inhibitory effect on inflammation, which was comparable to TN14003 (48%). Compared with the low success rate and high cost of the development of peptide drugs [36], small molecule CXCR4 inhibitors have practical advantages. After being treated with IIn, the inflammation induced in the left paw was clearly suppressed (Figure 6). As shown in histological analysis in Figure 7, the normal mouse paw tissue exhibits a dermis tightly connected to the epidermis through a basement membrane, and the papillary region is composed of loose areolar connective tissue (A1–3). However, the carrageenan-induced inflammatory tissue showed intense dermal papillae edema, and a dense infiltration of inflammatory cells (B1–3). Compound IIn significantly attenuated the mouse paw inflammation and damage in histological assay. Both the edema volume and the number of inflammatory cells decreased observably (C1–3). These data confirm that this selected anti-CXCR4 candidate can inhibit inflammation as anticipated.
Figure 5.
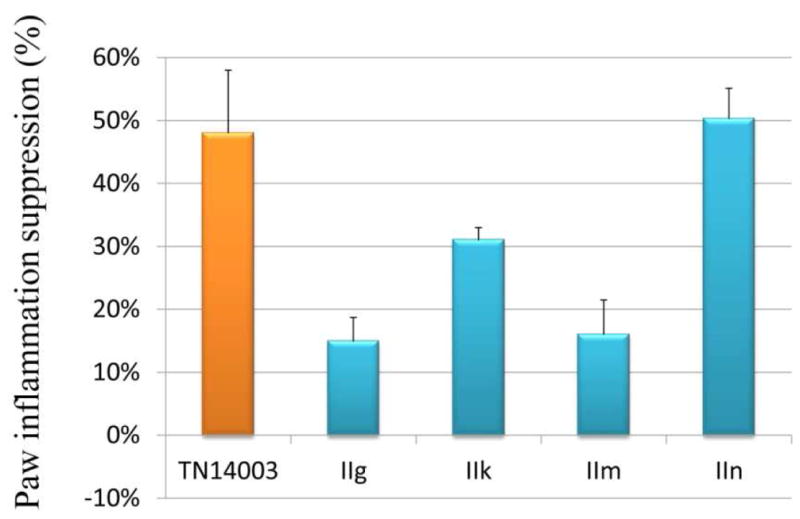
In vivo anti-inflammatory activity of compounds IIg, IIk, IIm and IIn.
Figure 6.
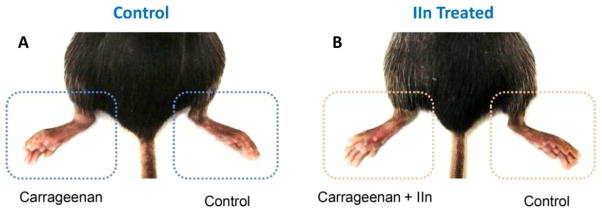
Suppression effect of anti-CXCR4 compound IIn on carrageenan-induced mouse paw inflammation. (A) Control mouse with left paw induced inflammation by carrageenan. (B) IIn treated mouse with left paw induced inflammation by carrageenan with about 50% suppression.
Figure 7.
Compound IIn significantly attenuated the mouse paw inflammation and damage in histological assay. Paw tissue sections were stained with H&E. The whole tissue slices were scanned/digitized by NanoZoomer 2.0 HT. Software NDP.view 2 was used to zoom in. Compared to the normal tissue (A1–3), carrageenan-induced skin inflammation exhibited intense dermal papillae edema, and a dense infiltration of inflammatory cells (B1–3). After being treated with IIn, both the edema volume and the number of inflammatory cells (dark purple) decreased observably (C1–3).
Since compound IIn, which possesses a pyridine pharmacophore, had the best suppression effect in vivo, we modified the terminal ring and synthesized compounds Io, Ip, IIo and IIp, bearing a nitrogen atom at the 3′ or 4′ position. Unfortunately, these compounds exhibited poor ECs in the binding affinity assay (> 1000 nM). Hence we stopped the further investigation of these compounds.
2.5. Discussion of paw edema results
Carrageenan-induced paw edema animal model is one of the most popular in vivo tests to screen anti-inflammatory properties of potential drugs. Complex sequential pathways for the inflammatory response to inter dermal injection of carrageenan were described by Vinergar R. et al [37]. Briefly, it consisted of a nonphagocytic inflammatory response followed by a phagocytic inflammatory response. The dermal nonphagocytic inflammatory response comprised edema, hyperemia, and hyperalgesia followed by hypoalgesia. The hyperalgesia and part of the edema were sensitive to cyclooxygenase (COX) inhibitors. The dermal phagocytic inflammatory response consisted of mobilization of neutrophils, monocytes, fibroblasts, and endothelial cells. While COX inhibitors reduce edema formation and hyperalgesia by blocking arachidonic acid metabolism, CXCR4 inhibitors reduce carrageenan-induced paw edema by blocking the recruitment of various inflammatory cells that are CXCR4-positive. In addition, CXCR4 has been shown to modulate NFκB that is the master switch of inflammation response [38].
Developing anti-CXCR4 drugs for inflammation indication provides an exciting avenue to target different pathways from COX which has been the major, if not the only one, target of anti-inflammation drugs. By combining drugs targeting different pathways, the efficacy will be enhanced without increasing dosage of a drug which is associated with unwanted side effects.
Previous reports on drug testing against inflammation using carrageenan-induced paw edema model were geared toward prevention by treating animals 30 minutes before carrageenan injection, which is clinically irrelevant [18, 39]. Patients present to hospital after the fact. In our evaluation, the compounds were treated after carrageenan injected.
2.6. Molecular modeling (docking) studies
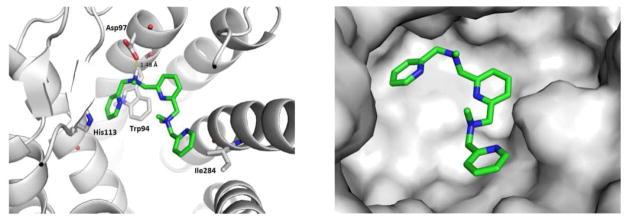
To better understanding the possible interaction between IIn and CXCR4, Schrodinger Maestro Package was performed based on the available crystal structure of CXCR4 (PDB code: 3ODU [40]). Figure 8 illustrated the binding pose with the best docking score. A hydrogen bond is formed between Asp97 and one of the nitrogen atoms of IIn. One pyridine ring shows a π-π stacking with both His113 and Trp94. The other pyridine ring demonstrates a partially hydrophobic interaction with Ile284. These favorable interactions are likely contributing to the binding of IIn with CXCR4.
Figure 8.
A low energy predicted binding pose for compound IIn in the CXCR4 X-ray structure (grey). The best docking pose is illustrated by two different visualizations: ribbon (left) and protein surface (right) representations.
3. Conclusion
On the basis of the promising previously prepared CXCR4 inhibitor 2, a series of novel symmetrical bis-tertiary amines, non-peptidic anti-CXCR4 small molecules, were designed and synthesized. The central benzene ring linker and side chains were modified and optimized to study the structure-activity relationship. This symmetrical bis-tertiary amine framework successfully maintained the significant CXCR4 antagonistic effect. Ten substances displayed potent binding affinity, with EC values from 1 to 100 nm. They were all comparatively more effective than the reference drug, AMD3100 (1000 nM). Most of the compounds with an EC lower than 1000 nM performed well in the invasion assay. Compounds Ie, IIb, IIc, IIg, IIk, IIm and IIn significantly blocked the tumor cell invasion. The inhibitory rates ranged from 65.3% to 100%, which were superior to AMD3100 (62.0%). Compound IIn showed a 50.3% suppressive effect against an inflammation animal model, comparable to the positive control, TN14003 (48.0%). These promising results demonstrate that symmetrical bis-tertiary amines are unique CXCR4 inhibitors with high potency.
4. Experimental section
4.1. Chemistry
4.1.1. General information
Proton and carbon NMR spectra were recorded on INOVA-400 (400 MHz) or VNMR-400 spectrometers at Emory NMR Research Center. The spectra obtained in deuteriochloroform (CDCl3) were referenced to the residual solvent peak. Chemical shifts (δ) are reported in parts per million (ppm) relative to residual undeuterated solvent as an internal reference. The following abbreviations were used to explain the multiplicities: s = single; d = doublet, t = triplet, q = quartet, dd = doublet of doublets, dt = doublet of triplets, m = multiplet, br = broad. Mass spectra were recorded on a JEOL spectrometer at Emory University Mass Spectrometry Center. Analytical thin layer chromatography (TLC) was performed on pre-coated glass backed plates from Scientific Adsorbents Incorporated (Silica Gel 60 F254; 0.25 mm thickness). Plates were visualized using ultraviolet radiation.
4.1.2. General Procedure for Synthesis of Compounds Ia-p
To a solution of 1,4-bis(bromomethyl)benzene (1 mmol) in anhydrous acetonitrile (10 mL) was added potassium carbonate (3 mmol) and the corresponding secondary amine (2.2 mmol). The reaction mixture was heated at reflux for 3 hours. The resulting mixture was diluted with water (20 mL) and extracted with ethyl acetate (15 mL) three times. The combined organic layers were washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo. The crude was purified by column chromatography with methanol/dichloromethane (150:1, v/v) to give the title compound as a colorless liquid or a white solid.
4.1.2.1. 1,4-Bis(pyrrolidin-1-ylmethyl)benzene (Ia)
Colorless liquid, yield 95%. 1H NMR (400 MHz, CDCl3) δ 7.27 (s, 4H), 3.59 (s, 4H), 2.48 – 2.53 (m, 8H), 1.76–1.80 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 137.95, 128.86, 60.54, 54.21, 23.49. HRMS calcd for C16H25N2 245.20123 [M + H]+, found 245.20084.
4.1.2.2. 1,4-Bis(piperidin-1-ylmethyl)benzene (Ib)
White solid, yield 92%, m.p. 84–86 °C. 1H NMR (400 MHz, CDCl3) δ 7.25 (s, 4H), 3.45 (s, 4H), 2.37 (s, 8H), 1.54–1.59 (m, 8H), 1.40–1.45 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 137.31, 129.19, 63.87, 54.70, 26.19, 24.61. HRMS calcd for C18H29N2 273.23253 [M + H]+, found 273.23243.
4.1.2.3. 1,4-Bis(morpholinomethyl)benzene (Ic)
White solid, yield 88%, m.p. 113–115 °C. 1H NMR (400 MHz, CDCl3) δ 7.27 (s, 4H), 3.70–3.72 (m, 8H), 3.48 (s, 4H), 2.43–2.45 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 136.83, 129.31, 67.20, 63.38, 53.80. HRMS calcd for C16H25O2N2 277.19105 [M + H]+, found 277.19089.
4.1.2.4. N,N′-(1,4-Phenylenebis(methylene))bis(N-methylaniline) (Id)
White solid, yield 83%, m.p. 77–79 °C. 1H NMR (400 MHz, CDCl3) δ 7.18–7.24 (m, 4H), 7.17 (s, 4H), 6.68– 6.76 (m, 6H), 4.51 (s, 4H), 3.00 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 149.87, 137.80, 129.32, 127.13, 116.67, 112.51, 56.51, 38.66. HRMS calcd for C22H25N2 317.20123 [M + H]+, found 317.20070.
4.1.2.5. N,N′-(1,4-phenylenebis(methylene))bis(4-fluoro-N-methylaniline) (Ie)
White solid, yield 80%, m.p. 78–80 °C. 1H NMR (400 MHz, CDCl3) δ 7.16 (s, 4H), 6.88–6.95 (m, 4H), 6.63–6.69 (m, 1H), 4.45 (s, 4H), 2.96 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 156.78, 154.44, 146.65, 137.74, 127.26, 115.76, 115.54, 113.82, 113.74, 57.37, 39.23. HRMS calcd for C22H22N2F2 352.17456 [M]+, found 352.17423.
4.1.2.6. N,N′-(1,4-Phenylenebis(methylene))bis(1-(2-chlorophenyl)-N-methylmethanamine) (If)
Colorless liquid, yield 78%. 1H NMR (400 MHz, CDCl3) δ 7.56 (dd, J = 1.5, 0.9 Hz, 1H), 7.54 (dd, J = 1.5, 0.9 Hz, 1H), 7.34 (d, J = 1.5 Hz, 1H), 7.32 (s, 1H), 7.32 (s, 4H), 7.22 – 7.26 (m, 2H), 7.14 – 7.19 (m, 2H), 3.63 (s, 4H), 3.57 (s, 4H), 2.20 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 138.14, 137.14, 134.43, 130.90, 129.58, 129.01, 128.17, 126.80, 62.21, 58.67, 42.46. HRMS calcd for C24H27N2Cl2 413.15458 [M + H]+, found 413.15445.
4.1.2.7. N,N′-(1,4-Phenylenebis(methylene))bis(1-(3-chlorophenyl)-N-methylmethanamine) (Ig)
Colorless liquid, yield 80%. 1H NMR (400 MHz, CDCl3) δ 7.37 (s, 2H), 7.31 (4, 1H), 7.20–7.25 (m, 6H), 3.52 (s, 4H), 3.48 (s, 4H), 2.18 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 141.73, 137.93, 134.34, 129.67, 129.08, 127.32, 127.17, 110.22, 61.87, 61.38, 42.42. HRMS calcd for C24H27N2Cl2 413.15458 [M + H]+, found 413.15482.
4.1.2.8. N,N′-(1,4-Phenylenebis(methylene))bis(1-(2-fluorophenyl)-N-methylmethanamine) (Ih)
Colorless liquid, yield 92%. 1H NMR (400 MHz, CDCl3) δ 7.44 (td, J = 7.5, 1.9 Hz, 2H), 7.31 (s, 4H), 7.19–7.25 (m, 2H), 7.11 (td, J = 7.5, 1.3 Hz, 2H), 6.99–7.04 (m, 2H), 3.59 (s, 3H), 3.54 (s, 3H), 2.20 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 162.79, 160.35, 138.05, 131.52, 131.48, 129.01, 128.72, 128.63, 126.10, 125.95, 124.06, 124.02, 115.47, 115.24, 61.90, 54.30, 42.29. HRMS calcd for C24H27N2F2 381.21368 [M + H]+, found 381.21303.
4.1.2.9. N,N′-(1,4-Phenylenebis(methylene))bis(1-(3-fluorophenyl)-N-methylmethanamine) (Ii)
Colorless liquid, yield 85%. 1H NMR (400 MHz, CDCl3) δ 7.31 (s, 4H), 7.24–7.29 (m, 2H), 7.09–7.13 (m, 4H), 6.90– 6.95 (m, 2H), 3.51 (s, 4H), 3.49 (s, 4H), 2.18 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 164.39, 161.94, 142.49, 142.42, 138.04, 129.80, 129.72, 129.00, 124.50, 124.47, 115.80, 115.59, 114.06, 113.84, 61.86, 61.43, 42.47. HRMS calcd for C24H27N2F2 381.21368 [M + H]+, found 381.21330.
4.1.2.10. N,N′-(1,4-Phenylenebis(methylene))bis(1-(4-fluorophenyl)-N-methylmethanamine) (Ij)
White solid, yield 81%, mp 80–82 °C. 1H NMR (400 MHz, CDCl3) δ 7.28–7.33 (m, 8H), 6.97–7.03 (m, 4H), 3.49 (s, 4H), 3.47 (s, 4H), 2.16 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 163.34, 160.91, 138.11, 135.27, 130.57, 130.49, 129.01, 115.28, 115.07, 61.78, 61.23, 42.37. HRMS calcd for C24H27N2F2 381.21368 [M + H]+, found 381.21343.
4.1.2.11. N,N′-(1,4-Phenylenebis(methylene))bis(1-(3-methoxyphenyl)-N-methylmethanamine) (Ik)
Colorless liquid, yield 87%. 1H NMR (400 MHz, CDCl3) δ 7.31 (s, 4H), 7.21–7.26 (m, 2H), 6.93–6.95 (m, 4H), 6.79–6.81 (m, 1H), 6.78 (dd, J = 2.7, 1.0 Hz, 1H), 3.81 (s, 6H), 3.50 (s, 4H), 3.49 (s, 4H), 2.18 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 159.77, 141.22, 138.05, 129.30, 128.98, 121.41, 114.41, 112.56, 61.98, 61.71, 55.33, 42.49. HRMS calcd for C26H33N2O2 405.25365 [M + H]+, found 405.25331.
4.1.2.12. N,N′-(1,4-Phenylenebis(methylene))bis(N-benzylethanamine) (Il)
Colorless liquid, yield 78%.1H NMR (400 MHz, CDCl3) δ 7.36 – 7.39 (m, 4H), 7.29 – 7.33 (m, 8H), 7.20 – 7.24 (m, 2H), 3.57 (s, 4H), 3.55 (s, 4H), 2.50 (q, J = 7.1 Hz, 4H), 1.07 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 140.27, 138.55, 128.93, 128.73, 128.31, 126.86, 57.89, 57.65, 47.24, 12.07. HRMS calcd for C26H33N2 373.26383 [M + H]+, found 373.26385.
4.1.2.13. N,N′-(1,4-Phenylenebis(methylene))bis(N-benzyl-1-cyclopropylmethanamine) (Im)
Colorless liquid, yield 80%. 1H NMR (400 MHz, CDCl3) δ 7.38–7.41 (m, 4H), 7.32 (s, 4H), 7.28–7.32 (m, 4H), 7.19–7.24 (m, 2H), 3.65 (s, 4H), 3.64 (s, 4H), 2.33 (s, 2H), 2.31 (s, 2H), 0.88–0.97 (m, 2H), 0.43–0.48 (m, 4H), 0.01–0.05 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 140.42, 138.68, 128.89, 128.68, 128.29, 126.83, 58.52, 58.37, 58.16, 8.68, 4.17. HRMS calcd for C30H37N2 425.29513 [M + H]+, found 425.29508.
4.1.2.14. N,N′-(1,4-Phenylenebis(methylene))bis(N-methyl-1-(pyridin-2-yl)methanamine) (In)
Colorless liquid, yield 82%. 1H NMR (400 MHz, CDCl3) δ 8.54 (dd, J = 1.8, 0.9 Hz, 1H), 8.53 (dd, J = 1.8, 1.0 Hz, 1H), 7.66 (td, J = 7.6, 1.8 Hz, 2H), 7.50–7.52 (m, 2H), 7.33 (s, 4H), 7.13–7.16 (m, 2H), 3.69 (s, 4H), 3.57 (s, 4H), 2.24 (s, 8H). 13C NMR (100 MHz, CDCl3) δ 159.87, 149.09, 137.83, 136.54, 128.99, 123.08, 122.02, 63.56, 61.98, 42.67. HRMS calcd for C22H27N4 347.22302 [M + H]+, found 347.22275.
4.1.2.15. N,N′-(1,4-Phenylenebis(methylene))bis(N-methyl-1-(pyridin-3-yl)methanamine) (Io)
Colorless liquid, yield 79%. 1H NMR (400 MHz, CDCl3) δ 8.57 (d, 2H), 8.50 (d, J = 1.7 Hz, 1H), 8.49 (d, J = 1.7 Hz, 1H), 7.71–7.72 (m, 1H), 7.69–7.70 (m, 1H), 7.31 (s, 4H), 7.24–7.28 (m, 2H), 3.53 (s, 4H), 3.52 (s, 4H), 2.19 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 150.42, 148.63, 137.90, 136.78, 134.88, 129.04, 123.55, 61.82, 59.06, 42.40. HRMS calcd for C22H27N4 347.22302 [M + H]+, found 347.22288.
4.1.2.16. N,N′-(1,4-Phenylenebis(methylene))bis(N-methyl-1-(pyridin-4-yl)methanamine) (Ip)
Colorless liquid, yield 80%. 1H NMR (400 MHz, CDCl3) δ 8.54–8.55 (m, 4H), 7.31–7.33 (m, 8H), 3.53 (s, 4H), 3.51 (s, 4H), 2.20 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 149.80, 149.02, 137.87, 129.01, 123.94, 61.96, 60.72, 42.61. HRMS calcd for C22H27N4 347.22302 [M + H]+, found 347.22290.
4.1.3. General Procedure for Synthesis of Compounds IIa-p
To a solution of 2,6-bis(chloromethyl)pyridine (1 mmol) in anhydrous acetonitrile (10 mL) was added cesium carbonate (3 mmol) and the corresponding secondary amine (2.2 mmol). The reaction mixture was heated at reflux for 5 hours. The resulting mixture was diluted with water (20 mL) and extracted with ethyl acetate (15 mL) three times. The combined organic layers were washed with brine, dried over anhydrous sodium sulfate, and concentrated in vacuo. The crude was purified by column chromatography with methanol/dichloromethane (100:1, v/v) to give the title compound as a colorless liquid or a white solid.
4.1.3.1. 2,6-Bis(pyrrolidin-1-ylmethyl)pyridine (IIa)
Colorless liquid, yield 86%. 1H NMR (400 MHz, CDCl3) δ 7.58 (t, J = 7.7 Hz, 1H), 7.25 (s, 1H), 7.24 (s, 1H), 3.75 (s, 4H), 2.52–2.58 (m, 8H), 1.73 – 1.81 (m, 8H). 13C NMR (100 MHz, CDCl3) δ 158.77, 136.89, 121.15, 62.41, 54.43, 23.69. HRMS calcd for C15H24N3 246.19647 [M + H]+, found 246.19644.
4.1.3.2. 2,6-Bis(piperidin-1-ylmethyl)pyridine (IIb)
Colorless liquid, yield 80%. 1H NMR (400 MHz, CDCl3) δ 7.59 (t, J = 7.7 Hz, 1H), 7.29 (s, 1H), 7.27 (s, 1H), 3.60 (s, 4H), 2.42–2.44 (m, 8H), 1.55–1.61 (m, 8H), 1.40–1.46 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 158.71, 136.67, 121.20, 65.57, 54.99, 26.20, 24.52. HRMS calcd for C17H28N3 274.22777 [M + H]+, found 274.22757.
4.1.3.3. 2,6-Bis(morpholinomethyl)pyridine (IIc)
White solid, yield 90%, m.p. 93–95 °C. 1H NMR (400 MHz, CDCl3) δ 7.63 (t, J = 7.7 Hz, 1H), 7.32 (s, 1H), 7.31 (s, 1H), 3.73 (t, 8H), 3.66 (s, 4H), 2.51 (t, 8H). 13C NMR (100 MHz, CDCl3) δ 157.90, 136.86, 121.56, 67.14, 65.02, 53.92. HRMS calcd for C15H24O2N3 278.18630 [M + H]+, found 278.18562.
4.1.3.4. N,N′-(Pyridine-2,6-diylbis(methylene))bis(N-methylaniline) (IId)
White solid, yield 75%, m.p. 91–93 °C. 1H NMR (400 MHz, CDCl3) δ 7.49 (t, J = 7.7 Hz, 1H), 7.20–7.25 (m, 4H), 7.02 (s, 1H), 7.00 (s, 1H), 6.70–6.75 (m, 6H), 4.65 (s, 4H), 3.13 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 159.44, 149.41, 137.61, 129.41, 119.01, 116.82, 112.32, 59.00, 39.24. HRMS calcd for C21H24N3 318.19647 [M + H]+, found 318.19664.
4.1.3.5. N,N′-(Pyridine-2,6-diylbis(methylene))bis(4-fluoro-N-methylaniline) (IIe)
Colorless liquid, yield 70%. 1H NMR (400 MHz, CDCl3) δ 7.52 (t, J = 7.7 Hz, 1H), 7.03 (t, J = 0.8 Hz, 1H), 7.01 (t, J = 0.8 Hz, 1H), 6.90–6.95 (m, 4H), 6.63–6.66 (m, 4H), 4.59 (s, 4H), 3.08 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 159.34, 156.83, 154.49, 146.14, 137.64, 129.87, 128.55, 119.19, 115.86, 115.64, 113.45, 113.38, 59.63, 39.85. HRMS calcd for C21H22F2N3 354.17763 [M + H]+, found 354.17776.
4.1.3.6. N,N′-(Pyridine-2,6-diylbis(methylene))bis(1-(2-chlorophenyl)-N-methylmethanamine) (IIf)
Colorless liquid, yield 80%. 1H NMR (400 MHz, CDCl3) δ 7.63 (t, J = 7.7 Hz, 1H), 7.57 (d, J = 1.8 Hz, 1H), 7.55 (d, J = 1.8 Hz, 1H), 7.42 (s, 1H), 7.40 (s, 1H), 7.34 (d, J = 1.5 Hz, 1H), 7.33 (d, J = 1.5 Hz, 1H), 7.15–7.25 (m, 4H), 3.77 (s, 4H), 3.72 (s, 4H), 2.29 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 158.88, 137.10, 136.75, 134.51, 130.97, 129.63, 128.32, 126.79, 121.33, 63.86, 58.85, 42.77. HRMS calcd for C23H26N3Cl2 414.14983 [M + H]+, found 414.14968.
4.1.3.7. N,N′-(Pyridine-2,6-diylbis(methylene))bis(1-(3-chlorophenyl)-N-methylmethanamine) (IIg)
Colorless liquid, yield 77%. 1H NMR (399 MHz, CDCl3) δ 7.68 (t, J = 7.7 Hz, 1H), 7.39 – 7.41 (m, 4H), 7.20 – 7.25 (m, 6H), 3.70 (s, 4H), 3.55 (s, 4H), 2.24 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 158.91, 141.55, 137.11, 134.34, 129.65, 129.08, 127.34, 127.16, 121.27, 63.69, 61.60, 42.76. HRMS calcd for C23H26N2Cl2 414.14983 [M + H]+, found 414.14902.
4.1.3.8. N,N′-(Pyridine-2,6-diylbis(methylene))bis(1-(2-fluorophenyl)-N-methylmethanamine) (IIh)
Colorless liquid, yield 79%. 1H NMR (400 MHz, CDCl3) δ 7.65 (t, J = 7.7 Hz, 1H), 7.45 (td, J = 7.5, 1.9 Hz, 2H), 7.41 (s, 1H), 7.39 (s, 1H), 7.20–7.25 (m, 2H), 7.10 (td, J = 7.5, 1.3 Hz, 2H), 6.99–7.04 (m, 2H), 3.73 (s, 4H), 3.66 (s, 4H), 2.26 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 162.76, 160.31, 158.86, 136.98, 131.57, 131.53, 128.81, 128.73, 125.67, 125.52, 123.98, 123.94, 121.21, 115.46, 115.24, 63.59, 54.60, 54.58, 42.47. HRMS calcd for C23H26N3F2 382.20893 [M + H]+, found 382.20767.
4.1.3.9. N,N′-(Pyridine-2,6-diylbis(methylene))bis(1-(3-fluorophenyl)-N-methylmethanamine) (IIi)
Colorless liquid, yield 80%. 1H NMR (400 MHz, CDCl3) δ 7.68 (t, J = 7.7 Hz, 1H), 7.42 (s, 1H), 7.40 (s, 1H), 7.23–7.29 (m, 2H), 7.13–7.15 (m, 2H), 7.11–7.12 (m, 2H), 6.90–6.95 (m, 2H), 3.70 (s, 4H), 3.57 (s, 4H), 2.25 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 164.31, 161.87, 158.89, 142.10, 142.03, 137.07, 129.77, 129.69, 124.49, 124.47, 121.17, 115.78, 115.56, 114.08, 113.87, 63.58, 61.59, 61.58, 42.68. HRMS calcd for C23H26N3F2 382.20893 [M + H]+, found 382.20915.
4.1.3.10. N,N′-(Pyridine-2,6-diylbis(methylene))bis(1-(4-fluorophenyl)-N-methylmethanamine) (IIj)
Colorless liquid, yield 77%. 1H NMR (400 MHz, CDCl3) δ 7.66 (t, J = 7.7 Hz, 1H), 7.39 (s, 1H), 7.37 (s, 1H), 7.30–7.35 (m, 4H), 6.96–7.02 (m, 4H), 3.68 (s, 4H), 3.54 (s, 4H), 2.22 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 163.28, 160.84, 158.94, 136.96, 134.86, 134.83, 130.55, 130.47, 121.14, 115.21, 115.00, 63.44, 61.34, 42.54. HRMS calcd for C23H26N3F2 382.20893 [M + H]+, found 382.20773.
4.1.3.11. N,N′-(Pyridine-2,6-diylbis(methylene))bis(1-(3-methoxyphenyl)-N-methylmethanamine) (IIk)
Colorless liquid, yield 90%. 1H NMR (400 MHz, CDCl3) δ 7.65 (dd, J = 7.9, 7.4 Hz, 1H), 7.42 (s, 1H), 7.40 (s, 1H), 7.20–7.24 (m, 2H), 6.94 – 6.97 (m, 4H), 6.80 (dd, J = 2.7, 1.4 Hz, 1H), 6.78 (dd, J = 2.7, 1.4 Hz, 1H), 3.81 (s, 6H), 3.69 (s, 4H), 3.56 (s, 4H), 2.25 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 159.79, 159.13, 141.01, 137.03, 129.35, 121.45, 121.14, 114.49, 112.61, 63.63, 62.24, 55.38, 42.81. HRMS calcd for C25H32N3O2 406.24890 [M + H]+, found 406.24852.
4.1.3.12. N,N′-(Pyridine-2,6-diylbis(methylene))bis(N-benzylethanamine) (IIl)
Colorless liquid, yield 86%. 1H NMR (400 MHz, CDCl3) δ 7.62 (t, J = 7.7 Hz, 1H), 7.44 (s, 1H), 7.42 (s, 1H), 7.36– 7.39 (m, 4H), 7.26–7.32 (m, 4H), 7.19–7.23 (m, 2H), 3.72 (s, 4H), 3.62 (s, 4H), 2.55 (q, J = 7.1 Hz, 4H), 1.08 (t, J = 7.1 Hz, 6H). 13C NMR (100 MHz, CDCl3) δ 159.75, 139.86, 136.84, 128.88, 128.28, 126.89, 120.61, 59.87, 58.16, 47.80, 12.15. HRMS calcd for C25H32N3 374.25907 [M + H]+, found 374.25907.
4.1.3.13. N,N′-(Pyridine-2,6-diylbis(methylene))bis(N-benzyl-1-cyclopropylmethanamine) (IIm)
Colorless liquid, yield 72%. 1H NMR (400 MHz, CDCl3) δ 7.64 (dd, J = 8.1, 7.2 Hz, 1H), 7.50 (s, 1H), 7.48 (s, 1H), 7.40–7.42 (m, 4H), 7.28–7.33 (m, 4H), 7.20–7.24 (m, 2H), 3.84 (s, 4H), 3.71 (s, 4H), 2.40 (s, 2H), 2.38 (s, 2H), 0.89–0.99 (m, 2H), 0.43–0.48 (m, 4H), 0.01–0.07 (m, 4H). 13C NMR (100 MHz, CDCl3) δ 159.88, 140.08, 136.85, 128.88, 128.33, 126.92, 120.66, 60.34, 59.01, 58.71, 8.72, 4.15. HRMS calcd for C29H36N3 426.29037 [M + H]+, found 426.28990.
4.1.3.14. N,N′-(Pyridine-2,6-diylbis(methylene))bis(N-methyl-1-(pyridin-2-yl)methanamine) (IIn)
Colorless liquid, yield 75%. 1H NMR (400 MHz, CDCl3) δ 8.51 (dd, J = 1.8, 0.9 Hz, 1H), 8.50 (dd, J = 1.8, 0.9 Hz, 1H), 7.64 (d, J = 1.7 Hz, 1H), 7.62 (d, J = 1.7 Hz, 1H), 7.60 (d, J = 1.7 Hz, 1H), 7.49 (t, J = 7.8, 1.1 Hz, 1H), 7.47 (t, J = 7.8, 1.1 Hz, 1H), 7.38 (s, 1H), 7.36 (s, 1H), 7.13 (dd, J = 4.9, 1.2 Hz, 1H), 7.11 (dd, J = 4.9, 1.2 Hz, 1H), 3.74 (s, 4H), 3.73 (s, 4H), 2.27 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 159.31, 158.63, 149.17, 137.03, 136.60, 123.23, 122.15, 121.41, 63.70, 63.63, 42.83. HRMS calcd for C21H26N5 348.21827 [M + H]+, found 348.21800.
4.1.3.15. N,N′-(Pyridine-2,6-diylbis(methylene))bis(N-methyl-1-(pyridin-3-yl)methanamine) (IIo)
Colorless liquid, yield 74%. 1H NMR (400 MHz, CDCl3) δ 8.59 (d, J = 0.8 Hz, 1H), 8.59 (d, J = 0.8 Hz, 1H), 8.50 (d, J = 1.7 Hz, 1H), 8.49 (d, J = 1.7 Hz, 1H), 7.72 (t, J = 2.0 Hz, 1H), 7.70 (t, J = 2.0 Hz, 1H), 7.67 (t, J = 7.7 Hz, 1H), 7.40 (s, 1H), 7.38 (s, 1H), 7.28–7.23 (m, 2H), 3.71 (s, 4H), 3.59 (s, 4H), 2.25 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 158.80, 150.56, 148.73, 137.16, 136.75, 134.61, 123.48, 121.34, 63.60, 59.38, 42.70. HRMS calcd for C21H26N5 348.21827 [M + H]+, found 348.21814.
4.1.3.16. N,N′-(Pyridine-2,6-diylbis(methylene))bis(N-methyl-1-(pyridin-4-yl)methanamine) (IIp)
Colorless liquid, yield 81%. 1H NMR (400 MHz, CDCl3) δ 8.53–8.55 (m, 4H), 7.69 (t, J = 7.7 Hz, 1H), 7.42 (s, 1H), 7.40 (s, 1H), 7.32–7.34 (m, 4H), 3.71 (s, 4H), 3.59 (s, 4H), 2.27 (s, 6H). 13C NMR (100 MHz, CDCl3) δ 158.70, 149.88, 148.62, 137.25, 123.92, 121.35, 63.72, 60.97, 42.91. HRMS calcd for C21H26N5 348.21827 [M + H]+, found 348.21813.
4.2. Primary binding affinity screening
For binding affinity assay, 2 × 104 MDA-MB-231 cells in 300 μL of cell culture medium were seeded in an 8-well slide chamber 2 days before the experiments were conducted. Various concentrations of different compounds (1, 10, 100, or 1000 nM) were added to the separate wells and incubated for 10 minutes at room temperature, and then the cells were fixed in 4% ice-cold paraformaldehyde. The cells were rehydrated in phosphate-buffered saline (PBS). The slides were subsequently incubated for 30 minutes at room temperature with 0.05 μg/mL biotinylated TN14003, washed three times with PBS, and incubated in streptavidin-rhodamine (1:150 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) for 30 minutes at room temperature. Finally, the slides were washed with PBS and mounted in an anti-fade mounting solution (Molecular Probes, Eugene, OR), and the samples were analyzed on a Nikon Eclipse E800 microscope [31, 34].
4.3. Matrigel invasion assay
Matrigel invasion assay was performed by using a Matrigel invasion chamber from Corning Biocoat (Bedford MA). CXCL12α (200 ng/mL; R & D Systems, Minneapolis, MN) was added to the bottom chamber to induce the invasion of MDA-MB-231 cells through the Matrigel. The selected compounds or AMD3100 were added to the cells (100 nM) before the cells were seeded in the top chamber. The Matrigel invasion chamber was incubated for 22 hours in a humidified cell culture incubator. First, non-invading cells were removed from the top of the Matrigel with a cotton-tipped swab. Invading cells on the filter at the bottom of the Matrigel were fixed in methanol and stained with hematoxylin and eosin (H&E). The percent of invasion was determined by counting the H&E stained cells [31, 34].
4.4. Paw Inflammation Suppression Test
Acute inflammation was induced by subcutaneous injection of 50 μL of λ-carrageenan (1% w/v in saline) into one of the hind paws of the female C57BL/6J mice (Jackson Laboratories); the other hind paw was injected with 50 μL of saline, which was used as a non-inflammation control. The selected compounds were dissolved in 10% DMSO and 90% of 45% (2-hydroxypropyl)-β-cyclodextrin (CD) in PBS. In the treatment group, compounds IIg, IIk, IIm and IIn were administered intraperitoneally (i.p.) at 10 mg/kg daily, while TN14003 was administered at 300 μg/kg, i.p., daily, 30 minutes following carrageenan challenge. The rationale for using 300 μg/kg for TN14003 was that we found this concentration to be the minimum concentration needed to achieve the maximum efficacy of this compound in a breast cancer metastasis animal model [41]. Control animals received corresponding i.p. injections of the vehicle. The animals were sacrificed 74 hours after carrageenan challenge and 2 hours after the last injection of the selected compounds. The final paws were photographed and measured for thickness from the “palm” to the back of the paw with a caliper. These were compared to the volume of carrageenan-untreated contralateral paw to obtain the edema volume; the volume of the contralateral paw was subtracted from the volume of the carrageenan injected paw to obtain the edema volume. The inflammation-suppression percentage was calculated by comparing the drug-treated group to the control group. Five mice per group were used to determine the effect of the CXCR4 inhibitors as previously described [27, 31].
4.5. Molecular modeling (docking) studies
The docking experiment was performed by Schrodinger Maestro Package [42]. The crystal structure of CXCR4 (PDB code: 3ODU [40]) was processed following the established Protein Prepare Wizard workflow to obtain the receptor structure suitable for docking [43]. All water molecules were deleted and only Chain A was used to prepare the receptor. All residues beyond 20 Å of the ligand were removed to accelerate the calculation speed. The binding site was selected using the default Receptor Grid Generation procedure and the docking grid was generated around the co-crystalized small molecule ligand IT1t. The IIn ligand was initially drawn by ChemDraw as a 2D structure and then prepared by the Ligprep application to obtain the 3D conformers for docking [44]. The prepared ligand was then flexibly docked onto the receptor using Glide [45] and the default setting parameters with no constrains. 10 docking poses were obtained and the one with the best Glide score was chosen to study the interaction. The plot (Figure 8) was constructed using Pymol version 0.99 [46].
Supplementary Material
Highlights.
A series of novel bis-tertiary amines targeting CXCR4 were designed and synthesized.
The bis-tertiary amine framework maintained the potent anti-CXCR4 activity.
Ten compounds displayed potent binding affinity with EC values from 1 to 100 nM.
Five compounds exhibited stronger invasion inhibitory activity than AMD3100, a clinical lead compound.
Compound IIn showed a 50.3% suppressive effect in a paw inflammation animal model.
Acknowledgments
This study was financially supported by a research grant from NIH NCI (R01 CA165306). We thank Ms. Jessica Paulishen and Mr. Andrew Teodorescu for proof-reading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Debnath B, Xu S, Grande F, Garofalo A, Neamati N. Small molecule inhibitors of CXCR4. Theranostics. 2013;3:47–75. doi: 10.7150/thno.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Das D, Maeda K, Hayashi Y, Gavande N, Desai DV, Chang SB, Ghosh AK, Mitsuya H. Insights into the mechanism of inhibition of CXCR4: identification of Piperidinylethanamine analogs as anti-HIV-1 inhibitors. Antimicrob Agents Chemother. 2015;59:1895–1904. doi: 10.1128/AAC.04654-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poty S, Desogere P, Goze C, Boschetti F, D’Huys T, Schols D, Cawthorne C, Archibald SJ, Maecke HR, Denat F. New AMD3100 derivatives for CXCR4 chemokine receptor targeted molecular imaging studies: synthesis, anti-HIV-1 evaluation and binding affinities. Dalton Trans. 2015;44:5004–5016. doi: 10.1039/c4dt02972k. [DOI] [PubMed] [Google Scholar]
- 4.Blades MC, Manzo A, Ingegnoli F, Taylor PR, Panayi GS, Irjala H, Jalkanen S, Haskard DO, Perretti M, Pitzalis C. Stromal cell-derived factor 1 (CXCL12) induces human cell migration into human lymph nodes transplanted into SCID mice. J Immunol. 2002;168:4308–4317. doi: 10.4049/jimmunol.168.9.4308. [DOI] [PubMed] [Google Scholar]
- 5.Liao YX, Fu ZZ, Zhou CH, Shan LC, Wang ZY, Yin F, Zheng LP, Hua YQ, Cai ZD. AMD3100 reduces CXCR4-mediated survival and metastasis of osteosarcoma by inhibiting JNK and Akt, but not p38 or Erk1/2, pathways in in vitro and mouse experiments. Oncol Rep. 2015;34:33–42. doi: 10.3892/or.2015.3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zachariassen ZG, Thiele S, Berg EA, Rasmussen P, Fossen T, Rosenkilde MM, Våbenø J, Haug BE. Design, synthesis, and biological evaluation of scaffold-based tripeptidomimetic antagonists for CXC chemokine receptor 4 (CXCR4) Bioorg Med Chem. 2014;22:4759–4769. doi: 10.1016/j.bmc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 8.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 9.Burger M, Hartmann T, Krome M, Rawluk J, Tamamura H, Fujii N, Kipps TJ, Burger JA. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106:1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 10.Woodard LE, Nimmagadda S. CXCR4-based imaging agents. J Nucl Med. 2011;52:1665–1669. doi: 10.2967/jnumed.111.097733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 12.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 13.Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, Bianchi R, Basik M. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–283. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 14.Allegretti M, Cesta MC, Garin A, Proudfoot AE. Current status of chemokine receptor inhibitors in development. Immunol Lett. 2012;145:68–78. doi: 10.1016/j.imlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Burger M, Glodek A, Hartmann T, Schmitt-Graff A, Silberstein LE, Fujii N, Kipps TJ, Burger JA. Functional expression of CXCR4 (CD184) on small-cell lung cancer cells mediates migration, integrin activation, and adhesion to stromal cells. Oncogene. 2003;22:8093–8101. doi: 10.1038/sj.onc.1207097. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Wang J, Sun Y, Song W, Nor JE, Wang CY, Taichman RS. Diverse signaling pathways through the CXCL12/CXCR4 chemokine axis in prostate cancer cell lines leads to altered patterns of cytokine secretion and angiogenesis. Cell Signal. 2005;17:1578–1592. doi: 10.1016/j.cellsig.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 17.El Rayes T, Catena R, Lee S, Stawowczyk M, Joshi N, Fischbach C, Powell CA, Dannenberg AJ, Altorki NK, Gao D, Mittal V. Lung inflammation promotes metastasis through neutrophil protease-mediated degradation of Tsp-1. Proc Natl Acad Sci USA. 2015;112:16000–16005. doi: 10.1073/pnas.1507294112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang B, Huang X, Yao H, Jiang J, Wu X, Jiang S, Wang Q, Lu T, Xu J. Discovery of potential anti-inflammatory drugs: diaryl-1,2,4-triazoles bearing N-hydroxyurea moiety as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase. Org Biomol Chem. 2014;12:2114–2127. doi: 10.1039/c3ob41936c. [DOI] [PubMed] [Google Scholar]
- 19.Rahme E, Bernatsky S. NSAIDs and risk of lower gastrointestinal bleeding. Lancet. 2010;376:146–148. doi: 10.1016/S0140-6736(10)60839-2. [DOI] [PubMed] [Google Scholar]
- 20.Dajani EZ, Islam K. Cardiovascular and gastrointestinal toxicity of selective cyclo-oxygenase-2 inhibitors in man. J Physiol Pharmacol. 2008;59:117–133. [PubMed] [Google Scholar]
- 21.Fosslien E. Adverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci. 1998;28:67–81. [PubMed] [Google Scholar]
- 22.Solomon DH. Selective cyclooxygenase 2 inhibitors and cardiovascular events. Arthritis Rheum. 2005;52:1968–1978. doi: 10.1002/art.21132. [DOI] [PubMed] [Google Scholar]
- 23.Dogne JM, Supuran CT, Pratico D. Adverse cardiovascular effects of the coxibs. J Med Chem. 2005;48:2251–2257. doi: 10.1021/jm0402059. [DOI] [PubMed] [Google Scholar]
- 24.Hummel S, Van Aken H, Zarbock A. Inhibitors of CXC chemokine receptor type 4: putative therapeutic approaches in inflammatory diseases. Curr Opin Hematol. 2014;21:29–36. doi: 10.1097/MOH.0000000000000002. [DOI] [PubMed] [Google Scholar]
- 25.Choi WT, Duggineni S, Xu Y, Huang Z, An J. Drug discovery research targeting the CXC chemokine receptor 4 (CXCR4) J Med Chem. 2012;55:977–994. doi: 10.1021/jm200568c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Clercq E. The bicyclam AMD3100 story. Nat Rev Drug Discov. 2003;2:581–587. doi: 10.1038/nrd1134. [DOI] [PubMed] [Google Scholar]
- 27.Liang Z, Zhan W, Zhu A, Yoon Y, Lin S, Sasaki M, Klapproth JM, Yang H, Grossniklaus HE, Xu J, Rojas M, Voll RJ, Goodman MM, Arrendale RF, Liu J, Yun CC, Snyder JP, Liotta DC, Shim H. Development of a unique small molecule modulator of CXCR4. PloS One. 2012;7:e34038. doi: 10.1371/journal.pone.0034038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz MK, Wells TN. New therapeutics that modulate chemokine networks. Nat Rev Drug Discov. 2002;1:347–358. doi: 10.1038/nrd795. [DOI] [PubMed] [Google Scholar]
- 29.Scozzafava A, Mastrolorenzo A, Supuran CT. Non-peptidic chemokine receptors antagonists as emerging anti-HIV agents. J Enzyme Inhib Med Chem. 2002;17:69–76. doi: 10.1080/14756360290024227. [DOI] [PubMed] [Google Scholar]
- 30.Zhan W, Liang Z, Zhu A, Kurtkaya S, Shim H, Snyder JP, Liotta DC. Discovery of small molecule CXCR4 antagonists. J Med Chem. 2007;50:5655–5664. doi: 10.1021/jm070679i. [DOI] [PubMed] [Google Scholar]
- 31.Zhu A, Zhan W, Liang Z, Yoon Y, Yang H, Grossniklaus HE, Xu J, Rojas M, Lockwood M, Snyder JP, Liotta DC, Shim H. Dipyrimidine amines: a novel class of chemokine receptor type 4 antagonists with high specificity. J Med Chem. 2010;53:8556–8568. doi: 10.1021/jm100786g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mooring SR, Gaines T, Liang Z, Shim H. Synthesis of pyridine derivatives as potential antagonists of chemokine receptor type 4. Heterocycl Comm. 2014;20:149–153. doi: 10.1515/hc-2014-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y, Hazeldine ST, Li J, Oupicky D. Development of functional poly(amido amine) CXCR4 antagonists with the ability to mobilize leukocytes and deliver nucleic acids. Adv Healthc Mater. 2015;4:729–738. doi: 10.1002/adhm.201400608. [DOI] [PubMed] [Google Scholar]
- 34.Mooring SR, Liu J, Liang Z, Ahn J, Hong S, Yoon Y, Snyder JP, Shim H. Benzenesulfonamides: a unique class of chemokine receptor type 4 inhibitors. ChemMedChem. 2013;8:622–632. doi: 10.1002/cmdc.201200582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shu HK, Yoon Y, Hong S, Xu K, Gao H, Hao C, Torres-Gonzalez E, Nayra C, Rojas M, Shim H. Inhibition of the CXCL12/CXCR4-axis as preventive therapy for radiation-induced pulmonary fibrosis. PloS One. 2013;8:e79768. doi: 10.1371/journal.pone.0079768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Vinegar R, Truax JF, Selph JL, Johnston PR, Venable AL, McKenzie KK. Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed Proc. 1987;46:118–126. [PubMed] [Google Scholar]
- 38.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wan YJ, Badr MZ. Inhibition of Carrageenan-Induced Cutaneous Inflammation by PPAR Agonists Is Dependent on Hepatocyte-Specific Retinoid X ReceptorAlpha. PPAR Res. 2006;2006:96341. doi: 10.1155/PPAR/2006/96341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu B, Chien EY, Mol CD, Fenalti G, Liu W, Katritch V, Abagyan R, Brooun A, Wells P, Bi FC, Hamel DJ, Kuhn P, Handel TM, Cherezov V, Stevens RC. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science. 2010;330:1066–1071. doi: 10.1126/science.1194396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoon Y, Liang Z, Zhang X, Choe M, Zhu A, Cho HT, Shin DM, Goodman MM, Chen ZG, Shim H. CXC chemokine receptor-4 antagonist blocks both growth of primary tumor and metastasis of head and neck cancer in xenograft mouse models. Cancer Res. 2007;67:7518–7524. doi: 10.1158/0008-5472.CAN-06-2263. [DOI] [PubMed] [Google Scholar]
- 42.Schrödinger Release 2015–4: Maestro, version 10.4. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- 43.Protein Preparation Wizard. Schrödinger, LLC; New York, NY: 2012. [Google Scholar]
- 44.Ligprep, version 3.6. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- 45.Glide, version 6.9. Schrödinger, LLC; New York, NY: 2015. [Google Scholar]
- 46.Glide, version 0.99. Schrödinger, LLC; New York, NY: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.