Abstract
Background:
Though trabeculectomy is often performed on patients with medically refractive pigmentary glaucoma (PG), the clinical outcomes of surgical treatment on PG remain unknown. The aim of this study was to summarize the long-term efficacy and safety of trabeculectomy on PG.
Methods:
This was a prospective case series observational study. Eighteen consecutive PG patients were followed up for 8 years after trabeculectomy from May 2006 to April 2007. Visual acuity (VA), best-corrected visual acuity (BCVA), slit lamp biomicroscopy, intraocular pressure (IOP) measurement, Humphrey visual field analysis (VFA), and stereoscopic funduscopy were performed on admission and every 6 months after the surgery. Postoperative IOP, VA, BCVA, VFA, adjunctive anti-glaucoma medication, treatment-related side-effects, changes in blebs, and main clinical findings in the anterior segment of PG were recorded and compared with the baseline.
Results:
Eighteen PG eyes from 18 patients, with average preoperative IOP of 34.5 ± 4.7 mmHg (range: 21–47 mmHg, 1 mmHg=0.133 kPa) were enrolled in this study. All enrolled patients completed the follow-up visits and required examinations. Eight years after trabeculectomy, all surgical eyes (18/18) had satisfactory IOP control with an average of 13.7 ± 2.5 mmHg (range: 9–19 mmHg), which was significantly lower than baseline (P = 0.001). Majority (15/18) of the PG eyes had stable VA, BCVA, VFA, and optic disc cupping parameters. Functional blebs still existed in 12/18 of the PG eyes at the last follow-up visit. Unanimously, pigmentation in the anterior segment attenuated with time after surgical treatment. No severe side-effects were recorded in any of the surgical eyes.
Conclusions:
All surgical PG eyes in this study had satisfactory IOP control 8 years after the surgery with well-preserved visual function. The long-term efficacy and safety of trabeculectomy are promising in PG patients.
Keywords: Pigment Dispersion Syndrome, Pigmentary Glaucoma, Trabeculectomy
INTRODUCTION
Pigment dispersion syndrome (PDS) results from posterior bowing of the iris and rubbing between the lens zonules and epithelial layer of the iris, and usually affects myopic eyes in men during the third to fourth decade of life.[1,2,3,4,5,6,7,8,9,10,11] The literature indicated that Caucasians have the highest prevalence of PDS among all races,[12] with clinical characteristics including Krukenberg spindle, homogeneous trabecular meshwork (TM) pigmentation, and spoke-like mid-peripheral iris transillumination defects (ITDs), which are referred as a triad of PDS. Zonular and lenticular pigmentation on posterior lens surface are also common findings in PDS patients after mydriasis.[8,9,10,11,12,13,14,15,16,17,18,19,20]
Pigmentary glaucoma (PG) is usually inevitable if PDS is not detected and interrupted at an early stage. Gradual pigmentation on the TM may lead to increased aqueous humor outflow resistance, resultant elevated intraocular pressure (IOP), and glaucomatous neuropathy.[7,12,18] TM pigmentation has been widely accepted as the cause of PG, which is resultant from PDS.[21,22,23,24,25] As secondary open-angle glaucoma, PG has much in common with primary open-angle glaucoma (POAG) in clinical manifestations and treatment principle except for laser iridoctomy to eliminate the reverse pupillary block. Trabeculectomy is commonly applied in advanced PG patients when laser treatment and/or antiglaucoma medication fail to arrest the high IOP. The literature has numerous references addressing efficacy and safety of trabeculectomy on POAG, while it has quite limited references on clinical outcomes of PG. Because the prevalence of PG is much lower than that of POAG, the number of PDS/PG patients is rare in clinical practice. As a consequent, it is difficult to collect enough PG patients for scientific research in majority of hospitals. The aim of the current study was to summarize the long-term clinical outcomes of trabeculectomy in 18 consecutive Chinese patients with PG in North China, which can provide a valuable reference for ophthalmologists in the management of PG.
METHODS
Patient enrollment and inclusion criteria
All outpatients presenting for care at the glaucoma specialty clinic at Beijing Tongren Eye Center, Beijing from May 2006 to April 2007 were evaluated for signs of PDS: corneal endothelial pigmentation, anterior iris stromal pigment dusting, ITDs, posterior iris bowing, increased TM pigmentation, and pigment granule dusting on lens zonules or peripheral posterior surface. Detailed ophthalmic examinations included visual acuity (VA), best-corrected visual acuity (BCVA), IOP measurement, slit lamp biomicroscopy pre- and post-mydriasis, gonioscopy, funduscopic examination, and automated Humphrey Swedish interactive thresholding algorithm (SITA)-standard 30-2 visual field analysis (VFA). Systemic and ocular medical histories were also recorded. All clinicians attended lectures on the clinical features of PDS in different racial groups and how to detect subtle clinical signs of PDS in pigmented racial patients before the study.
TM pigmentation was graded according to Scheie's grading system. Corneal endothelial pigment dusting was described as: Krukenberg spindle, diffusive pattern, or none. If the patient had received antiglaucoma medication before the evaluation, the IOP measured before medication was taken as the initial IOP. Slit-lamp examination, gonioscopy, and funduscopic examination of the optic disc of all subjects were performed and graded by the same doctor (Dr. Guo-Ping Qing) in order to avoid inter-physician bias.
Diagnostic criteria for PDS in Chinese patients included at least two of the following three signs: Krukenberg spindle, homogenous moderate to heavy TM pigmentation (≥ Scheie II), and any degree of zonular and/or lenticular pigment granule dusting. Patients with a history of uveitis, trauma, previous ocular surgery or anterior segment laser treatment, or any evidence of exfoliation material were excluded. Diagnosis of PG was made if PDS patient had two or more of the following findings: initial IOP >21 mmHg, glaucomatous optic nerve damage (increased cupping or glaucomatous disk appearance), or visual field (VF) defect.
We certified that all applicable institutional and governmental regulations concerning the ethical use of human subjects were followed during this research. The research followed the tenets of the Declaration of Helsinki. Informed consent was obtained from each subject after explanation of the nature and possible consequences of the study, which was approved by the Institutional Review Board of Beijing Tongren Hospital, Capital Medical University.
Primary outcomes
Eighteen consecutive PG patients were identified and received trabeculectomy in at least one eye for medically refractory glaucoma. They received regular follow-up after surgical treatment. Baseline examination included VA, BCVA, IOP, slit-lamp biomicroscopy pre- and post-mydriasis, gonioscopy, funduscopic examination, and automated Humphrey SITA-standard 30-2 VFA. These exams were repeated every 6 months posttrabeculectomy. Postoperative IOP, VA, BCVA, VFA parameters, optic disc cupping, morphological changes in bleb, and characteristic clinical findings of PG were main outcome parameters and were compared to the baseline values. All enrolled patients completed the 8-year follow-up after trabeculectomy.
Statistical analysis
All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Data were shown as a mean ± standard deviation (SD). The paired-sample t-test was applied to compare postoperative IOP, VA, BCVA, and VFA parameters at the last follow-up visit with the baseline. A value of P < 0.05 was considered statistically significant.
RESULTS
Patients’ demographic characteristics
Eighteen patients (12 males and 6 females) out of 94 suspected PDS in the glaucoma specialty clinic from May 2006 to April 2007 were identified as having PG in at least one eye, according to the diagnostic criteria. The mean age of the PG patients at enrollment was 35.5 ± 7.0 years (range: 22–49 years). The mean ages for male and female subjects were 35.7 ± 6.9 (range: 22–48 years) and 35.2 ± 8.0 years (range: 26–49 years), respectively. The initial IOP of the research PG eyes were 30.8 ± 10.4 mmHg (range: 22–41 mmHg, 1 mmHg=0.133 kPa) before trabeculectomy, with mild to severe VF defect. Humphrey VFA at enrollment showed that mean deviation (MD) of the research eyes before surgery was −15.2 ± 6.6 dB (ranging from −30.4 dB to −2.6 dB).
All but two eyes of two patients had myopia of −0.5D or greater, with a mean refractive error of −5.20 ± 5.79 (ranging from −24.75 to +0.5) spherical equivalent diopters. These two eyes had hyperopia of 0.75D and 1.25D, respectively. Majority of the patients (14/18) had bilateral PG, while the other four patients had only one eye affected with PG. Of patients with bilateral PG, nine (9/14) received trabeculectomy in both eyes for medically refractory glaucoma, and one eye was chosen randomly for research. Of nine patients who were treated with trabeculectomy in only one eye with PG, all were enrolled for the study.
Intraocular pressure reduction and visual function preservation
All enrolled patients completed the follow-up visits and required examinations. Eight years after trabeculectomy, all surgical PG eyes (18/18) had satisfactory IOP control along with well-maintained VA and visual function. The mean IOP of research eyes was 13.7 ± 2.5 mmHg (range: 9–19 mmHg) at the last follow-up visit, which was statistically lower than preoperation level (t = −3.83, P = 0.001). Three eyes were receiving adjunctive prostaglandin analogs (PGA) eye drop daily to help maintain target IOP. Majority of the PG eyes (15/18) had stable VA and BCVA. Three eyes had worse VA and BCVA, and lost two lines or more of letters on Snellen chart at the last follow-up visit; among these three eyes, two of them were due to cataract formation, but did not need immediate cataract extraction; the third one had dislocated lens and had to receive cataract surgery during the follow-up period, who was a 33-year-old female and complained a sudden blurring of vision in the morning when she rose up from a bending down position. Biomicroscopy on unplanned visit revealed that the crystalline lens in the research eye had dislocated completely into the anterior chamber (AC). Lens extraction and intraocular lens suturing surgery were performed quickly to help restore vision. She had improved vision after cataract surgery and no fluctuation of IOP was detected after the cataract surgery. Three of 18 PG eyes had slight enlargement of the glaucomatous optic cupping, verified through stereoscopic optic disc photography, in which VFA showed mild deterioration of VF defect. VF in the majority (15/18) of the research eyes did not worsen compared with the baseline 8 years after trabeculectomy.
Postoperative changes in anterior segment
All but one of the PG eyes had various extent of posterior bowing of the mid-peripheral iris at enrollment, with a thin concave slit beam on the surface of the iris. The slit beam was projected vertically through the center of the pupil at 30–45°. Seventeen PG eyes have obviously concave irides under slit lamp. Only one PG eye had regular iris at initial diagnosis. None of the PG eyes exhibited diffuse anterior iris stromal pigment granule dusting, except for trace cluster of pigment granules on inferior surface of the iris in three PG eyes from three patients. After trabeculectomy, the concave iris became completely flat or regular in all research eyes. In the contralateral eyes of the enrolled patients, either trabeculectomy or laser peripheral iridoctomy was performed to eliminate reverse pupillary block, and the iris recovered to flat or regular. The change of iris configuration remained stable and unchanged throughout the postoperative period of study in both eyes of the patients.
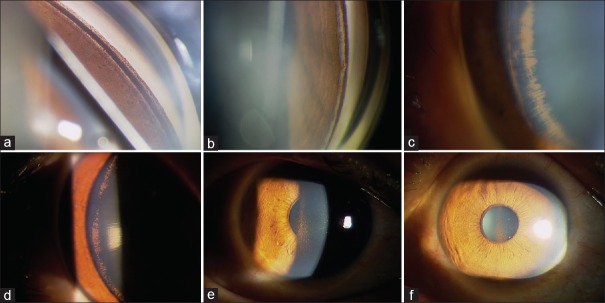
Functional blebs, symbols of successful trabeculectomy, still existed in 12 PG eyes at the last follow-up visit, though they were smaller and more constricted than the original size. Unanimously, pigment deposition in every part of the AC was found to be attenuated or decreased with time after trabeculectomy. Such findings were especially obvious when comparing the endpoint state with the baseline at enrollment [Figure 1a–1d].
Figure 1.
Anterior segment pigmentation attenuated after trabeculectomy during the follow-up visits. (a) Heavy homogeneous trabecular meshwork pigmentation at enrollment. (b) Trabecular meshwork pigmentation attenuated 8 years after trabeculectomy. (c) Lenticular pigmentation (Zentmayer ring) at enrollment. (d) Decreased lenticular pigmentation at last follow-up visit (8 years after surgery). (e) Krukenberg spindle at initial diagnosis. (f) Attenuated Krukenberg spindle at last follow-up visit.
Eleven patients (61.1%) had Krukenberg spindles, which were bilateral in eight and unilateral in three. The typical appearance of “Krukenberg spindle” in these patients was somewhat more like a “triangle”, rather than a spindle [Figure 1e]. Of the remaining seven, three had trace diffuse corneal endothelial pigmentation and four had no corneal pigment dusting, suggesting that Krukenberg spindle was not necessarily observed in all PG patients. During the 8-year follow-up period, all Krukenberg spindles were observed under biomicroscopy and photographed. It was commonly seen that the Krukenberg spindle became ambiguous and vague with time after trabeculectomy [Figure 1f].
Typical spoke-like radial ITDs, described in white PDS patients, were not discerned in any one of the patients. In two PG eyes of two enrolled patients, isolated short slit-like ITDs were visualized in large iris crypts. Moreover, no postoperative changes had been revealed during the following visits.
Heavy homogeneous TM pigmentation and pigment granule dusting on lens zonules and/or peripheral posterior lens surface were seen in all research eyes. Homogeneous TM pigmentation around the circumference of AC like a “mascara line” was visualized in all patients on gonioscopy, though the degrees were different. After mydriasis, the PG eyes showed different extent of pigment granule dusting on lens zonules and/or posterior peripheral surface referred to as Zentmayer ring or Scheie's line. Zonular and lenticular pigmentation were strong evidence for diagnosis of PDS/PG in that they were demonstrating the existence of irido-zonular rubbing, cause of pigment dispersion. When compared with baseline, it was striking to find that pigmentation in all parts of the AC became attenuated and lighter in all patients, after the configuration of the iris returned to normal after trabeculectomy.
To avoid inter-personal bias of the surgery, all trabeculectomies were performed by the same doctor (Dr. Ning-Li Wang). Functional blebs were observed and photographed during the postoperative visits. All enrolled PG eyes had functional blebs in the first 6 months after the filtration surgery. At the end point of the study, 16 patients still had functional blebs with satisfactory IOP control, with one receiving PGA eye drop daily at night. The remaining two patients had a scarred bleb and both needed PGA eye drop to keep IOP at target level.
Complications and side-effects
No serious surgical complications needing immediate surgical intervention or leading to negative outcome were recorded in anyone of the PG eyes during or after the filtration procedure. Leaking bleb was discovered in one PG eye and was successfully repaired through wearing a therapeutic soft contact lens.
DISCUSSION
Trabeculectomy is a classic surgery in the management of glaucoma. It has shown good IOP-lowering efficacy and safety in primary glaucoma and has been widely accepted in clinical practice for treatment of secondary glaucoma. PG is a type of secondary open-angle glaucoma, in which medication, laser, and trabeculectomy are all treatment options depending on different stages of the disease.[1,21] This study has summarized the long-term efficacy and safety of trabeculectomy on PG patients, which have not been reported in previous literature. The results have shown that trabeculectomy had a long IOP-lowering effect on PG eyes and promised safety as well. The 8-year outcome of the trabeculectomy has demonstrated that the visual function in PG eyes can be well-preserved after the filtration surgery.
PG is not common in clinical practice, especially in pigmented races. Yet, it is potentially sight-threatening with highly elevated IOP leading to irreversible glaucomatous neuropathy quickly.[10,23] Early detection, treatment of reverse papillary block, and sufficient lowering of IOP are critical in the management of PG. In trabeculectomy, all aspects of the treatment principle might be realized. The iridectomy breaks down the reverse pupillary block by communicating the AC and PC, after which the iris returns to be flat or regular and departs from the anterior surface of the crystalline lens and zonules, preventing more pigment granules to liberate from the IPE layer of the iris. At the same time, the artificial outflow pathway drains the aqueous humor out of the eyeball and reduces the elevated IOP significantly. A functional bleb is an important indicator for the success of trabeculectomy and serves as a clue for postoperative IOP level. In POAG eyes, functional blebs seldom survive more than 5 years because of tissue scarring around the operation site. It was striking to find that, in the PG eyes, 15/18 of the functional blebs survived 8 years after the surgery. Such high survival rate of functional blebs was accompanied with good IOP-control and preservation of visual function. The reason for a long survival rate of functional blebs in PG eyes after filtration surgery is still unknown. The main difference between POAG and PG eyes is that the latter has a lot of pigment granules deposited on anterior segment of the eyeball while the former does not. Thus, it is not unreasonable to postulate that pigment granules have a positive effect in preserving functional blebs, and such effect lasts long enough to keep the blebs survive 8 years. As the results have shown, the pigment granules still existed at the last follow-up visit 8 years after trabeculectomy, though the extent decreased.
The high survival rate of functional blebs was accompanied with sufficient IOP-lowering in the PG eyes. Eight-year outcome of trabeculectomy has shown that the treated eyes had well-controlled IOP, mostly with no adjunctive antiglaucoma medication at the end point of the study. At the last follow-up visit, the mean IOP of the treated eyes was 13.7 ± 2.5 mmHg (range: 9–19 mmHg). Only three PG eyes were receiving adjunctive PGA eye drop daily to help maintain the target IOP. Consequently, large majority of the PG eyes had stable VA, BCVA, and VF, as shown in the result section. There was no need for a second antiglaucoma surgery for any of the eyes during the study period. In the literature, the long-term IOP-reducing efficacy of trabeculectomy on POAG is not as promising yet. Majority of POAG eyes will face failure of trabeculectomy due to scarred bleb within 5 years. It is not uncommon that a second antiglaucoma surgery might be introduced if sufficient adjunctive antiglaucoma medication fails to reduce the IOP to target level. The mechanism for elevating resistance of aqueous humor outflow and high IOP in POAG is more complicated and substantial than that in PG. When trabeculectomy is performed in POAG, the artificial pass way assists the outflow of the aqueous humor and reduces the IOP. Such drainage assistance attenuates with time when tissue scarring occurs around the operation site, which results in a limited long-term IOP-lowering efficacy of trabeculectomy on POAG patients.
TM pigmentation is postulated to be the cause of elevated IOP in PG. The 8-year observation on PG eyes has demonstrated that TM pigmentation attenuated with time after the reverse pupillary block had been broken down after iridectomy, which prevented more pigment to disperse after the surgery. On the other hand, histological study of the TM tissue gained in trabeculectomy demonstrated that pigment granules in trabecular spaces might be phagocytozed by trabecular cells, which helped to clear the trabecular spaces. Moreover, small pigment granules can be drained out of the TM with aqueous humor convection. Such migration of pigment granule results in decreased number of pigment particles in the trabecular space and a compromised resistance of aqueous humor outflow. And such effect accumulates with time. As the results have shown [Figure 1], pigmentation in the anterior segment of the all research eyes attenuated after trabeculectomy. Both the long survival of functional blebs and the compromised TM pigmentation play an important role in the mechanism of IOP-lowering in PG eyes and might also help explain why trabeculectomy has a longer IOP-reducing efficacy in PG than POAG.
Side-effects of trabeculectomy were another major concern of this study. In the present study, we have found that the intra- and post-operative side-effects were quite similar between PG and those in POAG. No severe side-effects of the surgery were discovered on the follow-up visits of the PG eyes. No flat AC appeared in the patients after trabeculectomy. A leaking bleb was found in one of the PG eyes and healed after wearing of therapeutic soft contact lens.
In summary, we have characterized the long-term outcome of trabeculectomy on 18 PG eyes. The results have shown trabeculectomy was promising in controlling IOP of PG eyes. Functional blebs survived longer than that in POAG patients, and visual function was well-protected 8 years after the surgery. Trabeculectomy is a safe and effective treatment for PG eyes and should be introduced when medication and laser treatment fail to reduce the elevated IOP.
Financial support and sponsorship
Project supported by a grant from the National Natural Science Foundation of China (No. 81170845).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Xin Chen
REFERENCES
- 1.Becker B, Shin DH, Cooper DG, Kass MA. The pigment dispersion syndrome. Am J Ophthalmol. 1977;83:161–6. doi: 10.1016/0002-9394(77)90610-9. [DOI] [PubMed] [Google Scholar]
- 2.Gillies WE, Brooks TC. Clinical features at presentation of anterior segment pigment dispersion syndrome. Clin Experiment Ophthalmol. 2001;29:125–7. doi: 10.1046/j.1442-9071.2001.00391.x. [DOI] [PubMed] [Google Scholar]
- 3.Gottanka J, Johnson DH, Grehn F, Lütjen-Drecoll E. Histologic findings in pigment dispersion syndrome and pigmentary glaucoma. J Glaucoma. 2006;15:142–51. doi: 10.1097/00061198-200604000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Kupfer C, Kuwabara T, Kaiser-Kupfer M. The histopathology of pigmentary dispersion syndrome with glaucoma. Am J Ophthalmol. 1975;80:857–62. doi: 10.1016/0002-9394(75)90283-4. [DOI] [PubMed] [Google Scholar]
- 5.Lehto I, Vesti E. Diagnosis and management of pigmentary glaucoma. Curr Opin Ophthalmol. 1998;9:61–4. doi: 10.1097/00055735-199804000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Ong EL, Crowston J. The concave iris in pigment dispersion syndrome. Ophthalmology. 2011;118:66–70. doi: 10.1016/j.ophtha.2010.04.039. doi:10.1016/j.ophtha.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 7.Pavlin CJ. Ultrasound biomicroscopy in pigment dispersion syndrome. Ophthalmology. 1994;101:1475–7. doi: 10.1016/s0161-6420(13)32022-3. [DOI] [PubMed] [Google Scholar]
- 8.Ritch R. Pigment dispersion syndrome. Am J Ophthalmol. 1998;126:442–5. doi: 10.1016/s0002-9394(98)00270-0. [DOI] [PubMed] [Google Scholar]
- 9.Scheie HG, Cameron JD. Pigment dispersion syndrome: A clinical study. Br J Ophthalmol. 1981;65:264–9. doi: 10.1136/bjo.65.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui Y, Ten Hulzen RD, Cameron JD, Hodge DO, Johnson DH. What is the risk of developing pigmentary glaucoma from pigment dispersion syndrome? Am J Ophthalmol. 2003;135:794–9. doi: 10.1016/s0002-9394(02)02289-4. [DOI] [PubMed] [Google Scholar]
- 11.Sowka J. Pigment dispersion syndrome and pigmentary glaucoma. Optometry. 2004;75:115–22. doi: 10.1016/s1529-1839(04)70023-8. [DOI] [PubMed] [Google Scholar]
- 12.Ritch R, Steinberger D, Liebmann JM. Prevalence of pigment dispersion syndrome in a population undergoing glaucoma screening. Am J Ophthalmol. 1993;115:707–10. doi: 10.1016/s0002-9394(14)73635-9. [DOI] [PubMed] [Google Scholar]
- 13.Niyadurupola N, Broadway DC. Pigment dispersion syndrome and pigmentary glaucoma – A major review. Clin Experiment Ophthalmol. 2008;36:868–82. doi: 10.1111/j.1442-9071.2009.01920.x. doi:10.1111/j.1442-9071.2009.01920.x. [DOI] [PubMed] [Google Scholar]
- 14.Qing G, Wang N, Lu Q, Zhang S. Different transillumination property in Chinese and white irides. J Glaucoma. 2012;21:107–11. doi: 10.1097/IJG.0b013e318202794d. doi:10.1097/IJG.0b013e318202794d. [DOI] [PubMed] [Google Scholar]
- 15.Qing G, Wang N, Tang X, Zhang S, Chen H. Clinical characteristics of pigment dispersion syndrome in Chinese patients. Eye (Lond) 2009;23:1641–6. doi: 10.1038/eye.2008.328. doi:10.1038/eye.2008.328. [DOI] [PubMed] [Google Scholar]
- 16.Roberts DK, Chaglasian MA, Meetz RE. Clinical signs of the pigment dispersion syndrome in blacks. Optom Vis Sci. 1997;74:993–1006. doi: 10.1097/00006324-199712000-00020. [DOI] [PubMed] [Google Scholar]
- 17.Roberts DK, Chaglasian MA, Meetz RE. Iris transillumination defects in the pigment dispersion syndrome as detected with infrared videography: A comparison between a group of blacks and a group of nonblacks. Optom Vis Sci. 1999;76:544–9. doi: 10.1097/00006324-199908000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Roberts DK, Wernick MN. Infrared imaging technique may help demonstrate iris transillumination defects in blacks who show other pigment dispersion syndrome clinical signs. J Glaucoma. 2007;16:440–7. doi: 10.1097/IJG.0b013e3181405e72. [DOI] [PubMed] [Google Scholar]
- 19.Breingan PJ, Esaki K, Ishikawa H, Liebmann JM, Greenfield DS, Ritch R. Iridolenticular contact decreases following laser iridotomy for pigment dispersion syndrome. Arch Ophthalmol. 1999;117:325–8. doi: 10.1001/archopht.117.3.325. [DOI] [PubMed] [Google Scholar]
- 20.Roberts DK, Lukic AS, Yang Y, Moroi SE, Wilensky JT, Wernick MN. Novel observations and potential applications using digital infrared iris imaging. Ophthalmic Surg Lasers Imaging. 2009;40:207–16. doi: 10.3928/15428877-20090301-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alvarado JA, Murphy CG. Outflow obstruction in pigmentary and primary open angle glaucoma. Arch Ophthalmol. 1992;110:1769–78. doi: 10.1001/archopht.1992.01080240109042. [DOI] [PubMed] [Google Scholar]
- 22.Jacobi PC, Dietlein TS, Krieglstein GK. Effect of trabecular aspiration on intraocular pressure in pigment dispersion syndrome and pigmentary glaucoma. Ophthalmology. 2000;107:417–21. doi: 10.1016/s0161-6420(99)00091-3. [DOI] [PubMed] [Google Scholar]
- 23.Jewelewicz DA, Radcliffe NM, Liebmann J, Ritch R. Temporal evolution of intraocular pressure elevation after pupillary dilation in pigment dispersion syndrome. J Glaucoma. 2009;18:184–5. doi: 10.1097/IJG.0b013e318182edbf. doi:10.1097/IJG.0b013e318182edbf. [DOI] [PubMed] [Google Scholar]
- 24.Yip LW, Sothornwit N, Berkowitz J, Mikelberg FS. A comparison of interocular differences in patients with pigment dispersion syndrome. J Glaucoma. 2009;18:1–5. doi: 10.1097/IJG.0b013e31816f767b. doi:10.1097/IJG.0b013e31816f767b. [DOI] [PubMed] [Google Scholar]
- 25.Pavlin CJ, Macken P, Trope GE, Harasiewicz K, Foster FS. Accommodation and iridotomy in the pigment dispersion syndrome. Ophthalmic Surg Lasers. 1996;27:113–20. [PubMed] [Google Scholar]