Abstract
Objective:
To discuss the feasibility and clinical value of high-resolution magnetic resonance vessel wall imaging (HRMR VWI) for intracranial arterial stenosis.
Date Sources:
We retrieved information from PubMed database up to December 2015, using various search terms including vessel wall imaging (VWI), high-resolution magnetic resonance imaging, intracranial arterial stenosis, black blood, and intracranial atherosclerosis.
Study Selection:
We reviewed peer-reviewed articles printed in English on imaging technique of VWI and characteristic findings of various intracranial vasculopathies on VWI. We organized this data to explain the value of VWI in clinical application.
Results:
VWI with black blood technique could provide high-quality images with submillimeter voxel size, and display both the vessel wall and lumen of intracranial artery simultaneously. Various intracranial vasculopathies (atherosclerotic or nonatherosclerotic) had differentiating features including pattern of wall thickening, enhancement, and vessel remodeling on VWI. This technique could be used for determining causes of stenosis, identification of stroke mechanism, risk-stratifying patients, and directing therapeutic management in clinical practice. In addition, a new morphological classification based on VWI could be established for predicting the efficacy of endovascular therapy.
Conclusions:
This review highlights the value of HRMR VWI for discrimination of different intracranial vasculopathies and directing therapeutic management.
Keywords: Black Blood, High-resolution, Magnetic Resonance Images, Vessel Wall Imaging
INTRODUCTION
Intracranial arterial stenoses are common angiographic finding worldwide and contain a wide range of diseases.[1] Currently, conventional lumenography-based methods such as digital subtraction angiography (DSA), computed tomography angiography (CTA), magnetic resonance angiography (MRA) are used to detect the luminal stenosis.[2] However, those methods could not provide information about the vessel wall given the small diameter of intracranial arteries and limited spatial resolution of above imaging methods.[3,4] The pathological studies showed vessel abnormalities including intracranial atherosclerotic disease (ICAD),[5,6,7] dissection,[8] and vasculitis[9] mainly involved the vessel wall. High-resolution magnetic resonance (MR) vessel wall imaging (HRMR VWI) is the most promising technique for reliably imaging intracranial arterial wall duo to its superior soft tissue contrast and spatial resolution, has been applied to evaluate multiple intracranial arterial disease, both atherosclerotic[10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57] and nonatherosclerotic.[56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] In this review, we mainly discuss the clinical application of vessel wall imaging (VWI) for identifying different underlying pathologies of intracranial arterial stenoses.
TECHNOLOGY FOR HIGH-RESOLUTION MAGNETIC RESONANCE VESSEL WALL IMAGING
VWI has been popularly used to help identify vulnerable plaque in the internal carotid artery.[72,73] Comparatively, the wall features of intracranial arteries were less studied due to its poor accessibility and too small size to visualize with VWI in a conventional 1.5T MR scanner (Twinspeed, GE Healthcare, Waukesha, WI).[10,11,12] Recent studies had showed that VWI with a 3T or 7T MR might be expected to visualize the walls of intracranial arteries due to improved spatial resolution and/or signal-to-noise ratio.[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] To successfully image the intracranial vessel wall, black blood technique was popularly used by suppressing the blood signal with double inversion recovery[15] or preregional saturation pulses,[33] for obtaining sufficient image contrast. Early studies were mainly reported with two-dimensional technique including T1-, T2-, and proton density-weighted sequences.[10,11,12,13,14,15,16,17,18,19,20,21,22,23,25,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65] The intrinsic limitations of this technique include the inability to cover a large volume of intracranial artery due to time constraints and anisotropic spatial resolution with low spatial resolution in slice-select direction.[29] When different segments with variable orientations to the intracranial arteries need to be assessed, multiple two-dimensional slices need be scanned, with each slice oriented perpendicular to the local vessel orientation. A three-dimensional slices sequence with isotropic resolution might be superior because of its reconstruction possibilities that allow reconstruction of image planes perpendicular to the local vessel orientation.[24,26,27,28,29,34] Examples of 3-dimensional technique including flow-dephasing-prepared fast spoiled gradient recalled echo (FDP-FSPGR, Signa, TwinSpeed; GE Healthcare, USA),[27] Sampling Perfection with Application optimized Contrast by using different flip angle Evolutions (SPACE, Magnetom, Siemens Healthcare, Germany)[29] and Volume ISotropic Turbo spin echo Acquisition (VISTA, Achieve; Philips Healthcare, The Netherlands).[26]
ATHEROSCLEROTIC DISEASE
Remodeling pattern
Arterial vessels might respond to plaque growth by either positive remodeling (outward expansion of the vessel wall) or negative remodeling (vessel shrinkage).[74,75] Positive remodeling might be advantageous by avoiding luminal stenosis, but also be harmful because positive remodeling will make the plaque more vulnerable. On the contrast, negative remodeling will exacerbating rather than compensating for luminal loss, but might appear more stable.[33] Remodeling phenomenon was first described by Glagov et al.[74] in the study of coronary artery, and has been reported in the studies on middle cerebral arteries (MCAs)[30,31,32] and basilar arteries[18,33,34] recently. Although both the positive and negative remodeling could exit in the atherosclerotic intracranial arteries, the percentage of remodeling pattern might be various on the basis of different intracranial arteries.[18,30,31,32,33,34] Negative remodeling was not uncommon in the patients with symptomatic MCA stenosis.[32] Intracranial percutaneous transluminal angioplasty and stenting (PTAS) has been regarded as an important tool for patients with symptomatic stenosis.[76,77] When performing PTAS for the patients refractory to medical therapy, the remodeling pattern in ICAD should be considered when selecting the size of balloon and stent. Previous studies[75,78] on coronary showed patients with positive remodeling lesions faced major adverse cardiac events more frequently; however, patients with negative remodeling lesions had high rates of in-hospital complications, including postinterventional dissection. Remodeling pattern of ICAD might also be an important factor associated with periprocedural complications. In the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Arterial Stenosis (SAMMPRIS) study, patients were enrolled based on luminal narrowing severity without arterial wall imaging. The results of SAMMPRIS reminded us the importance of patient selection and stent selection, and VWI could be a valuable tool to that end.[76] Understanding of remodeling pattern will help to risk-stratify patients and direct stroke prevention and treatment. In clinical practice, more aggressive medical therapy would be need for lesions with positive remodeling due to plaque vulnerability than the lesions with negative remodeling.[75,78] In addition, when performing PTAS for the lesions with negative remodeling, experienced interventional neuroradiologists would select undersized balloons with gradual balloon inflation for decreasing the risk of vessel injury even rupture.[36] In the future, a prospective study was needed to assess the influence of local remodeling patterns on periprocedural complications.[32]
Plaque location
The characterization of plaque distribution on atherosclerotic arterial wall has important clinical implication.[35,36,37,38,39,40,41,42] The existence of a plaque close to branch vessel ostia has been shown to increase the risk of branch occlusion after coronary stenting.[79] Angioplasty or stenting would push the plaque outward against the wall of the artery, which can cause plaque shifting, resulting in branch or perforator occlusion. VWI could help display plaque location and the relationship between plaque and branch or perforator arteries [Figures 1 and 2]. In a study by Xu et al.[35] with 92 stenotic MCAs, plaques existed more frequently on the ventral (44.8%) and inferior (31.7%), compared with the superior (14.3%) and dorsal wall (9.0%, P < 0.001). For the symptomatic MCA stenosis, plaques existed on superior (P = 0.016) more frequently than asymptomatic stenosis. For the basilar artery's stenosis, Huang et al.[39] reported a study of 38 symptomatic patients, and found that plaques were more frequently located on the ventral wall (21.6%) than the dorsal (6.3%), left (4.6%) and right side (2.6%, P = 0.000). Imaging plaque location by VWI may help risk-stratify patients and individualization of treatment.[35,36] Jiang et al.[36] have already described cases with intracranial arterial stenoses by using VWI for helping guide the endovascular treatment. Although these data suggested the relationship between plaque location and branch or perforator occlusion, a prospective study with follow-up is still needed for further assessing implications of plaques location.
Figure 1.
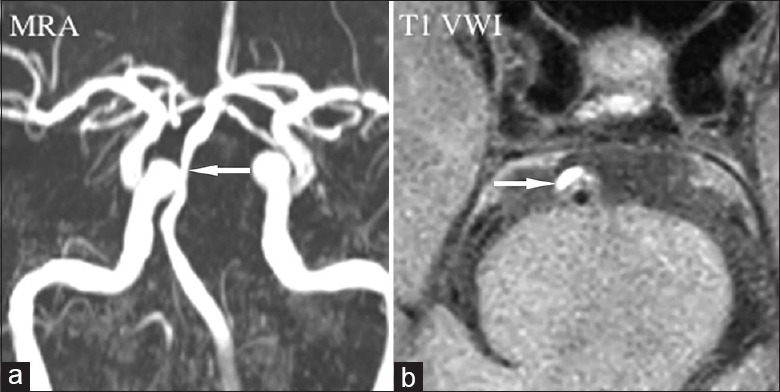
Atherosclerotic stenosis. Magnetic resonance angiography (a) showed luminal stenosis in basilar artery (arrow). T1-weighted vessel wall image displayed the eccentric plaque locating in the ventral wall with high signal indicating intraplaque hemorrhage (b, arrow). MRA: Magnetic resonance angiography; VWI: Vessel wall imaging.
Figure 2.
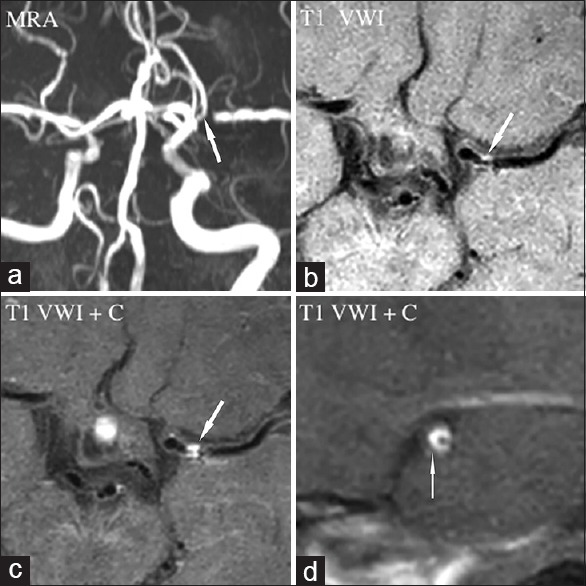
Atherosclerotic stenosis. Magnetic resonance angiography (a) showed luminal stenosis in left middle cerebral artery (arrow). Long-axis view of pre- (b, arrow) and post-contrast (c, arrow) T1-weighted vessel wall image displayed an eccentric plaque and enhancement. Short-axis view of postcontrast (d, arrow) T1-weighted vessel wall image displayed the eccentric-enhanced plaque locating in the ventral wall. MRA: Magnetic resonance angiography; VWI: Vessel wall imaging; VWI+C: VWI+Contrast.
Intraplaque hemorrhage
In the studies of extracranial carotid artery, intraplaque hemorrhage (IPH) was associated with symptom, and became a predictor of ischemic stroke.[72,73] VWI-defined IPH within carotid plaques strongly correlates with histology.[72] However, study of IPH of intracranial arteries was still limited given the technical difficulty of obtaining in vivo histology for comparison. With the definition as equal or higher than 150% of T1 signal of adjacent muscle [Figure 1], Turan et al.[43] reported a case with VWI-defined IPH in a patient with symptomatic MCA plaque. Xu et al.[44] used similar definition of IPH and found significantly different for the occurrence rate of VWI-defined IPH between symptomatic and asymptomatic MCA stenosis (19.6% vs. 3.2%, P = 0.01). Recently, by using a new sequence called magnetization-prepared rapid acquisition with gradient-echo sequence, Yu et al.[45] also reported a significantly different of VWI-defined IPH between symptomatic and asymptomatic basilar artery stenosis (80.0% vs. 48.8%, P < 0.01). In the future, prospective follow-up is needed for determining whether VWI-defined IPH predicts high stroke risk.
Plaque enhancement
VWI has been used for detecting the plaque enhancement by comparing precontrast and postcontrast T1-weighted images.[46,47,48,49,50,51,52,53,54,55,56,57] On the VWI, atherosclerotic plaque has characteristic finding as eccentric wall thickening and enhancement in most of the cases [Figure 2].[47] Recently, circumferential wall enhancement was also reported in a minority of patients, mimicking vasculitis.[61] Additional T2-weighted images would be needed for discrimination between the two diseases (T2 high signal on atherosclerotic plaque but not on vasculitis).[61] The value of plaque enhancement for suggesting plaque vulnerability has been suggested by many authors,[46,47,48,49,50,51,52,53,54,55,56,57] but not well established. Most of reported studies showed the plaque enhancement was strongly associated with recent ischemic stroke, and may serve as a marker of its stability.[46,47,48,49,50,51,52,53,54,55,56,57] However, the results of relationship between plaque enhancement and ischemic stroke may be affected by many factors. Ryu et al.[53] found the degree of stenosis instead of plaque enhancement was the only independent predictor of symptoms after multivariate analysis and inferred that previous reported relationship between plaque enhancement and stroke should be reconsidered and compared between symptomatic and asymptomatic patients with similar degree of stenosis. In addition, the optimal time point between contrast administration and peak enhancement needs to be determined. Most of postcontrast T1-weighted images were performed 5 min later after contrast material administration.[46,47,48,49,50,51,52,53,54,55,56,57] However, some author reported a contrast-to-noise ratio peak after 20 min of contrast material administration with 7T MR.[1] The pathophysiological mechanisms of contrast uptake also need to be further studied. Contrast enhancement was thought to relate with neovascularization and endothelial contrast permeablitiy, or as a result of vasa vasorum in the atherosclerotic plaques.[46,47,48,49,50,51,52,53,54,55,56,57]
NONATHEROSCLEROTIC DISEASE
Central nervous system vasculitis
Central nervous system (CNS) vasculitis[58,59,60,61] is generally diagnosed by conventional angiography with unspecific findings including lumen irregularities and stenosis. Those findings would confuse with other vascular disorders, such as atherosclerosis, reversible cerebral vasoconstriction syndrome (RCVS). VWI might be useful for the diagnosis of CNS vasculitis. CNS vasculitis was reported as diffuse concentric thickening and enhancement in a majority of patients or eccentric thickening and enhancement in a minority of patients on the VWI.[63] In addition, enhancement in CNS vasculitis generally persisted a long time with the median of seven months.[63]
Reversible cerebral vasoconstriction syndrome
Proposed diagnostic criteria for RCVS including:[80](1) angiography, MRA or CTA documenting multifocal segmental cerebral artery vasoconstriction; (2) no evidence of aneurysmal subarachnoid hemorrhage; (3) severe, acute headaches, with or without additional neurologic signs or symptoms; and (4) reversibility of angiographic abnormality within 12 weeks after onset or postmortem examination to rule out vasculitis, intracranial atherosclerosis, and aneurysmal subarachnoid hemorrhage. RCVS have overlapping clinical and imaging feature with CNS vasculitis.[61,62,63] Differential diagnosis between RCVS and CNS vasculitis is important given the different clinical course and treatment. RCVS is treated with observation or possibly calcium channel blockers, whereas CNS vasculitis is treated with steroids and immunosuppression.[62] Radiographic imaging is difficult to make discrimination for those vascular disorders. Recent studies showed that RCVS had different vessel wall characteristics with CNS vasculitis on VWI. RCVS generally demonstrated concentric wall thickening with negligible to mild enhancement,[61,62,63] and complete resolution of wall thickening on the follow-up VWI.[63]
Intracranial arterial dissection
Intracranial arterial dissection (IAD) is a relatively rare disease with less study available, compared with extracranial cervical arteries.[81] Patients can present with headache, ischemic stroke, subarachnoid hemorrhage, or mass effect. IAD was reportedly more common in Asian populations.[64,65,66,67,68] Radiological diagnosis of IADs is still difficult given the small size of intracranial arteries and subtle radiological signs. Pathognomonic radiological findings of intracranial artery dissection include intramural hematoma, intimal flap and double lumen.[81] Although DSA is still regarded as the gold standard for diagnosis of IAD, reliable signs including intimal flap or double lumen could only be observed in minority of the cases (about 30%). VWI could help detect pathognomonic findings of IADs [Figure 3]. Using two-dimensional VWI, Wang et al.[64] and Han et al.[65] detected the intramural hematoma in 60.5% and 54.3% of the IADs. However, using three-dimensional technique, Takano et al.[66] detected intramural hematoma in 87.5% of IADs. Compared with two-dimensional technique, three-dimensional HRMR VWI can provide isotropic spatial resolution images with higher spatial resolution in slice-selected direction, allowing visualization of the lesion in arbitrary orientations,[29] which is helpful to detect small hematoma.[66] VWI with three-dimensional black blood technique is regarded as optimum imaging tool for detecting intramural hematoma.[81] In addition, intimal flap could be identified in more than 90.0% of IADs on T2-weighted images or postcontrast T1-weighted images.[64,65]
Figure 3.
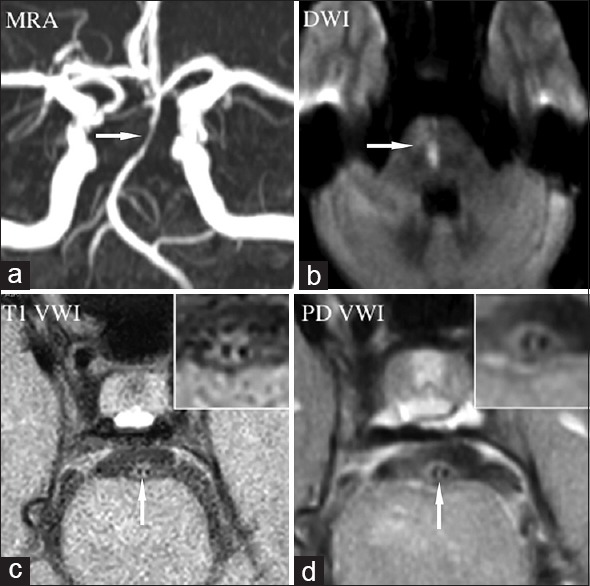
Intracranial arterial dissection. Magnetic resonance angiography (a) showed luminal stenosis in basilar artery (arrow). Diffusion-weighted imaging showed new pontine infarction due to perforator occlusion (b, arrow). T1- (c, arrow) and proton density- (d, arrow) weighted vessel wall image showed intimal flap and double lumen obviously (magnified view in the inset). MRA: Magnetic resonance angiography; DWI: Diffusion weighted imaging; VWI: Vessel wall imagin; PD VWI: Proton density VWI.
Moyamoya disease
Moyamoya disease (MMD) is an uncommon cerebrovascular disease characterized by progressive stenosis of the distal internal carotid artery or the proximal areas of its main braches, and abnormal vascular networks around the occlusive or stenotic arteries.[82] MMD has high incidence in East Asia, and has two peaks of age distribution at 5 years and at about 40 years.[82] For the old patients, differentiation of MMD from ICAD is not easy duo to the similar clinical presentations and angiographic features in some cases with concomitant vascular risk factors. MMD is generally treated with surgical revascularization, However, ICAD is treatment with aggressive medical treatment. VWI will provide information of wall characteristics. One case series reported by Kim et al.[70] showed a different wall features on HRMRI for the MMD and ICAD on the occluded segments. In the first large cohort study by Ryoo et al.[71] for comparing MMD (32 patients) with ICAD (16 patients) using HRMR VWI, MMD patients showed concentric enhancement on distal internal carotid arteries and MCAs regardless of symptoms or stages, whereas ICAD patients showed focal eccentric enhancement on the symptomatic segment. Furthermore, MMD had wall characteristics as MCA shrinkage [Figure 4], which is different with the findings of ICAD. HRMR VWI finding might help understand the pathogenesis of MMD characterized by hyperplasia intima and thinned media. On the VWI, the diffuse concentric enhancement could represent hyperproliferation of wall components, and diffuse wall thinning represent media shrinkage.[71] VWI could be used as a noninvasive diagnostic tool for MMD.
Figure 4.
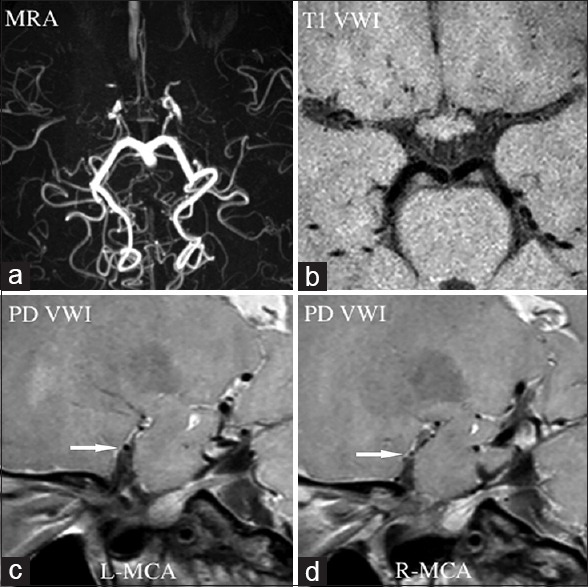
Moyamoya disease. Magnetic resonance angiography (a) showed luminal occlusion in bilateral anterior cerebral artery and middle cerebral artery, suggesting possible Moyamoya disease. Normal vessel structure of bilateral anterior cerebral artery and middle cerebral artery disappeared in T1-weighted vessel wall image (b). Short-axis view of proton density-weighted vessel wall image displayed shrinking middle cerebral artery (c, arrow), especially in the right side (d, arrow). MRA: Magnetic resonance angiography; VWI: Vessel wall imagin; PD VWI: Proton density VWI; L: Left; R: Right; MCA: Middle cerebral arteries.
Vertebrobasilar hypoplasia
Intracranial vertebral and basilar artery hypoplasia is presumably associated with posterior circulation infarctions.[24] The definition of hypoplasia is based on the luminograms when the diameter of target artery is less 2 mm or 3 mm.[24] However, similar luminal features might reflect diverse pathologies. Differential diagnosis between artery hypoplasia and acquired atherosclerotic stenosis might be difficult by luminal imaging including DSA, CTA, and MRA. VWI can display vessel wall and lumen simultaneously, and has been used for discrimination of artery hypoplasia and atherosclerotic stenosis.[24,57] On the VWI, hypoplastic vertebral or basilar artery can show narrowing lumen with normal or thick wall. In addition, hypoplastic arteries are more susceptible to prethrombotic or atherosclerotic processes than normal arteries.[24]
CLINICAL APPLICATION
Determining causes of stenosis
VWI could noninvasively differentiate between various intracranial vasculopathies by identifying wall thickening pattern or enhancement pattern [Table 1].[47,61,62,63]
Table 1.
HRMR VWI features of diverse intracranial vasculopathies
| Diverse vasculopathies | Features on HRMR VWI |
|---|---|
| Atherosclerosis | Eccentric wall thickening and enhancement, rarely circumferential wall thickening and enhancement; high signal on T2-weighted images |
| Central nervous system vasculitis | Concentric wall thickening and enhancement; rarely eccentric wall thickening and enhancement; no high signal on T2-weighted images. Enhancement persisted a long time with a median of 7 months |
| RCVS | Concentric wall thickening with negligible to mild enhancement, and complete resolution of wall thickening on the follow-up VWI |
| Intracranial arterial dissection (steno-occlusive pattern) | Intimal flap, double lumen, and intramural haemotoma |
| Moyamoya disease | Concentric enhancement on distal internal carotid arteries and middle cerebral arteries (MCAs) regardless of symptoms or stages; MCA shrinkage |
| Vertebrobasilar hypoplasia | Narrowing lumen with normal or thick vessel wall |
HRMR VWI: High-resolution magnetic resonance vessel wall imaging; RCVS: Reversible cerebral vasoconstriction syndrome
Identification of stroke mechanism
Stroke subtypes were principally classified by the Trial of Org 10172 in Acute Stroke Treatment criteria, including large artery atherosclerosis, cardioembolism, small-artery stroke, stroke with a determined cause, and stroke with an undetermined cause.[83] The mechanism of stroke is generally inferred based on clinical presentation and pattern of infarction,[83] resulting in mechanism of stroke ambiguously in many patients. Using HRMR VWI, culprit lesions could be detected, and mechanism of stroke could be better established.
In the patients with large artery atherosclerosis, vulnerable plaque was prone to rupture, leading to local vessel occlusion or artery-to-artery embolization. VWI will help find a vulnerable plaque with the features of IPH,[43,44,45] obvious plaque enhancement,[47,48,49,50,51,52,53,54,55,56,57] and positive remodeling.[18,30,31,32,33,34] Even for the stable plaque (e.g., plaque with a large fibrous cap and small lipid core, or plaque with negative remodeling),[31,32] severe stenosis will result in hypoperfusion and impaired clearance of emboli (washout), contributing to artery-to-artery thromboembolism,[83] especially in the border zones without adequate collateral flow.
Small-artery occlusion is another major cause of ischemic stroke, mainly associated with arterial hyalinization due to high blood pressure.[83] However, recent studies showed small-artery occlusion may be caused by branch atherosclerosis or growth of parental artery atherosclerotic plaque over the ostia of penetrating arteries.[37,38,39] Vessel wall MRI was able to display the arterial atherosclerotic plaque distribution and location in the parental artery, which was associated with perforator stroke.[35,36,37,38,39,40,41,42]
For the ischemic stroke with other determined cause, VWI is useful for definitive diagnosis of the stroke cause. For the IADs, VWI was the best tool for detecting intimal flap and intramural hematoma.[64,65] In the patients with vasculitis, diffuse concentric wall thickening and enhancement on VWIs would support the diagnosis.[58,59,60,61,62,63] For the ischemic stroke with undetermined cause, HRMR VWI would help investigate the underlying mechanism of stroke by the vessel wall morphological and signal features.
Directing intracranial percutaneous transluminal angioplasty and stenting
Intracranial arterial stenosis is the main cause of stroke worldwide and need more attention for prevention and intervention. First, recognizing and understanding the cause of stenosis and mechanism of stroke is essential, and can be helpful to select suitable therapy for individual patient. For the atherosclerotic stenosis, two strategies were available for the high-risk patients: aggressive medical management (double antiplatelet and management of vascular risk factors) and PTAS.[76,77] For the patients refractory to medical therapy, PTAS has been increasingly used in clinical practice. However, the intracranial arteries have many unique anatomical characteristics (absent of external elastic lamina between the media and adventitia, thin adventitia, and rich perforators) comparing with extracranial arteries. Periprocedural complication of PTAS was particularly high after stenting including parenchymal brain hemorrhage and perforator stroke.[76,77]
To increase the efficacy of PTAS, it is important to identify the patient with high-risk of complication and make individual therapeutic management for each patient. First, intracranial arterial stenosis with other pathogenesis (e.g., vasculits, or MMD) should be excluded from endovascular therapy. Given histopathologic evidence was generally unavailable, VWI was regarded as an alternative method for this purpose.[61,62,63,64] Second, patients that might benefit from endovascular treatment would be further selected based on the stroke mechanism. It is presumably that PTAS might benefit patients with hypoperfusion, but not with artery-to-artery embolism. VWI might help determine stroke mechanism and play a role in selecting suitable patients. Third, morphological features would affect the results of endovascular procedure. Patients with plaques located near the ostia of penetrating vessels are presumably prone to perforator stroke after stenting due to “snow-plowing” effect.[35,36,37,38,39,40,41,42] Furthermore, lesions with negative remodeling may face vessel injury more frequently comparing with lesions with positive remodeling as described in the coronary arteries, resulting in vessel dissection or hemorrhage.[32] A new morphological classification based on HRMRI could be established for global assessment of risk of endovascular therapy.
CONCLUSIONS
VWI could display both the vessel wall and lumen of intracranial artery simultaneously, and has been used for assessing morphological characteristics of various intracranial vasculopathies, which is useful for determining causes of stenosis, identification of stroke mechanism, risk-stratifying patients, and directing endovascular therapy. Further study was required to prospectively assess predictive value of VWI characteristics for various intracranial vasculopathies.
Financial support and sponsorship
This study was supported by grants from China-Japan Friendship Hospital Youth Science and Technology Excellence Project (No. 2014-QNYC-A-04), and National Natural Science Foundation of China (No. 81173595).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Dieleman N, van der Kolk AG, Zwanenburg JJ, Harteveld AA, Biessels GJ, Luijten PR, et al. Imaging intracranial vessel wall pathology with magnetic resonance imaging: Current prospects and future directions. Circulation. 2014;130:192–201. doi: 10.1161/CIRCULATIONAHA.113.006919. doi:10.1161/circulationaha.113.006919. [DOI] [PubMed] [Google Scholar]
- 2.Bash S, Villablanca JP, Jahan R, Duckwiler G, Tillis M, Kidwell C, et al. Intracranial vascular stenosis and occlusive disease: Evaluation with CT angiography, MR angiography, and digital subtraction angiography. AJNR Am J Neuroradiol. 2005;26:1012–21. [PMC free article] [PubMed] [Google Scholar]
- 3.Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TC. High-resolution magnetic resonance imaging: An emerging tool for evaluating intracranial arterial disease. Stroke. 2013;44:287–92. doi: 10.1161/STROKEAHA.112.664680. doi:10.1161/STROKEAHA.112.664680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandell DM, Shroff M. On MR imaging of the intracranial vessel wall. Can J Neurol Sci. 2011;38:4–5. doi: 10.1017/s0317167100011021. doi:10.1017/s0317167100011021. [DOI] [PubMed] [Google Scholar]
- 5.Turan TN, Rumboldt Z, Granholm AC, Columbo L, Welsh CT, Lopes-Virella MF, et al. Intracranial atherosclerosis: Correlation between in-vivo 3T high resolution MRI and pathology. Atherosclerosis. 2014;237:460–3. doi: 10.1016/j.atherosclerosis.2014.10.007. doi:10.1016/j.atherosclerosis.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Kolk AG, Zwanenburg JJ, Denswil NP, Vink A, Spliet WG, Daemen MJ, et al. Imaging the intracranial atherosclerotic vessel wall using 7T MRI: Initial comparison with histopathology. AJNR Am J Neuroradiol. 2015;36:694–701. doi: 10.3174/ajnr.A4178. doi:10.3174/ajnr.A4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majidi S, Sein J, Watanabe M, Hassan AE, de Van Moortele PF, Suri MF, et al. Intracranial-derived atherosclerosis assessment: An in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. Am J Neuroradiol. 2013;34:2259–64. doi: 10.3174/ajnr.A3631. doi:10.3174/ajnr.A3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ono H, Nakatomi H, Tsutsumi K, Inoue T, Teraoka A, Yoshimoto Y, et al. Symptomatic recurrence of intracranial arterial dissections: Follow-up study of 143 consecutive cases and pathological investigation. Stroke. 2012;44:126–31. doi: 10.1161/STROKEAHA.112.670745. doi:10.1161/strokeaha.112.670745. [DOI] [PubMed] [Google Scholar]
- 9.Elbers J, Halliday W, Hawkins C, Hutchinson C, Benseler SM. Brain biopsy in children with primary small-vessel central nervous system vasculitis. Ann Neurol. 2010;68:602–10. doi: 10.1002/ana.22075. doi:10.1002/ana.22075. [DOI] [PubMed] [Google Scholar]
- 10.Lam WW, Wong KS, So NM, Yeung TK, Gao S. Plaque volume measurement by magnetic resonance imaging as an index of remodeling of middle cerebral artery: Correlation with transcranial color Doppler and magnetic resonance angiography. Cerebrovasc Dis. 2004;17:166–9. doi: 10.1159/000075786. doi:10.1159/000075786. [DOI] [PubMed] [Google Scholar]
- 11.Klein IF, Lavallée PC, Schouman-Claeys E, Amarenco P. High-resolution MRI identifies basilar artery plaques in paramedian pontine infarct. Neurology. 2005;64:551–2. doi: 10.1212/01.WNL.0000150543.61244.06. doi:10.1212/01.wnl.0000150543.61244.06. [DOI] [PubMed] [Google Scholar]
- 12.Klein IF, Lavallée PC, Touboul PJ, Schouman-Claeys E, Amarenco P. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology. 2006;67:327–9. doi: 10.1212/01.wnl.0000225074.47396.71. doi:10.1212/01.wnl.0000225074.47396.71. [DOI] [PubMed] [Google Scholar]
- 13.Li ML, Xu WH, Song L, Feng F, You H, Ni J, et al. Atherosclerosis of middle cerebral artery: Evaluation with high-resolution MR imaging at 3T. Atherosclerosis. 2009;204:447–52. doi: 10.1016/j.atherosclerosis.2008.10.019. doi:10.1016/j.atherosclerosis.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 14.Klein IF, Lavallée PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: A high-resolution MRI study. Stroke. 2010;41:1405–9. doi: 10.1161/STROKEAHA.110.583534. doi:10.1159/000346577. [DOI] [PubMed] [Google Scholar]
- 15.Ma N, Lou X, Zhao TQ, Wong EH, Jiang WJ. Intraobserver and interobserver variability for measuring the wall area of the basilar artery at the level of the trigeminal ganglion on high-resolution MR images. AJNR Am J Neuroradiol. 2011;32:E29–32. doi: 10.3174/ajnr.A2049. doi:10.3174/ajnr.A2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turan TN, Rumboldt Z, Brown TC. High-resolution MRI of basilar atherosclerosis: Three-dimensional acquisition and FLAIR sequences. Brain Behav. 2012;3:1–3. doi: 10.1002/brb3.103. doi:10.1002/brb3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung GH, Kwak HS, Hwang SB, Jin TC. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur J Radiol. 2012;81:4069–74. doi: 10.1016/j.ejrad.2012.07.001. doi:10.1016/j.ejrad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Kim YS, Lim SH, Oh KW, Kim JY, Koh SH, Kim J, et al. The advantage of high-resolution MRI in evaluating basilar plaques: A comparison study with MRA. Atherosclerosis. 2012;224:411–6. doi: 10.1016/j.atherosclerosis.2012.07.037. doi:10.1016/j.atherosclerosis.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Yang Q, Xie G, Pang J, Fan Z, Li D. Improved black-blood imaging using DANTE-SPACE for simultaneous carotid and intracranial vessel wall evaluation. Magn Reson Med. 2015 doi: 10.1002/mrm.25785. Epub ahead of print. doi: 10.1002/mrm.25785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim TH, Choi JW, Roh HG, Moon WJ, Moon SG, Chun YI, et al. Atherosclerotic arterial wall change of non-stenotic intracracranial arteries on high-resolution MRI at 3.0T: Correlation with cerebrovascular risk factors and white matter hyperintensity. Clin Neurol Neurosurg. 2014;126:1–6. doi: 10.1016/j.clineuro.2014.08.010. doi:10.1016/j.clineuro.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Ryu CW, Kwak HS, Jahng GH, Lee TC. High-resolution MRI of intracranial atherosclerotic disease. Neurointervention. 2014;9:9–20. doi: 10.5469/neuroint.2014.9.1.9. doi:10.5469/neuroint.2014.9.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natori T, Sasaki M, Miyoshi M, Ohba H, Katsura N, Yamaguchi M, et al. Evaluating middle cerebral artery atherosclerotic lesions in acute ischemic stroke using magnetic resonance T1-weighted 3-dimensional vessel wall imaging. J Stroke Cerebrovasc Dis. 2014;23:706–11. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.025. doi:10.1016/j.jstrokecerebrovasdis.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 23.Yuan M, Liu ZQ, Wang ZQ, Li B, Xu LJ, Xiao XL. High-resolution MR imaging of the arterial wall in moyamoya disease. Neurosci Lett. 2015;584:77–82. doi: 10.1016/j.neulet.2014.10.021. doi:10.1016/j.neulet.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Zhu XJ, Wang W, Du B, Liu L, He XX, Hu LB, et al. Wall imaging for unilateral intracranial vertebral artery hypoplasia with three-dimensional high-isotropic resolution magnetic resonance images. Chin Med J. 2015;128:1601–6. doi: 10.4103/0366-6999.158314. doi:10.4103/0366-6999.158314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Kolk AG, Zwanenburg JJ, Brundel M, Biessels GJ, Visser F, Luijten PR, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke. 2011;42:2478–84. doi: 10.1161/STROKEAHA.111.620443. doi:10.1161/strokeaha.111.620443. [DOI] [PubMed] [Google Scholar]
- 26.Qiao Y, Steinman DA, Qin Q, Etesami M, Schär M, Astor BC, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging. 2011;34:22–30. doi: 10.1002/jmri.22592. doi:10.1002/jmri.22592. [DOI] [PubMed] [Google Scholar]
- 27.Lou X, Ma N, Shen H, Shi K, Jiang W, Ma L. Noninvasive visualization of the basilar artery wall and branch Ostia with high-resolution three-dimensional black-blood sequence at 3 tesla. J Magn Reson Imaging. 2013;39:911–6. doi: 10.1002/jmri.24222. doi:10.1002/jmri.24222. [DOI] [PubMed] [Google Scholar]
- 28.van der Kolk AG, Hendrikse J, Brundel M, Biessels GJ, Smit EJ, Visser F, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol. 2013;23:2996–3004. doi: 10.1007/s00330-013-2905-z. doi:10.1007/s00330-013-2905-z. [DOI] [PubMed] [Google Scholar]
- 29.Mugler JP., 3rd Optimized three-dimensional fast-spin-echo MRI. J Magn Reson Imaging. 2014;39:745–67. doi: 10.1002/jmri.24542. doi:10.1002/jmri.24542. [DOI] [PubMed] [Google Scholar]
- 30.Ryu CW, Jahng GH, Kim EJ, Choi WS, Yang TC. High resolution wall and lumen MRI of the middle cerebral arteries at 3 tesla. Cerebrovasc Dis. 2009;27:433–42. doi: 10.1159/000209238. doi:10.1159/000209238. [DOI] [PubMed] [Google Scholar]
- 31.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. 2010;212:507–11. doi: 10.1016/j.atherosclerosis.2010.06.035. doi:10.1016/j.atherosclerosis.2010.06.035. [DOI] [PubMed] [Google Scholar]
- 32.Zhu XJ, Du B, Lou X, Hui FK, Ma L, Zheng BW, et al. Morphologic characteristics of atherosclerotic middle cerebral arteries on 3T high-resolution MRI. AJNR Am J Neuroradiol. 2013;34:1717–22. doi: 10.3174/ajnr.A3573. doi:10.3174/ajnr.A3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, et al. Arterial remodeling of advanced basilar atherosclerosis: A 3-tesla MRI study. Neurology. 2010;75:253–8. doi: 10.1212/WNL.0b013e3181e8e714. doi:10.1212/WNL.0b013e3181e8e714. [DOI] [PubMed] [Google Scholar]
- 34.Zhu XJ, Jiang WJ, Liu L, Hu LB, Wang W, Liu TC. Plaques of nonstenotic basilar arteries with isolated pontine infarction on three-dimensional high isotropic resolution magnetic resonance imaging. Chin Med J. 2015;128:1433–7. doi: 10.4103/0366-6999.157633. doi:10.4103/0366-6999.157633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke. 2011;42:2957–9. doi: 10.1161/STROKEAHA.111.618132. doi:10.1161/STROKEAHA.111.618132. [DOI] [PubMed] [Google Scholar]
- 36.Jiang WJ, Yu W, Ma N, Du B, Lou X, Rasmussen TC. High resolution MRI guided endovascular intervention of basilar artery disease. J Neurointerv Surg. 2011;3:375–8. doi: 10.1136/jnis.2010.004291. doi:10.1136/jnis.2010.004291. [DOI] [PubMed] [Google Scholar]
- 37.Feng C, Xu Y, Bai X, Hua T, Li Q, Tang GY, et al. Basilar artery atherosclerosis and hypertensive small vessel disease in isolated pontine infarctions: A study based on high-resolution MRI. Eur Neurol. 2013;70:16–21. doi: 10.1159/000346577. doi:10.1159/000346577. [DOI] [PubMed] [Google Scholar]
- 38.Chung JW, Kim BJ, Sohn CH, Yoon BW, Lee TC. Branch atheromatous plaque: A major cause of lacunar infarction (high-resolution MRI study) Cerebrovasc Dis Extra. 2012;2:36–44. doi: 10.1159/000341399. doi:10.1159/000346577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang B, Yang WQ, Liu XT, Liu HJ, Li PJ, Lu HK. Basilar artery atherosclerotic plaques distribution in symptomatic patients: A 3.0T high-resolution MRI study. Eur J Radiol. 2013;82:e199–203. doi: 10.1016/j.ejrad.2012.10.031. doi:10.1016/j.ejrad.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Miyaji Y, Kawabata Y, Joki H, Seki S, Mori K, Kamide T, et al. High-resolution magnetic resonance imaging findings of basilar artery plaque in a patient with branch atheromatous disease: A case report. J Med Case Rep. 2014;8:395. doi: 10.1186/1752-1947-8-395. doi:10.1186/1752-1947-8-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao DL, Deng G, Xie B, Ju S, Yang M, Chen XH, et al. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci. 2015;22:700–4. doi: 10.1016/j.jocn.2014.10.018. doi:10.1016/j.jocn.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Sui B, Gao P, Lin Y, Jing L, Qin H. Distribution and features of middle cerebral artery atherosclerotic plaques in symptomatic patients: A 3.0T high-resolution MRI study. Neurol Res. 2015;37:391–6. doi: 10.1179/1743132815Y.0000000023. doi:10.1179/1743132815y.0000000023. [DOI] [PubMed] [Google Scholar]
- 43.Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz TC. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging. 2011;21:e159–61. doi: 10.1111/j.1552-6569.2009.00442.x. doi:10.1111/j.1552-6569.2009.00442.x. [DOI] [PubMed] [Google Scholar]
- 44.Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, et al. Middle cerebral artery intraplaque hemorrhage: Prevalence and clinical relevance. Ann Neurol. 2012;71:195–8. doi: 10.1002/ana.22626. doi:10.1002/ana.22626. [DOI] [PubMed] [Google Scholar]
- 45.Yu JH, Kwak HS, Chung GH, Hwang SB, Park MS, Park TC. Association of intraplaque hemorrhage and acute infarction in patients with basilar artery plaque. Stroke. 2015;46:2768–72. doi: 10.1161/STROKEAHA.115.009412. doi:10.1161/strokeaha.115.009412. [DOI] [PubMed] [Google Scholar]
- 46.Aoki S, Shirouzu I, Sasaki Y, Okubo T, Hayashi N, Machida T, et al. Enhancement of the intracranial arterial wall at MR imaging: Relationship to cerebral atherosclerosis. Radiology. 1995;194:477–81. doi: 10.1148/radiology.194.2.7824729. doi:10.1148/radiology.194.2.7824729. [DOI] [PubMed] [Google Scholar]
- 47.Swartz RH, Bhuta SS, Farb RI, Agid R, Willinsky RA, Terbrugge KG, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–34. doi: 10.1212/01.wnl.0000342470.69739.b3. doi:10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 48.Vergouwen MD, Silver FL, Mandell DM, Mikulis DJ, Krings T, Swartz TC. Fibrous cap enhancement in symptomatic atherosclerotic basilar artery stenosis. Arch Neurol. 2011;68:676. doi: 10.1001/archneurol.2011.89. doi:10.1001/archneurol.2011.89. [DOI] [PubMed] [Google Scholar]
- 49.Kim JM, Jung KH, Sohn CH, Moon J, Han MH, Roh TC. Middle cerebral artery plaque and prediction of the infarction pattern. Arch Neurol. 2012;69:1470–5. doi: 10.1001/archneurol.2012.1018. doi:10.1001/archneurol.2012.1018. [DOI] [PubMed] [Google Scholar]
- 50.Vakil P, Vranic J, Hurley MC, Bernstein RA, Korutz AW, Habib A, et al. T1 gadolinium enhancement of intracranial atherosclerotic plaques associated with symptomatic ischemic presentations. AJNR Am J Neuroradiol. 2013;34:2252–8. doi: 10.3174/ajnr.A3606. doi:10.3174/ajnr.a3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013;34:299–304. doi: 10.3174/ajnr.A3209. doi:10.3174/ajnr.a3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lou X, Ma N, Ma L, Jiang WJ. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol. 2013;34:513–7. doi: 10.3174/ajnr.A3241. doi:10.3174/ajnr.a3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryu CW, Jahng GH, Shin TC. Gadolinium enhancement of atherosclerotic plaque in the middle cerebral artery: Relation to symptoms and degree of stenosis. AJNR Am J Neuroradiol. 2014;35:2306–10. doi: 10.3174/ajnr.A4038. doi:10.3174/ajnr.a4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. 2014;271:534–42. doi: 10.1148/radiol.13122812. doi:10.1148/radiol.13122812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Power S, Matouk C, Casaubon LK, Silver FL, Krings T, Mikulis DJ, et al. Vessel wall magnetic resonance imaging in acute ischemic stroke: Effects of embolism and mechanical thrombectomy on the arterial wall. Stroke. 2014;45:2330–4. doi: 10.1161/STROKEAHA.114.005618. doi:10.1161/strokeaha.114.005618. [DOI] [PubMed] [Google Scholar]
- 56.Dieleman N, van der Kolk AG, van Veluw SJ, Frijns CJ, Harteveld AA, Luijten PR, et al. Patterns of intracranial vessel wall changes in relation to ischemic infarcts. Neurology. 2014;83:1316–20. doi: 10.1212/WNL.0000000000000868. doi:10.1212/wnl.0000000000000868. [DOI] [PubMed] [Google Scholar]
- 57.Chung JW, Kim BJ, Choi BS, Sohn CH, Bae HJ, Yoon BW, et al. High-resolution magnetic resonance imaging reveals hidden etiologies of symptomatic vertebral arterial lesions. J Stroke Cerebrovasc Dis. 2014;23:293–302. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.021. doi:10.1016/j.jstrokecerebrovasdis.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 58.Aoki S, Hayashi N, Abe O, Shirouzu I, Ishigame K, Okubo T, et al. Radiation-induced arteritis: Thickened wall with prominent enhancement on cranial MR images report of five cases and comparison with 18 cases of Moyamoya disease. Radiology. 2002;223:683–8. doi: 10.1148/radiol.2233010822. doi:10.1148/radiol.2233010822. [DOI] [PubMed] [Google Scholar]
- 59.Saam T, Habs M, Pollatos O, Cyran C, Pfefferkorn T, Dichgans M, et al. High-resolution black-blood contrast-enhanced T1 weighted images for the diagnosis and follow-up of intracranial arteritis. Br J Radiol. 2010;83:e182–4. doi: 10.1259/bjr/74101656. doi:10.1259/bjr/74101656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pfefferkorn T, Linn J, Habs M, Opherk C, Cyran C, Ottomeyer C, et al. Black blood MRI in suspected large artery primary angiitis of the central nervous system. J Neuroimaging. 2012;23:379–83. doi: 10.1111/j.1552-6569.2012.00743.x. doi:10.1111/j.1552-6569.2012.00743.x. [DOI] [PubMed] [Google Scholar]
- 61.Mossa-Basha M, Hwang WD, De Havenon A, Hippe D, Balu N, Becker KJ, et al. Multicontrast high-resolution vessel wall magnetic resonance imaging and its value in differentiating intracranial vasculopathic processes. Stroke. 2015;46:1567–73. doi: 10.1161/STROKEAHA.115.009037. doi:10.1161/strokeaha.115.009037. [DOI] [PubMed] [Google Scholar]
- 62.Mandell DM, Matouk CC, Farb RI, Krings T, Agid R, terBrugge K, et al. Vessel wall MRI to differentiate between reversible cerebral vasoconstriction syndrome and central nervous system vasculitis: Preliminary results. Stroke. 2012;43:860–2. doi: 10.1161/STROKEAHA.111.626184. doi:10.1161/strokeaha.111.626184. [DOI] [PubMed] [Google Scholar]
- 63.Obusez EC, Hui F, Hajj-Ali RA, Cerejo R, Calabrese LH, Hammad T, et al. High-resolution MRI vessel wall imaging: Spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol. 2014;35:1527–32. doi: 10.3174/ajnr.A3909. doi:10.3174/ajnr.a3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang Y, Lou X, Li Y, Sui B, Sun S, Li C, et al. Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: From the interventional neuroradiologists'view. Acta Neurochir (Wien) 2014;156:515–25. doi: 10.1007/s00701-013-1989-1. doi:10.1007/s00701-013-1989-1. [DOI] [PubMed] [Google Scholar]
- 65.Han M, Rim NJ, Lee JS, Kim SY, Choi JW. Feasibility of high-resolution MR imaging for the diagnosis of intracranial vertebrobasilar artery dissection. Eur Radiol. 2014;24:3017–24. doi: 10.1007/s00330-014-3296-5. doi:10.1007/s00330-014-3296-5. [DOI] [PubMed] [Google Scholar]
- 66.Takano K, Yamashita S, Takemoto K, Inoue T, Kuwabara Y, Yoshimitsu K. MRI of intracranial vertebral artery dissection: Evaluation of intramural haematoma using a black blood, variable-flip-angle 3D turbo spin-echo sequence. Neuroradiology. 2013;55:845–51. doi: 10.1007/s00234-013-1183-4. doi:10.1007/s00234-013-1183-4. [DOI] [PubMed] [Google Scholar]
- 67.Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: A high-resolution magnetic resonance imaging study. Stroke. 2015;46:697–703. doi: 10.1161/STROKEAHA.114.008181. doi:10.1161/strokeaha.114.008181. [DOI] [PubMed] [Google Scholar]
- 68.Hui FK, Zhu X, Jones SE, Uchino K, Bullen JA, Hussain MS, et al. Early experience in high-resolution MRI for large vessel occlusions. J Neurointerv Surg. 2015;7:509–16. doi: 10.1136/neurintsurg-2014-011142. doi:10.1136/neurintsurg-2014-011142. [DOI] [PubMed] [Google Scholar]
- 69.Kim SM, Ryu CW, Jahng GH, Kim EJ, Choi TC. Two different morphologies of chronic unilateral middle cerebral artery occlusion: Evaluation using high-resolution MRI. J Neuroimaging. 2013;24:460–6. doi: 10.1111/jon.12009. doi:10.1111/jon.12009. [DOI] [PubMed] [Google Scholar]
- 70.Kim JM, Jung KH, Sohn CH, Park J, Moon J, Han MH, et al. High-resolution MR technique can distinguish moyamoya disease from atherosclerotic occlusion. Neurology. 2013;80:775–6. doi: 10.1212/WNL.0b013e3182825162. doi:10.1212/wnl.0b013e3182825162. [DOI] [PubMed] [Google Scholar]
- 71.Ryoo S, Cha J, Kim SJ, Choi JW, Ki CS, Kim KH, et al. High-resolution magnetic resonance wall imaging findings of Moyamoya disease. Stroke. 2014;45:2457–60. doi: 10.1161/STROKEAHA.114.004761. doi:10.1161/strokeaha.114.004761. [DOI] [PubMed] [Google Scholar]
- 72.Saam T, Hatsukami TS, Takaya N, Chu B, Underhill H, Kerwin WS, et al. The vulnerable, or high-risk, atherosclerotic plaque: Noninvasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. doi:10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 73.Zhao X, Underhill HR, Zhao Q, Cai J, Li F, Oikawa M, et al. Discriminating carotid atherosclerotic lesion severity by luminal stenosis and plaque burden: A comparison utilizing high-resolution magnetic resonance imaging at 3.0 Tesla. Stroke. 2011;42:347–53. doi: 10.1161/STROKEAHA.110.597328. doi:10.1161/strokeaha.110.597328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–5. doi: 10.1056/NEJM198705283162204. doi:10.1056/nejm19∁283162204. [DOI] [PubMed] [Google Scholar]
- 75.Wexberg P, Gyöngyösi M, Sperker W, Kiss K, Yang P, Hassan A, et al. Pre-existing arterial remodeling is associated with in-hospital and late adverse cardiac events after coronary interventions in patients with stable angina pectoris. J Am Coll Cardiol. 2000;36:1860–9. doi: 10.1016/s0735-1097(00)00949-9. doi:10.1016/s0735-1097(00)00949-9. [DOI] [PubMed] [Google Scholar]
- 76.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. doi:10.1056/nejmx120039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: The VISSIT randomized clinical trial. JAMA. 2015;313:1240–9. doi: 10.1001/jama.2015.1693. doi:10.1001/jama.2015.1693. [DOI] [PubMed] [Google Scholar]
- 78.Varnava AM, Mills PG, Davies TC. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. 2002;105:939–43. doi: 10.1161/hc0802.104327. doi:10.1161/hc0802.104327. [DOI] [PubMed] [Google Scholar]
- 79.Park JJ, Chun EJ, Cho YS, Oh IY, Yoon CH, Suh JW, et al. Potential predictors of side-branch occlusion in bifurcation lesions after percutaneous coronary intervention: A coronary CT angiography study. Radiology. 2014;271:711–20. doi: 10.1148/radiol.14131959. doi:10.1148/radiol.14131959. [DOI] [PubMed] [Google Scholar]
- 80.Calabrese LH, Dodick DW, Schwedt TJ, Singhal TC. Narrative review: Reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34–44. doi: 10.7326/0003-4819-146-1-200701020-00007. doi:10.7326/0003-4819-146-1-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 81.Debette S, Compter A, Labeyrie MA, Uyttenboogaart M, Metso TM, Majersik JJ, et al. Epidemiology, pathophysiology, diagnosis, and management of intracranial artery dissection. Lancet Neurol. 2015;14:640–54. doi: 10.1016/S1474-4422(15)00009-5. doi:10.1016/s1474-4422(15)00009-5. [DOI] [PubMed] [Google Scholar]
- 82.Scott RM, Smith TC. Moyamoya disease and Moyamoya syndrome. N Engl J Med. 2009;360:1226–37. doi: 10.1056/NEJMra0804622. doi:10.1056/nejmra0804622. [DOI] [PubMed] [Google Scholar]
- 83.Adams HP., Jr Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. doi:10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]


